Abstract
Background
We previously found that African Americans and Native Hawaiians were at highest lung cancer risk compared with Japanese Americans and Latinos; whites were midway in risk. These differences were more evident at relatively low levels of smoking intensity, fewer than 20 cigarettes per day (CPD), than at higher intensity.
Methods
We apportioned lung cancer risk into three parts: age-specific background risk (among never smokers), an excess relative risk term for cumulative smoking, and modifiers of the smoking effect: race and years-quit smoking. We also explored the effect of replacing self-reports of CPD with a urinary biomarker—total nicotine equivalents—using data from a urinary biomarker substudy.
Results
Total lung cancers increased from 1979 to 4993 compared to earlier analysis. Estimated excess relative risks for lung cancer due to smoking for 50 years at 10 CPD (25 pack-years) ranged from 21.9 (95% CI = 18.0 to 25.8) for Native Hawaiians to 8.0 (95% CI = 6.6 to 9.4) for Latinos over the five groups. The risk from smoking was higher for squamous cell carcinomas and small cell cancers than for adenocarcinomas. Racial differences consistent with earlier patterns were seen for overall cancer and for cancer subtypes. Adjusting for predicted total nicotine equivalents, Japanese Americans no longer exhibit a lower risk, and African Americans are no longer at higher risk, compared to whites. Striking risk differences between Native Hawaiians and Latinos persist.
Conclusions
Racial differences in lung cancer risk persist in the Multiethnic Cohort study that are not easily explained by variations in self-reported or urinary biomarker-measured smoking intensities.
Lung cancer is the most common cause of cancer death in the United States (1). In an earlier analysis from the Multiethnic Cohort Study (MEC) (2), we found that, compared with African American and Native Hawaiian men, white men had a 40%, Japanese American men a 54%, and Latino men a 70% lower excess relative risk (ERR) of lung cancer for the same quantity of cigarettes smoked. For women, compared with African Americans, Native Hawaiians had a 20% lower risk of lung cancer and whites had a 17% lower risk, while Japanese Americans and Latinos had approximately 70% lower risk. These differences in ERR were more pronounced among those who smoked less intensely (<20 cigarettes per day [CPD]).
This update presents results based on 11 years of additional follow-up, additional questionnaire data, and biomarker data for a subset of current smokers (3). We added 3014 lung cancer cases, strengthening investigation of the risk of major lung cancer subtypes by race/ethnicity. The additional questionnaire data allow prospective study of quitting rates, which also may differ by race/ethnicity. Data from a biomarker substudy ertr used to estimate an internal smoking dose variable (total nicotine equivalents [TNE]), which is a more objective measure of smoking intensity than CPD (3).
Methods
Study Population and Follow-up
We investigated the risk of lung cancer using the MEC, a prospective cohort study established to investigate the association of lifestyle and dietary factors with chronic disease, within a diverse multiethnic population. See Kolonel et al. for study design details (4). In short, the cohort comprises greater than 215 000 men and women who were recruited in 1993–1996, were between the ages of 45 and 75 years at baseline, and self-identified as primarily belonging to one of the five racial/ethnic populations: African Americans, Japanese Americans, Latinos, Native Hawaiians, and whites. Potential participants were identified and recruited in Hawaii and California. Each participant completed an extensive self-administered questionnaire at baseline including questions about tobacco smoking. The University of Hawaii and the University of Southern California institutional review boards approved the study protocol, and all participants provided written informed consent.
The analytic cohort here comprises 183 656 MEC participants from the five main racial/ethnic groups without a prior lung cancer who provided adequate covariate data including smoking status, intensity, duration, and time of cessation. A total of 4993 lung cancers were identified prospectively between time of cohort entry and the end of 2012. Lung cancer cases were ascertained using linkage to the California and Hawaii Surveillance, Epidemiology, and End Results Program (SEER) tumor registries (5). Lung cancers were defined by the International Classification of Diseases for Oncology (ICD-O-3) (6) and ICD-10, C34 (7). Major lung cancer histological cell types were categorized using morphological codes found in Lewis et al. (8). Deaths were ascertained by regular linkage to state death certificate files and to the National Death Index.
Epidemiologic Data
The questionnaire data have been described previously (4). Briefly, all epidemiologic covariates were derived from the baseline questionnaire. Smoking data were updated using a second full follow-up questionnaire, which was obtained approximately 10 years after baseline (9).
Measure of TNE in the Subcohort
The subcohort of current smokers with smoking biomarkers available has been described previously (3). TNE was quantified as the sum of the urinary concentrations of total nicotine, total cotinine, total trans-3′-hydroxycotinine, and nicotine N-oxide, where “total” refers to the sum of the metabolite and its glucuronide conjugate. These biomarkers were measured using liquid chromatography tandem mass spectrometry (4) in 2239 MEC current smokers free of lung cancer who provided a urine sample. TNE is a superior biomarker to cotinine or nicotine of short-term internal smoking dose (3,10). While nicotine is not a carcinogen, TNE correlates with biomarkers of carcinogen uptake (11).
Statistical Methods
We used an ERR model for risk of incident lung cancer dependent on age, racial/ethnic group, pack-years of smoking, years-quit, and other covariates.
The ERR model we used is written as
| (Eq. 1) |
where the baseline term describes risk for never smokers. The ERR term splits into
In our simplest model the baseline risk (risk for never smokers) was assumed to be proportional to age t raised to a power, while the modifiers of the smoking (pack-year) effect included years since quit, racial/ethnic group, and interaction between smoking intensity and racial/ethnic group. We used Poisson regression for survival analysis (12) implemented in Epicure (13). Further modeling information is given in the Supplementary Methods (available online). The person-years table used in the Poisson regression is available from the authors upon request.
Because most smokers eventually quit, the pack-years variable is uncertain for current smokers at times beyond their last questionnaire contact. We adjusted smoking duration for quitting by assessing quitting rates among 11 630 current smokers at baseline who returned a valid follow-up questionnaire. Details of the quitting assessment and how it was used to modify smoking duration is given in the Supplementary Methods (available online).
We supplemented reported smoking intensity with data from a study of TNE in urine (14) for 2239 current smokers at the time of biospecimen collection. Differences in TNE levels for a given CPD may reflect differences in self-reporting accuracy or in smoking behavior that would also influence carcinogen exposure and underlie racial differences in risk (11).
As in regression calibration (15,16), we estimated a least–squares prediction model for urinary values of TNE in our biomarker substudy (3,11), including as explanatory variables CPD, race, sex, body mass index (BMI), and interactions between CPD and race and CPD and sex. We replaced CPD in some of the risk analyses with predicted TNE.
When estimating ratios of excess risk across racial ethnic groups for overall cancer and cancer subtypes (as in table 4 of our previous publication [2]), we set the parameters in the baseline portion of the model (Eq. 1) to those estimated using all the data (nonsmokers, ex-smokers, and current smokers at all smoking rates). The values used are given in Supplementary Table 3 (available online; first two listed for each subtype).
Table 4.
Ratios of smoking-related excess relative risk (ERR) among current and former smokers, according to the level of smoking
| Smoking level | African American | Native Hawaiian | Latino | Japanese American | White | Global P* |
|---|---|---|---|---|---|---|
| ≤ 10 CPD | ||||||
| ERR ratio (95% CI)† | 1 | 1.22 (0.94 to 1.59) | 0.35 (0.27 to 0.44) | 0.48 (0.38 to 0.61) | 0.62 (0.48 to 0.78) | |
| P‡ | − | 0.14 | <0.001 | <0.001 | <0.001 | <.001 |
| Cases of lung cancer | 410 | 88 | 193 | 172 | 161 | |
| No. of participants | 9876 | 2747 | 12 820 | 8375 | 7638 | |
| 11 to 20 CPD | ||||||
| ERR ratio (95% CI)† | 1 | 1.08 (0.90 to 1.30) | 0.56 (0.47 to 0.68) | 0.60 (0.52 to 0.70) | 0.69 (0.60 to 0.79) | |
| P‡ | − | 0.41 | <0.001 | <0.001 | <0.001 | <.001 |
| Cases of lung cancer | 496 | 176 | 203 | 433 | 423 | |
| No. of participants | 6505 | 3058 | 4923 | 10 678 | 9872 | |
| 21 to 30 CPD | ||||||
| ERR ratio (95% CI)† | 1 | 1.09 (0.83 to 1.44) | 0.75 (0.56 to 1.00) | 0.82 (0.65 to 1.02) | 0.87 (0.71 to 1.07) | .07 |
| P‡ | − | 0.54 | 0.06 | 0.07 | 0.19 | |
| Cases of lung cancer | 139 | 94 | 85 | 288 | 377 | |
| No. of participants | 1671 | 1429 | 1405 | 4710 | 6058 | |
| ≥31 CPD | ||||||
| ERR ratio (95% CI)† | 1 | 1.25 (0.89 to 1.74) | 0.71 (0.49 to 1.03) | 0.77 (0.58 to 1.04) | 0.86 (0.67 to 1.12) | .02 |
| P‡ | − | 0.20 | 0.08 | 0.08 | 0.28 | |
| Cases of lung cancer | 79 | 74 | 52 | 157 | 310 | |
| No. of participants | 758 | 788 | 800 | 2306 | 3968 |
Global P is calculated using a four-degree of freedom likelihood ratio test for heterogeneity of the ERRs by race/ethnicity. CI = confidence interval; CPD = cigarettes per day.
Excess risks are adjusted for the duration of smoking and time since quitting. For each cancer subtype, the parameters in the baseline model (risk in never smokers) were estimated from the fit of the baseline parameters in (Eq. 1) for each subtype; using all data, see explanation in Methods.
P values for single-degree of freedom tests computed using a two-sided Wald test.
P is computed from likelihood calculations (Wald and likelihood ratio tests) for survival analysis models and as t-tests for ordinary least squares regression. All tests were two-sided and P less than .05 was considered statistically significant.
Results
Study Demographics
Table 1 gives demographic information from the baseline questionnaire. We observed a wide range in levels of education, smoking prevalence, and smoking intensity within each race- and sex-defined group. The total number of lung cancers increased from 1979 to 4993 overall compared with our previous publication (2). Lung cancer totals by histological subtype are also shown.
Table 1.
Demographic, exposure, and lung cancer outcome summaries for the analytic cohort (N = 183 656)
| Variable | Men (N = 82 408) |
Women (N = 101 248) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| African American | Native Hawaiian | Latino | Japanese American | White | African American | Native Hawaiian | Latino | Japanese American | White | |
| No. of participants | 11 178 | 5801 | 19 465 | 24 968 | 20 996 | 19 865 | 7541 | 20 972 | 28 162 | 24 708 |
| Mean age (SD), y | 62.4 (8.9) | 57.1 (8.6) | 60.6 (7.7) | 61.6 (9.2) | 59.4 (9.1) | 61.4 (9.1) | 56.6 (8.7) | 59.7 (7.8) | 61.4 (9) | 59.3 (9) |
| Level of education, % | ||||||||||
| ≤8 y | 8.5 | 5.2 | 31.7 | 3.0 | 3.2 | 5.8 | 3.6 | 34.6 | 4.4 | 2.6 |
| 9–12 y | 32.7 | 44.8 | 31.4 | 34.1 | 18.8 | 34.4 | 51.0 | 36.6 | 37.6 | 27.1 |
| Completion of vocational school | 5.5 | 8.3 | 6.5 | 12.7 | 4.2 | 6.6 | 7.8 | 6.4 | 13.0 | 4.7 |
| Some college | 52.9 | 41.5 | 29.5 | 50.1 | 73.8 | 52.6 | 37.4 | 21.4 | 44.6 | 65.4 |
| Smoking status, % | ||||||||||
| Current smoker | 23.7 | 16.8 | 14.9 | 11.0 | 12.9 | 16.6 | 17.7 | 8.2 | 6.6 | 12.7 |
| Former smoker | 51.0 | 51.5 | 52.5 | 59.7 | 54.7 | 36.7 | 37.1 | 25.5 | 24.3 | 42.2 |
| Never smoked | 25.3 | 31.6 | 32.5 | 29.3 | 32.4 | 46.7 | 45.1 | 66.3 | 69.1 | 45.1 |
| Number of ever smokers total and by smoking intensity, %* | 8345 | 3965 | 13 135 | 17 663 | 14 186 | 10 587 | 4137 | 7073 | 8694 | 13 570 |
| ≤10 CPD | 43.4 | 25.2 | 59.1 | 22.8 | 21.0 | 60.2 | 44.2 | 75.2 | 53.2 | 36.0 |
| 11–20 CPD | 39.7 | 39.1 | 27.7 | 43.4 | 35.0 | 30.2 | 36.5 | 18.1 | 34.6 | 36.2 |
| 21–30 CPD | 11.1 | 22.1 | 8.4 | 22.2 | 25.1 | 7.0 | 13.4 | 4.3 | 9.2 | 18.4 |
| ≥31 CPD | 5.8 | 13.6 | 4.8 | 11.6 | 19.0 | 2.6 | 6.0 | 2.3 | 3.0 | 9.4 |
| Duration of smoking for ever smokers, %* | ||||||||||
| <20 y | 37.1 | 40.5 | 49.7 | 41.8 | 43.5 | 46.2 | 43.2 | 60.0 | 52.1 | 46.8 |
| 20–40 y | 43.9 | 46.2 | 36.3 | 44.1 | 42.0 | 42.1 | 47.3 | 31.5 | 41.0 | 41.0 |
| >40 y | 19.0 | 13.2 | 14.0 | 14.0 | 14.5 | 11.7 | 9.4 | 8.7 | 6.9 | 12.2 |
| Number of former smokers total and by time since quit, %† | 5698 | 2989 | 10 228 | 14 913 | 11 476 | 7287 | 2801 | 5356 | 6831 | 10 424 |
| ≤5 y | 28.1 | 27.3 | 25.5 | 20.9 | 23.5 | 35.0 | 38.6 | 34.1 | 29.3 | 27.6 |
| 6–15 y | 30.8 | 30.0 | 29.5 | 28.9 | 27.4 | 33.5 | 29.5 | 32.4 | 29.8 | 28.2 |
| >15 y | 41.1 | 42.7 | 45.0 | 50.2 | 49.1 | 31.5 | 31.9 | 33.5 | 40.9 | 44.2 |
| Mean (SD) BMI, kg/m2 | ||||||||||
| Current smoker | 25.7 (5.0) | 28.3 (5.8) | 26.7 (4.4) | 24.8 (3.7) | 25.9 (4.2) | 27.5 (6.8) | 27.7 (6.6) | 27.1 (5.7) | 23.4 (4.4) | 24.9 (5.6) |
| Former smoker | 27.2 (5.2) | 29.5 (5.6) | 27.7 (4.2) | 25.4 (3.4) | 26.8 (4.1) | 28.8 (7.0) | 29.2 (7.3) | 28.6 (6.1) | 24.1 (4.3) | 25.8 (5.6) |
| Never smoked | 27.1 (5.1) | 29.3 (5.6) | 27.2 (4.1) | 25.0 (3.2) | 26.3 (4.1) | 28.7 (7.0) | 28.4 (6.7) | 27.5 (5.5) | 23.2 (4.1) | 25.9 (5.6) |
| Cases of lung cancer, No. (%) | 576 (5.1) | 241 (4.1) | 399 (2.0) | 836 (3.3) | 669 (3.2) | 644 (3.2) | 225 (3.0) | 263 (1.3) | 430 (1.5) | 709 (2.9) |
| Histologic type, No. (%) | ||||||||||
| Adenocarcinoma | 197 (34.2) | 86 (35.7) | 127 (31.8) | 332 (39.7) | 242 (36.2) | 244 (37.9) | 80 (35.6) | 119 (45.2) | 225 (52.3) | 307 (43.3) |
| Squamous cell | 137 (23.8) | 61 (25.3) | 92 (23.1) | 192 (23.0) | 158 (23.6) | 137 (21.3) | 37 (16.4) | 31 (11.8) | 55 (12.8) | 102 (14.4) |
| Large cell | 28 (4.9) | 4 (1.7) | 22 (5.5) | 28 (3.3) | 17 (2.5) | 29 (4.5) | 3 (1.3) | 9 (3.4) | 9 (2.1) | 15 (2.1) |
| Small cell | 45 (7.8) | 36 (14.9) | 33 (8.3) | 74 (8.9) | 72 (10.8) | 64 (9.9) | 45 (20) | 27 (10.3) | 37 (8.6) | 78 (11.0) |
| Unspecified neoplasm and other cell types | 169 (29.3) | 54 (22.4) | 125 (31.3) | 210 (25.1) | 180 (26.9) | 170 (26.4) | 60 (26.7) | 77 (29.3) | 104 (24.2) | 207 (29.2) |
Current and former smokers from first questionnaire. BMI = body mass index; CPD = cigarettes per day; SD = standard deviation.
From first questionnaire.
Quitting Rates
Table 2 gives quitting totals for current smokers on the first questionnaire who returned the later questionnaire, stratified by racial/ethnic group and sex. After adjustment for the other factors (Supplementary Methods, available online), the quitting rates were lowest among African American smokers and highest among Japanese Americans, with Japanese Americans quitting at an absolute rate about 1.1% per year higher than African Americans for the same age and smoking intensity (see Supplementary Figure 1 and Supplementary Table 1, available online). The quitting rate also depended on age at cohort entry, CPD, and on time on study (which may in part reflect higher rates of quitting more recently compared with the past). We found that the rate of quitting ranged from zero for younger African American heavy smokers (CPD = 35) shortly after cohort entry to 10.3% per year for older Japanese American light smokers (CPD = 10) 10 years after cohort entry, with quit rates positively dependent on starting age and time on study and inversely dependent on smoking intensity. See Supplementary Table 1 and Supplementary Figure 1 (available online) for details of the quitting model.
Table 2.
Quitting among current smokers at baseline*
| Smoking group | Men (N = 15 027) No. (%) |
Women (N = 14 527) No. (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| African American | Native Hawaiian | Latino | Japanese American | White | African American | Native Hawaiian | Latino | Japanese American | White | |
| Total number of current smokers on QX1 | 3032 | 1270 | 3581 | 3730 | 3414 | 3975 | 1748 | 2199 | 2515 | 4090 |
| Total number and (%) of current smokers on QX1 who returned later questionnaire | 688 (22.7) | 534 (42.0) | 1140 (31.8) | 1811 (48.6) | 1438 (42.1) | 1170 (29.4) | 796 (45.6) | 798 (36.3) | 1246 (49.5) | 1856 (45.4) |
| Total number and (%) of current smokers on QX1 who returned later questionnaire and who reported continued smoking | 274 (39.8) | 218 (40.8) | 413 (36.3) | 753 (41.6) | 613 (42.6) | 451 (38.6) | 361 (45.4) | 286 (35.8) | 548 (44.0) | 820 (44.2) |
| Total number and (%) of current smokers on QX1 who reported quitting on later questionnaire | 414 (60.2) | 316 (59.2) | 727 (63.7) | 1058 (58.4) | 825 (57.4) | 719 (61.4) | 435 (54.6) | 512 (64.2) | 698 (56.0) | 1036 (55.8) |
*QX1 = first questionnaire
Supplementary Table 2 (available online) provides the demographic information of individuals who were alive as of 2006, indicating whether they returned the later questionnaire. Return rates were higher in whites, Japanese Americans, and Native Hawaiians compared with African Americans and Latinos. In addition, current smokers returned proportionately fewer questionnaires compared with former and never smokers.
Overall Risk of Lung Cancer and Lung Cancer Subtypes
In men, not smoking adjusted, African Americans and Native Hawaiians had the highest incidence of lung cancer (incidence rates of 252.1 per 100 000 population for both groups) (Table 3), while in women, the incidence per 100 000 population was similar across African Americans, Native Hawaiians, and whites, 146.8, 167.4, and 135.1, respectively. For overall lung cancer, Japanese American and Latino men and women had statistically significantly lower risk than African Americans, with relative risks ranging from 0.36 (95% CI = 0.33 to 0.45) for Latino women to 0.59 (95% CI = 0.52 to 0.65) for Japanese American men. These results are comparable to those reported previously (2) (Table 3), where the range of relative risks was from 0.29 for Latino women (compared with African Americans) to 0.46 for Japanese American men. The general pattern of ethnic/racial disparities in lung cancer risk is also seen in adenocarcinomas and squamous cell carcinomas. For small cell tumors, Native Hawaiians are at much higher risk than any other group (P < .001). Risk of large cell tumors is highest in African Americans.
Table 3.
Age-standardized incidence rates and relative risks of lung cancer among men and women according to ethnic or racial group, histologic cell type, and stage of disease
| Variable | African American | Native Hawaiian | Latino | Japanese American | White | Total No. |
|---|---|---|---|---|---|---|
| Variable sex men | ||||||
| No. of men | 11 178 | 5801 | 19 465 | 24 968 | 20 996 | 82 408 |
| Cases of lung cancer | 576 | 241 | 399 | 836 | 669 | 2721 |
| Incidence per 100 000* | 252.1 | 252.1 | 95.8 | 148.7 | 161.3 | |
| Relative risk (95% CI) | 1.00 (Referent) | 1.00 (0.86 to 1.16) | 0.38 (0.33 to 0.43) | 0.59 (0.52 to 0.65) | 0.64 (0.57 to 0.71) | |
| Women | ||||||
| No. of women | 19 865 | 7541 | 20 972 | 28 162 | 24 708 | 101 248 |
| Cases of lung cancer | 644 | 225 | 264 | 430 | 709 | 2272 |
| Incidence per 100 000* | 146.8 | 167.4 | 52.8 | 61.7 | 135.1 | |
| Relative risk (95% CI) | 1.00 (Referent) | 1.14 (0.98 to 1.35) | 0.36 (0.33 to 0.45) | 0.42 (0.37 to 0.48) | 0.92 (0.83 to 1.03) | |
| Histologic type† | ||||||
| Adenocarcinoma | ||||||
| No. | 441 | 166 | 247 | 557 | 549 | 1960 |
| Incidence per 100 000* | 66.4 | 72.4 | 27.9 | 44.5 | 59.1 | |
| Relative risk (95% CI) | 1.00 (Referent) | 1.09 (0.94 to 1.27) | 0.42 (0.37 to 0.49) | 0.67 (0.59 to 0.76) | 0.89 (0.80 to 0.99) | |
| Squamous cell | ||||||
| No. | 274 | 98 | 123 | 247 | 260 | 1002 |
| Incidence per 100 000* | 40.7 | 42.7 | 13.8 | 19.5 | 27.7 | |
| Relative risk (95% CI) | 1.00 (Referent) | 1.05 (0.83 to 1.32) | 0.34 (0.28 to 0.42) | 0.48 (0.40-0.57) | 0.68 (0.57 to 0.80) | |
| Small cell | ||||||
| No. | 109 | 81 | 60 | 111 | 150 | 511 |
| Incidence per 100 000* | 17.1 | 34.2 | 7 | 7.7 | 16.2 | |
| Relative risk (95% CI) | 1.00 (Referent) | 2.0 (1.51 to 2.7) | 0.41 (0.30 to 0.55) | 0.45 (0.42 to 0.71) | 0.95 (0.74 to 1.22) | |
| Large cell | ||||||
| No. | 57 | 7 | 31 | 37 | 32 | 164 |
| Incidence per 100 000* | 9.2 | 2.9 | 3.7 | 3.2 | 3.5 | |
| Relative risk (95% CI) | 1.00 (Referent) | 0.32 (0.15 to 0.71) | 0.40 (0.26 to 0.62) | 0.35 (0.23 to 0.53) | 0.38 (0.25 to 0.59) | |
| Other/unspecified cell types | ||||||
| No. | 280 | 98 | 171 | 278 | 321 | 1148 |
| Incidence per 100 000* | 41.4 | 44.3 | 19.5 | 21.5 | 34.4 | |
| Relative risk (95% CI) | 1.00 (Referent) | 1.07 (0.85 to 1.35) | 0.47 (0.39 to 0.57) | 0.52 (0.44 to 0.62) | 0.83 (0.71 to 0.98) |
Rates for African Americans were adjusted to the 2000 standard population. The incident rates for the other groups were computed by multiplying this figure by the relative risks. CI = confidence interval.
This category includes men and women.
Cigarette smoking is crucial to understanding lung cancer patterns. Table 4 gives ratios of lung cancer ERR between racial/ethnic groups by smoking rate for current and former smokers. Here we adjust for cumulative smoking (pack-years) and years since quitting as described in the Supplementary Methods (available online). The heterogeneity of racial/ethnic ERRs is stronger at lower smoking intensities (<20 CPD) where the risk differences are very statistically significant (P for heterogeneity <.001) than at higher smoking intensities (>21–30 CPD or ≥31, P for heterogeneity 0.07 and 0.02, respectively).
Examples of estimated ERRs among current smokers are given in Table 5. All groups of smokers show much higher rates of lung cancer compared with never smokers even at lower smoking intensities. Estimated ERRs for lung cancer due to smoking for 50 years at 10 CPD (25 pack-years) ranged from 21.9 (95% CI = 18.0 to 25.8) for Native Hawaiians to 8.0 (95% CI = 6.6 to 9.4) for Latinos over the five groups. Risk for all groups after smoking 20 CPD for 50 years are higher than at 10 CPD but are more similar to each other at the 20 CPD intensity than the 10 CPD intensity.
Table 5.
Estimated excess relative risk (ERR) of smoking-related lung cancer by histologic cell-types and race/ethnicity*
| Smoking level | African American ERR (95% CI) | Native Hawaiian ERR (95% CI) | Latino ERR (95% CI) | Japanese American ERR (95% CI) | White ERR (95% CI) |
|---|---|---|---|---|---|
| After accumulation of 25 pack-years at 10 CPD | |||||
| All lung cancers | 19.1 (16.9 to 21.3) | 21.9 (18.0 to 25.8) | 8.0 (6.6 to 9.4) | 10.1 (8.5 to 11.7) | 11.9 (10.0 to 13.8) |
| Adenocarcinoma | 8.3 (6.7 to 9.9) | 9.6 (6.6 to 12.6) | 2.8 (1.8 to 3.8) | 5.7 (4.3 to 7.1) | 5.7 (4.2 to 7.2) |
| Squamous cell | 77.8 (51.3 to 104.3) | 80.7 (44.6 to 116.8) | 31.4 (18.4 to 44.4) | 36.5 (21.4 to 51.5) | 42.4 (25.1 to 59.7) |
| Small cell | 104.2 (43.4 to 164.9) | 191.8 (67.5 to 316.1) | 59.4 (21.4 to 97.4) | 59.9 (20.1 to 99.7) | 76.7 (27.1 to 126.3) |
| Large cell | 75.6 (21.9 to 129.3) | 25.1 (-8.0 to 58.2) | 33.2 (5.0 to 61.4) | 14.4 (-1.9 to 30.7) | 26.9 (1.4 to 52.4) |
| After accumulation of 50 pack-years at 20 CPD | |||||
| All lung cancers | 31.7 (28.1 to 35.3) | 35.3 (30.5 to 40.1) | 20.0 (17.1 to 22.9) | 21.4 (16.0 to 23.8) | 24.0 (21.5 to 26.5) |
| Adenocarcinoma | 14.3 (11.7 to 16.9) | 14.7 (11.3 to 18.1) | 6.9 (5.0 to 8.8) | 9.3 (7.6 to 11.0) | 11.5 (11.2 to 12.4) |
| Squamous cell | 134.2 (88.5 to 179.9) | 142.6 (89.5 to 195.7) | 77.8 (47.8 to 107.8) | 91.9 (60.7 to 123.1) | 86.3 (57.3 to 115.3) |
| Small cell | 160.3 (65.4 to 255.2) | 365 (153.3 to 576.7) | 131.7 (49.6 to 213.8) | 144 (61.1 to 226.9) | 160.6 (69.9 to 251.3) |
| Large cell | 96.2 (24.7 to 167.7) | 33.8 (-3.4 to 71.1) | 85.2 (18.6 to 151.8) | 54.7 (12.8 to 96.6) | 38.8 (8.4 to 69.2) |
CI = confidence interval; CPD = cigarettes per day; ERR =excess relative risk.
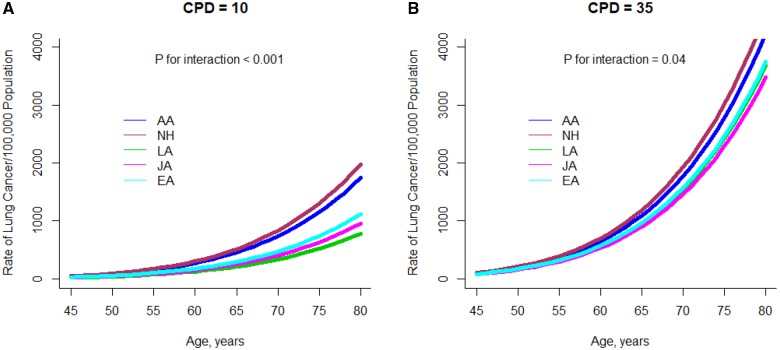
Figure 1 shows plots of the fitted absolute risks (baseline + excess) of all lung cancer by age for current smokers; plots for lung cancer subtypes are in Supplementary Figure 2 (available online). As in Table 5, Native Hawaiians and African Americans have a higher risk of all lung cancer, adenocarcinoma, and squamous cell carcinoma both at 10 CPD and 35 CPD, but racial/ethnic differences are less pronounced at 35 CPD than at 10.
Figure 1.
Plot of the model-predicted lung cancer risk for current smokers by age and racial/ethnic group and reported smoking intensity. A) Risk at 10 cigarettes per day and (B) at 35 cigarettes per day is shown. CPD = cigarettes per day; AA= African Americans; CPD = cigarettes per day; JA = Japanese Americans; LA = Latino Americans; NH = Native Hawaiians.
Baseline rates (estimated risk for never smokers) increased with age raised to the 5.2 power (95% CI = 5.0 to 5.5) in the analysis of overall lung cancer (Supplementary Table 3, available online). Restricting the analysis to never smokers, the increase with age was proportional to age raised to the 5.0 power (95% CI = 4.3 to 5.7). Baseline rate of disease did not differ by race/ethnicity (P = .49), or by sex (P = .96) among the never smokers.
The model described in the Supplementary Methods (available online) includes an effect of quitting smoking. The effect of quitting was quite stable in most models for lung cancer and lung cancer subtypes. For overall lung cancer, there was a 5.3% reduction (95% CI = 5.5% to −5.2%) in ERR of smoking for each year since quitting (see Supplementary Table 3, available online).
Additional Modifiers of Risk
As a model check, we investigated adequacy of the polynomial representation of lung cancer risk in never smokers and validity of the implied assumption that excess cancer risk is proportional to baseline risks at all ages. We added log(age)2 to the baseline portion of the model to allow for additional upward or downward curvature in the relationship between age and baseline risk. This addition was not statistically significant (P = .24). To investigate validity, we added log(age) as a modifier of the ERRs. This inclusion was not statistically significant (P = .07), and neither inclusion altered the basic relationships shown between race/ethnicity, smoking intensity, and cumulative smoking.
In the full model, sex was only weakly associated with modification of the ERR due to smoking; women had an estimated ERR of 6.8% less (95% CI = −13.2% to 0.0%, P = .06) than men (data not shown). In subtype analysis, female sex was statistically significantly protective for squamous cell carcinoma, having an ERR of 31.7% (95% CI = −40.9% to −21.1%) less than the men (P < .001). For small cell carcinomas, the ERR was 27.8% higher (95% CI = 5.7% to 54.6%) for women (P = .01).
Increased BMI was protective (P < .001) against lung cancer risk with a 2.3% decrease per unit BMI in the ERR due to smoking (95% CI = −3.0% to −1.6%). A one-year increase in years of schooling was associated with a 4.1% decrease in the ERR (95% CI = −5.4 to −3.0, P < .001). Employment and occupations deemed potentially exposed to lung carcinogens (farming, machine-working, factory, or crafts work) were not statistically significantly associated with risk either in the cohort as a whole (P = .16) or among never smokers (P = 0.50).
Application of TNE Estimation in the Lung Cancer Risk Analysis
Supplementary Table 4 (available online) gives demographic data for the participants of the calibration substudy. The regression parameters used for TNE prediction are shown in Supplementary Table 5 (available online), and the relationship between TNE and CPD is depicted in Supplementary Figure 3 (available online). Supplementary Table 6 (available online) displays the parameter estimates for the lung cancer risk model fit using TNE. Supplementary Table 7 (available online) provides additional information, updating Table 4 to use predicted TNE as the smoking intensity variable. When we fit a simple linear regression (Supplementary Table 5, available online) for urinary TNE on CPD race/ethnicity, sex, and BMI and interactions between CPD, and race, and sex, African Americans were estimated to have statistically significantly higher TNE than other groups. We also found that female sex (P < .0001) and higher BMI (P< .002) were statistically significantly associated with lower TNE after adjustment for CPD.
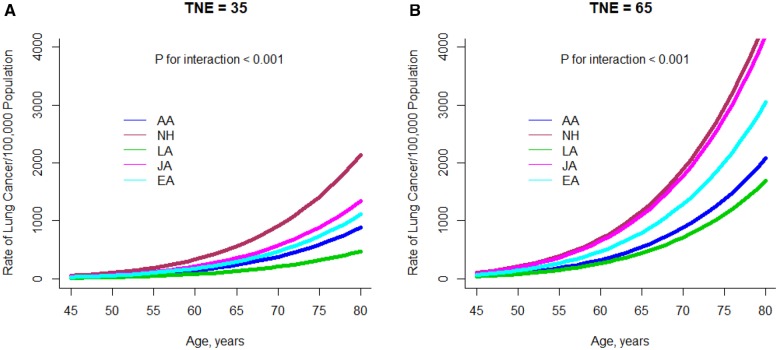
Replacing CPD with predicted TNE (using the parameter estimates in Supplementary Table 5, available online), racial/ethnic differences (Figure 2) are statistically significant both at low predicted TNE (TNE = 35 nmol/mL, df = 4, P < .001) and at high predicted TNE (TNE = 65 nmol/mL, df = 4, P < .001). The risk ranking of the ethnic groups is different from that seen with CPD in Figure 2, which is also evident in Supplementary Table 7 (available online): The Japanese Americans no longer show reduced risk compared with African Americans adjusting for predicted TNE at lower intensity and are very similar to the high-risk Native Hawaiians at high intensity. At TNE levels greater than 50 nmol/ml, the difference in the ERRs between Latinos and African Americans is attenuated, evident both in Supplementary Table 7 (available online) and Figure 2B.
Figure 2.
Plot of the model-predicted lung cancer risk by age for current smokers according to racial/ethnic group and predicted total nicotine equivalents. A) Risk at 35 nmol/mL and (B) at 65 nmol/mL is shown. TNE = total nicotine equivalents; AA= African Americans; CPD = cigarettes per day; JA = Japanese Americans; LA = Latino Americans; NH = Native Hawaiians; TNE = total nicotine equivalents.
We noted that two variables used in the calibration equation to predict TNE showed effects in the risk analysis based on TNE. Sex, which was not statistically significant in the risk analysis based on CPD, became statistically significant when using TNE. Women were at greater excess risk compared with males with excess risk higher by a factor of 1.32 (95% CI = 1.21 to 1.44; data not shown). This reflects the finding that women have lower TNE levels then men of the same ethnic group and CPD level (Supplementary Table 5, available online). When added as a modifier of the ERR for TNE, BMI was still protective, but the decrease in ERR per unit BMI was only half (1.3% decrease, 95% CI = 2.1% to 0.5%) that given above using CPD. These additions to the model did not meaningfully alter the order of the relationships between race/ethnicity and TNE-related ERR.
Discussion
With more than double the number of lung cancer cases, we find that many of the effects seen in our original report (2) persist: At lower CPD, Native Hawaiians and African American smokers have twice the excess risk of lung cancer compared with Japanese Americans and 2.7 times more than Latinos. At higher levels of cigarette consumption (≥ 31 CPD), risk differences are no longer as strong as those seen at low doses. Similar race/ethnicity patterns were observed for two of the subtypes: adenocarcinoma and squamous cell carcinomas. In contrast, results for small cell carcinomas showed the Native Hawaiians to be at the highest risk, especially at greater than or equal to 31 CPD compared with any of the other ethnic groups. This corresponds to an earlier SEER report that found Native Hawaiian/Pacific Islanders to have a higher incidence rate (not adjusted for smoking) of small cell carcinoma (17). For large cell carcinomas, African Americans clearly had the highest risk at lower intensity, but the sample size (164 cancers total) is rather limited to give more detailed information.
There are many factors that could underlie these risk differences, notably reporting accuracy, smoking behavior, and quitting behavior (18–20). We found that African Americans and Latinos had lower quit rates than the other groups, which has been reported before for African Americans (21) and Latinos (22). To account for quitting, we applied a model for quitting rates when computing smoking duration for current smokers. However, lack of information on the behavior of smokers not providing a second questionnaire is a potential weakness here, and current smokers were less likely to return the later questionnaire.
In the biomarker substudy, consistent with the direction of their smoking-related lung cancer risk, African Americans uptake greater amounts of nicotine per cigarette (as measured by urinary TNE) than any of the other four populations, while Japanese Americans uptake less (23). TNE is highly correlated with the uptake of known tobacco carcinogens such as nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (r = 0.70) and polycyclic aromatic hydrocarbons (r = 0.47) (24), as well as other compounds. The lower TNE observed in Japanese Americans is primarily a result of their lower activity of the enzyme CYP2A6, resulting in longer maintenance of nicotine in the body and less need to smoke intensely (10,25,26). To explore the possibility that the miss-measure of smoking intensity from self-reported data confounds our earlier findings, data from the biomarker substudy were used to estimate an objective measurement of nicotine uptake to replace self-reported smoking intensity for ever smokers. At fixed levels of predicted TNE, the apparent susceptibilities to lung cancer risk by racial/ethnic group are partially reordered. Both at low and high levels of predicted TNE (35 nmol/mL and 65 nmol/mL, respectively) the Japanese Americans are now higher than the whites, and the African Americans are similar to the whites (Figure 2). At high levels of predicted TNE (65 nmol/ml), the Japanese are similar to the high-risk Native Hawaiians, and African Americans and Latinos are at lower risk.
Of course, it is important to note that fewer Japanese American smokers have TNE as high as the other groups. In this sense, the Japanese Americans remain a lower risk group. In contrast, Native Hawaiians and Latinos remain the highest and lowest risk groups, indicating that they may truly have a high and low susceptibility to lung cancer due to smoking.
A primary limitation of our study is the reliance on self-reported quitting information for the computation both of duration and years since quit. Smoking amount is also self-reported but the biomarker substudy allows a more objective biomarker, TNE, to be imputed. However, the one-time nature of the biomarker study (only one measurement) and that only a subsample of current smokers could be accessed are other limitations.
In conclusion, an analysis with almost 5000 lung cancer cases reinforced earlier findings that at lower smoking intensity (10 CPD) African Americans and Native Hawaiians are at higher risk of smoking-related lung cancer than whites, Japanese Americans, and Latinos. Our biomarker data suggest that such differences in risk may may be only partially explained after accounting for internal smoking dose. Further study of other biomarkers to explain differences in lung cancer risk is warranted.
Funding
This work was supported by the National Cancer Institute (U01 CA164973, Understanding Ethnic Differences in Cancer: The Multiethnic Cohort Study and P01CA138338, Mechanisms of Ethnic/Racial Differences in Lung Cancer Due to Cigarette Smoking).
Notes
Affiliations of authors: Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (DOS, SLP, CAH, YP); Department of Biochemistry, Molecular Biology & Biophysics, University of Minnesota Medical School, Minneapolis, MN (SEM); Department of Laboratory Medicine and Pathology, University of Minnesota Medical School, Minneapolis, MN (SSH); Department of Epidemiology, University of Hawaii Cancer Center, Honolulu, Hawaii (LLM).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors have no disclosures.
We thank Dale Preston, Hirosoft Corporation, for help and advice on risk modeling using Epicure.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;671:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;3544:333–342. [DOI] [PubMed] [Google Scholar]
- 3. Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;3511:2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;1514:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975–2015. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 6. Percy C, Holten V, Muir CS, et al. International Classification of Diseases for Oncology Geneva: World Health Organization; 1990. [Google Scholar]
- 7. World Health Organization. ICD-10 International Classification of Diseases and Health Related Problems; 10th Revision. 2nd ed Geneva: World Health Organization; 2004. [Google Scholar]
- 8. Lewis DR, Check DP, Caporaso NE, et al. US lung cancer trends by histologic type. Cancer. 2014;12018:2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harmon BE, Boushey CJ, Shvetsov YB, et al. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: The Dietary Patterns Methods Project. Am J Clin Nutr. 2015;1013:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park SL, Murphy SE, Wilkens LR, et al. Association of CYP2A6 activity with lung cancer incidence in smokers: the Multiethnic Cohort Study. PLoS One. 2017;125:e0178435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park SL, Carmella SG, Ming X, et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol Biomarkers Prev. 2015;243:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laird N, Olivier D.. Covariance analysis of censored survival data using log-linear analysis technique. J Am Stat Assoc. 1981;76374:231–240. [Google Scholar]
- 13. Preston DL, Lubin JH, Pierce DA. Epicure User Guide. Seattle, WA: Hirosoft International Corporation; 1993. [Google Scholar]
- 14. Patel YM, Stram DO, Wilkens LR, et al. The contribution of common genetic variation to nicotine and cotinine glucuronidation in multiple ethnic/racial populations. Cancer Epidemiol Biomarkers Prev. 2015;241:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spiegelman D, McDermott A, Rosner B.. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(suppl 4): 1179S–1186S. [DOI] [PubMed] [Google Scholar]
- 16. Fraser GE, Stram DO.. Regression calibration in studies with correlated variables measured with error. Am J Epidemiol. 2001;1549:836–844. [DOI] [PubMed] [Google Scholar]
- 17. Cheng I, Le GM, Noone AM, et al. Lung cancer incidence trends by histology type among Asian American, Native Hawaiian, and Pacific Islander populations in the United States, 1990–2010. Cancer. Epidemiol Biomarkers Prev. 2014;2311:2250–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melikian AA, Djordjevic MV, Hosey J, et al. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res. 2007;93:377–387. [DOI] [PubMed] [Google Scholar]
- 19. Caraballo RS, Giovino GA, Pechacek TF, et al. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2001;1538:807–814. [DOI] [PubMed] [Google Scholar]
- 20. Cropsey KL, Leventhal AM, Stevens EN, et al. Expectancies for the effectiveness of different tobacco interventions account for racial and gender differences in motivation to quit and abstinence self-efficacy. Nicotine Tob Res. 2014;169:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones MR, Joshu CE, Navas-Acien A, et al. Racial/Ethnic differences in duration of smoking among former smokers in the National Health and Nutrition Examination Surveys. Nicotine Tob Res. 2018;20(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu SS, Kodl MM, Joseph AM, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008;177:1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SE, Park SL, Balbo S, et al. Tobacco biomarkers and genetic/epigenetic analysis to investigate ethnic/racial differences in lung cancer risk among smokers. NPJ Precis Oncol. 2018;2:17. [DOI] [PMC free article] [PubMed]
- 24. Patel YM, Park SL, Carmella SG, et al. Metabolites of the polycyclic aromatic hydrocarbon phenanthrene in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PLoS One. 2016;116:e0156203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SL, Tiirikainen MI, Patel YM, et al. Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risks of lung cancer and effect on their smoking intensity. Carcinogenesis. 2016;373:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derby KS, Cuthrell K, Caberto C, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;1712:3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.