Abstract
Bacterial invasion of synovial joints, as in infectious or septic arthritis, can be difficult to treat in both veterinary and human clinical practice. Biofilms, in the form of free-floating clumps or aggregates, are involved with the pathogenesis of infectious arthritis and periprosthetic joint infection (PJI). Infection of a joint containing an orthopedic implant can additionally complicate these infections due to the presence of adherent biofilms. Because of these biofilm phenotypes, bacteria within these infected joints show increased antimicrobial tolerance even at high antibiotic concentrations. To date, animal models of PJI or infectious arthritis have been limited to small animals such as rodents or rabbits. Small animal models, however, yield limited quantities of synovial fluid making them impractical for in vitro research. Herein, we describe the use of ex vivo equine and porcine models for the study of synovial fluid induced biofilm aggregate formation and antimicrobial tolerance. We observed Staphylococcus aureus and other bacterial pathogens adapt the same biofilm aggregate phenotype with significant antimicrobial tolerance in both equine and porcine synovial fluid, analogous to human synovial fluid. We also demonstrate that enzymatic dispersal of synovial fluid aggregates restores the activity of antimicrobials. Future studies investigating the interaction of bacterial cell surface proteins with host synovial fluid proteins can be readily carried out in equine or porcine ex vivo models to identify novel drug targets for treatment of prevention of these difficult to treat infectious diseases.
Introduction
Infectious or septic arthritis is an orthopedic emergency that results in substantial morbidity and mortality[1–3]. Staphylococcus aureus (S. aureus) is the most common bacterial organism isolated from infectious arthritis and also in periprosthetic joint infection (PJI), accounting for the highest treatment failure rates[2,4–7]. These high treatment failure rates are linked to the ability of Staphylococci to form robust biofilms[7–10]. The traditional definition of a biofilm is a community of bacteria within a polymeric matrix that is attached to an abiotic or biotic surface[11]. However, recent advancements in biofilm research suggests that bacteria do not need a surface for formation; rather bacteria may attach to one another or host-derived proteins to form a biofilm[12–16].
Current work in the infectious arthritis field has shown the ability of S. aureus to form free-floating biofilms in human synovial fluid both in vitro and in vivo[17–20]. Within that body of work, the authors evaluated both S. aureus laboratory strains and clinical isolates from human patients [17]. In addition, biofilm aggregates have been described in other locations within the body such as the lungs of cystic fibrosis (CF) patients, the middle ear, and on the skin[12,14,21,22]. These biofilm aggregates displayed antimicrobial tolerance to cefazolin and vancomycin in vitro[17–19]. The antimicrobial tolerance displayed by synovial fluid biofilm aggregates is similar to traditional biofilms and the biofilm aggregates that form the sputum of CF patients[13,23,24]. Continued in vitro investigations are critical for understanding this novel, free-floating bacterial phenotype in synovial fluid; however, these research efforts can be hampered as they rely on large volumes of synovial fluid which are difficult to source. Moreover, synovial fluid from human donors can be of variable quality due to underlying donor pathology.
To date, rodent and rabbit models have been at the forefront of infectious arthritis and PJI in vivo research[25,26]. However, rodent and rabbit cartilage biology as well as inflammatory responses are significantly different from those of humans[27–30]. Moreover, synovial fluid is difficult to obtain in large quantities from these species[31,32].
Large animals such as horses, pigs, goats, sheep and dogs have been successfully used to explore mechanisms of non-infectious joint disease, particularly osteoarthritis[33–35]. The advantage of large animal models is that their cartilage biology is more similar to that of humans than rodents and rabbits[33,36–38] and substantially larger volumes of synovial fluid can be collected. Of all the large animal models, horses and pigs are most commonly used for the study of osteoarthritis because cartilage thickness and response to injury, as well as their overall immune response, is similar to that of humans [33,35,39–41].
The objective of this study was to investigate if equine and porcine synovial fluid can be used as an ex vivo model system of human joint infection and to investigate how microbial-synovial fluid interactions limit antimicrobial activity. To achieve this goal, we first investigated whether synovial fluid induced aggregate formation would occur across the aforementioned species. Next, we asked if biofilm aggregate formation would also be observed with non-Staphylococcal species, i.e. Escherichia coli (E. coli), Streptococcus equi subspecies zooepidemicus (S. zooepidemicus) and Pseudomonas aeruginosa (P. aeruginosa). We then determined if biofilm aggregate formation in synovial fluid leads to antimicrobial tolerance as a function of antimicrobial class, bacterial species and synovial fluid source. Finally, we asked if enzymatic dispersal of biofilm aggregates could restore antimicrobial activity.
Results
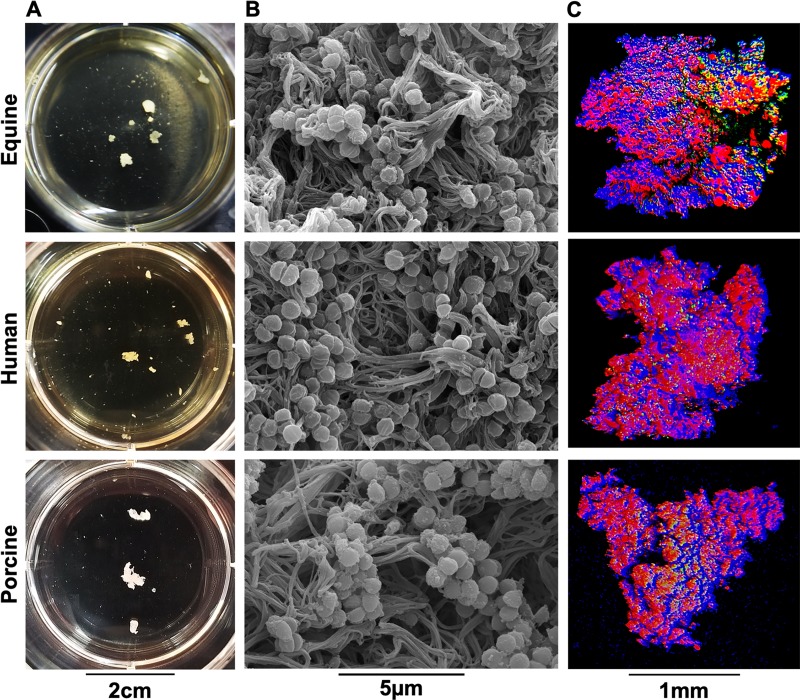
Staphylococcus aureus forms free-floating biofilm aggregates in equine, human and porcine synovial fluid
When grown in human or bovine synovial fluid, S. aureus or S. epidermidis, respectively, formed free-floating biofilm aggregates[17,20]. We first ascertained if S. aureus could form biofilm aggregates in equine and porcine synovial fluid with similar structure to those formed in human synovial fluid. Upon incubation of synovial fluid with S. aureus, aggregation was macroscopically evident within ~ 1–2 hours post-infection and biofilm aggregate size reached a plateau at ~16–18 hours post infection (S1 Fig). We found that S. aureus formed biofilm aggregates in all species by 24 hours post-infection (Fig 1A). Analysis using scanning electron microscopy (SEM) indicated that biofilm aggregates had similar ultrastructure in equine, porcine and human synovial fluid. In each species, we observed S. aureus contained within a polymeric, cord-like, extracellular matrix (Fig 1B). Using confocal microscopy three-dimensional (3D) reconstruction, we observed that synovial fluid biofilm aggregates exhibited a mixed protein (red, SYPRO) and carbohydrate (blue, WGA) extracellular matrix; nucleic acid/bacterial staining (green, SYTO9) was scattered throughout the aggregate in all three species (Fig 1C).
Fig 1. Staphylococcus aureus forms macroscopic biofilm aggregates in the synovial fluid of several different species.
Equine, human or porcine synovial fluid was infected at 1x106 CFU/mL with S. aureus (ATCC25923) and incubated overnight at 37°C in a microaerophilic chamber on a shaker at 120rpm to mimic the joint environment. (A) Macroscopic biofilm aggregates were observed in synovial fluid in all three species and photographed. (B) Aggregates were removed from the synovial fluid, fixed, dehydrated in ethanol, sputter coated and imaged with a scanning electron microscope with a FEI-Tecnai T12 microscope showing bacteria nested within a polymeric cord-like extracellular matrix. (C) Aggregates were stained with wheat germ agglutinin (WGA (blue)) for carbohydrates, SYTO9 for nucleic acids/bacteria (green), and SYPRO (red) for proteinaceous content. Confocal laser scanning microscopy (CLSM) was performed using a 12.5x upright lens on a Leica SP5 Multiphoton Microscope. CLSM images were generated as 3-D reconstructions by sequential Z-stacking and tile scanning with Velocity software.
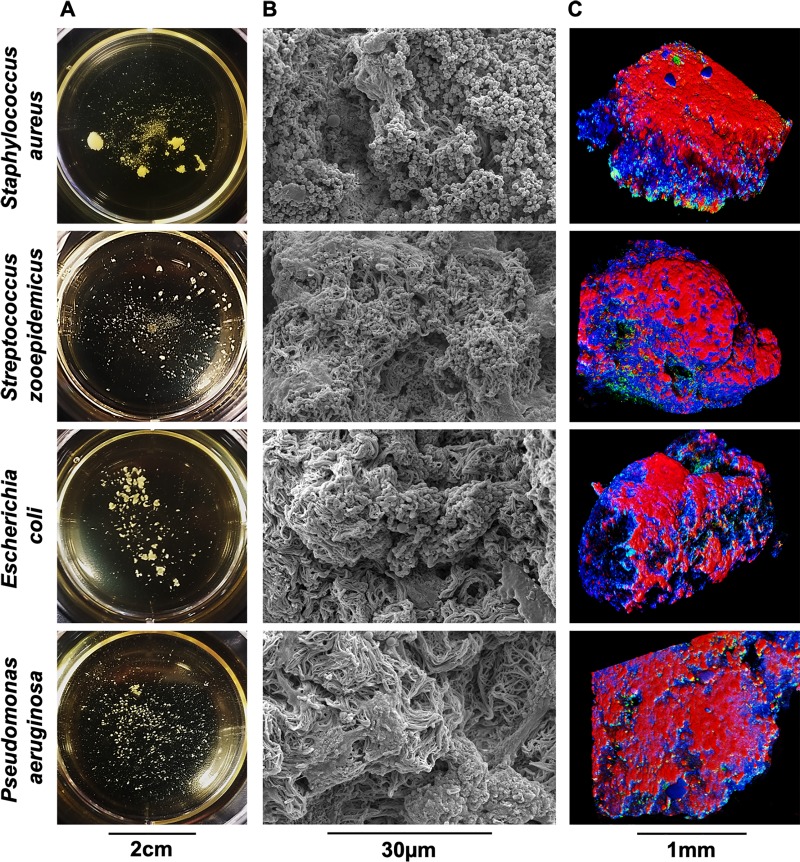
Non-Staphylococcal arthrotropic bacteria form biofilm aggregates in equine synovial fluid
Dastgheyb et al.[17] observed that methicillin-resistant (MRSA) and methicillin-sensitive S. aureus, from both laboratory-adapted strains and clinical isolates from cases of human septic arthritis, formed biofilm aggregates in synovial fluid. Therefore, we next asked if the biofilm aggregate phenotype that develops in synovial fluid was restricted to S. aureus. Using arthrotropic clinical isolates derived from equine septic arthritis cases, we infected synovial fluid with S. aureus, S. zooepidemicus, E. coli, or P. aeruginosa. These strains represent the most common isolates from equine infectious arthritis cases seen at the University of Pennsylvania George D. Widener Hospital Large Animal Hospital in the last five years[42]. By 24 hours, S. aureus formed large biofilm aggregates; S. zooepidemicus, E. coli, and P. aeruginosa also formed aggregates in synovial fluid, although the aggregates were smaller than those formed with S. aureus (Fig 2A). Micrograph analysis revealed that all biofilm aggregates were comprised of a polymeric, cord-like, extracellular matrix heavily colonized by bacteria whose morphology and size was consistent with bacterial strain (Fig 2B). Confocal microscopy 3D renderings of biofilm aggregates showed similarities between all isolates (compare Fig 2C to Fig 1C).
Fig 2. Gram-positive and gram-negative arthrotropic clinical isolates form macroscopic biofilm aggregates in equine synovial fluid.
Equine synovial fluid was infected at 1x106 CFU/mL for each clinical isolate (S. aureus, S. zooepidemicus, E. coli, and P. aeruginosa) and incubated overnight at 37°C in a microaerophilic chamber on a shaker at 120rpm to mimic the joint environment. (A) Macroscopic bacterial aggregates were observed in synovial fluid for all four strains and photographed. (B) Aggregates visualized by SEM as in Fig 1. (C) Aggregates visualized by confocal microscopy using WGA, Syto9 and SYPRO as in Fig 1.
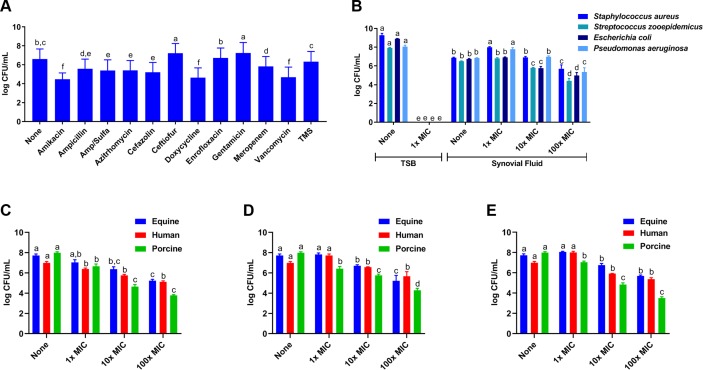
Synovial fluid biofilm aggregates display antimicrobial tolerance to several classes of drugs
We asked whether antimicrobial activity of several classes of antimicrobials against S. aureus would be altered when cultured in equine synovial fluid (MIC concentrations presented in Table 1). For all antimicrobials, planktonic bacteria in tryptic soy broth (TSB) were inhibited or killed at 100× MIC (S2 Fig). In equine synovial fluid, S. aureus biofilm aggregates persisted in vitro at 100× MIC (Fig 3A). Specifically, amikacin (an aminoglycoside), doxycycline (a tetracycline), and vancomycin (a glycopeptide) reduced S. aureus bacterial concentration by >2 log CFU/mL in equine synovial fluid (p<0.008; Fig 3A). As all four clinical isolates were susceptible to amikacin (based on antimicrobial susceptibility testing (Table 1)), we then used amikacin at 1×, 10× and 100× MIC to screen antimicrobial activity against biofilm aggregates in synovial fluid formed by these isolates. S. zooepidemicus, E. coli, and P. aeruginosa were also killed by amikacin at 1× MIC when grown in TSB planktonically (p<0.0001; Fig 3B). Little to no killing was seen when the drug was used at 1× or 10× MIC against aggregate-containing synovial fluid (Fig 3B); at 100× MIC, a modest decrease in bacterial concentration (~2–3 log CFU/mL) was observed (p<0.009; Fig 3B). Similarly, these bacteria in synovial fluid from equine, human and porcine sources were tolerant to amikacin (Fig 3C), doxycycline (Fig 3D) and vancomycin (Fig 3E) at 1×, 10× and 100× MIC.
Table 1. Median minimum inhibitory concentration of clinical isolates and ATCC25923 measured by antimicrobial susceptibility testing using the Sensititre Complete Automated AST System and the equine (Equine EQUIN1F Vet AST Plate) antimicrobial susceptibility panel.
| Antimicrobial | Range |
S. aureus (ATCC 25923) |
S. aureus | S. zooepidemicus | E. coli | P. aeruginosa |
|---|---|---|---|---|---|---|
| Amikacin | 4–32 | ≤ 4 | ≤ 4 | 8 | ≤ 4 | ≤ 4 |
| Ampicillin | 0.25–32 | 0.5 | ≤ 0.25 | ≤ 0.25 | 4 | ≥ 32 |
| Azithromycin | 0.25–4 | 0.5 | ≤ 0.25 | ≤ 0.25 | 2 | ≥ 4 |
| Cefazolin | 4–16 | ≤ 4 | ≤ 4 | ≤ 4 | 1 | 1 |
| Ceftazidime | 1–64 | 8 | 2 | ≤ 1 | 16 | 8 |
| Ceftiofur | 0.25–4 | 0.5 | 0.5 | ≤ 0.25 | 0.5 | 4 |
| Chloramphenicol | 4–32 | 8 | ≤ 4 | ≤ 4 | 8 | ≥ 32 |
| Clarithromycin | 1–8 | ≤ 1 | ≤ 1 | ≤ 1 | 8 | ≥ 8 |
| Doxycycline | 2–16 | ≤ 2 | ≤ 2 | ≤ 2 | ≤ 2 | ≥ 16 |
| Enrofloxacin | 0.25–2 | ≤ 0.25 | ≤ 0.25 | 1 | ≤ 0.25 | 1 |
| Erythromycin | 0.25–8 | 0.5 | ≤ 0.25 | ≤ 0.25 | 8 | ≥ 8 |
| Gentamicin | 1–8 | ≤ 1 | ≤ 1 | ≤ 1 | ≤ 1 | 2 |
| Imipenem | 1–8 | ≤ 1 | ≤ 1 | ≤ 1 | ≤ 1 | 1 |
| Oxacillin+2% NaCl | 0.25–4 | ≤ 0.25 | ≤ 0.25 | ≤ 0.25 | ≥ 4 | ≥ 4 |
| Penicillin | 0.06–8 | ≤ 0.06 | ≤ 0.06 | ≤ 0.06 | ≥ 8 | ≥ 8 |
| Rifampin | 1–4 | ≤ 1 | ≤ 1 | ≤ 1 | ≥ 4 | ≥ 4 |
| Tetracycline | 2–8 | ≤ 2 | ≤ 2 | 8 | 4 | ≥ 8 |
| Ticarcillin | 8–64 | ≤ 8 | ≤ 8 | ≤ 8 | 16 | 16 |
| Ticarcillin-clavulanate | 8/2-64/2 | ≤ 8/2 | ≤ 8/2 | ≤ 8/2 | ≤ 8/2 | 16/2 |
| Trimethoprim Sulfa | 0.5/9.5-4/76 | 1/19 | ≤ 0.5/9.5 | ≤ 0.5/9.5 | ≤ 0.5/9.5 | 4/76 |
Fig 3. Synovial fluid biofilm aggregates show antimicrobial tolerance against several different classes of antimicrobials.
(A) S. aureus (ATCC25923) biofilm aggregates were allowed to form in equine synovial fluid for 6 hours and this aggregate-containing synovial fluid was treated with a panel of different antimicrobials from several drug classes at 100× the minimum inhibitory concentration (MIC) as determined by in vitro antimicrobial susceptibility testing (Table 1). No concentration of any antimicrobial evaluated in this experiment was able to completely kill S. aureus grown in synovial fluid. (B) The four arthrotropic bacterial isolates from Fig 2 were grown in equine synovial fluid or in tryptic soy broth (TSB). Synovial fluid or TSB was subsequently challenged with 1×, 10× or 100× MIC amikacin, an antibiotic with broad spectrum activity; these isolates were susceptible to amikacin based on our in vitro antimicrobial susceptibility data. (C-E) S. aureus (ATCC25923) were incubated in equine, human or porcine synovial fluid and this bacterial aggregate-containing synovial fluid was subsequently treated with amikacin (C), doxycycline (D) or vancomycin (E) at 1×, 10× or 100× MIC. Bars are means and standard deviations of four biological replicates (i.e. synovial fluid from four individual horses, humans or pigs; n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
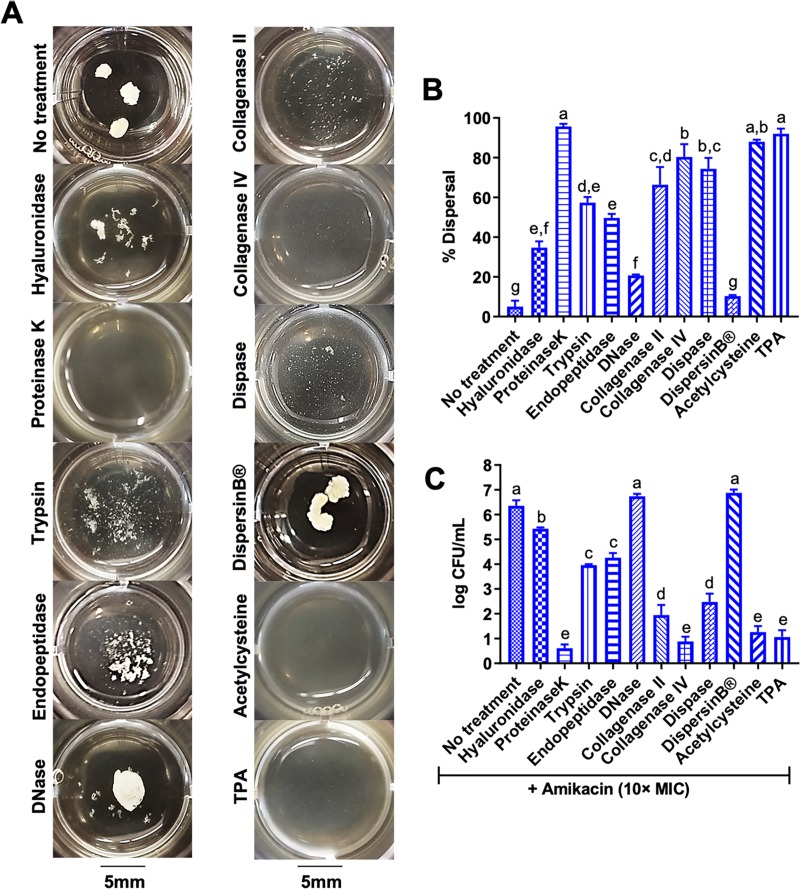
Enzymatic targeting of the proteinaceous matrix disperses synovial fluid biofilm aggregates
Since confocal microscopy showed that synovial fluid biofilm aggregates display a mixed sugar and protein matrix (Figs 1C and 2C) we tested the ability of different enzymes to hydrolyze the synovial fluid biofilm aggregate matrix, disperse the bacteria and improve antimicrobial activity. After S. aureus aggregate formation in synovial fluid, enzymes including DNase, DispersinB and proteinaseK, among others, were added to infected synovial fluid to target key molecules within the aggregate extracellular matrix. In agreement with Dastgheyb et al.[17], we showed that DispersinB and DNase did not disperse, while proteinaseK was able to completely disperse, S. aureus biofilm aggregates in synovial fluid (p<0.004; Fig 4A and 4B). Similar to Ibberson et al.[43], pre-treatment of synovial fluid with hyaluronidase, an enzyme that targets hyaluronic acid, the main carbohydrate in synovial fluid, prevented aggregate formation (S3A and S3B Fig); nevertheless, post-treatment with hyaluronidase only mildly dispersed biofilm aggregates in synovial fluid (p<0.02; Fig 4A and 4B). In addition, the proteolytic enzymes trypsin, endopeptidase (LysC), collagenase (type II), and dispase were also able to moderately disperse aggregates in synovial fluid (p<0.001; Fig 4A and 4B). Finally, collagenase (type IV), acetylcysteine and tissue plasminogen activator (TPA) were similar to proteinaseK in that they significantly dispersed aggregates in synovial fluid (p<0.0001; Fig 4A and 4B).
Fig 4. Enzymatic dispersal of synovial fluid biofilm aggregates restores antimicrobial efficacy.
(A) S. aureus (ATCC25923) biofilm aggregates in equine synovial fluid were treated with several enzymes in an attempt to breakdown the extracellular matrix and disperse the bacteria: hyaluronidase (1mg/mL), proteinaseK (200μg/mL), trypsin (200μg/mL), endopeptidase or LysC (200μg/mL), DNase (500μg/mL), collagenase type II (750μg/mL), collagenase type IV (750μg/mL), dispase (500μg/mL), DispersinB (1mg/mL), acetylcysteine (8mg/mL) and tissue plasminogen activator or TPA (1mg/mL). Synovial fluid containing biofilm aggregates was treated with the respective enzyme for 1 hour prior to macroscopic imaging. (B) Percent (%) dispersal was evaluated by measuring absorbance (600nm) and calculating a percentage compared to planktonic S. aureus grown in TSB to a similar CFU/mL. (C) After 1 hour of dispersion, amikacin was added at 10× MIC (40μg/mL) and log CFU/mL was measured with serial dilutions and colony counting 8 hours post-antimicrobial challenge. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
Enzymatic dispersal of synovial fluid biofilm aggregates restores antimicrobial activity
Several studies have reported that dispersal of biofilms restores the activity of several classes of antimicrobials[18,44–46]. To determine if dispersal of S. aureus biofilm aggregates restores antimicrobial activity, synovial fluid containing bacterial aggregates was treated with each enzyme for 1 hour, prior to challenge with amikacin at 10× MIC. Bacterial concentration (log CFU/mL) was measured by serial dilutions and plate counting 8 hours post-antimicrobial challenge. Control wells with enzymes alone did not alter bacterial concentration more than 1 log CFU/mL compared to untreated synovial fluid (S4 Fig). Trypsin, endopeptidase (LysC), collagenase (type II) and dispase treatment prior to challenge with amikacin moderately increased antimicrobial killing when compared to synovial fluid (containing aggregates) not treated with enzymes (p<0.01; Fig 4C). ProteinaseK, collagenase (type IV), acetylcysteine and TPA treatment prior to challenge with amikacin markedly increased antimicrobial activity as compared to biofilm aggregates in synovial fluid not treated with enzymes (p<0.0003; Fig 4C). Pre-treatment with hyaluronidase mildly increased antimicrobial killing (p<0.04) while no change was observed with DNase or DispersinB treatment (Fig 4C).
TPA disperses Staphylococcal and non-Staphylococcal biofilm aggregates and restores antimicrobial activity in synovial fluid from multiple species
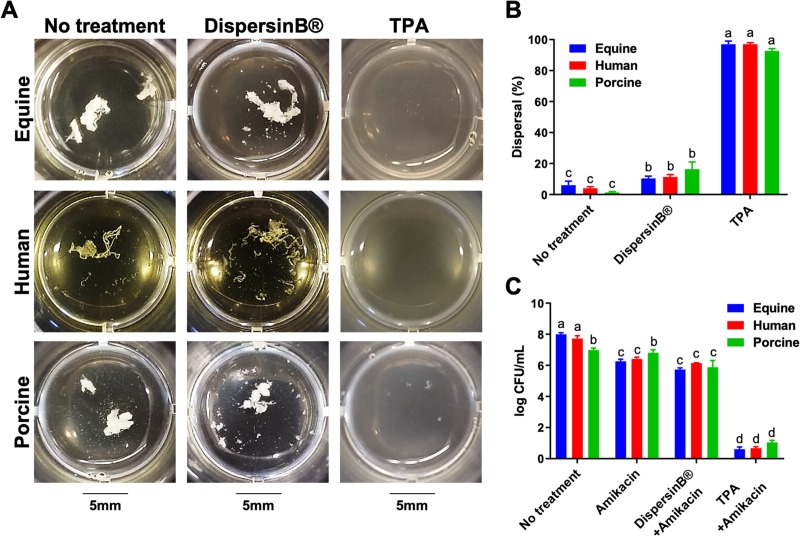
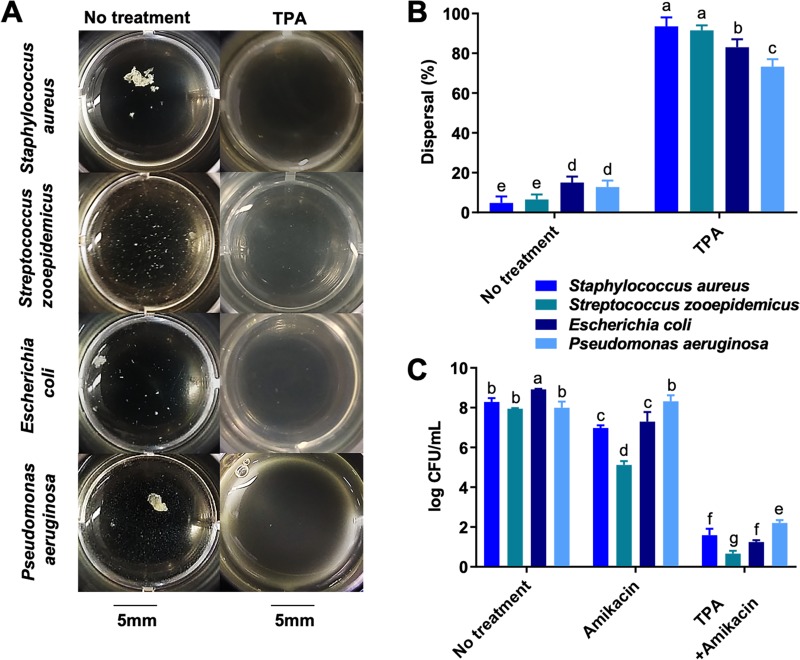
Because TPA most effectively dispersed S. aureus biofilm aggregates in equine synovial fluid (Fig 4A and 4B), we determined if it would also disperse S. aureus biofilm aggregates in human and porcine synovial fluid. We found that TPA was able to visibly disperse aggregates in equine, human, and porcine synovial fluid (p<0.0002; Fig 5A) and that dispersal was able to restore the antimicrobial activity of amikacin against S. aureus at 10× MIC (p<0.0004; Fig 5B). In addition, we showed that TPA could disperse aggregates of the equine clinical isolates (S. aureus, S. zooepidemicus, E. coli and P. aeruginosa) (Fig 6A), with restoration of antimicrobial activity against both gram-positive and gram-negative bacteria cultured in equine synovial fluid (p<0.001; Fig 6B).
Fig 5. Tissue plasminogen activator (TPA) disperses S. aureus biofilm aggregates and restores antimicrobial activity in equine, human and porcine synovial fluid.
(A) Equine, human and porcine synovial fluid containing S. aureus (ATCC25923) biofilm aggregates was left untreated or treated with DispersinB (1mg/mL) or TPA (1mg/mL) for 1 hour prior to macroscopic imaging. (B) Percent (%) dispersal was evaluated by measuring absorbance (600nm) and calculating a percentage compared to tryptic soy broth (TSB) containing planktonic S. aureus at a similar CFU/mL. (C) After 1 hour of dispersion, amikacin was added at 10× MIC (40μg/mL) and log CFU/mL was measured with serial dilutions and colony counting 8 hours post-antimicrobial challenge. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
Fig 6. TPA disperses synovial fluid biofilm aggregates and restores antimicrobial activity against both gram-negative and gram-positive aggregates.
(A) The four arthrotropic bacterial isolates from Fig 2 were grown in equine synovial fluid. Synovial fluid containing these aggregates bacteria was treated with TPA (1mg/mL) for 1 hour prior to macroscopic imaging. B) Percent (%) dispersal was evaluated by measuring absorbance (600nm) and calculating a percentage compared to planktonic S. aureus grown in TSB to a similar CFU/mL. (C) After 1 hour of dispersion, amikacin was added at 10× MIC (40μg/mL) to the infected synovial fluid containing biofilm aggregates and log CFU/mL was measured with serial dilutions and colony counting 8 hours post-antimicrobial challenge. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
Discussion
The purpose of this study was to examine the ability of S. aureus, the most commonly isolated bacteria from cases of infectious arthritis and periprosthetic joint infection[2,4–7], as well as non-Staphylococcal species, to aggregate and form free-floating biofilms in equine and porcine synovial fluid, a characteristic of infected human synovial fluid. We provide compelling evidence that biofilm aggregates are similar in structure and function across species indicating that both equine and porcine synovial fluid can be used as ex vivo model systems of human joint infection.
Biofilm aggregate formation in synovial fluid offers protection from traditional antimicrobial therapies[17,19] and the host immune system[47–49]. The results presented here show that antimicrobials can be used at 100× MIC in synovial fluid with little to no killing activity in vitro, while planktonic cells are easily killed at the same MIC. Based on the wildtype MIC of 4μg/mL used in this study, the clinical breakpoint for susceptible isolates of S. aureus, S. zooepidemicus, E. coli, and P. aeruginosa, and pharmacokinetic studies in horses[50], the aminoglycoside amikacin can achieve a concentration of ~5× MIC in synovial fluid after intravenous administration of a clinically relevant 10mg/kg dose. However, unlike systemic administration, local administration of amikacin to horses by regional limb perfusion or direct intra-articular injection can achieve a concentration in synovial fluid up to 100× MIC[51–53]. Conversely, local administration of amikacin to inflamed joints decreases the maximal concentration to ~50× MIC[52]. Therefore, concentrations between 1× to 100× MIC are considered within pharmacodynamic range of amikacin. Nevertheless, our studies show that we could not achieve a significant antibacterial effect even at 100× MIC of amikacin against bacteria cultured in synovial fluid due to their aggregation. These results indicate that clinical dosing of amikacin would be ineffective against biofilm aggregates in synovial fluid, which offers a possible explanation for persistent sepsis and increased severity of degenerative joint disease following infectious arthritis[54,55].
The antimicrobial tolerance displayed by bacteria in synovial fluid is similar to surface-attached biofilms and other biofilm aggregates such as those of P. aeruginosa in the sputum of CF patients[13,23,24]. In vitro models of P. aeruginosa aggregation in synthetic sputum or on alginate beads imparts significant antimicrobial tolerance[56–59], similar to that observed in this in vitro infectious arthritis model.
New methods to evaluate the pharmacodynamics of antimicrobial agents within biofilms are being developed[24,45]. One such method is called a minimum biofilm eradication concentration (MBEC), which is the biofilm equivalent to a planktonic minimum bactericidal concentration (MBC)[60]. The S. aureus MBC for aminoglycosides is within 1× to 4× MIC whereas the MBEC for aminoglycosides can reach concentrations greater than 1000× the MIC/MBC[61–63]. The MIC and MBC for amikacin can be achieved using clinical doses; however, the MBEC concentration is not within the therapeutic index of aminoglycosides. For example, concentrations higher than those currently achieved by local administration of amikacin are toxic to the articular cartilage and can cause nephrotoxicity or ototoxicity by systemic administration[64,65]. These observations correlate with pharmacokinetic studies reporting that MBECs are typically not achievable using clinical doses within the planktonic therapeutic index[66–68]. Therefore, new methods that combine tissue location-specific pharmacokinetic data and biofilm-specific pharmacodynamic data are vital to improve the clinical treatment of biofilm infections. The methods developed in this study could serve as a platform with improved translational fidelity to generate in vitro pharmacodynamics data within the articular-specific location.
In this study, we show dispersal of synovial fluid biofilm aggregates restores antimicrobial activity. This is similar to other reports of restoration of antimicrobial activity after dispersing surface-attached biofilms in vitro and in vivo[18,44–46]. Most biofilm in vitro studies rely heavily on traditional microbiological media, such as tryptic soy broth (TSB), which yields a biofilm matrix composed of bacterial-derived polysaccharide intercellular adhesion (PIA)[69–71]. In that regard, DispersinB, an enzyme that specifically targets PIA, has the ability to disperse S. aureus biofilms formed in vitro by traditional methods, whereas proteinaseK is unable to do so[72]. In contrast, this report shows that the biofilm aggregate matrix generated in synovial fluid is predominantly composed of proteins; therefore, treatment with proteinaseK, among other enzymes with proteolytic activity, but not DispersinB, dispersed aggregates. These results line up with previous work that reported PIA-independent biofilm aggregate formation in synovial fluid and dispersal with proteinaseK[17,43] In addition, other studies have noted that in vivo biofilms and biofilm aggregates tend to be embedded in a host-derived extracellular matrix versus a bacterial self-produced matrix such as PIA[12]. S. aureus in particular has several mechanisms to hijack host fibrinogen[48,73]. Since TPA was able to disperse synovial fluid biofilm aggregates, further investigation into the role of fibrinogen as an extracellular matrix component is warranted. Due to the ability of dispersion to restore antimicrobial activity, dispersal agents could decrease the MBEC of biofilm aggregates to fall within the therapeutic index of clinically relevant antimicrobial agents. This is a promising area of future study.
Human synovial fluid, particularly non-diseased, is difficult to obtain and identification of an alternative model that allows for movement between in vitro and in vivo components is critically needed to advance the field of biofilm aggregate research. Our findings show that both equine and porcine synovial fluid allow for robust biofilm aggregate formation with similar phenotypes to biofilm aggregates formed in human synovial fluid. Noteworthy, although rodent and rabbit models have been used extensively in infectious arthritis and PJI in vivo research[25,26,74], it is impractical to use these species for the ex vivo studies we have described here. The volume of synovial fluid able to be obtained from rodents is very small, ranging from 1–5μL per tibiofemoral joint in the mouse[32] up to 10–100μL per tibiofemoral joint in the rat[31], and 100–400μL per tibiofemoral joint in the rabbit[75]. Horses and pigs offer a distinct advantage in this regard since volumes of both normal and diseased synovial fluid range from 1.5-3mL per tibiofemoral joint in the pig [76] to 10-12mL per tibiotarsal joint in the horse[77]. In addition to these volume differences of up to four orders of magnitude, the cartilage biology of horses and pigs is very similar to humans[28,33,36–38]. Moreover, these animals are well supported by the FDA as pre-clinical models for osteoarthritis. Lastly, horses suffer from naturally occurring infectious arthritis that requires clinical treatment and rehabilitation protocols similar to that of humans [42,78,79]. Thus, the horse provides an ideal preclinical and clinical model for translational research.
This study offers a powerful alternative to traditional in vitro biofilm models to specifically study free-floating biofilm aggregates in physiological fluid. The complexity of host-derived fluids may influence the bacterial phenotype differently than traditional in vitro media such as TSB. Therefore, studying bacteria within the context of the infective environment, such as synovial fluid for infectious arthritis or sputum for cystic fibrosis, has the advantage of exploring the bacteria phenotype similar to what is encountered in vivo. By utilizing the microenvironment that bacteria encounter upon infection in vivo, the robust ex vivo model system described in this study offers an important advancement in benchtop biofilm research. Although the limitation of an ex vivo study is the lack of pressure from the host immune system or changes that occur within the biofluid during in vivo infection, such studies are imperative prior to performing costly long-term in vivo studies. By utilizing the equine and porcine model systems described here, we can study the mechanisms by which bacteria form biofilm aggregates in synovial fluid and become tolerant to antimicrobials. The findings from these in vitro studies demonstrate a higher degree of model fidelity as the research efforts transition from in vitro to in vivo model systems. Taken together, we hope that our investigations help advance new therapeutic modalities with the potential to decrease morbidity and mortality associated with infectious arthritis and periprosthetic joint infections.
Methods
Bacterial strains
The bacterial strains used in this study were clinical isolates derived from cases of equine septic arthritis collected by the Pennsylvania Animal Diagnostic Laboratory System (PADLS) New Bolton Center Clinical Microbiology Laboratory (S. aureus, S. zooepidemicus, E. coli and P. aeruginosa). In vitro antimicrobial susceptibility testing and microbial identification was performed using the Sensititre Complete Automated AST System and the equine (Equine EQUIN1F Vet AST Plate) antimicrobial susceptibility panel (Thermo Fisher Scientific, Waltham, MA). Breakpoint-associated minimum inhibitory concentrations (MIC) of each strain are presented in Table 1. Where indicated the laboratory strain of S. aureus, ATCC25923, was used as a well-characterized control strain. Antimicrobial susceptibility of this strain was determined as described for the clinical isolates. Bacteria were kept in frozen stocks on glycerol at -80°C. Blood agar plates were streaked from frozen stocks and used for in vitro experiments for a maximum of 1 week. Overnight cultures were made from the blood agar plates by taking one colony and adding to 30mL of tryptic soy broth (TSB); these cultures were made fresh for each experiment. On the day of an experiment, 100μL of an overnight culture was inoculated into 10mL of fresh TSB and grown to 0.5 McFarland (~3 hours) to ensure the bacteria were in the exponential phase of growth. Concentrations of cultures were confirmed using serial plate dilutions.
Synovial fluid collection
This study was approved by the Institutional Animal Care and Use Committees of The University of Pennsylvania and the North Carolina State University. Healthy horses free of orthopedic disease and free of medication for 48 hours prior to sampling were used for collection of synovial fluid. Synovial fluid samples were obtained from standing horses sedated with 0.005–0.01 mg/kg detomidine. All horses were well acclimated to standing under sedation for arthrocentesis, which is a short procedure. Both carpi were clipped and aseptically prepped along the dorsal aspect of the joints and 3–4 mL of synovial fluid was extracted from each joint. Following synovial fluid collection, 250mg of amikacin was injected into the joint through the same needle as a preventative measure, as is routinely performed in the clinical setting. Horses were monitored during the procedure and every 12 hours thereafter for 48 hours for signs of discomfort, pain/lameness, swelling at the site of collection, or other adverse effects, none of which were observed. Synovial fluid from both the right and left carpi were pooled among individual horses. Synovial fluid from pigs was collected post-mortem from healthy Yorkshires ~6 months of age. Pigs were part of an unrelated research study of an independent principal investigator at North Carolina State University and were euthanized with 60 mg/kg iv pentobarbital sodium following intramuscular sedation using xylazine (2mg/kg) and ketamine (20 mg/kg) and isofluorane until unconsciousness. Death was confirmed via auscultation. Human synovial fluid was purchased from Lee Biosolutions, Inc. (Maryland Heights, MO). Synovial fluid that was visually cloudy or otherwise abnormal was discarded. Synovial fluid was centrifuged at 1500g for 15 minutes to remove the cellular component and passed through a 40μM cell strainer to remove any large protein aggregates. The samples were stored at -20°C until use in the described experiments. All experiments were performed with four biological replicates (i.e. synovial fluid from four individual horses, humans or pigs).
Synovial fluid biofilm aggregates and planktonic growth conditions
Planktonic bacteria were grown in tryptic soy broth (TSB). Biofilm aggregates were grown in synovial fluid from the indicated mammalian species. All growth conditions were inoculated with 1x106 CFU/mL[17] of each bacterial strain in a microtiter plate (24-well or 6-well with 500μL or 2mL of media respectively) and incubated overnight (16–18 hours) in a microaerophilic chamber (AnaeroPack-MicroAero Gas Generator, Thermo Fisher Scientific, Waltham, MA) on a shaker at 120rpm at 37°C. Antimicrobial treatments and dispersal treatments were implemented during mid to late exponential phase or 6 hours post-infection (S4 Fig) and added to TSB or synovial fluid containing planktonic or biofilm aggregated bacteria respectively. Macroscopic images were taken with a Nikon D40 camera.
Confocal microscopy
Bacteria were stained with BacLight Green (Thermo Fisher Scientific, Waltham, MA) prior to inoculation of synovial fluid; after overnight culture in synovial fluid, macroscopic aggregates were gently removed from synovial fluid and washed three times with phosphate buffered saline (PBS). Aggregates were suspended in PBS and stained with wheat germ agglutinin (WGA, Thermo Fisher Scientific, Waltham, MA; 20μg/mL) for 15 min at room temperature in the dark. Supernatant containing WGA was removed and aggregates were stained with 1mL undiluted SYPRO (FilmTracer SYPRO Ruby Biofilm Matrix Stain, Thermo Fisher Scientific, Waltham, MA) for 30 min at room temperature in the dark. Thereafter, stain was removed and aggregates were washed three times with PBS before fixation in 2% paraformaldehyde. Aggregates were kept at 4°C until imaging. Imaging was performed using a Leica SP5 Confocal/Multiphoton Microscope at the PennVet Imaging Core.
Scanning electron microscopy (SEM)
Scanning electron microscopy images were attained by the staff at the Electron Microscopy Resource Laboratory (EMRL) at the University of Pennsylvania. In brief, aggregates were washed, fixed in glutaraldehyde, dehydrated, gold sputter coated and subsequently imaged with a scanning electron microscope at 3000x with a FEI-Tecnai T12 microscope at the PennMed Imaging Core.
Antimicrobial treatment
Results from the phenotypic susceptibility testing (Table 1) were used to estimate the MIC (μg/mL) by microbroth dilution of planktonic cultures using the Sensititre Complete Automated AST System and the equine (Equine EQUIN1F Vet AST Plate) antimicrobial susceptibility panel. The vancomycin MIC for S. aureus (ATCC25923) was 0.5μg/mL as determined by microbroth dilution following CLSI standards. Microtiter wells containing infected synovial fluid (biofilm aggregates) or TSB (planktonic) were treated with antimicrobials at 1×, 10× or 100× the reported MIC of each individual planktonic bacteria during mid to late exponential phase or 6 hours post-infection (S4 Fig). If an MIC was determined to be less than the lowest concentration evaluated, that evaluated concentration was used. For example, the MIC of Amikacin for S. aureus (ATCC25923) was ≤ 4μg/mL; therefore, bacteria was treated with amikacin at 4μg/mL for 1×MIC, 40μg/mL for 10×MIC, and 400μg/mL for 100×MIC. Antimicrobial treatments were carried out for 8 or 24 hours where indicated under the same growth conditions as the infective period. The infected TSB or synovial fluid was centrifuged at 8000g for 10 min and the supernatant was removed. The bacterial pellet was washed 3x with PBS and resuspended in 1mL of PBS containing 200μg/mL proteinaseK and incubated for 5–10 minutes on a shaker at 120rpm at 37°C to disperse the aggregates for enumeration of bacterial concentration by serial dilutions and plate counting of colony forming units (CFU/mL). This wash and proteinaseK step is critical for appropriate enumeration of bacteria as CFU/mL due to the inability to measure bacterial concentration within the biofilm aggregates. Dastgheyb et al. 2015 first showed the inaccuracy of measuring CFU/mL from intact aggregates and described the ability of proteinaseK to disperse aggregates for accurate enumeration of CFU/mL[17].
Dispersal treatment
ProteinaseK (200μg/mL), trypsin (200μg/mL), endopeptidase (200μg/mL), DNase (500μg/mL) collagenase type II (750μg/mL), collagenase type IV (750μg/mL), dispase (500μg/mL), DispersinB (100μg/mL), acetylcysteine (8mg/mL), and tissue plasminogen activator or TPA (1mg/mL) were used to test dispersal of biofilm aggregates. All enzymes were purchased from Sigma-Aldrich (St. Louis, MO) apart from DispersinB which was purchased from Kane Biotech (Winnipeg, Canada).The concentration of each enzyme was chosen as the highest concentration that did not exhibit bactericidal effects against planktonic bacteria grown in TSB. Each enzyme was incubated in the infected synovial fluid for 1 hour on a shaker at 120rpm at 37°C. Photographs of the dispersal treatment were taken with a Nikon D40 camera. Dispersal was evaluated by measuring optical density (OD) on a microtiter plate reader (Synergy 2, BioTek Instruments, Inc., Winooski, VT). Optical density was measured as an average of the absorbance (600nm) using a well-mode, or area scanning, method to ensure the entire well was measured. Dispersal was reported as a percentage compared to planktonic bacteria (each bacterial strain was used as its own internal control) in TSB at the same CFU/mL. Specifically, percentage dispersal was calculated as [(OD infected synovial fluid–OD uninfected synovial fluid)/(OD infected TSB–OD uninfected TSB)] X 100. This method was developed based on measurements and calculations of platelet aggregation[80–82]. Bacterial viability (CFU/mL) was measured post-dispersal and compared to the no treatment control to ensure that enzymatic treatment did not induce cell death. After 1 hour of dispersal, enzymatically treated synovial fluid samples containing biofilm aggregates were challenged with amikacin at 10× MIC for 8 hours. Bacterial viability (CFU/mL) was assessed post-dispersal and antimicrobial challenge using serial dilutions and colony counting as described above.
Statistics
Data was analyzed using 1-way non-parametric (Kruskal-Wallis Test) or 1-way/2-way ANOVA where applicable with Tukey's post hoc tests. Correlations were calculated using Spearman correlation coefficient. Analysis was performed using JMP Pro 11.0 software (SAS Institute Inc., Cary, NC). All graphs were generated using GraphPad Prism (GraphPad Software Inc., La Jolla California USA). For all comparisons, p<0.05 was considered statistically significant.
Supporting information
Equine synovial fluid was infected at 1x106 CFU/mL with S. aureus (ATCC25923) and incubated overnight at 37°C in a microaerophilic chamber on a shaker at 120rpm to mimic the joint environment. (A) S. aureus growth in synovial fluid over time was measured by treating synovial fluid with proteinaseK (20μg/mL) to disperse aggregates, followed by serial dilutions and plate counting for CFU/mL. (B) Biofilm aggregate formation was photographed at the same time as bacterial load determination.
(TIF)
S. aureus (ATCC25923) was grown planktonically in TSB for 6 hours and challenged with a panel of different antimicrobials from several drug classes at 100× the minimum inhibitory concentration (MIC) as determined by in vitro antimicrobial susceptibility testing (Table 1).
(TIF)
(A) Equine synovial fluid was either left untreated or pre-treated with hyaluronidase (1mg/mL) prior to infection with S. aureus (ATCC25923). Bacteria were added, incubated for 16 hours and either left untreated or post-treated with hyaluronidase (1mg/mL) for 1 hour. Thereafter, amikacin was added at 10× MIC (40μg/mL), incubated for 8 hours, and log CFU/mL was measured with serial dilutions and colony counting. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
(TIF)
Equine synovial fluid containing S. aureus (ATCC25923) biofilm aggregates were treated with: hyaluronidase (1mg/mL), proteinaseK (200μg/mL), trypsin (200μg/mL), endopeptidase or LysC (200μg/mL), DNase (500μg/mL), collagenase type II (750μg/mL), collagenase type IV (750μg/mL), dispase (500μg/mL), DispersinB (1mg/mL), acetylcysteine (8mg/mL) or tissue plasminogen activator or TPA (1mg/mL). Bacterial load (log CFU/mL) was measured with serial dilutions and colony counting 9 hours post-enzymatic treatment. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
(TIF)
Acknowledgments
The authors would like to thank Dr. Gordon Ruthel and the University of Pennsylvania School of Veterinary Medicine Microscopy Facility for the aid in acquisition of the confocal microscopy images, Dr. Ray Meade of the Biochemical Imaging Core Facility at the University of Pennsylvania for his assistance in generation of the scanning electron microscopy images, the North Carolina State University CVM Laboratory Animal Resources staff for their help with animal care and handling, and Ms. Anna Rogers in the Department of Population Health and Pathobiology at North Carolina State University CVM for her technical assistance with the microbiological assays.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by: (1) Raymond Firestone Trust (JMG and TPS), University of Pennsylvania Internal Grant; NO URL; (2) National Institutes of Health (NIH) under award number R01 AR072513 (NJH, IMS and TPS), https://www.niams.nih.gov/; (3) The Fund for Orthopedic Research in honor of Gus and Equine athletes (F.O.R.G.E; LVS), private foundation through The Schnabel Laboratory at North Carolina State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tong SYCC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. American Society for Microbiology (ASM); 2015;28: 603–661. 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. Elsevier; 2010;375: 846–855. 10.1016/S0140-6736(09)61595-6 [DOI] [PubMed] [Google Scholar]
- 3.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. American Society for Microbiology; 2002;15: 527–544. 10.1128/CMR.15.4.527-544.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466: 1710–1715. 10.1007/s11999-008-0209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. American Society for Microbiology (ASM); 2014;27: 302–345. 10.1128/CMR.00111-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, et al. A Large Multicenter Study of Methicillin–Susceptible and Methicillin–Resistant Staphylococcus aureus Prosthetic Joint Infections Managed With Implant Retention. Clin Infect Dis. Oxford University Press; 2013;56: 182–194. 10.1093/cid/cis746 [DOI] [PubMed] [Google Scholar]
- 7.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. NIH Public Access; 2008;322: 207–28. Available: http://www.ncbi.nlm.nih.gov/pubmed/18453278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. NIH Public Access; 2014;9: 987–1007. 10.2217/fmb.14.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65: 158–168. 10.1111/j.1574-695X.2012.00938.x [DOI] [PubMed] [Google Scholar]
- 10.Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. John Wiley & Sons, Ltd (10.1111); 2017;125: 353–364. 10.1111/apm.12687 [DOI] [PubMed] [Google Scholar]
- 11.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11: 1034–1043. 10.1111/j.1462-5822.2009.01323.x [DOI] [PubMed] [Google Scholar]
- 12.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, et al. The in vivo biofilm. Trends Microbiol. 2013;21: 466–474. 10.1016/j.tim.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. Webber MA, editor. PLoS One. Public Library of Science; 2011;6: e27943 10.1371/journal.pone.0027943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmølle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homøe P, et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59: 324–336. 10.1111/j.1574-695X.2010.00714.x [DOI] [PubMed] [Google Scholar]
- 15.Kragh KN, Hutchison JB, Melaugh G, Rodesney C, Roberts AEL, Irie Y, et al. Role of Multicellular Aggregates in Biofilm Formation. MBio. American Society for Microbiology (ASM); 2016;7: e00237 10.1128/mBio.00237-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay YDN, Labrie J, Chénier S, Jacques M. Actinobacillus pleuropneumoniae grows as aggregates in the lung of pigs: is it time to refine our in vitro biofilm assays? Microb Biotechnol. Wiley-Blackwell; 2017;10: 756–760. 10.1111/1751-7915.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211: 641–650. 10.1093/infdis/jiu514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dastgheyb SS, Villaruz AE, Le KY, Tan VY, Duong AC, Chatterjee SS, et al. Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Camilli A, editor. Infect Immun. 2015;83: 2966–2975. 10.1128/IAI.00394-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dastgheyb SS, Hammoud S, Ketonis C, Liu AY, Fitzgerald K, Parvizi J, et al. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob Agents Chemother. American Society for Microbiology; 2015;59: 2122–2128. 10.1128/AAC.04579-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez K, Patel R. Biofilm-like aggregation of Staphylococcus epidermidis in synovial fluid. J Infect Dis. 2015;212: 335–336. 10.1093/infdis/jiv096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post JC. Direct Evidence of Bacterial Biofilms in Otitis Media. Laryngoscope. 2001;111: 2083–2094. 10.1097/00005537-200112000-00001 [DOI] [PubMed] [Google Scholar]
- 22.Bay L, Kragh KN, Eickhardt SR, Poulsen SS, Gjerdrum LMR, Ghathian K, et al. Bacterial Aggregates Establish at the Edges of Acute Epidermal Wounds. Adv wound care. Mary Ann Liebert, Inc.; 2018;7: 105–113. 10.1089/wound.2017.0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landry RM, An D, Hupp JT, Singh PK, Parsek MR. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol Microbiol. 2006;59: 142–151. 10.1111/j.1365-2958.2005.04941.x [DOI] [PubMed] [Google Scholar]
- 24.Stewart PS. Antimicrobial Tolerance in Biofilms. Microb Biofilms, 2nd Ed. NIH Public Access; 2015;3: 269–285. 10.1128/microbiolspec.MB-0010-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrado A, Donato P, MacCari S, Cecchi R, Spadafina T, Arcidiacono L, et al. Staphylococcus aureus-dependent septic arthritis in murine knee joints: Local immune response and beneficial effects of vaccination. Sci Rep. Nature Publishing Group; 2016;6: 38043 10.1038/srep38043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Cheng LI, Helfer DR, Ashbaugh AG, Miller RJ, Tzomides AJ, et al. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci. National Academy of Sciences; 2017;114: 201703427 10.1073/pnas.1703427114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aigner T, Cook JL, Gerwin N, Glasson SS, Laverty S, Little CB, et al. Histopathology atlas of animal model systems—overview of guiding principles. Osteoarthr Cartil. 2010;18: S2–6. 10.1016/j.joca.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 28.Moran CJ, Ramesh A, Brama PAJ, O JM, O FJ, O’Byrne JM, et al. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. Springer; 2016;3: 1 10.1186/s40634-015-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker H V., Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. National Academy of Sciences; 2013;110: 3507–3512. 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mage RG, Esteves PJ, Rader C. Rabbit models of human diseases for diagnostics and therapeutics development. Dev Comp Immunol. Pergamon; 2019;92: 99–104. 10.1016/j.dci.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barton NJ, Stevens DA, Hughes JP, Rossi AG, Chessell IP, Reeve AJ, et al. Demonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee joints. J Inflamm (Lond). BioMed Central; 2007;4: 13 10.1186/1476-9255-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifer DR, Furman BD, Guilak F, Olson SA, Brooks SC, Kraus VB, et al. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthr Cartil. NIH Public Access; 2008;16: 1532–8. 10.1016/j.joca.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wayne Mcilwraith C, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2: 317–326. 10.1177/1947603511406531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCoy AM. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet Pathol. SAGE PublicationsSage CA: Los Angeles, CA; 2015;52: 803–818. 10.1177/0300985815588611 [DOI] [PubMed] [Google Scholar]
- 35.McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Jt Res. 2012;1: 297–309. 10.1302/2046-3758.111.2000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtig MB, Buschmann MD, Fortier LA, Hoemann CD, Hunziker EB, Jurvelin JS, et al. Preclinical studies for cartilage repair: Recommendations from the international cartilage repair society. Cartilage. 2011;2: 137–152. 10.1177/1947603511401905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCoy AM. Animal Models of Osteoarthritis. Vet Pathol. SAGE PublicationsSage CA: Los Angeles, CA; 2015;52: 803–818. 10.1177/0300985815588611 [DOI] [PubMed] [Google Scholar]
- 38.Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J Orthop Surg Res. BioMed Central; 2016;11: 19 10.1186/s13018-016-0346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horohov DW. The equine immune responses to infectious and allergic disease: A model for humans? Mol Immunol. Pergamon; 2015;66: 89–96. 10.1016/j.molimm.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 40.Mair KH, Sedlak C, Käser T, Pasternak A, Levast B, Gerner W, et al. The porcine innate immune system: An update. Dev Comp Immunol. 2014;45: 321–343. 10.1016/j.dci.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as Models in Biomedical Research and Toxicology Testing. Vet Pathol. SAGE PublicationsSage CA: Los Angeles, CA; 2012;49: 344–356. 10.1177/0300985811402846 [DOI] [PubMed] [Google Scholar]
- 42.Gilbertie JM, Schnabel LV, Stefanovski D, Kelly DJ, Jacob ME, Schaer TP. Gram-negative multi-drug resistant bacteria influence survival to discharge for horses with septic synovial structures: 206 Cases (2010–2015). Vet Microbiol. 2018;226 10.1016/j.vetmic.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 43.Ibberson CB, Parlet CP, Kwiecinski J, Crosby HA, Meyerholz DK, Horswill AR. Hyaluronan modulation impacts Staphylococcus aureus biofilm infection. Camilli A, editor. Infect Immun. 2016;84: 1917–1929. 10.1128/IAI.01418-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boles BR, Horswill AR. agr-mediated dispersal of Staphylococcus aureus biofilms. Cossart P, editor. PLoS Pathog. Public Library of Science; 2008;4: e1000052 10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H, Moser C, Wang H-ZZ, Høiby N, Song Z-JJ. Strategies for combating bacterial biofilm infections. Int J Oral Sci. Nature Publishing Group; 2015;7: 1–7. 10.1038/ijos.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleming D, Rumbaugh K. The Consequences of Biofilm Dispersal on the Host. Sci Rep. Nature Publishing Group; 2018;8: 10738 10.1038/s41598-018-29121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gries CM, Kielian T. Staphylococcal biofilms and immune polarization during prosthetic joint infection. J Am Acad Orthop Surg. 2017;25: S20–S24. 10.5435/JAAOS-D-16-00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Virulence Mechanisms of Bacterial Pathogens, Fifth Edition. NIH Public Access; 2016. pp. 529–566. 10.1128/microbiolspec.VMBF-0022-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanke ML, Kielian T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front Cell Infect Microbiol. Frontiers Media SA; 2012;2: 62 10.3389/fcimb.2012.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto N, Schumacher J, Taintor J, Degraves F, Duran S, Boothe D. Pharmacokinetics of amikacin in plasma and selected body fluids of healthy horses after a single intravenous dose. 2010; 10.1111/j.2042-3306.2010.00144.x [DOI] [PubMed] [Google Scholar]
- 51.Nieto JE, Trela J, Stanley SD, Yamout S, Snyder JR. Pharmacokinetics of a combination of amikacin sulfate and penicillin G sodium for intravenous regional limb perfusion in adult horses. Can J Vet Res. Canadian Veterinary Medical Association; 2016;80: 230–5. Available: http://www.ncbi.nlm.nih.gov/pubmed/27408337 [PMC free article] [PubMed] [Google Scholar]
- 52.Taintor J, Schumacher J, DeGraves F. Comparison of amikacin concentrations in normal and inflamed joints of horses following intra-articular administration. Equine Vet J. 2006;38: 189–91. Available: http://www.ncbi.nlm.nih.gov/pubmed/16536391 [DOI] [PubMed] [Google Scholar]
- 53.Murphey ED, Santschi EM, Papich MG. Regional intravenous perfusion of the distal limb of horses with amikacin sulfate. J Vet Pharmacol Ther. 1999;22: 68–71. Available: http://www.ncbi.nlm.nih.gov/pubmed/10211721 [DOI] [PubMed] [Google Scholar]
- 54.Walmsley EA, Anderson GA, Muurlink MA, Whitton RC. Retrospective investigation of prognostic indicators for adult horses with infection of a synovial structure. Aust Vet J. 2011;89: 226–231. 10.1111/j.1751-0813.2011.00720.x [DOI] [PubMed] [Google Scholar]
- 55.Taylor AH, Mair TS, Smith LJ, Perkins JD. Bacterial culture of septic synovial structures of horses: Does a positive bacterial culture influence prognosis? Equine Vet J. Blackwell Publishing Ltd; 2010;42: 213–218. 10.2746/042516409X480403 [DOI] [PubMed] [Google Scholar]
- 56.Kirchner S, Fothergill JL, Wright EA, James CE, Mowat E, Winstanley C. Use of Artificial Sputum Medium to Test Antibiotic Efficacy Against <em>Pseudomonas aeruginosa</em> in Conditions More Relevant to the Cystic Fibrosis Lung. J Vis Exp. MyJoVE Corporation; 2012; e3857 doi: 10.3791/3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haley CL, Colmer-Hamood JA, Hamood AN. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol. BioMed Central; 2012;12: 181 10.1186/1471-2180-12-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sønderholm M, Kragh KN, Koren K, Jakobsen TH, Darch SE, Alhede M, et al. Pseudomonas aeruginosa aggregate formation in an alginate bead model system exhibits in vivo-like characteristics. Appl Environ Microbiol. American Society for Microbiology (ASM); 2017;83 10.1128/AEM.00113-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Secor PR, Michaels LA, Ratjen A, Jennings LK, Singh PK. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc Natl Acad Sci. 2018;115: 10780–10785. 10.1073/pnas.1806005115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37: 1771–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/10325322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lorian V, Atkinson BA. Determination of the Range of Antibacterial Activity by Use of Viable Counts. Journal of Clinical Microbiology. 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen LK, Bjarnsholt T, Kragh KN, Aalbæk B, Henriksen NL, Blirup SA, et al. In Vivo Gentamicin Susceptibility Test for Prevention of Bacterial Biofilms in Bone Tissue and on Implants. Antimicrob Agents Chemother. American Society for Microbiology Journals; 2019;63: e01889–18. 10.1128/AAC.01889-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mottola C, Matias CS, Mendes JJ, Melo-Cristino J, Tavares L, Cavaco-Silva P, et al. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16: 119 10.1186/s12866-016-0737-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolt DM, Ishihara A, Weisbrode SE, Bertone AL. Effects of triamcinolone acetonide, sodium hyaluronate, amikacin sulfate, and mepivacaine hydrochloride, alone and in combination, on morphology and matrix composition of lipopolysaccharide-challenged and unchallenged equine articular cartilage explants. Am J Vet Res. 2008;69: 861–867. 10.2460/ajvr.69.7.861 [DOI] [PubMed] [Google Scholar]
- 65.Gatell JM, San Miguel JG, Zamora L, Araujo V, Bonet M, Bohé M, et al. Comparison of the nephrotoxicity and auditory toxicity of tobramycin and amikacin. Antimicrob Agents Chemother. 1983;23: 897–901. Available: http://www.ncbi.nlm.nih.gov/pubmed/6614894 10.1128/aac.23.6.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem on Mucoid and Nonmucoid Pseudomonas aeruginosa Biofilms. Antimicrob Agents Chemother. 2011;55: 4469–4474. 10.1128/AAC.00126-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. In Vivo Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem in Pseudomonas aeruginosa Biofilm Infection. Antimicrob Agents Chemother. 2012;56: 2683–2690. 10.1128/AAC.06486-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belfield K, Bayston R, Birchall JP, Daniel M. Do orally administered antibiotics reach concentrations in the middle ear sufficient to eradicate planktonic and biofilm bacteria? A review. Int J Pediatr Otorhinolaryngol. 2015;79: 296–300. 10.1016/j.ijporl.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 69.Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, et al. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. American Society for Microbiology; 2004;186: 4665–4684. 10.1128/JB.186.14.4665-4684.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Gara JP. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270: 179–188. 10.1111/j.1574-6968.2007.00688.x [DOI] [PubMed] [Google Scholar]
- 71.Arciola CR, Campoccia D, Ravaioli S, Montanaro L. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front Cell Infect Microbiol. Frontiers; 2015;5: 7 10.3389/fcimb.2015.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gawande P V., Leung KP , Madhyastha S. Antibiofilm and antimicrobial efficacy of Dispersinb®-KSL-w peptide-based wound gel against chronic wound infection associated bacteria. Curr Microbiol. 2014;68: 635–641. 10.1007/s00284-014-0519-6 [DOI] [PubMed] [Google Scholar]
- 73.Crosby HA, Kwiecinski J, Horswill AR. Staphylococcus aureus Aggregation and Coagulation Mechanisms, and Their Function in Host–Pathogen Interactions. Advances in Applied Microbiology. NIH Public Access; 2016. pp. 1–41. 10.1016/bs.aambs.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against Experimental Staphylococcus aureus Arthritis by Vaccination with Clumping Factor A, a Novel Virulence Determinant. J Infect Dis. Oxford University Press; 2001;184: 1572–1580. 10.1086/324430 [DOI] [PubMed] [Google Scholar]
- 75.Matsuzaka S, Sato S, Miyauchi S. Estimation of joint fluid volume in the knee joint of rabbits by measuring the endogenous calcium concentration. Clin Exp Rheumatol. 20: 531–4. Available: http://www.ncbi.nlm.nih.gov/pubmed/12175108 [PubMed] [Google Scholar]
- 76.Nakano T, Aherne FX, Thompson JR. Relative amounts of chondroitin sulfate and hyaluronic acid in synovial fluid from normal and osteochondrotic swine joints. [Internet]. Canadian journal of comparative medicine Canadian Veterinary Medical Association; October, 1984. pp. 434–6. Available: https://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC1236101&blobtype=pdf [PMC free article] [PubMed] [Google Scholar]
- 77.Van Pelt RW. Characteristics of normal equine tarsal synovial fluid. Can J Comp Med Vet Sci. Canadian Veterinary Medical Association; 1967;31: 342–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/4229934 [PMC free article] [PubMed] [Google Scholar]
- 78.Borg H, Carmalt JL. Postoperative Septic Arthritis After Elective Equine Arthroscopy Without Antimicrobial Prophylaxis. Vet Surg. 2013;42: 262–266. 10.1111/j.1532-950X.2013.01106.x [DOI] [PubMed] [Google Scholar]
- 79.Morton AJ. Diagnosis and treatment of septic arthritis. Vet Clin North Am—Equine Pract. 2005;21: 627–649. 10.1016/j.cveq.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 80.Gilbertie JM, Davis JL, Davidson GS, McDonald AM, Schirmer JM, Schnabel L V. Oral reserpine administration in horses results in low plasma concentrations that alter platelet biology. Equine Vet J. 2018; evj.13048 10.1111/evj.13048 [DOI] [PubMed] [Google Scholar]
- 81.Jarvis GE . Platelet Aggregation: Turbidimetric Measurements Platelets and Megakaryocytes. New Jersey: Humana Press; 2004. pp. 065–076. 10.1385/1-59259-782-3:065 [DOI] [PubMed] [Google Scholar]
- 82.Zhou L, Schmaier AH. Platelet aggregation testing in platelet-rich plasma: description of procedures with the aim to develop standards in the field. Am J Clin Pathol. 2005;123: 172–83. Available: http://www.ncbi.nlm.nih.gov/pubmed/15842039 10.1309/y9ec-63rw-3xg1-v313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Equine synovial fluid was infected at 1x106 CFU/mL with S. aureus (ATCC25923) and incubated overnight at 37°C in a microaerophilic chamber on a shaker at 120rpm to mimic the joint environment. (A) S. aureus growth in synovial fluid over time was measured by treating synovial fluid with proteinaseK (20μg/mL) to disperse aggregates, followed by serial dilutions and plate counting for CFU/mL. (B) Biofilm aggregate formation was photographed at the same time as bacterial load determination.
(TIF)
S. aureus (ATCC25923) was grown planktonically in TSB for 6 hours and challenged with a panel of different antimicrobials from several drug classes at 100× the minimum inhibitory concentration (MIC) as determined by in vitro antimicrobial susceptibility testing (Table 1).
(TIF)
(A) Equine synovial fluid was either left untreated or pre-treated with hyaluronidase (1mg/mL) prior to infection with S. aureus (ATCC25923). Bacteria were added, incubated for 16 hours and either left untreated or post-treated with hyaluronidase (1mg/mL) for 1 hour. Thereafter, amikacin was added at 10× MIC (40μg/mL), incubated for 8 hours, and log CFU/mL was measured with serial dilutions and colony counting. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
(TIF)
Equine synovial fluid containing S. aureus (ATCC25923) biofilm aggregates were treated with: hyaluronidase (1mg/mL), proteinaseK (200μg/mL), trypsin (200μg/mL), endopeptidase or LysC (200μg/mL), DNase (500μg/mL), collagenase type II (750μg/mL), collagenase type IV (750μg/mL), dispase (500μg/mL), DispersinB (1mg/mL), acetylcysteine (8mg/mL) or tissue plasminogen activator or TPA (1mg/mL). Bacterial load (log CFU/mL) was measured with serial dilutions and colony counting 9 hours post-enzymatic treatment. Bars are means and standard deviations of four biological replicates (n = 4), and significant differences (p<0.05) as determined by ANOVA with Tukey post-hoc are indicated by differing letters.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.