Abstract
Background
Genetic testing to determine BRCA status has been available for over two decades, but there are few population-based studies of test diffusion. We report 10-year trends in BRCAtesting in an integrated health-care system with long-standing access to genetic services.
Methods
A cohort of women aged 18 years and older was created to ascertain BRCA testing (n = 295 087). Annual testing rates between 2005 and 2015 were calculated in all women with and without incident (ie, newly diagnosed) breast and ovarian cancers and in clinically eligible subgroups by family cancer history, personal cancer history, and age at diagnosis. Secular trends were assessed using Poisson regression. Women tested early (2005–2008), midway (2009–2012), and late (2013–2015) in the study period were compared in cross-sectional analyses.
Results
Between 2005 and 2015, annual testing rates increased from 0.6/1000 person-years (pys) (95% confidence interval [CI] = 0.4 to 0.7/1000 pys) to 0.8/1000 pys (95% CI = 0.6 to 1.0/1000 pys) in women without incident breast or ovarian cancers. Rates decreased from 71.5/1000 pys (95% CI = 42.4 to 120.8/1000 pys) to 44.4/1000 pys (95% CI = 35.5 to 55.6/1000 pys) in women with incident diagnoses, despite improvements in provision of timely BRCA testing during this time frame. We found no evidence of secular trends in clinically eligible subgroups including women with family history indicating increased hereditary cancer risk, but no personal cancer history. At the end of the study period, 97.0% (95% CI = 96.6% to 97.3%) of these women remained untested.
Conclusion
Many eligible women did not receive BRCA testing despite having insurance coverage and access to specialty genetic services, underscoring challenges to primary and secondary hereditary cancer prevention.
Pathogenic BRCAvariants account for approximately 10% of breast cancer and 15% of ovarian cancer cases in the United States annually (1,2). Women with pathogenic variants have substantially increased breast and ovarian cancer risks, approximately 7-fold and 47-fold higher, respectively, than otherwise comparable women (3–5). Clinical genetic testing to determine BRCA variant status has been available since 1996 and allows women to consider more aggressive cancer prevention and treatment options (6). Numerous guidelines recommend offering testing with pretest genetic counseling to individuals with an elevated probability of carrying a pathogenic mutation (7–10). Specific risk factors indicating hereditary breast and ovarian cancer (HBOC) susceptibility include breast cancer diagnosed before age 50 years, ovarian cancer diagnosed at any age, and a family history of cancer with these features (7).
National BRCA testing rates have increased dramatically in the past two decades, but not necessarily among women most likely to benefit (11,12). Testing rates in women aged 18–64 years with employer-sponsored health insurance, for example, grew almost 10-fold between 2003 and 2014, from 2.68 per 100 000 person-years (pys) to 240.9 per 100 000 pys (13). Diffusion of BRCAtesting into routine practice has been attributed to expanding clinical guidelines, falling out-of-pocket costs, and increasing patient and provider awareness (13). However, population-based studies show that testing is still underused in many indicated groups and is increasingly occurring among average-risk women (14,15). Testing patterns among women eligible based on their family cancer history alone are largely unknown (14,15) and represent a critical gap in the literature.
We examined how BRCA test use evolved in a single organization over a 10-year period. Specifically, we describe testing trends in Kaiser Permanente Washington (KPWA), an integrated health-care delivery system in Washington State. In addition to calculating testing trends in women with and without incident (ie, newly diagnosed) breast and ovarian cancers, we examine rates in clinically eligible subgroups and compare characteristics of women tested early (2005–2008), midway (2009–2012), and late (2013–2015) in the study time frame.
Methods
Setting
KPWA provides health care and/or health insurance to approximately 690 000 members. Nearly two-thirds receive primary and specialty care at system-associated medical centers, similar to a traditional health maintenance organization model. KPWA has had a Department of Genetic Services since 2000. BRCA genetic testing has been covered with little to no cost-sharing for eligible women since 1996. Eligibility determinations have generally followed National Comprehensive Cancer Network and US Preventive Services Task Force practice guidelines (7,9). Medicare typically only covers testing for individuals with a personal cancer history, meaning some clinically eligible women may have had out-of-pocket costs (16). Since 2007, the health plan’s breast cancer screening guideline has recommended providers screen women aged 25 years and older for HBOC risk and refer those with personal and family histories suggestive of inherited susceptibility to the Department of Genetic Services or Medical Oncology for counseling and discussion of testing (17,18).
Study Population
We conducted a retrospective cohort study of female KPWA members aged 18 years and older from January 1, 2005, to September 30, 2015. To identify a cohort actively receiving care within the health system, women needed at least one year of continuous health plan enrollment (with an allowable 90-day coverage gap) and at least one in-person health system encounter for study inclusion. We excluded women who lived outside of the Seattle-Puget Sound Surveillance, Epidemiology, and End Results (SEER) tumor registry catchment area (19) and who did not have a KPWA primary care provider to minimize missing data on cancer diagnoses and care received outside the system. The Kaiser Permanente Washington Health Research Institute Institutional Review Board approved the study with a waiver of consent.
KPWA’s virtual data warehouse (VDW), which contains administrative, diagnostic, and procedure data for all health plan enrollees, was used to identify BRCA tests (20). We searched both internal procedure codes captured through the electronic health record and billing codes for evidence of testing ordered by health system providers or billed through the health plan (Supplementary Tables 1 and 2, available online). KPWA required the use of gene-specific billing codes for test reimbursement throughout the study time frame. Thus, we did not include methodology-based codes used to bill for nonspecific molecular diagnostic testing (21,22). For women who received BRCA tests, we also collected the following information from the VDW and/or SEER: test date; age when tested; ordering provider type; BRCA test type; and, for women with incident cancers, whether they were tested prior to their first-line surgery.
We determined women’s age, race/ethnicity, and length of prior health plan enrollment at study entry using the VDW. We identified incident breast (invasive and in situ) and nonepithelial ovarian cancers (including ovary, fallopian, and peritoneal) during the study period using SEER registry data (Supplementary Table 3, available online) (19). We characterized women’s breast cancer history prior to study entry using SEER registry data and self-reported data collected at health plan breast imaging facilities through the Breast Cancer Surveillance Consortium (BCSC) (23). As part of the BCSC, women are asked to complete a risk factor questionnaire that includes extensive personal and family cancer history information at each mammography visit (24). Thus, BCSC data was only available for women who had visited a health plan facility for breast imaging. Based on concerns about the quality of self-reported data on ovarian procedures (25) and lack of self-reported data on tumor histology to identify nonepithelial cancers, we used SEER data alone to characterize women’s ovarian cancer history prior to study entry. BCSC data was used to identify women self-reporting family history consistent with increased hereditary breast and ovarian cancer risk (Box 1
Box 1.
Family cancer history profiles identifying women eligible for genetic testing per health plan clinical guidelines (7–9)
| Self-report of any of the following: |
| ≥1 first-degree relative diagnosed with breast cancer before 50 years of age |
| ≥3 first- or second-degree relatives diagnosed with breast cancer |
| ≥2 second-degree relatives diagnosed with breast cancer before 50 years of age |
| ≥1 male relative diagnosed with breast cancer |
| ≥2 first- or second-degree relatives diagnosed with ovarian cancer |
| ≥1 first- or second-degree relative diagnosed with ovarian cancer AND ≥1 first- or second-degree relative diagnosed with breast cancer |
| ≥1 first-degree relative diagnosed with both ovarian and breast cancer |
Statistical Analysis
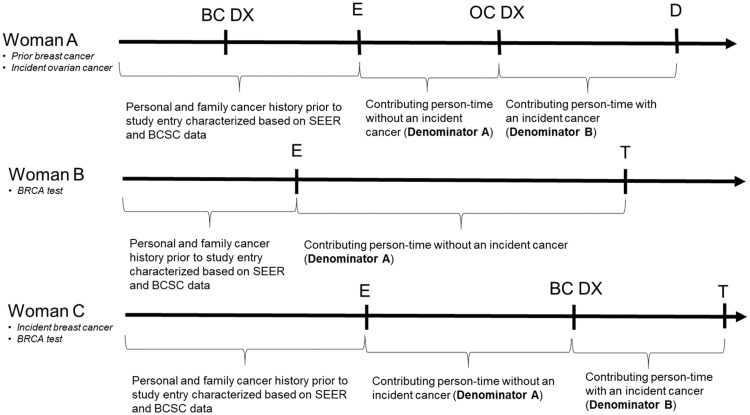
We excluded women with a BRCA test date prior to their study entry date (n = 409). We used descriptive statistics to characterize the study cohort including women who did and did not receive incident breast or ovarian cancer diagnoses during the study period. We calculated annual BRCA testing rates per 1000 person-years (pys) and 95% confidence intervals (CIs) in women with and without incident breast and ovarian cancers. These two denominator populations were not mutually exclusive (Figure 1). In the former analysis (denominator A), women began contributing person-time on January 1, 2005, or the earliest date they met all study eligibility criteria. For example, women with at least one year of health plan enrollment but no in-person encounters did not begin contributing person-time until the date of their first in-person interaction with the health system. Women were followed until September 30, 2015, with necessary censoring upon death, plan disenrollment, BRCAtesting, or receipt of an incident breast or ovarian cancer diagnosis. In the latter analysis (denominator B), women began contributing person-time on the date they received an incident breast or ovarian cancer diagnosis and were followed until September 30, 2015, with necessary censoring upon death, health plan disenrollment, or BRCAtesting.
Figure 1.
Personal and family cancer history and contributed person-time for three hypothetical women. BC = breast cancer; BCSC = Breast Cancer Surveillance Consortium; D = death; DX = diagnosis; E = study entry; OC = ovarian cancer; T = BRCA test; SEER = Surveillance, Epidemiology, and End Results Registry.
We calculated annual testing rates in women eligible for BRCA testing during the study period per health plan clinical guidelines, exclusively. For women without incident cancers (denominator A), eligible women were defined as those who entered the study with a prior breast cancer diagnosed before age 50 years or a family history consistent with increased HBOC risk (Box 1). Too few women entered the study with a prior ovarian cancer diagnosis to calculate rates in this subgroup. For women with incident cancer diagnoses (denominator B), eligible women were defined as those with any incident ovarian cancer or an incident breast cancer diagnosed before age 50 years. Linear trends in annual testing rates were evaluated using Poisson regression with calendar year fitted as a continuous variable in the model. In a sensitivity analysis, we restricted follow-up time for women diagnosed with incident cancers to one year better approximate trends in treatment-associated BRCA testing.
In cross-sectional analyses, we described women with and without incident breast and ovarian cancers who received BRCAtesting during the study period and compared characteristics of those tested in 2005–2008, 2009–2012, and 2013–2015 using χ2 and Fisher exact tests. All statistical tests were two-sided and a Pvalue of less than .05 was considered statistically significant. We performed selected chart review for evidence of BRCA testing in a stratified random sample of women identified as tested (n = 25) and untested, but eligible (n = 25) based on billing and procedures codes (Supplementary Tables 4 and 5, available online).
Results
The final cohort included 295 087 women (Table 1), who were mostly white and had a mean age of 43.6 years. About 2% had a previous breast cancer diagnosis at study entry; a small number of these were diagnosed prior to age 50 years. Family history information was available for 29.1% of the cohort. Overall, about 3.5% of the sample self-reported a family history suggestive of increased HBOC risk. A total of 3992 and 290 women, respectively, had an incident breast or ovarian cancer diagnosed during the study period. Of women with incident breast or ovarian cancer diagnoses, about 22.9% were under age 50 years and about 11.6% self-reported a family history indicating increased HBOC risk.
Table 1.
Characteristics of women at study entry, 2005–2015 (n = 295 087)
| Characteristics | Full cohort |
Incident cancer diagnosis during study period |
||
|---|---|---|---|---|
| Total | None | Breast cancer | Ovarian cancer | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Total No. | 295 087 | 290 805 | 3992 | 290 |
| Mean person-time (SD), y | 4.5 (3.8) | 4.5 (3.8) | 4.5 (3.1) | 4.3 (3.0) |
| Mean age (SD), y | 43.6 (17.9) | 43.4 (17.8) | 58.7 (12.5) | 61.6 (13.1) |
| Age group, y | ||||
| 18–29 | 79 609 (27.0) | 79 582 (27.4) | 23 (0.6) | 4 (1.4) |
| 30–49 | 105 278 (35.7) | 104 324 (35.9) | 907 (22.7) | 47 (16.2) |
| 50–69 | 84 300 (28.5) | 81 859 (28.1) | 2284 (57.2) | 160 (55.2) |
| ≥70 | 25 900 (8.8) | 25 040 (8.6) | 907 (22.7) | 47 (16.2) |
| Race/ethnicity | ||||
| Hispanic | 14 364 (4.9) | 14 106 (4.9) | 244 (6.1) | 14 (4.8) |
| White | 177 480 (60.1) | 174 052 (59.9) | 3182 (79.7) | 246 (84.8) |
| Asian | 27 230 (9.2) | 26 920 (9.3) | 293 (7.4) | 17 (5.9) |
| Black | 13 204 (4.5) | 13 068 (4.5) | 133 (3.3) | 3 (1.0) |
| Other* | 10 487 (3.6) | 10 339 (3.6) | 138 (3.5) | 10 (3.5) |
| Missing | 52 322 (17.7) | 52 320 (18.0) | 2 (0.1) | 0 (0.0) |
| Prior breast cancer | 5689 (1.9) | 5283 (1.8) | 388 (9.7) | 18 (6.2) |
| Prior breast cancer before age 50 years | 923 (0.3) | 851 (0.3) | 72 (1.8) | 0 (0.0) |
| FH indicating HBOC risk | ||||
| No | 75 616 (25.6) | 73 239 (25.2) | 2218 (55.6) | 159 (54.8) |
| Yes | 10 314 (3.5) | 9819 (3.4) | 464 (11.6) | 31 (10.7) |
| Missing | 209 157 (70.9) | 207 747 (71.4) | 1310 (32.8) | 100 (34.5) |
| Length of health plan enrollment, y | ||||
| 1–2 | 70 221 (23.8) | 70 150 (24.1) | 66 (1.7) | 5 (1.7) |
| 3–5 | 62 935 (21.3) | 62 679 (21.6) | 235 (5.9) | 21 (7.2) |
| 6–10 | 52 271 (17.7) | 51 722 (17.8) | 504 (12.6) | 45 (15.5) |
| ≥11 | 109 660 (37.2) | 106 254 (36.5) | 3187 (79.8) | 219 (75.5) |
*Includes individuals who identified as American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, and multiple race or ethnicity categories. FH = family history; HBOC = hereditary breast and ovarian cancer.
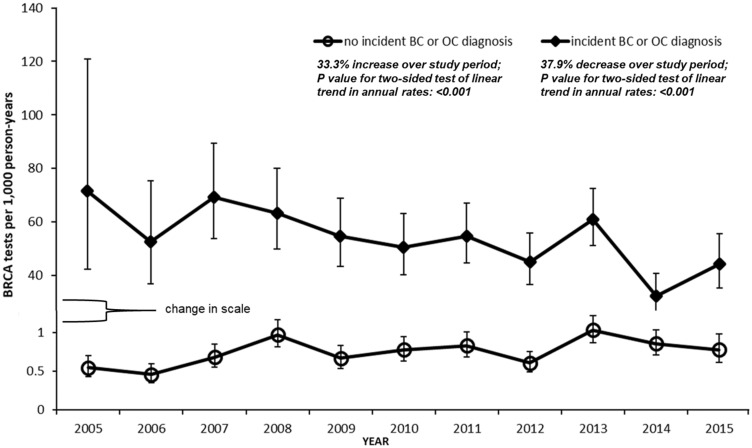
Annual testing rates steadily decreased during the study period in women with incident breast and ovarian cancers and steadily increased in women without incident cancers (Pfor both linear trend tests <.001). Specifically, annual testing rates fell 37.9% in women with incident breast and ovarian cancers, from 71.5/1000 pys in 2005 (95% CI = 42.4 to 120.8/1000 pys) to 44.4/1000 pys in 2015 (95% CI = 35.5 to 55.6/1000 pys), and grew 33.3% in women without cancer diagnoses, from 0.6/1000 pys in 2005 (95% CI = 0.4 to 0.7/1000 pys) to 0.8/1000 pys in 2015 (95% CI = 0.6 to 1.0/1000 pys) (Figure 2 ). The overall testing rate between 2005 and 2015 was about 73 times higher in women with an incident cancer diagnosis than in women without: 51.2/1000 pys (95% CI = 47.7 to 54.9/1000 pys) vs 0.7/1000 pys (95% CI = 0.7 to 0.8/1000 pys). When follow-up was restricted to one-year postdiagnosis for women with incident cancers, annual testing rates increased 268.4% over the study period (Supplementary Figure 1, available online; P for linear trend test <.001 ).
Figure 2.
Annual BRCAtesting rates in women with and without incident breast and ovarian cancer diagnoses, 2005–2015. Error bars indicate 95% confidence intervals for annual rates. BC = breast cancer; OC = ovarian cancer.
There was no evidence of secular testing trends in any of the clinically eligible subgroups (Supplementary Tables 6 and 7, available online). From 2005 to 2015, the overall BRCA testing rates in women who entered the study with a family history suggestive of increased HBOC risk or a prior breast cancer diagnosed before age 50 years were 4.5/1000 pys (95% CI = 4.1 to 5.1/1000 pys) and 18.7/1000 pys (95% CI = 15.5 to 22.5/1000 pys), respectively. Women younger than age 50 years diagnosed with an incident breast cancer had an overall testing rate of 225.2/1000 pys (95% CI = 200.9 to 252.5/1000 pys). The testing rate in women diagnosed with incident ovarian cancers was 130.9/1000 pys (95% CI = 105.7 to 162.1/1000 pys). Annual rates in women with incident diagnoses were unstable because of the small numbers of women diagnosed with breast and ovarian cancer each year. The proportion of women in each of these subgroups who remained untested at the end of the study period were as follows: 68.5% (95% CI = 65.4% to 71.5%) of women with an incident breast cancer diagnosed before age 50 years; 71.0% (95% CI = 65.4% to 76.2%) of women diagnosed with an incident ovarian cancer; 87.3% (95% CI = 84.9% to 89.5%) of women who entered the study with a prior breast cancer diagnosed before age 50 years; and 97.0% (95% CI = 96.6% to 97.3%) of women who entered the study with a family history indicating increased HBOC risk.
A total of 1761 women in the cohort received BRCA tests during the study period. Overall, 991 tests (56.3%) occurred in women without an incident cancer diagnosis. The remaining 770 tests occurred in women with incident breast or ovarian cancer diagnoses. Table 2 provides descriptive results comparing women tested without an incident breast or ovarian cancer in the beginning, middle, and end of the study period. Approximately 10.9% had a prior breast cancer diagnosed before age 50 years, although the proportion of women with this type of cancer history decreased over time. Women were almost exclusively tested through the Department of Genetic Services, but claims for BRCA tests ordered outside the health system started to appear in 2013. Most women received full sequencing and common deletion/duplication analyses, although the proportion decreased as integrated analyses (full sequencing and full duplication/deletion analyses) and hereditary cancer panels became available.
Table 2.
Characteristics of women tested without an incident breast or ovarian cancer diagnosis, by test year
| Characteristic | Overall | 2005–2008 | 2009–2012 | 2013–2015 | P * |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Total No. | 991 (100) | 322 (100) | 372 (100) | 297 (100) | |
| Mean age at testing (range), y | 51.7 (18–93) | 52.8 (18–86) | 52.1 (19–84) | 50.2 (19–93) | |
| Age group at testing, y | .11 | ||||
| 18–29 | 45 (4.5) | 12 (3.7) | 12 (3.2) | 21 (7.1) | |
| 30–49 | 338 (34.1) | 100 (31.1) | 130 (35.0) | 108 (36.4) | |
| 50–69 | 539 (54.4) | 183 (56.8) | 204 (54.8) | 152 (51.2) | |
| ≥70 | 69 (7.0) | 27 (8.4) | 26 (7.0) | 16 (5.4) | |
| Race/ethnicity | .03† | ||||
| Hispanic | 58 (5.9) | 13 (4.0) | 23 (6.2) | 22 (7.4) | |
| White | 799 (80.6) | 265 (82.3) | 298 (80.1) | 236 (79.5) | |
| Asian | 47 (4.7) | 12 (3.7) | 16 (4.3) | 19 (6.4) | |
| Black | 37 (3.7) | 11 (3.4) | 15 (4.0) | 11 (3.7) | |
| Other‡ | 33 (3.3) | 9 (2.8) | 16 (4.3) | 8 (2.7) | |
| Missing | 17 (1.7) | 12 (3.7) | 4 (1.1) | 1 (0.3) | |
| Prior breast cancer before age 50 years | 108 (10.9) | 47 (14.6) | 42 (11.3) | 19 (6.4) | .01 |
| FH indicating HBOC risk | |||||
| Yes | 464 (46.8) | 162 (50.3) | 178 (47.9) | 124 (41.8) | .86 |
| No | 294 (29.7) | 106 (32.9) | 107 (28.8) | 81 (27.3) | |
| Missing | 233 (23.5) | 54 (16.8) | 87 (23.4) | 92 (31.0) | |
| Ordering provider | <.001 | ||||
| Oncology | 22 (2.2) | 22 (6.8) | 0 (0.0) | 0 (0.0) | |
| Genetics | 936 (94.5) | 300 (93.2) | 369 (99.2) | 267 (89.9) | |
| Other internal | 4 (0.4) | 0 (0.0) | 3 (0.8) | 1 (0.3) | |
| External | 29 (2.9) | 0 (0.0) | 0 (0.0) | 29 (9.8) | |
| BRCA test type | <.001 | ||||
| Founder mutation panel | 102 (10.3) | 38 (11.8) | 47 (12.6) | 17 (5.7) | |
| Known mutation | 130 (13.1) | 43 (13.4) | 55 (14.8) | 32 (10.8) | |
| Sequencing, c. dup/del | 604 (60.9) | 232 (72.1) | 238 (64.0) | 134 (45.1) | |
| Rearrangement | 46 (4.6) | 8 (2.5) | 31 (8.3) | 7 (2.4) | |
| Sequencing, f. dup/del | 94 (9.5) | 1 (0.3) | 1 (0.3) | 92 (31.0) | |
| Hereditary cancer panel | 15 (1.5) | 0 (0.0) | 0 (0.0) | 15 (5.1) |
*Pvalue for two-sided Pearson χ2test using complete cases unless otherwise noted. c. dup/de l = common duplication/deletion; f. dup/del = full duplication/deletion; FH = family history; HBOC = hereditary breast and ovarian cancer.
†P value for two-sided Fisher exact test using complete cases.
‡Includes individuals who identified as American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, and multiple race or ethnicity categories.
Table 3 provides similar results for women tested after an incident breast or ovarian cancer diagnosis. By the end of the study period, fewer women had both an incident and a prior cancer diagnosis when tested. The proportion of women who received testing prior to their cancer surgery increased from 6.4% to 29.6% over the study period. Again, tests were primarily ordered through the Department of Genetic Services and claims for BRCAtests from external providers also began to appear in 2013. Most women received full sequencing and common deletion/duplication analysis, although this number also dropped with the introduction of integrated tests and hereditary cancer panels.
Table 3.
Characteristics of women tested after an incident breast or ovarian cancer diagnoses, by test year
| Characteristics | Overall | 2005–2008 | 2009–2012 | 2013–2015 | P * |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Total No. | 770 (100) | 171 (100) | 329 (100) | 270 (100) | |
| Mean age at test (range), y | 54.8 (24–92) | 53.3 (29–86) | 54.0 (24–92) | 56.8 (30–91) | |
| Age group at test, y | .02† | ||||
| 18–29 | 4 (0.5) | 1 (0.6) | 3 (0.9) | 0 (0.0) | |
| 30–49 | 277 (36.0) | 65 (38.0) | 133 (40.4) | 79 (29.3) | |
| 50–69 | 409 (53.1) | 93 (54.4) | 162 (49.2) | 154 (57.0) | |
| ≥70 | 80 (10.4) | 12 (7.0) | 31 (9.4) | 37 (13.7) | |
| Race/ethnicity | .21† | ||||
| Hispanic | 58 (7.5) | 8 (4.7) | 32 (9.7) | 18 (6.7) | |
| White | 618 (80.3) | 146 (85.4) | 259 (78.7) | 213 (78.9) | |
| Asian | 46 (6.0) | 10 (5.9) | 14 (4.3) | 22 (8.2) | |
| Black | 16 (2.1) | 3 (1.8) | 7 (2.1) | 6 (2.2) | |
| Other‡ | 32 (4.2) | 4 (2.3) | 17 (5.2) | 11 (4.1) | |
| Incident breast cancer before age 50 | 292 (37.9) | 67 (39.2) | 141 (42.9) | 84 (31.1) | .01 |
| Incident ovarian cancer | 81 (10.5) | 18 (10.5) | 33 (10.0) | 30 (11.1) | .91 |
| Prior breast cancer before age 50 | 98 (12.7) | 31 (18.1) | 47 (14.3) | 20 (7.4) | .01 |
| FH indicating HBOC risk | |||||
| Yes | 241 (31.1) | 64 (37.4) | 91 (27.7) | 86 (31.9) | .09 |
| No | 458 (59.5) | 98 (57.3) | 205 (62.3) | 155 (57.4) | |
| Missing | 71 (9.2) | 9 (5.3) | 33 (10.0) | 29 (10.7) | |
| Tested prior to cancer surgery | <.001† | ||||
| Yes | 177 (23.0) | 11 (6.4) | 86 (26.1) | 80 (29.6) | |
| No | 486 (63.1) | 120 (70.2) | 209 (63.5) | 157 (58.2) | |
| Same day | 2 (0.3) | 0 (0.0) | 1 (0.3) | 1 (0.4) | |
| No surgery | 80 (10.4) | 18 (10.5) | 33 (10.0) | 29 (10.7) | |
| Missing | 25 (3.3) | 22 (12.9) | 0 (0.0) | 3 (1.1) | |
| Ordering provider | <.001 | ||||
| Oncology | 20 (2.6) | 16 (9.4) | 2 (0.6) | 2 (0.7) | |
| Genetics | 706 (91.7) | 154 (90.1) | 324 (98.5) | 228 (84.4) | |
| Other internal | 3 (0.4) | 1 (0.6) | 2 (0.6) | 0 (0.0) | |
| External | 32 (4.2) | 0 (0.0) | 0 (0.0) | 32 (11.9) | |
| Unknown | 9 (1.2) | 0 (0.0) | 1 (0.3) | 8 (3.0) | |
| BRCA test type | <.001 | ||||
| Founder mutation panel | 39 (5.1) | 15 (8.8) | 20 (6.7) | 4 (1.5) | |
| Known mutation | 9 (1.2) | 1 (0.6) | 6 (2.0) | 2 (0.7) | |
| Sequencing, c. dup/del | 569 (76.9) | 152 (88.9) | 284 (85.0) | 163 (60.4) | |
| Rearrangement | 25 (3.2) | 3 (1.8) | 19 (6.4) | 3 (1.1) | |
| Sequencing, f. dup/del | 83 (11.2) | 0 (0.0) | 0 (0.0) | 83 (30.7) | |
| Hereditary cancer panel | 15 (1.9) | 0 (0.0) | 0 (0.0) | 15 (5.6) |
*Pvalue for two-sided Pearson χ2test using complete cases unless otherwise noted. c. dup/del = common duplication/deletion; f. dup/del = full duplication/deletion; FH = family history; HBOC = hereditary breast and ovarian cancer.
†P value for two-sided Fisher exact test using complete cases.
‡Includes individuals who identified as American Indian, Alaskan Native, Native Hawaiian, Pacific Islander, and multiple race or ethnicity categories.
Discussion
The utilization patterns we observed in this setting align with prior work demonstrating that BRCAtesting is increasing among insured women without new “test-triggering” cancer diagnoses (13,26). They also confirm gaps in testing eligible cancer survivors in the years following diagnosis—despite increasing rates of treatment-associated testing—suggesting a need to integrate discussion of hereditary risk into survivorship care (15,27). Throughout the study period, more women who received BRCA testing did so without an incident breast or ovarian cancer diagnosis. This pattern is opposite that observed in national studies, where prior to 2012, BRCA testing was primarily occurring in women with new cancers. Early leadership from KPWA providers aware of guidelines recommending testing for asymptomatic women (7,8) likely accounts for these differences. The proportion of women tested before receiving multiple cancer diagnoses and prior to their first-line treatment also increased during the study, indicating progress in providing cancer patients timely access to genetic services in this setting.
Previous studies of BRCA utilization have been limited in their ability to examine trends in clinically eligible denominator populations, particularly those eligible for testing based on family history alone. To address this gap, we used a combination of SEER registry data and self-reported family history data collected through breast imaging facilities to examine BRCA testing rates within four eligible subgroups. We found that overall testing rates during the study period were highest in women diagnosed with incident breast cancers before age 50 years, followed by women diagnosed with incident ovarian cancers. However, a substantial proportion of women with these clinical features remained untested at the end of the study period. Testing rates were even lower among women who entered the cohort with a prior breast cancer diagnosed before age 50 years and with a family history consistent with increased HBOC risk, with the majority of these women never receiving BRCA testing.
These findings reinforce what has become a familiar refrain: despite increasing BRCA testing rates, many eligible women are not receiving testing (28,29). That so few women with a family history indicating increased HBOC risk, but no personal cancer history, underwent testing in a setting with supportive policies and a history of testing asymptomatic women, specifically, is striking and an important contribution to the understanding of BRCAtest delivery. To contextualize findings in this system, a recent population-based study of breast cancer patients in Georgia and Los Angeles County reported that 53% of high-risk women received genetic testing (14). National Health Interview Survey data from 2005 to 2015 indicate lower testing rates: about 15% and 11% of eligible breast and ovarian cancer survivors, respectively (30). Efforts to identify and intervene on drivers of inappropriate variation in testing rates between regions, delivery settings, and clinical subpopulations are now needed.
Women began receiving hereditary cancer panels in 2012. Panel tests, which sequence multiple risk genes at one time, provide more information about inherited cancer risk than BRCA testing alone, but also lead to uncertain findings without clear implications for risk management (31). These tests appropriately accounted for a small proportion of all BRCA testing. Despite guidelines stating that eligible women should be referred to the Department of Genetic Services or Medical Oncology, women began receiving BRCA tests from providers outside of the health system in 2013. Growing demand may have led women to pursue testing through alternative clinical pathways, perhaps because of utilization controls or increasing wait times. We did not identify women referred to the Department of Genetic Services or who were deemed ineligible or were uninterested in pursuing testing. Given that genetic counseling and testing are preference-sensitive services, these quality indicators should be examined in future work.
Our study has several limitations. We were unable to identify women who received testing as members of other health plans. This may have led us to include previously tested women in our cohort, particularly women who entered the study in later years. Still, given that most women were long-term health plan enrollees and became newly eligible for testing because of a cancer diagnosis that occurred during the study, it is unlikely that this accounts for the low rates we observed. We also could not identify self-pay tests unless they were ordered by a health system provider, although testing was continuously covered for eligible women during the study period. Commercial companies offering testing through independent physicians were also uncommon and 23andMe stopped providing information about BRCA1 and BRCA2in 2013 based on pushback from the Food and Drug Administration, which only recently revised its stance (32). Finally, we had substantial missing data on family history and did not examine whether family history information was present in the electronic health record or used clinically during the study time frame. Despite these limitations, our study makes use of longitudinal data collected within a large cohort, detailed, self-reported family cancer history data collected through a well-established research consortium and robust ascertainment of BRCAtest use from procedure and billing data to fill important knowledge gaps about genetic test delivery.
During a period of dramatic national BRCA test expansion (13), a substantial proportion of clinically eligible women within an integrated health system did not receive testing, despite access and coverage of genetic service. Women eligible based on their family history alone had particularly low testing rates with little evidence of improvement over time. Pursing population-based delivery of hereditary cancer genetic services— increasingly the recommended approach—will require substantial resources to reach the estimated 1.2 million affected and 10.7 million unaffected women eligible for testing in the United States (33). These efforts must include workforce development and enhanced family history documentation as well as exploration of novel service delivery models and quality metrics (27,34,35). How to best deliver cancer genetic services, particularly in resource-limited settings, is a pressing issue for genomic medicine and cancer prevention and control.
Funding
This work was supported by the Agency for Healthcare Research and Quality (K12HS022982) and the National Institutes of Health (T32AG027677, P01CA154292, HHSN261201100031C, and U54CA163303).
Notes
Affiliations of authors: Department of Health Services, University of Washington School of Public Health, Seattle, WA (SK); Kaiser Permanente Washington Health Research Institute, Seattle, WA (SK, EJAB, DSMB, HG, KJW); Department of Genetic Services, Kaiser Permanente Washington, Seattle, WA (KAL).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors have no conflicts of interest.
Supplementary Material
References
- 1. Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;91:321–345. [DOI] [PubMed] [Google Scholar]
- 2. Norquist BM, Harrell MI, Brady MF et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;24:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;2511:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antoniou A, Pharoah PD, Narod S et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;725:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuchenbaecker KB, Hopper JL, Barnes DR et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;31723:2402–2416. [DOI] [PubMed] [Google Scholar]
- 6. Williams-Jones B. History of a gene patent: tracing the development and application of commercial BRCA testing. Health Law J. 2002;10:123–146. [PubMed] [Google Scholar]
- 7. U.S. Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;1604:271–281. [DOI] [PubMed] [Google Scholar]
- 8. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;1435:355–361. [DOI] [PubMed] [Google Scholar]
- 9. Daly MB, Pilarski R, Berry M et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;151:9–20. [DOI] [PubMed] [Google Scholar]
- 10. Robson ME, Bradbury AR, Arun B et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;3331:3660–3667. [DOI] [PubMed] [Google Scholar]
- 11. Kolor K, Chen Z, Grosse SD et al. BRCA genetic testing and receipt of preventive interventions among women aged 18–64 years with employer-sponsored health insurance in nonmetropolitan and metropolitan areas—United States, 2009–2014. MMWR Surveill Summ. 2017;6615:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg SM, Ruddy KJ, Tamimi RM et al. BRCA1 and BRCA2 mutation testing in young women with breast cancer. JAMA Oncol. 2016;26:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Z, Kolor K, Grosse SD et al. Trends in utilization and costs of BRCA testing among women aged 18–64 years in the United States, 2003–2014 Genet Med. 2018;204:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurian AW, Griffith KA, Hamilton AS et al. Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA. 2017;3175:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kehl KL, Shen C, Litton JK et al. Rates of BRCA1/2 mutation testing among young survivors of breast cancer. Breast Cancer Res Treat. 2016;1551:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Beattie MS, Ponce NA et al. Eligibility criteria in private and public coverage policies for BRCA genetic testing and genetic counseling. Genet Med. 2011;1312:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaiser Permanente Washington. Breast Cancer Risk Assessment and Screening Guideline. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/breast.pdf. Accessed September 18, 2018.
- 18. Pocobelli G, Chubak J, Hanson N et al. Prophylactic oophorectomy rates in relation to a guideline update on referral to genetic counseling. Gynecol Oncol. 2012;1262:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Surveillance Epidemiology and End Results Program. Seattle-Puget Sound Registry. https://seer.cancer.gov/registries/sps.html. Accessed September 18, 2018.
- 20. Ross TR, Ng D, Brown JS et al. The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Wash DC). 2014;21:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch J, Berse B. Methods to identify BRCA testing in claims data. Am J Obstet Gynecol. 2016;2151:133–134. [DOI] [PubMed] [Google Scholar]
- 22. Lynch JA, Berse B, Dotson WD et al. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017;198:890–899. [DOI] [PubMed] [Google Scholar]
- 23. Taplin SH, Thompson RS, Schnitzer F et al. Revisions in the risk-based breast cancer screening program at group health cooperative. Cancer. 1990;664:812–818. [DOI] [PubMed] [Google Scholar]
- 24. Taplin SH, Ichikawa L, Buist DS et al. Evaluating organized breast cancer screening implementation: the prevention of late-stage disease? Cancer Epidemiol Biomarkers Prev. 2004;132:225–234. [DOI] [PubMed] [Google Scholar]
- 25. Phipps AI, Buist DS. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;163:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo F, Hirth JM, Lin YL et al. Use of BRCA mutation test in the U.S., 2004–2014. Am J Prev Med. 2017;526:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samimi G, Bernardini MQ, Brody LC et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;3520:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellcross CA, Peipins LA, McCarty FA et al. Characteristics associated with genetic counseling referral and BRCA1/2 testing among women in a large integrated health system. Genet Med. 2015;171:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarthy AM, Bristol M, Fredricks T et al. Are physician recommendations for BRCA1/2 testing in patients with breast cancer appropriate? A population-based study. Cancer. 2013;11920:3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Childers CP, Childers KK, Maggard-Gibbons M et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;3534:3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurian AW, Ford JM. Multigene panel testing in oncology practice: how should we respond? JAMA Oncol. 2015;13:277–278. [DOI] [PubMed] [Google Scholar]
- 32. Annas GJ, Elias S. 23andMe and the FDA. N Engl J Med. 2014;37011:985–988. [DOI] [PubMed] [Google Scholar]
- 33. Hughes KS. Genetic testing: what problem are we trying to solve? J Clin Oncol. 2017;3534:3789–3791. [DOI] [PubMed] [Google Scholar]
- 34. Wood ME, Kadlubek P, Pham TH et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;328:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. Am J Med Genet C Genet. 2018;1781:24–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.