Abstract
Therapeutic brain stimulation has proven efficacious for treatment of nervous system diseases, exerting widespread influence via disease-specific neural networks. Activation or suppression of neural networks could theoretically be assessed by either clinical symptom modification (ie, tremor, rigidity, seizures) or development of specific biomarkers linked to treatment of symptomatic disease states. For example, biomarkers indicative of disease state could aid improved intraoperative localization of electrode position, optimize device efficacy or efficiency through dynamic control, and eventually serve to guide automatic adjustment of stimulation settings. Biomarkers to control either extracranial or intracranial stimulation span from continuous physiological brain activity, intermittent pathological activity, and triggered local phenomena or potentials, to wearable devices, blood flow, biochemical or cardiac signals, temperature perturbations, optical or magnetic resonance imaging changes, or optogenetic signals. The goal of this review is to update new approaches to implement control of stimulation through relevant biomarkers. Critical questions include whether adaptive systems adjusted through biomarkers can optimize efficiency and eventually efficacy, serve as inputs for stimulation adjustment, and consequently broaden our fundamental understanding of abnormal neural networks in pathologic states. Neurosurgeons are at the forefront of translating and developing biomarkers embedded within improved brain stimulation systems. Thus, criteria for developing and validating biomarkers for clinical use are important for the adaptation of device approaches into clinical practice.
Keywords: Deep brain stimulation, Biomarkers, Epilepsy, Parkinson disease, Adaptive brain stimulation, Beta hypersynchrony, Phase amplitude coupling, Evoked field potentials, Closed loop
ABBREVIATIONS
- AD
Alzheimer's disease
- aDBS
adaptive DBS
- BCI/BMI
brain computer/machine interface
- DBS
Deep brain stimulation
- ECoG
electrocorticography
- EEG
electroencephalogram
- EMG
electromyogram
- ECAP
evoked compound action potential
- FOG
freezing of gait
- GPi
globus pallidus internus
- IPG
internal pulse generator
- LFP
local field potential
- PD
Parkinson disease
- PAC
phase amplitude coupling
- SCS
spinal cord stimulation
- SCC
subcallosal cingulate
- STN
subthalamic nucleus
- TMS
transcranial magnetic stimulation
- TBI
traumatic brain injury
- TRD
treatment resistant depression
- VNS
vagal nerve stimulatorstimulation
- Vim
ventral intermediate nucleus of the thalamus
Both extracranial and intracranial brain stimulation can exert influence over multiple brain areas through modulation of disease- and patient-specific neural networks.1-4 Placement of intracranial brain stimulation and recording electrodes is currently executed via preoperative recordings and detailed imaging. In contrast, extracranial stimulation can be either diffusely applied to the skull or targeted to engage specific brain regions, such as in transcranial alternating current and transcranial magnetic stimulation (TMS),5-7 or lie outside of the cranium, such as vagal nerve stimulation (VNS)8 and spinal cord stimulation (SCS). Engagement of appropriate circuitry and treatment efficacy in all these forms of brain stimulation is typically verified by clinical assessment and symptom reduction as well as direct electrophysiological recordings with stimulation.
Clinical biomarkers or endpoints may also provide objective endpoints to therapy or elucidate why patients with optimal electrode position still have such variable clinical outcomes, including development of dose-response functions.9 The current clinical approach to programming stimulation parameters requires significant time and effort from both patient and clinician and can be subjective Use of biomarkers may facilitate objective and/or automated automatic adjustments,10 improved power efficiency, and fewer side effects by linking both underlying physiologic network activity and symptom relief. This may be achieved through intermittent or continuous, dynamic adjustment based on amplitude- or time-dependent changes.2,11
Neurosurgeons have been at the forefront of concept and device testing in clinical research in brain stimulation. The number of clinical and feasibility studies, usually in small pilot groups of patients for brief time frames (hours), indicate that this is an area moving rapidly from the laboratory to the bedside and eventual standard clinical applicability.12-16 A number of clinical trials for newly marketed and prototypical responsive, scheduled, and adaptive systems are ongoing at academic institutions. Here, we describe direct neurosurgery translation of intra- and extracranial feedback modulation of brain stimulation, updating an earlier, more comprehensive review.2
BIOMARKERS BY DISEASE
Characteristics of an ideal surrogate biomarker are noted in Table 1. The complexity of potential biomarkers and their barriers to implementation highlight the need for neural circuitry modeling. There are many avenues to analyze these biomarkers in initial clinical trials, ranging from intraoperative testing to verify appropriate electrode position, using percutaneous wires after surgery for short-term testing, or using one of the implanted sensing/recording devices for longer term data collection. Clinical implementation of biomarkers ranges from FDA approved devices to human trials with research devices to experimental biomarker identification pending clinical application. Table 2 provides a broad overview.
TABLE 1.
General Characteristics of a Desirable Biomarker
| Desired biomarker characteristic | Description |
|---|---|
| Directly correlates to clinical symptoms | Relevant in time course and extent to a measurable symptom |
| Constantly/dynamically tracks disease state | Dynamically changes as stimulation alters neural circuits to reflect symptom improvement |
| Minimal sampling error | Ability to differentiate location where best, representative signal obtained |
| Signal stability over time | Signal faithfully and consistently tracks disease state across multiple conditions, such as walking, sleeping and activity |
| Signal can be differentiated from background “noise” | The signal can be distinguished from ongoing spontaneous activity to be distinct and measurable |
| Confirmation of desired signal | Distinguish pathological signal from overlapping normal cortical signals |
| Adjustable | Ability to fine tune for patient to patient variability and across dynamic states |
A biomarker must do more than simply match clinical symptoms in order to be effectively implemented in a future adaptive or closed-loop device. This table highlights keep qualities necessary to make controls implementation and device design efficacious.
TABLE 2.
Possible Targets, Potential Surrogates, and Current Developmental Hurdles
| Disease | Stimulation targets | Surrogate/biomarker | Biomarker development challenges |
|---|---|---|---|
| Epilepsy | Anterior thalamic nucleus, CM thalamus, localized seizure focus, vagal nerve | Intracranial: abnormal synchrony and excitability noted on EEG, ECoG and depth electrodes | Ability to sense pre-ictal event, validate clinical efficacy in human trials |
| Extracranial: heart rate (VNS) | |||
| Parkinson (rigidity and bradykinesia) | STN, GPi | Beta hyper synchrony (Beta band oscillations) | Direct correlation with clinical symptom improvement |
| Phase amplitude coupling | Signal differentiation | ||
| Parksinon (dyskinesia) | STN + Gpi (dual electrode) | Gamma oscillations | |
| Parkinson (FOG) | Pedunculopontine nucleus | DBS: Increased beta frequency or cholinergic neuron action potentials | |
| SCS: Spinal cord evoked recordings or secondary sensory evoked recordings | |||
| Tourette | Centromedian nucleus of thalamus and GPi | Low frequency thalamic oscillations, cortical oscillations | Identify best control system mode (continuous versus adaptive versus responsive) |
| Essential tremor | Vim (of thalamus) | Internal: ECAP External: EMG, accelerometry | Signal stability over time; signal variability between patients; signal to noise ratio |
| Alzheimer's disease | Fornix, entorhinal cortex, hippocampus, cingulate, precuneous, frontal cortex | Electrophysiological: hippocampal evoked potentials chemical: cholinergic activity | Very theoretical, stimulation target/biomarker identification |
| Depression | Subcallosal cingulate (SCC) and | Imaging based: tractography | Leading biomarker identification |
| Area 25 (medial forebrain bundle), | intersection of 3 fiber bundles near | ||
| nucleus accumbens | SCC, frontal lobe evoked potentials |
These sites summarize the disease-based discussion in the text. (CM – centromedian nucleus (of the thalamus); PET – positron emission tomography; SPECT – single photon emission computed tomography; STN – substantia nigra; EEG – electroencephalogram; ECoG – electrocorticography.)
EPILEPSY
Intracranial Stimulation
Intracranial neurostimulation for medication resistant epilepsy includes the responsive NeuroPace system (NeuroPace Inc, Mountain View, California) or intermittent, scheduled stimulation via anterior thalamic (ANT) DBS (both now approved in the US – NeuroPace for responsive stimulation and ANT DBS for intermittent, open loop stimulation).17 ANT DBS, placed via microelectrode recording and frontal lobe scalp electroencephalogram (EEG), may modulate general frontal lobe networks to reduce seizure susceptibility.18 Scalp EEG in this setting could also be used as an intraoperative or permanent surrogate biomarker for both localization and to initiate stimulation in relation to onset of an epileptic event.19
The NeuroPace system is intermittently triggered by ictal events (or their precursor signatures) using detectable depth electrode or electrocorticography (ECoG) cortical ictal activity as the biomarker. Regions based on preoperative localization are stimulated to prevent seizure onset and propagation.20,21 NeuroPace's clinical limitations include accurate localization of both of both inputs and outputs, detection of pre-ictal events sufficiently far enough in advance of a seizure so that the patients does not detect clinical symptoms, and determining exact stimulation algorithms.
Extracranial Stimulation
Extracranial stimulation includes cervical VNS, likely affecting diffuse brain networks.8,22 Recent studies have indicated that direct electrocardiographic detection of tachycardia in the pre-ictal state may provide a key feedback signal to trigger scheduled intermittent stimulation. This signal is available since typically the internal pulse generator (IPG) for VNS is placed in the left chest region. Additionally, focused extracranial alternating current stimulation may also be activated (using intracranial sensing electrodes) to increase seizure threshold and/or treat ictal events.7 This is a promising new approach that may be able to take advantage of temporal interference to both spatial and temporal focusing of extracranial stimulation.5
PARKINSON DISEASE
Although ∼75% of Parkinson disease (PD) patients obtain some symptom improvement with DBS placement in the subthalamic nucleus (STN) or globus pallidus internus (GPi),23,24 there remains considerable variation in outcomes as the pathways and pathophysiology of the disease are not currently fully understood.25 It is possible that different biomarkers will likely be required for different PD symptoms, rather than attempting to treat the various disease phenotypes with one treatment biomarker.
Beta Band Oscillations
Spontaneous beta band oscillatory activity at 13 to 30 Hz spreads through the cortico-basal network upon synchronization of the cortex, basal ganglia, and thalamus.25 This beta band activity thought to be a marker of PD state in animal models and humans and a possible surrogate for treatment effect for bradykinesia (not tremor).26 Hypersynchrony lessens after therapeutic doses of dopaminergic medication and clinical levele DBS stimulation.27 Such synchrony can be recorded from motor cortex (ie, ECoG)28 or directly from STN or GPi DBS contacts.29-31 Whitmer et al25 recorded subdural cortical ECoG and spontaneous STN local field potential (LFPs) (in a clinical study of 13 humans). Several clinical studies have now shown proof of principle that adaptive adjustment of beta band oscillations may be equi-efficacious to continuous DBS and more efficient.12,32
Phase Amplitude Coupling
Based on primary motor cortex ECoG, de Hemptinne et al28,33 demonstrated that DBS reduces phase amplitude coupling (PAC) between beta oscillations and higher frequency superimposed oscillations characteristic of PD. PACs are thought to cooridinate timing between various cortical areas for execution of tasks.34 While they are seen in normal cortical activity, the increased PAC in PD possibly reflects neurons restricted to an inflexible pattern by PD, leading to the hallmark symptoms of rigidity and bradykinesia.33 Adaptive DBS (aDBS) using motor cortex sensing appears to be equally efficacious to continuous DBS in a few long-term patients.12,16
Evoked Potentials and Oscillations
In some cases, more than one DBS electrode may be needed for clinical efficacy, and an additional electrode at another anatomical site (ie, STN + GPi together) may prove to be beneficial. Instability and freezing of gait (FOG) are significant causes of morbidity in Parkinson patients.35 There is evidence of this in a few closed-loop systems described in the literature. For example, one study utilized a closed-loop system to alter GPi stimulation based on pedunculopontine nucleus potentials (correlated with FOG and instability) as the outputs.36 Dyskinesias after STN DBS are another application for dual electrode recordings by targeting STN together with GPi.37,38 Dual electrodes can allow for stimulation on one electrode and recording on another, although stimulation evoked potentials can also be recorded from STN DBS electrodes alone,39 showing an interesting resonant neural activity. Lastly, spontaneous gamma band oscillations (ie, 60-90 Hz) may also indicate hyperkinetic PD symptoms, (eg, dyskinesias) as another biomarker beyond beta band oscillations as discussed earlier.40
Spinal Cord Stimulation
SCS may improve gait in particular, and freezing episodes, possibly through direct activation of lower extremity circuits and/or secondarily through indirect activation of intracranial circuits.41,42 Biomarkers derived from SCS evoked recordings (ie, from stimulating one contact and recording on other contacts) or secondary sensory stimulation evoked recordings (ie, stimulating on a SCS contact and recording from sensory cortex) may be useful to titrate the level of SCS required (ie, above or below the level of paresthesia). This is an area of early clinical investigation.
TOURETTE SYNDROME
Tourette syndrome is an idiopathic neuropsychiatric disorder defined by motor and phonic tics43,44 and intraoperative thalamic recordings of the centromedian parafasicular complex suggest that low frequency bursts correlate with the clinical phenotype. Maling et al44 studied 5 Tourette patients implanted with the Neuropace device localized using cortical ECoG strips, CT-MRI fusion and intraoperative microelectrode recordings to delineate their anatomic CM target. Best symptom relief from stimulation correlated with increased gamma activity and eventual return to higher thalamic frequencies. Alternatively, low frequency oscillations in the GPi that precede electromyogram (EMG)-proven tic recordings by 50 to 2000 ms may be a possible anticipatory biomarker.13,45 Okun et al46 demonstrated long-term efficacy of scheduled stimulation in Tourette Syndrome as compared to purely continuous stimulation. They have also reported proof of concept for responsive DBS (where the spontaneous biomarker triggers onset of stimulation) for Tourette's pathology with improved battery life.15
ESSENTIAL TREMOR
Ventral intermediate nucleus of the thalamus (Vim) stimulation can help to treat essential tremor.47 Kent et al48 investigated the electrical stimulation-induced evoked compound action potential (ECAP) intraoperatively arising from neural elements (likely axons) near the lead.39 Neural activation appeared to correlate with programmatic stimulation adjustment and clinical tremor as measured by accelormeter.49 Specifically, low frequencies near 10 Hz worsened the tremor, but high frequencies closer to 130 Hz improved it. Unfortunately, ECAP signal has significant patient-to-patient variability, as significant as an order of magnitude between subjects.39,49 ECAP signal may also be altered over time by glial scarring, potentially hampering long-term applications.49 One idea to improve the signal to noise ratio during Vim DBS would be to record the electrically evoked field potential with a second electrode placed anteriorly in VOP to reduce stimulation artifact.43,48,50
Indeed, accelerometers for dynamic tremor measurement are examples of external biomarkers from wearable devices that can potentially provide closed-loop control.51 Cagnan et al52 developed a prototype device coupling the measured phase of tremor to the DBS IPG for intermittent, phase - specifica stimulation. Alternatively, Basu et al53 utilized surface EMG and accelerometry in an on/off adaptive control system incorporating a tremor-predictive algorithm.53 Another highly innovative tremor treatment approach utilizes the typical clinical observation that patients with essential tremor have minimal to no tremor at rest. By recording and interpreting a motor cortical ECoG signal indicating pending arm motor activity, Vim DBS may dynamically control tremor prior to and during activity with less power use.13
ALZHEIMER’S DISEASE
It is unclear the degree to which the abnormal circuits in Alzheimer's disease (AD) may be treated or affected by stimulation.54,55 Novel techniques such as DBS of the fornix in clinical trials have thus far shown limited efficacy.56,57 Large hippocampal evoked potentials could potentially become biomarkers both to adjust fornix stimulation amplitude (currently empirically determined) and to estimate plasticity following fornix stimulation. Another alternative site is the nucleus basalis of Meynert,58 although a cholinergic biomarker and associated dose-response curve might be required to titrate effectively such widespread and diffuse cholinergic enhancement.
Extracranial stimulation has also been implemented to improve59-61 and potentially to forestall worsening in AD. Extracranial stimulation may demonstrate the advantage of widespread circuitry activation, matching the nearly global pathology in more advanced AD. Intracranial markers may help adjust and define a dose-response curve for extracranial stimulation.62
DEPRESSION
DBS for treatment resistant depression (TRD) with numerous potential sites, including but not limited to subcallosal cingulate (SCC) white matter, Brodmann area 25 gray matter, and ventral capsule/ventral striatum.63,64 While small clinical trials have demonstrated even long-term efficacy,65 larger industry sponsored trials such as BROADEN (St. Jude) have not.66 Intraoperative testing is challenging as patient response is highly variable, personal, and may be delayed over weeks to months. TRD is a significant example of a disease state where nonelectrophysiologic biomarkers are the leading candidates. For example, when utilizing tractography to guide lead placement, autonomic effects (tachycardia and increases in skin conductance) correlate with intraoperative testing.67 SCC DBS lead placement for research studies was initially anatomically guided65 but therapeutic responses were widely variable.68 Prospective trials instead targeting white matter tract intersections near the SCC and interrogation of these individual white matter tracts with ECoG monitoring may be promising to better guide electrode placement and long term efficacy.69-71
Both VNS and TMS of the left frontal lobe have also been FDA approved for treatment of depression.72 However, a clear dose-response curve based on symptoms and intracranial responses will be critical to establish a circuitry basis for stimulation.
STIMULATION AND CONTROL MODES
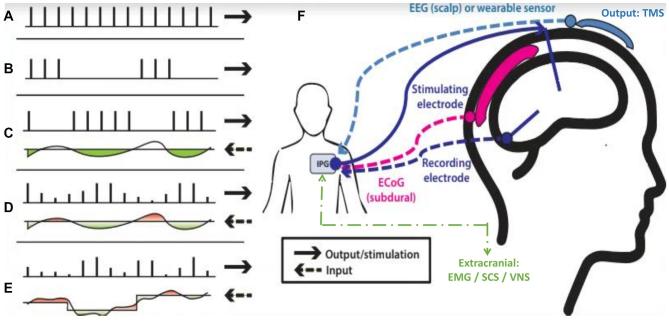
Time constants are a measure of the system's response time to an input.73 These can be significantly different. For example, tremor responses to thalamic DBS may stabilize within 10 to 20 s74 whereas subthalamic DBS for bradykinesia may initiate within seconds but require more than 30 min to stabilize.75 There are 5 major subtypes of systems control policy as related to stimulation (Figure and Table 3). The clinician's input decreases and system autonomy increases through the spectrum. In order of increasing complexity, the five subtypes include:
simple continuous (Figure A) with clinical adjustment;
intermittent or scheduled using a fixed schedule and fixed amplitude (Figure B), such as the 30 sec on/5 min off commonly used cycling in VNS;
responsive with a preset amplitude and width but requires a trigger for initiation (Figure C) from threshold changes in a few channels of input signal;
adaptive with flexibility of variable durations of stimulation in response to a single biomarker input threshold, or a variable amplitude (Figure D) typically set to minutes for on/off cycling;
closed-loop within the brain-machine interface context, this isa multidimensional input, rapid outputs from continuousprocessing of inputs (ie, 10 to 20 Hz), and appropriate feedback signal (Figure E).
FIGURE.
DBS stimulation control models show the input below each pulse sequence, reflecting continuous changes in the biomarker, and the upper reflects the actual system output in response to the input. The green color indicates stimulation on and the red color indicates stimulation off. The various modes include: A, continuous stimulation; B, scheduled intermittent stimulation; C, responsive stimulation. Each stimulation is the constant but requires a threshold to be met; D, adaptive stimulation. The number and amplitude of pulses may vary to achieve a control point value; E, closed-loop stimulation. A dynamic continuous output informs a continuous dynamic input. F, Depending on disease pathology, input may be from the DBS lead or secondary DBS, EEG, or ECoG electrodes. Output stimulation from the IPG is primarily though the DBS/parenchymal lead but future subdural or scalp hardware options are possible. Reprinted from: Hoang et al2 Copyright © 2017 Hoang, Cassar, Grill and Turner. CC BY.
TABLE 3.
Control Systems Description
| Control mode | Feedback type | Description of feedback | Time constant of activation |
|---|---|---|---|
| Continuous | Clinician observation | Clinician manual adjustment | Monthly or frequency of clinic visits |
| Scheduled Intermittent | None | Preset stimulation amplitude turned on or off at preset timing | Preset timing determined by system physiology or empirically |
| Responsive | Triggered by threshold event | Preset stimulation amplitude turned on or off by trigger (with defined lockouts) | 0.5-5 s, can be repeated |
| Adaptive | Single biomarker input, continuous monitoring | Stimulation output can be turned on or off, or scaled, for continuous adjustment | Tremor ∼ 10 s Rigidity, Gait ∼ 60-90 s |
| Closed-Loop | Multiple channels of input biomarkers for continuous analysis | Continuous prediction of brain intent for action | 20-50 ms for information update |
The use of biomarkers can be described in various approaches, including continuous and intermittent. Responsive and adaptive show progressively more flexibility in when to perform stimulation (ie, triggered by an event or threshold) and adaptive has inherently further flexibility in prolonged stimulation and levels of stimulation when on. Closed loop can apply to any scheme where a feedback signal is used to alter stimulation, but commonly is used in a brain-machine context. The chart gives the type of feedback which can be used, the nature of the feedback and time constants to be considered in delivering the feedback.
In these various modes the control policy can be defined internally (ie, a state transition map embedded within the device based on contingencies) or externally.22 Each state transition can then lead to a predefined stimulation change. Table 4 categorizes the devices and biomarkers previously described by the control subtypes discussed here.
TABLE 4.
Biomarkers Classified by Control Subtypes
| Control mode/class | Specific examples of biomarkers/devices | Description |
|---|---|---|
| Simple continuous | Open Loop DBS | All adjustments via clinician/direct patient observation |
| Intermittent | ANT DBS | Scheduled on preset timing, therefore no biomarkers used (exception: VNS and heart rate as biomarker preceding seizure event) |
| VNS | ||
| Responsive/triggered | Neuropace | Predetermined output in response to threshold |
| DBS for tourette | Conditional output in response to motor cortex patterned responses | |
| DBS for essential tremor | Output in response to motor cortex movement initiation signal | |
| Adaptive | DBS for Parkinson disease (eg, PC + S and RC + S) | Currently external; response tailored to continuous input signal. |
| Includes motor cortex/STN coherence (eg, PAC), spontaneous beta band, stimulation-evoked responses. | ||
| Closed Loop | Braingate system | Multiple inputs (∼100 channels) process to form contingent output |
| Other custom systems |
SCHEDULED INTERMITTENT STIMULATION
Epilepsy and Parkinson Disease
Rather than continuous stimulation (ie, Figure A), ANT DBS and VNS for epilepsy implement an intermittent, scheduled stimulation at a preset level17 (Figure B, Table 3). Scheduled intermittent stimulation or additional on-demand stimulation can reduce the amount of unnecessary stimulation and may preserve network sensitivity which can otherwise adapt or fade with constant stimulation. Phase-dependent stimulation intermittently dampens the network may critically dampen the system if the response is sufficiently rapid.52 There are criteria for a stimulation trigger with a “lockout” or safety mechanism preventing overstimulation. The pulse repetition frequency of DBS for epilepsy is critical as EEG synchrony caused by alternative frequencies can amplify seizures.21 The SANTE trial targeting the anterior nucleus of the thalamus (ANT) demonstrated reduction in seizure frequency, even over the long term, using scheduled, intermittent programming.18 Specifically, SANTE randomized participants to stimulation at either at 5 V or 0 V (no stimulation) with set parameters of 90 microseconds, 145 Hz, and a 1 min “on” and 5 min “off”.
Newer Medtronic DBS devices (ie, Medtronic PC + S and RC + S; Medtronic Inc, Dublin, Ireland) also feature lock-out, phase-in timing and adjustable response based on the specific input (Figure C).76-78 The RC + S has been implemented with more advanced features than the PC + S, including built in logic for contingent, incremental stimulation changes in response to a recorded signal, as well as a distributed network which can encompass both internal and external devices.
RESPONSIVE CONTROL
Epilepsy
Certain disease processes, like epilepsy, may have long asymptomatic or unpredictable periods between events where constant stimulation programming may not be ideal.20 The key difference from the scheduled intermittent control is the recording of network signals to initiate the stimulation. For the previously mentioned Neuropace system, stimulation at a preset level is delivered in a binary on/off fashion when upper or lower thresholds are reached, much as a thermostat controls a furnace.
ADAPTIVE CONTROL
Parkinson Disease
Arguably, the most advanced control systems concepts regarding brain stimulation are the last 2 types – aDBS and closed loop (Figure D and Figure E). Adaptive stimulation has adjustable or contingent stimulation in response to the external or internal biomarker, as compared to the fixed output of a responsive system. There is a growing body of evidence that aDBS is clinically feasible and safe.12,16,32 Little et al79,79 utilized STN aDBS via an externalized system on/off system linked to processing of LFPs (beta frequency amplitude). Notably the stimulation was dynamic with variable widths. They also compared continuous, scheduled intermittent, and adaptive systems directly and found statistically significant improvement with the adaptive system in motor scores, decreased speech side effects, reduction in stimulation time, and decreased energy utilization.80 However, longer-term data showed that the adaptive system demonstrated much improved efficiency but only a modest improvement in efficacy.81 Most recently, Arlotti et al12 studied neurophysiologic and clinical responses of LFP-based aDBS for advanced Parkinson for 8 h after implantation in 2 patients. Beta-band power correlated with clinical manifestations and aDBS automatically decreased DBS amplitude during “on” states effectively while preventing dyskinesia.12,82
A theoretical variation called a “scalar adaptive system” utilizes inputs of varying amplitudes to approach a desired set point.11,73 The difference between the desired set-point and the current value is noted and a larger difference generates a correspondingly larger change in stimulation, hence the term “scaled output”(Figure D). This slightly more sophisticated variation embodies classic control system principles by minimizing the amount of output oscillation and time to achieve a steady state. It also mitigates large or complete on/off changes which may cause uncomfortable side effects for the patient. A scalar response may also adapt to varying needs during task performance to dynamically control system response.
CLOSED LOOP
Motor and Psychiatric Disease
At the most complex end of the control spectrum, brain computer/machine interfaces (BCI/BMI) require constant or near-constant sensing, feedback parameter, and output for motor control.83 Whereas adaptive stimulation may have only a single setpoint, closed-loop stimulation can utilize dynamic or multiple setpoints, adjusted with the information from the rapidly updated feedback parameter.84
The initial work in BCI/BMI has largely focused on motor disorders. The previously mentioned Medtronic “Activa PC + S” has been used in a number of pertinent examples such a BCI in a locked-in patient with amyotrophic lateral sclerosis.85 More recently Widge and Sahay86 have also proposed extensions of closed-loop theory to psychiatric applications. Early work suggests that closed-loop feedback can uniquely modify neural network firing patterns with continued exposure to BCI training. This may have unique applicability to psychiatric disease which can fluctuate or clinically change over time.86 Additionally, a preliminary low frequency signal has been recorded in nucleus accumbens, which may be useful for measurement of impulsivity.87
Adaptive Stimulation with Extracranial Stimulation
There are a number of strategies to improve and focus extracranial stimulation and to develop contingent stimulation dependent upon intracranial biomarkers.7,8 VNS may also be contingent upon either direct patient triggering or electrocardiographic signals, such as tachycardia which may precede or accompany an aura or seizure onset.8 In this case, the VNS may be rapidly triggered to attempt to abort a seizure at the onset or in a precursor stage.
CONCLUSION
Identifying and determining efficacy of stimulation biomarkers will require considerable additional development, mainly in collaboration with neurosurgeons who can facilitate development, clinical testing and implantation of new systems. Electrophysiological measures, neurochemical or other markers may also be reasonable options beyond current imaging and electrophysiological measures. While treatment of disease can obviously be optimized, the potential to better understand the underlying circuitry on many pathologies is a more fundamental goal of this study. It is critical at this stage to identify the most promising biomarkers into proof of concept translational work, and well-designed clinical trials to demonstrate efficacy, safety, or noninferiority in comparison to conventional devices. Neurosurgeons are the driving force in many instances to implement and improve adaptive modulation systems as well as understand the underlying clinical diseases.
Disclosures
This work was supported by NIH R01 NS079312, NIH R37 NS040984, and NIH UH3 NS103468. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Henderson JM. “Connectomic surgery”: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Front Integr Neurosci. 2012;6:15. doi: 10.3389/fnint.2012.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoang KB, Cassar IR, Grill WM, Turner DA. Biomarkers and stimulation algorithms for adaptive brain stimulation. Front Neurosci. 2017;11:564. doi: 10.3389/fnins.2017.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406-424. [DOI] [PubMed] [Google Scholar]
- 4. Zamora-López G, Zhou C, Kurths J. Exploring brain function from anatomical connectivity. Front Neurosci. 2011;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grossman N, Bono D, Dedic N et al.. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell. 2017;169(6):1029-1041.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peterchev AV. Transcranial electric stimulation seen from within the brain. eLife. 2017;6:e25812. doi: 10.7554/eLife.25812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vöröslakos M, Takeuchi Y, Brinyiczki K et al.. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. 2018;9(1):483-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boon P, Vonck K, van Rijckevorsel K et al.. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure. 2015;32:52-61. doi: 10.1016/j.seizure.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 9. Trager MH, Koop MM, Velisar A et al.. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson's disease. Neurobiol Dis. 2016;96:22-30. doi: 10.1016/j.nbd.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 10. Parastarfeizabadi M, Kouzani AZ. Advances in closed-loop deep brain stimulation devices. J Neuroeng Rehabil. 2017;14(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosa M, Arlotti M, Ardolino G et al.. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord. 2015;30(7):1003-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arlotti M, Marceglia S, Foffani G et al.. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology. 2018;90(11):e971-e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunduz A, Foote KD, Okun MS. Reengineering deep brain stimulation for movement disorders: Emerging technologies. Curr Opin Biomed Eng. 2017;4:97-105. doi: 10.1016/j.cobme.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marceglia S, Rosa M, Servello D et al.. Adaptive Deep Brain Stimulation (aDBS) for Tourette Syndrome. Brain Sciences. 2017;8(1):E4. doi: 10.3390/brainsci8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molina R, Okun MS, Shute JB et al.. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J Neurosurg. 2017;129(2):308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swann NC, de Hemptinne C, Thompson MC et al.. Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J Neural Eng. 2018;15(4):046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. 2014;10(5):261-270. [DOI] [PubMed] [Google Scholar]
- 18. Salanova V, Witt T, Worth R et al.. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halpern CH, Samadani U, Litt B, Jaggi JL, Baltuch GH. Deep brain stimulation for epilepsy. Neurotherapeutics. 2008;5(1):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. [DOI] [PubMed] [Google Scholar]
- 21. Durand DM. Control of seizure activity by electrical stimulation: effect of frequency. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2375. doi: 10.1109/IEMBS.2009.5334993 [DOI] [PubMed] [Google Scholar]
- 22. Romero-Ugalde HM, Le Rolle V, Bonnet J-L et al.. Closed-loop vagus nerve stimulation based on state transition models. IEEE Trans Biomed Eng. 2018;65(7):1630-1638. [DOI] [PubMed] [Google Scholar]
- 23. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257-1272. [DOI] [PubMed] [Google Scholar]
- 24. Odekerken VJ, Boel JA, Schmand BA et al.. GPi vs STN deep brain stimulation for Parkinson disease. Neurology. 2016;86(8):755-761. [DOI] [PubMed] [Google Scholar]
- 25. Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuhn AA, Tsui A, Aziz T et al.. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380-387. [DOI] [PubMed] [Google Scholar]
- 27. Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson's disease? Ann N Y Acad Sci. 2012;1265(1):9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Hemptinne C, Swann NC, Ostrem JL et al.. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18(5):779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brocker DT, Swan BD, Turner DA et al.. Improved efficacy of temporally non-regular deep brain stimulation in Parkinson's disease. Exp Neurol. 2013;239:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gmel GE, Hamilton TJ, Obradovic M et al.. A new biomarker for subthalamic deep brain stimulation for patients with advanced Parkinson's disease–a pilot study. J Neural Eng. 2015;12(6):066013. [DOI] [PubMed] [Google Scholar]
- 31. Wang DD, de Hemptinne C, Miocinovic S et al.. Pallidal deep-brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in parkinson's disease. J Neurosci. 2018;38(19):4556-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pina-Fuentes D, Little S, Oterdoom M et al.. Adaptive DBS in a Parkinson's patient with chronically implanted DBS: A proof of principle. Mov Disord. 2017;32(8):1253-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Hemptinne C, Ryapolova-Webb ES, Air EL et al.. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci. 2013;110(12):4780-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aminoff MJ, Christine CW, Friedman JH et al.. Management of the hospitalized patient with Parkinson's disease: Current state of the field and need for guidelines. Parkinsonism Relat Disord. 2011;17(3):139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morita H, Hass CJ, Moro E, Sudhyadhom A, Kumar R, Okun MS. Pedunculopontine nucleus stimulation: where are we now and what needs to be done to move the field forward? Front Neurol. 2014;5:243. doi: 10.3389/fneur.2014.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cook RJ, Jones L, Fracchia G et al.. Globus pallidus internus deep brain stimulation as rescue therapy for refractory dyskinesias following effective subthalamic nucleus stimulation. Stereotact Funct Neurosurg. 2015;93(1):25-29. [DOI] [PubMed] [Google Scholar]
- 38. Matias CM, Silva D, Machado AG, Cooper SE. “Rescue” of bilateral subthalamic stimulation by bilateral pallidal stimulation: case report. J Neurosurg. 2016;124:417-421. doi: 10.3171/2015.1.jns141604 [DOI] [PubMed] [Google Scholar]
- 39. Sinclair NC, McDermott HJ, Bulluss KJ et al.. Subthalamic nucleus deep brain stimulation evokes resonant neural activity. Ann Neurol. 2018;83(5):1027-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swann NC, de Hemptinne C, Miocinovic S et al.. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in parkinson's disease. J Neurosci. 2016;36(24):6445-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pinto de Souza C, Hamani C, Oliveira Souza C et al.. Spinal cord stimulation improves gait in patients with Parkinson's disease previously treated with deep brain stimulation. Mov Disord. 2017;32(2):278-282. [DOI] [PubMed] [Google Scholar]
- 42. Samotus O, Parrent A, Jog M. Spinal cord stimulation therapy for gait dysfunction in advanced parkinson's disease patients. Mov Disord. 33(5):783-792. [DOI] [PubMed] [Google Scholar]
- 43. Almeida L, Martinez-Ramirez D, Rossi PJ, Peng Z, Gunduz A, Okun MS. Chasing tics in the human brain: development of open, scheduled and closed loop responsive approaches to deep brain stimulation for tourette syndrome. J Clin Neurol. 2015;11(2):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maling N, Hashemiyoon R, Foote KD, Okun MS, Sanchez JC. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with tourette's syndrome. PLoS One. 2012;7(9):e44215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol. 2013;245:77-86. doi: 10.1016/j.expneurol.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 46. Okun MS, Foote KD, Wu SS et al.. A trial of scheduled deep brain stimulation for tourette syndrome. JAMA Neurol. 2013;70(1):85-94. [DOI] [PubMed] [Google Scholar]
- 47. Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10(2):148-161. [DOI] [PubMed] [Google Scholar]
- 48. Kent AR, Grill WM. Recording evoked potentials during deep brain stimulation: development and validation of instrumentation to suppress the stimulus artefact. J Neural Eng. 2012;9(3):036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kent AR, Swan BD, Brocker DT, Turner DA, Gross RE, Grill WM. Measurement of evoked potentials during thalamic deep brain stimulation. Brain Stimul. 2015;8(1):42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. 2008;212(6):443-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herron JA, Thompson MC, Brown T, Chizeck HJ, Ojemann JG, Ko AL. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg. 2016:1-8. doi: 10.3171/2016.8.jns16536 [DOI] [PubMed] [Google Scholar]
- 52. Cagnan H, Pedrosa D, Little S et al.. Stimulating at the right time: phase-specific deep brain stimulation. Brain. 2017;140(1):132-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Basu I, Graupe D, Tuninetti D et al.. Pathological tremor prediction using surface electromyogram and acceleration: potential use in “ON-OFF” demand driven deep brain stimulator design. J Neural Eng. 2013;10(3):036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hardenacke K, Kuhn J, Lenartz D et al.. Stimulate or degenerate: deep brain stimulation of the nucleus basalis Meynert in Alzheimer dementia. World Neurosurg. 2013;80(S27):e35-e43. [DOI] [PubMed] [Google Scholar]
- 55. Hescham S, Lim LW, Jahanshahi A, Blokland A, Temel Y. Deep brain stimulation in dementia-related disorders. Neurosci Biobehav Rev. 2013;37(10):2666-2675. [DOI] [PubMed] [Google Scholar]
- 56. Lozano AM, Fosdick L, Chakravarty MM et al.. A Phase II study of fornix deep brain stimulation in mild alzheimer's disease. J Alzheimers Dis. 2016;54(2):777-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ponce FA, Asaad WF, Foote KD et al.. Bilateral deep brain stimulation of the fornix for Alzheimer's disease: surgical safety in the ADvance trial. J Neurosurg. 2015:1-10. doi: 10.3171/2015.6.JNS15716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hardenacke K, Kuhn J, Lenartz D et al.. Stimulate or degenerate: deep brain stimulation of the nucleus basalis Meynert in Alzheimer dementia. World Neurosurg. 2013;80(3-4):S27.e35-S27.e43. [DOI] [PubMed] [Google Scholar]
- 59. Antal A, Alekseichuk I, Bikson M et al.. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017;128(9):1774-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bystad M, Grønli O, Rasmussen ID et al.. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer's disease: a randomized, placebo-controlled trial. Alzheimers Res Ther. 2016;8(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gonsalvez I, Baror R, Fried P, Santarnecchi E, Pascual-Leone A. Therapeutic noninvasive brain stimulation in alzheimer's disease. Curr Alzheimer Res. 2017;14(4):362-376. [DOI] [PubMed] [Google Scholar]
- 62. Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26(16):2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crowell AL, Garlow SJ, Riva-Posse P, Mayberg HS. Characterizing the therapeutic response to deep brain stimulation for treatment-resistant depression: a single center long-term perspective. Front Integr Neurosci. 2015;9:41. doi: 10.3389/fnint.2015.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mayberg HS, Lozano AM, Voon V et al.. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660. [DOI] [PubMed] [Google Scholar]
- 65. Holtzheimer PE, Kelley ME, Gross RE et al.. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dougherty DD, Rezai AR, Carpenter LL et al.. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248. [DOI] [PubMed] [Google Scholar]
- 67. Riva-Posse P, Choi KS, Holtzheimer PE et al.. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215. [DOI] [PubMed] [Google Scholar]
- 69. Hartmann CJ, Lujan JL, Chaturvedi A et al.. Tractography activation patterns in dorsolateral prefrontal cortex suggest better clinical responses in OCD DBS. Front Neurosci. 2015;9:519. doi: 10.3389/fnins.2015.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lujan JL, Chaturvedi A, Choi KS et al.. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimulation. 2013;6(5):737-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martens HCF, Toader E, Decré MMJ et al.. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol. 2011;122(3):558-566. [DOI] [PubMed] [Google Scholar]
- 72. Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(02):225-239. [DOI] [PubMed] [Google Scholar]
- 73. Carron R, Chaillet A, Filipchuk A, Pasillas-Lépine W, Hammond C. Closing the loop of deep brain stimulation. Front Syst Neurosci. 2013;7:112. doi: 10.3389/fnsys.2013.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rehan M, Hong K-S. Modeling and automatic feedback control of tremor: adaptive estimation of deep brain stimulation. PLoS One. 2013;8(4):e62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Waldau B, Clayton DA, Gasperson LB, Turner DA. Analysis of the time course of the effect of subthalamic nucleus stimulation upon hand function in parkinson's patients. Stereotact Funct Neurosurg. 2011;89(1):48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Afshar P, Khambhati A, Stanslaski S et al.. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2012;6:117. doi: 10.3389/fncir.2012.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rouse AG, Stanslaski SR, Cong P et al.. A chronic generalized bi-directional brain-machine interface. J Neural Eng. 2011;8(3):036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stypulkowski PH, Stanslaski SR, Denison TJ, Giftakis JE. Chronic evaluation of a clinical system for deep brain stimulation and recording of neural network activity. Stereotact Funct Neurosurg. 2013;91(4):220-232. [DOI] [PubMed] [Google Scholar]
- 79. Little S, Beudel M, Zrinzo L et al.. Bilateral adaptive deep brain stimulation is effective in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2016;87(7):717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Little S, Pogosyan A, Neal S et al.. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tinkhauser G, Pogosyan A, Little S et al.. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson's disease. Brain. 2017;140(4):1053-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weiss D, Govindan RB, Rilk A et al.. Central oscillators in a patient with neuropathic tremor: evidence from intraoperative local field potential recordings. Mov Disord. 2011;26(2):323-327. [DOI] [PubMed] [Google Scholar]
- 83. Patil PG, Turner DA. The development of brain-machine interface neuroprosthetic devices. Neurotherapeutics. 2008;5(1):137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khobragade N, Graupe D, Tuninetti D. Towards fully automated closed-loop Deep Brain Stimulation in Parkinson's disease patients: A LAMSTAR-based tremor predictor. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2616-2619. doi: 10.1109/embc.2015.7318928 [DOI] [PubMed] [Google Scholar]
- 85. Vansteensel MJ, Pels EG, Bleichner MG et al.. Fully Implanted Brain-Computer Interface in a Locked-In Patient with ALS. N Engl J Med. 2016;375(21):2060-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Widge AS, Sahay A. Closing the Loop in Deep Brain Stimulation for Psychiatric Disorders: Lessons from Motor Neural Prosthetics. Neuropsychopharmacol. 2016;41(1):379-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu H, Miller KJ, Blumenfeld Z et al.. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc Natl Acad Sci USA. 2018;115(1):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]