Abstract
Vaccinia virus (VACV) possesses a great safety record as a smallpox vaccine and has been intensively used as an oncolytic virus against various types of cancer over the past decade. Different strategies were developed to make VACV safe and selective to cancer cells. Leading clinical candidates, such as Pexa-Vec, are attenuated through deletion of the viral thymidine kinase (TK) gene, which limits virus growth to replicate in cancer tissue. However, tumors are not the only tissues whose metabolic activity can overcome the lack of viral TK. In this study, we sought to further increase the tumor-specific replication and oncolytic potential of Copenhagen strain VACV ΔTK. We show that deletion of the anti-apoptosis viral gene F1L not only increases the safety of the Copenhagen ΔTK virus but also improves its oncolytic activity in an aggressive glioblastoma model. The additional loss of F1L does not affect VACV replication capacity, yet its ability to induce cancer cell death is significantly increased. Our results also indicate that cell death induced by the Copenhagen ΔTK/F1L mutant releases more immunogenic signals, as indicated by increased levels of IL-1β production. A cytotoxicity screen in an NCI-60 panel shows that the ΔTK/F1L virus induces faster tumor cell death in different cancer types. Most importantly, we show that, compared to the TK-deleted virus, the ΔTK/F1L virus is attenuated in human normal cells and causes fewer pox lesions in murine models. Collectively, our findings describe a new oncolytic vaccinia deletion strain that improves safety and increases tumor cell killing.
Introduction
Vaccinia virus (VACV) and closely related poxviruses have been used for centuries as vaccines for smallpox, culminating in the eradication of smallpox in the late 1970s. The eradication campaign showed that VACV has an impressive safety profile. To further improve on this attractive vaccine backbone, techniques were developed to engineer and rescue recombinant VACV.1 However, to date, more than half of the genome’s 200 genes have unknown or incompletely understood functions, leaving much to be learned about the intricate host-pathogen interaction of this virus. Interestingly, it was shown that several VACV genes can be knocked out with no observable in vitro or in vivo phenotype, revealing a complex genome with redundant and non-essential elements.2
In addition to its application as a vaccine vector, VACV also has intrinsic oncolytic properties.3 In particular, it shows preferential replication in tumor cells that display activated epidermal growth factor receptor (EGFR)/Ras/MAPK (mitogen-activated protein kinase) pathways,4 which are observed in many cancer types.5, 6 Beyond use as a potential mono-therapy agent, VACV has also long been used in combination with other cancer therapy agents.7, 8 For instance, oncolytic VACV strains were recently found to synergize well with immunotherapy9, 10 as well as induce anti-tumor immunity when encoding immune-stimulatory transgenes11 or tumor-associated antigens.12
The most advanced clinical VACV candidate is Pexa-Vec (JX-594). Pexa-Vec is a strain that has its viral thymidine kinase (TK) gene deleted, thus requiring the virus to utilize nucleotide pools produced in metabolically active tumor cells. The virus additionally expresses the GM-CSF (granulocyte-macrophage colony-stimulating factor) cytokine to activate immune cells at the tumor site.13 Pexa-Vec was shown in phase I and phase II clinical trial studies to be safe after intravenous delivery and capable of expressing the immunostimulatory gene.14 Currently, this virus is being assessed in a phase III clinical trial, testing its efficacy by intratumoral injection of patients suffering from hepatocarcinoma (ClinicalTrials.gov: NCT02562755). Although Pexa-Vec has shown minimal adverse side effects, the TK deletion may not fully preclude virus replication in non-cancerous metabolically active tissues, such as the liver. Furthermore, this attenuation may also hinder the virus’s capacity to proliferate in metabolically inactive tumor niches present in heterogeneous tumors.15 Therefore, there remains room for improved engineering of the virus as well as a need to seek alternative ways to increase the therapeutic window in a way that would target the widest possible range of cancer cells.
F1L is a VACV gene that inhibits infection-induced apoptosis and inflammation. F1L binds the pro-apoptotic Bcl-2 family members Bak and Bim,16 thus preventing apoptotic stimulus-induced loss of the inner mitochondrial membrane potential and the subsequent cytochrome c release.17, 18 The structure of F1L resembles a dimer-swapped Bcl-2 homolog with an atypical amino-terminal extension.19, 20 The amino terminus of F1L binds to and inhibits caspase-9 and the inflammasome component NLRP1.21 Overall, virulence of a F1L-deleted virus was attenuated in a mouse model, and this was attributed to the loss of NLRP1 activity of F1L.21 Deletion of F1L from the Copenhagen strain has shown an induction of apoptosis in fibroblast cells.18
Because non-cancerous cells use apoptosis as an antiviral defense mechanism, we hypothesized that disabling anti-apoptosis mechanisms in VACV would be beneficial for tumor selectivity. Furthermore, since apoptosis is only partially functional in tumor tissues, we ventured that our F1L-deleted Copenhagen can both replicate and induce faster cell death in cancer cells. In this study, we show that removing the F1L gene increases VACV's ability to induce immunogenic apoptosis and oncolytic activity in cancer cells.
Results
F1L KO Increases Immunogenic Apoptosis
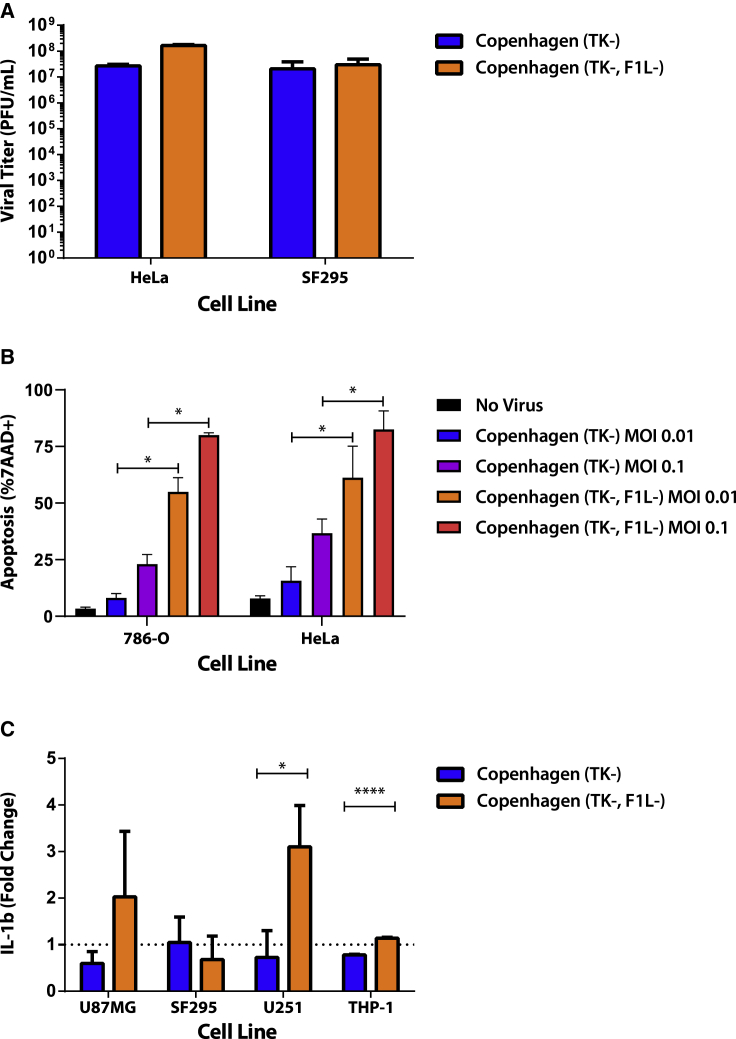
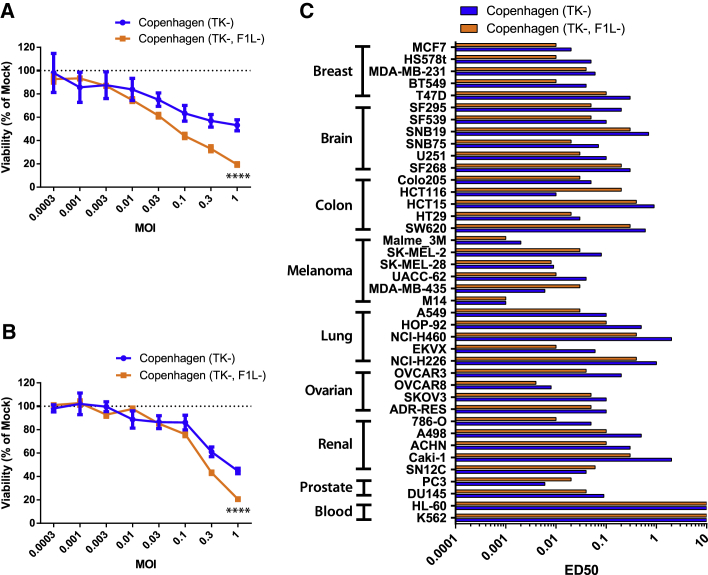
Here, we rescued an F1L deletion in a VacV Copenhagen ΔTK strain (Cop-ΔTK/F1L). Testing the ability of the virus to replicate, we found Cop-ΔTK/F1L to generate 10-fold more progeny virus, compared to Cop-ΔTK in HeLa cells (production cell line), but not SF295 (Figure 1A). Both 786-O and HeLa cells show a higher percentage of dead 7AAD-positive cells when infected with Cop-ΔTK/F1L, compared to Cop-ΔTK (Figure 1B). Furthermore, unlike Cop-ΔTK, Cop-ΔTK/F1L induces secretion of interleukin (IL)-1β cytokines in a variety of glioblastoma cells (Figure 1C). To assess whether these phenotypes are specific to certain tumor types, we screened 40 of the NCI-60 human cancer cell lines for virus-induced cell death. We found that, except for blood cancer, Cop-ΔTK/F1L induced faster cell death in all tumor types when compared to Cop-ΔTK (Figure 2).
Figure 1.
Deletion of F1L Increases Cancer Cell Death without Affecting Replication
(A) Viral replication of F1L deleted virus assessed through plaque assay in HeLa and SF295 cell lines at an MOI of 0.1 48 h post-infection. (B) Flow-cytometric analysis of 7-AAD apoptosis marker in 786-O and HeLa cells at various MOIs 24 h post-infection. (C) Induction of IL-1β levels as measured by ELISA in various cancer cell lines infected for 48 h at an MOI of 0.1. *p < 0.05; ****p < 0.0001. Data is shown as mean and SE.
Figure 2.
Vaccinia Delta F1L Induces Rapid Cancer Killing in a Wide Variety of Tumor Types
(A and B) Alamar blue viability of U87MG (A) and SF295 (B) cell lines measured as percentage of non-infected cells at various MOIs for a 48-h infection. Data is shown as mean and SE. (C) Alamar blue viability of various NCI-60 cell lines infected with either vaccinia for 48 h. ****p < 0.0001.
Removal of F1L Increases VACV Safety
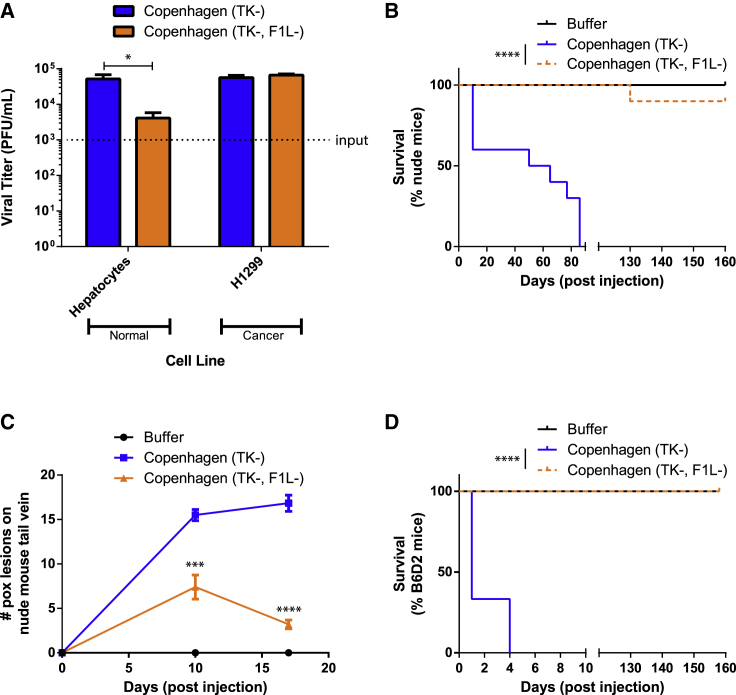
Interestingly, when we compared the two different Copenhagen mutant strains in normal human hepatocyte cells, we found Cop-ΔTK/F1L 10-fold less able to replicate than Cop-ΔTK/F1L (Figure 3A). These results suggest a role of F1L in VACV virulence and an ability to replicate in normal tissue. To evaluate whether this difference is significant in the presence of an immune system, we tested Cop-ΔTK/F1L anti-tumor activity in two mouse models. Strikingly, challenging immune-deficient mice intravenously at 1 × 108 plaque-forming units (PFUs) had a 90% survival rate for Cop-ΔTK/F1L and 0% for Cop-ΔTK (Figure 3B). Interestingly, injecting systemic Copenhagen in the tails of nude mice produced significantly more pox lesions at the site of injection by the Cop-ΔTK virus, compared to the double Cop-ΔTK/F1L (Figure 3C). Lastly, when deleting F1L in the Western Reserve (WR) neuro-adapted vaccinia, we saw less viral titer in the brain of nude mice after systemic injection of WR ΔTK/ΔF1L (Figure S1B).
Figure 3.
Deletion of F1L Increases the Safety and Tumor Selectivity of Vaccinia
(A) Viral replication in primary hepatocytes and H1299 cancer cells during a 72-h infection at an MOI of 0.001 as determined by plaque assay. (B and D) Nude mouse (B) and immunocompetent B6D2 mouse (D) survival monitoring following a dose (1 × 108 PFUs) of either VACV intravenously. Survival is shown as Kaplan-Meier survival curves. (C) Amount of pox lesions on mouse tails quantified at two time points during the experiment done in (B). (A and C) Data is shown as mean and SE.*p < 0.05; ***p < 0.001; ****p < 0.0001.
One disadvantage with VACV is the inability to use higher therapeutic doses of the virus when administering systemic intravenous injections to treat tumors. Indeed, injection of 1 × 108 PFUs of Cop-ΔTK systemically induces viral toxicity, and mice succumb within 4 days (Figure 3D). However, the same dose of Cop-ΔTK/F1L does not induce fatal toxicity and results in a survival rate of 100% (Figure 3D). Therefore, data suggest that Cop-ΔTK/F1L has a higher safety profile and is more efficacious.
Cop-ΔTK/F1L Is More Efficacious in Glioblastoma Tumors
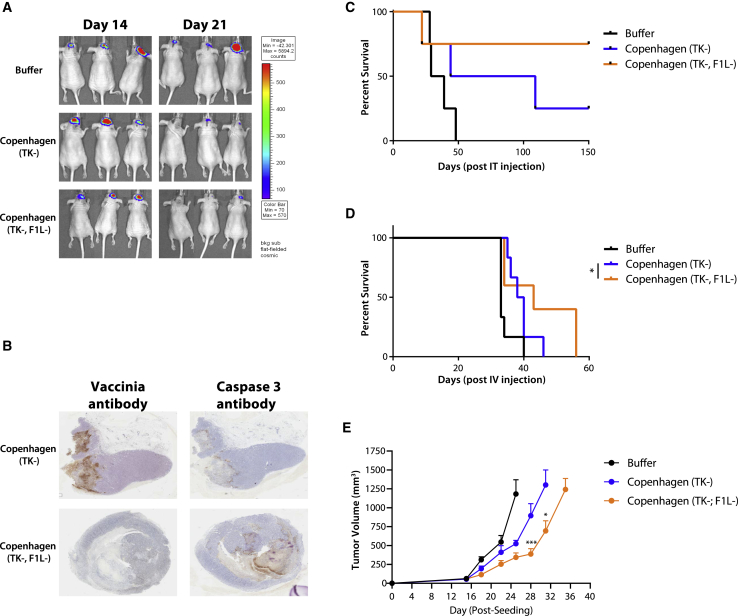
We have identified that Cop-ΔTK/F1L kills tumor cells faster and displays less off-target activity, even in mouse brain tissue. Since most oncolytic viruses cannot be administer intracranially at therapeutic doses without causing sickness and mouse death,22 the Cop-ΔTK/F1L safety profile makes it an appealing therapeutic candidate for glioblastoma treatment. To test the efficacy of Cop-ΔTK/F1L in a challenging glioblastoma model, we treated luciferase-expressing U87 xenografts implanted in CD-1 nude mouse brains. We observed the more tumor inhibition in Cop-ΔTK/F1L-treated mice as compared to Cop-ΔTK (Figure 4A), indicating improved oncolysis and tumor control. Immunohistochemical analysis also revealed increased caspase-3 straining in Cop-ΔTK/F1L-treated mice (Figure 4B). This suggests higher induction of tumor cell death in vivo and is consistent with the higher rates of apoptosis observed in vitro (Figure 1B). Indeed, Cop-ΔTK/F1L-mediated oncolysis translates to higher survival (85% versus 25%, compared to Cop-ΔTK) when treating U87 xenografts intracranially (Figure 4C). In line with these results, we also found that Cop-ΔTK/F1L administer intravenously extended mouse survival in the same model (Figure 4D).
Figure 4.
Treatment with Vaccinia Delta F1L Extends Survival in a Glioblastoma Model and Delays Tumor Growth in a Syngeneic Mouse Colon Model
(A) IVIS imaging of U87 luciferase-expressing tumors seeded intracranially in nude mice after one intracranial injection of either vaccinia or PBS vehicle. (B) Immunohistochemistry (IHC) on slices of U87 tumors treated intracranially with either vaccinia virus and stained with both vaccinia virus antibody and antibody for caspase-3. (C and D) Survival of nude mice seeded intracranially with U87 luciferase-expressing tumors and treated with one intracranial (C) or intravenous (D) dose of 1e7 PFUs of either vaccinia. Survival is shown as Kaplan-Meier survival curves. (E) Colon CT26-LacZ tumors were seeded subcutaneous (subQ) at 5e5 cells in BALB/c mice in a syngeneic model. Two weeks later, mice were treated with one intra-tumoral dose of 1e7 PFUs of either Copenhagen ΔTK or Copenhagen ΔTK/F1L virus. Tumor measurements are displayed. Data is shown as mean and SE. *p < 0.05; ***p < 0.001.
In order to test whether F1L deletion enhances the immunogenicity of the virus, we tested our virus in a syngeneic model. Upon treatment of CT26 tumors with either of our viruses, we found that Cop-ΔTK/F1L control tumor growth rates were better (Figure 4E). Interestingly, vaccinia mediated tumor control in this model was shown to be TLR4 dependent.9 Taken together, our data demonstrate that the F1L deletion in Cop-ΔTK improves efficacy of the oncolytic virus.
Discussion
VACV and other poxviruses have large genomes, encoding approximately 200 genes, of which ∼25% are considered virulence factors.23 Mammalian cells’ first line of defense against viral infection is interferon-stimulated antiviral signaling and apoptosis, both of which are targeted by VACV genes.24 Although these viral genes increase the pathogenic fitness of the virus, they have somewhat redundant functions, and their expression may compromise the oncolytic virotherapy’s safety. Cancer is a characterized by a series of defects, including impaired interferon signaling and upregulation of endogenous anti-apoptosis genes. The rationale of our study was to delete a major anti-apoptotic virulence factor in VACV due to the inherent ability of target cancer cells to evade apoptosis. We hypothesized that normal tissues would be better able to fight off viral infection, while cancerous tissue would succumb to oncolysis more efficiently.
This is the first study, to the best of our knowledge, to report improved anti-tumor efficacy of VACV-based oncolytic virus (OV) with a double deletion of both TK and F1L. The leading VACV-based clinical OV candidate, Pexa-Vec, encodes solely a TK deletion to drive tumor selectivity. This strategy aims to restrict VACV growth to metabolically active cells expressing high levels of TK, such as metabolically active cancerous tissue.25 This approach has some shortfalls, for instance some non-cancerous tissues such as immune cells and hepatocytes are continuously undergoing turnover, resulting in off-target effects. This is consistent with reported clinical incidences of pox lesions in patients receiving high doses of Pexa-Vec.26 This suggests that increased attenuation of the virus is required to improve VACV’s safety profile. Our addition of an F1L deletion significantly improved in vivo safety profile of the virus, as demonstrated by fewer pox lesions and increased survival in nude mice (Figure 3C). Collectively, our data suggest that the double deletion may improve safety in vivo.
In addition to increased safety, our study demonstrates that the F1L deletion conveys improved anti-tumor efficacy. The Cop-ΔTK/F1L virus maintained its ability to replicate in vitro, trigger a higher rate of apoptosis, and cause cytokine release upon cell death (Figure 1). An increased level of cytokines is positive in the context of a tumor microenvironment, as they can attract more lymphocytes to the tumor or activate an already existing one.27 This is consistent with a previous study reporting that deleting F1L in a non-replicating poxvirus vaccine vector increased its immunogenicity, generating larger immune responses to encoded HIV antigens.28 Furthermore, F1L deletion delays tumor growth in a syngeneic colon cancer murine model (Figure 4E). This particular model has been previously tested with other VACV, where control of tumor growth was shown to occur via TLR4 stimulation by HMGB1 release from infected cells.9 Interestingly, TLR4 stimulation results in, among many things, the production of IL-1β29 which, as we show, our F1L-deleted virus can induce in some cancer cells (Figure 1C). The advantage of the Cop-ΔTK/F1L virus is the ability to combine immunogenicity with oncolysis.
Several poxviruses encode anti-apoptotic factors to counteract cell death and increase viral persistence.30 Our work suggests that deletion of these factors may improve safety and anti-tumor efficacy of poxvirus-based OVs. Our results are consistent with the enhanced tumor selectivity of a VACV Western Reserve strain harboring a double deletion of two anti-apoptosis genes (SPI-1 and SPI-2).31 Other VACV strains encode other virulence factors with anti-apoptotic activity.32 Future investigations should examine the potential of deleting additional anti-apoptotic factors to improve poxvirus vectors as OVs.
Overall, our study has shown that the deletion of the F1L gene in VACV increases the safety profile and efficacy of the oncolytic potential of an existing clinical platform. We demonstrated that a single TK knockout is insufficient as a tumor-targeting mechanism. Our work highlights the need for more research into the therapeutic properties of additional VACV genes with unknown function, as their loss may further improve the use of VACV in oncolytic therapy.
Materials and Methods
Cell Lines
Cells were obtained from the American Type Culture Collection (ATCC) and passaged in DMEM supplemented with 5% fetal bovine serum (FBS).
Construction of Plasmids
Shuttle plasmid for deleting F1L was constructed using the DNA from the VV Copenhagen strain (GenBank: M35027). The DNA flanking regions of F1L were amplified by PCR. Primers of the F1L downstream flanking region (F1L-L sequence) were 5′- CGC GGA TCC ATC ATT TTT TCA CCA TTA CTT CT -3′ (BamHI site underlined) and 5′- CCG GAA TTC TTG TAG ATT ATA TAA CGG ACA TT -3′ (EcoRI site underlined). Primers for the F1L upstream region (F1L-R sequence) were 5′- GCC TGG CCA TTA GTG GAC TTG TCA AAT CTA T -3′ (MscI site underlined) and 5′- GCC CAG CTG ATG TTA TAT ACG TAA TGG GAG G -3′ (PvuII site underlined). The amplified DNA fragments were digested with restriction enzymes BamHI/EcoRI or MscI/PvuII then ligated into the corresponding sites in the PpolyIII plasmid.33 A repeat region of the downstream flanking region of F1L (F1L-L sequence) was amplified by PCR using the primers 5′- TCC CCC GGG TGA TGA TAT AGG GGT CTT CAT A -3′ (SmaI site underlined) and 5′- GCC GCA TGC TTG TAG ATT ATA TAA CGG ACA TT -3′ (SphI site underlined) and inserted in the pPolyIII plasmid. The repeat region was used to eliminate the selection cassette during the production of deleted viruses. The selection cassette GFP/GPT, a fusion of the gene encoding the GFP and the gene encoding guanine phosphoribosyltransferase (GPT), was positioned under the control of the pH5R vaccinia promoter and inserted into the SmaI/SphI site in the pPolyIII plasmid. The resulting plasmid is a shuttle plasmid named pΔF1L for the deletion of F1L gene.
Generation of Recombinant Vaccinia
All recombinant VACVs were derived from the Copenhagen strain. TK gene-deleted VACVs expressing the fusion gene FCU1 (VVΔTK-FCU1) under the control of the p7.5 promoter have been constructed and characterized previously.34 For generation of the double-deleted VVTKkoF1Lko-FCU1, chicken embryonic fibroblast (CEF) cells were infected with VVTKko-FCU1 at a MOI of 0.01, incubated at 37°C for 1 h, and then transfected with a CaCl2 precipitate of the shuttle plasmid pΔF1L (0.2 μg). The cells were incubated for 48 h at 37°C. Double recombination occurs between homologous regions (F1L-L and F1L-R) in the shuttle plasmid and the virus, resulting in the insertion of the gene cassette into the F1L-L locus of VACV. Recombinant VACVs expressing GPT are isolated in a selective medium containing hypoxanthine (15 μg/mL), xanthine (250 μg/mL), and mycophenolic acid (250 μg/mL). GPT+ plaques that displayed GFP fluorescence (easily detectable with a Leika MZ16 fluorescence binocular stereomicroscope) were isolated and submitted to several additional rounds of selection on CEF cells in the presence of GPT selection medium. To confirm that the VVΔTK-FCU1 was deleted of the targeted F1L regions, 40 PCR cycles were performed using primers within the F1L-deleted region and in the flanking region, external to the site of recombination. The doubly deleted virus was then used to infect CEF without any GPT selection to eliminate the GFP/GPT marker obtained after intragenic homologous recombination between the two F1L-L sequences flanking the GFP/GPT gene cassette. Non-fluorescent plaques were isolated and submitted to 2 additional plaque purification cycles in CEF. Virus structure was confirmed by multiple PCRs. Recombinant VACVs were amplified in CEF and purified over sucrose gradients, and virus stocks were titrated on CEF by plaque assay.
In Vitro Virus Yield
To evaluate viral replication between human tumor cells and human primary cells, human H1299 tumoral cells and human primary hepatocytes were infected in 12-well plates with VVΔTK-FCU1 and VVΔTK/ΔF1L-FCU1 at a MOI of 0.001 (1,000 PFUs per well). At 72 h post-infection, supernatant and cells were collected, freeze-thawed, and sonicated, and viral progeny were quantified on CEF by plaque assay.
Viral Pathogenicity
Viral pathogenicity was assessed by survival studies done on both Swiss nude mice (female, 6 weeks old from Charles River Laboratories) and immunocompetent B6D2 mice (female, 6 weeks old from Charles River Laboratories). High doses (1 × 108 PFUs) of the indicated vectors were injected intravenously by tail vein injection. Mice were observed daily throughout the course of the experiment. Pox lesions on tail were also measured and recorded after an intravenous (i.v.) injection of 1 × 108 PFUs of each virus in Swiss nude mice.
Mice and Tumor Models
Mice were from Charles River Laboratories (Wilmington, MA, USA). Imaging studies: U87 (1 × 106) tumors were established in the brain after surgery of 6-week-old CD1 female nude mice (N = 5). VACV were administered via intratumoral injection or i.v. (1 × 107 PFUs). Survival was followed. All experiments were performed in accordance with the guidelines of the University of Ottawa institutional review board for animal care.
In Vivo Imaging
Mice were injected with D-luciferin (Molecular Imaging Products, Ann Arbor, MI, USA) for firefly luciferase imaging. Mice were anesthetized under 3% isoflurane (Baxter, Deerfeld, IL, USA) and imaged with the IVIS 200 Series Imaging System (Xenogen, Hopkinton, MA, USA). Data acquisition and analysis were performed using Living Image v2.5 software. For each experiment, images were captured under identical exposure, aperture, and pixel binning settings, and bioluminescence was plotted on identical color scales.
Immunohistochemistry
Tissues were harvested, placed in optimal cutting temperature (OCT) mounting media (Tissue-Tek, Sakura Finetek, Torrance, CA, USA). Sectioned tissues were processed as previously described with anti-VV or Anti-Active Caspase-3. Tumor images were obtained with an Epson Perfection 2450 Photo Scanner (Epson, Toronto, ON, Canada), whereas magnifications were captured using a Zeiss AxioCam HRm inverted fluorescent microscope (Zeiss, Toronto, ON, Canada) and analyzed using AxioVision 4.0 software.
Statistical Analysis
Statistical significance is only shown when p values < 0.05. All reported tests compare Copenhagen ΔTK and Copenhagen ΔTK/F1L groups and are two-tailed. All data are shown as mean and SEM. For survival analysis, Kaplan-Meier survival curves were compared using a log-rank Mantel-Cox test. All statistical analysis was done using GraphPad Prism 5 software.
Author Contributions
A. Pelin, J.F., C.S., A. Postigo, M.W., P.E., F.L.B., and J.C.B. designed this study and planned the experiments. A. Pelin, J.F., J.P., F.G., M.F., C.S., and F.L.B. performed animal experiments and helped with tissue processing. V.A.J., L.J.S., and E.S. performed flow cytometry experiments. A. Pelin, J.F., R.S., M.H., C.S., E.S., B.L., and F.L.B. performed in vitro experiments. A. Pelin, R.S., F.L.B., and J.C.B. wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
J.C.B. is supported by the Terry Fox Foundation (TFF); the Canadian Cancer Society Research Institute (CCSRI); the Ontario Institute for Cancer Research (OICR); the Canadian Institutes of Health Research (CIHR); the Ottawa Regional Cancer Foundation; and the Ottawa Hospital Foundation. A. Pelin is supported by a Joseph Lebovic award admistered by OICR. R.S. is supported by CIHR funding.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.06.004.
Supplemental Information
References
- 1.Rintoul J.L., Wang J., Gammon D.B., van Buuren N.J., Garson K., Jardine K., Barry M., Evans D.H., Bell J.C. A selectable and excisable marker system for the rapid creation of recombinant poxviruses. PLoS ONE. 2011;6:e24643. doi: 10.1371/journal.pone.0024643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson B.M., Tscharke D.C. Redundancy complicates the definition of essential genes for vaccinia virus. J. Gen. Virol. 2015;96:3326–3337. doi: 10.1099/jgv.0.000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith E.R., Chiocca E.A. Oncolytic viruses as novel anticancer agents: turning one scourge against another. Expert Opin. Investig. Drugs. 2000;9:311–327. doi: 10.1517/13543784.9.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Postigo A., Martin M.C., Dodding M.P., Way M. Vaccinia-induced epidermal growth factor receptor-MEK signaling and the anti-apoptotic protein F1L synergize to suppress cell death during infection. Cell Microbiol. 2009;11:1208–1218. doi: 10.1111/j.1462-5822.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Tzahar E., Moyer J.D., Waterman H., Barbacci E.G., Bao J., Levkowitz G., Shelly M., Strano S., Pinkas-Kramarski R., Pierce J.H. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkins K.L., Ennist D.L., Winegar R.K., Weir J.P. In vivo delivery of interleukin-4 by a recombinant vaccinia virus prevents tumor development in mice. Hum. Gene Ther. 1994;5:809–820. doi: 10.1089/hum.1994.5.7-809. [DOI] [PubMed] [Google Scholar]
- 8.Martin N.T., Bell J.C. Oncolytic virus combination therapy: killing one bird with two stones. Mol. Ther. 2018;26:1414–1422. doi: 10.1016/j.ymthe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fend L., Yamazaki T., Remy C., Fahrner C., Gantzer M., Nourtier V., Préville X., Quéméneur E., Kepp O., Adam J. Immune checkpoint blockade, immunogenic chemotherapy or IFN-α blockade boost the local and abscopal effects of oncolytic virotherapy. Cancer Res. 2017;77:4146–4157. doi: 10.1158/0008-5472.CAN-16-2165. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z., Ravindranathan R., Kalinski P., Guo Z.S., Bartlett D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parviainen S., Ahonen M., Diaconu I., Hirvinen M., Karttunen Å., Vähä-Koskela M., Hemminki A., Cerullo V. CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther. 2014;21:195–204. doi: 10.1038/gt.2013.73. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y.Q., Tsai Y.C., Monie A., Wu T.C., Hung C.F. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 2010;18:692–699. doi: 10.1038/mt.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.H., Oh J.Y., Park B.H., Lee D.E., Kim J.S., Park H.E., Roh M.S., Je J.E., Yoon J.H., Thorne S.H. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 15.Pin R.H., Reinblatt M., Fong Y. Employing tumor hypoxia to enhance oncolytic viral therapy in breast cancer. Surgery. 2004;136:199–204. doi: 10.1016/j.surg.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Postigo A., Cross J.R., Downward J., Way M. Interaction of F1L with the BH3 domain of Bak is responsible for inhibiting vaccinia-induced apoptosis. Cell Death Differ. 2006;13:1651–1662. doi: 10.1038/sj.cdd.4401853. [DOI] [PubMed] [Google Scholar]
- 17.Zhai D., Yu E., Jin C., Welsh K., Shiau C.W., Chen L., Salvesen G.S., Liddington R., Reed J.C. Vaccinia virus protein F1L is a caspase-9 inhibitor. J. Biol. Chem. 2010;285:5569–5580. doi: 10.1074/jbc.M109.078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasilenko S.T., Banadyga L., Bond D., Barry M. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 2005;79:14031–14043. doi: 10.1128/JVI.79.22.14031-14043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvansakul M., Yang H., Fairlie W.D., Czabotar P.E., Fischer S.F., Perugini M.A., Huang D.C., Colman P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- 20.Postigo A., Way M. The vaccinia virus-encoded Bcl-2 homologues do not act as direct Bax inhibitors. J. Virol. 2012;86:203–213. doi: 10.1128/JVI.05817-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlic M., Faustin B., Postigo A., Yu E.C., Proell M., Gombosuren N., Krajewska M., Flynn R., Croft M., Way M. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc. Natl. Acad. Sci. USA. 2013;110:7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rainov N.G., Ren H. Oncolytic viruses for treatment of malignant brain tumours. Acta Neurochir. Suppl. (Wien) 2003;88:113–123. doi: 10.1007/978-3-7091-6090-9_17. [DOI] [PubMed] [Google Scholar]
- 23.Smith G.L., Benfield C.T., Maluquer de Motes C., Mazzon M., Ember S.W., Ferguson B.J., Sumner R.P. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013;94:2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 24.Perdiguero B., Esteban M. The interferon system and vaccinia virus evasion mechanisms. J. Interferon Cytokine Res. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- 25.Deng L., Fan J., Ding Y., Zhang J., Zhou B., Zhang Y., Huang B. Oncolytic efficacy of thymidine kinase-deleted vaccinia virus strain Guang9. Oncotarget. 2017;8:40533–40543. doi: 10.18632/oncotarget.17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cripe T.P., Ngo M.C., Geller J.I., Louis C.U., Currier M.A., Racadio J.M., Towbin A.J., Rooney C.M., Pelusio A., Moon A. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol. Ther. 2015;23:602–608. doi: 10.1038/mt.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo S.Y., Lee S.Y., Yoo N.C. Cytokine expression and cancer detection. Med. Sci. Monit. 2009;15:RA49–RA56. [PubMed] [Google Scholar]
- 28.Perdiguero B., Gómez C.E., Nájera J.L., Sorzano C.O., Delaloye J., González-Sanz R., Jiménez V., Roger T., Calandra T., Pantaleo G., Esteban M. Deletion of the viral anti-apoptotic gene F1L in the HIV/AIDS vaccine candidate MVA-C enhances immune responses against HIV-1 antigens. PLoS ONE. 2012;7:e48524. doi: 10.1371/journal.pone.0048524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eltom S., Belvisi M.G., Yew-Booth L., Dekkak B., Maher S.A., Dubuis E.D., Jones V., Fitzgerald K.A., Birrell M.A. TLR4 activation induces IL-1β release via an IPAF dependent but caspase 1/11/8 independent pathway in the lung. Respir. Res. 2014;15:87. doi: 10.1186/s12931-014-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor J.M., Barry M. Near death experiences: poxvirus regulation of apoptotic death. Virology. 2006;344:139–150. doi: 10.1016/j.virol.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z.S., Naik A., O’Malley M.E., Popovic P., Demarco R., Hu Y., Yin X., Yang S., Zeh H.J., Moss B. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 32.Nichols D.B., De Martini W., Cottrell J. Poxviruses utilize multiple strategies to inhibit apoptosis. Viruses. 2017;9:E215. doi: 10.3390/v9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathe R., Violette J.L., Clark A.J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57:193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- 34.Foloppe J., Kintz J., Futin N., Findeli A., Cordier P., Schlesinger Y., Hoffmann C., Tosch C., Balloul J.M., Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.