Abstract
Background
Cervical cancer is caused by persistent human papillomavirus (HPV) infection. US consensus management guidelines for a positive cervical screening result typically focus on the current screening result only. A negative testing history may alter risk of the following positive screening results, caused by a new HPV infection, and therefore its optimal management.
Methods
Women ages 30 years and older were screened with triennial HPV and cytology co-testing at Kaiser Permanente Northern California from 2003 to 2014. We estimated the subsequent 5-year risks of cervical intraepithelial neoplasia grade 3 or more severe diagnoses (CIN3+) in a cohort of 1 156 387 women following abnormal (atypical squamous cells of undetermined significance [ASC-US] or worse) cytology and/or positive HPV testing, when the test result followed 0 (n = 990 013), 1 (n = 543 986), 2 (n = 245 974), or 3 (n = 79 946) consecutive negative co-test(s). All statistical tests were two-sided.
Results
Following 0–3 successive negative co-tests, 5-year CIN3+ risks following a positive HPV test decreased progressively from 7.2% (95% CI = 7.0% to 7.4%) to 1.5% (95% CI = 0.7% to 3.4%) (Ptrend < .001). Similarly, risks following an abnormal (ASC-US or worse) cytology result decreased from 6.6% (95% CI = 6.4% to 6.9%) to 1.1% (95% CI = 0.5% to 2.3%) (Ptrend < .001). Risks following low-grade squamous intraepithelial lesion, the risk threshold for referral to colposcopy in the United States, decreased from 5.2% (95% CI = 4.7% to 5.7%) to 0.9% (95% CI = 0.2% to 4.3%). Risks following high-grade squamous intraepithelial lesion or more severe, a specific marker for the presence of precancerous lesions, decreased from 50.0% (95% CI = 47.5% to 52.5%) to 10.0% (95% CI = 2.6% to 34.4%).
Conclusions
Following one or more sequential antecedent, documented negative co-tests or HPV tests, women with HPV-positive ASC-US or low-grade squamous intraepithelial lesion might have sufficiently low CIN3+ risk that they do not need colposcopy referral and might instead undergo 6–12-month surveillance for evidence of higher risk before being referred to colposcopy.
Persistent human papillomavirus (HPV) infection is the cause of virtually all cervical cancer (1). New HPV infections are typically benign and usually clear, whereas failure to clear indicates increasing risk of progression to precancer (ie, cervical intraepithelial neoplasia grade 3 [CIN3] and adenocarcinoma in situ [AIS]) (2,3) and eventual cancer. Thus, the risk posed by a positive HPV test and related cytological abnormalities varies depending on how long the HPV infection has persisted, with much lower risks for newer infections (2,3). However, guidelines for the management of cervical screening abnormalities focus on current results, perhaps because past screening history is often unknown. In addition, because HPV-based screening is relatively new, there are extremely few long-term experiences in the United States to guide recommendations regarding its use in a longitudinal program of repeated screening rounds. Data from Kaiser Permanente Northern California (KPNC), which in 2003 initiated HPV and cytology co-testing every 3 years, now permit an examination of risks over multiple screening rounds.
Given the unique, long-term KPNC experience, we previously demonstrated that preceding negative screens augment the reassurance (further decrease the cancer risk) following a negative HPV test or HPV/cytology co-test (4). Here, we present for the first time the complementary analysis, by considering the impact of multiple negative co-tests (both HPV and cytology negative) on the risks of precancer following a newly positive screen, and the implications for clinical management that will be important to the ongoing revision of US consensus guidelines.
Methods
Population
From January 1, 2003 to December 31, 2014, a cohort of 1 156 387 women ages 30 years and older underwent HPV and cytology co-testing at KPNC (5). For each woman, the first available co-test in this study period was designated as her “baseline.” Cervical histopathology outcomes were collected for women through December 31, 2015. The KPNC institutional review board, National Institutes of Health Office of Human Subjects Research, and Albert Einstein College of Medicine institutional review board approved the use of these data without patient informed consent.
Screening and Clinical Management
Women ages 30 years and older were screened by triennial co-testing as previously described (6). Two cervical specimens from each woman undergoing co-testing were collected, the first for Pap testing and the second for high-risk HPV testing using Hybrid Capture 2 (Qiagen, Germantown, MD). Prior to 2009, conventional Pap slides underwent manual review that incorporated the BD Focal Point Slide Profiler (BD Diagnostics, Burlington, NC, USA). Starting in 2009, KPNC transitioned from conventional to liquid-based Pap using BD SurePath (BD Diagnostics, Burlington, NC). Cytological interpretations were based on the Bethesda System (7).
Screen-positive women were managed according to internal Kaiser guidelines, which were similar to US national guidelines at the time, in which women ages 30 years and older with definite cytological abnormalities (HPV-positive atypical squamous cells of undetermined significance [ASC-US], low-grade squamous intraepithelial lesion [LSIL], or more severe cytological abnormalities) were referred to colposcopy (8–12). Women with HPV-positive NILM (negative for intraepithelial lesion or malignancy) co-test results at KPNC were followed annually with co-testing and were referred to colposcopy if they had cytological abnormalities (from 2003 onward) or second HPV-positive NILM (from 2006 onward) on next co-test. A minority of younger women under the age of 40 years diagnosed with CIN2 chose follow-up with repeated colposcopy rather than immediate treatment (9,11).
Statistical Analyses
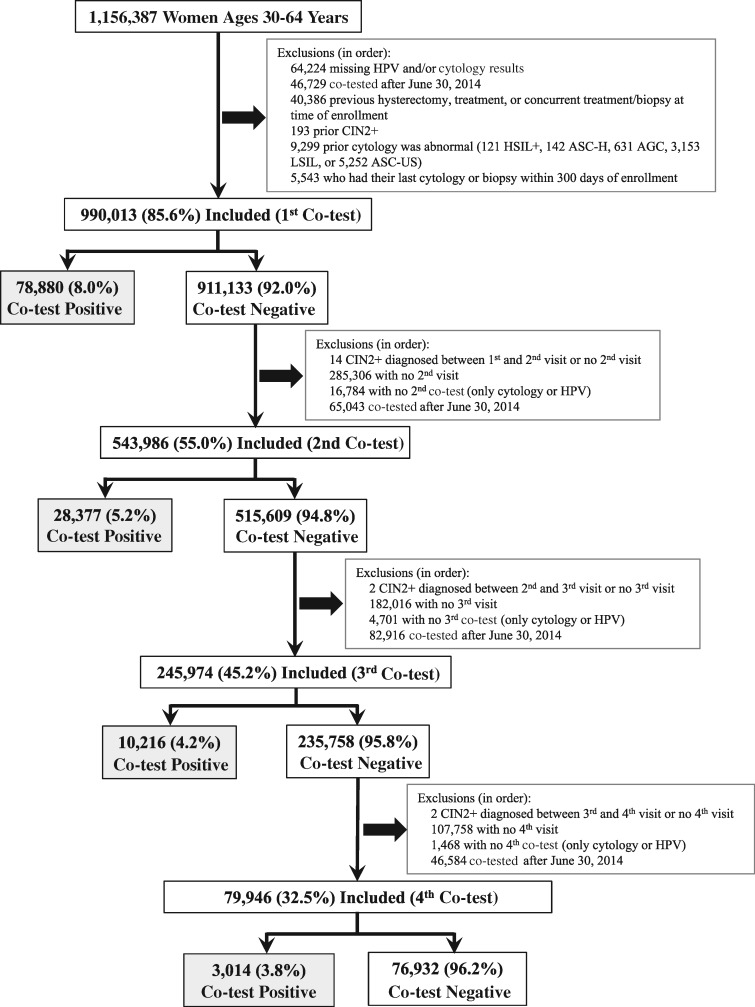
A diagram of the inclusions and exclusions by round of screening is shown in Figure 1. For each round, screen-positive women, those testing HPV positive and/or whose cytology was equivocal or definitively positive (ASC-US or more severe [ASC-US+]), were included in these analyses. Percentages for all combinations of positive HPV and/or ASC-US+ cytology results for the first, second, third, and fourth co-test following 0, 1, 2, and 3 consecutive negative co-test(s), respectively, were calculated with binomial exact 95% confidence intervals (95% CI). Analyses were restricted to women ages 30–64 years because routine screening is discontinued in women ages 65 years and older following a negative screening history.
Figure 1.
A diagram of the inclusions/exclusions by round of screening. A positive co-test was a positive HPV test and/or a positive cytology (ASC-US or more severe cytology) and a negative co-test was a negative HPV test and negative cytology. The gray-shaded boxes indicate the study groups of the first, second, third, and fourth positive co-tests following 0–3 consecutive negative co-tests, respectively. AGC = atypical glandular cells; ASC-H = atypical squamous cells, cannot rule out HSIL; ASC-US = atypical squamous cells of undetermined significance; CIN2 = cervical intraepithelial neoplasia grade 2 or more severe diagnoses (CIN2+); HPV = human papillomavirus; HSIL+ = high-grade squamous intraepithelial lesion (HSIL) or more severe; LSIL = low-grade squamous intraepithelial lesion.
Five-year cumulative detection (“risk”) of CIN3+ (5-year CIN3+ risk) was used as a surrogate for cancer risk. CIN3 and AIS are the immediate, most severe precursors to squamous cell carcinoma and adenocarcinoma, respectively, and a sizable minority of CIN3 (13) and even higher proportion of AIS will develop into cancer if untreated. The primary goal of cervical screening is to detect and treat CIN3 and AIS to prevent cervical cancer. Some analyses also included 5-year risk of invasive cervical cancer but only for three co-testing rounds because of few screen positives and no cancers following the fourth co-test. A 5-year follow-up rather than a shorter interval was used to account for differential follow-up (referral to colposcopy vs surveillance), late returns for visits, and imperfect disease ascertainment at any single screen. Three-year CIN3+ risks were also estimated to confirm the findings, as they are concordant with the per-protocol KPNC screening interval.
A logistic regression model for prevalent disease and a Weibull survival model for incident disease were combined (logistic-Weibull prevalence-incidence model) for risk estimation and 95% CI as previously described (14). A fuller explanation of our choice to use a logistic-Weibull model and our statistical approach are presented in the Supplementary Methods (available online).
Five-year CIN3+ risks were calculated for positive co-tests, HPV positive and/or ASC-US+, for the first, second, third, and fourth co-test following zero, one, two, and three consecutive negative co-tests, respectively. We also stratified risks using broad age groups, (30–39 years, 40–49 years, and 50 years and older) to explore whether there were age-modifying effects on these risks or that aging of the cohort itself explained the changes in risk by round of screening.
Diagnostic yield of CIN3+ with binomial exact 95% CI for each round was calculated as a simple measure of screening efficiency that takes into account the fraction of women who screen positive and, among those who screen positive, the fraction (risk) of those with CIN3+ diagnoses. Risk ratios of CIN2 to CIN3+ were also calculated because CIN2 is an equivocal diagnosis of precancer that is highly regressive (15). CIN2 is typically treated for safety, the benefits of which are uncertain because its treatment may represent overtreatment and therefore may contribute to the harms of screening. Treatment has been linked to negative reproductive outcomes including pre-term delivery (16,17). Variance for the risk ratios was estimated using the standard delta method to calculate the 95% CI while conservatively assuming independence between risk estimates for CIN2 and CIN3+ (18). However, for most part, CIN2 and CIN3+ are caused by the same HPV genotypes, and risk factors for CIN2 are similar to those for CIN3+.
Statistical analyses were done using R version 3.3. P values were two-sided. No P value was designated as a cut point for statistical significance due to possibility of false positive results from multiple comparisons.
Results
There were 990 013, 543 986, 245 974, and 79 946 women who underwent screening following 0–3 sequential preceding negative co-tests, respectively. Available demographics for the cohort for each round of co-testing are shown in Table 1. The logical aging of women followed longitudinally was evident. Those followed through many rounds were more likely to be white or Asian, and less likely to have unknown race. Screen-positive women who had follow-up tended to be older on average (mean) than those who did not for the first co-test (40.0 vs 39.2 years, respectively, P < .001) and second co-test (44.1 vs 42.9 years, respectively, P < .001) but not for the third co-test (47.1 vs 47.1 years, respectively, P = .99) or the fourth co-test (49.7 vs. 49.5 years, respectively, P = .40). The distribution of race/ethnicity differed by follow-up status (Supplementary Table 1, available online), especially for those whose race/ethnicity data were missing in the early rounds of co-testing. However, among those with non-missing race/ethnicity data, there were no meaningful differences in the distribution of race/ethnicity by follow-up status.
Table 1.
Demographics of the cohort at the first, second, third, and fourth co-test following 0–3 consecutive negative co-test(s), respectively
| Category | 1st co-test, No. (%) | 2nd co-test, No. (%) | 3rd co-test, No. (%) | 4th co-test, No. (%) |
|---|---|---|---|---|
| Age at co-test, y | ||||
| Mean | 43.9 | 47.7 | 50.1 | 52.2 |
| Median (IQR) | 42.0 (37.0 to 52.0) | 47.0 (40.0 to 55.0) | 50.0 (43.0 to 58.0) | 52.0 (46.0-59.0) |
| Age group at co-test, y | ||||
| 30–39 | 394 374 (39.8) | 124 114 (22.8) | 38 946 (15.8) | 3345 (4.2) |
| 40–49 | 293 357 (29.6) | 172 343 (31.7) | 82 732 (33.6) | 26 835 (33.6) |
| ≥50 | 302 282 (30.5) | 247 529 (45.5) | 124 296 (50.5) | 49 766 (62.2) |
| Race/ethnicity | ||||
| White | 371 676 (37.5) | 234 099 (43.0) | 114 106 (46.4) | 40 153 (50.2) |
| Missing* | 274 989 (27.8) | 102 946 (18.9) | 38 125 (15.5) | 11 450 (14.3) |
| Asian/Pacific Islander | 145 528 (14.7) | 90 188 (16.6) | 41 162 (16.7) | 12 453 (15.6) |
| Hispanic | 110 480 (11.2) | 65 012 (12.0) | 29 801 (12.1) | 9085 (11.4) |
| Black/African American | 58 040 (5.9) | 35 037 (6.4) | 15 393 (6.3) | 4522 (5.7) |
| Other† | 15 160 (1.5) | 8465 (1.6) | 3742 (1.5) | 1160 (1.5) |
| Unknown‡ | 10 772 (1.1) | 6318 (1.2) | 2814 (1.1) | 896 (1.1) |
| Native American/Aleutian/Eskimo | 3368 (0.3) | 1921 (0.4) | 831 (0.3) | 227 (0.28) |
No data on race/ethnicity were available. IQR = interquartile range.
Other races/ethnicity in small numbers.
Race/ethnicity reported as unknown.
Table 2 shows the decreasing likelihood of testing HPV positive, having ASC-US+ (abnormal) cytology, and specific combinations of testing HPV positive and/or having abnormal cytology results with successive rounds of co-testing following consecutive negative co-tests. Percentages of women who met the criteria for being referred to colposcopy (11) (HPV-positive ASC-US, LSIL, or more severe cytological interpretations) decreased with successive co-tests from 2.9% (95% CI = 2.8% to 2.9%) on the first co-test to 1.4% (95% CI = 1.3% to 1.5%) on the fourth co-test (Ptrend < .001). Likewise, percentages of women who tested HPV positive decreased with successful co-tests from 5.9% (95% CI = 5.9% to 6.0%) on the first co-test to 2.3% (95% CI = 2.2% to 2.4%) on the fourth co-test (Ptrend < .001). Percentages of women who had ASC-US+ cytology decreased with successful co-tests from 4.5% (95% CI = 4.5% to 4.6%) on the first co-test to 2.6% (95% CI = 2.5% to 2.7%) on the fourth co-test (Ptrend < .001).
Table 2.
Percentages for all combinations of positive human papillomavirus (HPV) and/or cytology co-testing results for the first, second, third, and fourth co-test following 0–3 consecutive negative co-test(s), respectively*
| Co-testing result | 1st co-test, % (95% CI) | 2nd co-test, % (95% CI) | 3rd co-test, % (95% CI) | 4th co-test, % (95% CI) | P trend |
|---|---|---|---|---|---|
| (n = 990 013) | (n = 543 986) | (n = 245 974) | (n = 79 946) | ||
| Co-test positive (HPV positive and/or ASC-US+) | 8.0 (7.9 to 8.0) | 5.2 (5.2 to 5.3) | 4.2 (4.1 to 4.2) | 3.8 (3.6 to 3.9) | <.001 |
| Immediate colposcopic referral† | 2.9 (2.8 to 2.9) | 1.7 (1.7 to 1.8) | 1.5 (1.5 to 1.6) | 1.4 (1.3 to 1.5) | <.001 |
| HPV-positive‡ | 5.9 (5.9 to 6.0) | 3.1 (3.1 to 3.2) | 2.6 (2.5 to 2.6) | 2.3 (2.2 to 2.4) | <.001 |
| ASC-US+§ | 4.5 (4.5 to 4.6) | 3.4 (3.4 to 3.5) | 2.8 (2.7 to 2.8) | 2.6 (2.5 to 2.7) | <.001 |
| HPV-positive ASC-US+ | 2.5 (2.4 to 2.5) | 1.4 (1.3 to 1.4) | 1.2 (1.1 to 1.2) | 1.1 (1.0 to 1.2) | <.001 |
| HPV-positive NILM | 3.4 (3.4 to 3.5) | 1.7 (1.6 to 1.7) | 1.4 (1.3 to 1.4) | 1.2 (1.1 to 1.3) | <.001 |
| HPV-negative ASC-US+ | 2.1 (2.0 to 2.1) | 2.1 (2.1 to 2.1) | 1.6 (1.5 to 1.6) | 1.5 (1.4 to 1.6) | <.001 |
| HPV-positive ASC-US | 1.2 (1.2 to 1.2) | 0.7 (0.7 to 0.7) | 0.6 (0.5 to 0.6) | 0.5 (0.5 to 0.6) | <.001 |
| HPV-positive LSIL | 0.9 (0.9 to 0.9) | 0.5 (0.5 to 0.6) | 0.5 (0.5 to 0.5) | 0.5 (0.4 to 0.5) | <.001 |
| HPV-positive ASC-H | 0.1 (0.1 to 0.1) | 0.05 (0.04 to 0.06) | 0.04 (0.04 to 0.05) | 0.04 (0.03 to 0.06) | <.001 |
| HPV-positive AGC | 0.05 (0.05 to 0.06) | 0.02 (0.01 to 0.02) | 0.02 (0.01 to 0.03) | 0.01 (0.00 to 0.01) | <.001 |
| HPV-positive HSIL+ | 0.2 (0.2 to 0.2) | 0.04 (0.04 to 0.05) | 0.03 (0.02 to 0.04) | 0.02 (0.01 to 0.04) | <.001 |
| HPV-negative ASC-US | 1.7 (1.6 to 1.7) | 1.7 (1.7 to 1.7) | 1.2 (1.2 to 1.3) | 1.2 (1.1 to 1.3) | <.001 |
| HPV-negative LSIL | 0.2 (0.2 to 0.2) | 0.1 (0.1 to 0.2) | 0.1 (0.1 to 0.2) | 0.1 (0.1 to 0.1) | <.001 |
| HPV-negative ASC-H | 0.05 (0.05 to 0.06) | 0.07 (0.06 to 0.07) | 0.05 (0.04 to 0.06) | 0.05 (0.04-0.07) | .48 |
| HPV-negative AGC | 0.1 (0.1 to 0.2) | 0.2 (0.2 to 0.2) | 0.1 (0.1 to 0.2) | 0.2 (0.1 to 0.2) | .23 |
| HPV-negative HSIL+ | 0.01 (0.01 to 0.01) | 0.01 (0.00 to 0.01) | 0.01 (0.00 to 0.01) | 0.01 (0.00 to 0.01) | <.001 |
Positive cytology was atypical squamous cells of undetermined significance (ASC-US) or more severe cytological interpretations (ASC-US+). AGC = atypical glandular cells; ASC-H = atypical squamous cells, cannot rule out HSIL); HSIL+ = high-grade squamous intraepithelial lesion or more severe; LSIL = low-grade squamous intraepithelial lesion; NILM = negative for intraepithelial lesion or malignancy (cytology negative); CI = confidence interval.
HPV-positive ASC-US, LSIL cytology, or more severe cytological interpretations, all of which are recommended for immediate referral to colposcopy for women ages 30 and older.
HPV positive regardless of the cytology result.
Cytology positive (ASC-US+) regardless of the HPV result.
Table 3 reports the main findings, ie, the change in clinical significance of positive screening results by past history of previous negative screenings. Following 0–3 successive negative co-tests, 5-year CIN3+ risks following a positive HPV test decreased progressively from 7.2% (95% CI = 7.0% to 7.4%) to 1.5% (95% CI = 0.7% to 3.4%) (Ptrend < .001). Similarly, risks following an abnormal (ASC-US+) cytology result decreased from 6.6% (95% CI = 6.4% to 6.9%) to 1.1% (95% CI = 0.5% to 2.3%) (Ptrend < .001).
Table 3.
Five-year cumulative detection (risks) for CIN3, AIS, or cancer (CIN3+) and for cervical cancer for human papillomavirus (HPV)-positive or cytology-positive (atypical squamous cells of undetermined significance or more severe [ASC-US+]) results for the first, second, third, and fourth co-test following 0–3 consecutive negative co-test(s), respectively
| Co-test result | Co-test No.† | Total, No. | CIN3+ |
Cervical cancer* |
||
|---|---|---|---|---|---|---|
| No. | 5-year risk‡, % (95% CI) | No. | 5-year risk‡, % (95% CI) | |||
| HPV positive§ | 1 | 58 446 | 3379 | 7.2 (7.0 to 7.4) | 245 | 0.5 (0.4 to 0.6) |
| HPV positive§ | 2 | 16 972 | 395 | 3.0 (2.7 to 3.4) | 19 | 0.1 (0.09 to 0.2) |
| HPV positive§ | 3 | 6290 | 98 | 2.3 (1.9 to 2.9) | 3 | 0.05 (0.04 to 0.1) |
| HPV positive§ | 4 | 1803 | 11 | 1.5 (0.7 to 3.4) | — | — |
| ASC-US+‖ | 1 | 44 861 | 2480 | 6.6 (6.4 to 6.9) | 199 | 0.5 (0.4 to 0.6) |
| ASC-US+‖ | 2 | 18 765 | 310 | 2.0 (1.8 to 2.3) | 17 | 0.1 (0.07 to 0.2) |
| ASC-US+‖ | 3 | 6765 | 72 | 1.5 (1.2 to 1.9) | 2 | 0.03 (0.01 to 0.1) |
| ASC-US+‖ | 4 | 2073 | 11 | 1.1 (0.5 to 2.3) | — | — |
Estimates of cervical cancer risk for the fourth co-test were not included because of small numbers of women overall and no cervical cancers were diagnosed. ASC-US+ = atypical squamous cells of undetermined significance or more severe cytological interpretations.
The co-test was preceded by the co-test number-1 of negative co-tests.
Risk estimates for HPV positive, ASC-US+, and HPV-negative ASC-US+ were obtained by estimating risks within subpopulations grouped by screening protocols. Point estimates are a weighted average of subpopulation risks, weighted by the frequency in which they occur. Confidence intervals are derived by using survey methodology approaches to combine the variance estimates of the subpopulation risks.
HPV positive regardless of the cytology result.
Cytology positive regardless of the HPV result.
Five-year CIN3+ risks for selected HPV and cytology results are shown in Table 4. Five-year CIN3+ risks decreased with subsequent rounds of co-testing (Ptrend < .001) for each of those results. For the two most common results leading to referral to colposcopy, LSIL and HPV-positive ASC-US cytology, similar decreases were observed. Five-year CIN3+ risks for LSIL cytology decreased from 5.2% (95% CI = 4.7% to 5.7%) for the first co-test to 0.9% (95% CI = 0.2% to 4.3%) on the fourth co-test. Five-year CIN3+ risks for HPV-positive ASC-US cytology decreased from 6.6% (95% CI = 6.1% to 7.1%) for the first co-test to 2.8% (95% CI = 1.0% to 8.1%) on the fourth co-test.
Table 4.
Five-year cumulative detection (risks) for CIN3, AIS, or cancer (CIN3+) for most important specific combinations of HPV-positive and/or cytology-positive results (atypical squamous cells of undetermined significance or more severe [ASC-US+]) by round of screening*
| Co-test result | Co-test No. † | Total No. | CIN3+ |
|
|---|---|---|---|---|
| No. | 5-year risk‡, % (95% CI) | |||
| HPV-positive NILM | 1 | 34 019 | 1025 | 3.9 (3.7 to 4.2) |
| HPV-positive NILM | 2 | 9612 | 120 | 1.8 (1.5 to 2.2) |
| HPV-positive NILM | 3 | 3451 | 33 | 1.7 (1.1 to 2.4) |
| HPV-positive NILM | 4 | 941 | 3 | 1.0 (0.3 to 3.3) |
| HPV-positive ASC-US | 1 | 11 663 | 610 | 6.6 (6.1 to 7.1) |
| HPV-positive ASC-US | 2 | 3810 | 93 | 3.1 (2.5 to 3.8) |
| HPV-positive ASC-US | 3 | 1418 | 26 | 2.8 (1.8 to 4.2) |
| HPV-positive ASC-US | 4 | 434 | 5 | 2.8 (1.0 to 8.1) |
| LSIL | 1 | 10 633 | 434 | 5.2 (4.7 to 5.7) |
| LSIL | 2 | 3723 | 77 | 2.7 (2.2 to 3.5) |
| LSIL | 3 | 1534 | 18 | 1.7 (1.0 to 2.9) |
| LSIL | 4 | 467 | 2 | 0.9 (0.2 to 4.3) |
| ASC-H | 1 | 1871 | 313 | 19.7 (17.8 to 21.8) |
| ASC-H | 2 | 629 | 39 | 7.3 (5.3 to 10.0) |
| ASC-H | 3 | 230 | 8 | 4.2 (2.0 to 8.8) |
| ASC-H | 4 | 77 | 0 | 0.0 (0.0 to 100.0) |
| AGC | 1 | 1984 | 136 | 7.8 (6.6 to 9.1) |
| AGC | 2 | 1017 | 22 | 2.4 (1.61 to 3.7) |
| AGC | 3 | 417 | 7 | 2.2 (1.0 to 4.7) |
| AGC | 4 | 131 | 2 | 2.5 (0.6 to 10.4) |
| HSIL+ | 1 | 2180 | 944 | 50.0 (47.5 to 52.5) |
| HSIL+ | 2 | 273 | 61 | 24.7 (19.5 to 30.8) |
| HSIL+ | 3 | 94 | 13 | 14.4 (8.7 to 23.6) |
| HSIL+ | 4 | 22 | 2 | 10.0 (2.6 to 34.4) |
For the 1st, 2nd, 3rd, and 4th co-test following 0, 1, 2, and 3 consecutive negative co-test(s), respectively. AGC = atypical glandular cells; ASC-H = atypical squamous cells, cannot rule out HSIL; LSIL = low-grade squamous intraepithelial lesion; NILM = negative for intraepithelial lesion or malignancy (cytology negative); HSIL+ = high-grade squamous intraepithelial lesion (HSIL) or more severe.
The co-test was preceded by the co-test number-1 of negative co-tests.
Risk estimates for HPV-positive ASC-US+, HPV negative ASC-US+ were obtained by estimating risks within subpopulations grouped by screening protocols. Point estimates are a weighted average of subpopulation risks, weighted by the frequency in which they occur. Confidence intervals are derived by using survey methodology approaches to combine the variance estimates of the subpopulation risks.
Five-year and three-year CIN3+ risks with the corresponding 95% CIs for all combinations of positive co-testing results (HPV positive and/or ASC-US+) are shown in Supplementary Table 2 (available online). Five-year CIN3+ risks decreased for all combinations. Risks following high-grade squamous intraepithelial lesion or more severe cytologic interpretations, a specific marker for the presence of precancerous lesions, decreased from 50.0% (95% CI = 47.5% to 52.5%) to 10.0% (95% CI = 2.6% to 34.4%). Similar findings were observed for 3-year CIN3+ risks as for 5-year CIN3+ risks.
Aging of the cohort did not explain the pattern of decreasing CIN3+ risks following increasing number of antecedent negative screens. Mildly abnormal screening results, defined as LSIL or HPV-positive ASC-US, were stratified by age group (30–39 years, 40–49 years, and 50 years and older) at the time of the positive result. In each age group, the CIN3+ risks by round decreased (Ptrend < .001) (data not shown). Using other age categorization demonstrated similar trends in decreasing risk by round of co-testing within each age category (data not shown).
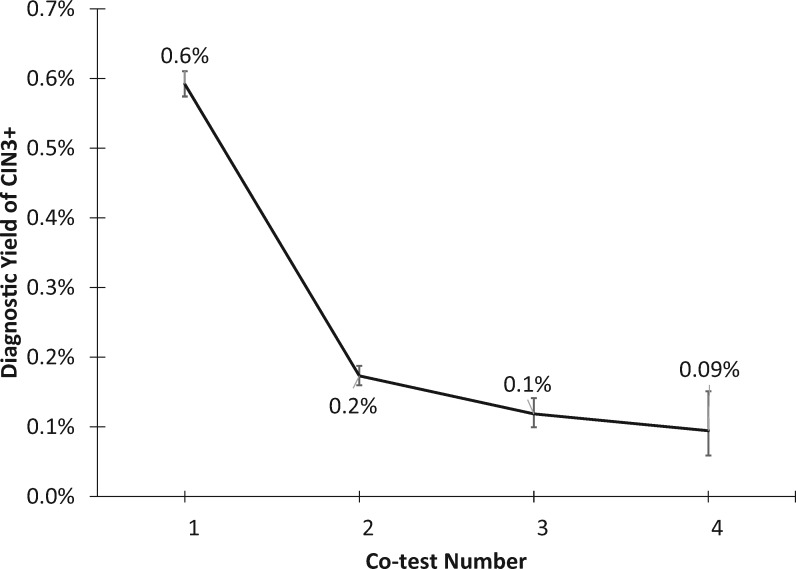
From a programmatic perspective, screening became less efficient following subsequent negative co-tests. The overall yield of the CIN3+ over 5 years decreased with each round of screening (Ptrend < .001) (Figure 2), from 0.6% (95% CI = 0.6% to 0.6%) in the first round to 0.09% (95% CI = 0.06% to 0.1%) in the fourth round of screening. In concert, 5-year risk ratios for CIN2/CIN3+ marginally increased with each round of screening (Ptrend = .06), from 1.45 (95% CI = 1.39 to 1.51) in the first round to 3.73 (95% CI = 2.23 to 6.27) in the fourth round.
Figure 2.
Diagnostic yield of CIN3+ by round of screening. Five-year cumulative detection (risk) of cervical intraepithelial neoplasia grade 3 (CIN3) or more severe diagnoses CIN3+, irrespective of screening result, for the first, second, third, and fourth co-test, following 0–3 negative co-tests. Bars show 95% CIs.
Discussion
Increasing numbers of consecutive negative co-tests, whose sensitivity is mainly due to incorporation of HPV testing, substantially lowered risks of CIN3+ following a subsequent positive screen. These decreases were observed for all combinations of positive HPV and/or cytology results and for different age groups. These findings are consistent with previous reports from much smaller and shorter-term studies (19,20). Thus, the clinical meaning of any positive HPV screening result changed depending on immediate past history, and guidelines might emphasize what is now often ignored, the importance of screening history in the management of any positive screening result.
The importance of HPV persistence (and the benign nature of newly detected infections) is not sufficiently recognized. Notably, a recent survey of primary-care physicians and obstetrician-gynecologists found that 30% of them would recommend asymptomatic women ages 30 years and older undergo annual screening with HPV testing (21). Our data show that annual screening that includes HPV testing of all women is excessive, given that the current interval recommendations for screening that includes HPV testing are 3–5 years in the general population (22,23) and these data show that the CIN3+ risk is much lower after even one negative co-test. Annual screening is in conflict with known HPV natural history, risking overmanagement of benign new infections, the most likely cause/indication of a positive screen following a negative co-test or HPV test. It could be argued, with some controversy, that even triennial screening including HPV testing of all women is more frequent than natural history data and programmatic efficiency (eg, diagnostic yields) support.
Modeling has estimated that most HPV infections that will cause cervical cancer are acquired before the age of 30 years (24). Therefore, cervical cancer screening of women 30 years and older is mostly finding and treating the precancers and cancers due to those early exposures. After the first round of HPV-based screening rules out a high proportion of prevalent, persistent infection and precancer, there are fewer HPV infections in the following rounds of screening and they are new, such that a smaller fraction will develop into CIN3/AIS. In fact, relatively more newly screen-positive women will be diagnosed and treated for CIN2 than CIN3. Although CIN2 is, the diagnostic threshold for treatment [except in women younger than 30 years (11)] to maximize safety, CIN2 is an equivocal diagnosis that likely represents a mixture of HPV infection and CIN3 (25). Given that treatment increases the risk of preterm delivery, it might be important to minimize unnecessary treatment. (16,17).
To increase the efficiency and maximize the benefits to harms of screening, a risk-based approach to screening and management of screen-positive women, using the principle of “equal management for equal risk” for clinical decision making, has been adopted in US consensus guidelines (26,27). Adhering to this principle, screening and management of a positive result or diagnosis following a history of negative co-testing or HPV testing could be adjusted to reduce unnecessary follow-up and medical procedures for minor cervical abnormalities due to benign HPV infection.
These data provide evidence for less aggressive management of several positive screening outcomes when immediately preceded by documentable negative screening history. Specifically, the two most common positive screening results, HPV-positive ASC-US and LSIL results, when immediately preceded by a negative screening history, might be followed by 6–12-month surveillance rather than being referred to colposcopy. The argument for this less aggressive management is based on the risks for these positive screening results falling well below the benchmark risk for colposcopic referral, ie, the risk associated with LSIL unqualified by screening history (10,11,26). Indeed, CIN3+ risks for women with HPV-positive ASC-US or LSIL are similar to those typically reported for ASC-US, HPV-negative LSIL, and HPV-positive NILM, all of which are followed for an interval of 6–12 months with retesting rather than referred to colposcopy immediately (11). Women in this risk group are followed until the underlying HPV infection has either resolved and they can be returned safely to routine screening, or persists and represents sufficiently elevated CIN3+ risk to warrant immediate colposcopy.
In the future, similar changes to management of abnormal screening results may be necessary in cohorts vaccinated against HPV16 and HPV18, the two most carcinogenic HPV genotypes (28). That is, CIN3+ risks associated with abnormal screening results in HPV-vaccinated cohorts may be sufficiently lower that they can be managed less aggressively: HPV-positive ASC-US and LSIL may not warrant immediate colposcopy (29). We have observed lower risks following an abnormal cytology in young women undergoing their first screening if they have been vaccinated against HPV16 and HPV18 before the age of 18 years (unpublished observations). Notably, women who have normal cytology and are positive for high-risk HPV but negative for HPV16 and HPV18 are managed differently than those positive for HPV16 and HPV18 (11). As a general rule, following negative screens, a longer interval to the next screen might be worth considering. Concurrent international guidelines recommend at least 5-year intervals with HPV testing (http://apps.who.int/iris/bitstream/10665/94830/1/9789241548694_eng.pdf) (30), recognizing that shorter intervals tend to find more transient, benign infections and related abnormalities that would be better not detected. We observed that the yield of CIN3+ with each round of screening decreased, indicating that the efficiency and the benefits of screening were also reduced. The US Preventive Services Task Force recently issued draft recommendations for a 5-year interval following negative HPV testing (https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2). The clinical pushback against such a long interval has been strong (21). When considering lengthened intervals, it might be worth taking into account whether women have had a negative screening history preceding any given result (4). The decision to extend screening intervals, and for what duration, will depend on the adherence to those intervals and the acceptability of the incrementally greater risk associated with longer intervals (31).
This analysis had a number of limitations. First, these data were from an observational cohort of women ages 30 years and older undergoing routine cervical cancer screening rather than a clinical trial. Thus, colposcopy was done based on screening outcomes and the concomitant risks, as it is done in routine screening, rather than systematically on all women, which affects the time at which CIN3+ is diagnosed in follow-up. To partially compensate, 5-year risks as the primary outcome were used to account for differential follow-up and the limitations of colposcopy to diagnose disease.
Another limitation, despite an effective call–recall program to minimize losses to follow-up, is that some women were lost to follow-up (left the KPNC membership or had follow-up after the cutoff date), which may have introduced some biases. Given that noncancerous abnormalities are asymptomatic, biases related to KPNC membership may be nondifferential by screening result. In a health-care setting that is unlike KPNC, ie, not an integrated health-care organization (health maintenance organization), the ability to incorporate screening history in clinical management will depend on the quality of the electronic medical records. If the data are not available, clinicians will have to rely only on the available information to make management decisions, but must recognize the limitations imposed by lack of clinical history, which turns out to be more important than previously known.
This analysis also did not address questions of complex screening histories of intermittent cytological abnormalities/HPV test positivity (eg, HPV negative and cytology negative, HPV-positive ASC-US, and HPV negative and cytology negative) vs a completely negative screening history (eg, three consecutive HPV-negative and cytology-negative results). The number of permutations and combinations of screening results after a few rounds of screening is great (11 different combinations of positive screening results per screening round) and will likely require complex modeling/computing, such as machine learning (32), to estimate the subsequent risks following each complex pattern.
In conclusion, these data show that in the new era of cervical cancer screening that includes HPV testing, screening history is an essential element in setting risk-based guidelines for management of a positive screen. Taking negative histories into account would mandate less aggressive management of a sizable fraction of screen-positive women (33). However, in the absence of documented data on past screening history, women with screen-positive results will need to be managed as before, based on the concurrent screening result only, to ensure patient safety.
Funding
The study was partially supported by the National Institutes of Health National Cancer Institute Intramural Research Program. PEC was supported in part by an Intergovernmental Personnel Act assignment from the National Institutes of Health National Cancer Institute.
Notes
Affiliations of authors: Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY (PEC, XX); Regional Laboratory, The Permanente Medical Group, Oakland, CA (WKK, NEP, TSL); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD (LCC, JCG, HAK, NW, MS).
The funding sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The National Cancer Institute (LCC, JCG, NW, HK, and MS) has received cervical screening test results from Roche and BD, for independent research. PEC has received cervical screening tests and diagnostics at a reduced or no cost for research from Roche, BD, Cepheid, and Arbor Vita Corporation. The other authors have no relevant relationships or conflicts of interest to disclose.
We gratefully acknowledge the seminal contributions of the late Ms Barbara Fetterman, who started this observational cohort. Without her leadership and vision on this project, this work would not have been possible. Requiescat in pace
Supplementary Material
References
- 1. Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;1891:12–19. [DOI] [PubMed] [Google Scholar]
- 2. Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;3392:b2569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kjær SK, Frederiksen K, Munk C, Iftner T.. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;10219:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer. Ann Intern Med. 2018;1681:20–29. [DOI] [PubMed] [Google Scholar]
- 5. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;127:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;1068:dju153.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;28716:2114–2119. [DOI] [PubMed] [Google Scholar]
- 8. Wright TC, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;1032:304–309. [DOI] [PubMed] [Google Scholar]
- 9. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D.. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007;114:223–239. [DOI] [PubMed] [Google Scholar]
- 10. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D.. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;114:201–222. [DOI] [PubMed] [Google Scholar]
- 11. Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. [DOI] [PubMed] [Google Scholar]
- 12. Wright TC Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ.. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;28716:2120–2129. [DOI] [PubMed] [Google Scholar]
- 13. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;95:425–434. [DOI] [PubMed] [Google Scholar]
- 14. Cheung LC, Pan Q, Hyun N, et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat Med. 2017;3622:3583–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tainio K, Athanasiou A, Tikkinen KAO, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ. 2018 Feb 27;360:k499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ. 2016;354:i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasieni P, Castanon A, Landy R, et al. Risk of preterm birth following surgical treatment for cervical disease: executive summary of a recent symposium. BJOG: Int J Obstet Gy. 2016;1239:1426–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casella G, Berger RL.. Statistical Inference. 2nd ed.Pacific Grove, CA: Duxbury/Thomson Learning; 2002. [Google Scholar]
- 19. Zorzi M, Frayle H, Rizzi M, et al. A 3-year interval is too short for re-screening women testing negative for human papillomavirus: a population-based cohort study. BJOG. 2017;12410:1585–1593. [DOI] [PubMed] [Google Scholar]
- 20. Kitchener HC, Gilham C, Sargent A, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer. 2011;476:864–871. [DOI] [PubMed] [Google Scholar]
- 21. Cooper CP, Saraiya M.. Primary HPV testing recommendations of US providers, 2015. Prev Med. 2015 Sep;78:33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;623:147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;1362:178–182. [DOI] [PubMed] [Google Scholar]
- 24. Burger EA, Kim JJ, Sy S, Castle PE.. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin Infect Dis. 2017;656:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;163:205–242. [DOI] [PubMed] [Google Scholar]
- 26. Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M.. Risk assessment to guide the prevention of cervical cancer. J Low Genit Tract Dis. 2008;121:1–7. [DOI] [PubMed] [Google Scholar]
- 28. Castle PE, Solomon D, Saslow D, Schiffman M.. Predicting the effect of successful human papillomavirus vaccination on existing cervical cancer prevention programs in the United States. Cancer. 2008;113(S10):3031–3035. [DOI] [PubMed] [Google Scholar]
- 29. Castle PE, Solomon D, Schiffman M, Wheeler CM.. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;9714:1066–1071. [DOI] [PubMed] [Google Scholar]
- 30. Jeronimo J, Castle PE, Temin S, et al. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2017;35:635–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinney WK, Huh WK.. Protection against cervical cancer versus decreasing harms from screening—what would U.S. patients and clinicians prefer, and do their preferences matter? Prev Med. 2017;98:31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baltzer N, Sundstrom K, Nygard JF, Dillner J, Komorowski J.. Risk stratification in cervical cancer screening by complete screening history: applying bioinformatics to a general screening population. Int J Cancer. 2017;1411:200–209. [DOI] [PubMed] [Google Scholar]
- 33. Schiffman M, Wentzensen N, Khan MJ, et al. Preparing for the next round of ASCCP-sponsored cervical screening and management guidelines. J Low Genit Tract Dis. 2017;212:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gage JC, Hunt WC, Schiffman M, et al. Risk stratification using human papillomavirus testing among women with equivocally abnormal cytology: results from a state-wide surveillance program. Cancer Epidemiol Biomarkers Prev. 2016;251:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schick A, Yu Q.. Consistency of the GMLE with mixed case interval-censored data. Scand J Stat. 2000;271:45–55. [Google Scholar]
- 36. Panageas KS, Ben-Porat L, Dickler MN, Chapman PB, Schrag D.. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;996:428–432. [DOI] [PubMed] [Google Scholar]
- 37. Dorey FJ, Little RJ, Schenker N.. Multiple imputation for threshold-crossing data with interval censoring. Stat Med. 1993;1217:1589–1603. [DOI] [PubMed] [Google Scholar]
- 38. Rucker G, Messerer D.. Remission duration: an example of interval-censored observations. Stat Med. 1988;711:1139–1145. [DOI] [PubMed] [Google Scholar]
- 39. Wellner JA, Zhan Y.. A hybrid algorithm for computation of the nonparametric maximum likelihood estimator from censored data. J Am Stat Assoc. 1997;92439:945–959. [Google Scholar]
- 40. Huang J. Efficient estimation for the proportional hazards model with interval censoring. Ann.Stat. 1996;242:540–568. [Google Scholar]
- 41. Goetghebeur E, Ryan L.. Semiparametric regression analysis of interval-censored data. Biometrics. 2000;564:1139–1144. [DOI] [PubMed] [Google Scholar]
- 42. Hyun N, Cheung L, Pan Q, Schiffman M, Katki H.. Flexible risk prediction models for left or interval-censored data from electronic health records. Ann Appl Stat. 2017;112:1063–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Armitage P, Doll R.. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954;81:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leung KM, Elashoff RM, Afifi AA.. Censoring issues in survival analysis. Annu Rev Public Health. 1997;18:83–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.