Abstract
Reactive oxygen and nitrogen species (ROS and RNS, respectively) activate the redox-sensitive Ras small GTPases. The three canonical genes (HRAS, NRAS, and KRAS) are archetypes of the superfamily of small GTPases and are the most common oncogenes in human cancer. Oncogenic Ras is intimately linked to redox biology, mainly in the context of tumorigenesis. The Ras protein structure is highly conserved, especially in effector-binding regions. Ras small GTPases are redox-sensitive proteins thanks to the presence of the NKCD motif (Asn116-Lys 117-Cys118-Asp119). Notably, the ROS- and RNS-based oxidation of Cys118 affects protein stability, activity, and localization, and protein-protein interactions. Cys residues at positions 80, 181, 184, and 186 may also help modulate these actions. Moreover, oncogenic mutations of Gly12Cys and Gly13Cys may introduce additional oxidative centres and represent actionable drug targets. Here, the pathophysiological involvement of Cys-redox regulation of Ras proteins is reviewed in the context of cancer and heart and brain diseases.
Keywords: Ras small GTPases, Cysteine 118, Redox-signalling
Abbreviations
- AII
Angiotensin II
- BAEC
bovine aortic endothelial cells
- eNOS
endothelial nitric oxide synthase
- GDP
guanosine-5′-triphosphate
- GTP
guanosine-5′-triphosphate
- HVR
carboxyl-terminal hypervariable region
- ICMT1
isoprenylcysteine carboxymethytransferase-1
- iNOS
inducible nitric oxide synthase
- MDS
myelodysplastic syndromes
- MPD
myeloproliferative diseases
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- PDEδ
phosphodiesterase-δ
- PKC
protein kinase-C
- RCE1
Ras-converting enzyme-1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Mn-SOD
manganese-containing superoxide dismutase
- SSc
systemic sclerosis
1. Introduction
Various studies support the notion that Ras GTPases are regulated by reactive oxygen and nitrogen species [[1], [2], [3], [4], [5], [6]]. Redox signalling is a form of signal transduction that progresses through the reversible oxidation of cysteines in proteins, mostly mediated by hydrogen peroxide as a second messenger. Few members of the small GTPase family are redox sensitive (H-Ras, N-Ras, K-Ras, E-Ras, Rap1A and Rap1B and some Rab proteins) and their known conserved redox-sensitive sequence, the NKCD amino acid sequence (Asn116-Lys117-Cys118-Asp119), is located in the G-domain and is virtually identical in all proteins containing this motif. Oxidation of specific amino acid residues in many small GTPases affects protein stability, activity, localization and protein-protein interactions [4]. Moreover, the observation that many oncogenes, such as RAS, and many tumour suppressor proteins, such as P53, can directly or indirectly alter the redox state of a cell [[7], [8], [9], [10]] reinforced the link between RAS redox circuitries and cancer.
Chemical modifications of Ras proteins consist of post-translational modifications occurring at the hypervariable region (HVR); in particular, Cys residues undergo farnesylation and palmitoylation. Other chemical modifications such as phosphorylation, ubiquitination and oxidation (S-glutathionylation, S-nitrosylation) involve the entire Ras sequence and are highly specific. These modifications induce distinct sensitivity, which is exhibited by individual Ras isoforms and their functional interactions with direct and/or downstream effectors. Notably, the direct effects of endogenous redox agents on these small GTPases perturb GTPase nucleotide-binding interactions that result in the enhancement of the guanine nucleotide exchange rate of these enzymes [11,12] (Table 1).
Table 1.
Major redox modifications on wild-type Ras protein.
| Ras isoform | Inducer | Chemical modification | Method | Cell type | Effects on Ras expression and phenotypic readouts | References |
|---|---|---|---|---|---|---|
| H-Ras | S-nitrosocysteine (NO) | S-Nitrosylation (Cys118) | Optical waveguide light-mode Spectrometry and GTP-bound activation |
Bovine brain cortex tissue and Lipid Bilayers | Decreases catalytic activity | [13] |
| H-Ras | S-nitrosocysteine (NO) | S-Nitrosylation (Cys118) | MS, immunoblot and immunofluorescence | Neural stem cells (NSC) and subventricular zone (SVZ) | Cell proliferation and neurogenesis | [14] |
| K-Ras4B | S-nitrosoglutathione (NO) | S-Nitrosylation (Cys118) | LC-MS/MS | Human cancer colon (CRC) | – | [15] |
| p21Ras full-lenghtb | oxidized low-density lipoproteins (oxLDL) (NO) | S-Nitrosylation (Cys118) | GTP-bound activation | Bovine aortic endothelial cells (BAEC) | Increased catalytic activity | [12] |
| p21Rasfragment 1-166 | S-nitrosocysteine (NO) | S-Nitrosylation (Cys118) | NMR, MS | Test-tube assay | No effectsa | [16] |
| N-Ras | eNOS-derived NO | S-Nitrosylation (Cys118) | Flow cytometry, Time-Lapse Fluorescence Microscopy, Immunoblotting | Mouse T cells | GTP-bound activation T cell apoptosis |
[17] |
| H-Ras | High palmitate/high glucose (HPHG) (ROS) | S-glutathionlyation (Cys181; Cys184) | MALDI-TOF-MS | Bovine aortic endothelial cells (BAEC) and cardiac tissue | Decreased palmitoylation | [18] |
| H-Ras | Angiotensin II (ROS) | S-glutathionlyation (Cys181; Cys184; Cys186; Cys80) | MALDI-TOF-MS, GTP-bound activation | Rat vascular smooth muscle cells (VSMC) | Increased catalytic activity | [11] |
| H-Ras | S-nitrosocysteine (NO) | S-Nitrosylation | Radiolabeling, Immunoprecipitation and immunoblotting | Mouse fibroblasts NIH-3T3 | Increased palmitate turnover | [19] |
| H-Ras | sodium nitroprusside (NO) hypoxia/normoxia |
S-Nitrosylation | Immunoprecipitation, immunoblotting and S-nitrosylation assay | Rat pheochromocytoma PC12 | Decreased S-nitrosylation | [20] |
| H-Ras | S-nitrosoglutathione (NO) | S-Nitrosylation | Live-fluorescence microscopy, GTP-bound activation, immunoblotting | Human umbilical vein endothelial (HUVEC) and HeLa cells | GTP-bound activation and compartmentalization | [21] |
| H-Ras | S-nitrosocysteine (NO) | S-nitrosylation and S-glutathionlyation | Immunoprecipitation and immunoblotting, Isoelectric focusing (IEF) | Mouse fibroblasts NIH-3T3 and in vitro assay | Lipid turnover | [22] |
| p21Ras full-lenght | Hischemic perfusion | S-glutathionlyation | MALDI-TOF-MS | Rat Ischemic heart | – | [23] |
Several excellent reviews have recently been published that describe the biochemistry of Ras redox signalling in deep detail [[3], [4], [5], [6],24]. This review focuses on the Cys80-, Cys118-, Cys181-, Cys184-, and Cys186-based redox signalling of the Ras small GTPases by highlighting the differences between the four Ras isoforms and their cellular consequences. Pathophysiological involvement of this redox regulation of native Ras proteins is discussed in the context of cancer and in heart and brain diseases. Moreover, oncogenic mutation of Gly12Cys and Gly13Cys may introduce additional oxidative centres and represent actionable drug targets. Downstream signalling, such as that involving IP3K or MAPK, and the in-depth chemical analysis of redox signalling are not a major focus of this review.
2. RAS proteins structure
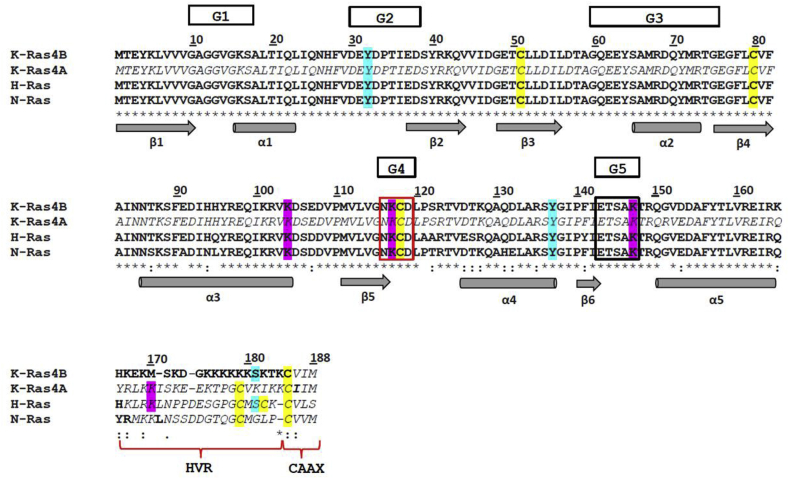
All Ras proteins consist of 188 or 189 amino acid residues with a molecular weight of approximately 21 kDa. K-Ras, H-Ras and N-Ras display two domains: (i) the catalytic domain (G domain), which corresponds to the amino acid residues 1–166; and (ii) the hypervariable region (HVR), which corresponds to residues 167–189 [25]. The G domain is characterized by five motifs (G1-G5). The G1-G3 motifs build up the effector lobe (amino acids 1–86) containing the Switch subdomains, I and II (residues 32–38 and 60–75, respectively); the sequence identity of G1-G3 is 100% in all family members. On the other hand, the G4 (NKCD; i.e., Asn116-Lys 117-Cys118-Asp119) and G5 (ETSAK; i.e., Glu143-Thr144-Ser145-Ala146-Lys 147) motifs build up the allosteric lobe (amino acids 87–166); the sequence identity is 82% among all family members [26]. The amino acid sequence of the C-terminal HVR domain (amino acids 167–189) is highly flexible and divergent among the Ras proteins, except for the CAAX motif (i.e., Cys185-Aaa186-Aaa187-Xxx188; where Aaa is an aliphatic amino acid residue, and Xxx is the C-terminal amino acid residue). Although all four Ras isoforms (i.e., K-Ras4A, K-Ras4B, N-Ras and H-Ras) display Cys residues at positions 51, 80 and 118 in the G-domain, the HVR differs in the Cys content [27,28]. In addition, the poly-Lys stretch (amino acids 175–180), which is believed to interact with the negatively charged head groups of plasma membrane lipids, is present only in K-Ras4B [27,29,30] (Fig. 1).
Fig. 1.
Multiple amino acid sequence alignment of K-Ras4B (PDB ID code: 5TAR, chain A) [30], K-Ras4A (UniProtKB entry: P01116-1) [32], H-Ras (PDB ID code: 5 X 9S) [33], and N-Ras (PDB ID code: 3CON) (Reid et al., 2017). The amino-acid sequences have been aligned with CLUSTAL omega [107] using the BLOSUM62 substitution matrix [108] to score both pairwise and multiple alignment. In bold are reported the amino acid residues building up the three-dimensional structures of K-Ras4B, H-Ras, and N-Ras respectively. The three-dimensional structure of K-Ras4A is not available. In italic are highlighted the amino acid residues of K-Ras4A and those of K-Ras4B, H-Ras, and N-Ras that are not solved in the three-dimensional structures. The amino acid residues that undergo post-translational modifications are indicated with different colors: Cys residues are in yellow, phosphorylated Tyr are in light blue, and ubiquitylated Lys are in pink. The NKCD (i.e., G4) and the ETSAK (i.e., G5) motifs have been boxed in red and black, respectively. The HVR domain and the CAAX motif are also shown. The G1-G5 loops that catalyze the GTP hydrolysis are evidenced. The arrows indicate the β-strands and the cylinders the α-helices. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
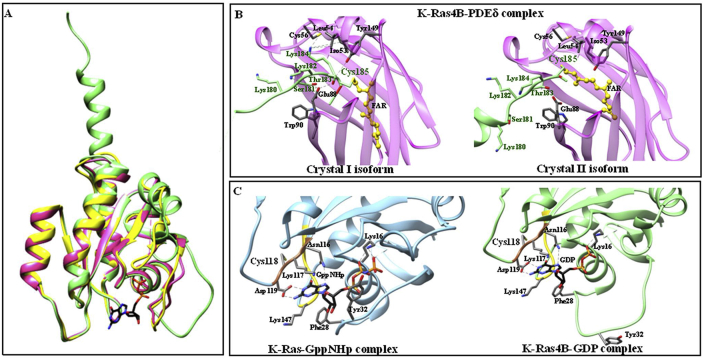
The structural organization of the Ras G domain, which is characterized by six β-strands, five α-helices and nine connecting loops, is highly conserved in all Ras small GTPases [31] (Fig. 2, Panel A). Only the G domain of most Ras small GTPases has been crystallized and the three-dimensional structure solved at atomic resolution [[32], [33], [34]]. The high flexibility of the Ras small GTPases C-terminus likely impairs protein crystallization. The three-dimensional structure of the α-helical K-Ras4B C-terminus, including the HVR region, has been determined only in complex with phosphodiesterase-δ (PDEδ); the protein:protein interaction induces the α-helical structural organization of the flexible HVR domain of K-Ras4B, which contains the highly reactive Cys residues [30] (Fig. 2, Panel B). These Cys residues at the C-terminal region are highly reactive towards oxidative species and their molecular environment could regulate their reactivity, not only by controlling the interactions with oxidative species but also by modifying the pKa of the thiol group [35].
Fig. 2.
Three-dimensional structures of RAS proteins. Panel A. Superimposition of K-Ras4B (in green, PDB ID code: 5TB5) [30], H-Ras (in yellow, PDB ID: 3KUD) [109], and N-Ras (in pink; PDB ID: 3CON). Panel B: Intermolecular contacts in the crystal I and II isoforms of the K-Ras4B-PDEδ complex (PDB ID code: 5TB5 and 5TAR, respectively) [30]. K-Ras4B is in green and PDEδ is in magenta. Amino acid residues Cys118 and Cys185 are in large characters. The pictures have been drawn with UCSF-Chimera package [110]. Ribbon representation highlights the amino acid residues topology surrounding the Cys185 and relative proximal residues. This residue has been farnesylated for crystallization purposes and the intermolecular contacts show two different geometry of bound compounds. Panel C. Ribbon representation highlights the amino acid residues topology surrounding the Cys118 and relative proximal residues. Amino acid residues surrounding GppNHp (in light blue, left panel; PDB ID code: 6GOD) [34] bound to K-Ras and GDP (in green, PDB ID code: 5TB5) [30] bound to K-Ras4B. The G4 and G5 motifs, involved in the guanine nucleotide-dependent allosteric transition of Ras proteins, are highlighted in gold and brown, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The Cys118 residue, located in the G4 motif, undergoes S-nitrosylation, which facilitates guanine nucleotide exchange [16]. Moreover, Arg128 and Arg135 side chains promote the electrostatic interaction between the G domain of H-Ras and the plasma membrane [36] (Fig. 2, Panel C).
The HVR domain contains residues that undergo post-translational modifications, which are essential for targeting Ras proteins to the cytosolic leaflet of cellular membranes. Moreover, all Ras proteins are farnesylated and carboxymethylated at the Cys186 (Cys185 numbering for K-Ras4B) residue of the CAAX motif [30,37]. Upon GTP binding to the G domain, K-Ras, H-Ras and N-Ras associate with the effector Ras-binding domains (RBDs) through the Switch I and II subdomains, which undergo conformational changes upon nucleotide hydrolysis (i.e., the conversion of GTP to GDP) [33,38,39].
3. RAS protein post-translational modifications
Under physiological and pathological conditions, H-Ras, N-Ras, K-Ras4A and K-Ras4B actions are modulated by farnesylation and palmitoylation, phosphorylation, ubiquitination, acetylation and oxidation (i.e., S-nitrosylation and S-gluthationylation). Post-translational modifications of the HVR domain dictate the binding mode of Ras proteins to membranes. In turn, differences in the membrane association of Ras proteins have been shown to regulate effector recognition [[40], [41], [42]]. The C-terminal CAAX motif (containing the invariant Cys186 residue) is subjected to a series of post-translational modifications [39,43]. In particular, farnesylation and palmitoylation modulate the subcellular distribution of H-Ras, N-Ras, K-Ras4A and K-Ras4B, in which the Cys residues are the major players [29,44]. In detail, a farnesyl group is covalently attached to Cys186 in the CAAX motif by farnesyltransferase. This prenylation reaction is followed by (i) the proteolytic removal of the AAX tripeptide (i.e., Ile/Val-Ile/Leu/Val-Xxx) by Ras-converting enzyme-1 (RCE1), and (ii) the subsequent carboxymethylation of the resulting C-terminal Cys by isoprenylcysteine carboxymethytransferase-1 (ICMT1). In addition, the palmitoylation of Cys residue(s) adjacent to the CAAX motif by a palmitoyltransferase stabilizes the membrane anchoring of farnesylated H-Ras, N-Ras, and K-Ras4A (Hancock et al., 2003). The palmitoylation-depalmitoylation process of the C-terminal residues of H-Ras modulates the protein transit from the Golgi to the plasma membrane [45]. Notably, phosphorylation, ubiquitination and acetylation play a minor role on the subcellular localization of H-Ras, N-Ras, K-Ras4A and K-Ras4B [[46], [47], [48], [49], [50]]. In particular, K-Ras is monoubiquitinated at Lys147 [48] and phosphorylated at Ser 181 [28,51,52], H-Ras is ubiquitinated at Lys117 [53], K-Ras4B is acetylated at Lys104 [47], and N-RAS is phosphorylated at Tyr32 [49]. However, the poly-Lys stretch of the HVR region of K-Ras4B allows its recruitment to the inner leaflet of the acidic plasma membrane by electrostatic interactions [27,54].
4. Cysteine-based redox regulation of RAS isoforms
The oxidation of Ras protein Cys residue(s) affects protein stability, activity, localization and protein-protein interactions (Table 1, Table 2).
Table 2.
Major redox modifications on oncogenic Ras protein.
| Ras isoform | Inducer | Chemical modification | Method | Cell type | Phenotypic read-out | References |
|---|---|---|---|---|---|---|
| KRASG12C (Gly12→Cys) | RNS (NO) | S-Nitrosylation (Cys12) | Biochemical analysis | Human Lung | Tumorigenic activity | [55] |
| KRASC118S (Cys118→Ser) | – | – | Tumour xenograft analysis Biochemical analysis |
Mouse Lung | Decreased tumorigenesis | [56] |
| KRASC118S (Cys118→Ser) | – | – | Tumour xenograft analysis Biochemical analysis |
Mouse Lung | Decreased tumorigenesis | [57] |
|
NRASG12C (Gly12→Cys) HRASG12V (Gly12→Val) |
Retroviral expression | – | Cell survival, proliferation, and cell-cycle analysis | Human haematopoietic progenitors | Increased proliferation | [58] |
Footnote: Cysteine residues sites of redox modification are listed in the table with principal redox-inducer and experimental model specifications (reactive oxygen or nitrogen species; type of chemical modification; phenotypic cellular read-outs; analytical methods). This list focuses on aspects relevant to the current paper and is not intended to be truly comprehensive. a No effects means no measurable effect on the protein structure, GTPase activity, intrinsic and GEF-mediated guanine nucleotide dissociation rate, or the ability to bind an effector; b The recombinant p21ras was obtained from BioMol (Plymouth Meeting, PA).
4.1. Cys118
Although the Cys118 residue (present in the NKCD region, located in the invariant N-terminal domain up to amino acid 166) is considered the primary target of ROS and RNS [[12], [13], [14], [15], [16], [17]], the involvement of this residue in the redox-based modulation is controversial. Conflicting results likely reflect the use of different methodological approaches, redox inducers, and cell lines (Table 1).
In 1995, nitric oxide (NO) was reported, for the first time, to modulate Ras activation in human T cells, by Cys118 S-nitrosylation, promoting guanine nucleotide exchange [59]. This finding was later confirmed by structural and biochemical studies on the purified H-Ras protein [16,60]. Further, eNOS has been reported to selectively activate N-Ras, but not K-Ras, at the Golgi of T cells via Cys118 S-nitrosylation. Notably, the activation at the Golgi has been shown to facilitate the positive selection of T cells, whereas N-Ras activation at the plasma membrane has been reported to induce the negative selection of T cells [17]. Further, in mouse brain development, the iNOS-dependent S-nitrosylation of Cys118 of H-Ras has been shown to induce neurogenic effects on neuronal stem cells (NSC) [14]. Importantly, the mutation of Cys118 to Ser (Cys118Ser) renders the G-domain of H-Ras insensitive to (i) free radical activation, (ii) GTPase activity, (iii) the intrinsic and GEF-mediated guanine nucleotide dissociation rate, and (iv) the ability to bind effectors [[60], [61], [62], [63], [64], [65]].
Moreover, in bovine aortic endothelial cells (BAEC) treated with oxidized low-density lipoproteins (oxLDL), MALDI-TOF mass spectrometry was used to identify S-glutathionylated Cys residues. Specifically, ROS-induced Cys118-glutathionylation increases the catalytic activity of H-Ras involving this residue, which is a mechanism of insulin resistance [12] (Table 1).
S-nitrosylation of soluble cytoplasmic and membrane-bound H-Ras was also investigated in a model membrane system by optical waveguide light-mode spectroscopy. Cys-nitrosylation of H-Ras leads to the stimulation of the intrinsic GTPase activity that shortens the lifetime of the active GTP-bound state of the protein. Only the membrane-bound H-Ras is nitrosylated at Cys118 and this modification decreases the rate of GTP hydrolysis [13]. Last, mass spectrometry analysis provided evidence that the endogenous Cys118-nitrosylation of the K-Ras4B isoform occurs in colon cancer cells and primary tissues [15].
In contrast, in a rat model of cardiac hypertrophy, vascular smooth muscle cells (VSMC) treated with angiotensin II (AII) (an indirect inducer of redox modification) did not show evidence of Cys118 S-nitrosylation, as assessed by MALDI-TOF mass spectrometry [11]. Moreover, stable Cys118-nitrosylation of H-Ras fragment 1–166 does not cause any significant perturbation in the secondary or tertiary structure, as demonstrated by structural and biochemical studies [16].
The pivotal role of Cys118 in redox modulation has also been reported in lower eukaryotes (i.e., Saccharomyces cerevisiae and Dictyostelium), which express homologues of mammalian Ras [[66], [67], [68]]. In particular, superoxide facilitates GDP exchange by targeting the Cys118 residue present in the NKCD motif of Dictyostelium Ras proteins (RasG); as shown in mammals, the Cys118Ala mutation impairs redox sensitivity [63,67]. Furthermore, in Saccharomyces cerevisiae, mitochondrial dysfunction reduces yeast replicative lifespan by elevating Ras-dependent ROS production by the ER-localized NADPH oxidase Yno1 [68]. It has been well established that Ras 2 (i) activates Saccharomyces cerevisiae adenylyl cyclase (CYR1) depending on the glucose level and (ii) regulates PKA activation by controlling cAMP levels [69].
4.2. Other Cys residues
As the primary mediator of membrane attachment, the HVR domain has a significant influence on isoform-specific behaviour. Although Cys181, Cys184 and Cys186 are known to be modified by attached lipid molecules in intact cells, these reactive Cys residues may undergo oxidation and nitrosylation during normal and pathological cellular events. In particular, S-glutathionylation of H-Ras plays a critical role in Angiotensin II (AII)-induced hypertrophic signalling of cultured vascular smooth muscle cells (VSMC). MALDI-TOF mass spectrometry provided evidence that three modification sites are present on H-Ras at the C-terminal Cys80, Cys181, Cys184, and Cys186. However, Cys118-nitrosylation was not detected by mass spectrometry probably because NO is not involved in AII signalling in VSMCs [11]. In addition, ROS directly alters protein palmitoylation by oxidizing the C-terminal Cys residues in a mouse model of metabolic syndrome. Specifically, in a high-fat-diet animal model, decreased H-Ras palmitoylation reflected the oxidation of the Cys181 and Cys184 residues. This induces H-Ras inactivation by trapping it at the Golgi and preventing physiological signalling through RAF-1 kinase [18]. The isoform specificity is a critical issue from the structural viewpoint as the HVR has been only partially characterized. In fact, the oxidation of Cys residues 181, 184 and 186 was identified by MALDI-TOF analysis only in H-Ras [11,18]. No other information concerning Cys residues is available for other Ras isoforms.
4.3. Unidentified Cys residues
Redox regulation has a profound impact on Ras localization [[19], [20], [21], [22],70]. Cell-based studies show that different signalling outputs and phenotypic responses are associated with Ras oxidation, albeit without Cys identification. Studies with fluorescent probes in human cells show that the NO-mediated activation of H-Ras in different subcellular compartments regulates different downstream signalling pathways, which lead to various outcomes in proliferation, differentiation and apoptosis. In particular, in human HUVECs and HeLa cells, H-Ras is activated both at the plasma membrane and at the Golgi by low concentrations of S-nitrosoglutathione, leading to the activation of the Akt and ERK1/2MAPK kinases and promoting cell proliferation [21].
S-nitrosothiols may competitively impair the palmitoylation of H-Ras in mouse fibroblast NIH-3T3 cells, thus preventing its localization to the plasma membrane [19,22]. Similarly, in prostate cancer cells, hypoxia/hyperoxia regulates prenylation of H-Ras. Exogenous hyperoxia increases the intracellular oxygen concentration and induces the rapid H-Ras translocation from cytosol to the membrane; however, this effect is completely reversed by mevalonate, which is well known to induce Ras upregulation [70,71].
5. Redox-sensitive RAS proteins in pathogenesis
5.1. Oncogenic Ras
Oncogenic-RAS activation induces tumorigenesis by intracellular ROS levels, i.e., superoxide [10,58,72,73], ROS-generating NADPH-oxidase NOX4 [74], ROS-generating NADPH-oxidase NOX1 [75], unspecified intracellular ROS [9,[76], [77], [78], [79]], and mitochondrial-derived hydrogen peroxide [80,81].
Oncogenic H-Ras has been shown to promote ROS production in a variety of human cell types, including embryonic lung cells [72], human fibroblasts (Lee et al., 1996), immortalized keratinocytes [79], and mouse and human macrophages and monocytes [82]. In particular, the constitutive expression of the active form of v-H-Ras in immortal rat kidney epithelial cells caused cellular transformation and led to the production of a significantly larger amount of superoxide radicals with respect to wildtype cells. The overexpression of antioxidant enzymes, such as manganese-containing superoxide dismutase (Mn-SOD), effectively inhibited Ras-induced transformation indicating that Ras induces cellular transformation through ROS production [73]. Ectopic expression of mutant H-Ras (Gly12Val) in human diploid fibroblasts increased ROS production [80], and this effect was mainly regulated by NADPH-oxidase (NOX) [7]. Furthermore, the constitutive activation of HRAS in haematopoietic progenitor and cancer cells dramatically increases ROS production via activation of the NADPH oxidase complex [58,74,77].
The induction of ROS (i.e., superoxide) mediated by NOX4 is the key step of K-Ras-driven cellular transformation, genomic instability and tumorigenesis [7,10]. K-Ras orchestrates ROS production by promoting the localization of the NOX1 component p47phox to the plasma membrane. This facilitates the interaction of p47phox with protein kinase-C (PKC) isoforms to mediate cellular transformation [75]. KRAS activation is believed to increase ROS production [81]. In turn, high intracellular levels of ROS appear to contribute to the selective oxidative death of KRAS mutant cells. The conditional expression of the oncogenic KRAS G12D mutant increases the level of cellular ROS favouring the selective killing of KRAS mutant cells [78]. In contrast, oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis in mouse embryonic fibroblasts (MEFs), and in NIH-3T3 cells, it lowers cellular ROS [9]. The association of K-Ras with Bcl-XL on mitochondria promotes apoptosis [83].
Oncogenic NRAS is not directly associated with redox imbalance in cancer. Although oncogenic NRAS is associated with melanoma and lymphoid cancers [50], the quantitative relationship between oncogenic N-Ras expression and ROS production has only been described in myelodysplastic syndromes, myeloproliferative diseases [76], and in CD34 + haematopoietic progenitor cells [58]. Notably, oncogenic NRAS (Gly12Asp) expression promotes the proliferative response mediated by increased ROS production in normal human CD34 + progenitor cells [58], although to a lower extent than HRAS (Gly12Val). Interestingly, NRAS mutations in myelodysplastic syndromes (MDS)/myeloproliferative diseases (MPD) result in ROS production [76].
The role of the redox-sensitive Cys118 has been investigated in mouse tumorigenesis. Both urethane-induced lung tumorigenesis [56] and oncogenic HRAS-driven tumorigenesis (Huang et al., 2015) are sensitive to loss of redox-dependent reactions with Cys118 (Table 2). Specifically, mutation of Cys118 (Cys118Ser) in a mouse model of tumorigenesis reduced lung tumour growth. These studies are of paramount importance in understanding the functional implications of Cys oxidation in cancer and focus on personalized therapy with Cys-directed covalent drugs, specifically in the case of oncogenic KRAS (Gly12Cys) [[84], [85], [86]]. Indeed, S-nitrosylation of the Ras Gly12Cys mutant affects the tumorigenic growth of human and murine cell lines [55] (Table 2). Two research groups have recently targeted the Gly12Cys mutation in the oncoprotein K-Ras-Gly12Cys [84,85]. The Gly12Cys mutation induces conformational changes that favour GTP rather than GDP binding. They have independently found K-Ras-Gly12Cys inhibitors, now in phase one clinical trials, that bind to a “cryptic binding” site in the so-called switch II region, which is located in the vicinity of mutated Cys12. This binding site is distinct from the GTP pocket. One compound allosterically locks K-Ras-Gly12Cys in the GDP-bound, and hence inactive, state and impairs the binding of K-Ras to its downstream target, RAF.
5.2. Non-oncogenic Ras
Compared with cancer, considerably less is known about the involvement of non-oncogenic Ras in redox-based pathology. The role of redox reactions in the pathogenesis of autoimmunity has been largely discussed and several different mechanisms of oxidative damage in several different autoimmune diseases have been reported [87]. Endogenous H-Ras is chronically activated by ROS in skin fibroblasts from patients with systemic sclerosis (SSc), establishing a loop between increased ROS, the Ras/MAPK signalling cascade and the complex phenotype of scleroderma fibroblasts in vivo [88].
Moreover, the involvement of oxidative stress in diabetes-induced retinal degeneration has also been investigated, demonstrating that the accumulation of ROS considerably affects all phases of diabetic retinopathy pathogenesis. In mouse models of this disease, translocation of H-Ras has been indicated as a plausible mechanism responsible for accelerated apoptosis of retinal capillary cells. The administration of simvastatin (an inhibitor of Ras protein prenylation) and silencing of H-Ras inhibit protein translocation to the membrane and pathology development. Further, the overexpression of Mn-SOD in mice prevented diabetes-induced activation of H-Ras [[89], [90], [91]]. In addition, another member of the Ras family, R-Ras, has been reported to control retinal vessel maturation and stabilization in ischaemic retinopathy [92].
Ischaemia and hypoxia are major players in heart and brain diseases. NO plays a key role in tissue function during hypoxia by regulating the S-nitrosylation of target proteins [93,94], such as Ras and Ras-related GTPases [64]. In particular, under hypoxic conditions, p21Ras is activated by S-nitrosylation initiating the translocation of H-Ras in the cytosol [95]. Interestingly, the endogenous S-nitrosylation of membrane-bound H-Ras does not change during hypoxia in rat pheochromocytoma PC12 cells [20]. Ras protein has been reported to undergo S-thiolation during reperfusion of ischaemic rat heart tissue [23].
In the brain, ischaemia and hypoxia rely on astrocyte function. In fact, astrocytes are specialized CNS oxygen sensors tuned for rapid detection of physiological changes in brain oxygenation [96]. Interestingly, among the mammalian tissues, the highest level of Ras proteins (K-Ras mainly) was detected in the brain [97,98], albeit the protein levels in neurons or associated glial cells are unknown. The exposure of primary astrocytes to exogenous hydrogen peroxide induces an intracellular burst of ROS that mediates different transcriptional, translational and post-translational actions of H-Ras and K-Ras proto-oncogenes [[99], [100], [101]]. Furthermore, trophic deprivation (the main inducer of autophagy) results in the initial increase of intracellular ROS levels [102] and acute H-Ras induction in primary astrocytes [99]. These studies examined the role of two members of the small GTP-binding proteins family, H-Ras and K-Ras, which are highly expressed in astrocytes, albeit not fully characterized, and suggest that astrocytic Ras-dependent pathways are important mediators for neuroprotective adaptive response to oxidative stress.
Similarly, transcriptional induction of HRAS, NRAS, and KRAS in cultured primary astrocytes is induced by interferon-γ treatment, which is the most potent inducer of ROS-RNS formation in target cells [103]. Moreover, adult human astrocytes stimulated with interferon-γ exert potent neurotoxic effects in vitro [104], possibly reflecting the transient upregulation of HRAS in cultured astrocytes by ROS.
6. Conclusions
The oxidation and reduction of Cys residues provide a mechanism that rapidly and reversibly alters protein functions, albeit at physiological ROS levels. The Cys-based oxidative modification of H-Ras can be extensive in vivo, both S-nitrosylated and S-glutathionylated forms may be important, and oxidation may occur on reactive Cys residues that are usually targeted for lipid-modification reactions. Thus, these chemical modifications could act as antagonists of the physiological processing of the proteins and subsequent proper subcellular localization. Moreover, studies on Cys-directed therapy have increasingly gained attention in personalized cancer therapeutics [86].
Isoform-distinct Ras biological roles in normal and neoplastic cells are increasingly appreciated, but biochemical studies of native proteins are currently lacking. Indeed, the crystallographic structure of full-length proteins is still lacking, as only the structure of K-Ras4B in complex with PDEδ has recently been reported [30]. Many studies use a single isoform (namely, H-Ras); therefore, the lack of evidence for other isoforms does not exclude their involvement in redox biology.
Moreover, site-directed mutagenesis is often employed to determine whether specific Cys residues (mainly Cys118) have redox-regulated functional roles, but this approach provides no information on the oxidation status of the endogenous protein. The generation of oxidized Ras by adding bolus oxidants to cells and the overexpression of recombinant and/or truncated proteins represent significant caveats for the investigation of the redox biology of Ras. Moreover, cell-based studies should be performed with endogenous ROS sources and redox state characterization and be absent of GTPase overexpression. Indeed, redox-sensitive probes targeted to different cellular compartments will revolutionize our understanding of in vivo redox signalling in both time and space [105].
More recently, reactive cysteines have been targeted by high-throughput screening and fragment-based ligand discovery efforts [106]. Specific Cys residues function as redox-dependent switches in most ROS-dependent Ras signalling events. Whether the oxidation state of thiol groups can be harnessed to develop a unique class of redox-sensitive small molecule drugs remains to be seen.
7. Credit authorship contribution statement
Samantha Messina: Conceptualization, writing of the original draft, and funding acquisition. Giovanna De Simone: Structural analysis and writing of the original draft. Paolo Ascenzi: Review and supervision.
Conflicts of interest
The authors declare that there are no competing interests associated with this manuscript.
Acknowledgements Funding
The Grant of Excellence Departments, MIUR (Articolo 1, Commi 314–337 Legge 232/2016), is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101282.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cross J.V., Templeton D.J. Regulation of signal transduction through protein cysteine oxidation. Antioxidants Redox Signal. 2006;8:1819–1827. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T. Intracellular redox regulation by the family of small GTPases. Antioxidants Redox Signal. 2006;8:1857–1863. doi: 10.1089/ars.2006.8.1857. [DOI] [PubMed] [Google Scholar]
- 3.Davis M.F., Vigil D., Campbell S.L. Regulation of Ras proteins by reactive nitrogen species. Free Radic. Biol. Med. 2011;51:565–575. doi: 10.1016/j.freeradbiomed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxidants Redox Signal. 2011;14:689–724. doi: 10.1089/ars.2009.2984. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell L., Hobbs G.A., Aghajanian A., Campbell S.L. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxidants Redox Signal. 2013;18:250–258. doi: 10.1089/ars.2012.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro E., Goitre L., Baldini E., Retta S.F., Trabalzini L. Ras GTPases are both regulators and effectors of redox agents. Methods Mol. Biol. 2014;1120:55–74. doi: 10.1007/978-1-62703-791-4_5. [DOI] [PubMed] [Google Scholar]
- 7.Irani K., Xia Y., Zweier J.L., Sollott S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Nicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., Scrimieri F., Winter J.M., Hruban R.H., Iacobuzio-Donahue C., Kern S.E., Blair I.A., Tuveson D.A. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogrunc M., Di Micco R., Liontos M., Bombardelli L., Mione M., Fumagalli M., Gorgoulis V.G., d'Adda di Fagagna F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adachi T., Pimentel D.R., Heibeck T., Hou X., Lee Y.J., Jiang B., Ido Y., Cohen R.A. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 12.Clavreul N., Adachi T., Pimental D.R., Ido Y., Schöneich C., Cohen R.A. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 13.Shanshiashvili L., Narmania N., Barbakadze T., Zhuravliova E., Natsvlishvili N., Ramsden J., Mikeladze D.G. S-nitrosylation decreases the adsorption of H-Ras in lipid bilayer and changes intrinsic catalytic activity. Cell Biochem. Biophys. 2011;59:191–199. doi: 10.1007/s12013-010-9132-x. [DOI] [PubMed] [Google Scholar]
- 14.Santos A.I., Carreira B.P., Izquierdo-Álvarez A., Ramos E., Lourenço A.S., Filipa Santos D., Morte M.I., Ribeiro L.F., Marreiros A., Sánchez-López N., Marina A., Carvalho C.M., Martínez-Ruiz A., Araújo I.M. S-nitrosylation of Ras mediates nitric oxide-dependent post-injury neurogenesis in a seizure model. Antioxidants Redox Signal. 2018;28:15–30. doi: 10.1089/ars.2016.6858. [DOI] [PubMed] [Google Scholar]
- 15.Ntai, Fornelli L., DeHart C.J., Hutton J.E., Doubleday P.F., LeDuc R.D., van Nispen A.J., Fellers R.T., Whiteley G., Boja E.S., Rodriguez H., Kelleher N.L. Precise characterization of KRAS4b proteoforms in human colorectal cells and tumors reveals mutation/modification cross-talk. Proc. Natl. Acad. Sci. U.S.A. 2018;115:4140–4145. doi: 10.1073/pnas.1716122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams J.G., Pappu K., Campbell S.L. Structural and biochemical studies of p21Ras S-nitrosylation and nitric oxide-mediated guanine nucleotide exchange. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6376–6381. doi: 10.1073/pnas.1037299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibiza S., Perez-Rodriguez A., Ortega A., Martinez-Ruiz A., Barreiro O., Garcia-Dominguez C.A., Serrador J.M. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10507–10512. doi: 10.1073/pnas.0711062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgoyne J.R., Haeussler D.J., Kumar V., Ji Y., Pimental D.R., Zee R.S., Costello C.E., Lin C., McComb M.E., Cohen R.A., Bachschmid M.M. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 2012;26:832–841. doi: 10.1096/fj.11-189415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker, Booden M.A., Buss J.E. S-nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J. Biol. Chem. 2000;275:22037–22047. doi: 10.1074/jbc.M001813200. [DOI] [PubMed] [Google Scholar]
- 20.Barbakadze T., Goloshvili G., Narmania N., Zhuravliova E., Mikeladze D. Subcellular distribution of S-nitrosylated H-Ras in differentiated and undifferentiated PC12 cells during hypoxia. Cell. 2017;19:443–451. doi: 10.22074/cellj.2017.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batista W.L., Ogata F.T., Curcio M.F., Miguel R.B., Arai R.J., Matsuo A.L., Monteiro H.P. S-nitrosoglutathione and endothelial nitric oxide synthase-derived nitric oxide regulate compartmentalized ras S-nitrosylation and stimulate cell proliferation. Antioxidants Redox Signal. 2013;18:221–238. doi: 10.1089/ars.2011.4455. [DOI] [PubMed] [Google Scholar]
- 22.Mallis R.J., Buss J.E., Thomas J.A. Oxidative modification of H-ras: S-thiolation and S-nitrosylation of reactive cysteines. Biochem. J. 2001;355:145–153. doi: 10.1042/0264-6021:3550145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton P., Byers H.L., Leeds N., Ward M.A., Shattock M.J. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J. Biol. Chem. 2002;277 doi: 10.1074/jbc.M111454200. 9806-1981. [DOI] [PubMed] [Google Scholar]
- 24.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 25.Hancock J.F. Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 26.Vetter I.R. The structure of the G domain of the Ras superfamily. In: Wittinghofer A., editor. Ras Superfamily Small G Proteins: Biology and Mechanisms 1. Springer-Verlag; Wien: 2014. [Google Scholar]
- 27.Hancock J.F., Paterson H., Marshall C.J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 28.Chavan T.S., Jang H., Khavrutskii L., Abraham S.J., Banerjee A., Freed B.C., Johannessen L., Tarasov S.G., Gaponenko V., Nussinov R., Tarasova N.I. High-Affinity interaction of the K-Ras4B hypervariable region with the ras active site. Biophys. J. 2015;109:2602–2613. doi: 10.1016/j.bpj.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang H., Abraham S.J., Chavan T.S., Hitchinson B., Khavrutskii L., Tarasova N.I., Nussinov R., Gaponenko V. Mechanisms of membrane binding of small GTPase K-Ras4B farnesylated hypervariable region. J. Biol. Chem. 2015;290:9465–9477. doi: 10.1074/jbc.M114.620724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dharmaiah S., Bindu L., Tran T.H., Gillette W.K., Frank P.H., Ghirlando R., Nissley D.V., Esposito D., McCormick F., Stephen A.G., Simanshu D.K. Structural basis of recognition of farnesylated and methylated KRAS4b by PDEδ. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E6766–E6775. doi: 10.1073/pnas.1615316113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittinghofer A., Vetter I.R. Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 2011;80:943–971. doi: 10.1146/annurev-biochem-062708-134043. [DOI] [PubMed] [Google Scholar]
- 32.Sun Q., Burke J.P., Phan J., Burns M.C., Olejniczak E.T., Waterson A.G., Lee T., Rossanese O.W., Fesik S.W. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew. Chem. Int. Ed. Engl. 2012;51:6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke H., Matsumoto S., Murashima Y., Taniguchi-Tamura H., Miyamoto R., Yoshikawa Y., Tsuda C., Kumasaka T., Mizohata E., Edamatsu H., Kataoka T. Structural basis for intramolecular interaction of post-translationally modified H-Ras•GTP prepared by protein ligation. FEBS Lett. 2017;591:2470–2481. doi: 10.1002/1873-3468.12759. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Migoni A., Canning P., Quevedo C.E., Bataille C.J.R., Bery N., Miller A., Russell A.J., Phillips S.E.V., Carr S.B., Rabbitts T.H. Structure-based development of new RAS-effector inhibitors from a combination of active and inactive RAS-binding compounds. Proc. Natl. Acad. Sci. U.S.A. 2019;116:2545–2550. doi: 10.1073/pnas.1811360116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defelipe L.A., Lanzarotti E., Gauto D., Marti M.A., Turjanski A.G. Protein topology determines cysteine oxidation fate: the case of sulfenyl amide formation among protein families. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Prakash P., Gorfe A.A., Hancock J.F. Ras and the plasma membrane: a complicated relationship. Cold Spring Harb. Perspect. Med. 2018;8:a031831. doi: 10.1101/cshperspect.a031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid T.S., Terry K.L., Casey P.J., Beese L.S. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J. Mol. 2004;343:417–433. doi: 10.1016/j.jmb.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 38.Vetter I.R., Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 39.Castellano E., Santos E. Functional specificity of ras isoforms: so similar but so different. Genes Cancer. 2011;2:216–231. doi: 10.1177/1947601911408081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abankwa D., Gorfe A.A., Hancock J.F. Mechanisms of Ras membrane organization and signalling: ras on a rocker. Cell Cycle. 2008;7:2667–2673. doi: 10.4161/cc.7.17.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abankwa D., Gorfe A.A., Inder K., Hancock J.F. Ras membrane orientation and nanodomain localization generate isoform diversity. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1130–1135. doi: 10.1073/pnas.0903907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker J.A., Mattos C. The Ras-membrane interface: isoform-specific differences in the catalytic domain. Mol. Cancer Res. 2015;13:595–603. doi: 10.1158/1541-7786.MCR-14-0535. [DOI] [PubMed] [Google Scholar]
- 43.Konstantinopoulos P.A., Karamouzis M.V., Papavassiliou A.G. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat. Rev. Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 44.Cox A.D., Der C.J., Philips M.R. Targeting RAS membrane association: back to the future for anti-RAS drug discovery? Clin. Cancer Res. 2015;21:1819–1827. doi: 10.1158/1078-0432.CCR-14-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocks O., Peyker A., Kahms M., Verveer P.J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P.I. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki A.T., Carracedo A., Locasale J.W., Anastasiou D., Takeuchi K., Kahoud E.R., Haviv S., Asara J.M., Pandolfi P.P., Cantley L.C. Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci. Signal. 2011;4:ra13. doi: 10.1126/scisignal.2001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M.H., Nickerson S., Kim E.T., Liot C., Laurent G., Spang R., Philips M.R., Shan Y., Shaw D.E., Bar-Sagi D., Haigis M.C., Haigis K.M. Regulation of RAS oncogenicity by acetylation. Proc. Natl. Acad. Sci. U.S.A. 2012;109:10843–10848. doi: 10.1073/pnas.1201487109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker R., Wilkerson E.M., Sumita K., Isom D.G., Sasaki A.T., Dohlman H.G., Campbell S.L. Differences in the regulation of K-Ras and H-Ras isoforms by monoubiquitination. J. Biol. Chem. 2013;288:36856–36862. doi: 10.1074/jbc.C113.525691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunda S., Heir P., Srikumar T., Cook J.D., Burrell K., Kano Y., Lee J.E., Zadeh G., Raught B., Ohh M. Src promotes GTPase activity of Ras via tyrosine 32 phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E3785–E3794. doi: 10.1073/pnas.1406559111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobbs G.A., Der C.J., Rossman K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballester R., Furth M.E., Rosen O.M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J. Biol. Chem. 1987;262:2688–2695. [PubMed] [Google Scholar]
- 52.Zhang S.Y., Sperlich B., Li F.Y., Al-Ayoubi S., Chen H.X., Zhao Y., Li Y.M., Weise K., Winter R., Chen Y.X. Phosphorylation weakens but does not inhibit membrane binding and clustering of K-Ras4B. ACS Chem. Biol. 2017;12:1703–1710. doi: 10.1021/acschembio.7b00165. [DOI] [PubMed] [Google Scholar]
- 53.Jura N., Scotto-Lavino E., Sobczyk A., Bar-Sagi D. Differential modification of Ras proteins by ubiquitination. Mol. Cell. 2006;21:679–687. doi: 10.1016/j.molcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Hancock J.F., Cadwallader K, Paterson H., Marshall C.J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crowe M. Biochemical and tumorigenic effects of redox modification of Ras-G12c by nitric oxide. Redox Biol. 2015;5:414. doi: 10.1016/j.redox.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Huang L., Carney J., Cardona D.M., Counter C.M. Decreased tumorigenesis in mice with a Kras point mutation at C118. Nat. Commun. 2014;5:5410. doi: 10.1038/ncomms6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L., Counter C.M. Reduced HRAS G12V-driven tumorigenesis of cell lines expressing KRAS C118S. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hole P.S., Pearn L., Tonks A.J., James P.E., Burnett A.K., Darley R.L., Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115:1238–1246. doi: 10.1182/blood-2009-06-222869. [DOI] [PubMed] [Google Scholar]
- 59.Lander H.M., Ogiste J.S., Teng K.K., Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J. Biol. Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 60.Heo J., Campbell S.L. Mechanism of p21Ras S-nitrosylation and kinetics of nitric oxide-mediated guanine nucleotide exchange. Biochemistry. 2004;43:2314–2322. doi: 10.1021/bi035275g. [DOI] [PubMed] [Google Scholar]
- 61.Lander H.M., Milbank A.J., Tauras J.M., Hajjar D.P., Hempstead B.L., Schwartz G.D., Kraemer R.T., Mirza U.A., Chait B.T., Burk S.C., Quilliam L.A. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 62.Lander H.M., Hajjar D.P., Hempstead B.L., Mirza U.A., Chait B.T., Campbell S., Quilliam L.A. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J. Biol. Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 63.Mott H.R., Carpenter J.W., Campbell S.L. Structural and functional analysis of a mutant Ras protein that is insensitive to nitric oxide activation. Biochemistry. 1997;36:3640–3644. doi: 10.1021/bi962790o. [DOI] [PubMed] [Google Scholar]
- 64.Raines K.W., Bonini M.G., Campbell S.L. Nitric oxide cell signaling: S-nitrosation of Ras superfamily GTPases. Cardiovasc. Res. 2007;75:229–239. doi: 10.1016/j.cardiores.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Hobbs G.A., Bonini M.G., Gunawardena H.P., Chen X., Campbell S.L. Glutathiolated Ras: characterization and implications for Ras activation. Free Radic. Biol. Med. 2013;57:221–229. doi: 10.1016/j.freeradbiomed.2012.10.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tisi R., Belotti F., Martegani E. Yeast as a model for Ras signalling. Methods Mol. Biol. 2014;1120:359–390. doi: 10.1007/978-1-62703-791-4_23. [DOI] [PubMed] [Google Scholar]
- 67.Castillo B., Kim S.H., Sharief M., Sun T., Kim L.W. SodC modulates ras and PKB signaling in Dictyostelium. Eur. J. Cell Biol. 2017;96:1–12. doi: 10.1016/j.ejcb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Yi D.G., Hong S., Huh W.K. Mitochondrial dysfunction reduces yeast replicative lifespan by elevating RAS-dependent ROS production by the ER-localized NADPH oxidase. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shima F., Okada T., Kido M., Sen H., Tanaka Y., Tamada M., Hu C.-D., Yamawaki-Kataoka Y., Kariya K., Kataoka T. Association of yeast adenylyl cyclase with cyclase-associated protein CAP forms a second Ras-binding site which mediates its Ras-dependent activation. Mol. Cell. Biol. 2000;20:26–33. doi: 10.1128/mcb.20.1.26-33.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim A., Davis R., Higuchi M. Intracellular oxygen determined by respiration regulates localization of Ras and prenylated proteins. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holstein S.A., Wohlford-Lenane C.L., Hohl R.J. Consequences of mevalonate depletion. Differential transcriptional, translational, and post-translational up-regulation of Ras, Rap1a, RhoA, and RhoB. J. Biol. Chem. 2002;277:10678–10682. doi: 10.1074/jbc.M111369200. [DOI] [PubMed] [Google Scholar]
- 72.Liu R., Li B., Qiu M. Elevated superoxide production by active H-ras enhances human lung WI-38VA-13 cell proliferation, migration and resistance to TNF-alpha. Oncogene. 2001;20:1486–1496. doi: 10.1038/sj.onc.1204214. [DOI] [PubMed] [Google Scholar]
- 73.Yang J.Q., Li S., Huang Y., Zhang H.J., Domann F.E., Buettner G.R., Oberley L.W., Ha-Ras V.- Overexpression induces superoxide production and alters levels of primary antioxidant enzymes. Antioxidants Redox Signal. 2001;3:697–709. doi: 10.1089/15230860152543032. [DOI] [PubMed] [Google Scholar]
- 74.Weyemi U., Lagente-Chevallier O., Boufraqech M., Prenois F., Courtin F., Caillou B., Talbot M., Dardalhon M., Al Ghuzlan A., Bidart J.M., Schlumberger M., Dupuy C. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2012;31:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park M.T., Kim M.J., Suh Y., Kim R.K., Kim H., Lim E.J., Yoo K.C., Lee G.H., Kim Y.H., Hwang S.G., Yi J.M., Lee S.J. Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death Differ. 2014;21:1185–1197. doi: 10.1038/cdd.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rassool F.V., Gaymes T.J., Omidvar N., Brady N., Beurlet S., Pla M., Reboul M., Lea N., Chomienne C., Thomas N.S., Mufti G.J., Padua R.A. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007;67:8762–8771. doi: 10.1158/0008-5472.CAN-06-4807. [DOI] [PubMed] [Google Scholar]
- 77.Adachi Y., Shibai Y., Mitsushita J., Shang W.H., Hirose K., Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27:4921–4932. doi: 10.1038/onc.2008.133. [DOI] [PubMed] [Google Scholar]
- 78.Shaw A.T., Winslow M.M., Magendantz M., Ouyang C., Dowdle J., Subramanian A., Lewis T.A., Maglathin R.L., Tolliday N., Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zamkova M., Khromova N., Kopnin B.P., Kopnin P. Ras-induced ROS upregulation affecting cell proliferation is connected with cell type-specific alterations of HSF1/SESN3/p21Cip1/WAF1 pathways. Cell Cycle. 2013;12:826–836. doi: 10.4161/cc.23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee A.C., Fenster B.E., Ito H., Takeda K., Bae N.S., Hirai T., Yu Z.X., Ferrans V.J., Howard B.H., Finkel T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 81.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Bessler W.K., Hudson F.Z., Zhang H., Harris V., Wang Y., Mund J.A., Stansfield B.K. Neurofibromin is a novel regulator of Ras-induced reactive oxygen species production in mice and humans. Free Radic. Biol. Med. 2016;97:212–222. doi: 10.1016/j.freeradbiomed.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bivona T.G., Quatela S.E., Bodemann B.O., Ahearn I.M., Soskis M.J., Mor A., Miura J., Wiener H.H., Wright L., Saba S.G., Yim D., Fein A., Pérez de Castro I., Li C., Thompson C.B., Cox A.D., Philips M.R. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol. Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim S.M., Westover K.D., Ficarro S.B., Harrison R.A., Choi H.G., Pacold M.E., Carrasco M., Hunter J., Kim N.D., Xie T., Sim T., Jänne P.A., Meyerson M., Marto J.A., Engen J.R., Gray N.S. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. Int. Ed. Engl. 2014;53:199–204. doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Visscher M., Arkin M.R., Dansen T.B. Covalent targeting of acquired cysteines in cancer. Curr. Opin. Chem. Biol. 2016;30:61–67. doi: 10.1016/j.cbpa.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffmann M.H., Griffiths H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic. Biol. Med. 2018;125:62–71. doi: 10.1016/j.freeradbiomed.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Svegliati S., Cancello R., Sambo P., Luchetti M., Paroncini P., Orlandini G., Discepoli G., Paterno R., Santillo M., Cuozzo C., Cassano S., Avvedimento E.V., Gabrielli A. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J. Biol. Chem. 2005;280:36474–36482. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- 89.Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowluru R.A., Kanwar M. Translocation of H-Ras and its implications in the development of diabetic retinopathy. Biochem. Biophys. Res. Commun. 2009;387:461–466. doi: 10.1016/j.bbrc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kowluru R.A. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Investig. Ophthalmol. Vis. Sci. 2010;51:4320–4326. doi: 10.1167/iovs.09-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vähätupa M., Prince S., Vataja S., Mertimo T., Kataja M., Kinnunen K., Marjomäki V., Uusitalo H., Komatsu M., Järvinen T.A., Uusitalo-Järvinen H. Lack of R-Ras leads to increased vascular permeability in ischemic retinopathy. Investig. Ophthalmol. Vis. Sci. 2016;57:4898–4909. doi: 10.1167/iovs.16-19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foster M.W., McMahon T.J., Stamler J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 94.Ho J.J., Man H.S., Marsden P.A. Nitric oxide signaling in hypoxia. J. Mol. Med. (Berl.) 2012;90:217–231. doi: 10.1007/s00109-012-0880-5. [DOI] [PubMed] [Google Scholar]
- 95.Wainwright M.S., Brennan L.A., Dizon M.L., S.M. Black, p21ras activation following hypoxia-ischemia in the newborn rat brain is dependent on nitric oxide synthase activity but p21ras does not contribute to neurologic injury. Brain Res. Dev. Brain Res. 2003;146:79–85. doi: 10.1016/j.devbrainres.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Angelova P.R., Kasymov V., Christie I., Sheikhbahaei S., Turovsky E., Marina N., Korsak A., Zwicker J., Teschemacher A.G., Ackland G.L., Funk G.D., Kasparov S., Abramov A.Y., Gourine A.V. Functional oxygen sensitivity of astrocytes. J. Neurosci. 2015;35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Furth M.E., Aldrich T.H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987;1:47–58. [PubMed] [Google Scholar]
- 98.Mizoguchi A., Ueda T., Ikeda K., Shiku H., Mizoguti H., Takai Y. Localization and subcellular distribution of cellular ras gene products in rat brain. Brain Res. Mol. Brain Res. 1989;5:31–44. doi: 10.1016/0169-328x(89)90015-6. [DOI] [PubMed] [Google Scholar]
- 99.Messina S., Molinaro G., Bruno V., Battaglia G., Spinsanti P., Di Pardo A., Nicoletti F., Frati L., Porcellini A. Enhanced expression of Harvey ras induced by serum deprivation in cultured astrocytes. J. Neurochem. 2008;106:551–559. doi: 10.1111/j.1471-4159.2008.05420.x. [DOI] [PubMed] [Google Scholar]
- 100.Messina S., Frati L., Porcellini A. Oxidative stress posttranslationally regulates the expression of Ha-Ras and Ki-Ras in cultured astrocytes, Oxid. Med Cell. Longev. 2012:792705. doi: 10.1155/2012/792705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Messina S., Di Zazzo E., Moncharmont B. Early and late induction of KRAS and HRAS proto-oncogenes by reactive oxygen species in primary astrocytes. Antioxidants. 2017;6:48. doi: 10.3390/antiox6030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scherz-Shouval R., Elazar Z. Monitoring starvation-induced reactive oxygen species formation. Methods Enzymol. 2009;452:119–130. doi: 10.1016/S0076-6879(08)03608-2. [DOI] [PubMed] [Google Scholar]
- 103.Rubio N. Interferon-gamma protects astrocytes from apoptosis and increases the formation of p21ras-GTP complex through ras oncogene family overexpression. Glia. 2001;33:151–159. doi: 10.1002/1098-1136(200102)33:2<151::aid-glia1014>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 104.Hashioka S., Klegeris A., Schwab C., McGeer P.L. Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol. Aging. 2009;30:1924–1935. doi: 10.1016/j.neurobiolaging.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Maulucci G., Bačić G., Bridal L., Schmidt H.H., Tavitian B., Viel T., Utsumi H., Yalçın A.S., De Spirito M. Imaging reactive oxygen species-induced modifications in living systems. Antioxidants Redox Signal. 2016;24:939–958. doi: 10.1089/ars.2015.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Backus K.M. Applications of reactive cysteine profiling. Curr. Top. Microbiol. Immunol. 2019;420:375–417. doi: 10.1007/82_2018_120. [DOI] [PubMed] [Google Scholar]
- 107.Sievers F., Higgins D.G. Clustal omega. Curr. Protoc. Bioinformatics. 2014;48:3. doi: 10.1002/0471250953.bi0313s48. 13.1-3. 13.16. [DOI] [PubMed] [Google Scholar]
- 108.Henikoff S., Henikoff J.G. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Filchtinski D., Sharabi O., Rüppel A., Vetter I.R., Herrmann C., Shifman J.M. What makes Ras an efficient molecular switch: a computational, biophysical, and structural study of Ras-GDP interactions with mutants of Raf. J. Mol. Biol. 2010;399:422–435. doi: 10.1016/j.jmb.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 110.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.