Abstract
The genus Sporothrix contains both species pathogenic to humans and animals as well as environmental fungi. S. humicola, a member of the latter S. pallida clade, has previously been reported only from soil. We have isolated this dimorphic fungus from multiple cutaneous lesions in two endangered marsupials native to Tasmania. Clinical appearance resembled cutaneous sporotrichosis, highlighting the principle pathogenic potential. Identification was based on partial ITS, β-tubulin and calmodulin gene sequencing.
Keywords: Sporothrix humicola, Eastern quoll, Dasyurus viverrinus, Dermatomycosis, MALDI-TOF MS
1. Introduction
Despite nomenclatural debate concerning the unequivocal affiliation of taxa to the genera Sporothrix (S.) and Ophiostoma, recent molecular studies revealed a well separated monophyletic clade for the 51 species of Sporothrix, now containing only sexual morphs within six species complexes [1]. Besides the pathogenic clade containing S. brasiliensis, S. schenckii, S. globosa and S. luriei the remaining species represent environmental fungi from soil, hardwoods and plant infructescences [1]. For some of the pathogenic species, animal reservoirs and zoonotic transmission may play an important role for human and animal infections, e.g. in S. brasiliensis caused by cat scratches or bites [2,3]. However, certain members of the environmental clades might also serve as facultative pathogens as they are capable of inducing clinical signs of disease, especially in immunocompromised hosts and following traumatic inoculation. Here, we document two cases of S. humicola infection in two endangered eastern quolls (Dasyurus viverrinus) from a zoological collection that suffered from multiple skin lesions. Quolls (Dasyurus spp.) are unique marsupials endemic to Australia. Few studies have implicated pathogen status in Dasyurus spp., including wildlife infections with Trypanosoma copemani in chuditches (Dasyurus geoffroii) [4], Babesia thylacis in northern quolls (Dasyurus hallucatus) [5], Hepatozoon spp. [6] and various nematodes [7,8] as well as a case series with atypical mycobacteria in a captive group of tiger quolls (Dasyurus maculatus) [9]. To the best of our knowledge, this is the first report on an isolation of S. humicola from a mycotic lesion in an animal species. Together with the uncommon microorganism and its host, but distinct clinical signs it is mandatory to properly identifying the etiologic agent to species level to rule out possible involvement of highly pathogenic dimorphic fungi.
2. Case
Eastern quolls (Dasyurus viverrinus) are an endangered marsupial species, restricted to Tasmania that are maintained in an ex situ breeding programme. In August 2016, a pair of 4-year-old quolls displayed crustaceous skin alterations and alopecia, whereupon a biopsy of the female was submitted on 09.08.2016 for microbiology and parasitology (sample ID 161009975). Standard bacterial and fungal cultures followed a previously described methodology [10] and were incubated up to 21 days. A mixed bacterial culture of Staphylococcus xylosus and Bacillus sp. could be isolated according to Table 1, but fungal cultures on Sabouraud glucose agar with gentamicin and chloramphenicol revealed growth of a filamentous-like fungus. Following DNA extraction from pure culture using a commercial kit (DNeasy® Blood & Tissue Kit, Qiagen, Hilden, Germany) according to the manufacturer's recommendations, sequencing of the ITS1-5.8S-ITS2 (ITS) region of the rRNA gene cluster was carried out using primers according to Table 2 [11]. Amplicons have been purified using a commercial PCR purification kit (E.N.Z.A. MicroElute™ Cycle Pure Kit, Omega Bio-tek Inc., Norcross, USA [distributed by VWR International, Darmstadt, Germany]) as recommended by the manufacturer and sequenced by Seqlab Sequence Laboratories (Göttingen, Germany). The sequences were analysed using BLAST N database (http://blast.ncbi.nlm.nih.gov/) and revealed the highest query coverage (99%) for S. mexicana (acc. no. LT799730.1) and S. schenckii (KX440160.1). Selective culture attempts to grow strictly anaerobic microbiota as well as the examination for ectoparasites did not yield any positive results.
Table 1.
Origin of Sporothrix humicola field isolates from eastern quolls investigated in this study as well as concomitant microbiota results from respective cases (M: male, F: female).
| Sample ID | Sex | Tissue | Sporothrix humicola proof and quantity+ | Concomitant microbiota* |
|---|---|---|---|---|
| 161009975 | F | skin biopsy | + | Bacillussp. +++, Sta. xylosus++ |
| 161011194 | M | liver | – | α-haemolysing streptococci +++, Sta. aureus +, Proteus sp. +, motile Aeromonas sp. +, Enterococcus sp. ++ |
| 161011194 | M | spleen | – | α-haemolysing streptococci ++, Sta. aureus +, Proteus sp. +, E. coli +, Sta. sciuri ++ |
| 161011194 | M | kidney | – | α-haemolysing streptococci ++, Sta. aureus +, Proteus sp. +++, E. coli +, Enterococcus sp. ++ |
| 161011194 | M | lung | – | α-haemolysing streptococci ++, Sta. sciuri +, Aspergillus fumigatus +, Enterococcus sp. ++ |
| 161011194 | M | small intestine | – |
E. coli +++, K. pneumoniae +++, Proteus sp. +, B. cereus + |
| 161011194 | M | large intestine | – |
E. coli ++, Proteus sp. +++, H. alvei +++, K. pneumoniae + |
| 161011194 | M | bone marrow | – | None |
| 161011194 | M | testicle | – | α-haemolysing streptococci ++, Sta. sciuri +, Proteus sp. +, Enterococcus sp. ++ |
| 161011194 | M | skin, foot 1 | + | Sta. sciuri ++, Aspergillus fumigatus + |
| 161011194 | M | skin, foot 2 | + | Sta. sciuri +++, B. cereus +, Aspergillus fumigatus + |
| 161011194 | M | skin, neck | + | K. pneumoniae ++, Sta. sciuri +++, Aspergillus sp. +, Aspergillus fumigatus + |
| 161011194 | M | skin, scrotum | – | Sta. sciuri +, B. cereus +, Str. thoraltensis + |
| 161011194 | F | liver | – | L. garvieae ++, E. coli ++, H. alvei ++, Proteus sp. ++ |
| 161011194 | F | spleen | – | L. garvieae ++, CNS ++, H. alvei + |
| 161011194 | F | kidney | – | L. garvieae +++, E. coli +, H. alvei + |
| 161011194 | F | lung | – | L. garvieae +++, E. coli +, H. alvei ++ |
| 161011194 | F | small intestine | – |
E. coli +, H. alvei ++, B. cereus +, Enterococcus sp. ++, Geotrichum candidum + |
| 161011194 | F | large intestine | + | E. coli +++, B. cereus ++, H. alvei ++, Mucor sp. +, |
| 161011194 | F | bone marrow | – | None |
| 161011194 | F | skin, neck | + | Sta. kloosii ++, |
| 161011194 | F | skin, right trunk | + | Sta. kloosii +, B. cereus +, Aspergillus fumigatus + |
| 161011194 | F | skin, left trunk | – | Sta. kloosii +, B. cereus + |
| 161011194 | F | skin, teats | – | Sta. kloosii + |
+: 1–10 colony forming units (cfu), ++: 11–50 cfu, +++: 51–200 cfu, ++++: >200 cfu, *B.: Bacillus, CNS: Coagulase-negative staphylococci, E.: Escherichia, H.: Hafnia, K.: Klebsiella, L.: Lactococcus, Str.: Streptococcus, Sta.: Staphylococcus (all bacterial identifications were carried-out by MALDI-TOF MS).
Table 2.
Oligonucleotide primer sequences and PCR conditions of the target genes used for the detection of Sporothrix humicola.
| Target gene | Sequence (5′-3′) | PCR program† | Reference |
|---|---|---|---|
| ITS1-5.8S-ITS2 (rRNA gene) | ITS-1: TCC GTA GGT GAA CCT GCG G ITS-4: TCC TCC GCT TAT TGA TAT GC |
1 1 |
[11] |
| beta-tubulin | bt2a: GGT AAC CAA ATC GGT GCT GCT TTC bt2b: ACC CTC AGT GTA GTG ACC CTT GGC |
2 2 |
[17] |
| calmodulin | CL1: GAR TWC AAG GAG GCC TTC TC CL2: TTT TTG CAT CAT GAG TTG GAC |
3 3 |
[18] |
†: PCR program.
1: ×1 (95 °C, 900 secs), ×40 (95 °C, 60s, 53 °C, 30s, 72 °C, 90s), ×1 (72 °C, 600 secs).
2: ×1 (94 °C, 240 secs), ×40 (94 °C, 45s, 58 °C, 45s, 72 °C, 90s), ×1 (72 °C, 600 secs).
3: ×1 (95 °C, 240 secs), ×40 (95 °C, 45s, 54 °C, 45s, 72 °C, 90s), ×1 (72 °C, 600 secs).
Due to deterioration of other age-related health issues (ankylosis of the vertebral column, confirmed by x-rays, data not shown) and a general life expectancy of five years maximum, euthanasia was performed according to AVMA guidelines (https://www.avma.org/KB/Policies/Documents/euthanasia.pdf). Briefly, pentobarbital-sodium 300 mg/mL (Release, Wirtschaftsgenossenschaft deutscher Tierärzte, Garbsen, Germany) was administered intraperitoneally and both quolls were subsequently submitted for necropsy. Following gross pathology (on 12.09.2016; sample ID 161011194), tissue specimens from skin, liver, spleen, kidney, lung, small and large intestine, bone marrow and gonads were both processed for microbiological culture and fixed in 4% neutral buffered formalin and embedded in paraffin for pathohistological examinations. Sections of 3 μm were cut and routinely stained with hematoxylin and eosin (HE). Additionally, sections were stained with Periodic Acid-Schiff (PAS) and Ziehl-Neelsen (ZN). Gross pathology of the male included a focal papillary skin proliferation on the neck. Multifocal erosive to ulcerative inflammations (up to 30 mm in diameter) of the skin of neck, trunk, tail and teats were predominantly found in the female, associated with pasty subcutaneous oedema and focal crusts (Fig. 1).
Fig. 1.
Female eastern quoll (Dasyurus viverrinus) with skin lesions, from which Sporothrix humicola could be isolated.
In the male, non-purulent perivascular inflammation of the lungs with accumulation of macrophages and acute alveolar haemorrhages with adjacent blistered emphysema were observed. Skin samples revealed a mild to moderate nodular pyoderma with focal erosions, ulcers and predominantly a purulent-necrotizing to rarely pyogranulomatous inflammation up to the subcutis in the male and similar, but high-grade and multifocal lesions in the female that were exacerbated by an inflammation of hair follicles, subcutaneous fatty tissue and dilatations of sweat glands. Tributary lymph nodes and all other internal organs as well as ZN- and PAS-slides were unremarkable in both quolls.
Microbiological examinations revealed a high-grade growth of various bacteria and fungi according to Table 1. Selective culture attempts to grow Brucella spp. or Salmonella spp. as well as the parasitological examination did not yield any positive results. Fungal isolates resembled members of the genus Sporothrix as was based on morphological features. Briefly, colonies at 20 °C had a flattened morphology with a smooth texture after 3–5 days of incubation [12], whereas colonies at 37 °C appeared cream-coloured, waxy and much more tiny (Fig. 2A and B). None of these forms showed any darkening with age as has been described for clinical S. schenckii isolates [12]. Colonies were also prepared in triplicate for MALDI-TOF mass spectrometry (MS; Microflex LT [BrukerDaltonics, Bremen, Germany]) analysis using an ethanol-formic acid preparation protocol for filamentous fungi provided by the manufacturer and MBT compass software (version V4.1) for identification. The commercial database used (DB 468 [Filamentous Fungi Library V2.0, Bruker]) comprised 24 spectra from one S. schenckii strain. The software considers MALDI score values > 2.3 and > 2.0 as secure species and genus identification levels, respectively. The Sporothrix isolates under study did not give conclusive identifications by MALDI-TOF MS. Although the database indeed suggested S. schenckii as the most probable matching pattern, the score values of 1.3–1.5 were not reliable for proper identification, even at genus level. By adding spectral information from the well-characterized, quality-controlled isolates from this study to the MALDI-TOF database it was possible to unequivocally identify this fungus to species level (score values 2.36; data not shown).
Fig. 2.
Colonies of Sporothrix humicola, incubated for 48 hours on Sabouraud glucose agar with gentamicin and chloramphenicol. A. mycelial phase at 20 °C; B. yeast-like phase at 37 °C.
Nevertheless, partial sequencing of the ribosomal operon again confirmed a member of Sporothrix as well as of other mycota that could predominantly be found – together with Aspergillus fumigatus – in the skin lesions in both animals. Highest similarity (99%) was found for S. mexicana (LT799730.1) and S. pallida (KP017076.1). To unequivocally identify the involved Sporothrix species, one each isolate from sample nos. 161009975 and 161011194 were submitted to the National Reference Center for Invasive Mycoses (NRZMyk, Jena, Germany). Here, genomic DNA was extracted from cultures grown on 4% malt extract agar (MEA; Difco) following a protocol [13] that was modified. Briefly, fungal material was homogenised for 5 min using a vortex adapter in a tube containing 1 mL lysis buffer (50 mM Tris, 50 mM sodium EDTA, 3% sodium dodecyl sulphate; pH 8) and acid-washed glass beads.
After 1 h at 68 °C, the tubes were centrifuged for 10 min at 16,000 relative centrifugal force (RCF). The supernatant was precipitated using 99.9% ethanol and the DNA pellet was washed twice with 70% ethanol, dried, resuspended in 50 μL distilled water and stored at −20 °C.
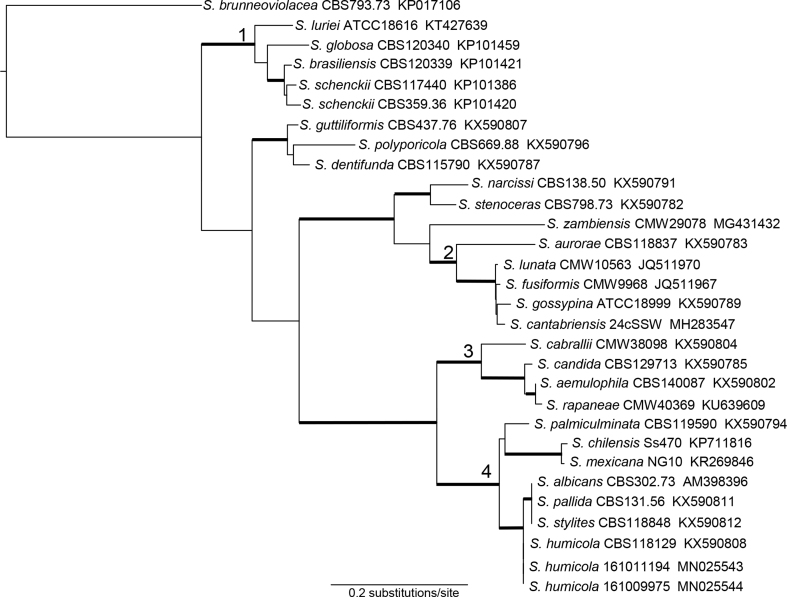
The 50 μL PCR mixture contained 0.2 μM of each primer, 10 μL of 5x MyTaq reaction buffer (Bioline GmbH, Luckenwalde, Germany) and 1 unit of MyTaq DNA polymerase (Bioline) and approximately 100 ng DNA. PCR products were purified and sent for sequencing to GATC Biotech (Cologne, Germany). Sequences were manually checked and edited using Chromas Lite (http://www.technelysium.com.au). Sequences, together with reference sequences downloaded from NCBI GenBank, were aligned using an online version of MAFFT 7 [14] within the CIPRES portal (www.phylo.org [15]). The datasets were subjected to Maximum Likelihood analyses (RAxML v8; [16]) using default settings with 1000 bootstrap iterations. Both isolates were identified as S. humicola based on partial sequencing of the ITS, β-tubulin (BT2) and calmodulin (CAL) genes (s. Table 2 and Fig. 3 for PCR details and a phylogenetic tree, respectively). Isolates have been stored in our strain collections under the following numbers: JMRC:NRZ:0556Vet-2016-015 and LHL 3648 (for isolate 161011194) and JMRC:NRZ:0557Vet-2016-016 and LHL 3602 (for isolate 161009975).
Fig. 3.
Phylogram derived from the calmodulin dataset. Both isolates from this study cluster within the S. pallida complex (4). The aligned sequences of all three S. humicola isolates are identical. The following complexes as described in de Beer et al., 2016 [1] are well supported with bootstrap values > 75%: pathogenic clade (1), S. gossypina complex (2), S. candida complex (3), S. pallida complex (4). The dataset contained 30 taxa with 905 characters. Bootstrap support >75% is indicated with bold branches.
2.1. Nucleotide sequence accession numbers
The GenBank database accession numbers (https://www.ncbi.nlm.nih.gov) for partial ITS1-5.8S-ITS2, calmodulin and beta-tubulin genes of both S. humicola isolates are MN053045, MN025543, MN025545 (for 161011194) and MN05344, MN025544, MN025546 (for 161009975), respectively.
3. Discussion
Sporotrichosis is the only dimorphic fungal disease that seems to have substantial zoonotic transmission [16]. A number of animal species have been linked with reservoirs for thermodimorphic fungi of the genus Sporothrix, but previously published work merely concentrates on the role of animals with respect to zoonotic infections due to the pathogenic clade. To this end, human infections could be attributed predominantly to cats and armadillos, but rats, dogs, squirrels, dolphins, birds, snakes, fish as well as invertebrates such as mosquitoes, ants and spiders have been documented to harbor members of Sporothrix [3,16,19]. In contrast, very few articles document animal carriage or human infection based on Sporothrix members of the environmental clades. Interestingly, soil species of the S. pallida clade, namely S. chilensis, S. mexicana and S. pallida, as well as S. stenoceras have been isolated especially from cutaneous lesions with low-virulence to the warm-blooded, vertebrate host [[19], [20], [21]]. S. humicola, also a member of the S. pallida clade [1], has been described in 2008, originally isolated from South African soil in an effort to differentiate so called ‘environmental isolates of S. schenckii’ from true pathogens [12] and has so far never been attributed with pathologies in animals. Conversely, a member most closely related to S. humicola, S. pallida and S. stylites was found as the predominant of several fungi isolated from the peel pedicle of banana fruits called ‘fuzzy pedicle’ [22]. Here, we give first evidence of the pathological potential of S. humicola also as a vertebrate pathogen. The investigations of the present study show that this fungus could repeatedly be isolated from cutaneous alterations very similar to lesions in pathogenic congener species. However, samples were usually poly-microbial that might reflect concomitant or even synergistic microbiota of the skin from which at least some are well-known pyogenic bacteria (e.g. Staphylococcus aureus) and thus could have exacerbated the clinical state. Furthermore, the senescence of these two individuals might have caused a subtle immune-impairment and thus rendered them susceptible for opportunistic infections. Very little is known about the role of this microorganism with respect to the very rare host species, because quolls are seldom kept as zoo animals and the captive bred group from this study never had any contact with conspecifics from the wild. No such lesions have been reported from other maintenances or from the wild, respectively, although one can imagine that this savanna and dry forest dwelling species will regularly be exposed to soil microbiota. Clinical signs were mild and restricted to the external skin. The only internal proof from the large intestine in the female quoll was not associated with extracutaneous pathologies, nor were any other internal tissues affected. Unfortunately, it was not possible to demonstrate yeast-like cells in the affected cutaneous lesions. On the other hand, this is in considerable congruence with findings in the pathogenic clade where a relatively low sensitivity for histological investigations was found, even when using fungus specific stains [19]. This might also be explained by the seemingly lower conversion rate of environmental isolates into yeast-like cells in vitro compared to the pathogenic clade (Fig. 4A and B). The deficient morphological mycelium-to-yeast conversion has, nevertheless, been linked to other environmental Sporothrix species and deviant pathogenicity in the murine model [19] and may thus explain also a low pathogenicity in S. humicola.
Fig. 4.
Microscopic images of Sporothrix humicola on Sabouraud glucose agar with gentamicin and chloramphenicol. A. mycelial phase, incubated for 48 hours at 20 °C; B. yeast-like phase, incubated for 6 days at 37 °C (400× magnification).
With respect to a proper species identification that is also essential for the assessment of epidemiology and reservoir range and to rule-out highly pathogenic fungi it might be necessary to use protein coding gene in addition to ITS region gene sequences for the recognition of cryptic species, especially in the S. pallida complex [19]. This was true also in the present report where sequencing of the ITS region alone or in combination with the ribosomal large subunit [23] did not succeed to identify the correct Sporothrix species. Only the combination together with BT2 and CAL genes facilitated unequivocal species identification. Phenotypical identification by MALDI-TOF MS suffers from a deficient database, because only the type species, S. schenckii, has been included based on one single strain entry, yet. This can be improved by the addition of spectra of well-characterized isolates to the database used. By adding spectral information proceeded by a quality control protocol provided by the manufacturer improved the quality of identification to a secure species level in our case [24].
Concluding, besides three strains from soil in South Africa and The Netherlands the isolates from this study seem to represent the only two further members of this species that have been stored to date. These represent also the first isolates of S. humicola involved in a clinical mycosis in a vertebrate species.
Conflict of interest
There are none.
Ethical form
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animal husbandry fulfilled ethical standard guidelines according to the code of ethics and animal welfare of the world association of zoos and aquariums (WAZA; http://ethics.iit.edu/ecodes/node/3013).
Acknowledgements
The authors like to thank Katharina Engel, Anna Eckert, Marie-Luise Sonneborn, Andreas Junck, Walter Lang, Sassan Schwarz and Carmen Karkowski for excellent technical assistance. The LHL is supported by the Hessian Ministry for the Environment, Climate Change, Agriculture and Consumer Protection. Work in the German National Reference Center is supported by the Robert Koch Institute from funds provided by the German Ministry of Health (grant 1369-240).
References
- 1.de Beer Z.W., Duong T.A., Wingfield M.J. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud. Mycol. 2016;83:165–191. doi: 10.1016/j.simyco.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della Terra P.P., Rodrigues A.M., Fernandes G.F., Nishikaku A.S., Burger E., de Camargo Z.P. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Neglected Trop. Dis. 2017;11(8) doi: 10.1371/journal.pntd.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues A.M., de Hoog G.S., de Camargo Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12(7) doi: 10.1371/journal.ppat.1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C., Keatley S., Northover A., Gofton A.W., Brigg F., Lymbery A.J. Next generation sequencing reveals widespread trypanosome diversity and polyparasitism in marsupials from western Australia. Int J Parasitol Parasites Wildl. 2018;7(1):58–67. doi: 10.1016/j.ijppaw.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangs, Purnomo M.J. Babesia thylacis (apicomplexa: babesiidae) in a northern quoll, Dasyurus hallucatus (marsupialia: dasyuridae), from western Australia. Comp. Parasitol. 1996;63:266–268. [Google Scholar]
- 6.Barbosa A., Reiss A., Jackson B., Warren K., Paparini A., Gillespie G. Prevalence, genetic diversity and potential clinical impact of blood-borne and enteric protozoan parasites in native mammals from northern Australia. Vet. Parasitol. 2017;238:94–105. doi: 10.1016/j.vetpar.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Chabaud A.G., Seureau C., Beveridge I., Bain O., Durette-Desset M.C. [Studies on the echinonematinae (nematoda)] - in French. Ann. Parasitol. Hum. Comp. 1980;55(4):427–443. [PubMed] [Google Scholar]
- 8.Smales L.R. Linstowinema, smales, 1997 (nematoda: seuratidae) from dasyurids (marsupialia: dasyuridae) from Australia. Syst. Parasitol. 1999;43(1):29–39. doi: 10.1023/a:1006127915855. [DOI] [PubMed] [Google Scholar]
- 9.Raymond J.T., Tell L., Bush M., Nichols D.K., Schulman F.Y., Montali R.J. Subcutaneous atypical mycobacteriosis in captive tiger quolls (Dasyurus maculatus) Vet Pathol. 2000;37(2):137–142. doi: 10.1354/vp.37-2-137. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg T., Seeger H., Kasuga T., Eskens U., Sauerwald C., Kaim U. Detection and characterization of Histoplasma capsulatum in a German badger (Meles meles) by ITS sequencing and multilocus sequencing analysis. Med. Mycol. 2013;51(4):337–344. doi: 10.3109/13693786.2012.723831. [DOI] [PubMed] [Google Scholar]
- 11.White T.J., Bruns T.D., Lee S., Taylor J. Academic Press; New York: 1990. Analysis of Phylogenetic Relationships by Amplification and Direct Sequencing of Ribosomal RNA Genes. PCR Protocols: a Guide to Methods and Applications. [Google Scholar]
- 12.de Meyer E.M., de Beer Z.W., Summerbell R.C., Moharram A.M., de Hoog G.S., Vismer H.F. Taxonomy and phylogeny of new wood- and soil-inhabiting Sporothrix species in the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia. 2008;100(4):647–661. doi: 10.3852/07-157r. [DOI] [PubMed] [Google Scholar]
- 13.Möller E.M., Bahnweg G., Sandermann H., Geiger H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992;20(22):6115–6116. doi: 10.1093/nar/20.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M.A., Pfeiffer W., Schwartz T., editors. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov 2010, New Orleans, LA. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. [Google Scholar]
- 16.Chakrabarti A., Bonifaz A., Gutierrez-Galhardo M.C., Mochizuki T., Li S. Global epidemiology of sporotrichosis. Med. Mycol. 2015;53(1):3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 17.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61(4):1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell K., Kistler H.C., Tacke B.K., Casper H.H. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. U. S. A. 2000;97(14):7905–7910. doi: 10.1073/pnas.130193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orofino-Costa R., Macedo P.M., Rodrigues A.M., Bernardes-Engemann A.R. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017;92(5):606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison A.S., Lockhart S.R., Bromley J.G., Kim J.Y., Burd E.M. An environmental Sporothrix as a cause of corneal ulcer. Med Mycol Case Rep. 2013;2:88–90. doi: 10.1016/j.mmcr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues A.M., Cruz Choappa R., Fernandes G.F., de Hoog G.S., de Camargo Z.P. Sporothrix chilensis sp. nov. (Ascomycota: ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016;120(2):246–264. doi: 10.1016/j.funbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Tarnowski T.L., Perez-Martinez J.M., Ploetz R.C. Fuzzy pedicel: a new postharvest disease of banana. Plant Dis. 2010;94(5):621–627. doi: 10.1094/PDIS-94-5-0621. [DOI] [PubMed] [Google Scholar]
- 23.Sting R., Eisenberg T., Hrubenja M. Rapid and reasonable molecular identification of bacteria and fungi in microbiological diagnostics using rapid real-time PCR and Sanger sequencing. J. Microbiol. Methods. 2019;159:148–156. doi: 10.1016/j.mimet.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Rau J., Eisenberg T., Männig A., Wind C., Lasch P., Sting R. 2016. MALDI-UP – an internet platform for the exchange of MALDI-TOF mass spectra; pp. 1–17.http://maldi-up.ua-bw.de/ User guide for. Aspects of food control and animal health (eJournal) [Google Scholar]