Abstract
Objectives
Hepacare® is a widely marketed herbal formulation in Nigeria for treating chronic liver ailments. This study evaluated the safety, as well as pro-inflammatory and genotoxicity effects, of Hepacare® in mice.
Methods
The effect of the formulation was estimated in a 28-day study where 25 mice were divided into five groups, and Hepacare® was orally administered at 250, 500, 750 and 2500 mg/kg body weight. The biochemical and haematological parameters were determined, organ weights were estimated and histopathology was also conducted. mRNA expression of the pro-inflammatory cytokines, TNF-α and IL-6 was estimated by RT-PCR in acute toxicity experiments.
Results
The LD50 was calculated at 3807.89 mg/kg body weight in mice. There was a significant increase (p < 0.05) in the ALP activity in the 750 mg/kg treated group, while the 2500 mg/kg group exhibited significant increases in their AST, ALT, ALP, total bilirubin and total protein levels compared with the control group. However, there was a significant dose related increase in monocytes counts in the groups treated with 750 and 2500 mg/kg. There was no significant difference (p > 0.05) in TNF-α and IL-6 mRNA expression in the genotoxicity studies in all of the treatment groups compared with the control. However, several hepatic and nephro-pathological derangements were observed in the groups treated with higher doses of the formulation.
Conclusions
The study established that the herbal formulation may not induce significant pro-inflammatory toxic responses and genotoxic effects, but prolonged intake of higher doses may cause severe biochemical and clinical abnormalities.
Keywords: Genotoxicity, Hepacare, Herbal formulation, Pro-inflammation, Toxicity studies
الملخص
أهداف البحث
إن ”هيباكير“ مستحضر عشبي واسع التسويق في نيجيريا لعلاج أمراض الكبد المزمنة. قَيَّمت هذه الدراسة سلامة استخدام ”هيباكير“ بالإضافة إلى آثاره المحفزة للإلتهاب وسُميتة الوراثية في الفيران.
طرق البحث
قُدِّر تأثير المستحضر في دراسة دامت ٢٨ يوما تم فيها تقسيم ٢٥ فأرا إلى خمس مجموعات وأُعطي ”هيباكير“ عن طريق الفم بتركيز ٢٥٠ و٥٠٠ و٧٥٠ و٢٥٠٠ مجم/كجم من وزن الفأر. حُددت المعلمات الكيميائية الحيوية والدموية وقُدرت أوزان الأعضاء، كما أُجريت التحاليل النسيجية. تم تقدير تعبير الحمض النووي الريبي المرسال بالنسبة للسيتوكاينات المحفزة للإلتهاب وعامل نخر الورم- ألفا وإنترلوكن- 6 باستخدام تفاعل البلمرة المتسلسل بالنسخ العكسي في تجربة التسمم الحاد.
النتائج
تم احتساب الجرعة المميتة للنصف عند ٣٨٠٧.٨٩ مجم/كجم من وزن الفأر. كانت هناك زيادة ذات قيمة في نشاط الفوسفاتيز القلوي في المجموعة التي أُعطيت ٧٥٠ مجم/كجم في حين أن المجموعة التي أُعطيت ٢٥٠٠ مجم/كجم أظهرت زيادة ذات قيمة في مستويات كل من ناقلة أسبارتات وناقلة ألانين والفوسفاتيز القلوي والبيليروبين الكلي والبروتين الكلي، مقارنة مع مجموعة التحكم. ولكن كانت هناك زيادة ذات قيمة ومرتبطة بالجرعة في تعداد الخلايا البيضاء ذات النواة أحادية الفص في المجموعات التي أُعطيت ٧٥٠ و٢٥٠٠ مجم/كجم. لم يكن هناك فرق ذا قيمة في تعبير الحمض النووي الريبي المرسال بالنسبة لكل من عامل نخر الورم -ألفا وإنترلوكن -6 والسُمية الوراثية في جميع المجموعات العلاجية مقارنة مع مجموعة التحكم. إلا أنه تمت ملاحظة بعض الاضطرابات الكبدية والكلوية في المجموعات المُعطاة جرعات أعلى من المستحضر.
الاستنتاجات
أكدت الدراسة بأن المستحضر العشبي قد لا يُنتج ردود فعل سامة محفزة للإلتهاب وآثارا سمية وراثية ذات قيمة، إلا أن تناول جرعات أعلى لفترات طويلة قد يسبب اضطرابات كيميائية حيوية وسريرية شديدة.
الكلمات المفتاحية: هيباكير, مستحضر عشبي, محفز للإلتهاب, سمية وراثية, دراسات السمية
Introduction
Plants, extracts and their formulations have been used for centuries in therapy for various ailments and conditions. Plants contain phytochemical and secondary metabolites, which form the active compounds responsible for their therapeutic property. Despite these plants and extracts' derived benefits, inappropriate use of medicinal plants or drugs from herbal origins may lead to an overdose of these plant products, which contain these phytochemicals, a number of which may cause toxic reactions to animals or even human beings.1, 2 However, most herbal medicines lack proper documentation for their potential toxicity and they rarely require a professional to prescribe their use.3 The reason why plant drugs are seemingly without restriction for use and do not undergo the rigours of testing that other drug classes undergo is because plants fall under the food class4 and they are mostly marketed as supplements.5 Fortunately, plants, especially those without immediate, observable harm after consumption, rarely ever have side effects.3, 6
There are numerous traditional plant formulations available for the treatment of various diseases. A botanical market review revealed that remedies from natural origins account for a $1.8 billion market in the United States. For example, a single herbal preparation, Silymarin, used almost exclusively for liver diseases, accounted for $180 million in Germany alone.7 Approximately 600 commercial herbal formulations with claimed hepatoprotective activities are being sold all over the world. From these mixtures, more than 170 phytoconstituents isolated from 110 plants belonging to 55 families have been reported and ascertained to possess hepatoprotective activity. Some of these herbal preparations exist as standardized extracts with major known ingredients or even pure compounds that are being evaluated.7 However, only a small proportion of hepatoprotective plants as well as other formulation classes used in traditional medicine are pharmacologically evaluated for their safety and efficacy.8 Hepacare® is a formulation prepared using a blend of indigenous African herbal ingredients. These include Olax subscorpioidea and Capsicum frutescens. Although used as a supplement, it is also used as an alternative medical approach to treat some liver disorders, such as hepatitis and jaundice. Although there is recent scientific evidence to support the hepatoprotective properties of this herbal formulation, a proper toxicological assessment for the drug has not been performed to date.9
Recently, the Organization for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals called for molecular toxicological assessments to be made on substances of exposure in addition to other mainstream toxicological procedures.10 This motivated us to expand our research to include inflammation and genotoxicity studies that could complement other indicators of toxicity studies such as, biochemical, haematological and histopathological assessments. Proper toxicological evaluation of safety of herbal formulation use is necessary to establish the benefits of the use of herbal formulations, which forms the primary motivation of this study.
Materials and Methods
Chemicals
Drugs and chemicals, including distilled water (DW), formalin, diethyl ether, agarose, low melting point (LMP) agarose, boric acid, sodium chloride (NaCl), ethylenediaminetetraacetic acid (EDTA), trizma base, Triton-x, sodium hydroxide (NaOH), hydrochloric acid (HCl) and ethidium bromide were purchased from Sigma Aldrich, St. Louis MO, USA. Cyclophosphamide (Cytoxan) was purchased from a Health Plus Pharmacy in Lagos, Nigeria.
Hepacare® sample preparation
Hepacare® herbal formulation capsules were purchased from a pharmaceutical shop in Lagos, Nigeria. The content of the capsule was ground, dissolved in DW and adjusted to 5 ml/kg body weight for mice to minimize administration trauma in experimental animals.
Source of animals and housing
Fifty (50) healthy male adult mice weighing between 18 and 22 g were purchased from the University of Agriculture, Abeokuta, Ogun State, Nigeria. The mice were spaced and securely caged and maintained to acclimatize at room temperature between 22 and 24 °C with a 12 h light/dark cycle in a well-ventilated, pathogen-free animal facility for seven days before the commencement of the experiment. Mice were given feed and water ad libitum during the acclimatization period and for the duration of the experiment daily. After the experiment, the animals were euthanized. Ethical approval for the use of animals was obtained from the Department of Biological Sciences Research Ethics Committee of Covenant University. All procedure and handling were performed in compliance with the National Institutes for Health (NIH) Guide for the Care and Use of Laboratory Animals (1984).
Acute toxicity evaluation of herbal formulation
The method as described by Lorke11 was used in the determination of the median lethal dose (LD50) of the herbal formulation in mice with several modifications. A total of 18 mice were used for this experiment. The experiment for the determination of the LD50 of the herbal formulation was divided into two phases. In the first phase, the mice were weighed and randomly divided into four groups. Each group contains three mice. Three groups received herbal drug (by gavage) at doses of 10, 100 and 1000 mg/kg. The control group was treated only with distilled water, which was also orally administered. In the second phase of the experiment, three groups of three mice per group were weighed and administered with doses of 1600, 2900 and 5000 mg/kg, respectively. In both phases, the mice were observed for 72 h for delayed mortality, any abnormal behaviour and other toxic signs. The LD50 was calculated using a method reported by Lorke11 as the geometric mean between the minimal lethal dose and the maximal sub-lethal dose. At the end of the experiment, the surviving animals were euthanized according to ethical prescriptions.
Acute pro-inflammatory evaluation
The mRNA from liver tissue obtained from mice was used for experiments, as described in the RNeasy Mini Kit protocol (Qiagen Inc., Valencia, CA). The kit technology combined the selective binding properties of a silica-gel-based membrane with microspin technology and consists of a high-salt buffer system that permits RNA of up to 200 bases to bind to a silica-gel membrane. The total RNA binds to the membrane and contaminants are effectively separated. The procedure favours enrichment for mRNA, as most RNAs less than 200 nucleotides (such as smaller rRNA, and tRNAs) are selectively excluded. Liver samples from each group (control, 250, 500, and 2500 mg/kg) were lysed and homogenized in the presence of a highly denaturing guanidine thiocyanate-containing buffer, which was used to inactivate RNases to ensure purification of intact RNA. Ethanol was added to provide appropriate binding conditions. The RNA concentration contained in 2 μl was determined using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE) and the volume of each sample was normalized to 1 μg of RNA per PCR combination. Primers of Mouse tumour necrosis factor α (TNF-α, ID No., X02611), Mouse interleukin 6 (IL-6, ID No., NM_031168) and Mouse GAPDH (ID No., M32599) were purchased from Qiagen (Valencia, CA). Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was carried out using the Transgen Reverse Transcriptase one-step PCR kit (Transgen Biotech Co, Beijing China) according to the manufacturer's manual, using a Bio-rad C1000 Touch™ Thermal Cycler (Bio-rad Laboratories, Hercules, CA, USA). GAPDH, TNF-α and IL-6 mRNA expression levels and quantification were performed with the ImageJ (version 1.48) software (National Institutes of Health, WI, USA) as described in Schneider et al.12
Sub-chronic evaluation of the herbal formulation
Twenty-five male mice were randomly distributed into five groups with five animals each. The herbal formulation was orally administered daily for 28 days in single doses of 250, 500, 750, and 2500 mg/kg. The control mice received only vehicle (DW) and were fed with food and water. Signs of toxicity and mortality were also recorded daily throughout the study period. At the end of the experiment, all mice were anesthetized in diethyl ether, and blood samples were collected via cardiac puncture into non-heparinized and EDTA-containing tubes for biochemical and haematological analysis, respectively. The liver and kidneys were excised and stored in 10% formalin for histopathology examination.13
Blood sample analysis
Serum was prepared by separating it from whole blood and centrifuged at 2500 rpm for 10 min. Biochemical indices, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein and bilirubin, were determined using an automated Tc Matrix Tc-3100 Analyser (Teco Diagnostics, CA, United States). Haematological indices, including packed cell volume (PCV), total white blood cell (WBC), neutrophil, lymphocyte and monocyte counts were also quantified using a Sysmex 8000i Automated Hematology Analyzer (Sysmex Corporation, Kobe, HYG, Japan).
Histopathological examinations
Histological examinations were performed at the University College Hospital, Ibadan, Nigeria by a licensed pathologist who was not informed of details of the treatment groups to eliminate bias. Kidney and liver tissues were embedded in paraffin, and cut into 5 μm thick sections using a rotary microtome (RM2125, Leica Microsystems Inc., Bannockburn, IL, USA). The sections were stained with haematoxylin-eosin dye and evaluated under a light microscope with 40× and 400× resolution. Photomicrographs of the tissue sections were taken and reported.13
Genotoxicity studies
Twelve male mice weighing between 20 and 25 g were used for the single-cell electrophoresis (comet) assay. The mice were divided into four groups containing three animals each. Two of the groups received a single dose of 100 and 1000 mg/kg b.w. of the formulation dissolved in DW. The control group received only DW. The fourth group was administered with reconstituted cyclophosphamide at 100 mg/kg body weight. The animals were anaesthetized in diethyl ether after 24 h of administration of the treatments. The femur was obtained for bone marrow and processed to obtained cell suspensions. The “alkaline” comet assay was performed as described by Tice et al.14 Slides were pre-coated on one side in 1% normal agarose (pre-heated to 100 °C) by dipping the slide in agarose and wiping one slide clean. The slides were left to dry for 24 h. Cells were suspended in 1% low melting point agarose (maintained alternatively in water baths of 30 and 70 °C) and were subsequently used to coat the previously coated slides with a micro pipette. After the agarose had solidified, the slides were placed in cell lysis buffer (100 mM EDTA; 2.5 M NaCl; 1% Triton-X100, 10 mM Trizma base; pH = 10) for 1 h, after which the slides were transferred to an alkaline buffer (1 mM EDTA; 300 mM NaOH; pH > 13) for the DNA unwinding process. The slides were then subjected to electrophoresis at 25 V and 300 mA for 20 min. The comets were visualized and captured with the 40× objective of an EVOS® FL Cell Imaging System. The extent of DNA damage was calculated by image analysis with the OpenComet plugin using the software ImageJ (version 1.48) software.
Statistical analysis
All results are reported as the means and standard error of the mean (mean ± SEM). Statistical evaluations of the data were initially tested by the homogeneity of variances. Comparisons between groups were performed using one way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests using the R statistical programming language (version 3.1.1). All comparisons were made relative to untreated controls (positive control in the case of the genotoxic assessment), and differences with a p value <0.05 were considered to be significant. Data were visualized in figures with Graph pad prism version 6.
Results
LD50 value
The median lethal dose (LD50) result for Hepacare is shown in Table 1. The LD50 value was estimated to be 3807.89 mg/kg b.w.
Table 1.
LD50 value estimation for Hepacare® herbal formulation in mice.
| Hepacare® | Intragastric dose (mg/kg) | ||
|---|---|---|---|
| Phase I | 10 | 100 | 1000 |
| Mortality | 0/3 | 0/3 | 0/3 |
| Phase II | 1600 | 2900 | 5000 |
| Mortality | 0/3 | 0/3 | 2/3 |
| LD50 | 3807.89 | ||
Acute pro-inflammation results
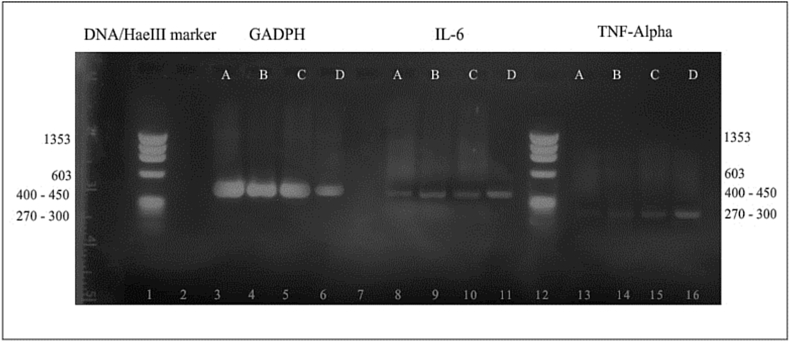
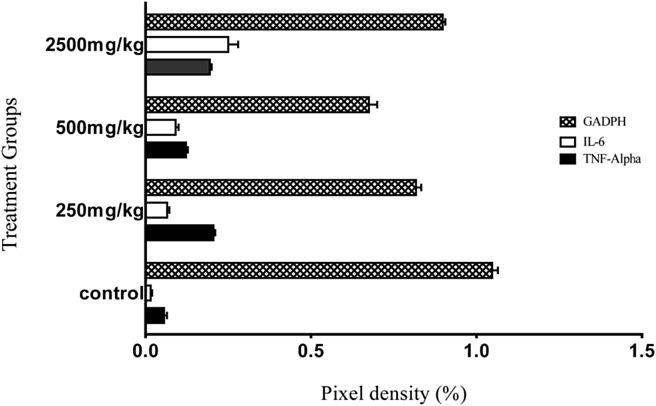
The acute effect of the herbal formulation on IL-6 and TNF-α mRNA expression levels is as shown in Figure 1, Figure 2. Expression of the quantification by pixel intensity measurements of the visualized lanes of stained mRNA after electrophoresis (Figure 1) showed that there was no significant (p > 0.05) difference between the mRNA expression of either TNF-α or IL-6 in the groups treated with 250 and 500 mg/kg dose of the drug in mice when compared with the group that received no treatment after 24 h of oral administration of the drug. We also noted a significant (p < 0.05) increase in the mRNA expression level in mouse liver homogenates of both IL-6 and TNF-α in the group treated with 2500 mg/kg b.w. compared with the negative control group. After normalization with a housekeeping gene (GADPH), which was used as a positive expression control, increased values were noted for both IL-6 and TNF-α mRNA expression in the 2500 mg/kg b.w. treated group; however, these increased levels were not statistically significant (p > 0.05) (Figure 2).
Figure 1.
Effects of Hepacare® on IL-6 and TNF-α gene expression against a DNA/HaeIII marker (72 bp–1353 bp) in bone marrow cells of mice following 24 h of treatment. Lanes A = control; B = 250 mg/kg; C = 500 mg/kg treatment; D = 2500 mg/kg treatment group.
Figure 2.
Band pixel density (%) after normalization with a housekeeping gene (GADPH) expressed as the mean ± SEM of 3 replicate measurements.
Sub-chronic toxicity studies
Physical and weight examination of organs
Our findings showed that Hepacare® did not significantly increase or decrease the relative heart, liver, kidney, spleen or lung weights (p > 0.05) after 28 days of administration in any of the tested doses when compared with the negative control group. There were also no noticeable visible pathological changes in the organs (Table 2).
Table 2.
Effects of Hepacare® on the relative organ weights of mice after 28-day sub-chronic treatment.
| Sample | Control | 250 mg/kg | 500 mg/kg | 750 mg/kg | 2500 mg/kg |
|---|---|---|---|---|---|
| Mean group weight | 22.45 ± 1.54 | 23.26 ± 2.87 | 21.34 ± 0.55 | 22.43 ± 2.87 | 21.26 ± 3.34 |
| Liver body/organ weight | 65.45 ± 1.87 | 63.04 ± 2.92 | 66.84 ± 3.87 | 65.48 ± 2.41 | 68.80 ± 2.50 |
| Kidney body/organ weight | 10.27 ± 0.32 | 9.65 ± 0.32 | 9.52 ± 0.45 | 9.78 ± 0.51 | 9.65 ± 0.14 |
| Spleen body/organ weight | 2.53 ± 0.10 | 2.45 ± 0.13 | 2.52 ± 0.12 | 2.54 ± 0.07 | 2.31 ± 0.20 |
| Heart body/organ weight | 5.53 ± 0.63 | 6.64 ± 0.65 | 6.52 ± 0.74 | 5.65 ± 0.54 | 6.43 ± 2.64 |
Values represent mean ± SEM of 5 replicates. p > 0.05.
Biochemical parameters
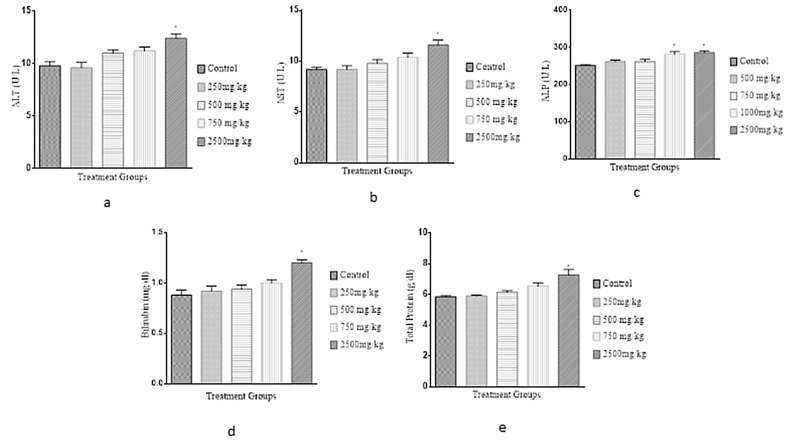
Our results, after 28 days of oral treatment of the herbal formulation in mice, are shown in Figure 3. We observed a significant increase (p < 0.05) in ALP activity in the 750 mg/kg bw treated group compared with the control group. There was also a significant increase in the ALT, AST, and ALP activity in the 2500 mg/kg treated group compared with the control group. However, there was no significant difference (p > 0.05) observed for ALT, AST and ALP in the 250 and 500 mg/kg treatment groups. For the non-enzymatic specific markers, there was a significant (p < 0.05) elevation of bilirubin and total proteins in the 2500 mg/kg b.w. treated group. There was no significant (p > 0.05) elevation in either of these parameters in the groups treated with doses lower than 2500 mg/kg b.w.
Figure 3.
Effect of Hepacare® on biochemical parameters in mice, including (a) alanine aminotransferases (ALT), (b) aspartate aminotransferases (AST), (c) alkaline phosphatase (ALP), (d) total bilirubin (Bilirubin) and (e) total protein. Values represent mean ± SEM of 5 replicates; *p < 0.05.
Haematological parameters
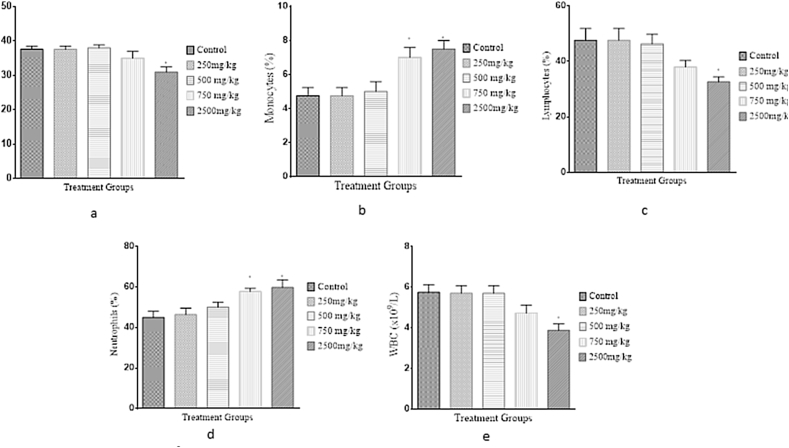
Findings from our haematological analysis of blood sampled from mice after the 28-day sub-chronic oral treatment with the herbal drug are presented in Figure 4. There was a significant increase (p < 0.05) in monocyte counts in the 750 and 2500 mg/kg treatment groups when compared with the control group. However, there was no significant difference (p > 0.05) observed between the treatment and control groups regarding the PCV, WBC, neutrophil and lymphocyte levels.
Figure 4.
Effect of Hepacare® on haematological parameters in mice, including (a) pack cell volume (PCV), (b) monocytes, (c) lymphocytes, (d) neutrophils and total white blood cells (WBC). Values represent the mean ± standard error of mean (SEM) of 5 replicates; *p < 0.05.
Histological liver and kidney tissue examinations
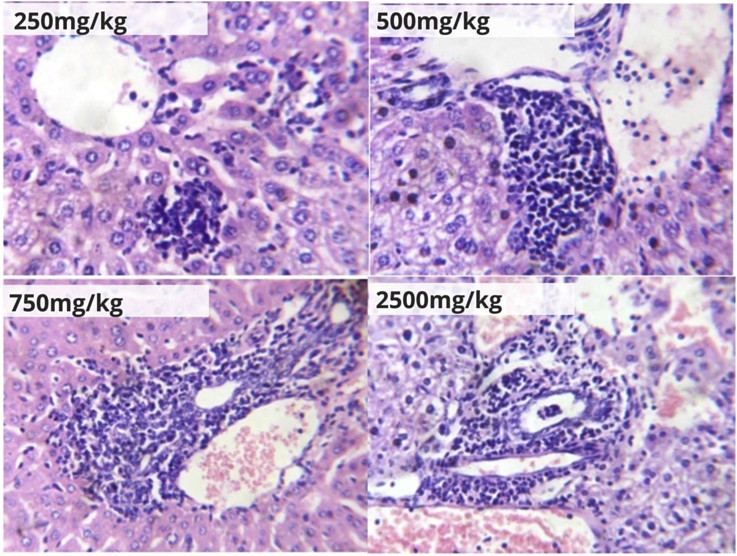
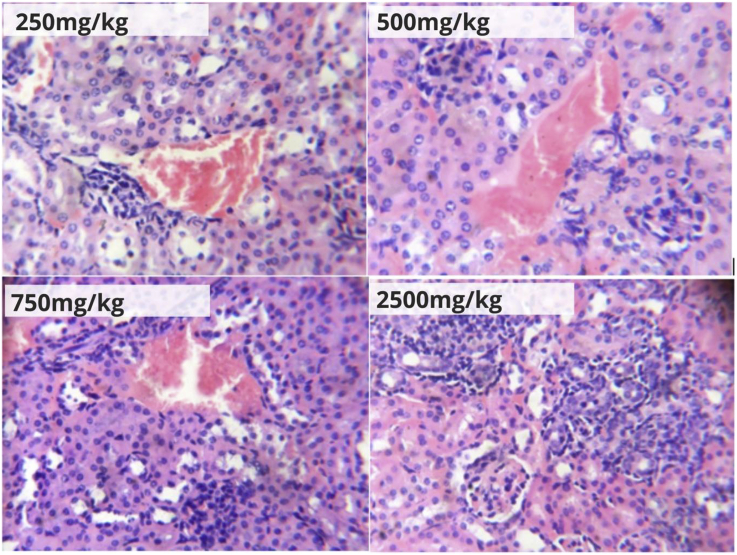
Photomicrographs of hepatic and renal tissues of mice after the 28-day treatment with 250, 500, 750 and 2500 mg/kg b.w. of the herbal formulation are shown in Figure 5, Figure 6. The observed effects of the formulation after this period included macrovesicular steatosis, which progressed from moderate (in the 250 and 500 mg/kg b.w. treated groups) to severe (in the 750 and 2500 mg/kg treated groups); congestion of blood vessels, which progressed from mild (in the 250 mg/kg b.w. treated group), marked (in the 500 mg/kg b.w. treated group), to severe (in the 750 and 2500 mg/kg b.w. treated groups); and periportal inflammation, which ranged from mild (in the 250 mg/kg b.w. treated group), moderate (in the 500 and 750 mg/kg b.w. treated groups) to severe (in the 2500 mg/kg b.w. treated group) (Figure 5). Renal histological examination in this study also showed similar patterns of progressive damage (Figure 6) with increasing concentrations of the herbal formulation in mice. This included mild (in the 250 and 500 mg/kg b.w. treated groups) to moderate (in the 750 and 2500 mg/kg b.w. treated groups) congestion, as well as mild (in the 250 and 500 mg/kg treated groups) to moderate (in the 750 mg/kg b.w. treated group) interstitial inflammation. Multiple zones of severe inflammation, granular tissue formation and mild blood congestion were seen in the renal tissue of the 2500 mg/kg treated group.
Figure 5.
Hepatic tissue photomicrographs (H&E ×400) of mice orally treated with Hepacare® at 250, 500, 750 and 2500 mg/kg b.w.
Figure 6.
Renal tissue photomicrographs (H&E ×400) of mice orally treated with Hepacare® at 250, 500, 750 and 2500 mg/kg body weight.
Genotoxicity studies
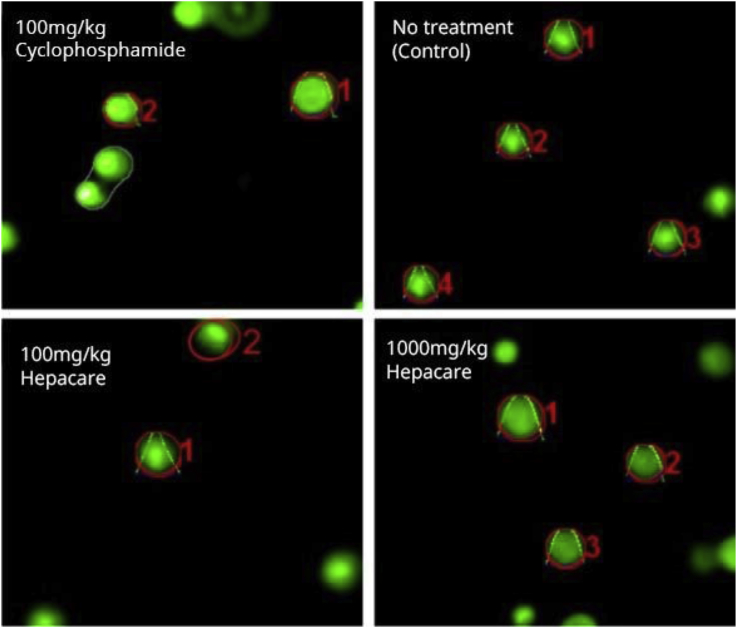
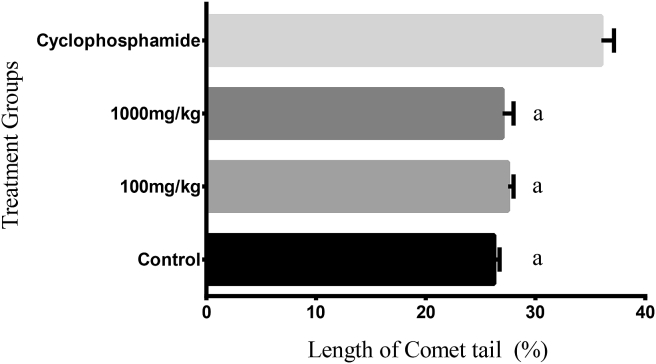
The results of our comet assay are shown in Figure 7, Figure 8. The lengths of the comet tails were quantified by pixel intensity measurements of photomicrographs of stained DNA after electrophoresis (Figure 7). Our results showed a significant difference (p < 0.05) in tail lengths between the positive control-group treated with cyclophosphamide (a known genotoxic agent) and all other treatment groups (Figure 8).
Figure 7.
Pictographs of stained DNA in bone marrow cells after single-cell gel electrophoresis analysed after treatment with Hepacare® in mice dosed with 100 or 1000 mg/kg compared with cyclophosphamide and a group receiving no treatment after 24 h in a single dose treatment experiment. Red markings show software capture of cells by OpenComet in ImageJ (NIH, USA).
Figure 8.
Comet tail lengths from a comets assay analysed with OpenComet in ImageJ (NIH, USA) showing the effect of Hepacare® in mice compared with cyclophosphamide and a group receiving no treatment after 24 h at single dose treatments. Values represent mean ± standard error of mean (SEM) of 5 replicates; ap < 0.05.
Discussion
Acute toxicity of Hepacare
Toxicological assessments are necessary tools used to evaluate the possible health risks in humans that may be caused by drug compounds or herbs.3, 15 Herbal medicines, which are often used without formal professional prescription, may occasionally result in abuse and overdose.3 The median lethal dose is an appropriate estimation of the safety of a chemical or drug that has been useful in providing information for consumer safety.16 From our results (Table 1), the LD50 of the herbal formulation places it within the category of substances with “low” oral toxicity in the OECD globally harmonized classification system.15 It is very unlikely that the herbal formulation would be harmful when taken at the regimen indicated on the packaging of the herbal formulation in which 1 capsule (250 mg) is expected to be taken in three separate doses daily.
Effect of Hepacare on pro-inflammatory markers for toxicity
Inflammation is a tightly regulated biological response to adverse stimuli. Acute exposure to chemical substances originating from plants can lead to acute cytokine-mediated inflammatory responses.17 TNF-α and IL-6 are two pro-inflammatory cytokines that are implicated in acute inflammation events that lead to tissue damage and organ failure, a direct response of the increase in mRNA expression of these cytokines during the onset of inflammation.18, 19 In our study, RT-PCR was used to assess the effects of the herbal formulation on the TNF-α and IL-6 mRNA expression levels in mouse liver tissue. Our results showed no significant (p > 0.05) increase in the mRNA expression of either TNF-α and IL-6 dosed with 250, 500 or 750 mg/kg b.w. after a 24 h period. However, there were some pro-inflammatory modulation activity changes in mice given the 2500 mg/kg treatment, but this increase was not significant (p > 0.05) when normalized with GADPH (Figure 2). In a similar study, C. frutescens was demonstrated to increase the IL-6 and TNF-α levels significantly.20, 21, 17 However, it was also shown in other investigations that capsaicin, which was isolated from C. frutescens, is a potent anti-inflammatory substance.22 This suggests that capsaicin may not be activated unless it is in a pure state. It is therefore possible that herb–herb interactions that have been described in previous investigations of other polyphenols present in the herbal mixture may activate capsaicin's anti-inflammatory properties, or other polyphenols and flavonoids with anti-inflammatory activities are present that mitigate the active principles that induce acute inflammation in the C. frutescens component of the herbal mixture.23
Sub-chronic toxicity of Hepacare in mice
This herbal formulation is currently sold and employed for the treatment of chronic liver diseases among others ailments, as discussed in Ishola et al.9 However, it has been suggested that long-term exposure of the herbal formulation can lead to chronic ailments in users.24 Therefore, a chronic toxicity study of the herbal formulation is also of necessary importance for the assessment of the drug's safety. Changes in the relative organ weights form one such assessment of organ weights affected by alterations in physiology that is caused by toxic chronic events.25 There were no significant (p > 0.05) changes in the relative heart, liver, kidney, spleen and lung tissue weights following macroscopic examination post mortem of the mice after the 28 day treatments (Table 2). However, organ injuries, including the liver and kidney, may be microscopic in nature and can also be observed for disease manifestation in sub chronic events.25 In the liver, hepatocyte injury by herbal formulation may impair normal processes of synthesis and/or elimination of triglycerides, which constitute other important liver functions. These lead to increased accumulation of lipids in the macrovesicles (macrovesicular steatosis), which give rise to inflammation and associated physiological responses.26 Kidneys are also involved in xenobiotic processing and are also susceptible to damage by medicinal herbs. Haemorrhage has been linked to high concentrations of the herbal medicine's secondary metabolites.3 In our study, we found evidence of progressive damage in the liver and the kidney with increasing concentrations of the herbal formulation given to mice over a 28-day period, and they were statistically significant at higher doses (Figure 5, Figure 6). However, contrary observations were made in a previous study conducted by Adebayo et al.13 where Olax subsorpioidea extracts dosed in albino Wistar rats at 250, 500 and 750 mg/kg per body weight revealed no hepatic or renal damage after treatment after the same duration. Some studies have described herb–herb interactions that lead to the potentiation of harmful phytochemicals within formulations that can counteract beneficial (or harmful) effects in co-administration, which may be a reason for this discrepancy.3 As common mechanisms for damage are as a result of high concentrations of phytochemicals in tissue cells, it is unlikely that polyphenols and flavonoids would elicit significant damage to either liver or renal tissue at lower concentrations in mice.
Effects of Hepacare on biochemical serum markers of toxicity
The liver is susceptible to injury following chronic exposure to drugs and xenobiotics. Aspartate aminotransferase (AST or serum glutamic oxaloacetic transaminase, SGOT), alanine aminotransferase (ALT or serum glutamic pyruvic transaminase, SGPT) and alkaline phosphatase (ALP) are serum markers of hepatic injury and hepatocellular necrosis that have been used to assess the extent of liver damage following chronic exposure to harmful xenobiotics.27, 28 It may be important to note that AST and ALP are also found in cells other than the liver and may indicate injury to other tissues.27, 28, 29 Results from the 28 day sub-chronic experiment in this study showed a significant (p < 0.05) elevation of serum AST and ALT levels in the 2500 mg/kg dose group and significant (p < 0.05) elevations of serum ALP in the 750 and 2500 mg/kg dose groups in mice compared with the control. No significant (p > 0.05) elevations in serum AST, ALT or ALP levels were observed at lower doses (Figure 3a–c). A similar study conducted on O. subsorpioidea in Wistar rats also showed a significant (p < 0.05) increase in serum AST, ALT and ALP levels when dosed at 1000 mg/kg b.w. Large amounts of bilirubin may be found in the blood when it accumulates and re-enters circulation due to intrahepatic obstruction and this can lead to severe liver or kidney injury.30 High total protein levels in the blood may also indicate liver or kidney injury due to chronic inflammation.31 Our results also showed a significant (p < 0.05) elevation of bilirubin and total protein in the serum of the 2500 mg/kg b.w. treated group. There was no significant (p > 0.05) increase in bilirubin and total protein in the lower treatment groups (Figure 3d–e). Adebayo et al.13 also demonstrated that a lower dose (500 mg/kg) significantly (p < 0.05) increased the total bilirubin levels. It is unlikely that liver damage would occur at moderate doses of the herbal drug at even moderately high doses over extended treatment periods in murine subjects (i.e., <500 mg/kg).
Effects of Hepacare on haematological serum markers for toxicity
Packed cell volume (PCV) is relevant in diagnosing anaemia and polycythaemia that are linked to the presence of heavy metals (e.g., lead and cadmium) and other chemical impurities (e.g., from insecticides and herbicides) in herbs and herbal medicines.32, 3 In our study, there was no significant difference (p > 0.05) observed in the PCV values when all of the treatment groups were compared with the control; therefore, it is unlikely that the herbal formulation drug possess toxic blood impurities (Figure 4a). Other blood cellular elements (i.e., WBCs, which include neutrophils, eosinophils, basophils, lymphocytes and monocytes) protect the body against infection and foreign bodies and may be elevated in the serum in these events. Elevated serum monocytes may be linked to chronic inflammation and stress amongst other diseases.33, 34 Our results (Figure 4b) showed significantly (p < 0.05) elevated monocyte levels in the 750 and 2500 mg/kg treatment groups. Our histopathological observation in this study, which showed marked inflammation in hepatic tissues of these two treatment groups, is consistent with this finding. Lymphocytes (B cells, T cells and natural killer cells) constitute the adaptive immune system and are usually elevated in recognition to infection by pathogens. Neutrophils are the most abundant leucocyte and are usually the first responders to inflammation and are usually elevated in acute inflammation.33, 34 There was no significant increase (p > 0.05) in the lymphocyte or neutrophil percentages or the white blood cell counts in the serum levels of any treatment group compared with the controls (Figure 4c–e). In a study conducted by Adebayo et al.13 lymphocyte and neutrophil percentages also showed no significant changes when one of the ingredients of the herbal formulation was tested in Wistar albino rats. It is thus improbable that the use of the herbal drug would cause or support infection.
Genotoxicity assessment by single-cell gel electrophoresis
The comet single cell gel assay is a sensitive method for quantifying DNA damage in low amounts in almost all mammalian cells.35, 36 In this study, the comet single cell gel assay demonstrated no evidence that the herbal formulated drug was potentially genotoxic (Figure 7, Figure 8). Certain plants have been demonstrated to be genotoxic due to the content of certain phytochemicals present in them that form adducts with DNA that interfere with normal DNA repair mechanism in various cells.37, 38, 39, 40, 41 In previous studies, capsaicin, which is linked to C. frutescens, has been demonstrated to be carcinogenic in humans.42 In other reports, however, C. frutescens was used as an effective treatment for cancers and this was largely attributed to the antioxidant properties of the plant.20 Herb–herb interactions were described by Brantley et al.23 wherein may lie the reason for this paradox. The presence of other herb components in the herbal formulated drug that protect DNA and stimulate repair may act in mitigating capsaicin's DNA damaging effect, if any, or be involved in keeping capsaicin in its inactive state.
Conclusions
Owing to the lack of experimental data on Hepacare's® safety profile, a toxicological evaluation was performed using mice as an experimental model. For the first time, this study provides relevant data and additional evidence to support the herbal formulation usage as a therapeutic remedy on a broader scale. Additionally, this study reveals that although the herbal formulation at high doses may cause a significant increase in serum levels of certain biochemical parameters, namely, AST, ALT, ALP, total bilirubin and total protein, and significant histological changes in both hepatic and renal tissue, it is unlikely to do so at doses below 500 mg/kg b.w. in mice. Furthermore, from our pro-inflammatory cytokine mRNA expression data, namely, IL-6 and TNF-α, this study shows that acute inflammation is an unlikely mechanism for tissue injury in the liver at high dose treatments of the drug. However, the haematology results showed that inflammation may be important in chronic conditions. The results in this study also show that the drug may not be genotoxic. This study also suggests the interplay of possible polyphenol active principles in herb–herb interactions that seem to control the general properties of the drug blend as a whole, which sometimes is contrasting to the properties exhibited for each individual ingredient studied in isolation. It is recommended that further studies be carried out to isolate and identify the active components and determine their individual activities as well as molecular modes of action and explain the possible harmful (or beneficial) interactions between them. In conclusion, Hepacare® may not induce significant toxic effects when administered in mice below 500 mg/kg b.w. in both acute and sub-chronic conditions and thus may be safe for use at the recommended therapeutic dose; however, patients who have prolonged liver diseases should exercise caution to avoid further complications.
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contributions
AHA conceived and designed the study and reviewed and approved the final draft of the manuscript. EEA conducted the research, provided the research materials, collected and organized the data and wrote the first draft. OFY wrote the final draft of the manuscript and interpreted the data. OO analysed the data and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgements
The author thanks the Department of Biological Sciences for providing the laboratory space to carry out the experiments.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jtumed.2017.02.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kempinas W., Carvalho T.L. A method for estimating the concentration of spermatozoa in the rat cauda epididymidis. Braz J Med Biol Res. 1988;22:154–156. doi: 10.1258/002367788780864547. [DOI] [PubMed] [Google Scholar]
- 2.Newall C.A., Anderson L.A., Phillipson J.D. The Pharmaceutical Press; 1996. Herbal medicines. A guide for health-care professionals. [Google Scholar]
- 3.Chau F., Fung K., Koon C., Lau K., Wei S., Leung P. Bioactive components in herbal medicine experimental approaches. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal medicine: biomolecular and clinical aspects. CRC Press; Boca Raton: 2011. [PubMed] [Google Scholar]
- 4.FDA Complementary and alternative medicine products and their regulation by the food and drug administration guidance for industry – complementary and alternative medicine products and their regulation by the food and drug administration. Draft Guidance. 2006:1–17. [Google Scholar]
- 5.World Health Organization (WHO) 2010. Traditional medicine. [Google Scholar]
- 6.Zeng Z., Chau F., Chan H., Cheung C., Lau T., Wei S., Mok D.K., Chan C., Liang Y. Recent advances in the compound-oriented and pattern-oriented approaches to the quality control of herbal medicines. Chin Med. 2008;3:9. doi: 10.1186/1749-8546-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuppan D., Jia J.D., Brinkhaus B., Hahn E.G. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology (Baltimore, Md.) 1999;30:1099–1104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 8.Dhiman R.K., Chawla Y.K. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50:1807–1812. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 9.Ishola I.O., Akinyede A.A., Robert A.K., Omilabu S.A. Hepatoprotective and antioxidant activities of Hepacare®, a herbal formulation against carbon tetrachloride-induced liver injury. Drug Res. 2005;65:30–39. doi: 10.1055/s-0034-1371829. [DOI] [PubMed] [Google Scholar]
- 10.OECD . 2014. OECD guidelines for testing chemicals: in vivo mammalian alkaline comet assay. [Google Scholar]
- 11.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 12.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adebayo A.H., Adegbite O.S., Olugbuyiro J.A.O., Famodu O.O., Odenigbo K.B. Toxicological evaluation of extract of Olax subsorpioidea on albino Wistar rats. Afr J Pharm Pharmacol. 2014;8:570–578. [Google Scholar]
- 14.Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Pratt I.S. Global harmonisation of classification and labelling of hazardous chemicals. Toxicol Lett. 2002;128:5–15. doi: 10.1016/s0378-4274(01)00529-x. [DOI] [PubMed] [Google Scholar]
- 16.OECD . 2002. Guideline for the testing of chemicals: avian acute oral toxicity test; pp. 1–15. [Google Scholar]
- 17.Lee S.Y., Kim Y.-S., Lim J.Y., Chang N., Kang M.-H., Oh S.-Y., Lee H.-J., Kim H., Kim Y. Effects of plant-based Korean food extracts on lipopolysaccharide-stimulated production of inflammatory mediators in vitro. Nutr Res Pract. 2014;8:249–256. doi: 10.4162/nrp.2014.8.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.W., Chen S.W.C., Kim M., Brown K.M., Kolls J.K., D'Agati V.D., Lee H.T. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Investig J Tech Methods Pathol. 2011;91:63–84. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Lu Y., Dong Y., Zhang G., Zhang Y., Xu Z., Culley D.J., Crosby G., Marcantonio E.R., Tanzi R.E., Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory cytokine TNF-α, IL-6 and IL-1β. Neurobiol Aging. 2012;33:1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surh Y.J., Lee S.S. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 1995;56:1845–1855. doi: 10.1016/0024-3205(95)00159-4. [DOI] [PubMed] [Google Scholar]
- 21.Reilly C.A., Taylor J.L., Lanza D.L., Carr B.A., Crouch D.J., Yost G.S. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci. 2003;73:170–181. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis C.L., Tomporowski P.D., Boyle C.A., Waller J.L., Miller P.H., Naglieri J.A. Effects of aerobic exercise on overweight children's cognitive functioning: a randomized controlled trial. Res Q Exerc Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantley S.J., Argikar A.A., Lin Y.S., Nagar S., Paine M.F. Herb-drug interactions: challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;42:301–317. doi: 10.1124/dmd.113.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farsi E., Shafaei A., Hor S.Y., Ahamed M.B.K., Yam M.F., Asmawi M.Z., Ismail Z. Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics (São Paulo, Brazil) 2013;68:865–875. doi: 10.6061/clinics/2013(06)23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuttle A.H. University of Virginia; Anderson Bros: 1898. The principles of histology, descriptive and practical: book I. Descriptive histology. [Google Scholar]
- 26.Marshall W.J., Bangert S.K. Churchill Livingstone/Elsevier; Edinburgh: 2008. Clinical biochemistry: metabolic and clinical aspects. [Google Scholar]
- 27.Fariss M.W. Cadmium toxicity: unique cytoprotective properties of alpha tocopheryl succinate in hepatocytes. Toxicology. 1991;69:63–77. doi: 10.1016/0300-483x(91)90154-s. [DOI] [PubMed] [Google Scholar]
- 28.Rej R., Horder M. Aspartate aminotransferase. In: Bergmeyer H.U., Bergmeyer J., Grassl M., editors. vol. 3. Verlag Chemie; Weinheim: 1983. pp. 416–433. (Methods of enzymatic analysis). [Google Scholar]
- 29.Rej R., Bretaudiere J.P. Effects of metal ions on the measurement of alkaline phosphatase activity. Clin Chem. 1980;26:3423–3428. [PubMed] [Google Scholar]
- 30.Pratt D.S., Kaplan M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 31.Klein C.J., Moser-Veillon P.B., Schweitzer A. Magnesium, calcium, zinc, and nitrogen loss in trauma patients during continuous renal replacement therapy. JPEN J Parenter Enteral Nutr. 2002;26(2):77–92. doi: 10.1177/014860710202600277. [DOI] [PubMed] [Google Scholar]
- 32.Lipowsky H.H., Usami S., Chien S., Pittman R.N. Hematocrit determination in small bore tubes by differential spectrophotometry. Microvasc Res. 1982;24:42–55. doi: 10.1016/0026-2862(82)90041-3. [DOI] [PubMed] [Google Scholar]
- 33.Janeway C.A., Travers P., Walport M., Shlomchik M.J. Garland Science; 2001. Innate immunity. [Google Scholar]
- 34.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.-L., Kohler R.H., Chudnovskiy A., Waterman P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki Y.F., Kawaguchi S., Kamaya A., Ohshita M., Kabasawa K., Iwama K., Taniguchi K., Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res Genet Toxicol Environ Mutagen. 2002;519:103–119. doi: 10.1016/s1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi S., Nakamura T., Yamamoto A., Honda G., Sasaki Y.F. Is the comet assay a sensitive procedure for detecting genotoxicity? J Nucleic Acids. 2010 doi: 10.4061/2010/541050. 8 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanherweghem J.L., Depierreux M., Tielemans C., Abramowicz D., Dratwa M., Jadoul M., Richard C., Vandervelde D., Verbeelen D., Vanhaelen-Fastre R. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 38.Schmeiser H.H., Bieler C.A., Wiessler M., van Ypersele de Strihou C., Cosyns J.P. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res. 1996;56:2025–2028. [PubMed] [Google Scholar]
- 39.Kohara A., Suzuki T., Honma M., Ohwada T., Hayashi M. Mutagenicity of aristolochic acid in the lambda/lacZ transgenic mouse (MutaMouse) Mutat Res. 2002;515:63–72. doi: 10.1016/s1383-5718(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 40.Fang Z.-Z., Zhang Y.-Y., Wang X.-L., Cao Y.-F., Huo H., Yang L. Bioactivation of herbal constituents: simple alerts in the complex system. Expert Opin Drug Metab Toxicol. 2011;7:989–1007. doi: 10.1517/17425255.2011.586335. [DOI] [PubMed] [Google Scholar]
- 41.Hwang Y.-H., Kim T., Cho W.-K., Yang H.J., Kwak D.H., Ha H., Song K.H., Ma J.Y. In vitro and in vivo genotoxicity assessment of Aristolochia manshuriensis Kom. Evid Based Complement Alternat Med. 2012:412736. doi: 10.1155/2012/412736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archer V.E., Jones D.W. Capsaicin pepper, cancer and ethnicity. Med Hypotheses. 2002;59:450–457. doi: 10.1016/s0306-9877(02)00152-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.