Abstract
IN BRIEF With the introduction of intermittently scanned continuous glucose monitoring (CGM) systems to the marketplace, providers and patients now have several options to continuously monitor glucose levels. This article addresses appropriate patient selection criteria for using patient- or practice-based CGM systems and the barriers to achieving optimal benefits from this technology. The authors have developed a flowchart to guide clinicians and patients in decision-making regarding the most appropriate type of CGM to use in various circumstances.
Since the development of qualitative urinary glucose testing in the mid-1800s, great advances in diabetes self-monitoring have been achieved (1). The first U.S. Food and Drug Administration–approved continuous glucose monitoring (CGM) device was released in the United States in 1999 (2), and since that time, technological advances have improved the accuracy and ease of use of CGM systems.
Diabetes professional organizations now recommend that CGM be considered for patients with type 1 diabetes (3) and patients with type 2 diabetes who are on insulin therapy and not meeting glycemic targets (4) because CGM has been shown to lower A1C with regular use (5,6). A recent consensus conference of the American Association of Clinical Endocrinologists/American College of Endocrinology concluded that “evidence supports the benefits of CGM in type 1 diabetes and that these benefits are likely to apply whenever intensive insulin therapy is used, regardless of diabetes type” (4). Participants in this conference also urged expanded CGM coverage by government and private health care payers (4). The American Diabetes Association’s (ADA’s) Standards of Medical Care in Diabetes—2019 state that real-time CGM (rtCGM) systems, which continuously transmit glucose data to a receiver or other compatible device, can be useful for patients with type 1 diabetes who are not meeting glycemic targets and patients with hypoglycemia unawareness or frequent hypoglycemia episodes. These guidelines also suggest that intermittently scanned CGM (isCGM) systems, with which users scan a sensor to receive glucose data, is an acceptable substitute for self-monitoring of blood glucose (SMBG) for adults with diabetes who require frequent glucose monitoring (7).
With the addition of isCGM devices to the marketplace, providers and patients are faced with multiple products and features when choosing a personal CGM device for patients. In addition, professional CGM systems can be purchased by a clinic and used intermittently by patients as prescribed by their clinicians. In this article, we aim to outline appropriate patient selection criteria for both patient-based (personal) and practice-based (professional) CGM use and the barriers to achieving optimal benefits from CGM. We have developed a basic flowchart to guide both clinicians and patients in decision-making regarding the best type and use of CGM in various circumstances.
Understanding the advantages and disadvantages of the available personal and professional CGM systems is necessary. A thorough review of the accuracy and performance of current CGM technologies was recently published in Diabetes Care (8). Rather than providing another detailed review of available devices, we will instead discuss the device features that may benefit different patients and patient populations.
Personal CGM Systems
Personal CGM systems are recommended for appropriate patients with type 1 or type 2 diabetes who are treated with intensive insulin therapy and are not achieving glucose targets or are experiencing problematic hypoglycemia (9). Personal rtCGM devices continuously transmit glucose data to a personal receiver, smartphone, smartwatch, or other compatible device and can sound alerts and alarms in response to rising or falling glucose levels. Most of these devices also allow users to share their data remotely with family members, caregivers, and clinicians. Sharing data with caregivers and family members can be particularly reassuring for all parties and allows patients to share the burden of daily diabetes management.
The only isCGM system currently on the market is the FreeStyle Libre (Abbott, Alameda, CA). This device requires users to scan their sensor to obtain blood glucose data and does not have alerts or alarms. However, trend arrows and 8 hours of retrospective blood glucose data are available at each scan. Two versions of FreeStyle Libre are available: a 10-day-wear sensor and receiver with a 12-hour blinded warm-up period and a 14-day-wear sensor compatible with receiver and iPhone with 1-hour blinded warm-up period. The receivers for each version are not interchangeable (10).
The FreeStyle Libre isCGM and Dexcom G6 rtCGM systems (Dexcom, San Diego, CA) do not require calibration with fingerstick blood glucose test results. Some rtCGM systems require patients to calibrate by entering their fasting blood glucose level into the sensor (or into their pump when using pump/CGM integrated technology) to generate sensor glucose readings. This ease of monitoring has been shown to increase the frequency of blood glucose monitoring. One study of patients with type 1 diabetes demonstrated that users of the FreeStyle Libre scanned their sensor an average of 15 times per day (10).
Both real-time and intermittently scanned CGM systems have advantages and disadvantages, and it is crucial for clinicians to guide their patients in choosing an appropriate system. Table 1 presents a summary of the basic features of five currently available devices (11–15).
TABLE 1.
Comparison of Personal CGM Devices
| FreeStyle Libre | Dexcom G6 | Dexcom G5 | Medtronic Guardian Sensor 3 | Eversense | |
|---|---|---|---|---|---|
| Type of system | isCGM | rtCGM | rtCGM | rtCGM | rtCGM |
| Approved for insulin dosing? | Yes | Yes | Yes | No | No |
| Calibration requirements | None | None | At least twice daily | At least twicedaily | At least twice daily |
| Compatibility with mobile devices | Reader, Apple smartphone (14-day system only) | Receiver, Android and Apple smartphones, smartwatches, and Tandem t:slim X2 insulin pump | Receiver, Android and Apple smartphones, smartwatches, and Tandem t:slim X2 insulin pump | Guardian Connect app on Apple iOS devices | Android and Apple iOS smartphones, smartwatches, and other devices |
| Real-time remote data sharing? | No | Yes | Yes | Yes | Yes |
| Integration with insulin pump? | No | Yes (Tandem t:slim X2) | Yes (Tandem t:slim X2) | Yes (Minimed 630G and 670G) | No |
| Sensor wear | 14 or 10 days | 10 days | 7 days | 7 days | Currently approved in the Untied States for up to 90 daysafter implantation in upper arm (under local anesthesia; in-office procedure) |
| Warm-up time | 14-day system: 1 hour 10-day system: 12 hours |
2 hours | 2 hours | 2 hours | 24 hours |
| Predictive low glucose alert? | No | Yes | Yes | Yes | Yes |
| Covered by Medicare? | Yes, for people who take insulin at least three times daily and perform SMBG four times daily | To be covered in late 2019 | Yes, for people who take insulin at least three times daily and perform SMBG four times daily | No | No |
| MARD | 10-day system: 9.7% 14-day system: 9.4% (11) |
9% (12) | 9% (13) | 9.6–10.5%* (14) | 8.8% (15) |
| Transmitter charging needed? | No | No | No | Yes, weekly with sensor change | Yes, daily |
Based on two versus three to four calibrations per day using CONTOUR NEXT Link 2.4 meter.
It should be noted that CGM devices are not indicated for use by patients who are receiving dialysis, are pregnant, are critically ill, or have implanted medical devices because they have not been adequately studied in these groups (11–15). However, ADA guidelines state that rtCGM may be beneficial if used effectively to improve A1C levels and neonatal outcomes in pregnant women with diabetes (7). In addition, the Eversense CGM system (Eversense, Germantown, Md.), an implantable rtCGM discussed below, carries an additional warning against use in patients receiving immunosuppressant therapy, chemotherapy, or anticoagulant therapy; those with another active implantable device (e.g., an implantable defibrillator); and those with known allergies to or who are using systemic glucocorticoids (15).
Considerations for Selecting a Personal CGM System
Multiple factors should be considered when deciding which personal CGM system may be most appropriate for a given patient.
If severe hypoglycemia or hypoglycemia unawareness is an issue, rtCGM is imperative due to the benefit of alerts and alarms, which can notify patients before or in the setting of a hypoglycemic event (16). However, patients who are averse to CGM use due to frustration with alarms or alarm fatigue may benefit from isCGM. Patients with alarm fatigue may also benefit from using an rtCGM with alarm features that can be disabled or modified. Several of the psychological issues with CGM, including alarm fatigue, are discussed in more detail below.
Insulin pump integration is also of major importance when choosing a CGM system. For patients who are already using an insulin pump and are happy with it, choosing an rtCGM device that integrates with their pump is important (Table 1). For example, if a patient uses the Tandem t:slim X2 insulin pump, then a Dexcom G5 or G6 CGM system may be preferred. If a patient is interested in the Medtronic Minimed 670G insulin pump, then the Medtronic Guardian Sensor 3 is indicated. It is important to keep in mind that insulin pump and CGM integration, as well as purchasing and upgrading programs, are rapidly changing.
If integration with an insulin pump is not important to a given patient, the next consideration is whether to select an rtCGM or an isCGM system. The only published head-to-head comparison to date of which the authors are aware involved adult patients with type 1 diabetes who were using a multiple daily injection (MDI) insulin regimen and had hypoglycemia unawareness (16). In this study, rtCGM reduced time spent in hypoglycemia (P = 0.006); there was no meaningful improvement in time spent in hypoglycemia with isCGM. When study participants were moved from isCGM to rtCGM, a significant beneficial impact on hypoglycemia outcomes was seen, and continued use of rtCGM maintained the reduction in risk of hypoglycemia (17).
isCGM systems may be beneficial for patients with diabetes who struggle with performing SMBG as recommended because of either dexterity issues, problematic neuropathies, or circulatory issues associated with diabetes. Two major published trials have investigated the use of isCGM compared to SMBG. The first was an unblinded, prospective, randomized controlled trial (RCT) examining patients with well-controlled type 1 diabetes (A1C <7.5%) (10). The second was also an unblinded, prospective RCT comparing isCGM to SMBG in patients with type 2 diabetes (A1C 7.5–12%) who were on intensive insulin therapy (defined as prandial, prandial and basal, or insulin pump therapy) (18). Both studies showed similar reductions in hypoglycemia for patients using isCGM without worsening of A1C compared to SMBG alone (10,18). Mean time in hypoglycemia was reported to decrease by 38% in the intervention group versus the control group (P <0.0001) in the type 1 diabetes trial (10) and by 43% (P = 0.0006) in the study of patients with type 2 diabetes (18).
Among the rtCGM devices, there are several distinguishing features that should be considered when counseling patients on their options. Traditional CGM, whether real-time or intermittently scanned (i.e., the Dexcom G4, G5, and G6; Guardian Sensor 3; and FreeStyle Libre) involves the placement of a subcutaneous sensor with transmitter attached, which is held in place with an adhesive patch and worn continuously for a certain number of days (currently up 14 days) (9–12). However, the Eversense CGM features an implanted sensor that is changed every 3 months in an in-office procedure involving local anesthesia and a removable transmitter that is worn on the skin over the implanted sensor (13).
Additional factors to consider include:
Ease of insertion of sensor and transmitter.
Continuous wear. Patients who desire a removable option may want to consider the Eversense system because its transmitter can be intermittently removed if patients want to be discreet about wearing a CGM.
Required calibration. Patients struggling with or bothered by calibrations with SMBG who nonetheless desire rtCGM may benefit from the Dexcom G6 system.
Adhesive sensitivities. Patients concerned about skin sensitivities may want to have a trial of a professional CGM product to assess for skin reactions before purchasing a personal system. Some patients may want to consider the Eversense system, which has a removable transmitter and uses a silicone-based adhesive patch.
Current or future insulin delivery desires. Patients seeking hybrid closed-loop therapy in the near future may consider the Medtronic Guardian Sensor 3, which is compatible with the only available hybrid closed-loop system to date. Patients considering near-future use of the Tandem t:slim X2 insulin pump may want to consider the Dexcom G5 or Dexcom G6 CGM systems, which are compatible with the t:slim X2 pump.
Expectations for performance. Clinicians should discuss performance of the various systems, explaining the performance measurement of mean absolute relative difference (MARD) between glucose meter blood glucose values and CGM data and the various systems’ precision in the settings of hypoglycemia and rapidly changing blood glucose levels. Reviewing performance in different glucose states (i.e., how the systems perform during hypoglycemia and hyperglycemia) may be helpful for some patients.
Professional CGM Systems
Professional CGM involves patients wearing a CGM device provided by their health care provider’s clinic for a short period of time (up to 2 weeks). Patients return the sensor and equipment to the clinic, and data are downloaded and analyzed. Professional CGM can be a useful patient teaching tool and can provide important data for pattern identification and insulin dose adjustments. This intervention has been shown to assist patients in reaching their glycemic targets and to improve physical activity and promote other positive behavioral changes (19,20).
One study of 52 patients with type 2 diabetes demonstrated that using professional CGM as a part of diabetes education resulted in higher levels of physical activity (P <0.05) and decreases in BMI and A1C compared to patients not using professional CGM (19). Professional CGM has also been shown to be beneficial when used in patients with low socioeconomic status, with 66% of patients showing improvement in A1C with a median decrease of 0.8% (mean A1C 9.2% before and 8.77% after, P = 0.13) when using blinded professional CGM for 14 days (21).
Blinded Versus Unblinded Professional CGM
One key distinction among these products is the option for blinded versus unblinded data. A 2013 review by Teodoro de Oliveira et al. (22) addressed the use of blinded CGM as an educational tool in the primary care setting. The authors reported that professional CGM used in primary care assisted “both providers and patients to understand why starting insulin (either basal or premeal) is necessary by giving them a clear and personalized visual image of the specific problem.” Some argue that blinded CGM may yield more realistic data captured in a “regular day” where behavior is not influenced by the awareness of blood glucose data and therefore more representative of patients’ typical patterns of diet, exercise, and medication use (23). Older professional CGM systems limit data collection to 3 days, making standard day and trend analysis more difficult due to the vast day-to-day glycemic variability typical in diabetes. Also, the role of the “Hawthorne effect” (the tendency of individuals to modify their behavior in response to their awareness of being observed) can be an issue (24). However, the option now available for 14 days of blinded professional CGM might mitigate concerns about both variability and the Hawthorne effect.
On the other hand, rtCGM can provide real-time feedback to patients, allowing them to observe the effects of food, activity, stress, alcohol, and other factors on their blood glucose levels. It can also serve as an opportunity for hesitant patients to try CGM before committing to purchasing a personal CGM device. However, it is worth noting that the professional rtCGM systems available today use older-model sensors with higher MARD values compared to more contemporary personal models and therefore may not be useful for patients who are skeptical or anxious about the reliability and accuracy of the devices.
Ahn et al. (23) suggest that un-blinded professional CGM should replace blinded CGM in clinical practice as the standard of care for patients with type 1 or type 2 diabetes (23). They argue that unblinded CGM is an intervention in itself, and the inherent glycemic variability of diabetes precludes the accurate “regular day” data that blinded devices attempt to measure.
Potential Applications for Professional CGM in Clinical Practice
Professional CGM can be beneficial for patients who do not wish to pursue or do not qualify for personal CGM. With rapidly improving insurance coverage, clinics can consider implementing professional CGM into their regular practice models. For instance, clinics could place a professional CGM sensor on patients who are not reaching glycemic targets 1–2 weeks before their scheduled clinic visit so data will be available at the time of visit for medication and lifestyle adjustment. Or, patients can begin wearing a sensor before their visit and continue to wear it after the visit, to aid in assessing the effects of any regimen changes made during the visit. The FreeStyle Libre Pro system (described in more detail below), which does not require advance planning and scheduling of shared equipment, can be applied at the time of a clinic visit if interest is expressed.
As previously mentioned, professional CGM can also serve as a trial for patients considering purchasing a personal CGM system. For instance, for patients who are fearful of having the device connected to their body, a short trial may prove this fear unjustified. Professional rtCGM could also allow patients and providers to more rapidly and safely titrate diabetes medications during the days the device is worn.
Professional CGM can also be used for more individualized patient needs. For example, it can help to gauge glycemic control for patients with confirmed or suspected inaccuracies in their A1C due to renal disease or hemoglobinopathies (23). It can also be used to confirm that there is no prohibitive hypoglycemia for patients seeking a driver’s or professional license. Similarly, it can help to ensure adequate preoperative glycemic control for elective surgeries.
Available Professional CGM Systems
Three professional CGM systems are currently available in the United States for purchase by medical clinics.
The FreeStyle Libre Pro is a blinded CGM sensor; the patient receives no feedback or blood glucose readings while wearing the sensor (25). A quarter-sized sensor is applied on the arm in the clinic, and the patient wears it for up to 14 days (26). The single-use sensor, which holds data indefinitely, is then returned to the clinic, and the data are downloaded using FreeStyle LibreView software and analyzed by the clinician (26). The sensor is then discarded. No calibration with SMBG is required (26).
The Medtronic iPro2 is a blinded professional CGM system that uses the Medtronic Enlite sensor and can store up to seven 24-hour periods of data (27). Patients are advised to perform SMBG four times per day and to record their SMBG data on a log sheet (27). When a patient returns the device, the SMBG calibration readings are entered into CareLink iPro therapy management software (27), and a report is generated for analysis by the clinician. The iPro2 professional CGM system can be used by multiple patients with proper disinfection (27).
The Dexcom G4 Platinum professional CGM system is an rtCGM device available for professional use. This system can be used in the unblinded mode, providing wearers with real-time feedback or can be blinded when clinically indicated. Patients can wear the device for up to 7 days, and the transmitter and receiver can be used by multiple patients after proper disinfection (28). Manual SMBG calibrations are required every 12 hours, as well as 2 hours after sensor insertion (28). In the unblinded mode, patients are able to view data on a receiver, and alarms can be customized to each patient (28). When using the system in blinded mode, patients will still calibrate the system twice daily but will not have access to glucose data values, and alarms will be inactive. At the end of the 7-day period, a patient returns the device to the clinic, data from the receiver are downloaded into Dexcom Studio software, and a report is generated for analysis by the patient and clinician (28).
CGM-Related Psychological Considerations
CGM use has been shown to improve diabetes-specific quality of life measures, including reducing diabetes distress and increasing hypoglycemia confidence compared to SMBG (29). Numerous individuals have noted the ease of use of CGM systems and the increased awareness of blood glucose levels they afford. Whereas SMBG provides a snapshot of blood glucose at a single point in time, CGM provides insights into glucose trends and allows patients to act preemptively to avoid hyperglycemia or hypoglycemia. This functionality results in increased time spent in the target glycemic range and decreased variability and instills higher confidence for patients to go about their normal activities (30–32). Patients may freely experiment with various foods and receive real-time feedback on the effects of those foods on their blood glucose levels, which in turn can facilitate diet modification and promote efficacious mealtime insulin boluses (32).
Current rtCGM technology includes modifiable alarms that alert users to rapidly rising or falling blood glucose levels or levels that are out of the target range. Many rtCGM systems also offer predictive alerts warning of blood glucose levels trending toward high or low limits. Such information could prove to be life-saving. Some individuals have noted that this feature provides reassurance while they are driving and also permits safer exercise (32).
Conversely, frequent alarms could make patients feel as if their diabetes controls their life and that they are failing to reach their target blood glucose levels, leading to increased anxiety (30,31). The term “alarm fatigue” describes situations in which patients receive so many CGM alarms that they become less likely to respond appropriately. Alarm fatigue can also lead to less-than-optimal use of rtCGM and may even lead some patients to discontinue CGM use (31,33), thus negating all of the positive benefits afforded by consistent CGM use.
How can patients maximize the benefit of these alarms without interrupting their day-to-day activities and sleep? With rtCGM, high and low alert thresholds can be customized to avoid oversaturation with alarms. Alert schedules can be customized for time and day of the week, which can be useful in limiting alarms during school or work activities or during sleep. Alarm sounds can be customized to be more discreet. If alarms become a burden, then isCGM can be considered to allow patients the ability to retrieve their blood glucose data at will by scanning their sensor.
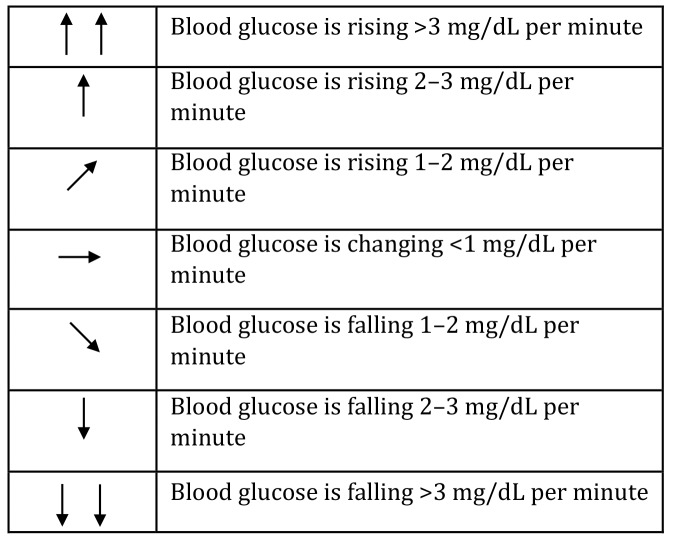
CGM systems also feature trend arrows to give context to current glucose levels. This feature can be invaluable for patients when making treatment decisions and increase their confidence in dosing decisions, particularly for patients who are especially fearful of hypoglycemia or hyperglycemia. Each CGM system has a different visual representation of the trend arrows but generally represent blood glucose levels changing at a rate of anywhere from <1 mg/dL per minute (horizontal arrow) to up to >2–3 mg/dL per minute (single or double vertical arrow pointed upward) with the inverse for declining blood glucose levels (Figure 1). The Endocrine Society has published practical guides for using the trend arrows on the Dexcom G6 or FreeStyle Libre CGM systems (34,35). These guides assist patients in adjusting their insulin dosing in the context of their current glucose trend.
FIGURE 1.
Sample CGM trend arrows. (See the manuals of individual devices for the specific ways they depict trends.)
Some patients who use CGM systems express concern about having a device connected to their body, which increases their self-consciousness about their diabetes and advertises their diagnosis to others (33). Suggestions for discreet placement of the device, including on the abdomen, may alleviate some of these concerns and allow for less visible monitoring of blood glucose levels in the workplace compared to traditional SMBG (32). Inopportune alarms can further exacerbate feelings of being different, and strategies to minimize alarm burden have been outlined previously.
The Provider’s Role in Facilitating CGM Use
Providing Education
Providers’ attitudes toward CGM are crucial in shaping patients’ willingness to try these novel technologies and get the maximal benefit from their use. There are numerous barriers to CGM use in the health care setting, including time constraints, limitations in provider knowledge, and lack of appropriate software to download data (36). Working within a multidisciplinary team of providers that includes certified diabetes educators (CDEs) will facilitate the most thorough training of individuals who wish to use CGM.
According to the ADA’s Standards of Care, “Robust diabetes education, training, and support are required for optimal continuous glucose monitor implementation and ongoing use” (7). In contrast to past practices, patients are often directed to online videos/tutorials and illustrated instructions rather than direct face-to-face training by device companies. As a result, it is imperative for the providers to assess each patient’s ability to adopt a new technology and provide in-office training with a CDE when appropriate.
Explaining Accuracy
Discussion with and education of patients regarding common misconceptions about the accuracy and precision of CGM versus SMBG are important to ensure optimal use of CGM systems and thorough understanding of CGM data. CGM technology currently measures glucose in the interstitial fluid (ISF), unlike blood glucose meters, which measure glucose in capillary blood. These are different physiological compartments that follow different dynamics (37). For example, the physiological time delay from ISF glucose level to blood glucose level is assumed to be 5–10 minutes, but the exact time delay can differ between patients and depending on the state of glucose levels. For instance, rapidly rising or falling blood glucose levels can increase this delay (38).
Device manufactures have focused on MARD as a measure of accuracy of CGM systems. MARD is defined as the mean absolute relative difference of sensor readings compared to simultaneously measured laboratory glucose values. However, as Ajjan et al. (37) point out, this definition may be oversimplified, as MARD calculations are lowest and most reliable when glucose readings are stable but change based on the rate of change of blood glucose levels and are subject to larger errors as glucose falls toward the hypoglycemic range (37). Therefore, it is paramount to educate patients about the decreased precision of CGM data in the setting of hypoglycemia or rapidly changing blood glucose levels. However, reinforcing the utility of trend arrows and predictive alarms to help support patient management decisions can empower patients to best interpret and act on data obtained from CGM, even in settings of rapidly changing blood glucose levels or hypoglycemia.
It is also worth addressing that the MARD in relation to laboratory glucose values of 17 current blood glucose meters was found to range from 5 to 22%, with higher MARD values in the settings of hypoglycemia and hyperglycemia (39). This is an important teaching point for patients who may be frustrated if glucose levels reported by their CGM device differ from those obtained through SMBG.
Analyzing and Interpreting Data
Using software or Web-based applications for data downloading is important to ensure a streamlined process of data collection when patients arrive for their appointments. Fully using separate billable professional services for CGM interpretation can also alleviate some of the time constraints associated with this additional work. As of 2018, Current Procedural Terminology (CPT) code 95251 can be used for analysis and interpretation of a minimum of 72 hours of data from ambulatory CGM (40). This is distinct from an evaluation and management (E/M) service, and the modifier 25 may need to be added to the E/M code if reported on the same day of service. However, it is important to note that the interpretation and report of CGM data cannot be used in the medical decision-making portion of the E/M (40). An appropriate CGM analysis, interpretation, and report should include the following (40):
Patient name
Date of birth
Medical record number
Indication for the device placement
Name and type of device placed
Sensor placement date and removal date
Date of printout of data
Analysis of data
Interpretation of data
Signature of interpreting physician or other qualified health care professional
CPT code 95250 can continue to be used for office-provided equipment with sensor placement, hook-up, calibration of monitor, patient training, removal of sensor, and printout of recording (40).
CGM provides an abundance of data that may be quite overwhelming to patients; thus, the onus is on providers to help patients use this information appropriately to adjust insulin doses and avoid becoming overwhelmed with the data. Providing patients with practical approaches to using trend arrows and other CGM data for insulin adjustment can be particularly helpful for reaching glycemic targets and preventing hypoglycemia (34,35,41).
Supporting patients’ positive coping mechanisms, including self-control in dealing with frustrations associated with CGM alarms and inaccuracies may lead to improved blood glucose control (33). Demonstrating retrospective data review is an important part of patient visits, as individuals who regularly use retrospective data may also see a reduction in A1C (33).
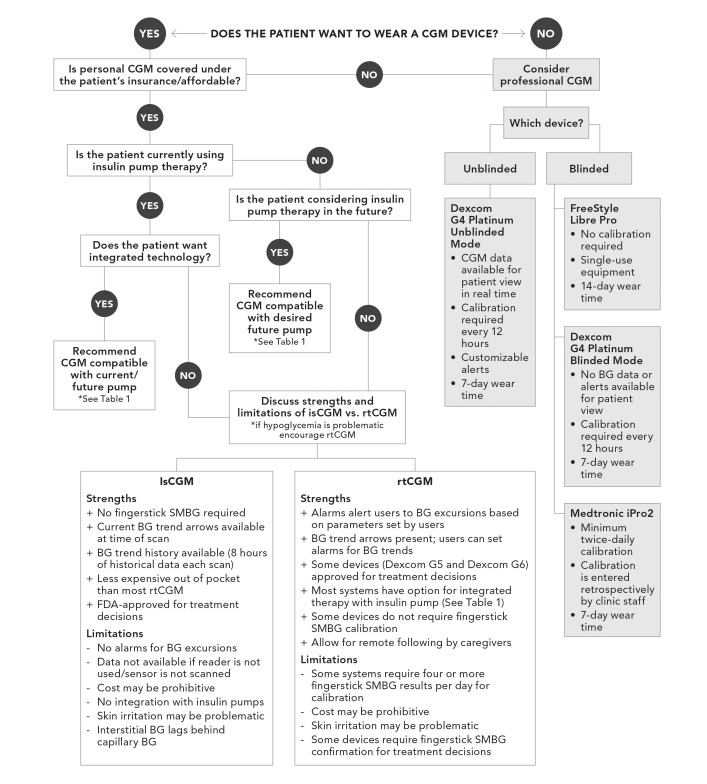
Guiding the Selection of a CGM System
The flowchart depicted in Figure 2 was developed to provide a basic, step-by-step guide to selecting a CGM system for a given patient. Asking about patient preferences is crucial to determining the ideal device for each patient both at this present time and in the near future. It has also been shown that there may be a discrepancy between patients’ own wishes and the professionals' perceptions and goals (42). Therefore, having a conversation regarding each patient’s desire to wear a CGM device is imperative. If a provider feels that CGM would be beneficial but the patient is skeptical, professional CGM can serve as an effective trial, allowing the patient to experience daily life with a CGM device (43), keeping in mind that current unblinded professional CGM technology uses older-model sensors and may be less accurate than currently available personal CGM sensors. Cost remains a major barrier for many patients interested in using CGM technology (44). The use of intermittent professional CGM may be a more affordable or better-covered option for patients who are unable to afford a personal CGM system.
FIGURE 2.
Flowchart for guiding patient selection of a CGM system. BG, blood glucose.
There are multiple options for the integration of CGM systems and insulin pumps. Some patients may not be interested in integrated technologies because of both modifiable and nonmodifiable factors such as cost, alarm annoyance, perceptions of accuracy, body image issues, and perceived hassle (45). Therefore, isCGM may be the most appropriate therapy for some patients who are on insulin pump therapy despite its lack of integration.
As previously mentioned, patients with hypoglycemia unawareness, nocturnal hypoglycemia, fear of hypoglycemia, or history of severe hypoglycemia may be best directed toward rtCGM, which has been demonstrated to reduce the duration of hypoglycemia (46), improve hypoglycemia awareness, and reduce the burden of problematic hypoglycemia (47). A trial of professional CGM (either blinded or unblinded) may be helpful to identify undetected hypoglycemia (48).
Case Studies
Case 1. Professional CGM
When faced with an A1C that does not correlate well with patient-reported blood glucose levels or the more objective data from a blood glucose meter, professional CGM may be helpful to assess a patient’s proximity to glucose targets and appropriately adjust diabetes therapy accordingly. To illustrate this point, we present the case of D.S., a 57-year-old woman with type 2 diabetes and subsequent insulin deficiency after pancreatic surgery for the treatment of pancreatic cancer. She was initially treated with oral medications after her diagnosis of diabetes at around the age of 45 years, and she transitioned to a basal-bolus insulin regimen after surgery, when there was evidence of insulin deficiency.
Despite careful titration of her insulin doses, her A1C remained in the range of 8–10%. SMBG results recorded in her blood glucose logbook did not correlate with her A1C, as the vast majority of those results were in the 100 mg/dL range. She did endorse late-morning hypoglycemia on occasion. There were glimpses of hyperglycemia at various times throughout the day, but no specific patterns were noted.
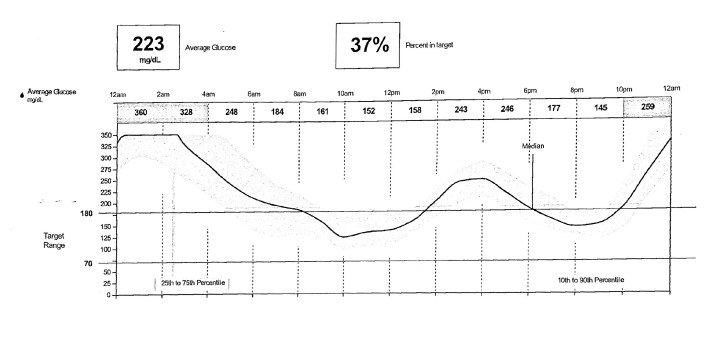
D.S. agreed to the placement of a FreeStyle Libre Pro CGM device. By relying solely on fingerstick SMBG data, post-lunch hyperglycemia and the more impressive overnight hyperglycemia were entirely missed (Figure 3). The average sensor glucose of 223 mg/dL correlated very well with her elevated A1C. The new data provided by professional CGM allowed for more accurate titration of her insulin regimen, including a reduction in basal insulin and an increase in prandial insulin doses with lunch and dinner. It also illustrated the effects of her frequent evening snacks and allowed for counseling on appropriate dietary changes.
FIGURE 3.
Case 1: D.S.’s professional CGM graph.
Case 2. Intermittently Scanned CGM
G.K. is a 37-year-old man with a 22-year history of type 1 diabetes without complications. He had an A1C of 5.9%; however, blood glucose levels checked at clinic appointments and via venous blood draws were in the range of 200–350 mg/dL. These data led his health care provider to believe that his A1C was discordant with is blood glucose concentrations.
Although G.K. understood that he was not likely reaching his glycemic targets, he refused to perform SMBG except on random occasions. He reported having a needle phobia and being quite bothered by the pain involved in fingersticks for SMBG. He took his bolus insulin based on his sensation of hypoglycemia or hyperglycemia and would often skip bolus doses when eating lower-carbohydrate meals (<45 g).
He and his health care provider had previously discussed rtCGM, but G.K. was not willing to check his blood glucose levels as required for calibration at the time. (All rtCGM systems then available required calibration.) With the introduction of blinded, no-calibration professional CGM, he agreed to a trial, which demonstrated an average blood glucose of 298 mg/dL, significant hyperglycemia, and occasional nocturnal hypoglycemia.
Based on this information and his experience of wearing the sensor, G.K. decided to obtain his own isCGM system. Three months later, downloaded data from his personal isCGM revealed an average blood glucose of 201 mg/dL, 0% hypoglycemia, and an average of 5 scans/day. G.K. is now taking his mealtime and correction insulin boluses more regularly and is motivated to reach his glucose targets. Most importantly, he reports an improved relationship with his diabetes and a greater sense of empowerment.
Case 3. rtCGM
R.J. is a 59-year-old man who has had type 2 diabetes since the age of 29 years, now complicated by stage 2 CKD and peripheral neuropathy. His A1C is 7.8%, and he uses an MDI insulin regimen and estimates his mealtime insulin based on his current blood glucose and estimations of the carbohydrate content of his meals. He has hypoglycemia sporadically several times per week and has had multiple instances in which emergency medical services were contacted because of severe hypoglycemia episodes.
R.J. has been educated regarding options for CGM, but he has refused because he was concerned that it would interfere with his job as a landscaper and felt that wearing a device continually would be burdensome. However, his family was concerned about his hypoglycemia, and he reported marital difficulties because of disagreements regarding his diabetes. Thus, he agreed to undergo 1 week of unblinded professional CGM.
He returned after the study reporting an improved sense of security at work and decreased stress felt by his wife. In addition, he was surprised to find that the device did not dislodge during the trial and that, after a few days, he was not bothered by wearing the sensor.
R.J. has now obtained his own rtCGM system and has an A1C of 7.6% with significantly less hypoglycemia.
Summary
CGM can be a useful tool for helping patients with diabetes reach their glucose targets, prevent hypoglycemia, improve their quality of life, and ease some of their burden of managing the daily demands of diabetes. It is imperative for clinicians to keep abreast of the latest technological advances so they can help to guide patients to the right therapies to monitor and manage their diabetes.
Acknowledgments
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
R.L. and S.S. researched data and wrote and edited the manuscript. R.L. is the guarantor of this work and, as such, had full access to the researched material and takes responsibility for the integrity of the content.
References
- 1.Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci 2012;69:83–93 [PubMed] [Google Scholar]
- 2.Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr 2018;12:181–187 [DOI] [PubMed] [Google Scholar]
- 3.Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology: continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:3922–3937 [DOI] [PubMed] [Google Scholar]
- 4.Fonseca VA, Grunberger G Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract 2016;22:1008–1021 [DOI] [PubMed] [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 6.Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2008;82:73–79 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association 7. Diabetes technology: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S71–S80 [DOI] [PubMed] [Google Scholar]
- 8.Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 2018;41:2265–2274 [DOI] [PubMed] [Google Scholar]
- 9.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 11.Abbott Diabetes Care FreeStyle Libre Flash glucose monitoring system provider information. Available from provider.myfreestyle.com. Accessed 4 February 2019
- 12.Dexcom Dexcom G6 Continuous Glucose Monitoring System User Guide. 2018. Available from s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf?_ga=2.206598675.1037310784.1544407431-1650225887.1541987205. Accessed 9 December 2018
- 13.Dexcom User Guide for Dexcom G5 Mobile Continuous Glucose Monitoring (CGM) System. 2018. Available from s3-us-west-2.amazonaws.com/dexcompdf/G5-Mobile-Users-Guide-Touchscreen-Receiver.pdf?_ga=2.203176081.1037310784.1544407431-1650225887.1541987205. Accessed 9 December 2018
- 14.Medtronic Guardian Sensor (3) User Guide. 2018. Available from www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian%20Sensor%203%20User%20Guide%20-%20June-%202018.pdf. Accessed 9 December 2018.
- 15.Eversense User Guide. 2018. Available from www.eversensediabetes.com/wp-content/uploads/2018/08/LBL-1602-01-001-Rev-D_Eversense-User-Guide_mgdL_R1-2.pdf. Accessed 9 December 2018
- 16.Reddy M, Jugnee N, El Laboui A, Spanudakis E, Anatharaja S, Olver N. A randomized controlled pilot study of continuous glucose monitor and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycemia. Diabet Med 2018;35:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy M, Jugnee N, Anatharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Technol Ther 2018;20:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behavior of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2008;80:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra JA, Shah M, Gill MS, et al. Clinical and economic benefits of professional CGM among people with type 2 diabetes in the United States: analysis of claims and lab data. J Med Econ 2018;21:225–230 [DOI] [PubMed] [Google Scholar]
- 21.Hashmi S, Mon S. Benefits of use of professional CGM in low socioeconomic population. Diabetes 2018;67(Suppl. 1):903-P [Google Scholar]
- 22.Teodoro de Oliveira AO, Barholomew K, Lavin-Tompkins J, Sperl-Hilen J. Use of continuous glucose monitoring as an educational tool in the primary care setting. Diabetes Spectr 2013;26:120–123 [Google Scholar]
- 23.Ahn D, Pettus J, Edelman S. Unblinded CGM should replace blinded CGM in the clinical management of diabetes. J Diabetes Sci Technol 2016;110:793–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiesa M, Hobbs S. Making sense of social research: how useful is the Hawthorne effect? Eur J Soc Psychol 2008;38:67–74 [Google Scholar]
- 25.Riddiesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther 2018;20:314–316 [DOI] [PubMed] [Google Scholar]
- 26.Abbott Diabetes Care FreeStyle Libre Pro flash glucose monitoring system. Available from provider.myfreestyle.com/pdf/Simulator-FreeStyle-Libre-Pro.pdf Accessed 9 December 2018
- 27.Medtronic iPro2 User Guide. 2016. Available from www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/iPro2_User_Guide-en-US.pdf. Accessed 9 December 2018
- 28.Dexcom Dexcom G4 Platinum Professional Continuous Glucose Monitoring System User's Guide. 2014. Available from s3-us-west-2.amazonaws.com/dexcompdf/HCP_Website/LBL-012206+Rev+05+User's+Guide%2C+G4+PLATINUM+Pro+US+Web.pdf. Accessed 9 December 2018
- 29.Polonsky WH, Hessler D, Ruedy KJ, Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]
- 30.Lawton J, Blackburn M, Allen J, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with type 1 diabetes. Diabet Med 2018;35:409–418 [DOI] [PubMed] [Google Scholar]
- 32.Pickup JC, Holloway MF, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care 2015;38:544–550 [DOI] [PubMed] [Google Scholar]
- 33.Ritholz MD, Atakov-Castillo A, Beste M, et al. Psychosocial factors associated with use of continuous glucose monitoring. Diabet Med 2010;27:1060–1065 [DOI] [PubMed] [Google Scholar]
- 34.Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM for the management of adults with diabetes. J Endocr Soc 2017;1:1445–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre Flash glucose monitoring system in adults. J Endocr Soc 2018;2:1320–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James S, Perry L, Gallagher R, Lowe J. Diabetes educators: perceived experiences, supports and barriers to use of common diabetes-related technologies. J Diabetes Sci Technol 2016;10:1115–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajjan RA, Cummings MH, Jennings P, Leelarathna L, Rayman G, Wilmot EG. Accuracy of flash glucose monitoring and continuous glucose monitoring technologies: Implications for clinical practice. Diab Vasc Dis Res 2018;15:175–184 [DOI] [PubMed] [Google Scholar]
- 38.Schmelzeisen-Redeker G, Schoemaker M, Kirchsteiger H, Freckmann G, Heinemann L, Re L. Time delay of CGM sensors: relevance, cause and countermeasures. J Diabetes Sci Technol 2015;9:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekhlaspour L, Mondesir D, Lautsch N, et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol 2017;11:558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Association of Clinical Endocrinologists New and updated codes for continuous glucose monitoring (CGM) in 2018. Revised 2 July 2018. Available from www.aace.com/files/socioeconomics/new_revised_codes_2018.pdf. Accessed 1 February 2019
- 41.Pettus J, Edelman SV. Recommendation for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol 2017;11:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hortensius J, Kars MC, Wierenga WS, Kleefstra N, Bilo H, J van der Bijil J. Perspectives of patients with type 1 or insulin-treated type 2 diabetes on self-monitoring of blood glucose: a qualitative study. BMC Public Health 2012;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz S, Scheiner G. The role of continuous glucose monitoring in the management of type-1 and type-2 diabetes. In Evidence Based Management of Diabetes Vora J, Buse J, Eds. Nr. Shrewsbury, U.K., TFM Publishing, 2012, p. 91–111 [Google Scholar]
- 44.Tanenbaum ML, Adams RN, Hanes SJ, et al. Optimal use of diabetes devices: clinical perspectives on barriers and adherence to device use. J Diabetes Sci Technol 2017;11:484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanenbaum ML, Hanes SJ, Miller KM, Diana N, Benson R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanenberg R, Bode B, Lane W, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc 2004;79:1521–1526 [DOI] [PubMed] [Google Scholar]
- 47.Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab 2018;103:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsirona S, Pappas C, Kandaraki E, Kassi G, Chronopoulou G, Diamanti-Kandarakis E. Detection of hypoglycemia in type 2 diabetic patients. Poster presented at the 18th European Congress of Endocrinology in Munich, Germany, 28–31 May 2016. Endocrine Abstracts 2016;41:EP527 [Google Scholar]