Abstract
Background
Posttraumatic stress disorder is a prevalent mental health condition with substantial impact on daily functioning that lacks sufficient treatment options. Here we evaluate six phase 2 trials in a pooled analysis to determine the study design for phase 3 trials of MDMA-assisted psychotherapy for PTSD.
Methods
Six randomized, double-blind, controlled clinical trials at five study sites were conducted from April 2004 to February 2017. Active doses of MDMA (75–125 mg, n = 72) or placebo/control doses (0–40 mg, n = 31) were administered to individuals with PTSD during manualized psychotherapy sessions in two or three 8-h sessions spaced a month apart. Three non-drug 90-min therapy sessions preceded the first MDMA exposure, and three to four followed each experimental session.
Results
After two blinded experimental sessions, the active group had significantly greater reductions in CAPS-IV total scores from baseline than the control group [MMRM estimated mean difference (SE) between groups − 22.0 (5.17), P < 0.001]. The between-group Cohen’s d effect size was 0.8, indicating a large treatment effect. After two experimental sessions, more participants in the active group (54.2%) did not meet CAPS-IV PTSD diagnostic criteria than the control group (22.6%). Depression symptom improvement on the BDI-II was greatest for the active group compared to the control group, although only trended towards significant group differences [MMRM, estimated mean difference (SE) between groups − 6.0 (3.03), P = 0.053]. All doses of MDMA were well tolerated, with some expected reactions occurring at greater frequency for the active MDMA group during experimental sessions and the 7 days following.
Conclusions
MDMA-assisted psychotherapy was efficacious and well tolerated in a large sample of adults with PTSD. These studies supported expansion into phase 3 trials and led to FDA granting Breakthrough Therapy designation for this promising treatment.
Trial registration
ClinicalTrials.gov Identifier: NCT00090064, NCT00353938, NCT01958593, NCT01211405, NCT01689740, NCT01793610.
Electronic supplementary material
The online version of this article (10.1007/s00213-019-05249-5) contains supplementary material, which is available to authorized users.
Keywords: MDMA, MDMA-assisted psychotherapy, Posttraumatic stress disorder, Anxiety, Psychedelic
Introduction
Posttraumatic stress disorder (PTSD) is a serious debilitating disorder with lifetime prevalence estimated at nearly 4% globally and over 8% in the USA (Kilpatrick et al. 2013; Koenen et al. 2017). Symptoms of PTSD include intrusive thoughts and memories, negative effects on cognition and mood, hyperarousal and reactivity, and avoidance that do not remit for at least 1 month subsequent to exposure to a traumatic event (Koenen et al. 2017). Individuals with PTSD may experience a substantial reduction in quality of life and relationships, and the disability resulting from PTSD can have further negative consequences such as obesity (Scott et al. 2008), hypertension (Kibler et al. 2009), comorbid mental health conditions, and suicidality (Dorrington et al. 2014; Tarrier and Gregg 2004). In addition to these profound costs to individuals with PTSD, the disorder also exerts a substantial economic toll through lost productivity and treatment costs (Marshall et al. 2000).
Widely used treatments for PTSD include psychotherapies and medications. A recent review identified trauma-focused psychotherapies as first-line treatments for PTSD (Lee et al. 2016); however, while a substantial proportion of individuals with PTSD respond to psychotherapies [e.g., cognitive processing therapy (Monson et al. 2006; Resick et al. 2008) and prolonged exposure therapy (Foa et al. 2007)], these therapies may be difficult to access and are ineffective for many (Koenen et al. 2017; Steenkamp et al. 2015). A variety of medications have also been used to address PTSD symptoms, but only two drugs—sertraline and paroxetine—are approved by the FDA for PTSD. Extant pharmacotherapies, however, are ineffective for many individuals with PTSD, with an estimated 40–60% of patients not responding adequately (Bradley et al. 2005; Brady et al. 2000; Steenkamp et al. 2015). They may have problematic side effects and generally require long-term use to maintain effectiveness (Lee et al. 2016). In sum, the sizable proportion of cases of PTSD are persistent (Koenen et al. 2017) and the shortcomings of currently available treatments make the development of novel PTSD treatments a research priority.
A promising approach to the treatment of PTSD is the combination of psychotherapy with pharmacotherapy using 3,4-methylenedioxymethamphetamine (MDMA). Interest in the therapeutic potential of MDMA for trauma-related psychopathology developed in the context of the broader potential for MDMA to catalyze psychotherapeutic processes by facilitating communication and connection between therapists and patients (Nichols 1986). MDMA was first synthesized in 1912 by Merck, but it was not until the early 1970s that MDMA was first used in combination with psychotherapy. Case reports from that period described therapeutic benefits, although no clinical trials were conducted at that time. Recreational use of “Ecstasy,” tablets purported to contain MDMA, became popular in the 1980s, leading to its classification as a Schedule 1 controlled substance in 1985. The scheduling of MDMA made its use in therapy illegal and created obstacles to clinical research. A non-profit organization, the Multidisciplinary Association for Psychedelic Studies (MAPS), filed a Drug Master File (DMF) application in 1986, followed by an Investigational New Drug (IND) application in 2001, embarking on the FDA drug development process to study the safety and efficacy of MDMA as an adjunct to psychotherapy for PTSD (Greer and Tolbert 1986; Grof 2001; Mithoefer 2011, 2017; Mithoefer et al. 2018).
After nonclinical toxicity studies and an investigator-initiated phase 1 study of MDMA were completed (Frith et al. 1987; Grob et al. 1996, 1998), six phase 2 randomized trials of MDMA-assisted psychotherapy for treatment of PTSD were conducted from 2004 to 2017. Active doses of MDMA (75–125 mg) or control doses of inactive placebo or low-dose MDMA (25–40 mg) were combined with manualized inner-directed psychotherapy (Mithoefer 2017) in which participants were supported by a male and female therapy team (Mithoefer et al. 2011, 2018). The therapeutic model described in the Treatment Manual was based upon initial work with classic psychedelics (Grof 2001; Mithoefer 2017) and early reports of MDMA in a therapeutic setting (Greer and Tolbert 1986). Four of these MAPS-sponsored studies have been published (Mithoefer et al. 2011, 2013, 2018; Oehen et al. 2013; Ot’alora et al. 2018), and all six studies demonstrated acceptable safety and promising efficacy results. The MDMA doses selected for phase 2 trials (control—0 mg, 25 mg, 30 mg, 40 mg; active—75 mg, 100 mg, 125 mg) were based on tolerability and subjective effects reported in several prior phase 1 studies (Cami et al. 2000; de la Torre et al. 2000; Grob et al. 1998; Harris et al. 2002; Liechti et al. 2001). Low doses (25 mg, 30 mg, 40 mg) produce some changes in subjective effects that could presumably enhance blinding as an active placebo but would be inadequate for a therapeutic response (Harris et al. 2002). The FDA, after reviewing all available data in 2016, granted Breakthrough Therapy Designation in 2017 and approved the designs of two phase 3 trials that started in 2018.
To optimize the design of the phase 3 trials, we pooled data from six phase 2 trials that had similar study objectives and designs. We aimed to determine how many MDMA sessions are needed to achieve a clinically significant response, what demographic and other baseline variables might impact outcomes, which safety parameters are essential, the optimal dose, and how best to minimize bias and enhance blinding. To that end, the aim of this paper is to present pooled data from randomized clinical trials at different study sites that evaluated the efficacy and safety profile of MDMA-assisted psychotherapy among individuals with PTSD from a range of causes.
Methods
Setting
Six randomized, double-blind phase 2 studies took place at five sites. The sites were located in the USA (MP-1, MP-8, MP-12), Canada (MP-4), Switzerland (MP-2), and Israel (MP-9). Five sites were private practices and one was a psychiatric clinic. Data were collected from April 2004 to March 2017. Studies were approved by the Western-Copernicus Institutional Review Board (Research Triangle or Cary, NC; MP-1, MP-8, MP-12), IRB Services/Chesapeake (Aurora ON; MP-4), Ethics Committee of Solothurn (Switzerland; MP-2), and Helsinki Committee of Beer Yaakov Hospital (Israel; MP-9).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants
Participants were recruited through internet advertisements, referrals by health professionals, and by word of mouth. Candidates had chronic PTSD with symptoms lasting longer than 6 months and Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) scores ≥ 50 (all studies except MP-4) or ≥ 60 (MP-4) upon enrollment (see eTable 1 for individual study criteria). Studies enrolled men and women, including civilians and veterans/first responders, aged 18 and older with previous inadequate response to at least one pharmacotherapy and/or psychotherapy. An inadequate response to previous treatment was concluded if participants had a CAPS-IV total score indicating moderate to extreme PTSD at screening.
Participants underwent extensive screening by independent examiners, including psychological assessments, physical examinations, laboratory testing, and ECG to identify any possible contraindications to receiving MDMA. The Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version (SCID-I-RV) or the SCID-II was used during screening to detect comorbid disorders, and medical and therapy records from outside providers were reviewed. Participants were not excluded for meeting criteria for anxiety disorders or depression but were excluded if they met criteria for past or current psychotic disorder or Bipolar Disorder 1, or for current borderline personality disorder, or eating disorder with active purging. Other exclusion criteria included significant medical diagnoses (contraindications for MDMA), pregnancy or lactation, and weight under 48 kg. Cardiovascular or cerebrovascular disease was excluded, except in one study where candidates with well-controlled hypertension and no other evidence of vascular disease could enroll after additional screening with nuclear stress test and carotid ultrasound. All therapists maintained a Basic Life Support certification, and a study physician was available by telephone throughout the study. In order to be enrolled, individuals had to meet all inclusion/exclusion criteria and agree to comply with all planned study visits. All participants confirmed comprehension of study procedures and gave written informed consent.
Participants could not have a diagnosis of substance abuse disorders within 60 days of screening for five studies and within 6 months for one study. Psychiatric medications were tapered and discontinued prior to commencing experimental sessions. Anxiolytics and sedative hypnotics were used as-needed between experimental sessions.
Protocols and treatments
After screening and enrollment, participants were randomized through a web-based system (MP-8, MP-12) or a list generated by a blinded randomization monitor (MP-1, MP-2, MP-4, MP-9) to receive blinded doses of placebo/control (0 mg placebo; 25 mg, 30 mg, or 40 mg MDMA) or active doses of MDMA (75 mg, 100 mg, or 125 mg MDMA) at approximately 1:2 ratio. Doses were administered during two 8-h psychotherapy sessions spaced 3–5 weeks apart. The initial dose was followed approximately 1.5–2.5 h later by an optional supplemental dose equal to half the initial dose. Participants could accept or decline the supplemental dose, and could discuss the choice with the therapy team. The team could withhold the supplemental dose if there were contraindicating circumstances. Participants underwent two to three non-drug 90-min therapy sessions prior to the first experimental session. Fifty participants who received 100 mg or 125 mg had a third experimental session, either open label or blinded depending on the study, and one 75 mg participant had a blinded third session before a protocol amendment changed the crossover to occur after two sessions. The control groups subsequently had the option to receive two to three open-label sessions with active dose MDMA in a crossover segment (data not shown). MDMA was synthesized by David Nichols at Purdue University. Gelatin capsules were compounded with lactose to produce equivalent-weight capsules across dose groups.
The same male/female therapy team was present for all therapy sessions for a given participant. There were 18 therapy teams across the six studies. All but one team (MP-2) were trained in the MAPS Therapy Training Program based on the method described in the MDMA-assisted Psychotherapy Treatment Manual (Mithoefer 2017). The method includes periods of introspection alternating with periods of communication between therapists and the participant. The method is aimed at allowing participants to revisit traumatic experiences while staying emotionally engaged even during intense feelings of anxiety, pain, or grief without feeling overwhelmed. The relatively non-directive approach is intended to allow for processing of other psychological, interpersonal, or behavioral aspects of the participants’ lives that are likely to arise spontaneously in addition to processing the traumatic memories that led to PTSD.
Experimental sessions took place in a designated area that contained a futon or sofa, as well as artwork or other objects intended to make the space esthetically pleasing. Participants had the option of wearing eye shades and listening to mostly instrumental music during the parts of experimental sessions when they were focused inward. After the 8-h experimental sessions, participants remained at the study site overnight with a supportive attendant. On the following day, they met with the therapists in a 90-min integration session to address and process material that arose during the experimental session. Two to three more integration sessions occurred during the month after each experimental session. For 7 days following each experimental session, the therapy team checked in with the participants in brief telephone calls to assess wellbeing and safety.
Assessments
Assessments were administered at baseline and at follow-up visits occurring 1 to 2 months after the second and third experimental sessions and at additional time points in some studies. Blinded independent raters not present during therapy sessions administered the CAPS-IV. Safety data were collected throughout treatment. Here, we present a limited set of assessments to support the rationale of this paper.
Primary outcome
The CAPS-IV is a semi-structured interview addressing PTSD symptom clusters (re-experiencing, avoidance, negative mood or cognition, and increased arousal) as recognized by DSM-IV (Blake et al. 1995; Nagy et al. 1993; Weathers et al. 2001). The CAPS-IV contains frequency and intensity scores for each of the three symptom clusters that are summed to produce a total severity score, the primary outcome for these studies. The CAPS-IV has a dichotomous diagnostic score assigned on the basis of meeting PTSD diagnostic criteria.
Secondary outcome
The Beck Depression Inventory—II (BDI-II), an established 21-item measure of self-reported depression symptoms (Beck et al. 1996), was administered in four of the six studies (MP-4, MP-8, MP-9, and MP-12). Responses are made on a four-point Likert scale and summed to produce an overall score.
Safety outcomes
Safety was assessed by tracking the rates of spontaneously reported reactions (subset of adverse events (AEs) that could be expected based on findings from published studies in healthy volunteers) during experimental sessions and 7 days following, and by recording treatment-emergent AEs (TEAEs), which were not collected on the spontaneously reported reactions list, or were reactions that continued for 7 days or more after experimental sessions. Blood pressure and heart rate were measured in intervals of 15 to 30 min, and body temperature every 60 to 90 min during experimental sessions. Suicidal ideation and behavior were collected at all visits and twice during the 7 days of contact in four of the six studies (MP-4, MP-8, MP-9, and MP-12) using the clinician-administered Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2007, 2011), a structured interview addressing presence and intensity of suicidal ideation and behavior. Participants completed the Paced Auditory Serial Addition Task (PASAT) (Gronwall 1977) and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph 1998) at baseline and 2-month follow-up to determine whether changes in cognitive function had occurred after two sessions with placebo dose or active dose MDMA in specific studies (MP-1, MP-4, and MP-12).
Statistical analysis
Data were pooled across the six studies. Participants who received MDMA (75, 100, 125 mg) were combined into an active dose group; participants who received MDMA (0, 25, 30, 40 mg) were combined for the control group. The modified intent-to-treat set included randomized participants who completed at least one blinded experimental session and a post-baseline assessment. Missing data were not imputed. The safety set included all participants exposed to at least one dose of study drug or placebo.
Group differences in baseline characteristics and demographics were evaluated with χ2 tests or independent-samples t tests. The primary efficacy evaluation was made with a mixed-effect repeated measure model (MMRM) on change in CAPS-IV total score from baseline to post second experimental session endpoint, and the post third experimental endpoint. The base model included treatment (active/control), baseline CAPS-IV score, and study as a fixed effect, and participant was specified as a random effect. To assess the relationship between outcome measures and age, PTSD duration, sex, race, and prior self-reported “ecstasy” use (substances assumed to contain MDMA), these variables were added to the base model one at a time. BDI-II scores were analyzed the same way. AEs were categorized with the Medical Dictionary for Regulatory Activities (MedDRA) in System Organ Classes and preferred terms. AEs, reactions, and suicidal ideation and behavior were summarized descriptively. Independent-samples t tests compared peak vital signs during experimental sessions between groups. Between-group effect size was calculated with Cohen’s d (Kadel and Kip 2012). SAS software version 9.3 (SAS Institute Inc., Cary, NC) was used for analyses.
Results
Sample
eFigure 1 illustrates flow of participants for these studies. From the 488 telephone-screened, 105 were enrolled and randomized [mean (SD) age, 40.5 (10.5); the majority were white/Caucasian participants (87.6%); and nearly sex balanced (females 58.1%)]. Table 1 displays the characteristics of the sample. Demographic characteristics were approximately matched between treatment arms, and no significant differences were found between groups for demographic and baseline characteristics presented in Table 1. The mean (SD) duration of PTSD was 215.3 (190.3) months, with trauma from various causes. Many participants had a lifetime history of positive suicidal ideation (86.8%) and/or behavior (30.9%). The optional supplemental dose was taken in 179/197 (90.9%) of blinded experimental sessions. The dropout rate was 7.6% (8/105), with six participants terminating early, but having completed at least one experimental session and follow-up assessment.
Table 1.
Demographics and baseline characteristicsa
| Control (n = 31) |
Active (n = 74) |
Total (n = 105) |
|
|---|---|---|---|
| Age, mean (SD), years | 40.4 (8.5) | 40.5 (11.4) | 40.5 (10.6) |
| Sex, n (%) | |||
| Male | 12 (38.7) | 32 (43.2) | 44 (41.9) |
| Female | 19 (61.3) | 42 (56.8) | 61 (58.1) |
| Race, n (%) | |||
| White/Caucasian | 27 (87.1) | 65 (87.8) | 92 (87.6) |
| Latino/Hispanic | 1 (3.2) | 2 (2.7) | 3 (2.9) |
| Native American | 1 (3.2) | 1 (1.4) | 2 (1.9) |
| Middle Eastern | 1 (3.2) | 1 (1.4) | 2 (1.9) |
| Other/biracial | 1 (3.2) | 5 (6.8) | 6 (5.7) |
| BMI, mean (SD) | 26.2 (6.1) | 26.1 (5.4) | 26.1 (5.6) |
| Duration of PTSD, mean (SD), months | 197.9 (139.1) | 222.6 (208.5) | 215.3 (190.3) |
| Pre-study PTSD medicationsb, n (%) | |||
| Sertraline | 10 (32.3) | 25 (33.8) | 35 (33.3) |
| Paroxetine | 4 (12.9) | 14 (18.9) | 18 (17.1) |
| Pre-study therapy, n (%) | |||
| CPT, IPT | 0 | 4 (5.4) | 4 (3.8) |
| Other CBT | 24 (77.4) | 34 (45.9) | 68 (64.8) |
| EMDR | 11 (35.5) | 22 (39.7) | 33 (31.4) |
| Group therapy | 4 (12.9) | 18 (24.3) | 22 (21.0) |
| PE | 3 (9.7) | 5 (6.8) | 8 (7.6) |
| Psychodynamic | 9 (29.0) | 14 (18.9) | 23 (21.9) |
| Insight | 6 (19.4) | 15 (20.3) | 21 (20.0) |
| Other | 18 (58.1) | 49 (66.2) | 67 (63.8) |
| None | 0 | 2 (2.7) | 2 (1.9) |
| Prior ecstasy use, n (%) | |||
| Yes | 7 (22.6) | 24 (32.4) | 31 (29.5) |
| No | 24 (77.4) | 50 (67.6) | 74 (70.5) |
| Lifetime C-SSRSc, n (%) | |||
| Positive ideation | 14 (77.8) | 45 (90.0) | 59 (86.8) |
| Serious ideation | 4 (22.2) | 21 (42.0) | 25 (36.8) |
| Positive behavior | 6 (33.3) | 15 (30.0) | 21 (30.9) |
| CAPS-IV total score | |||
| Baseline, mean (SD) | 81.3 (15.9) | 85.8 (19.3) | 84.5 (18.4) |
| BDI-II total scored | |||
| Baseline, mean (SD) | 26.1 (10.6) | 30.2 (11.6) | 29.1 (11.4) |
BMI, body mass index; CPT, cognitive processing therapy; IPT, interpersonal therapy; CBT, cognitive-behavioral therapy; EMDR, eye movement desensitization and reprocessing therapy; PE, prolonged exposure therapy; C-SSRS, Columbia–Suicide Severity Rating Scale; CAPS-IV, Clinician-Administered PTSD Scale; BDI-II, Beck Depression Inventory
aThere were no significant group differences (χ2 or independent t tests) for any variables presented in this table
bSertraline and paroxetine are the only two FDA-approved medications for PTSD. Participants took many other medications for symptom management pre-study that are not presented here. Twelve participants took both sertraline and paroxetine
cLifetime accounts for all suicidal ideation and behavior prior to study, according to participant recall and medical records. According to the C-SSRS scoring guide, scores of four or five on the suicidal ideation category are considered serious ideation, and scores of one or greater are considered positive behavior or ideation. Four phase 2 studies administered the C-SSRS (control group n = 18 and active MDMA group n = 50)
dFor BDI-II, active group (n = 50) and control group (n = 18)
Primary outcome
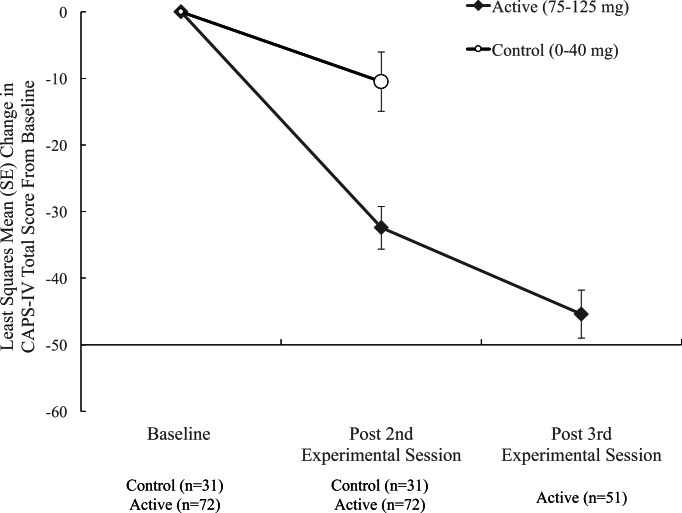
The change in CAPS-IV total score (Fig. 1) from Baseline to after the second experimental session was significantly different [t(95) = − 4.25, P < 0.0001] between control (0–40 mg) and active (75–125 mg) groups (Table 2). The active group had the greatest estimated mean (SE) drop in scores − 30.4 (3.20) compared to the control group − 10.5 (4.46). The between-group Cohen’s d effect size was 0.8, indicating a large treatment effect. Study, age, PTSD duration, sex, race, and prior “ecstasy” use did not predict outcome in this model.
Figure 1.
CAPS-IV total score least squared mean estimates at endpoints. The change in scores from baseline to post two experimental sessions were significantly different between MDMA and control groups (***P < 0.0001). After the third MDMA session, the active dose group showed further improvement compared to post two MDMA sessions (***P < 0.0001)
Table 2.
Outcome measuresa
| Control (n = 31) |
Active MDMA (n = 72) |
Mean difference (control vs. active) | |
|---|---|---|---|
| CAPS-IV total score | |||
| Post 2 experimental sessions, LS (SE) changeb | − 10.47 (4.46) | − 32.43 (3.20) | – |
| Difference (active − control) | – | – | − 21.95 (5.17) |
| P value | 0.0208 | < 0.0001 | < 0.0001 |
| Post 3 experimental sessions, LS (SE) changeb | – | − 45.39 (3.61)c | – |
| P value | – | < 0.0001 | – |
| Difference post 3 − post 2, LS (SE) change | – | − 12.97 (2.89)c | – |
| P value | – | < 0.0001 | – |
| CAPS-IV PTSD diagnostic criteria met, n (%) | |||
| Post 2 experimental sessions | |||
| Yes | 24 (77.4%) | 33 (45.8%) | – |
| No | 7 (22.6%) | 39 (54.2%) | – |
| Post 3 experimental sessionsc | |||
| Yes | – | 24 (47.1%) | |
| No | – | 27 (52.9%) | – |
| BDI-II total score | |||
| Post 2 experimental sessions, LS (SE) changeb | − 6.46 (2.69) | − 12.44 (1.84) | – |
| Difference (active − control) | – | – | − 5.97 (3.03) |
| P value | 0.019 | < 0.0001 | 0.0534 |
| Post 3 experimental sessions, LS (SE) changeb | – | − 17.36 (1.89) | – |
| P value | – | < 0.0001 | – |
| Difference post 3 − post 2, LS (SE) change | – | − 9.40 (5.66) | – |
| P value | – | 0.1019 | – |
CAPS-IV, Clinician Administered PTSD Scale; BDI-II, Beck Depression Inventory-II; LS, least square mean estimates; SE, standard error
aAll outcomes are based on intent-to-treat set
bCompared to baseline
cActive MDMA group (n = 51 for CAPS) post 3 experimental sessions, control group crossed over after 2 blinded sessions, except for MP2 study (data not included)
Secondary outcomes
According to CAPS-IV assessment at the endpoint 1–2 months post two experimental sessions (Table 2), more participants in the active group (54.2%) did not meet PTSD diagnostic criteria than the control group (22.6%). Depression symptom improvement on the BDI-II was greatest for the active dose group, estimated mean (SE) change active − 12.4 (1.84) versus control group − 6.5 (2.69), with the difference between groups trending toward significance [t(61) = − 1.97, P = 0.053].
Depending on the study, after two blinded experimental sessions, most participants in the active dose group had one additional open-label (MDMA 100–125 mg, n = 42) or blinded session (MDMA 75–125 mg, n = 9). The estimated mean change (SE) from baseline to post third session on CAPS-IV for the active dose group was − 45.4 (3.61) with a significant further decline from second to third session [t(95) = − 12.58, P < 0.0001]. The within-participant pre-test (baseline) to post-test Cohen’s d effect size increased from 1.4 (post two sessions) to 1.9 (post three sessions). Due to the crossover, there is no between-group comparison for the post third session time point.
Safety and tolerability
Treatment-emergent adverse events (TEAEs) during the blinded treatment segment most commonly reported across all doses included events in the following MedDRA System Organ Classes (SOC): psychiatric disorders, gastrointestinal disorders, and general disorders (eTable 2). The most frequently reported psychiatric TEAEs (Table 3) were anxiety, depressed mood, irritability, and panic attack. On the day of blinded experimental sessions, reactions reported by ≥ 40% of participants in either group were anxiety, dizziness, fatigue, headache, jaw clenching/tight jaw, lack of appetite, and nausea. The majority of expected reactions were rated mild or moderate, and the frequency of reports decreased over the 7 days following an experimental session (eTables 5 and 6). No changes in neurocognitive function were detected (eTable 4).
Table 3.
Treatment-emergent adverse events during the blinded treatment segment and expected reactions during two blinded MDMA sessions
| Control (n = 31) |
Active MDMA (n = 72) |
Total (n = 103) |
|
|---|---|---|---|
| Top reactions during experimental sessions, n (%)a | |||
| Anxiety | 15 (48.39) | 52 (72.22) | 67 (65.05) |
| Dizziness | 6 (19.35) | 29 (40.28) | 35 (34.00) |
| Fatigue | 18 (58.06) | 35 (48.61) | 53 (51.46) |
| Headache | 22 (70.97) | 38 (52.78) | 60 (58.25) |
| Jaw clenching, tight jaw | 6 (19.35) | 46 (63.89) | 52 (50.49) |
| Lack of appetite | 7 (22.58) | 35 (48.61) | 42 (40.78) |
| Nausea | 6 (19.35) | 29 (40.28) | 35 (33.98) |
| Psychiatric TEAEs, n (%)b | |||
| Anxiety | 3 (9.7) | 17 (23.6) | 20 (19.4) |
| Depressed mood | 1 (3.2) | 6 (8.3) | 7 (6.8) |
| Irritability | 0 | 3 (5.6) | 3 (2.9) |
| Panic attack | 0 | 3 (5.6) | 3 (2.9) |
TEAE, treatment-emergent adverse event
aFrequency of subjects who reported an expected, spontaneously reported reaction collected during blinded experimental sessions 1 and 2 (only reactions reported by ≥ 40% of participants in any group are displayed; see supplemental for full list of reactions)
bFrequency of subjects who self-reported psychiatric adverse events after first drug administration until the day before experimental session 3 (only AEs reported by three or more subjects in either group displayed)
There were no unexpected MDMA-related SAEs. Four SAEs were reported during the blinded treatment period, including one instance of suicidal ideation (30 mg) (Mithoefer et al. 2018); one SAE of exacerbation of ventricular extrasystoles was reported during an open-label session (125 mg) (Mithoefer et al. 2018) and one SAE of suicidal behavior prior to MDMA exposure in the first experimental session.
There was no suicidal behavior during the treatment period after dosing (eTable 3). At baseline, prior to any drug dosing, the active dose group (46%) had much higher rates of positive suicidal ideation than the control group (16.7%), but the lifetime reports (Table 1) were similar between groups. During the treatment phase, suicidal ideation transiently increased in some participants and was more common in the active MDMA group (eTable 3), although the causal relationship to the psychotherapeutic processing of traumatic memories or to MDMA itself, or to random group differences could not be determined.
Discussion
By pooling data across six phase 2 trials, we found significant symptom reductions in a large sample of participants with PTSD treated with active doses of MDMA combined with psychotherapy. The results informed the design of two phase 3 trials (one now ongoing the other to follow) that were approved through a Special Protocol Assessment by the FDA. The reproducible findings attained by various therapy teams in participants with PTSD arising from different types of traumatic experiences demonstrate the generalizability of this manualized drug-therapy approach and the applicability of the MAPS MDMA Therapy Training Program. Overall, the treatment was safe and efficacious for civilians and veterans/first responders with chronic PTSD who had previously failed to respond to pharmacotherapies and/or psychotherapy. More than half of the participants had previously undergone first-line trauma-focused psychotherapies, and all but two participants had received some type of psychotherapy prior to study enrollment. MDMA-assisted psychotherapy was effective for these individuals, suggesting a different mechanism of action for MDMA for reducing PTSD symptoms.
The data show that when using the “gold standard” measure of PTSD (CAPS-IV) as a primary outcome measure, with blinded raters, for participants with highly refractory PTSD (mean duration 215.3 months), there was a significant effect after two blinded active doses of MDMA adjunctive with psychotherapy versus psychotherapy with control doses. Notably, more participants in the active dose group (54.2%) no longer met PTSD diagnostic criteria compared to the control group (22.6%). The between-group effect size was large with Cohen’s d equal to 0.8. The effect size was used in power calculations for phase 3 trials. Planned enrollment is 100 participants in each phase 3 trial, with an interim analysis and option for sample size adjustment after 60% of participants have completed the primary endpoint. In addition, depression symptoms trended toward greater improvement in participants receiving active MDMA compared with the control group.
After a third experimental session, symptoms on average improved further for the active dose group. The interpretation is limited because the third session was open label for most participants, and there was no control group for comparison due to the open-label crossover after two blinded sessions for most participants. However, it appears that while many people respond adequately after two MDMA sessions, an additional session leads to more participants reaching clinically significant symptom reductions and greater drops in CAPS-IV scores. For this reason, phase 3 trials will include three blinded experimental sessions to maximize response at the primary endpoint (2 months post third experimental session, i.e., 18 weeks post baseline).
Phase 2 data showed that 75 mg MDMA produced significant improvement (Mithoefer et al. 2018), yet the sample was quite small (n = 7); therefore, we do not know what the optimal dose is, 75 mg, 100 mg, or 125 mg. There is individual variation in subjective effects of MDMA, and fixed-dose regimens do not account for differences in body weight. To gather more information about optimal dosing, phase 3 trials from 15 sites in the USA, Canada, and Israel will employ a flexible dose regimen. Participants will be randomized to receive equal-weight blinded capsules of inactive placebo or MDMA (80 mg) plus supplemental half-dose unless contraindicated in experimental session one, and then have the choice to escalate the dose to 120 mg with optional supplemental dose (or stay at 80 mg) in the next two sessions. An inactive placebo plus the same psychotherapy will be used as the control group, with the same option to escalate the dose. To minimize bias, a blinded independent rater (IR) pool will administer the primary outcome measure (CAPS-5) to participants across all sites based on availability of IRs. Consecutive assignments to the same IR will not be permitted. Independent raters will remain blinded to the number or timing of CAPS measurements in the study; therefore, we cannot reveal this information until the trials are complete.
The safety and tolerability of limited doses of MDMA in highly controlled therapeutic settings in a PTSD population was adequate, consistent with previous phase 1 studies. There was a dose effect for mean increase in vital signs during MDMA sessions (eTable 7), with values returning or trending toward baseline by the end of the 8-h session. Because vital sign increases did not reach clinically concerning ranges, the frequency of required vital measurements will be reduced in phase 3 trials to baseline, pre-supplemental dose, and session end. Vital signs at the pre-supplemental dose reading will be taken into consideration before administering the additional half-dose. Neurocognitive measures will not be employed during phase 3 because phase 2 studies showed no evidence of cognitive impairment after two doses of MDMA (eTable 4).
MDMA at all doses tested was well tolerated, as demonstrated by the low rate of TEAEs and expected reactions. Most were mild to moderate, resolving as MDMA effects dissipated or during the week following (eTables 5 and 6). During experimental sessions, the active MDMA group had higher incidences of some reactions, including anxiety, dizziness, jaw clenching/tight jaw, lack of appetite, and nausea. Whether reactions are due to the pharmacological effects of MDMA or from augmented trauma processing catalyzed by MDMA effects cannot be determined from the data collected in these studies, but phase 1 studies in healthy individuals report similar reactions to MDMA. During the 7 days following experimental sessions, some reactions occurred more often in the active dose group for the first few days before declining by the end of the week. For this reason, the number of telephone contacts after an experimental session will be less frequent for phase 3 trials, which will require four telephone contacts over 7 days. In accordance with FDA guidance for all psychiatric drugs under development, the C-SSRS will be given at each in-person visit. In phase 2 trials, there were no related deaths or incidence of suicidal behavior after MDMA. The low dropout rate (7.6%) in MDMA trials compared to other PTSD treatments (approximately 17–36%) (Bradley et al. 2005; Marshall et al. 2001; Steenkamp et al. 2015) could be related to the propensity of MDMA to make trauma processing more tolerable with rapid symptom improvements in the days and weeks following. Participants in the placebo/control group had the opportunity to cross over to receive three open-label (100–125 mg) sessions of MDMA-assisted psychotherapy if they complete the blinded segment which likely motivated participants to complete treatment.
MDMA in the context of psychotherapy was found to have a low potential for abuse. There were no AEs or treatment discontinuation related to “ecstasy” seeking or craving, and no reports of use outside the study through the post third session endpoint. Indeed, many participates anecdotally reported that the experimental sessions were not particularly pleasurable experiences, but rather difficult therapeutic work delving into their traumatic memories. Overall, safety outcomes were favorable for use of MDMA in individuals with PTSD in a supportive environment with trained mental health professionals.
Limitations
There are limitations of these trials and the associated pooled data analyses. The sample was nearly gender balanced, but participants and therapists were predominantly White/Caucasian. Phase 3 studies will evaluate the generalizability to individuals from more diverse ethnic and cultural backgrounds. Across the six trials, there were variations in study design, such as differences in timing of outcome measures, doses tested, number of blinded experimental sessions, and number of participants in each dose group. Drawbacks of pooled data analyses are that multiple doses tested were combined into two groups—control group and active dose group—and that the third experimental session was blinded or open-label full-dose MDMA, depending on the study. Also, there was no control group for a between-group comparison of the post third session; therefore, response after three sessions was limited to a within-subject analysis. Due to small sample sizes, reliability of effect size estimates from individual studies is unknown. Blinding of treatment assignment for psychoactive substances is a recognized challenge. Both psychological and vital sign changes during experimental sessions can be clues to the group assignment. To reduce bias, blinded independent raters who were not present during therapy sessions administered the CAPS-IV. However, participants and therapists often, but not always, accurately guessed dose assignment (Mithoefer et al. 2011, 2018; Oehen et al. 2013; Ot’alora et al. 2018)—a recognized limitation in clinical trials of all drugs with perceivable effects and in all psychotherapy studies where there is no possibility of effective blinding.
Conclusions
Based on the promising safety and efficacy results from these six phase 2 trials, we have designed multi-site, placebo-controlled phase 3 trials that started in late 2018 to evaluate MDMA-assisted psychotherapy in approximately 200 participants with PTSD. Limitations discussed here will be addressed, and if findings are significant and no new safety concerns arise, MDMA could become an FDA-approved treatment for PTSD in the context of psychotherapy by 2021.
Electronic supplementary material
(DOCX 111 kb)
(PDF 663 kb)
Acknowledgments
We sincerely thank the study participants for their willingness to volunteer for these clinical trials, and staff whose hard work made the trials possible: Rebecca Matthews, BA and Ben Shechet, BA for monitoring data; Colin Hennigan, MA for clinical database management and randomization monitoring; Allison Wilens, BS and John Poncini, BS for supporting video data collection; and to acknowledge study therapists: Michael Mithoefer, MD; Anne Mithoefer, BSN; Peter Oehen, MD; Verena Widmer, RN; Ingrid Pacey, MBBS, FRCP[C]; Hayden Rubensohn, MD; Richard Yensen, PhD; Donna Dryer, MD, FRCP[C]; Keren Tzarfaty, Naftali Halberstadt, PhD; Tali Nachshoni, MD; Daniel Dogan, LCSW; Ido Siemion, PhD; Sergio Marchevsky; Marcela Ot’alora G, MA, LPC; Bruce Poulter, RN, MPH; Jim Grigsby, PhD; Will Van Derveer, MD; Sandra Van Derveer, MA; Saj Razvi, LPC, MA; Sara Gael Giron; Alison McQueen, MA, LPC; study coordinators: Sarah Sadler, AA; Katrina Blommaert, MPH; Dafna Bornstein, MA; Peggy Ivers; independent raters: Mark Wagner, PhD; Joy Wymer, PhD; Rafael Traber, MD; Zach Walsh, PhD; Annie-Maria Ullman, PhD; Carla Clements, PhD, LPC; medical monitor Julie Holland MD; and all other site personnel including the physicians, pharmacists, adherence raters, and night attendants.
Author contributions
S.H. is responsible for integrity of the data and accuracy of data analysis.
Study concept and design: M.C.M., A.M., L.J., A.E., B.Y.-K., R.D., M.W.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: A.A.F., L.J., M.C.M., M.W., Z.W.
Critical revision of the manuscript for important intellectual content: all authors.
Obtained funding: R.D.
Study supervision: M.C.M., A.M.
Funding/sponsor
These six phase 2 studies were sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) nonprofit organization. MAPS provided the MDMA and fully funded this study from private donations. MAPS Public Benefit Corporation (MAPS PBC), wholly owned by MAPS, was the trial organizer.
Role of the funder/sponsor
MAPS and MAPS PBC assisted with study design; monitoring of study data; analysis, management, and interpretation of data; preparation, review, and approval of manuscript; and decision to submit the manuscript for publication. The funder had no role in the collection of data or conduct of the study.
Compliance with ethical standards
Conflict of interest
M.C.M. received salary support from MAPS PBC as a clinical investigator and clinical trial medical monitor as well as for training and supervision of research psychotherapists.
A.A.F. received salary support for full-time employment with MAPS PBC.
L.J. received salary support for full-time employment with MAPS PBC.
A.M. received salary support from MAPS PBC as a clinical investigator and for training and supervision of research psychotherapists.
M.W. received research support to conduct study assessments.
Z.W. received research support to conduct study assessments.
S.H. received salary support from MAPS PBC as a biostatistician.
B.Y.-K. received salary support for full-time employment with MAPS.
A.E. received salary support for full-time employment with MAPS PBC.
R.D. received salary support for full-time employment with MAPS.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s00213-024-06666-x"
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/10/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s00213-024-06666-x
References
- Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 67:588–597 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995) The development of a clinician-administered PTSD scale. J Trauma Stress 8:75–90 [DOI] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D (2005) A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 162:214–227 10.1176/appi.ajp.162.2.214 [DOI] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM (2000) Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 283:1837–1844 10.1001/jama.283.14.1837 [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, San L, de la Torre R (2000) Human pharmacology of 3,4-methylenedioxymethamphetamine ("ecstasy"): psychomotor performance and subjective effects. J Clin Psychopharmacol 20:455–466 10.1097/00004714-200008000-00010 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J (2000) Pharmacology of MDMA in humans. Ann N Y Acad Sci 914:225–237 10.1111/j.1749-6632.2000.tb05199.x [DOI] [PubMed] [Google Scholar]
- Dorrington S, Zavos H, Ball H, McGuffin P, Rijsdijk F, Siribaddana S, Sumathipala A, Hotopf M (2014) Trauma, post-traumatic stress disorder and psychiatric disorders in a middle-income setting: prevalence and comorbidity. Br J Psychiatry 205:383–389 10.1192/bjp.bp.113.141796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Rothbaumn BO (2007) Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences: therapist guide. Oxford University Press, New York, NY [Google Scholar]
- Frith CH, Chang LW, Lattin DL, Walls RC, Hamm J, Doblin R (1987) Toxicity of methylenedioxymethamphetamine (MDMA) in the dog and the rat. Fundam Appl Toxicol 9:110–119 10.1016/0272-0590(87)90158-8 [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R (1986) Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 18:319–327 10.1080/02791072.1986.10472364 [DOI] [PubMed] [Google Scholar]
- Grob CS, Greer GR, Mangini M (1998) Hallucinogens at the turn of the century: an introduction. J Psychoactive Drugs 30:315–319 10.1080/02791072.1998.10399707 [DOI] [PubMed] [Google Scholar]
- Grob CS, Poland RE, Chang L, Ernst T (1996) Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res 73:103–107 10.1016/0166-4328(96)00078-2 [DOI] [PubMed] [Google Scholar]
- Grof S (2001) LSD psychotherapy, 4th edn. Multidisciplinary Association for Psychedelic Studies, Ben Lomond, CA [Google Scholar]
- Gronwall DM (1977) Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 44:367–373 10.2466/pms.1977.44.2.367 [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT (2002) Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 162:396–405 10.1007/s00213-002-1131-1 [DOI] [PubMed] [Google Scholar]
- Kadel R & Kip K (2012). A SAS macro to compute effect size (Cohen’s d) and its confidence interval from raw survey data. In Proceedings of the Annual Southeast SAS Users Group Conference
- Kibler JL, Joshi K, Ma M (2009) Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med 34:125–132 10.3200/BMED.34.4.125-132 [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ (2013) National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26:537–547 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, Atwoli L, Petukhova M, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Bunting B, Ciutan M, de Girolamo G, Degenhardt L, Gureje O, Haro JM, Huang Y, Kawakami N, Lee S, Navarro-Mateu F, Pennell BE, Piazza M, Sampson N, Ten Have M, Torres Y, Viana MC, Williams D, Xavier M, Kessler RC (2017) Posttraumatic stress disorder in the world mental health surveys. Psychol Med 47:2260–2274 10.1017/S0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW (2016) Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety 33:792–806 10.1002/da.22511 [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology 154:161–168 10.1007/s002130000648 [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R (2001) Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 158:1982–1988 10.1176/appi.ajp.158.12.1982 [DOI] [PubMed] [Google Scholar]
- Marshall RP, Jorm AF, Grayson DA, O'Toole BI (2000) Medical-care costs associated with posttraumatic stress disorder in Vietnam veterans. Aust N Z J Psychiatry 34:954–962 10.1080/000486700269 [DOI] [PubMed] [Google Scholar]
- Mithoefer M (2017). A manual for MDMA-assisted psychotherapy in the treatment of posttraumatic stress disorder; Version 8. http://www.maps.org/research/mdma/mdma-research-timeline/4887-a-manual-for-mdma-assisted-psychotherapy-in-the-treatment-of-ptsd. Accessed 2 April 2019
- Mithoefer MC, Mithoefer AT, Feduccia AA, Jerome L, Wagner M, Wymer J, Holland J, Hamilton S, Yazar-Klosinski B, Emerson A, Doblin R (2018) 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 5:486–497 10.1016/S2215-0366(18)30135-4 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R (2011) The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 25:439–452 10.1177/0269881110378371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, Michel Y, Brewerton TD, Doblin R (2013) Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol 27:28–39 10.1177/0269881112456611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP (2006) Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol 74:898–907 10.1037/0022-006X.74.5.898 [DOI] [PubMed] [Google Scholar]
- Nagy LM, Morgan CA 3rd, Southwick SM, Charney DS (1993) Open prospective trial of fluoxetine for posttraumatic stress disorder. J Clin Psychopharmacol 13:107–113 10.1097/00004714-199304000-00004 [DOI] [PubMed] [Google Scholar]
- Nichols DE (1986) Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs 18:305–313 10.1080/02791072.1986.10472362 [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U (2013) A randomized, controlled pilot study of MDMA (+/− 3,4-methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic post-traumatic stress disorder (PTSD). J Psychopharmacol 27:40–52 10.1177/0269881112464827 [DOI] [PubMed] [Google Scholar]
- Ot’alora GM, Grigsby J, Poulter B, Van Derveer JW, Giron SG, Jerome L, Feduccia AA, Hamilton S, Yazar-Klosinski B, Emerson A, Mithoefer M, Doblin R (2018) 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized controlled trial. J Psychopharmacol 32:1295–1307 10.1177/0269881118806297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The Columbia–suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M (2007) Columbia classification algorithm of suicide assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164:1035–1043 10.1176/ajp.2007.164.7.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C (1998) Repeatable battery for the assessment of neuropsychological status manual. In: The psychological corporation. TX, San Antonio [Google Scholar]
- Resick PA, Galovski TE, Uhlmansiek MO, Scher CD, Clum GA, Young-Xu Y (2008) A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol 76:243–258 10.1037/0022-006X.76.2.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK (2008) Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65:220–231 10.1001/archgenpsychiatry.2007.34 [DOI] [PubMed] [Google Scholar]
- Steenkamp MM, Litz BT, Hoge CW, Marmar CR (2015) Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA 314:489–500 10.1001/jama.2015.8370 [DOI] [PubMed] [Google Scholar]
- Tarrier N, Gregg L (2004) Suicide risk in civilian PTSD patients—predictors of suicidal ideation, planning and attempts. Soc Psychiatry Psychiatr Epidemiol 39:655–661 10.1007/s00127-004-0799-4 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR (2001) Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13:132–156 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 111 kb)
(PDF 663 kb)