Highlights
-
•
Many factors confer increased risk of recurrent endocarditis.
-
•
Often many of these factors co-exist in the same host.
-
•
There is always the risk of residual infected endocardial tissue despite repeated debridement.
-
•
The traditional 4–6 week parenteral course in endocarditis accounts for bacterial tolerance and antimicrobial resistance.
-
•
The decision on antimicrobial therapy and duration are supported by evidence but should be individualized.
Keywords: Corynebacterium jeikeium endocarditis, Infective endocarditis, Recurrent endovascular infection
Abstract
A 53-year-old woman with end-stage renal impairment on hemodialysis was evaluated for recurrent episodes of Corynebacterium jeikeium bacteremia and endocarditis. Her recurrences occurred despite surgical debridement and extended courses of culture-directed antimicrobial therapy. The clinical course was complicated by the requirement of various endovascular prostheses for vascular access. To our knowledge this degree of relapse with guideline medical and surgical therapy is rare and challenges current practice and understanding of this group of infection.
Introduction
Infective endocarditis is a biofilm associated disease. These biofilms can contribute to antimicrobial resistance and have been the probable cause of many recurrent implant-associated infections [1]. The Corynebacterium species was traditionally classified as culture contaminants, but have become associated with life-threatening infections in immunocompromised hosts [2,3]. Our case highlights many of the challenges with treating recurrent endovascular infections.
Case presentation
A 53-year-old woman was referred to our Emergency department (ED) after developing fever and rigors with worsening dyspnea three hours following an outpatient fluoroscopic Port-A-Cath® placement. This was to facilitate outpatient weekly transfusion of blood products. The patient denied any prior subjective fever, upper respiratory or gastrointestinal symptoms. Her co-morbidities included: end-stage renal disease related to focal segmental glomerulosclerosis (FSGS) on thrice weekly hemodialysis via a left brachia-axial polytetrafluoroethylene (PTFE) AV graft (created 5 years prior), native aortic and mitral, followed by subsequent prosthetic aortic valve Corynebacterium jeikeium endocarditis (six and three months prior) and a transfusion-dependent autoimmune hemolytic anemia and thrombocytopenia on daily prednisone 10 mg.
On arrival to the ED her blood pressure was 138/94 mmHg, pulse rate 144bpm, respiratory rate 36 breaths/min, oral temperature 98.5 °F and an oxygen saturation 98% on 3 L/min oxygen (90% on room air). Her clinical examination was consistent with decompensated biventricular failure with respiratory failure. The Port-A-Cath® site was clean and dry and the AV graft site was without erythema or discoloration. Complete blood count revealed a hemoglobin 7.3 g/dL, white blood cell (WBC) count 6,000 cells/μL with 85% neutrophils and a platelet count 46,000 cells/μL. Her comprehensive metabolic panel was at baseline approaching next hemodialysis session. Our patient was commenced on empiric antimicrobials with vancomycin and meropenem for possible health-care associated infection before being admitted to a critical care unit.
Admission peripheral blood cultures showed gram positive rods which later were identified as C. jeikeium. Transesophageal echocardiogram (TEE) revealed evidence of a 0.9 cm × 0.6 cm prosthetic aortic valve abscess. There was severe mitral regurgitation with a mobile mass present on the posterior mitral valve leaflet (Fig. 1). Clearance of bacteremia was achieved within 72 h. Antibiotics were deescalated to vancomycin monotherapy. Cardiothoracic surgical intervention was delayed pending investigation for a source of intermittent bacteremia. Indium-111 WBC scan showed abnormally increased uptake in the region of the heart in-keeping with infective endocarditis, without abnormal uptake in the area of the AV graft.
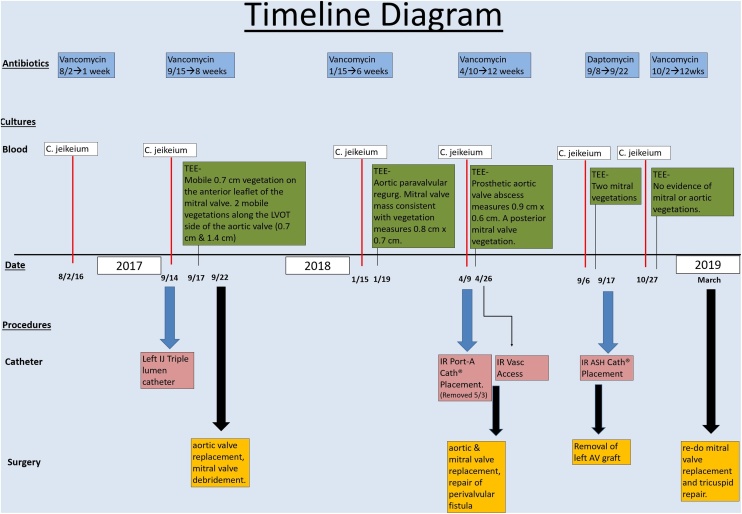
Fig. 1.
Timeline diagram outlining patient’s clinical course over two [2] year period.
Ultimately, she underwent repeat surgery involving aortic valve replacement with a 21 mm Inspiris Edwards® biological valve, repair of perivalvular fistula with homologous pericardial patch, and replacement of mitral valve with a 25 mm Magna Ease® mitral valve prosthesis. Her post-operative course was complicated by thrombosis of her AV graft with subsequent successful thrombectomy. She required an internal jugular Ash Split Cath™ for interim hemodialysis (HD) and was discharged home to complete 6 weeks of vancomycin with HD.
Four months later, she re-presented with evidence of decompensated heart failure. One week into her hospitalization she was persistently febrile and blood cultures grew C. jeikeium. She was started on daptomycin 6 mg/kg every 48 h (Fig. 1). TEE showed two mobile echogenic masses on the bioprosthetic mitral valve, most consistent with vegetation. Her bacteremia cleared within 72 h. The cardiothoracic team decided there was a high risk of re-infection of a replacement valve without removal of the AV graft. Final AV graft tissue cultures did not isolate bacteria.
Isolate referral of her C. jeikeium species was sent to Yale New Haven Hospital, CT laboratory for susceptibility testing. The organism was identified as Corynebacterium jeikeium which was resistant to ciprofloxacin (MIC = 8 mcg/mL), ceftriaxone (MIC = 8mcg/mL), but sensitive to daptomycin (MIC = 0.38 mcg/mL), doxycycline (MIC = 0.50 mcg/mL) and vancomycin (MIC = 0.75 mcg/mL). She later completed a 6-week daptomycin course.
Four weeks later blood cultures drawn while undergoing HD via a tunneled Ash Split Cath™ isolated gram-positive rods. Our patient was admitted and switched to vancomycin. TEE showed no vegetations. MRI of the lumbar spine showed no evidence of diskitis, osteomyelitis or epidural abscess. After confirmation of bacteremia clearance her dialysis catheter was changed. She was discharged on intravenous vancomycin with HD, with the aim of transitioning to oral suppressive therapy with doxycycline. However, after a 12-week course of vancomycin with negative cultures, she was observed for one month off antimicrobials before proceeding with surgery.
At the time of writing this report, she has undergone re-do mitral valve replacement and tricuspid repair. The post-op course has been uneventful and valve tissue cultures have not isolated bacteria.
Discussion
Coryneform bacteria, or ‘diphtheroid’ species are pleomorphic, aerobic, non-sporulating gram-positive rods which are found as constituents of normal skin flora and have been traditionally regarded as contaminants when isolated in clinical specimens [4,5]. Corynebacterium endocarditis has a predilection for left-sided valves and over a third of patients have pre-existing valvular heart disease. Additional risk factors are previous episodes of endocarditis, end-stage-renal disease, presence of prosthetic devices, intravascular catheters and atrial-ventricular cerebrospinal fluid shunts [6,7].
It is often uncertain when the same organism is isolated on multiple occasions whether it represents relapse or reinfection [8]. It is reasonable given the time course between recurrences, that our patient has had ‘relapse’ of the same C. jeikeium bacteremia. A hypothesis is that despite extended courses of antimicrobial therapy, our patient maintained an endovascular site of bacterial colonization, resulting in repeated inoculation of prosthetic material. There is always the risk of residual infected tissue despite repeated debridement, and negative culture specimens. This is compounded by an almost non-existent ‘prosthetic-free interval’. This case generates a few questions: what is the source of relapse?; where was the original inoculum? what is the appropriate duration of antimicrobial therapy? and is there a role for life-long suppression therapy?
Intraoperative contamination remains the most common cause of vascular graft infections (VGIs). One of the least common causes is bacteremia, and the risk of hematogenous seeding decreases after >2 months due to endothelization [9]. One hypothesis was that the PTFE graft was a nidus for recurrent bacterial seeding in our patient’s case. A negative tissue culture does not eliminate this possibility. However, the IE recurrence after graft removal raises the possibility of an alternative explanation.
Many C. jeikeium species have shown susceptibility to vancomycin and daptomycin, which is similar to the sensitivity profile in our case. C. jeikeium species have shown resistance to many beta-lactam antimicrobials, doxycycline, ciprofloxacin, clindamycin and imipenem [10]. This resistance pattern makes the choice of a chronic oral suppression regimen very difficult. Two of the challenges in treating IE are bacterial tolerance and antimicrobial resistance [11]. Tolerance describes the survival of more resilient bacterial phenotypes in the presence of antimicrobial exposure. The traditional 4–6 week parenteral requirement for therapy was designed to account for the slow bactericidal effects of antimicrobials as well as the risk of tolerance.
Conclusion
Endovascular infections present unique clinical challenges. The timing of any surgical intervention, as well as the decision on antimicrobial therapy and duration are supported by evidence but are often individualized. Patients with endovascular prostheses, immunocompromised states or frequent exposure to nosocomial pathogens, add to treatment complexity. Although our case is uncommon in its time course and relapse rate, it highlights many of the fundamental approaches to treatment as well as raises potential new considerations in this patient population.
Funding source
The authors have no funding source to declare.
Ethical approval
Ethics approval was not applicable.
Author statement
All authors have read and approved the final version of the manuscript for submission.
CRediT authorship contribution statement
John-Ross D. Clarke: Conceptualization, Writing - original draft. Manal Abdur Rahman: Writing - review & editing. Zane Saul: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.Neguț A.C., Streinu-Cercel A., Moțoi M.M., Bradu L., Berciu I., Streinu-Cercel O. Recurrent infective endocarditis due to probable biofilm formation on cardiac stimulator probe. BMC Infect Dis. 2013;13(Suppl. 1) [Google Scholar]
- 2.Murray B.E., Karchmer A.W., Moellering R.C., Jr. Diphtheroid prosthetic valve endocarditis. A study of clinical features and infecting organisms. Am J Med. 1980;69(6):838–848. doi: 10.1016/s0002-9343(80)80009-x. [DOI] [PubMed] [Google Scholar]
- 3.Berbari E.C.F., Steckelberg J. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 4.von Graevenitz A., Punter V., Gruner E., Pfyffer G.E., Funke G. Identification of coryneform and other gram-positive rods with several methods. APMIS. 1994;102:381–389. doi: 10.1111/j.1699-0463.1994.tb04887.x. [DOI] [PubMed] [Google Scholar]
- 5.Funke G., von Graevenitz A., Clarridge J.E., 3rd, Bernard K.A. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10(1):125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao C.T., Huang J.W., Yen C.J. A rare and under-recognized pathogen in peritoneal dialysis peritonitis: Corynebacterium jeikeium. Perit Dial Int. 2013;33(5):580–581. doi: 10.3747/pdi.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knox K.H.A. Nosocomial endocarditis caused by Corynebacterium emycolatum and other nondiphtheriae corynebacteria. Emerg Infect Dis. 2002;1:97–99. doi: 10.3201/eid0801.010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu V.H., Sexton D.J., Cabell C.H., Reller L.B., Pappas P.A., Singh R.K. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis. 2005;41(3):406–409. doi: 10.1086/431590. [DOI] [PubMed] [Google Scholar]
- 9.Wilson W.R., Bower T.C., Creager M.A., Amin-Hanjani S., O’Gara P.T., Lockhart P.B. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134(20) doi: 10.1161/CIR.0000000000000457. e412–e60. [DOI] [PubMed] [Google Scholar]
- 10.Soriano F. Eva nieto antimicorbial susceptibilities of Corynebacterium species and other non-Spore-Forming gram-positive bacilli to 18 antimicocrobial agents. Antimicrob Agents Chemother. 1995;39:208–214. doi: 10.1128/aac.39.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill T.J., Baddour L.M., Habib G., Hoen B., Salaun E., Pettersson G.B. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69(3):325–344. doi: 10.1016/j.jacc.2016.10.066. [DOI] [PubMed] [Google Scholar]