Highlights
-
•
Healthy non-diabetic adults who report hypoglycemic symptoms may experience frequent clinically significant hypoglycemia.
-
•
This condition may complete criteria for Whipple’s Triad.
-
•
Hypoglycemic symptoms encourage potentially obesogenic compensatory behaviors.
-
•
Hypoglycemic symptoms in the absence of diabetes remains understudied.
Keywords: Hypoglycemia, Hypoglycemic symptoms, Continuous glucose monitor, Obesity
Abstract
Aims
Clinical visits of non-diabetic patients reporting hypoglycemic symptoms are common in endocrinology practices, but remain understudied and lack clinical definition and evidence-based recommendations for diagnosis or treatment. Our goal was to pilot test the concordance of hypoglycemic symptoms with low glucose values in young non-diabetic individuals.
Methods
We recruited eight individuals who reported regularly experiencing symptoms consistent with hypoglycemia to wear a blinded Dexcom continuous glucose monitor and report symptoms for seven days. We excluded individuals with diabetes or other known causes of hypoglycemia or similar symptoms.
Results
Participants were all women with an average age of 29 years. 25% were African American and 25% had obesity. All participants experienced glucose values ≤ 70 mg/dL and half (4/8) experienced glucose ≤ 54 mg/dL for at least 15 min or 3 consecutive readings. Average time between last meal and reported symptoms was 4.4 h. Lower glucose values were significantly associated with higher odds of experiencing hypoglycemic symptoms 1.15 (CI: 1.07–1.24) for every −5mg/dL, (p < 0.001) from mixed effects models for repeated measures adjusted for age, race, and body mass index. All participants also reported engaging in potentially obesogenic behaviors in order to avoid symptoms.
Conclusions
Individuals with hypoglycemic symptoms in the absence of diabetes experience clinical hypoglycemia, indicating the need to understand the etiology, behavioral responses, and other health risks that might be associated with this understudied condition.
Introduction
Hypoglycemia in the absence of diabetes, sometimes referred to as reactive, postprandial, or idiopathic hypoglycemia [1], is a regular complaint by young non-diabetic patients referred for endocrinology evaluation. Despite this prevalent medical concern and the estimate that 38% of women in the UK may experience this condition [2], there are currently no standard diagnostic criteria for this syndrome [3], few estimates of its prevalence in the general population, and little evidence about its potential health implications. In fact, the health relevance, and indeed existence, of this condition has been controversial for decades [4], [5], [6].
The current paradigm assumes that low glucose outside the context of clinical hypoglycemia is healthy. As such, this group is largely ignored by the health care system, despite frequent, expensive, and largely unfruitful visits to providers and specialists. Individuals who experience hypoglycemic symptoms also report lower quality of life [7], [8], and may therefore become frustrated with the lack of a medical solution [8], and turn to alternative medicine. Many websites target this group with recommendations for untested diets, herbs, or other supplements. For example, The Hypoglycemia Support Foundation Inc, a 501(C)(3) non-profit, specifically focuses on this frustration with the tag line “Your symptoms may not be in your head.” and claims to have had more than a million visitors to their website [9].
Until recently, technical challenges to clinically addressing this understudied condition included difficulties in measuring glucose specifically during symptoms, in order to complete Whipple’s Triad of symptoms with a recorded low glucose value that respond promptly to feeding. Previous work has focused primarily on investigating reactive hypoglycemia in the laboratory setting. While some evidence of reactive hypoglycemia being tied to obesity and an exaggerated insulin response exists, Brun et al. conclude that the majority of cases are actually in the context of high insulin sensitivity accompanied by defects in counterregulation, most likely a blunted glucagon response [10]. The role of epinephrine and other counterregulatory hormones in reactive hypoglycemia and the relation with other potentially heterogeneous hypoglycemic conditions remain mostly theoretical. Brun et al. summarizes the criticisms of the field more generally, stating that the most commonly used approach, the oral glucose tolerance test, is “not suitable” for diagnosis and neither is the mixed meal test. They go further, calling hypoglycemia during oral glucose tolerance tests an “artifact” due to the “unphysiologic stress, seldom encountered outside the laboratory setting”. In line with Brun et al.’s recommendations that glucose must be measured concordantly with symptoms during “everyday life”, the updated technology and improved accuracy of continuous glucose monitors (CGM) have provided new opportunities to advance investigation of this condition. Taking advantage of the increased usability of CGM, we hypothesized a priori that reported symptoms would be significantly associated with lower glucose values. We further hypothesized that participants would report that symptoms resulted in lower quality of life and that they engaged in obesogenic behaviors specifically to avoid symptoms.
Materials and methods
In order to establish the concordance of low glucose values with hypoglycemic symptoms in the absence of diabetes, we conducted a pilot study in a convenience sample of volunteers. Volunteers were identified via a website designed for patients to enroll in active studies in an area of their interest [11]. We screened participants by phone to ensure that they had not been previously diagnosed with any of the following conditions by self-report: diabetes, clinical hypoglycemia, heart arrhythmia, insulinoma, or gestational diabetes or other known reason for their symptoms. We recruited 8 participants between the ages of 18 and 35 who reported regular hypoglycemic symptoms in the absence of diabetes to wear a blinded Dexcom G4 continuous glucose monitor for 7 days. The Dexcom G4 reported glucose measurements every 5 min. We blinded the monitors so that they did not report glucose values to participants, in order to avoid changes in normal everyday participant behavior. Regular hypoglycemic symptoms were defined by a screening response of “yes” to both of the following questions: 1. “Do you ever have symptoms of low blood sugar when you are fasting or between meals? That means any of the following beyond just feeling hungry: Headache, trouble concentrating or confusion, irritability, blurred vision, weakness, fatigue, nervousness, palpitations, tremor or shakiness, or dizziness. Please ONLY answer “yes” if these symptoms occur when you have not eaten in more than 3 h.” and 2. “Do you have these symptoms regularly or frequently when you are fasting or between meals? By frequently, I mean 2 or more times a week?”. We matched self-reported timing of hypoglycemic symptoms, and meal times to glucose measurements from the Dexcom G4. All participants provided written informed consent and the project was approved by the Wake Forest IRB.
We calculated baseline characteristics using mean and standard deviation, described regular experience of symptoms and age of symptom onset, and estimated the percentage of participants reporting different symptoms or behavior change in response to symptoms. We reported frequency of hypoglycemia measured during continuous glucose monitoring and time of symptom onset since previous meal. We defined hypoglycemia by two different cut-points: 1. Hypoglycemia: Glucose ≤ 70 mg/dL, as 70 mg/dL is generally considered the lower limit of the normal glucose range, and 2. Clinically significant hypoglycemia: Glucose ≤ 54 mg/dL, as 54 mg/dL was recently determined by the International Hypoglycaemia Study Group as the threshold that should be used for reporting of hypoglycemia as an adverse event in clinical trials for diabetes [12]. Classification of hypoglycemia was dependent on readings below these minimum values persisting for at least 15 min or 3 consecutive continuous glucose readings. We used mixed effects logistic regression to assess the association between continuous glucose values from the CGM as the exposure and reported symptoms (yes/no) as the outcome. This approach allowed us to account for the correlation between repeated measures on the same individual in order to estimate confidence intervals more accurately. Marginal probabilities were produced directly by the software from the mixed effects model as a post regression option. We adjusted for age, race, and body mass index as they have been shown to be associated with glucose, although predominantly in the context of diabetes. Analysis was conducted using Stata 14 [13].
Results
Participants were 100% female with an average age of 29 years. Six were Caucasian and two were African American. Six had normal weight (18.5 < BMI < 25.0 kg/m2) and two had obesity (BMI greater than 30 kg/m2) calculated from height and weight. Participants reported 15 years old as the average age of symptom onset. During the enrollment survey, the most common symptoms reported were fatigue and irritability, followed by weakness, dizziness, and palpitations. Three out of five participants also reported having experienced difficulty concentrating, blurred vision, and tremor or shakiness. 63% (5/8) of participants reported that their symptoms limited their ability to do the things they wanted to do or lowered their quality of life. Participants unanimously reported having altered their behavior in response to symptoms, with all eight stating that they had changed the timing of their meals and “snacked between meals when not hungry” to avoid symptoms (Table 1). In addition, 38% (3/8) reported that they had avoided exercise as a result of or to prevent symptoms.
Table 1.
Characteristics of study participants (n = 8) at enrollment and during study period (7 days).
| Characteristic | Mean | Standard Deviation |
|---|---|---|
| Age (years) | 28.5 | 4.06 |
| Female (%) | 100 | – |
| African American (%) | 25 | – |
| Body mass index (kg/m2) | 24.7 | 6.5 |
| Age at first symptom (years) | 15.1 | 4.01 |
| Usual frequency of symptoms | 2–3 times a week | – |
| Participants reporting: | ||
| Symptoms have lowered quality of life? (%) | 100 | – |
| Altered behavior to avoid symptoms? (%) | 100 | – |
| Changed the size of your meals? (%) | 75 | – |
| Changed the timing of your meals? (%) | 100 | – |
| Changed what types of foods you eat? (%) | 75 | – |
| Snacked between meals when not hungry? (%) | 100 | – |
| Continued to eat past full? (%) | 50 | – |
| Avoided exercise? (%) | 37.5 | – |
| Continuous glucose monitor results | ||
| Time wearing continuous glucose monitor (days) | 7.1 | 0.6 |
| Total readings (#) | 1994 | 433 |
| Average glucose (mg/dL) | 108.4 | 9.05 |
| First waking glucose (mg/dL) | 97.5 | 7.94 |
| Minimum glucose (mg/dL) | 51.9 | 8.67 |
| Participants with hypoglycemia: glucose ≤ 70 mg/dL (%) | 100 | – |
| Participants with clinically significant hypoglycemia: glucose ≤ 54 mg/dL (%) | 50 | – |
| Time out of range ≤ 70 mg/dL (%) | 6.2 | 7.9 |
| Time out of range ≤ 54 mg/dL (%) | 1.3 | 2.6 |
| Time out of range ≥ 180 mg/dL(%) | 1.1 | 0.7 |
| Time in range: between 70 and 180 mg/dL (%) | 92.7 | 8.3 |
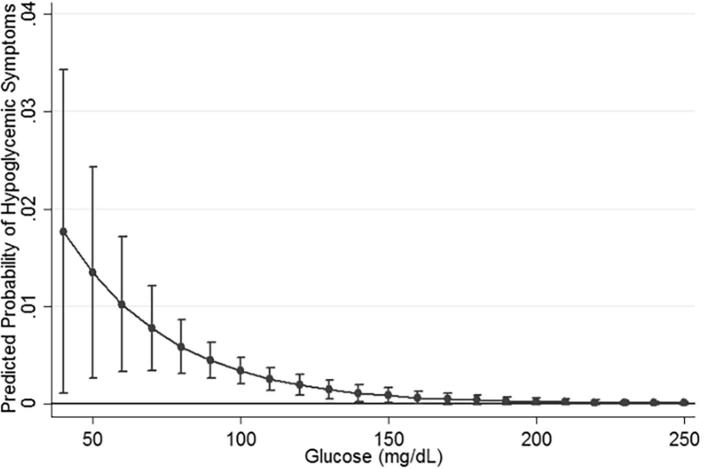
An average of 1994 (1767–2799) glucose values were measured from each participant across the 7-day follow-up period, except for one participant who ended the study one day early due to uncomfortable placement of the CGM device (1229 glucose measurements across 6 days). Participants unanimously reported complete compliance calibrating the CGM device (with a finger stick twice a day) and this is validated by the device reports (calibrations averaged 1.99 per day). During the study period, three participants reported experiencing symptoms daily, two participants experienced symptoms four times during the week, and two participants experienced symptoms twice a week. This frequency is similar to the rate of symptomatic hypoglycemia experienced by those with Type 1 Diabetes [14]. It is generally believed that hypoglycemia is rare in individuals without treated diabetes [14]; however, all participants in our pilot study had minimum glucose readings lower than 70 mg/dL and half (4/8) had a minimum glucose reading lower than 50 mg/dL (Table 1), the value considered to be “The generic nondiabetic glycemic threshold for impairment of cognitive function” [12]. Participants with hypoglycemia defined as ≤54 mg/dL had an average of 1.75 days with at least one occurrence of a minimum glucose below that threshold. The average number of days with minimum readings lower than 70 mg/dL was 3.4. The average minimum glucose reading was 51.9 mg/dL (39, 40, 48, 49, 57, 57, 58, and 67 respectively). The average time between last meal and symptom onset was 4.4 h. The odds ratio for symptom occurrence was 1.15 (CI: 1.07–1.24p = 0.001) for every 5 mg/dL lower glucose value, adjusted for age, race, and body mass index. Adjustment did not attenuate the estimate (unadjusted OR = 1.14 (CI: 1.06–1.23 p = 0.003)). Including the participant who did not complete the CGM also did not change this estimate. Fig. 1 shows the marginal continuous association between glucose values and reported hypoglycemic symptoms.
Fig. 1.
Marginal probability and 95% confidence intervals of reported hypoglycemic symptoms by continuous glucose value in 8 non-diabetic women aged 18–35. From mixed effects logistic regression (p < 0.001 for glucose coefficient).
Discussion
In our pilot study of individuals reporting regularly experiencing hypoglycemic symptoms, all participants had measured hypoglycemia (≤70 mg/dL) and half had clinically significant hypoglycemia (≤54md/dL) during the 7 days of blinded continuous glucose monitoring. Lower glucose levels were significantly associated with reported hypoglycemic symptoms, as was length of time since previous meal, and participants unanimously reported engaging in obesogenic behaviors to avoid symptoms.
In choosing a threshold for hypoglycemic reporting in clinical trials, the International Hypoglycaemia Study Group specifically sought to choose a cut-point with “immediate and long-term danger to the individual” and specify that values below 54 mg/dL “are distinctly low glucose concentrations that do not occur under physiological conditions in nondiabetic individuals” [12]. Despite other reports confirming the rarity of hypoglycemic readings from CGM in non-diabetic reference cohorts [15], [16], our pilot data contradict this statement, showing that young non-diabetic women who report symptoms may also experience these “unequivocally hypoglycemic values” [12]. The Study Group goes on to specify that glucose values in this range “cause defective glucose counterregulation and impaired awareness of hypoglycemia” and have been associated with cardiac arrhythmias and mortality in individuals with type 2 diabetes [12], [17], [18]. Since values in this range are unexpected in individuals without diabetes [12], [14], similar estimates in this group are rare. Moreover, despite these concerningly low glucose readings it is unlikely that these participants would receive a diagnosis of hypoglycemia under the current clinical paradigm since their average fasting glucose after waking was 97.5 mg/dL. These fasting glucose values solidly in the normal range at the time point when glucose is most likely to be measured for clinical decision making strongly suggest that individuals with this condition are regularly being overlooked in the clinic despite the potential for hypoglycemic pathology. Separate from the concordance with hypoglycemia, this condition should be a target for obesity prevention given the high prevalence of obesogenic behaviors reported by participants specifically to avoid symptoms. The strong association between lower glucose and symptoms suggests that this condition warrants further investigation from both behavioral and clinical perspectives.
This study has many of the limitations inherent to a pilot study: small sample size and limited diversity of measurements. Despite the primary limitation of small sample size, the large amount of data collected from CGM and the temporal congruity between time-stamped glucose measurements and reported symptoms is striking. Similarly, one participant did not wear the CGM for the complete study period (CGM removed on day 6); however, exclusion of data from this participant did not influence the results. This participant also had the lowest glucose values in the study suggesting that any bias from the missing data would create an underestimate. Second, the limited variables collected for this study leave open the possibility that other factors may better explain this relationship than glucose; however, adjustment for age, race, and BMI did not attenuate the results. While we did not measure insulin, the potential that the experience of these participants can be explained by undiagnosed insulinoma is highly unlikely given that the prevalence of insulinoma is 1–4 out of every million people, and fasting hypoglycemia is more characteristic of this condition [19]. Similarly, despite the low occurrence of glucose values above 180 mg/dL suggesting that participants did not have diabetes, impaired fasting glucose could not be fully ruled out due to the lack of blood collection. Other conditions that might explain our findings are also similarly rare, especially in young adult women. A stronger possibility is that symptoms are associated with epinephrine release triggered by low glucose [1], [20]. While it was not possible to measure epinephrine in this pilot study, this hypothesis would provide explanation for delays between hypoglycemia and symptom occurrence as glucose would already have started to rise by the time individuals felt the effects of epinephrine. Finally, there has been concern about using CGM as an indicator for hypoglycemia. While the lower range accuracy of older CGM models may have been questionable, the newer generation models have mostly resolved this problem. For instance, the Dexcom G4 used in this study has high overall accuracy [21], with a mean absolute difference in glucose level of 6 mg/dL for values below 70 mg/dL [22]. Further, these devices have been used specifically to avoid clinical hypoglycemia in patients with Type 1 Diabetes and hypoglycemia unawareness with much success [23], [24]. The current state of CGM technology combined with our approach of classifying hypoglycemia by repeated measurements below the minimum value, and the concurrence of these readings with reported symptoms (Fig. 1), provide strong support for our findings of clinical hypoglycemia in these young women without diabetes.
The limitations of this pilot study are offset by several strengths. Firstly, to our knowledge this is the first study to use the new CGM technology to investigate the concordance of hypoglycemic symptoms with low glucose in individuals without diabetes. Without the use of CGM, studies on this topic have primarily been relegated to short observations in the laboratory setting [1], [25], [26], [27], [28], [29], [30], [31], [32], where it has been challenging to capture symptoms. Additionally, the use of blinded CGM ensured that normal daily behavior and symptoms reporting was not influenced by glucose readings. Secondly, we focused study inclusion on young adults who were least likely to have comorbidities that could explain symptomology, and excluded participants with diabetes, gestational diabetes, and other diagnosed conditions known to cause similar symptoms. Finally, this study provides novel evidence that individuals with this condition engage in obesogenic behaviors specifically to avoid symptoms that may increase their long-term cardiometabolic risk.
These data, while from a small and heterogeneous sample, suggest that those who report experiencing hypoglycemic symptoms are experiencing clinical hypoglycemia on a regular basis and indicate a physiological explanation for symptoms. Moreover, these results provide initial progress towards completing Whipple’s Triad for this condition by showing: 1. Reported symptoms correspond to a fasting state, 2. Lower glucose values are concurrent with hypoglycemic symptoms, and 3. Participants unanimously reported that they changed eating behavior to avoid symptoms suggesting that symptoms resolve with food. These findings further elucidate the potential risk and pathology of this condition that has previously not been investigated. Our preliminary results implicate glucose dysregulation as an important driver of hypoglycemic symptoms in the absence of diabetes, with consequent detriments to quality of life and initiation of obesogenic behaviors.
Our results support the longstanding clinical observations of endocrinologists who regularly see young non-diabetic patients complaining of hypoglycemic symptoms, and contradict the belief that self-reported hypoglycemic symptoms are unreliable indicators of glucose level. To our knowledge, this is the first study to show this concordance between clinical hypoglycemia and symptoms in this population, and a significant association between lower glucose and symptom onset. New technology has allowed for the feasibility of concurrent measurement and reduced participant burden, and offers an opportunity for future investigation to fill the large gaps remaining in this area. We hope these findings will be a first step towards answering patient questions about this potentially highly prevalent condition, and understanding the control and implications of glucose regulation more generally. Hypoglycemic symptoms in the absence of diabetes are an indicator of potential clinically significant hypoglycemia that warrants further investigation and understanding.
Acknowledgments
Acknowledgements:
Funding: This work was supported by a Wake Forest Clinical and Translational Science Institute Ignition Fund Pilot Award by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health [UL1TR001420]. The authors also gratefully acknowledge use of the services and facilities of the Clinical Research Unit and Study Coordinator Pool, funded through the same grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Chalew S.A., McLaughlin J.V., Mersey J.H., Adams A.J., Cornblath M., Kowarski A.A. The use of the plasma epinephrine response in the diagnosis of idiopathic postprandial syndrome. JAMA, J Am Med Assoc. 1984;251(5):612–615. [PubMed] [Google Scholar]
- 2.Simpson E.J., Holdsworth M., Macdonald I.A. Prevalence of self-reported symptoms attributed to hypoglycaemia within a general female population of the UK. J Psychosom Res. 2006;60:403–406. doi: 10.1016/j.jpsychores.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Service FJ, Nathan DM, Mulder JE. Postprandial (reactive) hypoglycemia. UpToDate 2013; Oct 19, 2012.
- 4.Service F.J. Hypoglycemic Disorders. N Engl J Med. 1995;332(17):1144–1152. doi: 10.1056/NEJM199504273321707. [DOI] [PubMed] [Google Scholar]
- 5.Ng C.L. Hypoglycaemia in nondiabetic patients - an evidence. Aust Fam Physician. 2010;39(6):399–404. [PubMed] [Google Scholar]
- 6.Yager J. Non-Hypoglycemia is an Epidemic Condition. N Engl J Med. 1974;291(17):907–908. doi: 10.1056/NEJM197410242911713. [DOI] [PubMed] [Google Scholar]
- 7.Berlin I., Grimaldi A., Landault C., Cesselin F., Puech A.J. Suspected postprandial hypoglycemia is associated with beta-adrenergic hypersensitivity and emotional distress. J Clin Endocrinol Metab. 1994;79(5):1428–1433. doi: 10.1210/jcem.79.5.7962339. [DOI] [PubMed] [Google Scholar]
- 8.Pourmotabbed G., Kitabchi A.E. Hypoglycemia. Obstet Gynecol Clin North Am. 2001;28(2):383–400. doi: 10.1016/s0889-8545(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 9.The Hypoglycemia Support Foundation Inc. http://hypoglycemia.org/. Accessed 8/2/2017. http://hypoglycemia.org/ (accessed 8/28/2017.
- 10.Brun J.F., Fedou C., Mercier J. Postprandial reactive hypoglycemia. Diabetes Metab. 2000;26(5):337–351. [PubMed] [Google Scholar]
- 11.Wake Forest Baptist Medical Center. Be Involved. http://www.wakehealth.edu/beinvolved/ (accessed 3/31/2018.
- 12.Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care 2017; 40(1): 155-7. [DOI] [PubMed]
- 13.StataCorp. 2009. Stata Statistical Software Release 11. College Station, TX: StataCorp LP.
- 14.Service FJ, Cryer PE, Vella A. Hypoglycemia in adults: Clinical manifestations, definition, and causes. 3/14/2017 2018. https://www.uptodate.com/contents/hypoglycemia-in-adults-clinical-manifestations-definition-and-causes (accessed 3/31/2018.
- 15.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Variation of Interstitial Glucose Measurements Assessed by Continuous Glucose Monitors in Healthy, Nondiabetic Individuals. Diabetes care 2010; 33(6): 1297-9. [DOI] [PMC free article] [PubMed]
- 16.Hill N.R., Oliver N.S., Choudhary P., Levy J.C., Hindmarsh P., Matthews D.R. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow E., Bernjak A., Williams S. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 18.Bonds D.E., Miller M.E., Bergenstal R.M. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ (Clinical research ed) 2010;340 doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant C.S. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19(5):783–798. doi: 10.1016/j.bpg.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Cameron O.G., Buzan R., McCann D.S. Symptoms of insulin-induced hypoglycemia in normal subjects. J Psychosom Res. 1988;32(1):41–49. doi: 10.1016/0022-3999(88)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K., Balo A. The Accuracy and Efficacy of the Dexcom G4 Platinum Continuous Glucose Monitoring System. J Diabetes Sci Technol. 2015;9(5):1021–1026. doi: 10.1177/1932296815577812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyser T.A., Nakamura K., Price D., Bohnett L.C., Hirsch I.B., Balo A. Hypoglycemic Accuracy and Improved Low Glucose Alerts of the Latest Dexcom G4 Platinum Continuous Glucose Monitoring System. Diabetes Technol Ther. 2015;17(8):548–554. doi: 10.1089/dia.2014.0415. [DOI] [PubMed] [Google Scholar]
- 23.van Beers C.A.J., DeVries J.H., Kleijer S.J. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. doi: 10.1016/S2213-8587(16)30193-0. [DOI] [PubMed] [Google Scholar]
- 24.Heinemann L., Freckmann G., Ehrmann D. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–1377. doi: 10.1016/S0140-6736(18)30297-6. [DOI] [PubMed] [Google Scholar]
- 25.Brun J.F., Fedou C., Bouix O., Raynaud E., Orsetti A. Evaluation of a standardized hyperglucidic breakfast test in postprandial reactive hypoglycaemia. Diabetologia. 1995;38(4):494–501. doi: 10.1007/BF00410289. [DOI] [PubMed] [Google Scholar]
- 26.Palardy J., Havrankova J., Lepage R. Blood glucose measurements during symptomatic episodes in patients with suspected postprandial hypoglycemia. New England J Med. 1989;321(21):1421–1425. doi: 10.1056/NEJM198911233212101. [DOI] [PubMed] [Google Scholar]
- 27.Leonetti F., Foniciello M., Iozzo P. Increased nonoxidative glucose metabolism in idiopathic reactive hypoglycemia. Metab Clin Exp. 1996;45(5):606–610. doi: 10.1016/s0026-0495(96)90031-1. [DOI] [PubMed] [Google Scholar]
- 28.Simpson E.J., Holdsworth M., Macdonald I.A. Interstitial glucose profile associated with symptoms attributed to hypoglycemia by otherwise healthy women. Am J Clin Nutr. 2008;87(2):354–361. doi: 10.1093/ajcn/87.2.354. [DOI] [PubMed] [Google Scholar]
- 29.Snorgaard O., Binder C. Monitoring of blood glucose concentration in subjects with hypoglycaemic symptoms during everyday life. BMJ (Clinical research ed) 1990;300(6716):16–18. doi: 10.1136/bmj.300.6716.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snorgaard O., Lassen L.H., Rosenfalck A.M., Binder C. Glycaemic thresholds for hypoglycaemic symptoms, impairment of cognitive function, and release of counterregulatory hormones in subjects with functional hypoglycaemia. J Intern Med. 1991;229(4):343–350. doi: 10.1111/j.1365-2796.1991.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 31.Tamburrano G., Leonetti F., Sbraccia P., Giaccari A., Locuratolo N., Lala A. Increased insulin sensitivity in patients with idiopathic reactive hypoglycemia. J Clin Endocrinol Metab. 1989;69(4):885–890. doi: 10.1210/jcem-69-4-885. [DOI] [PubMed] [Google Scholar]
- 32.Vexiau P., Legoff B., Cathelineau G. Insulin and cortisol secretion during OGTT in patients with reactive hypoglycaemia with or without clinical symptoms. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1983;15(9):419–421. doi: 10.1055/s-2007-1018744. [DOI] [PubMed] [Google Scholar]