Abstract
We retrospectively assessed association of stromal tumor-infiltrating lymphocytes (sTILs) with clinical outcomes and molecular variables reportedly predictive of trastuzumab-benefit in National Surgical Adjuvant Breast and Bowel Project B-31 (N = 2130). sTILs were assessed in 1581 eligible B-31 cases utilizing all available hematoxylin and eosin slides. Mean concordance between main reviewer and six other pathologists was 90.8% in 100 cases. Cox regressions were used to calculate hazard ratios (HRs). In chemotherapy and trastuzumab added to chemotherapy arms, increases in sTILs, as a semicontinuous variable (combined arms HR = 0.42, 95% confidence interval = 0.27 to 0.64, two-sided P < .001) or as lymphocyte-predominant breast cancer with more than 50% sTILs (combined arms HR = 0.65, 95% confidence interval = 0.49 to 0.86, two-sided P = .003) were statistically significantly associated with improved disease-free survival. There was no association of sTILs with trastuzumab benefit. However, higher sTILs were statistically significantly associated with higher trastuzumab benefit groups by 8-gene prediction model (two-sided P < .001). Neither PIK3CA mutations nor Fc-gamma-receptor polymorphisms were associated with sTILs. sTILs may have utility as a prognostic biomarker identifying HER2-positive early breast cancer at low recurrence risk.
Evidence has been presented demonstrating that the host immune system and microenvironment interactions play an important role in tumorigenesis and clinical outcomes of HER2-positive breast cancer as well as triple-negative breast cancer (1–3). Higher stromal tumor-infiltrating lymphocytes (sTILs) in tumors are associated with improved prognosis in HER2-positive disease (4–6). However, in NCCTG N9831 (7), which tested the benefit of adding trastuzumab to adjuvant standard chemotherapy in early-stage HER2-positive breast cancer, the prognostic association of sTILs was statistically significant only in the chemotherapy alone (C) arm, and not in the trastuzumab added to chemotherapy (CT) arm. As a result, high sTILs were statistically significantly associated with lack of adjuvant trastuzumab benefit. Given these contradictory results, we evaluated the prognostic and predictive value of sTILs in the NRG Oncology/National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 (trial registration: NCT00004067), which was a large randomly assigned trial testing the value of trastuzumab added to adjuvant chemotherapy in the treatment of HER2-positive early-stage breast cancer. We assessed sTILs utilizing all available hematoxylin and eosin (H&E) slides from the entire B-31 cohort and evaluated associations with other molecular variables. The current study was performed with approvals from the National Cancer Institute Cancer Therapy Evaluation Program and institutional review board for NSABP/NRG Oncology. Patients signed informed consent, approved by the Human Investigations Committee in accordance with Department of Health and Human Services (DHHS), to permit future use of tissue.
sTILs were assessed by a pathologist (R. S. Kim) in all 1581 cases with full-face sectioned H&E slides out of 1931 eligible cases among the entire B-31 cohort (N = 2130) (Supplementary Figure 1, available online). Percentages of sTILs were assessed as the proportion of stromal areas occupied with morphological mononuclear cells including lymphocytes and plasma cells within invasive tumor nests based on guidelines standardized by the International Immuno-oncology Working Group (8).
To assess the performance of the main pathologist/reviewer (R. S. Kim), a ring study of sTILs evaluation was performed by six pathologists who independently performed assessments on whole slide scanned images in 100 cases. The mean concordance of R. S. Kim with other pathologists in sTILs percentage scores was 90.8%. A previous in-silico ring study showed prediction by different sTILs levels was stable even in the lower range of intra-class correlation coefficients (9).
The prognostic value of sTILs for disease-free survival (DFS) was assessed using a multivariable Cox proportional hazards model adjusted by clinicopathological variables including ER, node, and tumor size. The predictive value of sTILs was assessed using Cox models with an interaction term between treatment and sTILs. The proportional hazards assumption was verified using the Schoenfeld residual plot and test. Associations of sTILs with other variables were assessed using analysis of variance tests. All statistical tests were two-sided and a P value of less than .05 was considered statistically significant.
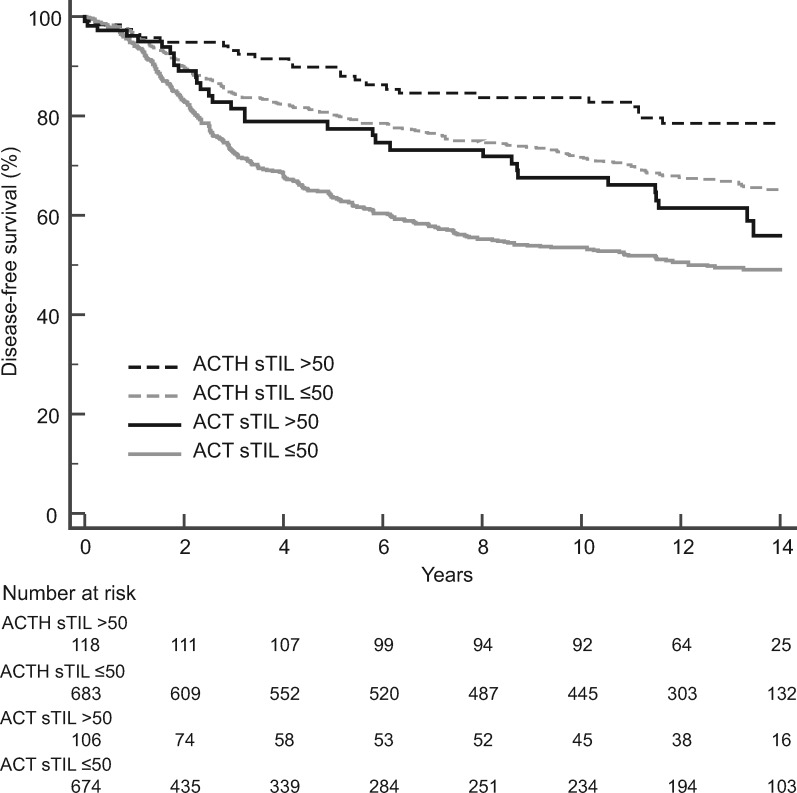
Higher sTILs were statistically significantly associated with improved DFS in the combined arms (hazard ratio = 0.42, 95% confidence interval = 0.27 to 0.64, P < .001) (Supplementary Table 1, available online). However, there was no association of sTILs with the degree of trastuzumab benefit (interaction P = .65). When sTILs were dichotomized (lymphocyte-predominant breast cancer >50% sTILs), similar results were observed. sTILs were statistically significantly associated with improved DFS (combined arms: hazard ratio = 0.65, 95% confidence interval = 0.49 to 0.86, P = .003) (Figure 1 ; Supplementary Table 1, available online), but there was no association of sTIL levels with the degree of trastuzumab benefit (interaction P = .50).
Figure 1.
Prognostic value of stromal tumor-infiltrating lymphocytes (sTILs) in both the chemotherapy and trastuzumab added to chemotherapy arms. Kaplan-Meier analysis of disease-free survival of patients treated with doxorubicin, cyclophosphamide, and taxol chemotherapy (ACT) vs patients treated with trastuzumab added to ACT (ACTH).
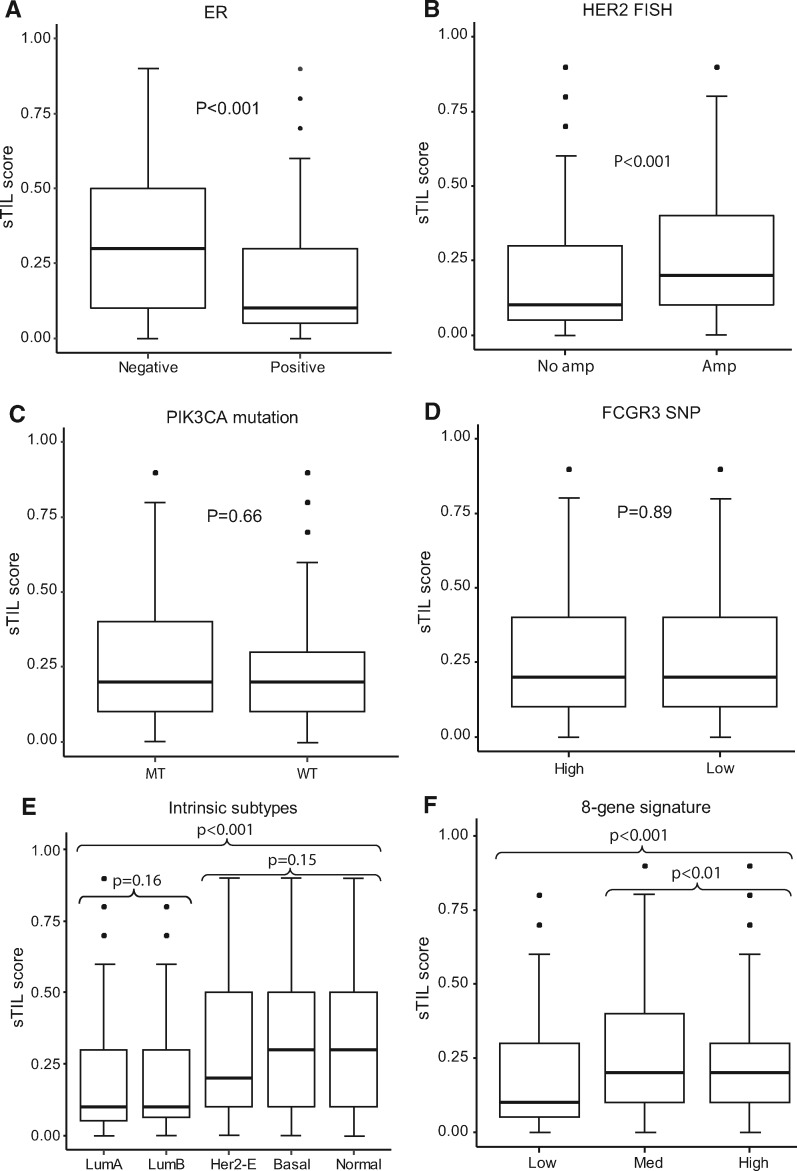
We examined the association of sTILs scores with clinicopathological variables (Supplementary Table 2, available online). High sTILs were statistically significantly associated with HER2 amplification by immunohistochemistry (P < .001) and Fluorescence in situ hybridization (P < .001). ER status by immunohistochemistry was inversely associated with sTILs (P < .001) (Figure 2), which is consistent with results from other studies (4, 5, 10–12).
Figure 2.
Distribution of stromal tumor-infiltrating lymphocytes (sTILs) segregated by estrogen receptor status, HER2 amplification, phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations, FCGR3A-158V/F genotyping, intrinsic subtype, and 8-gene signature groups. A) ER-negative (n = 905) vs ER-positive (n = 1024). B) HER2 human epithelial growth factor receptor 2 Fluorescence in Situ Hybridization (FISH) no amplification (n = 210) vs amplification (n = 1584). C) PIK3CA mutant (MT; n = 162) vs wild type (WT; n = 493). D) FCGR3A high-affinity (n = 726) vs low-affinity (n = 608). E) Luminal A (LumA; n = 424) vs Luminal B (LumB; n = 219) vs HER2 enriched (n = 726) vs basal (n = 98) vs normal-like (n = 74); F) 8-gene signature low (n = 178) vs medium (n = 685) vs high (n = 678) benefit group. Two-sided analysis of variance test was used to calculate P values. Error bars represent first quantile – IQR, and third quantile + IQR, where IQR is the range between first and third quantiles. FCGR3A = Fc fragment of IgG receptor IIIa; HER2-E = HER2-enriched; SNP = single nucleotide polymorphism.
In addition to tumor microenvironment, tumor intrinsic subtypes have been reported as associated with clinical outcomes of HER2-positive breast cancer in other trials (13, 14). Therefore, we tested the association of sTILs with PAM50 intrinsic subtypes. PAM50 subtypes had no trastuzumab-predictive value in our previous B-31 study (15). When we tested sTILs in the B-31 subtypes data set, high levels of sTILs were statistically significantly associated with basal, HER2-enriched, and normal-like intrinsic subtypes (P < .001) (Figure 2; Supplementary Table 2, available online). Other clinical trial results also demonstrated that basal and HER2-enriched subtypes tend to have more sTILs compared with luminal subtypes (13).
Unique to this study is our observation that sTILs were associated with the 8-gene trastuzumab predictive signature based on nCounter gene expression profiling of formalin-fixed paraffin-embedded tumor blocks (16). The 8-gene model was tested because to date it is the only gene expression signature independently validated in both B-31 and N9831 that is able to predict trastuzumab benefit (15–17). The 8-gene signature categorizes patients into one of three groups with regard to trastuzumab benefit: high, medium, or low. Higher levels of sTILs were statistically significantly associated with high- and medium-benefit groups (P < .001) (Figure 2; Supplementary Table 2, available online).
PIK3CA mutations have been associated with a lack of pCR in the neoadjuvant setting and with poor prognosis in the metastatic setting in HER2+ patients treated with anti-HER2 therapy (18–20). Nonetheless, we did not detect an association of PIK3CA mutations with sTILs in B-31 (Supplementary Table 2, available online).
Previously, we showed that patients with one or two high-binding alleles (FCGR3A-158V) for the single nucleotide polymorphism FCGR3A-158V/F were associated with greater benefit from trastuzumab than were patients who were homozygous for the low-binding allele (FCGR3A-158F) (21). However, there was no association between sTILs and polymorphisms in Fc gamma receptors when FCGR3A SNPs were assessed in the same way or in three different genotypes (Supplementary Table 2, available online; Figure 2). Further analysis of sTILs adjusting clinicopathologicial and molecular variables showed sTILs were associated with improved DFS in trastuzumab added to doxorubicin, cyclophosphamide, and taxol chemotherapy (Supplementary Table 3, available online) and across all arms. However, sTILs were not associated with trastuzumab benefit (interaction P = .25).
This is a retrospective study that was not prospectively powered for biomarker discovery. Nonetheless, our study confirmed that the quantity of sTILs is an independent prognostic marker in early-stage HER2-positive breast cancer. More than one-half of the patients in both the N9831 C and CT arms (arm A: 625 of 1081; arm C: 457 of 946) were excluded from sTILs assessment; it may be worthwhile to assess missing cases to confirm the current results (7). Furthermore, correlative studies confirmed sTILs levels are different across intrinsic subtypes, and high levels of sTILs are associated with high-benefit groups defined by 8-gene trastuzumab predictive signature, although sTILs alone are not predictive of trastuzumab benefit. Lack of interaction between trastuzumab benefit and global immune reaction defined by sTILs on H&E slides may be different when specific immune markers are examined. However, in this large-cohort randomly assigned study, high sTILs identified a subset of patients with excellent outcomes treated with CT who will derive potentially little absolute benefit from enhanced anti-HER2 therapies. Further pooled analyses might prove sTILs useful as a prognostic biomarker stratifying patients with HER2-positive breast cancer for potential combination therapies.
Funding
Funding was provided by NCI U10CA180868, -180822, UG1-189867, and U24-196067; The Pennsylvania Department of Health, and the Breast Cancer Research Foundation.
The Pennsylvania Department of Health specifically disclaims responsibility for any analysis, interpretations, or conclusions. The study sponsors played no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit for publication.
Notes
Affiliations of authors: Department of Pathology (RSK; PGG), NSABP/NRG Oncology, Pittsburgh, PA (NSABP Legacy trials are now part of the NRG Oncology portfolio) (RSK, NS, PGG, HB, PR, LF, EPM, SMS, DLW, JPC, SP, NW, CEGJ, PCL, KLPG); Department of Pathology, GZA, Antwerp, Belgium (RS, GGGMVdE); Department of Pathology, Division of Research, Peter MacCallum Cancer Centre, Melbourne, Australia (RS); Department of Biostatistics (JPC), The University of Pittsburgh, Pittsburgh, PA (HB, JPC); Department of Pathology and Laboratory Medicine, University of Ottawa, Ottawa, Canada (ZK); Laboratory of Translational Cell & Tissue Research, Department of Imaging and Pathology, KU Leuven- University of Leuven, Leuven, Belgium (GF); Department of Pathology, KU Leuven- University Hospitals Leuven, Leuven, Belgium (GF); Professor of Pathology and Laboratory Medicine; Department of Pathology and Laboratory Medicine, Indiana University, Indianapolis, IN (SB); Department of Radiation Oncology, and Pathology and Laboratory Medicine, Weill Cornell Medical College, New York, NY (SD); Department of Oncology, University of Pittsburgh Cancer Institute, Pittsburgh, PA (PR); Department of Oncology, Kaiser Permanente Oncology Clinical Trials Northern California, Vallejo, CA (LF); Department of Surgery, UF Health Cancer Center, Orlando Health, Orlando, FL (EPM); Department of Medicine, Georgetown University Medical Center, Washington, DC (SMS); Department of Human Oncology, Allegheny Health Network Cancer Institute, Pittsburgh, PA (DLW, NW); Yonsei University College of Medicine, Seoul, Republic of South Korea (SP); Virginia Commonwealth University Massey Cancer Center, Richmond, VA (CEGJ); Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA (PCL).
The authors declare the following potential conflict(s) of interest: Dr Salgado reports support from a grant from the Breast Cancer Research Foundation (BCRF, NY, US). Dr Demaria reports grants and personal fees from Lytix Biopharma, grants from Nanobiotix, and personal fees from AbbVie and AstraZeneca, outside the submitted work. Dr Mamounas reports personal fees from Genentech, during the conduct of the study. Dr Lucas reports ownership of stock in Amgen. Dr Paik reports grants from NCI during the conduct of the study. Dr Pogue-Geile reports grants from National Cancer Institute and the Pennsylvania Dept of Health, during the conduct of the study. All other authors declare no other potential conflict(s) of interest.
Supplementary Material
References
- 1. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;1445:646–674. [DOI] [PubMed] [Google Scholar]
- 2. Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL.. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;603:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianchini G, Gianni L.. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;152:e58–68. [DOI] [PubMed] [Google Scholar]
- 4. Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015; 33(9):983–991. [DOI] [PubMed] [Google Scholar]
- 5. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab. JAMA Oncol. 2015;14:448.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;181:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: recommendations by an International TILS Working Group 2014. Ann Oncol. 2015;262:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52(Pt2):16–25. [DOI] [PubMed] [Google Scholar]
- 10. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;317:860–867. [DOI] [PubMed] [Google Scholar]
- 11. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;258:1544–1550. [DOI] [PubMed] [Google Scholar]
- 12. Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;281:105–113. [DOI] [PubMed] [Google Scholar]
- 13. Dieci MV, Prat A, Tagliafico E, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann Oncol. 2016;2710:1867–1873. [DOI] [PubMed] [Google Scholar]
- 14. Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;346:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pogue-Geile KL, Song N, Jeong JH, et al. Intrinsic subtypes, PIK3CA mutation, and the degree of benefit from adjuvant trastuzumab in the NSABP B-31 trial. J Clin Oncol. 2015;3312:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pogue-Geile KL, Kim C, Jeong JH, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst. 2013;10523:1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pogue-Geile KL, Song N, Serie DJ, Thompson EA.. The NSABP/NRG 8-gene signature accurately predicts degree of benefit from trastuzumab in Alliance/NCCTG N9831: validation of the 8-gene signature in an independent clinical trial. Cancer Res 2018; 78(4) Supplement DOI: 10.1158/1538-7445.SABCS17-PD3-18. [Google Scholar]
- 18. Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;3312:1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baselga J, Cortés J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;3233:3753–3761. [DOI] [PubMed] [Google Scholar]
- 20. Loibl S, Von Minckwitz G, Schneeweiss A, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (HER2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;3229:3212–3220. [DOI] [PubMed] [Google Scholar]
- 21. Gavin PG, Song N, Kim RS, et al. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast cancer analysis of the NSABP B-31 trial. JAMA Oncol. 2017;33:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.