Abstract
Background
Data are limited regarding the risk of heart failure (HF) requiring hospital-based care after early stage breast cancer (EBC) and its relationship to other types of cardiovascular disease (CVD).
Methods
We conducted a population-based, retrospective cohort study of EBC patients (diagnosed April 1, 2005–March 31, 2015) matched 1:3 on birth-year to cancer-free control subjects. We identified hospitalizations and emergency department visits for CVD through March 31, 2017. We used cumulative incidence function curves to estimate CVD incidence and cause-specific regression models to compare CVD rates between cohorts. All statistical tests were two-sided.
Results
We identified 78 318 EBC patients and 234 954 control subjects. The 10-year incidence of CVD hospitalization was 10.8% (95% confidence interval [CI] = 10.5% to 11.1%) after EBC and 9.1% (95% CI = 8.9% to 9.2%) in control subjects. Ischemic heart disease was the most common reason for CVD hospitalization after EBC. After regression adjustment, the relative rates compared with control subjects remained statistically significantly elevated for HF (hazard ratio [HR] = 1.21, 95% CI = 1.14 to 1.29, P < .001), arrhythmias (HR = 1.31, 95% CI = 1.23 to 1.39, P < .001), and cerebrovascular disease (HR 1.10, 95% CI = 1.04 to 1.17, P = .002) hospitalizations. It was rare for HF hospital presentations (2.9% of cases) to occur in EBC patients without recognized risk factors (age >60 years, hypertension, diabetes, prior CVD). Anthracycline and/or trastuzumab were used in 28 950 EBC patients; they were younger than the overall cohort with lower absolute rates of CVD, hypertension, and diabetes. However, they had higher relative rates of CVD in comparison with age-matched control subjects.
Conclusions
Atherosclerotic diagnoses, rather than HF, were the most common reasons for CVD hospitalization after EBC. HF hospital presentations were often preceded by risk factors other than chemotherapy, suggesting potential opportunities for prevention.
Despite increasing concern about cardiovascular disease (CVD) after early stage breast cancer (EBC) (1,2), most cardio-oncology research has focused on heart failure (HF) using outcome definitions based on outpatient recognition of reduced left ventricular ejection fraction (3–5). There are fewer data on clinically overt HF requiring hospital-based care, which are necessary to better understand the impact of HF on cancer survivors. Although many patients experience subclinical cardiac injury within a year of cardiotoxic cancer therapy (4,6–8), additional cardiac insults may be needed before they develop clinically overt HF (1). However, there are limited data on other forms of CVD and their relationship to the development of HF after EBC. The median age at EBC diagnosis exceeds 60 years, and ischemic heart disease (IHD) is an important health concern for older women. The risk of IHD after EBC may be further increased by left-sided radiation therapy and the transition to aromatase inhibitors as the preferred endocrine therapy for postmenopausal patients (9–12). Cancer patients may also be at higher risk for arrhythmias such as atrial fibrillation (AF) (13].
Accordingly, we used a population-based cohort of women with EBC to identify all cardiovascular hospital presentations over long-term follow-up with comparison to an age-matched cohort of cancer-free women. We studied categories of CVD other than HF, along with their risk factors, to understand their incidence and contribution to the development of HF requiring hospital-based care after EBC diagnosis.
Methods
Study Cohort
Residents of Ontario, Canada receive universal coverage for medically necessary physician services through the Ontario Health Insurance Plan (OHIP). Prescription medications are reimbursed for residents aged over 65 years through the Ontario Drug Benefit program (14). The Ontario Cancer Registry stores data on residents diagnosed with cancer or who died of it (15). The Cancer Activity Level Reporting database records chemotherapy and radiation treatment information at regional cancer centers. The New Drug Funding Program database tracks use of high-cost agents, including trastuzumab and epirubicin, thus allowing determination of cancer treatment details (5,16). Hospitalization data are recorded in the Canadian Institute for Health Information Discharge Abstract Database. The National Ambulatory Care Reporting System collects data on emergency department (ED) and hospital-based ambulatory care. These datasets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES). It is important to note that these data sources do not provide information on important risk factors such as body mass index, smoking, dyslipidemia, and left ventricular ejection fraction. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
We identified all patients diagnosed with breast cancer in Ontario between April 1, 2005 and March 31, 2015. The index date was that of cancer diagnosis. We excluded patients with: (1) missing or invalid key data (OHIP number, age, or sex); (2) non-Ontario residence; (3) cancer diagnosis at/after death; (4) male sex; (5) age 18 years or younger at diagnosis; (6) age over 105 years at diagnosis; (7) OHIP ineligibility in the year preceding the index date (to allow baseline data collection); (8) in situ and stage IV breast cancer at diagnosis. For each EBC patient, we identified three female control subjects of the same birth year without a cancer diagnosis preceding the index date of the matched woman with cancer. Cancer-free control subjects were subject to similar eligibility criteria and assigned the same index date of the cancer patient to whom they were matched. We also identified a subgroup of cardiotoxin-exposed women who were documented to have received anthracyclines and/or trastuzumab within the 3 months preceding the index date or the 12 subsequent months. We used established algorithms to determine the presence of HF, myocardial infarction, IHD, cerebrovascular disease, AF, hypertension, diabetes, chronic obstructive pulmonary disease, and chronic kidney disease (CKD) (17–25) preceding the index date. The Ontario Drug Benefit database was used to determine prescription medication exposure for patients ages 66 years and older.
The primary outcome was hospitalization for which CVD was the most responsible diagnosis. We identified all hospitalizations during available follow-up and categorized them based on the underlying International Statistical Classification of Diseases and Related Health Problems codes (see Supplementary Table 1, available online) into five mutually exclusive categories: 1) HF, 2) IHD, 3) cerebrovascular disease, 4) arrhythmias, or 5) other CVD diagnoses. We also examined a composite outcome of hospitalization or ED visits (henceforth referred to as hospital presentations) for HF and AF, because these diagnoses can be treated in the ED without necessitating hospitalization. Death prior to a CVD event was treated as a competing risk. The date of last follow-up was March 31, 2017.
Statistical Analysis
Baseline characteristics were compared between the EBC and control cohorts. Continuous variables were summarized using the median (with interquartile ranges) and differences compared using the Wilcoxon rank-sum test. Counts (with percentages) were used for discrete variables and differences compared using McNemar’s test. The magnitude of difference between groups was compared using standardized differences, which describe the difference as a proportion of the pooled SD. This statistic is not influenced by sample size. Standardized differences less than 0.1 indicate a minimal magnitude of difference between the groups.
Overall survival was estimated using the Kaplan-Meier method. The cumulative incidence function (CIF) was used to describe the incidence (or probability) over time of hospitalization for any CVD while accounting for the competing risk of death (26,27). This was repeated for each CVD category without treating preceding CVD events from alternate categories as competing risks. We studied the relationship between EBC and the rate of each CVD category by estimating the cause-specific hazard ratio (HR) (26,27) from a regression model with cancer status as the predictor variable and time to cardiovascular hospitalization as the outcome. The regression model used a robust variance estimator to account for the matching of samples. Year of cohort entry was incorporated as a stratification variable within models. For each outcome, we also estimated the cause-specific HR in a multivariable model additionally incorporating rural residence and median neighborhood income as well as a history of IHD, HF, cerebrovascular disease, peripheral vascular disease, diabetes, hypertension, diabetes, chronic obstructive pulmonary disease, and CKD. The model also accounted for AF diagnoses in hospitalizations preceding the index date.
Subsequently, we identified all women with a hospital presentation for HF after the index date. We surveyed the period between the index date and the first HF event to identify any hospital presentations for CVD preceding the first HF event. For the EBC cohort, we also determined the proportion of women with HF hospital presentations who had the following nontreatment risk factors: age over 60 years, hypertension or diabetes at cancer diagnosis, or CVD diagnosed prior to the HF presentation (28).
These analyses were conducted in the full cohort and repeated for the cardiotoxin-exposed subgroup using SAS Version 9.4 (SAS Institute Inc., Cary, NC). Because the study used administrative datasets from a universal health-care system encompassing the entire population, we assumed missing data were negligible unless otherwise stated. All statistical tests were two-sided. Statistical significance was defined as a two-sided P value less than .05.
Results
Cohort Characteristics
As illustrated in Supplementary Figure 1 (available online), we studied 78 318 women with EBC along with 234 954 age-matched, cancer-free control subjects. Their baseline characteristics are summarized in Table 1. The median age was 61 (interquartile range [IQR] = 51–72) years. Compared with cancer-free control subjects, EBC patients were more likely to have a documented history of CVD, CKD, hypertension, or diabetes with greater use of corresponding medications. Overall survival at 10 years was 71.1% after EBC and 79.7% in control subjects. Median available follow-up in the EBC cohort was 5.7 (IQR = 3.5–8.4) years. Despite a higher risk of death among women with EBC, their incidence of CVD hospitalization was higher than cancer-free control subjects. At 10 years, the cumulative incidence of CVD hospitalization was 10.8% (95% confidence interval [CI] = 10.5% to 11.1%) after EBC and 9.1% (95% CI = 8.9% to 9.2%) in control subjects. The cause-specific HR for CVD hospitalization was 1.30 (95% CI = 1.26 to 1.34) for EBC patients relative to age-matched control subjects. After accounting for differences in baseline characteristics, the multivariable-adjusted HR was 1.15 (95% CI = 1.11 to 1.18).
Table 1.
Baseline characteristics of women with EBC and age-matched women without a history of cancer (control subjects)
| Characteristic | EBC, No. (%) | Control subjects, No. (%) | Standardized difference | P * |
|---|---|---|---|---|
| Total No. | 78 318 (100.0) | 234 954 (100.0) | ||
| Median age (IQR), y | 61 (51–72) | 61 (51–72) | <0.01 | .92 |
| Rural residence | 9803 (12.5) | 27 987 (11.9) | 0.02 | <.001 |
| Median neighborhood income | <.001 | |||
| Quintile 1 (lowest) | 13 492 (17.2) | 43 151 (18.4) | 0.03 | — |
| Quintile 2 | 15 069 (19.2) | 44 767 (19.1) | <0.01 | — |
| Quintile 3 | 15 357 (19.6) | 43 811 (18.6) | 0.02 | — |
| Quintile 4 | 16 565 (21.2) | 45 610 (19.4) | 0.04 | — |
| Quintile 5 (highest) | 17 561 (22.4) | 46 499 (19.8) | 0.06 | — |
| Long-term care resident | 1082 (1.4) | 4354 (1.9) | 0.04 | <.001 |
| HF | 3424 (4.4) | 8619 (3.7) | 0.04 | <.001 |
| IHD | 6240 (8.0) | 16 273 (6.9) | 0.04 | <.001 |
| Cerebrovascular disease | 2112 (2.7) | 5825 (2.5) | 0.01 | <.001 |
| Peripheral vascular disease | 1475 (1.9) | 3621 (1.5) | 0.03 | <.001 |
| AF | 1600 (2.0) | 3896 (1.7) | 0.03 | <.001 |
| Diabetes | 13 029 (16.6) | 34 559 (14.7) | 0.05 | <.001 |
| Hypertension | 36 805 (47.0) | 95 160 (40.5) | 0.13 | <.001 |
| Chronic obstructive pulmonary disorder | 3827 (4.9) | 9769 (4.2) | 0.04 | <.001 |
| CKD | 2329 (3.0) | 6037 (2.6) | 0.02 | <.001 |
| Medications in the year preceding cancer diagnosis† | ||||
| Angiotensin converting enzyme inhibitors | 7647 (24.9) | 18 635 (20.2) | 0.11 | <.001 |
| Angiotensin receptor blockers | 6120 (19.9) | 14 295 (15.5) | 0.12 | <.001 |
| Beta-blockers | 6422 (20.9) | 15 776 (17.1) | 0.10 | <.001 |
| Calcium channel blockers | 7595 (24.7) | 18 755 (20.4) | 0.10 | <.001 |
| Thiazide diuretics | 8736 (28.4) | 20 425 (22.2) | 0.14 | <.001 |
| HMG-CoA reductase inhibitors (statins) | 11 162 (36.3) | 28 048 (30.5) | 0.12 | <.001 |
| Loop diuretics | 2324 (7.6) | 5461 (5.9) | 0.07 | <.001 |
| Mineralocorticoid receptor antagonists | 1165 (3.8) | 2526 (2.7) | 0.06 | <.001 |
| Digoxin | 894 (2.9) | 1799 (2.0) | 0.06 | <.001 |
| Endocrine therapy in the year following cancer diagnosis† | ||||
| Aromatase inhibitor | 1965 (46.3) | — | — | — |
| Tamoxifen | 420 (9.9) | — | — | — |
The P value was calculated using Wilcoxon rank-sum test for age and McNemar’s test for remaining variables. All statistical tests were two-sided. AF = atrial fibrillation; CKD = chronic kidney disease; EBC = early stage breast cancer; HF = heart failure; IHD = ischemic heart disease; IQR = interquartile range.
Assessed for 30 712 EBC patients ages 66 years and older. Endocrine therapy was not assessed in the corresponding matched control subjects.
Anthracyclines or trastuzumab use was documented in 28 950 women from the EBC cohort (37.0%), and radiation therapy was documented for 51 145 women (65.3% of cohort). The baseline characteristics of women exposed to anthracyclines and/ or trastuzumab, along with their 86 850 cancer-free control subjects, are listed in Table 2. This subgroup was younger than the overall cohort, with a median age of 53 (IQR = 46–61) years. As in the overall cohort, EBC patients were more likely to have hypertension and diabetes than control subjects. In contrast to the overall cohort, EBC women receiving cardiotoxic chemotherapy were less likely to have preexisting CVD and CKD compared with control subjects. Anthracycline-treated women were younger than those treated with trastuzumab alone, with less baseline CVD, hypertension, and diabetes (see Supplementary Table 2, available online). Overall survival at 10 years was 76.7% in cardiotoxin-exposed EBC patients and 95.4% in their matched control subjects. The incidence of CVD hospitalization was lower in this younger subset, but cardiotoxin-exposed women remained at increased CVD risk compared with control subjects. The 10-year cumulative incidence of CVD hospitalization was 6.0% (95% CI = 5.6% to 6.3%) after EBC and 4.9% (95% CI = 4.7% to 5.1%) in control subjects. The age-matched, cause-specific HR was 1.41 (95% CI = 1.33 to 1.51) relative to age-matched control subjects, and the multivariable-adjusted HR was 1.39 (95% CI = 1.30 to 1.49).
Table 2.
Baseline characteristics of the cardiotoxin-exposed subgroup of women with EBC and their age-matched, cancer-free control subjects
| Characteristic | EBC, No. (%) | Control subjects, No. (%) | Standardized difference | P * |
|---|---|---|---|---|
| Total No. | 28 950 (100.0) | 86 850 (100.0) | — | — |
| Median age (IQR), y | 53 (46-61) | 53 (46-61) | <0.01 | .93 |
| Rural residence | 3737 (12.9) | 9761 (11.2) | 0.05 | <.001 |
| Median neighborhood income | <.001 | |||
| Quintile 1 (lowest) | 4685 (16.2) | 15 582 (17.9) | 0.05 | — |
| Quintile 2 | 5370 (18.5) | 16 216 (18.7) | <0.01 | — |
| Quintile 3 | 5734 (19.8) | 16 055 (18.5) | 0.03 | — |
| Quintile 4 | 6454 (22.3) | 17 134 (19.7) | 0.06 | — |
| Quintile 5 (highest) | 6597 (22.8) | 17 465 (20.1) | 0.07 | — |
| Long term care resident | 21 (0.1) | 293 (0.3) | 0.06 | <.001 |
| HF | 260 (0.9) | 1060 (1.2) | 0.03 | <.001 |
| IHD | 1064 (3.7) | 3371 (3.9) | 0.01 | .02 |
| Cerebrovascular disease | 280 (1.0) | 893 (1.0) | 0.01 | .20 |
| Peripheral vascular disease | 161 (0.6) | 644 (0.7) | 0.02 | .001 |
| AF | 99 (0.3) | 443 (0.5) | 0.03 | <.001 |
| Diabetes | 3329 (11.5) | 9141 (10.5) | 0.03 | <.001 |
| Hypertension | 9032 (31.2) | 23 457 (27.0) | 0.09 | <.001 |
| Chronic obstructive pulmonary disease | 630 (2.2) | 1907 (2.2) | <0.01 | .78 |
| CKD | 334 (1.2) | 1183 (1.4) | 0.02 | <.001 |
| Medications in the year preceding cancer diagnosis† | ||||
| Angiotensin converting enzyme inhibitors | 921 (21.7) | 2260 (17.8) | 0.10 | <.001 |
| Angiotensin receptor blockers | 727 (17.1) | 1897 (14.9) | 0.06 | <.001 |
| Beta-blockers | 639 (15.0) | 1879 (14.8) | 0.01 | .59 |
| Calcium channel blockers | 827 (19.5) | 2241 (17.6) | 0.05 | .001 |
| Thiazide diuretics | 1169 (27.5) | 2756 (21.7) | 0.14 | <.001 |
| HMG-CoA reductase inhibitors (statins) | 1408 (33.2) | 3760 (29.6) | 0.08 | <.001 |
| Loop diuretics | 90 (2.1) | 432 (3.4) | 0.08 | <.001 |
| Mineralocorticoid receptor antagonists | 120 (2.8) | 288 (2.3) | 0.04 | .003 |
| Digoxin | 42 (1.0) | 127 (1.0) | <0.01 | .80 |
| Cardiotoxin exposure | ||||
| Anthracyclines without trastuzumab | 19 618 (67.8) | — | — | — |
| Trastuzumab without anthracyclines | 3154 (10.9) | — | — | — |
| Anthracyclines and trastuzumab | 6178 (21.3) | — | — | — |
| Radiation | 22 856 (78.9) | — | — | — |
| Endocrine therapy in the year following cancer diagnosis† | ||||
| Aromatase inhibitor | 1965 (46.3) | — | — | — |
| Tamoxifen | 420 (9.9) | — | — | — |
The P value was calculated using Wilcoxon rank-sum test for age, and McNemar’s test for remaining variables. All statistical tests were two-sided. AF = atrial fibrillation; CKD = chronic kidney disease; EBC = early stage breast cancer; HF = heart failure; IHD = ischemic heart disease; IQR = interquartile range.
Assessed for 4 246 EBC patients and matched controls ages 66 years and older.
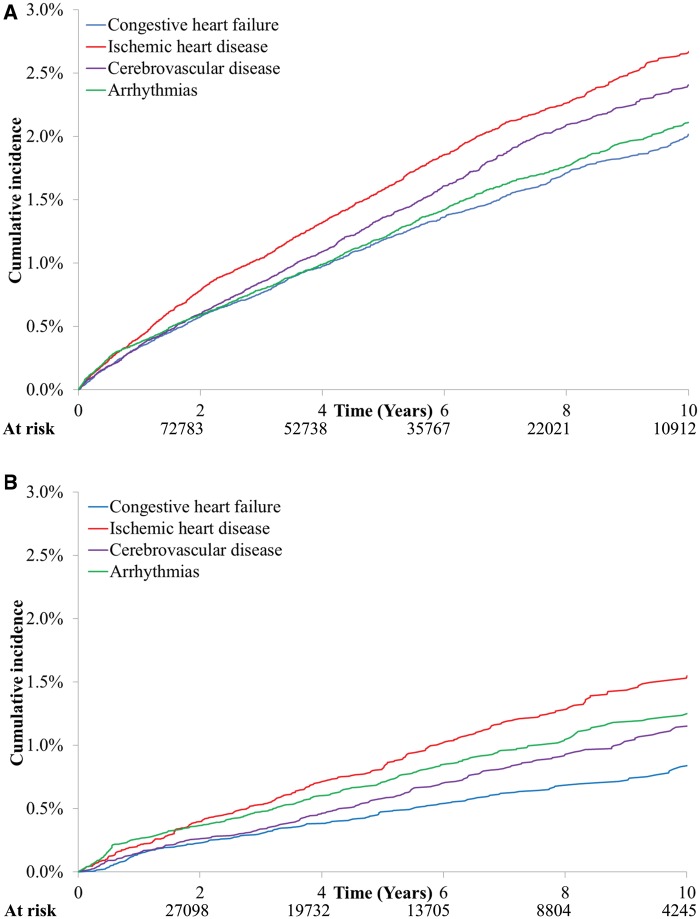
Incidence of CVD by Category
Figure 1 illustrates the cumulative incidence of diagnostic categories responsible for the first CVD hospitalization after EBC diagnosis. Panel A displays data for the full cohort, whereas panel B is limited to cardiotoxin-exposed women. The absolute probabilities were higher in panel A, which encompasses older women with a greater prevalence of preexisting CVD and associated risk factors. In both groups, the most common category for the first CVD event was IHD. The 5-year cumulative incidence was 2.7% (95% CI = 2.5% to 2.8%) in the full cohort, with 76% of these hospitalizations being documented as acute coronary syndromes. Among cardiotoxin-exposed women, the 10-year cumulative incidence of IHD was 1.6% (95% CI = 1.4% to 1.7%), with 72% of hospitalizations attributed to acute coronary syndromes. We observed similar patterns after stratifying women by their specific cardiotoxic exposure, as described in Supplementary Figure 2 (available online). The corresponding CIF curves for the age-matched, cancer-free control subjects are provided in Supplementary Figure 3 (available online).
Figure 1.
Cumulative incidence of diagnoses responsible for first cardiovascular hospitalization after early stage breast cancer diagnosis. A) Data from the full cohort. B) Data limited to patients who received anthracyclines or trastuzumab.
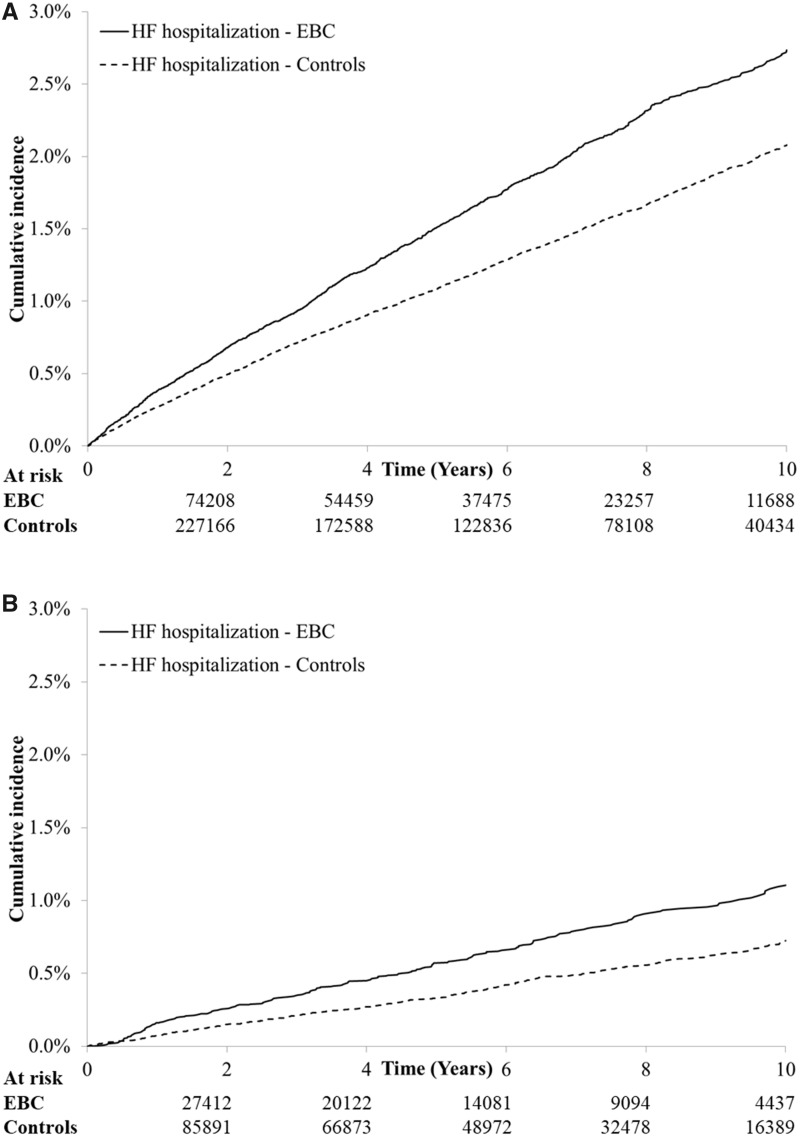
Table 3 summarizes the cumulative incidence of hospitalization for different CVD categories without treating preceding hospitalizations of alternate categories as competing events, along with cause-specific HRs for each category relative to age-matched control subjects. Supplementary Table 3 (available online) presents corresponding data on HF hospital presentations and AF hospitalizations with or without ED visits. Although IHD incurred the highest absolute risk for CVD after EBC, the relative rate was comparable to cancer-free control subjects after adjusting for baseline characteristics. In contrast, the relative rates of hospitalization for HF (HR = 1.21, 95% CI = 1.14 to 1.29, P < .001), arrhythmias (HR = 1.31, 95% CI = 1.23 to 1.39, P < .001), cerebrovascular disease (HR = 1.10, 95% CI = 1.04 to 1.17, P < .002), and AF (HR = 1.27, 95% CI = 1.17 to 1.38, P < .001) were higher for EBC patients compared with cancer-free control subjects. Women treated with cardiotoxic exposures had higher relative rates of CVD than the overall cohort, though the absolute risk was lower in this younger subgroup with less baseline comorbidity. The CIF curves for HF hospitalization are depicted in Figure 2, and the corresponding curves for HF hospital presentations are illustrated in Supplementary Figure 4 (available online). Despite a modestly steeper slope in year 1, the curves steadily diverge throughout follow-up, suggesting a persistently increased HF risk for the EBC cohort.
Table 3.
Cumulative incidence of hospitalization for different categories of CVD in women with EBC and age-matched, cancer-free women without treating preceding hospitalizations from another cardiovascular category as a competing risk
| Cardiovascular outcome | Full EBC cohort |
EBC patients receiving cardiotoxic therapy* |
||
|---|---|---|---|---|
| Cumulative incidence, % (95% CI) |
Cumulative incidence, % (95% CI) |
|||
| EBC | Control subjects | EBC | Control subjects | |
| HF | ||||
| 3 years | 0.93 (0.87 to 1.00) | 0.71 (0.68 to 0.75) | 0.35 (0.29 to 0.42) | 0.21 (0.18 to 0.24) |
| 5 years | 1.51 (1.42 to 1.61) | 1.08 (1.04 to 1.13) | 0.58 (0.49 to 0.67) | 0.33 (0.29 to 0.37) |
| 10 years | 2.73 (2.58 to 2.89) | 2.08 (2.00 to 2.16) | 1.12 (0.96 to 1.29) | 0.73 (0.65 to 0.81) |
| Age-matched HR (95% CI)† | 1.41 (1.33 to 1.50) | 1.69 (1.44 to 1.99) | ||
| P ‡ | <.001 | <.001 | ||
| Multivariable adjusted HR (95% CI)§ | 1.21 (1.14 to 1.29) | 1.81 (1.53 to 2.13) | ||
| P ‖ | <.001 | <.001 | ||
| IHD | ||||
| 3 years | 1.15 (1.08 to 1.23) | 1.03 (0.99 to 1.07) | 0.56 (0.48 to 0.66) | 0.55 (0.50 to 0.60) |
| 5 years | 1.76 (1.67 to 1.86) | 1.64 (1.58 to 1.69) | 0.87 (0.76 to 0.99) | 0.91 (0.85 to 0.98) |
| 10 years | 3.10 (2.94 to 3.26) | 2.97 (2.88 to 3.07) | 1.71 (1.52 to 1.92) | 1.88 (1.76 to 2.01) |
| Age matched HR (95% CI)† | 1.12 (1.07 to 1.19) | 1.04 (0.93 to 1.18) | ||
| P ‡ | <.001 | .49 | ||
| Multivariable adjusted HR (95% CI)§ | 0.99 (0.94 to 1.05) | 1.03 (0.91 to 1.16) | ||
| P ‖ | .76 | .63 | ||
| Cerebrovascular disease | ||||
| 3 years | 0.91 (0.84 to 0.98) | 0.77 (0.74 to 0.81) | 0.36 (0.30 to 0.44) | 0.32 (0.29 to 0.36) |
| 5 years | 1.49 (1.40 to 1.58) | 1.26 (1.21 to 1.31) | 0.62 (0.53 to 0.72) | 0.53 (0.49 to 0.59) |
| 10 years | 2.76 (2.61 to 2.91) | 2.46 (2.37 to 2.54) | 1.26 (1.09 to 1.44) | 1.16 (1.06 to 1.26) |
| Age-matched HR (95% CI)† | 1.23 (1.16 to 1.30) | 1.23 (1.06 to 1.42) | ||
| P ‡ | <.001 | .005 | ||
| Multivariable adjusted HR (95% CI)§ | 1.10 (1.04 to 1.17) | 1.20 (1.04 to 1.39) | ||
| P ‖ | .002 | .01 | ||
| Arrhythmias | ||||
| 3 years | 0.88 (0.82 to 0.95) | 0.59 (0.56 to 0.62) | 0.51 (0.44 to 0.60) | 0.23 (0.20 to 0.27) |
| 5 years | 1.39 (1.31 to 1.48) | 0.95 (0.91 to 0.99) | 0.79 (0.69 to 0.91) | 0.39 (0.35 to 0.44) |
| 10 years | 2.51 (2.37 to 2.65) | 1.88 (1.81 to 1.96) | 1.40 (1.23 to 1.58) | 0.95 (0.86 to 1.05) |
| Age-matched HR (95% CI)† | 1.49 (1.40 to 1.59) | 1.93 (1.68 to 2.23) | ||
| P ‡ | <.001 | <.001 | ||
| Multivariable adjusted HR (95% CI)§ | 1.31 (1.23 to 1.39) | 1.89 (1.63 to 2.18) | ||
| P ‖ | <.001 | <.001 | ||
Cardiotoxic therapy refers to anthracyclines and/or trastuzumab. CI = confidence interval; CVD = cardiovascular disease; EBC = early stage breast cancer; HF = heart failure; HR = hazard ratio; IHD = ischemic heart disease.
Overall cause-specific HRs from a regression model with EBC status as the only variable.
The P value was calculated using a univariable cause-specific regression model. The P value is two-sided.
Overall cause-specific HRs from a multivariable regression model (covariates described in Methods).
The P value was calculated using a multivariable cause-specific regression model. The P value is two-sided.
Figure 2.
Cumulative incidence of heart failure (HF) hospitalizations among women with early stage breast cancer (EBC) and age-matched, cancer-free control subjects. A) Data for the full cohort. B) Data limited to women receiving anthracyclines and/or trastuzumab.
Risk Factors in Women Developing HF
We identified 2344 women who had a hospital presentation for HF after EBC. Of these, 755 women (32.2%) had a hospital presentation for another cardiovascular diagnosis between their cancer diagnosis and the first HF episode. For comparison, 5264 cancer-free control subjects had a HF hospital presentation after the index date, of whom 1824 (34.7%) had an intervening hospital presentation for another cardiovascular diagnosis. It was rare for EBC patients to present to hospital with HF without a recognizable risk factor: only 67 women (2.9%) with a HF hospital presentation after EBC were ages 60 years or younger at cancer diagnosis without prior CVD, hypertension, or diabetes. The most common predisposing factors were age older than 60 years (87.2% of women with HF hospital presentation after EBC), hypertension (81.5%), and diabetes (39.7%).
Within the cardiotoxin-exposed subgroup, 352 EBC patients had a HF hospital presentation after the index date. Of these, 95 (27.0%) women had an intervening hospital presentation for another cardiovascular diagnosis between their cancer diagnosis and first HF episode. For comparison, 724 control subjects had a HF hospital presentation after the index date, of whom 267 (36.9%) had another CVD hospital presentation between the index date and the first HF episode. Only 56 women from the cardiotoxin-exposed subgroup presented to hospital with HF in the absence of a predisposing factor, the most common of which were hypertension (61.5% of EBC patients with HF hospital presentation), age older than 60 years (52.1%), and diabetes (25.7%).
Discussion
Using a population-based, matched cohort study, we demonstrated that EBC patients had a statistically significantly higher risk of CVD hospitalization compared with age-matched, cancer-free women. They were also more likely to have preexisting CVD, hypertension, or diabetes than age-matched control subjects. IHD was the most common reason for CVD hospitalization after EBC, though it occurred at a similar rate within cancer-free women. In contrast, the relative rate of HF, arrhythmias, and cerebrovascular disease was statistically significantly higher in EBC survivors compared with control subjects. HF hospital presentations after EBC rarely occurred without a predisposing factor (age >60 years, hypertension, diabetes, or prior CVD). Women treated with anthracyclines and/or trastuzumab were younger than the overall cohort; they had a higher prevalence of hypertension and diabetes, but less preexisting CVD or CKD, than age-matched control subjects. The absolute risks of CVD hospitalization were lower in cardiotoxin-exposed women, but the cause-specific HRs relative to age-matched control subjects were higher than the overall cohort.
Our observations confirmed the higher long-term risk of HF after EBC, even when analyses are limited to hospital-based episodes. The relative increase in the rate of HF was higher with anthracyclines or trastuzumab, as we previously demonstrated (5). This supports the recommendation that EBC survivors with cardiotoxic exposures be considered in stage A HF (29–31). Most EBC patients with a hospital presentation for HF were older than 60 years or had preexisting CVD, hypertension, or diabetes. The increased risk of HF hospitalization in the EBC cohort persisted through prolonged follow-up, even though most LV dysfunction occurs within a year of chemotherapy (4). Others have similarly demonstrated that older women treated with anthracyclines for EBC remain at an increased risk of HF through more than 10 years of follow-up (32). Collectively, these observations are in keeping with the “multiple hit hypothesis” for development of HF after EBC (1).
Most trials of primary prevention of chemotherapy-related cardiac dysfunction have had limited success in the era of contemporary therapy with lower anthracycline doses (33–37). Our observations suggest that a possible alternate approach to reduce long-term HF risk is to target other “hits” by controlling blood pressure (29,33), lipids (preferably with statins) (33), and other CVD risk factors. Moreover, atheroembolic diseases (IHD and cerebrovascular disease) accounted for nearly half of first CVD hospitalizations after EBC. The risk of IHD may increase further with longer follow-up of women who received left-sided radiation or aromatase inhibitors (9–12). It is important to recognize the discrepancy between the absolute and relative risks of different categories of CVD after EBC (38–40). Interventions to reduce the risk of CVD (such as blood pressure reduction and statin use) are generally geared towards a patient’s absolute estimated CVD risk (41,42). Similarly, CVD preventative interventions in EBC survivors should be tailored towards their absolute risk of all preventable forms of CVD. The demonstration of higher risk for most forms of CVD provides additional motivation for exercise therapy, which is associated with improved cardiovascular health, quality of life, and overall survival after EBC (43–48).
Most prior studies on CVD after EBC have focused on older women, specific treatments, or specific types of CVD, limiting direct comparisons to our data (3,5,11,12,32,49). It was recently reported that the Framingham risk score underestimates CVD risk in HER2-positive breast cancer (50). This may reflect the higher CVD rate in cardiotoxin-treated patients compared with cancer-free women. Similar to our data, Park et al. (51) demonstrated that women with incident EBC in the Women’s Health Initiative had a comparable rate of coronary heart disease to women without cancer. In contrast to us, they reported a similar stroke risk between the two groups. However, their analysis was limited to 1164 patients who were diagnosed with EBC 5 years after study entry. They also did not examine arrhythmias.
We noted a higher risk for cerebrovascular disease but not for IHD in women with EBC. This divergence in risk may be mediated by a higher risk of AF. Several studies have reported an association between AF and cancer (13), but there are fewer data specific to EBC (52,53). A study of patients admitted to a surgical ward reported 2% prevalence of AF in presurgery electrocardiograms for EBC patients vs 0.6% in control subjects admitted for noncancer surgery (52). However, most AF is diagnosed and managed out of hospital. Our observations regarding AF after EBC should only be considered hypothesis-generating and require corroboration with a dedicated analysis that accounts for AF in the outpatient setting.
A key strength of our study is that we accounted for competing risks within a relatively closed system with minimal loss to follow-up. We limited our outcome definitions to hospital-based events where CVD was the most responsible diagnosis to focus on clinically meaningful events whose implications can be appreciated by clinicians and patients. This also minimizes detection bias due to differential health-care contact, because patients are less likely to be hospitalized for stable CVD. However, we may have underestimated CVD risk after EBC by prioritizing specificity over sensitivity. Another limitation is that EBC patients are more likely to be documented with preexisting CVD and risk factors than cancer-free control subjects given greater health system contact around the time of diagnosis. Thus, analyses comparing pretreatment data between the two cohorts may be biased, and multivariable analyses may underestimate the HR of CVD. Moreover, patients who left the province would be lost to follow-up.
Women with EBC had a higher risk of hospitalization for most categories of CVD compared with age-matched, cancer-free women despite a higher competing risk from death. The absolute risk was highest for IHD, rather than HF, even for cardiotoxin-exposed women. In contrast, the relative rate compared with cancer-free women was highest for HF and arrhythmias such as AF. There was a long-term increase in the risk of HF, but hospital presentations for it rarely occurred in patients without prior CVD or risk factors thereof. These data highlight the potential to reduce HF after EBC by expanding our focus to the full spectrum of CVD and modifiable risk factors in women treated for EBC.
Funding
This work was supported by a grant in aid from the Heart and Stroke Foundation of Canada; Canadian Institutes of Health Research Fellowship (personnel support award to HAQ); University of Toronto Clinician-Scientist Training Program (to HAQ); Canadian Institutes of Health Research New Investigator Award (to PT); Heart and Stroke Foundation of Canada mid-career research award (to DSL); Ted Rogers Chair in Heart Function Outcomes (to DSL); Heart and Stroke Foundation Mid-Career Investigator Award (to PCA); Tier 1 Canada Research Chair in Health Services Research (to JVT); and an Eaton Scholar award (to JVT).
This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s) and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Notes
Affiliations of authors: Department of Medicine, Women’s College Hospital, Toronto, ON, Canada (HAQ); Division of Cardiology, Peter Munk Cardiac Centre and the Ted Rogers Centre for Heart Research, University Health Network, Toronto, ON, Canada (HAQ, PT, DSL); Institute for Clinical Evaluative Sciences, Toronto, ON, Canada (HAQ, PCA, DSL, JVT, KF, GMA); University of Toronto, Institute of Health Policy, Management, and Evaluation, Toronto, ON, Canada (HAQ, PCA, DSL, EA, JVT); Division of Medical Oncology, Princess Margaret Cancer Centre, Toronto, ON, Canada (EA); Department of Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada (JVT).
The authors have no conflicts of interest to disclose.
The study funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
This manuscript is dedicated to the memory of Dr. Jack V. Tu (March 1, 1965 - May 30, 2018).
Supplementary Material
References
- 1. Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;5015:1435–1441. [DOI] [PubMed] [Google Scholar]
- 2. Abdel-Qadir H, Austin PC, Lee DS, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;21:88–93. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;6024:2504–2512. [DOI] [PubMed] [Google Scholar]
- 4. Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;13122:1981–1988. [DOI] [PubMed] [Google Scholar]
- 5. Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;3419:2239–2246. [DOI] [PubMed] [Google Scholar]
- 6. Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;55:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;10922:2749–2754. [DOI] [PubMed] [Google Scholar]
- 8. Thavendiranathan P, Poulin F, Lim KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy. A systematic review. J Am Coll Cardiol. 2014;63(25 pt A):2751–2768. [DOI] [PubMed] [Google Scholar]
- 9. Jaworski C, Mariani JA, Wheeler G, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;6123:2319–2328. [DOI] [PubMed] [Google Scholar]
- 10. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;36811:987–998. [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Qadir H, Amir E, Fischer HD, et al. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Cancer. 2016;68:11–21. [DOI] [PubMed] [Google Scholar]
- 12. Goldvaser H, Barnes TA, Seruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;1101. [DOI] [PubMed] [Google Scholar]
- 13. Farmakis D, Parissis J, Filippatos G.. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;6310:945–953. [DOI] [PubMed] [Google Scholar]
- 14. Levy AR, O’Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;102:67–71. [PubMed] [Google Scholar]
- 15. Robles SC, Marrett LD, Clarke EA, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J. Clin. Epidemiol. 1988;415:495–501. [DOI] [PubMed] [Google Scholar]
- 16. Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. Risk-imaging mismatch in cardiac imaging practices for women receiving systemic therapy for early-stage breast cancer: a population-based cohort study. J Clin Oncol. 2018:Jco2018779736. doi:10.1200/JCO.2018.77.9736. [DOI] [PubMed] [Google Scholar]
- 17. Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;432:182–188. [DOI] [PubMed] [Google Scholar]
- 18. Vermeulen MJ, Tu JV, Schull MJ.. ICD-10 adaptations of the Ontario acute myocardial infarction mortality prediction rules performed as well as the original versions. J Clin Epidemiol. 2007;609:971–974. [DOI] [PubMed] [Google Scholar]
- 19. Tu K, Mitiku T, Lee DS, et al. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can J Cardiol. 2010;267:e225–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;253:512–516. [DOI] [PubMed] [Google Scholar]
- 21. Tu K, Wang M, Young J, et al. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;2911:1388–1394. [DOI] [PubMed] [Google Scholar]
- 22. Tu K, Nieuwlaat R, Cheng SY, et al. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;3212:1561–1565. [DOI] [PubMed] [Google Scholar]
- 23. Tu K, Campbell NR, Chen ZL, et al. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;11:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 24. Fleet JL, Dixon SN, Shariff SZ, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gershon AS, Warner L, Cascagnette P, et al. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;3789795:991–996. [DOI] [PubMed] [Google Scholar]
- 26. Abdel-Qadir H, Fang J, Lee DS, et al. Importance of considering competing risks in time-to-event analyses: application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2018;117:e004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC, Lee DS, Fine JP.. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;1336:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;358:893–911. [DOI] [PubMed] [Google Scholar]
- 29. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;706:776–803. [DOI] [PubMed] [Google Scholar]
- 30. Kenigsberg B, Wellstein A, Barac A.. Left ventricular dysfunction in cancer treatment: is it relevant? JACC Heart Fail. 2018;62:87–95. [DOI] [PubMed] [Google Scholar]
- 31. Abdel-Qadir H, Amir E, Thavendiranathan P.. Prevention, detection, and management of chemotherapy-related cardiac dysfunction. Can J Cardiol. 2016;327:891–899. [DOI] [PubMed] [Google Scholar]
- 32. Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;2525:3808–3815. [DOI] [PubMed] [Google Scholar]
- 33. Abdel-Qadir H, Ong G, Fazelzad R, et al. Interventions for preventing cardiomyopathy due to anthracyclines: a Bayesian network meta-analysis. Ann Oncol. 2017;283:628–633. [DOI] [PubMed] [Google Scholar]
- 34. Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;3721:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cardinale D, Ciceri F, Latini R, et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society-one trial. Eur J Cancer. 2018;94:126–137. [DOI] [PubMed] [Google Scholar]
- 36. Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;7120:2281–2290. [DOI] [PubMed] [Google Scholar]
- 37. Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2017;358:870–877. [DOI] [PubMed] [Google Scholar]
- 38. Malenka DJ, Baron JA, Johansen S, et al. The framing effect of relative and absolute risk. J Gen Intern Med. 1993;810:543–548. [DOI] [PubMed] [Google Scholar]
- 39. Barratt A, Wyer PC, Hatala R, et al. Tips for learners of evidence-based medicine: 1. Relative risk reduction, absolute risk reduction and number needed to treat. CMAJ. 2004;1714:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sedgwick JE. Absolute, attributable, and relative risk in the management of coronary heart disease. Heart. 2001;855:491–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 42. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;37322:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Irwin ML, McTiernan A, Manson JE, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res (Phila). 2011;44:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones SB, Thomas GA, Hesselsweet SD, et al. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev Res (Phila). 2013;62:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scott JM, Iyengar NM, Nilsen TS, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer. 2018;12412:2552–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott JM, Nilsen TS, Gupta D, et al. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;13711:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scott JM, Zabor EC, Schwitzer E, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;3622:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh B, Spence RR, Steele ML, et al. A systematic review and meta-analysis of the safety, feasibility and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil. 2018;9912:2621–2636. [DOI] [PubMed] [Google Scholar]
- 49. Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300, 000 women in US SEER cancer registries. Lancet Oncol. 2005;68:557–565. [DOI] [PubMed] [Google Scholar]
- 50. Law W, Johnson C, Rushton M, et al. The Framingham risk score underestimates the risk of cardiovascular events in the HER2-positive breast cancer population. Curr Oncol. 2017;245:e348–e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park NJ, Chang Y, Bender C, et al. Cardiovascular disease and mortality after breast cancer in postmenopausal women: results from the Women’s Health Initiative. PLoS One. 2017;129:e0184174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guzzetti S, Costantino G, Vernocchi A, et al. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;33:227–231. [DOI] [PubMed] [Google Scholar]
- 53. Yaylali YT, Saricopur A, Yurtdas M, et al. Atrial function in patients with breast cancer after treatment with anthracyclines. Arq Bras Cardiol. 2016;1075:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.