Abstract
IN BRIEF Insulin pump therapy is advancing rapidly. This article summarizes the variety of insulin pump technologies available to date and discusses important clinical considerations for each type of technology.
Continuous subcutaneous insulin infusion (CSII), more commonly referred to as insulin pump therapy, is one of the most notable advancements in diabetes technology in the past 50 years. The first commercial insulin pumps were on the market as early as the 1970s; however, rapid uptake of insulin pump technology did not occur until the early 2000s, after the conclusion of the landmark Diabetes Control and Complications Trial (DCCT) in the early 1990s. The DCCT demonstrated the importance of intensive insulin therapy to maintain tight glycemic control and prevent diabetes complications such as retinopathy, neuropathy, nephropathy, and cardiovascular disease (1–4).
Since the conclusion of the DCCT, insulin pump technology has advanced rapidly in an attempt to more closely mimic physiologic insulin secretion and help patients achieve tight glycemic control while minimizing the risk of hypoglycemia. As a result, use of insulin pumps has increased dramatically in the United States from <7,000 users in 1990 to nearly 100,000 users in 2000 (3) and >350,000 users today (5). The majority of insulin pump users have type 1 diabetes, although ∼10% have type 2 diabetes (5). According to the T1D Exchange registry, >60% of individuals within the T1D Exchange use an insulin pump (6) instead of a multiple daily injection (MDI) regimen for intensive insulin therapy. Additionally, the use of insulin pump therapy for individuals with type 2 diabetes is increasing (7,8).
There are many advantages to using an insulin pump compared to an MDI regimen. Insulin pump therapy allows for more precise and flexible insulin dosing with fewer injections. Many individuals with type 1 diabetes report using insulin pumps because they want improved glycemic control and a more flexible lifestyle than is afforded with MDI therapy, especially around meals and social situations (9). Many studies and systematic reviews have demonstrated improved glycemic control and a reduction in hypoglycemia with insulin pump therapy compared to MDI in pediatric and adult populations with type 1 diabetes (10–17). Although some randomized controlled trials have shown no difference in glycemic control in young children (<7 years of age) when comparing insulin pump therapy to MDI (18,19), parental satisfaction with insulin pump therapy is high (20). Further, insulin pumps offer many advantages in managing unpredictable eating habits and low insulin requirements in the youngest children (21), suggesting that insulin pump therapy may be an ideal option for many young children with type 1 diabetes and their families.
Overall, insulin pump technology is evolving at an extraordinary rate, with new technologies becoming available every year. The integration of insulin pumps with continuous glucose monitoring (CGM) systems has drastically expanded the insulin pump market with “smarter” insulin pumps that suspend insulin for hypoglycemia or even automate some insulin delivery, all with the goal of helping individuals meet glycemic targets with less burden (22,23). However, this rapid technological progression can be overwhelming for individuals with diabetes and their health care providers. Thus, the purposes of this article are 1) to provide an overview of insulin pump technologies, from simple, disposable pumps designed for those with type 2 diabetes to complex automated insulin delivery systems and 2) to discuss the clinical implications of these insulin pump technologies.
Conventional Insulin Pump Therapy
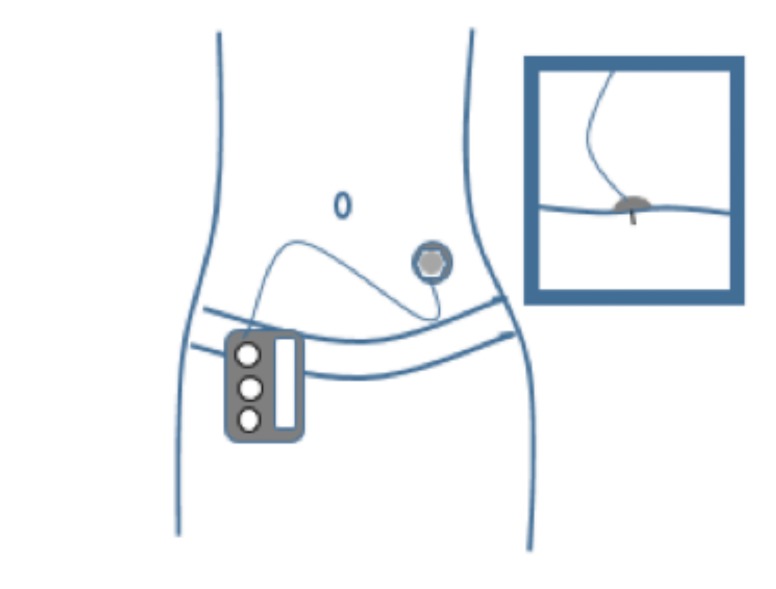
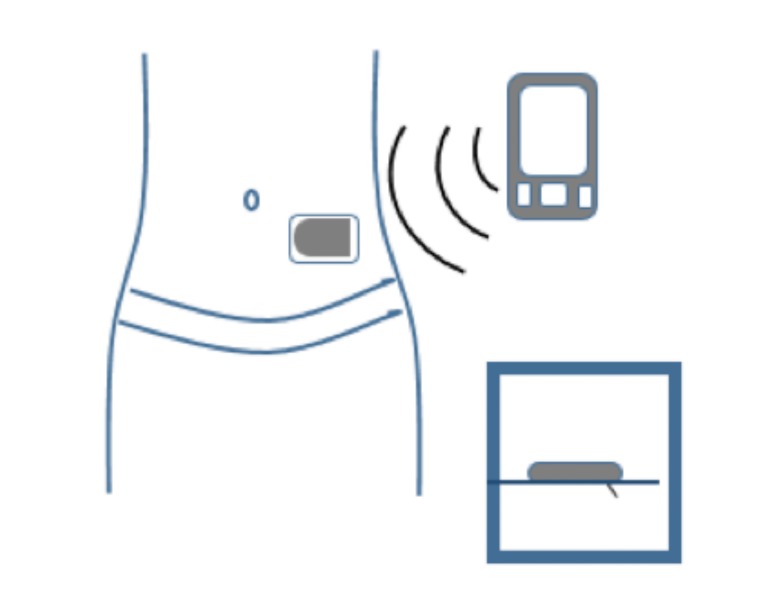
An insulin pump is a small, digital device that continuously delivers rapid-acting insulin through a small catheter inserted into the subcutaneous tissue and secured in place on the skin with adhesive (referred to as an “infusion set” or “infusion cannula”). In most insulin pumps, the infusion set connects to the pump by plastic tubing, and insulin infuses from the pump through the tubing to the infusion set cannula and into the subcutaneous tissue (Figure 1). Some pumps, referred to as “patch pumps,” do not use tubing and instead adhere directly to the skin. Patch pumps deliver insulin through the infusion cannula and are programmed from a remote device using wireless technology (Figure 2) (24).
FIGURE 1.
Insulin pump with tubing. The tubing connects the insulin pump, which contains the reservoir where the insulin is held, to the infusion cannula inserted in the subcutaneous tissue.
FIGURE 2.
Insulin pump without tubing (“patch pump”). Tubeless patch pumps contain the insulin reservoir and the infusion cannula and adhere directly to the skin. A handheld device is used to program insulin delivery and insert the infusion cannula.
Insulin pumps generally use rapid-acting insulin formulations (i.e., insulin lispro, aspart, or glulisine). Lispro and aspart are approved by the U.S. Food and Drug Administration (FDA) for use in a pump insulin reservoir for up to 144 hours, but glulisine should be replaced every 48 hours due to a risk of crystallization. Regular insulin is also FDA-approved for use in pumps and is sometimes used instead of rapid-acting formulations because of its lower cost. Concentrated insulins (e.g., U200 or U500), dilute insulin (e.g., U50 or U10), and ultra-rapid-acting insulin analogs (e.g., Fiasp) are undergoing studies but are not yet FDA-approved for use in pumps.
Insulin pumps deliver insulin in two primary ways: a continuous infusion of rapid-acting insulin throughout the day and night (basal), and discrete, one-time doses of rapid-acting insulin given by the user for meals or high blood glucose correction (bolus). Basal insulin delivery replaces the use of the longer-acting exogenous insulin formulations used in MDI regimens. A multitude of factors influence basal insulin needs, including physiology, developmental life stage (i.e., puberty or growth), activity level, time of day, and sleep schedule. Insulin pumps deliver basal insulin in increments as small as 0.01 unit/hour and permit multiple rates of basal infusion throughout the day and night to best optimize glycemic control and individualize therapy, mimicking nondiabetes physiology. Additionally, many insulin pumps include a temporary basal feature, allowing users to temporarily increase or decrease basal delivery by a percentage relative to the programmed basal rate or by programming a new basal rate. The temporary basal feature is useful for situations in which insulin needs may change drastically, but for a confined period, such as during acute illness or when exercising.
Users can program larger, discrete bolus doses of insulin for carbohydrate consumption and high blood glucose corrections. Most insulin pumps contain a bolus calculator with which the pump calculates a bolus dose recommendation based on the current blood glucose value, current insulin on board (remaining active insulin from previous bolus doses), and total grams of carbohydrates that the user enters into the pump. Some pumps have an extended bolus option, which delivers a portion of the total bolus dose immediately and extends the delivery of the remainder of the dose over a longer period (usually 2–3 hours) to help prevent delayed postprandial hyperglycemia. This option can be helpful when consuming high-fat meals or for individuals with gastroparesis. Pumps deliver bolus doses in increments as small as 0.025 units, allowing for more precise insulin dosing than is possible with insulin pens or syringes. A variety of different insulin pump options are commercially available to individuals with type 1 or type 2 diabetes, and the spectrum of options consistently evolves with new technologies arriving on the market each year (Table 1).
TABLE 1.
Commercially Available Insulin Pumps, United States, as of March 2019
| Insulin Pump Brand and Manufacturer | ||||
|---|---|---|---|---|
| V-Go Valeritas, Inc. (Bridgewater, NJ) | Omnipod Insulet Corporation (Billerica, MA) | t:slim Tandem Diabetes Care, Inc. (San Diego, CA) | Minimed Medtronic Minimed, Inc. (Northridge, CA) | |
| Pump models | • V-Go 20 (pre-set for 20 units/day basal) | • Omnipod system | • t:slim X2 | • Minimed 530G (discontinued new sales 2018) |
| • V-Go 30 (30 units/day basal) | • Omnipod Dash | • Minimed 630G | ||
| • V-Go 40 (40 units/day basal | • Minimed 670G | |||
| Description | • Tubeless patch pump indicated for type 2 diabetes only | • Omnipod system: tubeless, patch pump (“pod”) operated by a handheld device called the “PDM” that is wirelessly connected by Bluetooth | • Touchscreen pump with color screen | • Battery-operated pump |
| • FDA approval in 2010; marketed in United States since 2012 | • Omnipo Dash: tubeless, patch pump (“pod”) operated by a touchscreen locked-down Android PDM that is wirelessly connected by Bluetooth | • Rechargeable battery; charged via micro-USB port | • 300-unit reservoir | |
| • Pre-set basal delivery | • Pods are disposable: worn for up to 3 days then discarded | • Water-resistant (IPX7): 3 feet for 30 minutes | • Operated by buttons on front of pump | |
| • Up to 36 units of on-demand bolus dosing in 2-unit increments | • PDM is used to program pump settings and deliver bolus doses wirelessly through the pod | • Contains Bluetooth wireless technology | • Waterproof (IPX8): 12 feet for up to 24 hours | |
| • Disposable: worn for 24 hours then discarded | • Waterproof (IP28): 25 feet for 60 minutes | • 300-unit insulin reservoir | ||
| • Pod holds up to 200 units of insulin | • Updatable software: can update pump features using a personal computer (e.g., can add Basal-IQ functionality) | |||
| Infusion sets | • 4.6-mm, 90-degree, stainless steel cannula | • 6.5-mm, 45-degree angle, soft cannula | • 6-mm, 9-mm, flexible, 90-degree cannula with inserter device | • 6-mm, 9-mm, flexible, 90-degree cannula with inserter device |
| • Infusion cannula integrated into patch pump | • Infusion cannula integrated into pod | • 13-mm, 17-mm angled, soft cannula; can be inserted manually or with inserter device | • 13-mm, 17-mm angled, soft cannula; can be inserted manually or with inserter device | |
| • Cannula auto-inserted after placing pump on body via button press | • Cannula auto-inserted after placing pod on body and following steps on PDM | • 6-mm Teflon cannula, 90-degree with manual insertion | • 6-mm Teflon cannula, 90-degree with manual insertion | |
| Minimum basal increments, units/hour | Pre-set, dependent on total daily basal dose of 20, 30, or 40 units | 0.05 | 0.01 | 0.025 |
| Minimum bolus increments, units | Pre-set at 2 units per button press | 0.05 | 0.05 | 0.025 |
| Blood glucose meter pairing | No | Omnipod system: PDM has built-in blood glucose meter; uses FreeStyle Lite test strips | No | Contour Next Link 2.4 blood glucose meter for 630G and 670G |
| Omnipod Dash: PDM pairs with Contour Next One blood glucose meter | Contour Next Link blood glucose meter for 530G | |||
| CGM system pairing | No | Omnipod system: no direct CGM pairing to PDM | Dexcom G5 and Dexcom G6 | Minimed 530G: Enlite CGM discontinued sales in 2018 |
| Omnipod Dash system has mobile applications that allow simultaneous viewing of Dexcom G5 mobile app data with pump data on PDM | Minimed 630G and 670G: Guardian 3 CGM | |||
| Hypoglycemia suspension | No | No | PLGS (Basal IQ) (8) with Dexcom G5 or G6 pairing | Minimed 530G: LGS (“threshold suspend”) |
| Minimed 630G: LGS (“suspend on low”) | ||||
| Minimed 670G in manual mode: | ||||
| LGS (“suspend on low”) or PLGS (“suspend before low”) | ||||
| Automated insulin delivery | No | HCL in development (Omnipod Horizon) (23,45,48) | HCL in clinical trials (Control IQ) (45,49) | Minimed 670G HCL (“auto mode”): automated basal insulin delivery with user-delivered mealtime boluses using bolus calculator |
PDM, personal diabetes manager.
Insulin Pump Therapy for Type 2 Diabetes
Insulin pump therapy was originally developed for use in type 1 diabetes. However, it may also benefit those with type 2 diabetes who require insulin therapy (7,8). Several studies have demonstrated improved glycemic control for individuals with suboptimally controlled type 2 diabetes treated with multiple oral diabetes medications or an MDI insulin regimen who discontinue all oral medications other than metformin and initiate insulin pump therapy. These studies have reported a reduction in A1C of 1.0% or more with lower total daily insulin requirements, reduced risk of hypoglycemia, and higher treatment satisfaction compared to MDI (25–28). These benefits were obtained using relatively simple insulin dosing regimens. Participants required only one or two basal rates, and use of the bolus calculator to determine bolus doses was not associated with reduction in A1C (28). This result suggests that individuals with type 2 diabetes may not require the complex pump features that are beneficial for the management of type 1 diabetes (29) and that cited barriers to conventional insulin pump therapy for individuals with type 2 diabetes, such as extensive educational requirements and high cost compared to MDI, could be reduced with simpler devices (30).
Thus, simplified pump technology has been developed specifically targeting the needs of people with type 2 diabetes. These novel pumps for type 2 diabetes are small, disposable, patch pumps that adhere to the body with adhesive and consist of an insulin reservoir and an infusion cannula that auto-inserts with the press of a button. The pumps come pre-programmed for basal delivery based on total daily basal dose and permit bolus dosing, via a bolus button, with pre-set bolus delivery increments (i.e., 2-unit insulin delivery per each button press). Currently, these pumps do not have a bolus calculator or any of the other advanced features of conventional insulin pumps.
There are a few patch pumps specifically indicated for individuals with type 2 diabetes; however, only the V-Go (31) is commercially available in the United States. The V-Go is prescribed as V-Go 20, 30, or 40, with the numbers referring to the fixed amount of basal insulin that will be delivered in 24 hours (i.e., V-Go 20 will deliver 20 units of insulin in 24 hours at a single rate of 0.83 units/hour). The V-Go contains a bolus button for meals that permits up to 36 units of bolus insulin delivery per day, in 2-unit increments.
Clinical studies using these novel patch pumps for type 2 diabetes management have demonstrated improve glycemic control, high patient satisfaction, reduced barriers to insulin pump treatment, and cost savings when compared to MDI therapy (32–34).
Sensor Augmented Pump Therapy
The development of CGM systems in the early 2000s was followed by the advent of the sensor augmented pump (SAP), which combines a CGM and insulin pump in one system. A CGM device consists of three components: 1) a thin, flexible sensor, which is inserted into the subcutaneous tissue and continuously measures glucose levels in the interstitial fluid, 2) a transmitter that sends the sensor glucose data to a receiver, and 3) a receiver, which displays the glucose values. In an SAP system, the insulin pump pairs to a CGM system and acts as the receiver, displaying CGM sensor glucose data on the pump’s home screen and thus allowing users easy access to the sensor glucose information. SAP systems have shown a greater reduction in A1C (−0.8 ± 0.8% vs. −0.2 ± 0.9%, P <0.001) after 12 months when compared to MDI therapy (35). The STAR 3 study, a randomized controlled trial involving 82 children and adolescents, found that participants using SAP therapy were more likely to meet glycemic targets than those using MDI, and SAP users had reduced glycemic variability after 12 months (36). Those wearing the sensor more consistently in the SAP group were more likely to meet glycemic targets, suggesting that easy access to CGM data on the pump helps individuals respond more readily to high and low glucose values, thus reducing glucose variability (35).
Insulin Pumps With Hypoglycemia Suspension
After the advent of SAP, further integration of pumps with CGM devices occurred with hypoglycemia suspension technology. In hypoglycemia suspension systems, the insulin pump not only displays the sensor glucose values, but also automatically suspends insulin delivery in response to hypoglycemia or anticipated hypoglycemia, based on CGM data, in an effort to prevent low blood glucose levels (37). This additional layer of protection against hypoglycemia is vital, as severe hypoglycemia remains the most concerning acute complication of intensive insulin therapy (38,39).
The first insulin pump system with hypoglycemia suspension technology was the Minimed 530G, which suspends insulin delivery when hypoglycemia occurs, a function referred to as “threshold suspend” or “low glucose suspend” (LGS). Studies using LGS have demonstrated a 40–50% reduction in hypoglycemia (<70 mg/dL), without an increase in A1C or mean sensor glucose values compared to SAP therapy alone (40,41). More recently, two more systems have become available in the United States that contain predictive low glucose suspend (PLGS) technology: the Minimed 670G (“suspend before low”) and the t:slim X2 system (Basal-IQ). These systems automatically suspend insulin delivery 30 minutes before hypoglycemia is predicted to occur, based on CGM data. Studies on the effectiveness of PLGS for reducing exposure to nocturnal hypoglycemia have demonstrated a 50–80% reduction in hypoglycemia overnight, without increasing the risk of ketosis (42,43), and an overall 31–50% reduction in hypoglycemia when using PLGS compared to SAP alone, with no increase in mean glucose value or hyperglycemia (44,45).
Automated Insulin Delivery
Hyperglycemia remains a significant challenge in diabetes management, even with the use of an insulin pump. Automated insulin delivery technologies (also referred to as “artificial pancreas” or “closed-loop” systems) aim to reduce hypoglycemia and hyperglycemia, thus improving overall glycemic control and increasing time spent in the glucose target range (70–180 mg/dL). An automated insulin delivery system consists of an insulin pump, a CGM device, and a control algorithm that calculates and dynamically adjusts insulin delivery in real time, based on the CGM sensor glucose values and trends (i.e., as sensor glucose values increase or decrease, insulin delivery increases or decreases as well). The first generation of automated insulin delivery is the hybrid closed-loop (HCL) system, which dynamically modulates basal insulin delivery but still requires users to deliver bolus doses for meals using the bolus calculator. Clinical trials using a variety of automated insulin delivery systems in children and adults have consistently demonstrated improved glycemic control, evidenced by reduction in A1C, increased sensor time in target range, reduced glycemic variability, and reduction in hypoglycemia (46–51).
In 2016, the Minimed 670G became the first automated insulin delivery system to be approved by the FDA for use in children and adults with type 1 diabetes. This system operates in “manual mode” or “auto mode” and pairs with the Guardian 3 CGM device. Manual mode refers to the conventional pump mode, through which basal insulin delivery is dictated by basal rates programmed into the pump. The 670G system in manual mode also contains LGS and PLGS technologies when the pump is paired with CGM. Auto mode refers to the HCL system, through which the pump calculates basal delivery every 5 minutes based on the sensor glucose trends. To use auto mode, the CGM must be active and sensor adequately calibrated. In both modes, the user delivers bolus doses for meals and high blood glucose corrections.
Results from the pivotal trial in adolescents and adults demonstrated safety and effectiveness of the system for a cohort of 30 adolescents and 94 adults (all previous insulin pump users) using the HCL feature (auto mode). A1C decreased by ∼0.5%, and sensor time in range increased by ∼8% after 3 months of use, with no occurrences of severe hypoglycemia or diabetic ketoacidosis (52). Adolescents spent less time in the HCL mode compared to the adults in the trial, indicating that adolescents may have a more difficult time adhering to system requirements to maintain time in the HCL mode (auto mode), such as responding to system alerts and maintaining sensor calibration.
Several other HCL systems are undergoing clinical testing and are expected to become commercially available in the next few years. In addition, the “Do-It-Yourself” (DIY) diabetes community has developed a closed-loop algorithm, used by many individuals with diabetes to essentially build their own closed-loop device. DIY closed-loop systems use commercially available CGM systems and insulin pumps and an open source algorithm run on a smartphone app to automate insulin delivery (53).
Clinical Indications for Insulin Pump Therapy
Recent clinical guidelines from diabetes organizations worldwide, including the American Diabetes Association, the International Society for Pediatric and Adolescent Diabetes, the Endocrine Society, and the American Association of Clinical Endocrinologists/American College of Endocrinology state that insulin pump therapy may be beneficial for all individuals with type 1 diabetes, regardless of age (54–57). Individuals with type 1 diabetes who are not meeting glycemic targets or have high rates of hypoglycemia or hypoglycemic unawareness may benefit the most from pump therapy. Individuals with gastroparesis may also benefit from pump therapy, specifically from the ability to extend bolus delivery to manage the delayed rise in glucose from meals that occurs with gastroparesis. Finally, even individuals who are meeting their glycemic targets with an MDI regimen but who desire more flexibility in their type 1 diabetes management may find improvements in quality of life and treatment satisfaction when switching to insulin pump therapy. Insulin pump therapy is recommended for individuals with type 2 diabetes who are not meeting glycemic targets with MDI, oral medication, and lifestyle modifications (56).
As advancing insulin pump technologies more fully incorporate CGM, it is important to consider which individuals will benefit from CGM as well when counseling patients on the optimal type of insulin pump therapy for them. SAP systems and automated insulin delivery systems will benefit individuals who can manage CGM and those who are willing to relinquish some control of insulin dosing to the automated pump system. Table 2 provides extensive detail on clinical considerations for different types of insulin pump technologies.
TABLE 2.
Clinical Considerations and Ideal Attributes for Insulin Pump Therapy
| Clinical Considerations for Insulin Pump Therapies | |
|---|---|
| • Not meeting glycemic targets | • Gastroparesis |
| • Frequent and/or nocturnal hypoglycemia | • Desire more flexibility in diabetes management |
| • Hypoglycemia unawareness | • Desire fewer injections |
| • Dawn phenomenon | • Unpredictable eating habits |
| • Pregnancy | • Variable schedules or work shifts |
| • Require small doses of insulin (i.e., pediatric patients) | |
| Ideal Attributes for Insulin Pump Candidates | |
| • Willing to wear device on body and able to tolerate adhesive on skin | |
| • Motivated to complete education and frequent follow-up with health care team | |
| • Views insulin pump as a tool to improve care but not as a “cure” for diabetes | |
| • Adequate insurance coverage for the device and ongoing supplies or ability to pay out of pocket | |
| • Sufficient dexterity and vision to operate insulin pump or has a care partner willing to operate the pump | |
| • Willing and able to check blood glucose four to six times daily | |
| • Willing and able to bolus for meals and high blood glucose correction, as needed | |
| • Willing and able to count carbohydrates* | |
| • Willing and able to use the bolus calculator* | |
| Additional attributes for SAP technologies | |
| • Willing to wear a sensor and infusion set on body | |
| • Willing to check blood glucose as needed and when prompted for CGM calibration | |
| • Able to manage additional information from CGM | |
| • Willing to respond to alerts from CGM and work with provider to personalize alert settings based on goals | |
| Additional attributes for hypoglycemia suspend technologies | |
| • Comfortable with pump suspending automatically, potentially without user knowledge | |
| • Understanding that, if hypoglycemia does occur, system has likely already suspended insulin delivery; therefore, may require treatment with fewer grams of carbohydrates (i.e., 5–10 g) to prevent rebound hyperglycemia | |
| Additional attributes for HCL systems | |
| • Willing to respond to additional alerts related to HCL functionality | |
| • Willing to bolus for all meals and snacks | |
| • Comfortable with relinquishing some control of insulin dosing to the device (i.e., user cannot influence basal insulin dosing in HCL systems) | |
For individuals with type 1 diabetes only; individuals with type 2 diabetes may not require advanced pump features or carbohydrate counting while on pump therapy.
To ensure successful adoption of insulin pump therapies, individuals must be willing to wear an insulin pump and CGM device (when applicable). Additionally, individuals and their caregivers should have appropriate expectations of their insulin pump technology and be able to adhere to the self-care tasks required for success with their chosen technology. This is true for all insulin pump technologies, from the simplest pumps to automated insulin delivery systems. Further, individuals and their caregivers must be cognitively and emotionally able to manage the insulin pump device and solve problems that may arise, such as infusion set malfunctions. Finally, patients must be motivated to complete all of the necessary education on their therapy and follow up regularly with their health care team.
Clinical Considerations for Insulin Pump Therapy
Wearing Devices
When assessing an individual’s readiness for insulin pump technologies, the individual’s willingness to wear a device on the body and ability to cope with the device’s presence over time are among the most important clinical considerations. In fact, in a study surveying >1,500 adults with type 1 diabetes, one of the most commonly endorsed barriers to device uptake was the hassle of wearing a device and disliking having devices on one’s body (58). Further, a recent review summarizing biopsychosocial factors related to sustained device use reported that body image concerns are a major barrier to device use for both adolescents and adults (59). Individuals have reported feeling self-conscious during intimacy or feeling like a “cyborg”’ being “shackled” to multiple devices (58,60–62). Clinicians should discuss these concerns with their patients regularly and assist patients in increasing their problem-solving capacity and social support to reduce these barriers and facilitate sustained device use. Ultimately, insulin pump and CGM manufacturers need to continue working to reduce the size and improve the discreetness of insulin pumps and CGM systems to increase the uptake of diabetes devices and reduce the risk of discontinuation among patients who try them (63,64).
In addition to the psychosocial concerns about wearing devices, there are also numerous practical issues involved in wearing devices. Many people struggle to keep devices adhered to the body, which is especially true for young children, individuals who participate in sports or other physical activities, and individuals who experience heavy sweating independent of activity level. Further, adverse skin reactions to infusion set and CGM adhesives are a common reason for discontinuation of insulin pump and CGM therapies. Clinicians should assess skin integrity and tolerance to adhesives and work with their patients to overcome these barriers (65). Many products are available to help protect the skin from irritation, such as barrier films and hypoallergenic over tapes; such products may help people keep their devices in place while also reducing the incidence of skin reactions (65). Finally, clinicians should educate their patients on the importance of site selection and site rotation to avoid other skin issues with chronic device wear, such as lipohypertrophy.
Ensuring Appropriate Expectations
One of the most important roles clinicians play in optimizing their patients’ success with insulin pump therapy is setting appropriate expectations for the devices. Clinicians should assess their patients’ expectations of the therapy, including why they desire to use a particular insulin pump, what they expect the system to be like, and what type of self-care they think is required of the user for the device to operate properly. A balanced discussion of the potential benefits and drawbacks of the preferred system should occur. Unrealistic expectations of any diabetes technology increase the risk for dissatisfaction, suboptimal glycemic control, and discontinuation of device use (60,61,66–68). Individuals with diabetes and their caregivers must understand the limitations of insulin pump technologies and the potential problems they may encounter, such as infusion set failure, pump malfunction, skin irritation, and alarm fatigue. Use of CGM requires responding to alarms and managing difficulties such as lost sensor signals or errors in calibration. Further, it is imperative that individuals conceptualize insulin pump technologies as a tool to help them improve their diabetes management and not as a panacea that will “cure” their diabetes or eliminate the need for self-care.
Expectation-setting is even more important for automated insulin delivery systems (69). Many individuals with diabetes expect these systems to take over their diabetes care for them (64), and, to date, this is not a realistic expectation. Early HCL systems available today, such as the Minimed 670G, may require users to check blood glucose levels, calibrate the CGM device, count carbohydrates, administer boluses for meals, and respond to system alerts.
Adherence to Self-Care Behaviors
Performing self-care behaviors is especially important for glycemic control and safety while using an insulin pump. Several studies have shown that missed meal boluses (70–72) and lack of correction boluses (73) in response to hyperglycemia predict suboptimal glycemic control, especially for adolescents with type 1 diabetes. Further, consistent monitoring of glucose values is highly correlated to improved glycemic control (74) and remains an important behavior for success with all insulin pump technologies. It is important that clinicians communicate clearly about the importance of adherence to self-care with insulin pump therapy and work with individuals to overcome any barriers to self-care.
Educational Needs
Initiating insulin pump therapy requires extensive education and frequent follow-up with the health care team. Education should be ongoing and individualized to teach advanced skills over time (i.e., the use of extended boluses, hypoglycemia suspension, and HCL features), based on individuals’ specific diabetes management needs. Individuals with diabetes should be encouraged to complete the necessary education, and their families/caregivers must also be integrated into the educational process. Further, health care providers must be able to provide their patients with expert education on using insulin pump therapy and adequate clinical follow-up.
The American Association of Diabetes Educators provides guidance and practical tips on how to assess individual readiness for pump therapy, educate individuals on the basics of insulin pump therapy, and provide sufficient follow-up to support success (75). All pump education programs should include instruction on basic pump operation, including inserting the infusion set, changing the insulin reservoir, programming insulin pump settings, and delivering boluses. Initial pump education should also include device troubleshooting and guidance on managing persistent hyperglycemia, including when and how to check ketone levels, give a subcutaneous injection, and change the infusion set (76). Finally, individuals need frequent contact with their health care team in the initial weeks to optimize basal and bolus insulin pump settings.
When initiating advanced insulin pump therapies such as SAP or automated insulin delivery systems, the educational approach must be individualized. For those new to CGM or insulin pump therapy or those who struggled using either technology in the past, initiating both the pump and CGM at once may be overwhelming. Likewise, ensuring success and confidence in the basics of each technology is paramount before adding advanced features such as hypoglycemia suspension or automated insulin delivery. Thus, completing education on each component of a system separately, before integrating the technologies, may increase success with advancing therapies (77).
Conclusion
There is no one-size-fits-all approach to insulin pump therapy, and fortunately, there are many options for clinicians to consider with each patient with diabetes. Insulin pump technologies are advancing at an extraordinary rate and have potential to improve diabetes outcomes for individuals of all ages with type 1 or type 2 diabetes. However, individuals with diabetes must be able to overcome any barriers to device wear, have realistic expectations of their particular device, perform self-care, and complete extensive education and clinical follow-up to realize success with insulin pump therapies. It is important for clinicians to work with individuals with diabetes and their caregivers to optimize use of insulin pump technologies initially and into the future.
Acknowledgments
Duality of Interest
C.B. is a contracted product trainer for Medtronic and has received speaking honoraria from Insulet. L.H.M. is a contracted product trainer for Medtronic and a consultant for Tandem Diabetes, Clinical Sensors, and Capillary Biomedical. G.P.F. receives research support from Abbott, Beta Bionics, Dexcom, Insulet, Medtronic, Tandem, and Type Zero. He has served as a speaker/consultant for Dexcom, Medtronic, and Tandem. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
C.B. wrote the manuscript and conducted the literature review, L.H.M. and G.P.F. reviewed/edited the manuscript. C.B. is the guarantor of this work, and, as such, takes responsibility for the integrity of the manuscript and the accuracy of the information presented.
References
- 1.DCCT Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Fry A. Insulin delivery device technology 2012: where are we after 90 years? J Diabetes Sci Technol 2012;6:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode BW, Sabbah HT, Gross TM, Fredrickson LP, Davidson PC. Diabetes management in the new millennium using insulin pump therapy. Diabetes Metab Res Rev 2002;18(Suppl. 1):S14–S20 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al. ; DCCT/EDIC Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams BH, Rizvi AA. An overview of insulin pumps and glucose sensors for the generalist. J Clin Med 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller KM, Foster NC, Beck RW, et al.; TID Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 7.Reznik Y, Cohen O. Insulin pump for type 2 diabetes: use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S219–S225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther 2010;12(Suppl. 1):S17–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsaleh FM, Smith FJ, Taylor KM. Experiences of children/young people and their parents, using insulin pump therapy for the management of type 1 diabetes: qualitative review. J Clin Pharm Ther 2012;37:140–147 [DOI] [PubMed] [Google Scholar]
- 10.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079–1087 [DOI] [PubMed] [Google Scholar]
- 11.Burckhardt MA, Smith GJ, Cooper MN, Jones TW, Davis EA. Real-world outcomes of insulin pump compared to injection therapy in a population-based sample of children with type 1 diabetes. Pediatr Diabetes 2018;19:1459–1466 [DOI] [PubMed] [Google Scholar]
- 12.Mameli C, Scaramuzza AE, Ho J, Cardona-Hernandez R, Suarez-Ortega L, Zuccotti GV. A 7-year follow-up retrospective, international, multicenter study of insulin pump therapy in children and adolescents with type 1 diabetes. Acta Diabetol 2014;51:205–210 [DOI] [PubMed] [Google Scholar]
- 13.Brorsson AL, Viklund G, Ortqvist E, Lindholm Olinder A. Does treatment with an insulin pump improve glycaemic control in children and adolescents with type 1 diabetes? A retrospective case-control study. Pediatr Diabetes 2015;16:546–553 [DOI] [PubMed] [Google Scholar]
- 14.Litton J, Rice A, Friedman N, Oden J, Lee MM, Freemark M. Insulin pump therapy in toddlers and preschool children with type 1 diabetes mellitus. J Pediatr 2002;141:490–495 [DOI] [PubMed] [Google Scholar]
- 15.McMahon SK, Airey FL, Marangou DA, et al. Insulin pump therapy in children and adolescents: improvements in key parameters of diabetes management including quality of life. Diabet Med 2005;22:92–96 [DOI] [PubMed] [Google Scholar]
- 16.Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1C. Diabetes Care 2004;27:2590–2596 [DOI] [PubMed] [Google Scholar]
- 17.Pankowska E, Blazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 2009;10:52–58 [DOI] [PubMed] [Google Scholar]
- 18.DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. J Pediatr 2004;145:380–384 [DOI] [PubMed] [Google Scholar]
- 19.Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care 2005;28:1277–1281 [DOI] [PubMed] [Google Scholar]
- 20.Churchill JN, Ruppe RL, Smaldone A. Use of continuous insulin infusion pumps in young children with type 1 diabetes: a systematic review. J Pediatr Health Care 2009;23:173–179 [DOI] [PubMed] [Google Scholar]
- 21.Weinzimer SA, Swan KL, Sikes KA, Ahern JH. Emerging evidence for the use of insulin pump therapy in infants, toddlers, and preschool-aged children with type 1 diabetes. Pediatr Diabetes 2006;7(Suppl. 4):15–19 [DOI] [PubMed] [Google Scholar]
- 22.Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and progress towards the artificial pancreas. J Pediatr 2016;169:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015;38:1036–1043 [DOI] [PubMed] [Google Scholar]
- 24.Ly TT, Layne JE, Huyett LM, Nazzaro D, O'Connor JB. Novel Bluetooth-enabled tubeless insulin pump: innovating pump therapy for patients in the digital age. J Diabetes Sci Technol 2019;13:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther 2010;12:627–633 [DOI] [PubMed] [Google Scholar]
- 26.Vigersky RA, Huang S, Cordero TL, et al.; OpT2mise Study Group . Improved HbA1c, total daily insulin dose, and treatment satisfaction with insulin pump therapy compares to multiple daily injections in patients with type 2 diabetes irrespective of baseline C-peptide levels. Endocr Pract 2018;24:446–452 [DOI] [PubMed] [Google Scholar]
- 27.Aronson R, Reznik Y, Conget I, et al. ,; OpT2mise Study Group . Sustained efficacy of insulin pump therapy compared with multiple daily injections in type 2 diabetes: 12-month data from the OpT2mise randomized trial. Diabetes Obes Metab 2016;18:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reznik Y, Cohen O, Aronson R, et al. ; OpT2mise Study Group . Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 2014;384:1265–1272 [DOI] [PubMed] [Google Scholar]
- 29.King AB, Clark D, Wolfe GS. The number of basal rates required to achieve near-normal basal glucose control in pump-treated type 2 diabetes. Diabetes Technol Ther 2012;14:900–903 [DOI] [PubMed] [Google Scholar]
- 30.Skyler JS, Ponder S, Kruger DM, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clin Diabetes 2007;25:50–56 [Google Scholar]
- 31.Knutsen PG, Voelker CQ, Nikkel CC. Clinical insights into a new, disposable insulin delivery device. Diabetes Spectr 2015;28:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilly LC, Mader JK, Warner J. Developing a simple 3-day insulin delivery device to meet the needs of people with type 2 diabetes. J Diabetes Sci Technol 2019:13: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermanns N, Lilly LC, Mader JK, et al. Novel simple insulin delivery device reduces barriers to insulin therapy in type 2 diabetes: results from a pilot study. J Diabetes Sci Technol 2015;9:581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mader JK, Lilly LC, Aberer F, et al. Improved glycaemic control and treatment satisfaction with a simple wearable 3-day insulin delivery device among people with type 2 diabetes. Diabet Med 2018;35:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 36.Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes 2012;13:6–11 [DOI] [PubMed] [Google Scholar]
- 37.Steineck I, Ranjan A, Norgaard K, Schmidt S. Sensor-augmented insulin pumps and hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol 2017;11:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DCCT/EDIC Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC Study 30-year follow-up. Diabetes Care 2016;39:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awoniyi O, Rehman R, Dagogo-Jack S. Hypoglycemia in patients with type 1 diabetes: epidemiology, pathogenesis, and prevention. Curr Diab Rep 2013;13:669–678 [DOI] [PubMed] [Google Scholar]
- 40.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 41.Danne T, Kordonouri O, Holder M, et al. Prevention of hypoglycemia by using low glucose suspend function in sensor-augmented pump therapy. Diabetes Technol Ther 2011;13:1129–1134 [DOI] [PubMed] [Google Scholar]
- 42.Maahs DM, Calhoun P, Buckingham BA, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care 2014;37:1885–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckingham BA, Raghinaru D, Cameron F, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care 2015;38:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG Trial. Diabetes Care 2018;41:2155–2161 [DOI] [PubMed] [Google Scholar]
- 45.Abraham MB, Nicholas JA, Smith GJ, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2018;41:303–310 [DOI] [PubMed] [Google Scholar]
- 46.Buckingham BA, Christiansen MP, Forlenza GP, et al. Performance of the Omnipod personalized model predictive control algorithm with meal bolus challenges in adults with type 1 diabetes. Diabetes Technol Ther 2018;20:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forlenza GP, Cameron FM, Ly TT, et al. Fully closed-loop multiple model probabilistic predictive controller artificial pancreas performance in adolescents and adults in a supervised hotel setting. Diabetes Technol Ther 2018;20:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zisser H, Renard E, Kovatchev B, et al. Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites. Diabetes Technol Ther 2014;16:613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckingham BA, Forlenza GP, Pinsker JE, et al. Safety and feasibility of the OmniPod hybrid closed-loop system in adult, adolescent, and pediatric patients with type 1 diabetes using a personalized model predictive control algorithm. Diabetes Technol Ther 2018;20:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown S, Raghinaru D, Emory E, Kovatchev B. First look at Control-IQ: a new-generation automated insulin delivery system. Diabetes Care 2018;41:2634–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 52.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis D. History and perspective on DIY closed looping. J Diabetes Sci Technol. Epub ahead of print on 22 October 2018 (doi: 10.1177/1932296818808307) [DOI] [PMC free article] [PubMed]
- 54.12. Children and adolescents: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl 1):S126–S136 [DOI] [PubMed] [Google Scholar]
- 55.Sherr JL, Tauschmann M, Battelino T, et al. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes 2018;19(Suppl. 27):302–325 [DOI] [PubMed] [Google Scholar]
- 56.Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology: continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:3922–3937 [DOI] [PubMed] [Google Scholar]
- 57.Grunberger G, Handelsman Y, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology 2018 position statement on integration of insulin pumps and continuous glucoe monitoring in patients with diabetes mellitus. Endocr Pract 2018;24:302–308 [DOI] [PubMed] [Google Scholar]
- 58.Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forlenza GP, Messer LH, Berget C, Wadwa RP, Driscoll KA. Biopsychosocial factors associated with satisfaction and sustained use of artificial pancreas technology and its components: a call to the technology field. Curr Diab Rep 2018;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashotte J, Tousignant K, Richardson C, et al. Living with sensor-augmented pump therapy in type 1 diabetes: adolescents' and parents' search for harmony. Can J Diabetes 2014;38:256–262 [DOI] [PubMed] [Google Scholar]
- 61.Ritholz MD, Atakov-Castillo A, Beste M, et al. Psychosocial factors associated with use of continuous glucose monitoring. Diabet Med 2010;27:1060–1065 [DOI] [PubMed] [Google Scholar]
- 62.Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with type 1 diabetes. Diabet Med 2018;35:409–418 [DOI] [PubMed] [Google Scholar]
- 63.Naranjo D, Suttiratana SC, Iturralde E, et al. What end users and stakeholders want from automated insulin delivery systems. Diabetes Care 2017;40:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iturralde E, Tanenbaum ML, Hanes SJ, et al. Expectations and attitudes of individuals with type 1 diabetes after using a hybrid closed loop system. Diabetes Educ 2017;43:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther 2018;20(Suppl. 2):S254–S264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofer SE, Heidtmann B, Raile K, et al. Discontinuation of insulin pump treatment in children, adolescents, and young adults: a multicenter analysis based on the DPV database in Germany and Austria. Pediatr Diabetes 2010;11:116–121 [DOI] [PubMed] [Google Scholar]
- 67.Low KG, Massa L, Lehman D, Olshan JS. Insulin pump use in young adolescents with type 1 diabetes: a descriptive study. Pediatr Diabetes 2005;6:22–31 [DOI] [PubMed] [Google Scholar]
- 68.Hirsch IB. Clinical review: realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab 2009;94:2232–2238 [DOI] [PubMed] [Google Scholar]
- 69.Messer LH. Why expectations will determine the future of artificial pancreas. Diabetes Technol Ther 2018;20(Suppl. 2):S265–S268 [DOI] [PubMed] [Google Scholar]
- 70.Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004;113:e221–r224 [DOI] [PubMed] [Google Scholar]
- 71.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes 2009;10:142–148 [DOI] [PubMed] [Google Scholar]
- 72.Patton SR, Driscoll KA, Clements MA. Adherence to insulin pump behaviors in young children with type 1 diabetes mellitus. J Diabetes Sci Technol 2017;11:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westen SC, Warnick JL, Albanese-O'Neill A, et al. Objectively measured adherence in adolescents with type 1 diabetes on multiple daily injections and insulin pump therapy. J Pediatr Psychol 2019;44:21–31 [DOI] [PubMed] [Google Scholar]
- 74.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics 2009;124:e1171–e1179 [DOI] [PubMed] [Google Scholar]
- 75.American Association of Diabetes Educators AADE practice paper: continuous subcutaneous insulin infusion (CSII) without and with sensor integration. Available from www.diabeteseducator.org/docs/default-source/default-document-library/continuous-subcutaneous-insulin-infusion-2018-v2.pdf?sfvrsn=0. Accessed 14 March 2019
- 76.Heinemann L, Fleming GA, Petrie JR, Holl RW, Bergenstal RM, Peters AL. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs. A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia 2015;58:862–870 [DOI] [PubMed] [Google Scholar]
- 77.Berget C, Thomas SE, Messer L, et al. A clinical training program for hybrid closed loop therapy in a pediatric diabetes clinic. J Diabetes Sci Technol. Epub ahead of print on 12 March 2019 (doi: 10.1177/1932296819835183) [DOI] [PMC free article] [PubMed]