Abstract
Background
High intensity treatments such as hematopoietic cell transplantation (HCT) can be curative for patients with hematologic malignancies, but this needs to be balanced by the high risk of nonrelapse mortality (NRM) during the first 2 years after HCT. Sarcopenia (low muscle mass) is associated with physical disability and premature mortality in individuals with nonmalignant diseases and may be a predictor of NRM and poor overall survival in patients undergoing HCT.
Methods
This was a retrospective cohort study of 859 patients with acute leukemia or myelodysplastic syndrome who underwent a first HCT as adults (≥18 years) between 2007 and 2014. Sarcopenia was assessed from pre-HCT abdominal computed tomography scans. Two-year cumulative incidence of NRM was calculated, with relapse/progression considered as a competing risk event. Fine-Gray subdistribution hazard ratio estimates and 95% confidence intervals (CI) were obtained and adjusted for relevant covariates. Kaplan-Meier method was used to examine overall survival. All statistical tests were two-sided.
Results
Median age at HCT was 51 years (range = 18–74 years); 52.5% had a high [≥3] HCT-comorbidity index; 33.7% had sarcopenia pre-HCT. Sarcopenia was an independent predictor of higher NRM risk (hazard ratio = 1.58, 95% CI = 1.16 to 2.16) compared with patients who were not. The 2-year incidence of NRM approached 30% in patients with sarcopenia and high (≥3) HCT-comorbidity index. Patients with sarcopenia had on average a longer hospitalization (37.2 days vs 31.5 days, P < .001) and inferior overall survival at 2 years (55.2%, 95% CI = 49.5% to 61.0% vs 66.9%, 95% CI = 63.0% to 70.8%, P < .001).
Conclusions
Sarcopenia is an important and independent predictor of survival after HCT, with potential additional downstream impacts on health-economic outcomes. This information can be used to facilitate treatment decisions prior to HCT and guide interventions to decrease the risk of treatment-related complications after HCT.
Allogeneic hematopoietic cell transplantation (HCT) is the treatment of choice for a growing number of adults with acute leukemia (lymphoblastic, myeloid [AML]) or myelodysplastic syndrome (MDS) (1,2). However, the potent antileukemic effect of HCT needs to be balanced by the high risk of nonrelapse mortality (NRM) during the first 2 years after HCT. Pre-HCT risk calculators such as the HCT-comorbidity index (HCT-CI) facilitate stratification of NRM risk, but high interuser variability and limited predictive power has curtailed the routine use of this tool in the clinical setting (3–5). Sarcopenia (loss of lean muscle mass) is an important cause of age-related functional decline and is associated with physical disability, increased length of hospital stay, and premature mortality in individuals with nonmalignant disease (6–8). Studies conducted mostly in patients with gastrointestinal cancers (eg, esophageal, hepatobiliary, colorectal) (9–12) have highlighted the independent and prognostic impact of sarcopenia on treatment-related toxicity and overall survival (9–14). There is a paucity of information regarding the influence of pre-HCT sarcopenia on outcomes after allogeneic HCT, a complex and costly procedure (cost for the first 100 days approximately $2.5 billion per year in the United States) (15) with associated downstream societal costs in the subsequent years due to high lifelong morbidity and mortality (16).
Computerized tomography (CT) is a gold standard measure of body composition, allowing accurate characterization of muscle mass in a number of clinical settings (17,18). For the current study, we utilized archival CT images to perform population-based assessment of muscle mass in patients prior to allogeneic HCT, using a software (SliceOmatic; Tomovision, Quebec, Canada) that allows researchers to objectively measure, segment, and analyze skeletal muscle from scans performed for routine diagnostic purposes. The overall aims of this study were to describe the prevalence of sarcopenia prior to HCT and to evaluate its impact on clinically relevant outcomes such as length of hospitalization (LOS), NRM, and overall survival. This information may help providers refine risk reduction strategies prior to HCT (eg, myeloablative vs reduced-intensity conditioning, prehabilitation) and develop interventions to optimize patient management (eg, dietary optimization, increase physical/occupational therapy services) during and after HCT.
Methods
Population Cohort and Data Definitions
This was a retrospective cohort study of patients who underwent allogenic HCT for acute leukemia or MDS as adults (≥18 years old) at City of Hope (COH) between January 1, 2007 and December 31, 2014. This study was approved by the COH institutional review board as a minimal-risk study (medical record review of standard clinical assessments, informed consent was waived). Medical records were abstracted for demographic data (age at HCT, sex, race/ethnicity), cytomegalovirus (CMV) serostatus, diagnosis (AML, ALL, MDS, other), variables necessary to derive HCT-CI, Karnofsky performance score (KPS), HCT details (donor source, conditioning), severity of acute graft versus host disease (GVHD), neutrophil engraftment, LOS, and rehospitalization less than 30 days of discharge. Information on vital status and cause of death was obtained from the National Death Index and COH medical records.
Patients with acute leukemia in first complete remission, MDS with refractory anemia, or refractory anemia with ringed sideroblasts were considered at low risk of relapse at HCT; all others were considered high risk. Myeloablative conditioning was defined as receipt of 8 or more Gy of fractionated body/marrow irradiation, or busulfan (>8 mg/kg) with cyclophosphamide (120 mg/kg) or with clofarabine (40 mg/m2) (19). GVHD prophylaxis was tacrolimus/sirolimus-based or tacrolimus with a short course of methotrexate (20). CMV seropositivity was defined as antigenemia in graft recipients irrespective of donor status. Grading of severity of acute GVHD was per established criteria (21). High HCT-CI was defined as having a pre-HCT comorbidity-age index of 3 or more (3,5). Time to neutrophil engraftment was determined as time from stem cell infusion (day 0) to the first of three consecutive days with neutrophil count of at least 500/µL. Hospital LOS was determined as time from day 0 to discharge or death, whichever came first.
CT Image Analysis
All patients who underwent allogeneic HCT at COH between 2007 and 2014 had screening CT scans of the chest, abdomen, and pelvis as part of a preemptive strategy to rule out occult infection before initiation of conditioning therapy. Cross-sectional images obtained from a spiral CT scanner were used to quantify muscle area. CT scans completed at 90 or fewer days of stem cell infusion were deemed to accurately represent patients’ muscle status at HCT and were therefore selected for analysis. Three adjacent axial images within the same series at the third lumbar vertebra were selected for quantification of total muscle cross-sectional area (square centimeters) (18,22). Mean muscle cross-sectional area was calculated for each patient by taking the sum of measurements and dividing it by the total number (9,12,22,23). Directly ascertained mean muscle area (square centimeters) of the total third lumbar vertebra skeletal muscle has been shown to be linearly correlated (r > 0.90) to whole-body muscle mass and was therefore normalized for height in meters squared (22,24), reported as lumbar skeletal muscle index (cm2/m2) per conventional body composition measurements (9,12,18,22).
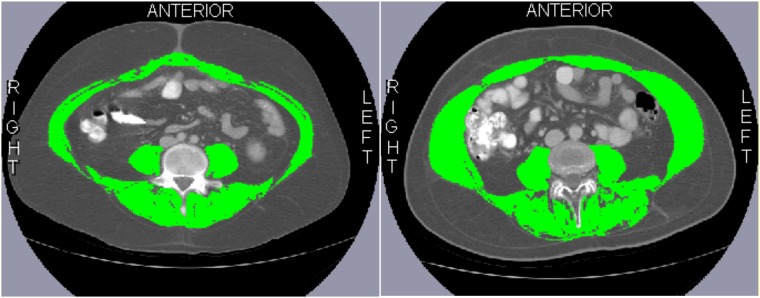
Research staff (KM, BL, SL, AI) were trained to correctly identify and quantify lumbar vertebrae, and the following muscles were included in their measurements: psoas, paraspinal, transversus abdominis, external/internal obliques, and rectus abdominis (Figure 1). An interobserver coefficient of less than 1.5% (n = 40) was required, consistent with similar reports in the literature (9,12,14,25). All observers were blinded to patient characteristics and outcomes. Muscles were quantified within a Hounsfield unit (range = −29–150) using SliceOmatic (v.5.0) (18,22,26). Of note, the intraclass coefficients between this and other commercial softwares have been shown to be 0.979–1.000 (P < .001) (17,18), with excellent intra- and interobserver agreement for measurement of muscle mass (≥0.999) (17,18). We used an a priori definition for sarcopenia (9,18) (<43 cm2/m2 [male, BMI <25 kg/m2], or <53 cm2/m2 [male, BMI ≥25 kg/m2], or <41 cm2/m2 [female, regardless of BMI]).
Figure 1.
Axial computed tomography images of the third lumbar vertebra region for two different patients. Skeletal muscle is highlighted in green. Characteristics of both patients at hematopoietic cell transplantation (HCT): Hispanic male, age 25 years; body mass index = 28 kg/m2; diagnosis: ALL; pre-HCT relapse risk: high risk; HCT-comorbidity index score: 3; HCT with myeloablative conditioning. Patient on the left was sarcopenic (skeletal muscle index [SMI] = 41.2 cm2/m2) whereas the patient on the right was not (SMI = 65.4 cm2/m2).
Statistical Analysis
Univariate analyses were performed to compare demographics, diagnosis, risk of relapse at HCT, CMV serostatus, HCT-CI severity, stem cell source, conditioning intensity, hospital LOS, time to neutrophil engraftment, rehospitalization rate, and severity of acute GVHD between patients who were sarcopenic and those who were not, using two-sided χ2 for categorical or two-sided two-sample Student t tests (comparison of means) and two-sided Wilcoxon test (comparison of medians) for continuous variables. The primary study outcome was NRM at 2 years, defined as death not attributable to relapse/progression. Relapse/progression was considered as a competing event. Relapse-related mortality (RRM) was defined as death attributable to relapse/progression, and all-cause mortality was defined as death due to NRM or RRM. Time was calculated from date of HCT to date of death, relapse/progression, or last contact. Patients alive at last contact were censored at 2 years.
Cumulative incidence of NRM and RRM was calculated taking into consideration competing risk of death for right-censored data (27). Gray’s test (27) was used to compare the cumulative incidence of NRM or RRM between various subgroups. Fine-Gray proportional subdistribution hazard models were used to estimate the relationship between clinically relevant variables and NRM, taking RRM into account as competing risk. Hazard ratios and their 95% confidence intervals (CI) were determined to quantify magnitude of risk. Due to high collinearity between diagnosis and relapse risk at HCT, diagnosis was not included in the regression model. Two separate multivariable regression models were created and included the following: Model 1: sarcopenia (no [referent], yes), HCT-CI risk (low [<3, referent], high [≥3]), race/ethnicity (non-Hispanic white [referent], Hispanic, Asian, other), +relapse risk (low [referent], high), KPS (continuous), stem cell source (matched related [referent], unrelated, cord), age at HCT (continuous), and acute GVHD severity (none/grade I [referent], grades II-IV); Model 2: combined sarcopenia + HCT-CI risk (no sarcopenia, low HCT-CI [referent]; no sarcopenia, high HCT-CI; sarcopenia, low HCT-CI; sarcopenia, high HCT-CI). An additional analysis was conducted using propensity scores as a method to adjust for potential imbalance in baseline variables between sarcopenia and no sarcopenia groups (Supplementary Methods, available online).
The Kaplan-Meier method was used to examine the effect of each variable on overall survival; log-rank tests were used to compare survival curves. Cox proportional hazards models were used to estimate the relationship between clinically relevant variables and overall survival. Proportionality was verified by visual inspection of the Kaplan-Meier plot and Schoenfeld residuals plot and using Schoenfeld’s global goodness-of-fit test (28). The variables included in the multivariable model for all-cause mortality risk were the same as those included in the NRM model. All statistical analyses were two-sided, and a P value less than .05 was considered statistically significant.
Results
Patient Characteristics
Of 1012 patients who underwent allogeneic HCT for acute leukemia or MDS as adults between 2007 and 2014, 72 were excluded from the current study because they had undergone a previous HCT, and an additional 81 were excluded because abdominal CT images were more than 90 days of HCT (n = 73) or because of the poor quality of the obtained images (n = 8). The clinical characteristics of the 859 patients (91.4% of patients who underwent a first HCT) that were included in the study are detailed in Table 1. Median time from CT scan to HCT was 26 days (range = 0–86 days), and 650 (75.6%) had a CT scan performed within 30 days of HCT. The median age at HCT was 51 years (range = 18–74 years), and the majority of patients were male (54.0%), non-Hispanic white (56.9%), and CMV seropositive (90.1%), had a diagnosis of AML (54.1%), and were considered at high risk for relapse (54.4%); 52.5% had a high (≥3) HCT-CI. Furthermore, the majority of patients received their stem cells from an unrelated donor (matched unrelated [50.8%], cord [5.7%]), and myeloablative conditioning was used in 51.1% of individuals.
Table 1.
Demographic and clinical characteristics of HCT patients
| Characteristics | Entire cohort (N = 859) No. (%) | Sarcopenic (N = 290)No. (%) | Nonsarcopenic (N = 569)No. (%) | P * |
|---|---|---|---|---|
| Median age at HCT (range), y | 51.0 (18.0–74.0) | 53.9 (18.0–73.0) | 49.0 (18.0–74.0) | .002 |
| Sex | ||||
| Male | 464 (54.0) | 143 (49.3) | 321 (56.4) | |
| Female | 395 (46.0) | 147 (50.7) | 248 (43.6) | .048 |
| Race/ethnicity | ||||
| Non-Hispanic white | 489 (56.9) | 186 (64.1) | 303 (53.3) | |
| Hispanic | 203 (23.6) | 49 (16.9) | 154 (27.1) | |
| Asian | 103 (12.0) | 36 (12.4) | 67 (11.8) | |
| Other | 64 (7.5) | 19 (6.6) | 45 (7.5) | .005 |
| CMV serostatus | ||||
| Negative (donor −/recipient −) | 85 (9.9) | 34 (11.7) | 51 (9.0) | |
| Positive (donor or recipient +) | 774 (90.1) | 256 (88.3) | 518 (91.0) | .20 |
| Risk of relapse† | ||||
| Low | 392 (45.6) | 123 (42.4) | 269 (47.3) | |
| High | 467 (54.4) | 167 (57.6) | 300 (52.7) | .18 |
| HCT-CI | ||||
| 0-2 | 409 (47.6) | 127 (43.8) | 282 (49.6) | |
| ≥3 | 450 (52.4) | 163 (56.2) | 287 (50.4) | .11 |
| KPS | ||||
| >80 | 647 (75.3) | 197 (67.9) | 450 (79.1) | |
| ≤80 | 212 (24.7) | 93 (32.1) | 119 (20.9) | <.001 |
| Diagnosis | ||||
| AML | 465 (54.1) | 155 (53.4) | 310 (54.4) | |
| Acute lymphoid leukemia | 219 (25.5) | 68 (23.4) | 151 (26.5) | |
| MDS | 164 (19.1) | 65 (22.4) | 99 (17.4) | |
| Other | 11 (1.3) | 3 (1.0) | 8 (1.4) | .31 |
| Donor source | ||||
| Matched sibling | 374 (43.5) | 115 (39.7) | 259 (45.5) | |
| Matched unrelated | 436 (50.8) | 159 (54.8) | 277 (48.7) | |
| Cord blood | 49 (5.7) | 16 (5.5) | 33 (5.8) | .23 |
| HCT conditioning | ||||
| Reduced intensity/nonmyeloablative | 420 (48.9) | 163 (56.2) | 257 (45.2) | |
| Myeloablative | 439 (51.1) | 127 (43.8) | 312 (54.8) | .002 |
| Acute GvHD | ||||
| None/grade I | 485 (56.5) | 155 (53.4) | 330 (58.0) | |
| Grades II-IV | 374 (43.5) | 135 (46.6) | 239 (42.0) | .20 |
| None/grades I-II | 723 (84.2) | 242 (83.4) | 481 (84.5) | |
| Grades III-IV | 136 (15.8) | 48 (16.6) | 88 (15.5) | .68 |
P values assess the statistical significance of the difference between values for sarcopenic vs nonsarcopenic patients; two-sided χ2 test was used for categorical variables and two-sided Wilcoxon test for comparison of medians. AML = acute myeloid leukemia; CMV = cytomegalovirus; GvHD = graft versus host disease; HCT = hematopoietic cell transplantation; HCT-CI = hematopoietic cell transplantation-comorbidity index; KPS = Karnofsky performance score; MDS = myelodysplastic syndrome.
Low disease risk for relapse included acute leukemia in first complete remission, myelodysplastic syndromes with refractory anemia, or refractory anemia with ringed sideroblasts. High disease risk for relapse included all other disease statuses.
The prevalence of pre-HCT sarcopenia was 33.7%. As shown in Table 1, sarcopenic patients were statistically significantly more likely to be older at HCT (median age = 53.9 years vs 49.0 years; P = .002), female (50.7% vs 43.6%; P = .048), and non-Hispanic white (64.1% vs 53.3%; P = .005) and to have a lower (≤80) KPS at HCT (32.1% vs 20.9%; P < .001) and to have received nonmyeloablative conditioning (56.2% vs 45.4%; P = .002). There was no difference in CMV serostatus, relapse risk, HCT-CI severity, diagnosis, or stem cell source between the two groups.
Health Outcomes by Sarcopenia
Average LOS was statistically significantly longer for patients who were sarcopenic compared with those who were not (37.2 [24.1] days vs 31.5 [15.6] days; P < .001). On the other hand, there was no difference in time to neutrophil engraftment (16.2 [5.0] days vs 16.0 [5.5] days; P = .66), prevalence of acute GVHD (grades II–IV = 46.6% vs 42.0%; P = .20; grades III–IV = 16.6% vs 15.8%; P = .68; Table 1), and 30-day rehospitalization rate (21.4% vs 25.6%; P = .18) between the two groups.
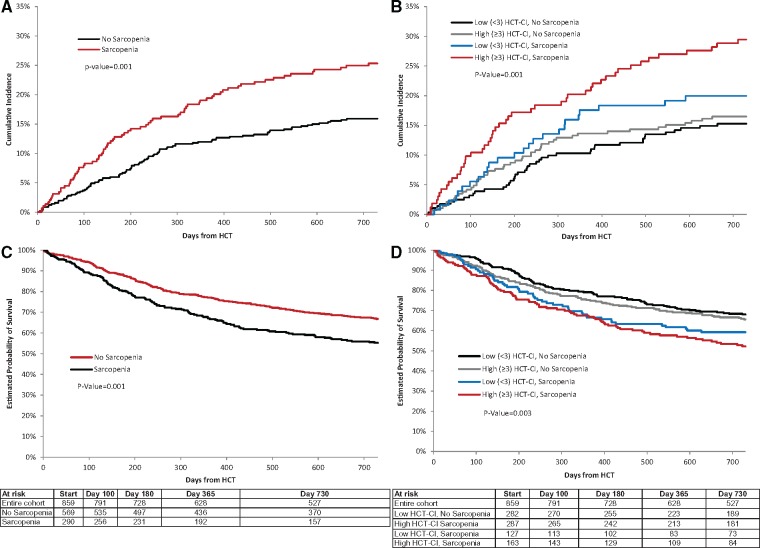
One hundred sixty-three patients (19.0% of the entire cohort) died from nonrelapse-related causes within the first 2 years after HCT. The 2-year incidence of NRM was statistically significantly higher in patients who were sarcopenic compared with those who were not (25.3%, 95% CI = 20.5% to 30.5% vs 15.9%, 95% CI = 13.0% to 19.0%; P = .001; Table 2; Figure 2A), corresponding to a 1.6-fold increased risk of NRM (hazard ratio [HR] = 1.58, 95% CI = 1.16 to 2.16; P = .004; Table 2). Similar results were obtained by propensity score analysis (HR = 1.58, 95% CI = 1.13 to 2.19; P = .01; Supplementary Tables 1–3, available online). There was an incremental increase in the cumulative incidence of NRM by the following categories: not sarcopenic, low HCT-CI (15.3%, 95% CI = 11.4% to 19.8%); not sarcopenic, high HCT-CI (16.5%, 95% CI = 12.4% to 21.0%); sarcopenic, low HCT-CI (20.0%, 95% CI = 13.5% to 27.4%); sarcopenic, high-HCT-CI (29.5%, 95% CI = 22.6% to 36.6%; P = .001) (Table 2,Figure 2B). The highest risk of NRM was in patients who were sarcopenic and had a high HCT-CI (HR = 2.11, 95% CI = 1.38 to 3.22; Table 2).
Table 2.
Cumulative incidence and risk of NRM
| Sarcopenia status | No. events/No. at risk | Cumulative incidence, % (95% CI) |
Unadjusted |
Adjusted* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 100 | Day 180 | Day 365 | Day 730 | P † | HR (95% CI) | P ‡ | HR (95% CI) | P ‡ | ||
| Model 1 | ||||||||||
| Not sarcopenic | 90/569 | 3.7 (2.4 to 5.5) | 6.5 (4.7 to 8.7) | 12.0 (9.5 to 14.8) | 15.9 (13.0 to 19.0) | 1.0 (reference) | 1.0 (reference) | |||
| Sarcopenic | 73/290 | 8.0 (5.2 to 11.4) | 12.8 (9.3 to 17.0) | 19.4 (15.1 to 24.2) | 25.3 (20.5 to 30.5) | .001 | 1.68 (1.32 to 2.28) | .001 | 1.58 (1.16 to 2.16) | .004 |
| Model 2 | ||||||||||
| Not sarcopenic, HCT-CI score | ||||||||||
| Low (0–2) | 43/282 | 3.2 (1.6 to 5.7) | 4.6 (2.6 to 7.5) | 10.3 (7.1 to 14.2) | 15.3 (11.4 to 19.8) | 1.0 (reference) | 1.0 (reference) | |||
| High (≥3) | 47/287 | 4.2 (2.3 to 7.0) | 8.4 (5.5 to 12.0) | 13.6 (10.0 to 17.9) | 16.5 (12.4 to 21.0) | 1.12 (0.69 to 1.73) | 1.15 (0.75 to 1.75) | |||
| Sarcopenic, HCT-CI score | ||||||||||
| Low (0–2) | 25/127 | 5.5 (2.4 to 10.5) | 9.6 (5.2 to 15.5) | 17.6 (11.5 to 24.7) | 20.0 (13.5 to 27.4) | 1.41 (0.83 to 2.16) | 1.27 (0.78 to 2.08) | |||
| High (≥3) | 48/163 | 9.8 (5.9 to 15.0) | 15.3 (10.3 to 21.3) | 20.9 (15.0 to 27.4) | 29.5 (22.6 to 36.6) | .001 | 2.13 (1.44 to 3.23) | .001 | 2.11 (1.38 to 3.22) | .003 |
Cox regression. Models were adjusted for variables as follows: Model 1: HCT-CI risk group, race or ethnicity, risk of relapse at HC, stem cell source, acute graft versus host disease severity; Model 2: race or ethnicity, risk of relapse at HCT, stem cell source, acute graft versus host disease severity. CI = confidence interval; HCT-CI = hematopoietic cell transplantation-comorbidity index; HR = hazard ratio; NRM = nonrelapse mortality.
Gray’s k-sample two-sided test for equality of cumulative incidence functions.
Two-sided Fine-Gray proportional subdistribution hazard model.
Figure 2.
Outcomes following hematopoietic cell transplantation (HCT). A and B) Two-year cumulative incidence of nonrelapse mortality according to pre-HCT sarcopenia (A), and the combination of sarcopenia and HCT-comorbidity index severity (HCT-CI) (B). C and D) Kaplan-Meier plots of overall survival according to pre-HCT (C), and the combination of sarcopenia and HCT-CI severity (D). Gray’s k-sample two-sided test was used to examine equality of cumulative incidence functions; two-sided log-rank tests were used to compare survival curves.
The most common cause of NRM was GVHD (68.7%), followed by infection (15.9%) and organ failure (8.6%); cause-specific analysis revealed a higher risk of death due to infection or organ failure (HR = 2.34, 95% CI = 1.19 to 4.34) in patients who were sarcopenic compared with those who were not. Separately, there was no statistically significant difference in the incidence of NRM by BMI at HCT (Supplementary Table 4; Supplementary Figure 1, available online).
The 2-year overall survival rate for the cohort was 63%; relapse or progression of disease accounted for 48.6% of deaths. Patients who were sarcopenic had statistically significantly worse 2-year overall survival (55.2%, 95% CI = 49.5% to 61.0% vs 66.9%, 95% CI = 63.0% to 70.8%; P = .001; Figure 2C), and the survival rate was lowest among patients with sarcopenia and high HCT-CI (52.2%, 95% CI = 44.5% to 59.8%; Figure 2D). Sarcopenia was associated with a 1.4-fold (HR = 1.42, 95% CI = 1.09 to 1.78; P = .003) risk of all-cause mortality compared with those without sarcopenia. There was no statistically significant difference in the incidence of RRM between the two groups (not sarcopenic: 17.3%, 95% CI = 15.4% to 20.2% vs sarcopenic: 19.5%, 95% CI = 17.1% to 22.3%; P = .41), suggesting the association between sarcopenia and all-cause mortality was largely driven by NRM.
Discussion
In this large and contemporary cohort of patients who underwent allogeneic HCT for acute leukemia or MDS, we found a statistically significantly higher risk of NRM in patients who were sarcopenic pre-HCT compared with patients who were not. The 2-year incidence of NRM approached 30% in patients with sarcopenia and a high HCT-CI. Interestingly, patients who had a high HCT-CI but no sarcopenia had a marginally higher incidence of NRM compared with patients with a low HCT-CI and no sarcopenia, and we found no association with NRM and commonly used surrogates of body composition such as BMI, highlighting the importance of objective quantification of muscle mass. Patients with sarcopenia had on average a longer hospitalization and statistically significantly lower overall survival, largely due to their higher incidence of NRM. The findings from this study speak to the importance of comprehensive screening for sarcopenia in patients prior to HCT, allowing for implementation of prevention and treatment strategies to mitigate adverse outcomes after HCT.
For the current study, we relied on archived CT images obtained as part of clinical care to measure muscle mass, a strategy that has been successfully utilized in studies examining outcomes in other oncology and nononcology populations (9,12,22,23). We were also able to successfully access archived images from more than 90% of eligible patients, providing us with near-complete assessment of muscle mass for this cohort. To date, studies examining the prognostic impact of sarcopenia have been largely limited to patients with gastrointestinal cancers (9–12) due to their close link with undernutrition. In these studies, pretreatment sarcopenia has been statistically significantly and independently associated with postoperative complications, chemotherapy-related toxicity, and lower survival (9–12). To our knowledge, only one other study has examined the impact of sarcopenia in patients with hematologic malignancies (29). In 121 patients with lymphoma who underwent autologous HCT, low muscle mass was associated with a greater number of hospitalization days, and there was a 2.4-fold increased risk of NRM (29). The cumulative incidence of NRM at 1 year was 4.2% (29), which is lower than the 1-year NRM (15.2%, 95% CI = 12.4% to 18.3%) seen in the current study of allogeneic HCT patients, speaking to the differential burden of morbidity and mortality by transplant type.
The loss of muscle mass in cancer patients is often multi-factorial, resulting from undernutrition, low physical activity, comorbid health conditions (eg, gonadal dysfunction, growth hormone deficiency), chronic inflammation, or treatment-related myopathy (eg, systemic corticosteroids) (10,30,31). It is important to note that an overwhelming majority of patients who underwent allogeneic HCT in our cohort likely received systemic chemotherapy for disease control prior to HCT, representing a heavily pretreated population. One in three patients was sarcopenic prior to HCT, which is striking given the relatively young age (median age at HCT = 51 years) of this population. Sarcopenia was associated with a greater than twofold risk of NRM due to infection or organ failure, highlighting their physiologic vulnerability to treatment-related toxicity. The implications from our study extend beyond the immediate post-HCT period to long-term (≥2 years after HCT) survivorship, because sarcopenia is a precursor to frailty and a well-established phenotype of aging in the general population (6,7). In a recent study (32) of younger (<65 years) HCT survivors, frail participants had a threefold increased risk of subsequent mortality compared with nonfrail participants (32), which is similar to the downstream consequences of frailty seen in the general population such as adverse health outcomes (33) and early mortality (34,35).
Studies conducted mostly in older individuals without cancer have found that multi-disciplinary interventions that target physical activity, nutrition optimization, and management of comorbid conditions can result in improvement in muscle performance and delay of the frailty phenotype (8,36–38). For patients planning to undergo HCT, there is often a period of 2 to 4 weeks prior to HCT where there is no administration of anticancer therapy, representing a window to screen for and initiate prehabilitation strategies in patients with sarcopenia. In a meta-analysis of 14 studies investigating the effects of preoperative exercise in lung cancer patients, exercise decreased hospital stay and statistically significantly reduced postoperative complications (39), a strategy worth pursuing in HCT patients. This prehabilitation approach may be strengthened with additional interventions to optimize nutrition and better management of comorbidities, strategies that have been effective for patients with chronic pulmonary disorders (40–42) and those affected by human immunodeficiency virus-related muscle wasting (42–44).
The findings from this study have to be considered in the context of its limitations. We relied on retrospectively archived CT images that were obtained for purposes other than measurement of muscle mass. That said, we used strict quality control to minimize interobserver variability (coefficient <1.5%) and relied on standardized and validated skeletal muscle index cutoffs to define sarcopenia. We acknowledge that the practice of whole body CT to rule out occult infection is no longer considered standard of care prior to HCT. Future studies will need to examine the utility of other platforms (eg, ultrasound based, bioelectrical impedance) to screen for low muscle mass using a risk-stratified approach that balances their accessibility and accuracy, keeping in mind differences in interpretation of muscle health. Importantly, it is not known whether practical in-person functional assessments (eg, grip strength, walking speed) could supplant the advanced imaging platforms discussed in the current study. This remains an active area of investigation for researchers seeking to develop accessible yet accurate risk prediction tools that can be used before HCT.
In conclusion, this is the first study to comprehensively examine the association between sarcopenia and outcomes after allogeneic HCT. We found that a one-time pre-HCT measure of muscle mass was a statistically significant and independent predictor of NRM after HCT. The downstream impact of pre-HCT sarcopenia may extend beyond NRM to include health-economic considerations such as LOS and the burden of chronic morbidity in long-term survivors. The information from the current study can be used to inform treatment decisions prior to HCT (eg, choice of conditioning regimen and intensity, outpatient vs inpatient) and facilitate the implementation of early interventions to decrease the risk of treatment-related complications after HCT. The growing number of patients undergoing HCT [approximately 25 000 per year in the United States (2)] makes the development of personalized strategies for transplantation imperative to ensure that that these patients live long and healthy lives well beyond the immediate HCT period.
Funding
This work was supported, in part, by grants from the Lymphoma/Leukemia Society Scholar Award for Clinical Research (2315–17 to SA) and the National Institutes of Health/National Cancer Institute (CA196854 to SA).
Notes
Affiliations of authors: Department of Population Sciences, City of Hope, Duarte, CA (SHA, MX, JBT, BL, HAC, KM, SL, AL, JJX, FLW); Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY (JMS, LWJ); Department of Hematology and Hematopoietic Cell Transplantation, City of Hope, Duarte, CA (SJF, RN).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors declare no conflict of interest.
Author contributions: SHA designed the research, collected and assembled the data, analyzed and interpreted the data, and wrote the paper. MX and FLW analyzed and interpreted the data, and contributed to the writing of the paper. JBT, BL, HAC, KM, SL, AI, and JJJ collected and assembled the data and contributed to the writing of the paper. JMS, LWJ, SJF, and RN analyzed and interpreted the data and contributed to the writing of the paper.
Supplementary Material
References
- 1. Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;516:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pasquini M, Zhu X.. Current Uses and Outcomes of Hematopoietic Stem Cell Transplantation: CIBMTR Summary Slides CIBMTR; 2015. http://www.cibmtr.org/. Accessed on December 12, 2017.
- 3. Elsawy M, Sorror ML.. Up-to-date tools for risk assessment before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2016;5110:1283–1300. [DOI] [PubMed] [Google Scholar]
- 4. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;1068:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;3229:3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cederholm T. Overlaps between frailty and sarcopenia definitions. Nestle Nutr Inst Workshop Ser. 2015;83:65–69. [DOI] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;394:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;124:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;3112:1539–1547. [DOI] [PubMed] [Google Scholar]
- 10. Pamoukdjian F, Bouillet T, Levy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2017;37(4):1101–1113. [DOI] [PubMed] [Google Scholar]
- 11. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;3412:1339–1344. [DOI] [PubMed] [Google Scholar]
- 12. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;97:629–635. [DOI] [PubMed] [Google Scholar]
- 13. Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 14. Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3(12):e172319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Majhail NS, Mothukuri JM, Brunstein CG, et al. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;155:564–573. [DOI] [PubMed] [Google Scholar]
- 16. Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;11617:3129–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Vugt JL, Levolger S, Gharbharan A, et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle. 2017;82:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prado CM, Birdsell LA, Baracos VE.. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;34:269–275. [DOI] [PubMed] [Google Scholar]
- 19. Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;1512:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodriguez R, Nakamura R, Palmer JM, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;1155:1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;156:825–828. [PubMed] [Google Scholar]
- 22. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;976:2333–2338. [DOI] [PubMed] [Google Scholar]
- 23. Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;335:997–1006. [DOI] [PubMed] [Google Scholar]
- 24. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;851:115–122. [DOI] [PubMed] [Google Scholar]
- 25. Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;26:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Irving BA, Weltman JY, Brock DW, et al. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring). 2007;152:370–376. [DOI] [PubMed] [Google Scholar]
- 27. Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;163:1141–1154. [Google Scholar]
- 28. Schoenfeld D. Residuals for the proportional hazards regresssion model. Biometrika. 1982;691:239–241. [Google Scholar]
- 29. Caram MV, Bellile EL, Englesbe MJ, et al. Sarcopenia is associated with autologous transplant-related outcomes in patients with lymphoma. Leuk Lymphoma. 2015;5610:2855–2862. [DOI] [PubMed] [Google Scholar]
- 30. Argiles JM, Busquets S, Felipe A, et al. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol. 2005;375:1084–1104. [DOI] [PubMed] [Google Scholar]
- 31. Mohammed J, Akomolafe T, Aljurf M, et al. ‘To treat or not to treat’: raising awareness on the effects of graft versus host disease drugs on musculoskeletal system. Bone Marrow Transplant. 2018;53(7):909–912. [DOI] [PubMed] [Google Scholar]
- 32. Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the bone marrow transplant survivor study. JAMA Oncol. 2016;2(10):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rockwood K, Mitnitski A.. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;271:17–26. [DOI] [PubMed] [Google Scholar]
- 34. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;563:M146–M156. [DOI] [PubMed] [Google Scholar]
- 35. Hogan DB, MacKnight C, Bergman H.. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(suppl 3):1–29. [PubMed] [Google Scholar]
- 36. Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fairhall N, Langron C, Sherrington C, et al. Treating frailty—a practical guide. BMC Med. 2011;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fairhall N, Sherrington C, Lord SR, et al. Effect of a multifactorial, interdisciplinary intervention on risk factors for falls and fall rate in frail older people: a randomised controlled trial. Age Ageing. 2014;435:616–622. [DOI] [PubMed] [Google Scholar]
- 39. Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;233:486–497. [DOI] [PubMed] [Google Scholar]
- 40. Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;1708:870–878. [DOI] [PubMed] [Google Scholar]
- 41. Strollo F, Strollo G, More M, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;162:33–37. [DOI] [PubMed] [Google Scholar]
- 42. Vellas B, Fielding R, Bhasin S, et al. Sarcopenia trials in specific diseases: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging. 2016;54:194–200. [DOI] [PubMed] [Google Scholar]
- 43. Esposito JG, Thomas SG, Kingdon L, et al. Growth hormone treatment improves peripheral muscle oxygen extraction-utilization during exercise in patients with human immunodeficiency virus-associated wasting: a randomized controlled trial. J Clin Endocrinol Metab. 2004;8910:5124–5131. [DOI] [PubMed] [Google Scholar]
- 44. Moyle GJ, Schoelles K, Fahrbach K, et al. Efficacy of selected treatments of HIV wasting: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2004;37(suppl 5):S262–S276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.