Abstract
The effects of Enterococcus faecium on growth, intestinal barrier function, and immune response in Escherichia coli O78-challenged broiler chickens were investigated. Three hundred eight 1-day-old Ross male chickens were randomly assigned into three treatment groups: negative control (C), E. coli O78-infected positive (EP), and E. coli O78-infected with 200 mg/kg E. faecium dietary supplementation (EF). E. faecium significantly increased the body weight on day 10 (P < 0.05) and day 15. Furthermore, these birds had a greater average daily gain compared with the other groups during days 1–10 (P < 0.05). The death rate of the EF chickens dramatically declined. E. faecium supplementation improved the jejunal villus height and the ratio of villus height to crypt depth (P < 0.05) 3 and 7 days post-infection. The mRNA expression of claudin-1 significantly increased by E. faecium (P < 0.05) 3 and 7 days post-infection, and Mucin2 was markedly enhanced (P < 0.05) 3 days post-infection. E. faecium upregulated the mRNA expression of PPAR-γ and IL-10 (P < 0.05) and downregulated that of NF-κB, TLR4, and IL-1β (P < 0.05) in the spleen 3 and 7 days post-infection. Lipopolysaccharide stimulation index was markedly enhanced in the EF group (P < 0.05) 3 days post-infection. The increased liver E. coli number caused by the E. coli O78 challenge was significantly reversed by E. faecium (P < 0.05). E. faecium improved growth and reduced the death rate by regulating the immune response and maintaining the intestinal integrity in E. coli O78-challenged broiler chickens.
Keywords: Broilers, Enterococcus faecium, Escherichia coli O78, Immune response, Intestinal barrier function
Introduction
Avian colibacillosis, caused by specific serotypes or opportunistically pathogenic Escherichia coli, is one of the crucial bacterial diseases of poultry [1, 2]. Young birds, in which the protective immune system is not fully developed, are more vulnerable. E. coli serotypes O78:K80, O1:K1, and O2:K1 are the most commonly found in domestic breeds with colibacillosis [3]. Although various antibiotics are typically used to prevent and control colibacillosis, cumulative reports have demonstrated that drug resistance of E. coli has increased owing to the spreading of resistance genes such as extended-spectrum beta-lactamases (ESBL) and/or plasmid-mediated Amp-C beta-lactamases (Amp-C) [4, 5]. Therefore, potential antibiotic alternatives to reduce antimicrobial drug usage in poultry production are urgently needed.
Enterococcus faecium, a lactic acid-producing Gram-positive bacterium found in the intestine of healthy animals and humans, is a probiotic that may be beneficial for animal health [6, 7]. E. faecium increases the concentration of organic acids and bacteriocins, which are important for the alimentary tract because of their nutritional benefits for enterocytes and their inhibitory effects on pathogens [8]. Previous studies have indicated that E. faecium improves the metabolism of macronutrients [7], promotes growth performance [6, 9], inhibits pathogen proliferation [1, 10], improves intestinal morphology [6, 11], and enhances the immune response [12]. Additionally, E. faecium prevents E. coli-induced intestinal disorders and manipulates the cecal microflora [1]. E. faecium can also elicit protective immune responses by inducing cytokines, and T and B lymphocytes against Salmonella spp. [8, 13]. However, there are limited published reports on the effects of E. faecium on E. coli-challenged broiler chickens. Hence, the present study was designed to investigate the effects of E. faecium on growth performance, intestinal barrier function, and the innate immune response in broilers challenged with E. coli O78.
Methods
Experimental Design
The experimental animal protocol in this study was approved by the Animal Care and Use Committee of China Agricultural University (permit number 20121209–1). A total of 216 1-day-old birds were randomly assigned into three groups with six replicates of each. Each replicate consisted of 12 birds. Treatments were set as follows: negative control birds were fed a basal diet and injected with sterile saline (0.2 ml) in the left thoracic air sac (C); positive control birds were fed a basal diet and challenged at 11 days of age with E. coli O78 [0.2 ml, 104 colony-forming unit (CFU)/ml] injected into the left thoracic air sac (EP) [2]; probiotic birds were fed a basal diet containing E. faecium and challenged with 0.2 ml, 104 CFU/ml E. coli O78 that was injected into the left thoracic air sac (EF). The basal diet formula met or exceeded the nutrient requirements for broiler chickens recommended by the National Research Council (1994) and the diet composition is listed in Table 1. The total feeding period was 20 days.
Table 1.
Composition and nutrient level of diet
| Items (%) | Content |
|---|---|
| Ingredient | |
| Corn | 56 |
| Wheat | 2 |
| Soybean meal | 34.5 |
| Soybean oil | 3.2 |
| Dicalcium phosphate | 1.85 |
| Limestone | 1.2 |
| DL-Met | 0.2 |
| l-Lys-HCl | 0.25 |
| Sodium chloride | 0.3 |
| Choline chloride (50%) | 0.2 |
| Santoquin | 0.05 |
| Maifanite | 0.03 |
| Vitamin premixa | 0.02 |
| Trace mineral premixb | 0.2 |
| Nutrient and energy level | |
| ME (Mcal/kg) | 2.96 |
| Protein | 19.98 |
| Calcium | 0.97 |
| Lysine | 1.19 |
| Methionine | 0.51 |
| Available phosphorus | 0.43 |
aThe vitamin premix supplied the following (per kg of diet): vitamin A, 12500 IU; vitamin D3, 2500 IU; vitamin E 18.75 IU; vitamin K3, 2.65 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B6, 6 mg; vitamin B12, 0.025 mg; d-biotin, 0.0325 mg; folic acid, 1.25 mg; d-calcium pantothenate, 12 mg; nicotinic acid 50 mg
bThe trace mineral premix supplied the following (per kg of diet): copper, 8 mg; zinc,75 mg; iron, 80 mg; manganese,100 mg; selenium, 0.15 mg; iodine,0.35 mg
E. faecium NCIMB11181 Preparation
The E. faecium NCIMB11181 preparation used in this study was a commercial product purchased from Probiotics International Ltd. (Stoke Sub Hamdon, Somerset, UK), which contained a total bacteria count ≥ 2.00 × 1012 CFU/kg. Sample testing showed that the bacteria count was 8.2 × 1012 CFU/kg. The E. faecium product (200 mg/kg) was carefully mixed into the basal diet. The diets in this experiment were manufactured at the feed mill of China Agricultural University. Finally, the actual diet contained 5.1 × 1010 CFU/kg E. faecium.
E. coli O78 Preparation
The E. coli O78 (CVCC1555) used in this study was purchased from China General Microbiological Culture Collection Center, Beijing, China. The strain was aerobically incubated in Luria-Bacterial liquid medium for 24 h at 37 °C with shaking (120 rpm). Before the challenge, the bacteria were centrifuged at 2800×g for 10 min and washed three times with phosphate-buffered saline (PBS). The bacterial concentration was measured with a spectrometer at 600 nm. PBS was used to adjust the suspension to the desired bacterial concentration.
Sample Collection
On days 15 and 20, one bird of each replicate was randomly selected and euthanized by sodium pentobarbital (30 mg/kg). The small intestine was removed and gently flushed with PBS. Jejunum samples were collected for intestinal morphology, and mucosal antibody and mRNA expression. Liver samples for bacterial count and spleen samples for mRNA expression were taken. All samples for mRNA expression were immediately frozen in liquid nitrogen.
Growth Performance
Body weight (BW) for each replicate was measured on d1, d10 (the day before the challenge), d15 (3 days post-infection), and d20 (7 days post-infection). The average daily gain (ADG) was calculated during d1–10, d10–15, and d10–20. The death rate was calculated during d1–10 and d10–20.
Intestinal Morphology
Jejunum segments were fixed with 4% paraformaldehyde and embedded in paraffin after 48 h. For morphological examination, tissue sections (5 μm) were stained with hematoxylin and eosin. Nine complete villi were measured in each section. The villus height was measured from the tip of the villus to the villus-crypt junction, and crypt depth was measured from the bottom of the villus to the lamina propria. Then, the villus height/crypt depth ratio was calculated. All the observations and measurements were performed with an Olympus optical microscope and ProgRes CapturePro software (version 2.7; Jenoptik, Jena, Germany).
Mucosal IgA
Mucosal scraping was collected with sterile slides from two 5-cm jejunal samples and homogenized in saline. The secretory IgA (sIgA) concentration was measured by an ELISA kit (Bethyl Laboratories Inc., Montgomery, TX, USA) and the total protein of the mucosal homogenate was determined by a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturers’ protocols. The sIgA concentration is presented as milligrams per gram of protein.
Liver Bacterial Count
Two hundred micrograms of liver tissue per sample was placed in 0.2 ml sterile saline and homogenized by a stomacher. Each homogenate was diluted from 10−1 to 10−6 with sterile saline. Each diluted sample (0.1 ml) was incubated on a MacConkey agar plate (Land Bridge Technology, Beijing, China) at 37 °C for 24 h. Plates containing 30–300 bacterial colonies were selected to count. The final number is shown as log10 (CFU/g tissue).
Peripheral Blood Mononuclear Cell Isolation
Blood was collected into heparin anticoagulant tubes on d15 and d20. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll solution (Histopaque1077; Sigma-Aldrich co., St. Louis, MO, USA). Uncoagulated blood was diluted with Hanks solution at 1:1 (no calcium and magnesium, Thermo Fisher Scientific) and layered on top of the Ficoll solution in a 10-ml centrifuge tube (2:1). After centrifugation for 30 min at 1000×g (20 °C), the PBMCs at the plasma-Ficoll interface were collected carefully. Then, cold RPMI-1640 medium (containing 5.0% inactivated fetal bovine serum, 0.0599 mg/ml penicillin, 100 μg/ml streptomycin, and 24 mM HEPES) was used to wash the PBMCs three times with centrifugation at 250×g for 10 min (4 °C). The PBMC count was evaluated by trypan blue staining.
Flow Cytometry for Lymphocyte Subpopulation Analysis
Peripheral lymphocytes contained in PBMC fraction as prepared above were stained with chicken CD3 (SPRD, clone: CT-3), CD4 (FITC, clone: CT-4), and CD8 (PE, clone: CT-8), and incubated in a water bath for 30 min. All the antibodies used in the study were purchased from SouthernBiotech (Birmingham, AL, USA). Then, lymphocytes were washed twice with Hanks solution and fixed with 3% paraformaldehyde solution. The analysis was conducted by a multi-channel cytometer (Beckman-Coulter, Carlsbad, CA, USA). The results are presented as the percentage of positive lymphocyte subpopulation with the specific antibody.
Lymphocyte Proliferative Responses
The method for PBMC isolation was described above. Trypan blue staining was used to evaluate cell count and viability. The proliferative response of T and B lymphocytes was measured by the MTT assay, after stimulation with Concanavalin A (Con A; from Canavalia ensiformis; Sigma-Aldrich) and lipopolysaccharides (LPS; from E. coli; Sigma-Aldrich), respectively. Results are expressed as stimulation index (SI).
Total RNA Extraction and Real-time Quantitative PCR
Total RNA of spleen and jejunal tissues was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The RNA concentration was measured by a nanodrop spectrophotometer (ND-2000, Thermo Fisher Scientific) at 260 and 280 nm. The total RNA purity was verified by the 260 nm/280 nm ratio; the results of all samples were between 1.8 and 2.0. Then, 2 μg of total RNA was used for reverse transcription by a commercial kit (Takara Biotechnology Co. Ltd., Tokyo, Japan) following the manufacturer’s protocol. The complementary DNA was stored at − 20 °C.
The expression of inflammation-related genes and tight junction (TJ) genes in the spleen and jejunum were determined by real-time quantitative PCR (RT-PCR). Gene primer sequences are presented in Table 2. RT-PCR was performed on an Applied Biosystems 7500 Fast Real-time PCR System (Applied Biosystems, Carlsbad, CA, USA) using a commercial SYBR Green kit (Takara Biotechnology Co. Ltd.). According to the manufacturer’s protocol, the initial denaturation phase was set at 95 °C for 5 min followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s during annealing and extension. Melting curve analysis was used to evaluate the specificity of the amplified products. All the genes in this study were analyzed using GAPDH as an endogenous reference gene. The average gene expression level relative to GAPDH of each sample was calculated using the 2−ΔΔCt method.
Table 2.
Primers for real-time quantitative PCR assay
| Gene | Primer sequence (5′–3′) | Accession no. |
|---|---|---|
| Mucin-2 | F: TTCATGATGCCTGCTCTTGTG | XM_421035 |
| R: CCTGAGCCTTGGTACATTCTTGT | ||
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC | XM_413773 |
| R: CTGTGGTTTCATGGCTGGATC | ||
| Claudin-1 | F: CATACTCCTGGGTCTGGTTGGT | AY750897.1 |
| R: GACAGCCATCCGCATCTTCT | ||
| Occludin | F: ACGGCAGCACCTACCTCAA | D21837.1 |
| R: GGGCGAAGAAGCAGATGAG | ||
| NF-κb | F: GTGTGAAGAAACGGGAACTG | NM205129 |
| R: GGCACGGTTGTCATAGATGG | ||
| PPARγ | F: GACCTTAATTGTCGCATCCAT | AF163811 |
| R: CGGGAAGGACTTTATGTATGA | ||
| TLR4 | F: AGTCTGAAATTGCTGAGCTCAAAT | NM_001030693 |
| R: GCGACGTTAAGCCATGGAAG | ||
| TNF-α | F: GAGCGTTGACTTGGCTGTC | NM_204267 |
| R: AAGCAACAACCAGCTATGCAC | ||
| IL-1β | F: ACTGGGCATCAAGGGCTA | NM_204524 |
| R: GGTAGAAGATGAAGCGGGTC | ||
| IL-6 | F: TTTATG GAGAAGACCGTGAGG | NM_204628 |
| R: TGTGGCAGATTGGTAACAGAG | ||
| IL-10 | F: GCTGTCACCGCTTCTTCACCT | EF554720.1 |
| R: GGCTCACTTCCTCCTCCTCATC |
F, forward; R, reverse
Statistical Analysis
Data were analyzed by one-way ANOVA using SPSS 17.0 software (version 17.0, SPSS Inc., Chicago, IL, USA). Statistical differences among treatments were examined by Duncan’s multiple range test. Results are presented as the mean ± SE. Differences were considered statistically significant at P < 0.05, and 0.05 < P < 0.1 was regarded as a trend towards significance.
Results
Growth Performance
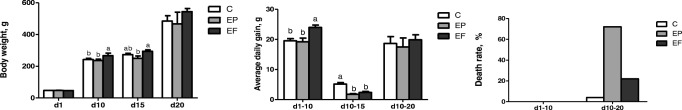
The growth performance parameters are shown in Fig. 1. Compared with the control group, E. faecium supplementation significantly increased the BW of chickens on d10 (P < 0.05) right before the E. coli challenge, and on d15 (3 days after the E. coli challenge). The EF birds had a greater ADG than the other birds during d1–10 (P < 0.05). The death rate was dramatically lower in the EF group compared with that in the EP group. However, the E. coli challenge markedly decreased the BW on d15 (P < 0.05) and the ADG during d10–15 (P < 0.05). The death rate of the EP group was the highest among all three groups. No significant differences were observed in BW on d1 and 20, and in the ADG from d10 to d20.
Fig. 1.
Effects of dietary Enterococcus faecium on growth performance (body weight, average daily gain, and death rate) of broilers. C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF = birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78. Bars with letters (a–c) suggested significant difference among different treatments (P < 0.05)
Intestinal Morphology
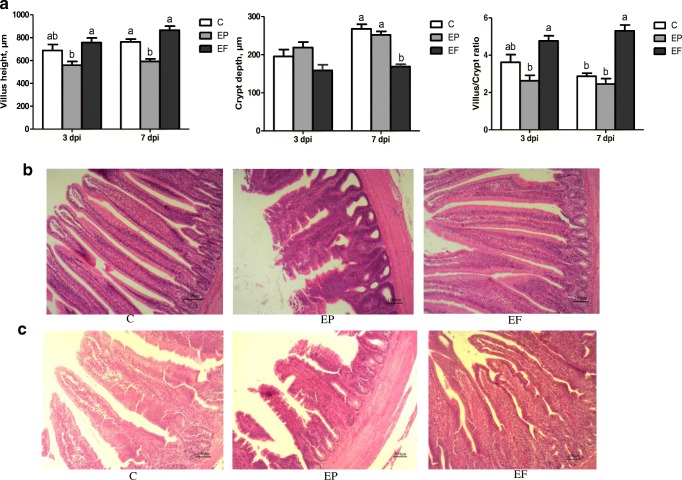
According to Fig. 2(A), 3 and 7 days post-infection, the E. coli challenge significantly decreased the jejunal villus height (P < 0.05), and the birds in the EF group had a markedly higher jejunal villus height than those in the other groups (P < 0.05). The E. coli infection significantly increased the crypt depth 3 and 7 days post-infection (P < 0.05). E. faecium markedly increased the villus/crypt ratio (P < 0.05) 3 and 7 days post-infection. Histopathological changes (Fig. 2(B, C)) showed that the E.coli infection caused shedding and swelling of villus tip and increased crypt depth 3 and 7 days post-infection. The supplementation of E. faecium maintained intact structural of jejunum and increased villus height 3 and 7 days post-infection.
Fig. 2.
Effects of Enterococcus faecium on the jejunum morphology of broilers. (A) Statistic data of villus height, crypt depth, and villus/crypt ratio (V/C). (B) and (C) Photomicrographs of jejunum contained from C, EP, and EF group, on day 3 and 7 post-infection, respectively (× 100). C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF, birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78. Bars with small letters (a–c) suggested significant difference among different treatments (P < 0.05)
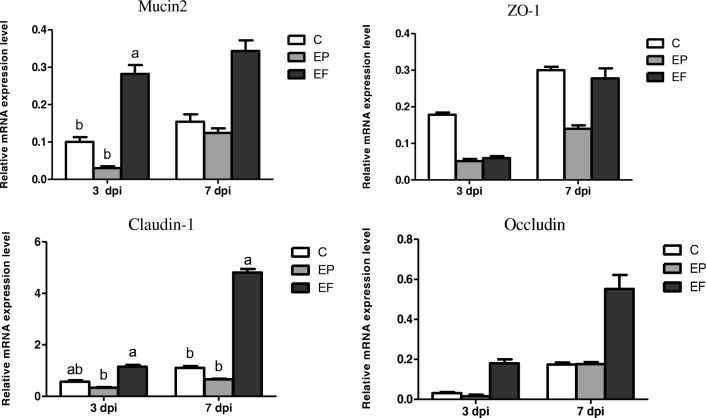
Relative mRNA Expression of TJ Proteins and Mucin
The relative mRNA expression of different intestinal TJ proteins (zonula occludens-1, claudin-1, and occludin) and the Mucin2 gene is shown in Fig. 3. Mucin2 mRNA expression in the jejunum significantly decreased after E. coli infection (P < 0.05). However, the EF group showed a significant increase in Mucin2 mRNA 3 days post-infection (P < 0.05) and an increased tendency 7 days post-infection (P = 0.083). The E. coli infection and the E. faecium supplementation did not affect the zonola occludens-1 (ZO-1) mRNA expression 3 and 7 days post-infection (P > 0.05). E. faecium addition markedly increased claudin-1 mRNA expression 3 and 7 days post-infection (P < 0.05). The E. faecium supplementation also tended to upregulate occludin mRNA expression 3 and 7 days post-infection (P = 0.053 and P = 0.051, respectively).
Fig. 3.
The expression of intestinal tight junction proteins and mucin2 gene in the jejunum. C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF = birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78. Bars with letters (a–c) suggested significant difference among different treatments (P < 0.05)
Evaluation of Cellular and Humoral Immunity
The peripheral blood lymphocyte phenotypes of the different treatments are summarized in Table 3. No remarkable differences were observed in the percentage of CD3+, CD4+, and CD8+ cells, as well as the ratio of CD4+/CD8+ among the three treatments 3 and 7 days post-infection (P > 0.05).
Table 3.
Phenotyping of lymphocytes in peripheral blood of broilers in different treatments
| Items | Treatments | SEM | P value | ||
|---|---|---|---|---|---|
| C | EP | EF | |||
| 3 dpi | |||||
| CD3+ | 52.1 ± 5.69 | 59.58 ± 2.96 | 53.53 ± 4.28 | 2.521 | 0.481 |
| CD4+ | 31.78 ± 3.38 | 36.5 ± 2.11 | 34.6 ± 2.81 | 1.582 | 0.515 |
| CD8+ | 19.28 ± 2.34 | 20.43 ± 0.82 | 17.13 ± 2.05 | 1.052 | 0.472 |
| CD4+/CD8+ | 1.66 ± 0.06 | 1.79 ± 0.11 | 2.16 ± 0.41 | 0.145 | 0.375 |
| 7 dpi | |||||
| CD3+ | 86.43 ± 2.16 | 84.35 ± 2.58 | 87.38 ± 0.96 | 1.122 | 0.577 |
| CD4+ | 60.1 ± 1.38 | 55.9 ± 1.79 | 59.38 ± 1.42 | 0.976 | 0.176 |
| CD8+ | 25.58 ± 1.49 | 27.05 ± 1.43 | 29.33 ± 0.3 | 0.784 | 0.142 |
| CD4+/CD8+ | 2.38 ± 0.15 | 2.08 ± 0.08 | 2.03 ± 0.06 | 0.073 | 0.094 |
C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF, birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78
The function of peripheral blood lymphocytes among the three treatments 3 days post-infection was tested in vitro (Table 4). The proliferative response of T lymphocytes was not affected by E. coli infection or E. faecium supplementation (P > 0.05), as evidenced by ConA SI. However, E. faecium significantly increased the LPS SI (P < 0.05), which reflects the active response of B lymphocyte proliferation in the EF group.
Table 4.
The function of peripheral blood lymphocytes of broilers in different treatments on day 3 post-infection
| Items | Treatments | SEM | P value | ||
|---|---|---|---|---|---|
| C | EP | EF | |||
| ConA SI | 0.7 ± 0.070 | 0.66 ± 0.020 | 0.78 ± 0.040 | 0.030 | 0.212 |
| LPS SI | 0.73 ± 0.05b | 0.68 ± 0.02b | 1.15 ± 0.14a | 0.088 | 0.023 |
a, bMeans in the same row, values with different small letter superscripts, show significant difference (P < 0.05)
C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF, birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78
Intestinal Immune Responses (sIgA)
The concentration of the jejunum sIgA was not influenced by either the E. coli challenge or the E. faecium supplementation 3 and 7 days post-infection (P > 0.05) (data not shown).
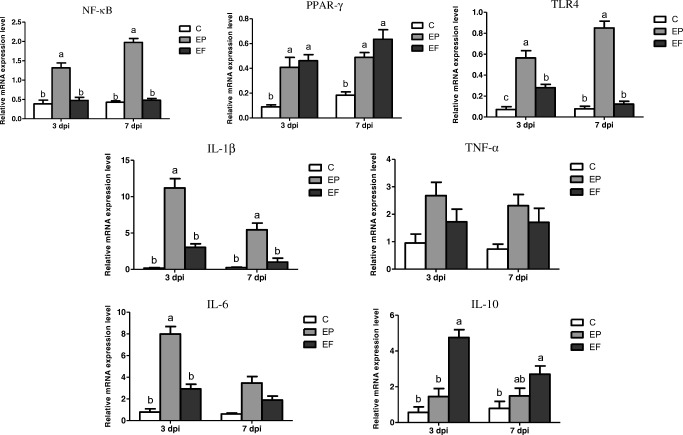
Spleen Inflammation-Related Gene Expression
The relative mRNA expression levels of spleen immune cytokines are presented in Fig. 4. Three days post-infection, the E. coli challenge significantly increased the expression of nuclear factor-κB (NF-κB), peroxisome proliferator-activated receptor-γ (PPAR-γ), toll-like receptor 4 (TLR4), IL-1β, and IL-6 (P < 0.05) and tended to increase the expression of TNF-α (P = 0.052). The E. faecium supplementation markedly decreased the expression of NF-κB, TLR4, IL-1β, and IL-6 (P < 0.05), and significantly enhanced the expression of PPAR-γ and IL-10 (P < 0.05). Seven days post-infection, the EP birds had remarkably higher expression levels of NF-κB, PPAR-γ, TLR4, and IL-1β (P < 0.05). Furthermore, the E. coli infection tended to increase the expression of TNF-α and IL-6 (P = 0.057 and P = 0.092, respectively). Dietary addition of E. faecium significantly elevated the expression of NF-κB, PPAR-γ, and IL-10 (P < 0.05), and downregulated the expression of TLR4 and IL-1β (P < 0.05). The birds in the EF group had a relatively lower TNF-α and IL-6 expression (P = 0.057 and P = 0.092, respectively).
Fig. 4.
Relative mRNA expression levels of spleen inflammation-related cytokines of broilers in different treatments. C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF, birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78. Bars with letters (a–c) suggested significant difference among different treatments (P < 0.05)
Liver Bacterial Translocation
As presented in Fig. 5, E. coli infection caused considerable liver bacterial translocation in broiler chickens. The E. coli challenge significantly increased the liver E. coli number (P < 0.05) 3 and 7 days post-infection. Compared with the EP group, the EF group had a markedly lower number of liver E. coli (P < 0.05) 3 and 7 days post-infection.
Fig. 5.
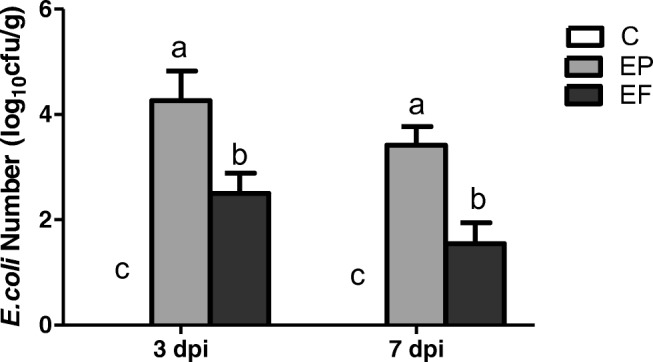
Liver bacterial translocation of E. coli in broilers (log10 cfu/g). C, birds fed with basal diet; EP, birds fed with basal diet and challenged with E. coli O78; EF, birds fed a basal diet supplemented with E. faecium and challenged with E. coli O78. Bars with letters (a–c) suggested significant difference among different treatments (P < 0.05)
Discussion
Probiotics have been proven to be beneficial for broiler breeding [14, 15]. E. faecium is a Lactobacillus genus that shows many positive effects on broiler growth and immunity [6, 7, 11, 12].
E. coli is a Gram-negative bacterium and its core pathogenic element is LPS [5, 16]. LPS can trigger system inflammation and cause death. Inflammation limits the synthesis of muscle protein and mobilizes energy to support the immune response, resulting in poor growth [16]. In the present study, chickens infected with E. coli O78 had a lower BW and ADG 3 days post-infection and the highest death rate during the entire feeding period. Many studies have shown that E. faecium can improve broiler performance [6, 9]. Cao et al. [1] have indicated that E. faecium enhanced chicken growth performance after an E. coli K88 challenge. Similarly, we showed that dietary E. faecium supplementation increased BW and ADG both before and 3 days post-infection and decreased the death rate.
Intestinal morphology reflects the health and integrity of the alimentary tract. A decreasing crypt size indicates the reconstruction of intestinal villus by accelerating the regenerative rate of enterocytes [14]. Therefore, the gut can resist devastating damage by pathogens or toxins. However, increased villus suggests the expansion of the intestinal absorption area, accumulation of mature enterocytes, and strengthening of the absorption and digestion ability [17]. Zhang et al. [5] have found that an E. coli K88 challenge disrupted intestinal morphology. Furthermore, E. faecium efficiently improved the intestinal mucosal architecture by increasing the villus height and decreasing the crypt depth [6]. According to Jin et al. [10], E. faecium can inhibit the adhesion of E. coli to enterocytes potentially through modifying the lumen pH and altering steric hindrance. Cao et al. [1] have reported that E. faecium was beneficial for the jejunal morphology of E. coli K88-challenged broilers compared with the control and antibiotic groups. Consistently, we showed that the addition of E. faecium improved the intestinal histomorphology with an increased V:C ratio and villus height in the challenged birds 3 and 7 days post-infection.
The intestinal mechanical barrier, which is composed of enterocytes and TJs, plays a key role in the natural defense against pathogen invasion [18, 19]. The major TJ proteins, including occludin, claudin and zonula occludens (ZO), and junctional adhesion molecule (JAM) [20], play an important role in maintaining intestinal permeability and the mucosal barrier function by sealing the extracellular space between the epithelial cells. In this study, the E. coli O78 challenge significantly reduced the expression of claudin-1 3 and 7 days post-infection, which is consistent with the findings of Gadde et al. [21] and Lee et al. [22], who showed that LPS treatment decreased the expression of TJ proteins. Probiotics have been proven to enhance TJ protein excretion and strengthen the mucosal barrier [23]. Feeding chickens with E. faecium upregulated the expression of claudin-1 3 and 7 days post-infection. The mucosal layer above the gastrointestinal tract is the chemical barrier of the intestine and mucins are the fundamental components [24]. Mucin-2, acting as a major mucin gene in the small intestine, was significantly increased 3 days post-infection by the E. faecium supplementation in the present study. This result is consistent with Gadde et al. [21], who revealed that probiotics increased the expression of the Mucin2 gene in LPS-challenged chickens. These results suggest that E. faecium addition counteracted the detrimental effects of E. coli, and significantly enhanced claudin-1 and Mucin2 expression, especially at the early infection stage. However, E. faecium did not affect the gene expression of other TJ proteins.
The spleen, a vital immune organ involved in both cellular and humoral immune responses, is important for lymphocyte generation, maturation, and storage [25]. Thus, immune-mediated spleen gene expression is considered to be an indicator of system immunity [26]. LPS is the primary cytoderm component of Gram-negative bacteria, and the LPS endotoxin serves as an activator of the innate immune response [27]. E. coli O78 can release LPS. Pattern recognition receptors (PRRs) have been selected to recognize the conserved elements of pathogens during the process of evolution [28]. TLRs are crucial members of PRRs and TLR4 can recognize LPS [29]. After binding to LPS, avian TLR4 triggers a cascade of inflammation responses via the myeloid differentiation factor 88 (MyD88)-dependent signaling pathway, which results in NF-κB activation [30]. NF-κB is the key factor in the regulation of the secretion of various cytokines and inflammatory mediators [31]. Many studies have used LPS injection to trigger a pro-inflammatory cytokine response in broiler chickens [16, 27, 31, 32], which resulted in activated TLR4. Similarly, in the current study, the infection of E. coli O78 significantly increased the expression of TLR4 and NF-κB 3 and 7 days post-infection. PPARs are key regulators of inflammatory and immune responses [16]. Cumulative research has proven that PPAR-γ ligands can inhibit major inflammation signaling pathways such as NF-κB, which implies the anti-inflammatory effect of PPAR-γ [33]. We found that E. faecium supplementation downregulated the expression of TLR4 and NF-κB, and upregulated PPAR-γ expression 3 and 7 days post-infection, which is consistent with the results by Gadde et al. [21].
Pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α regulate the immune response by inducing differentiation and proliferation of leukocytes to eliminate pathogens [34]. However, excessive secretion leads to organ damage and exacerbated energy consumption [34]. Thus, the suppression of IL-1β, IL-6, and TNF-α by dietary E. faecium supplementation in the current study may alleviate system inflammation. Conversely, Cao et al. [1] have indicated that E. faecium had no effect on the immune response to an E. coli K88 challenge. Different challenge approaches and measuring methods may be the reasons for this discrepancy. However, IL-10, which is a critical anti-inflammatory cytokine, acts as an inflammation feedback factor to modulate the immune response [35]. An LPS challenge suppressed the expression of anti-inflammatory cytokines [16, 21]. Our study found the same downregulation of IL-10 expression after E. coli O78 infection. Siepert et al. [12] have suggested that E. faecium supplementation elevated IL-10 expression. In the present study, addition of E. feacium markedly increased the IL-10 gene expression. Hence, we assume that E. feacium might regulate the NF-κB pathway by interacting with TLR4 and PPAR-γ, to alleviate the system inflammation caused by E. coli O78 infection.
Cellular and humoral immunity is the primary defense mechanism to obliterate pathogens [13]. CD4+ and CD8+ T cells play an important role in the cellular immune response. CD4+ T lymphocytes enhance the intercellular killing by macrophages and promote the expansion of cytotoxic T lymphocytes. CD8+ T lymphocytes are mainly involved in the elimination of antigens. CD3+ presents a surface marker of mature T lymphocytes [36]. The ratio of CD4+/CD8+ indicates the level of cell immunity [8]. The effect of E. faecium on animal immunity is controversial. Wang et al. [37] have demonstrated that the E. faecium supplementation elevated the activation of T helper lymphocytes and cytotoxic T lymphocytes in the peripheral blood of piglets infected with swine influenza. Levkut et al. [8] have indicated that E. faecium addition increased the number of CD3+, CD4+, and CD8+ cells in the peripheral blood of broiler chickens challenged with Salmonella enteritidis. In contrast, Kreuzer et al. [38] have found that E. faecium had no effect on the cellular immune response in lymph nodes and blood of piglets subjected with Salmonella enterica. In the current study, the differences in CD3+, CD4+, CD8+, and CD4+/CD8+ were not obvious among the three treatments, both 3 and 7 days post-infection. This result suggests that E. faecium may have a limited effect on immune mediation. Alternatively, it may be that cellular immunity has a minor effect on extracellular bacteria such as E. coli. At the early infection stage, the result of lymphocyte proliferative responses indicated that dietary E. faecium supplementation had no effect on T lymphocytes, as shown by ConA SI, whereas it significantly elevated B lymphocyte proliferation, as shown by LPS SI. Therefore, these results further indicate that the protective immune response evoked by E. faecium mainly focused on humoral immunity rather than cellular immunity. Interestingly, the E. coli O78 challenge reduced the LPS SI. According to Shini et al. [39], the endotoxins released by LPS induce degeneration and destruction of lymphocytes in birds. SIgA is a crucial immunoglobulin that serves as the first line of defense against pathogens [40]. Several studies have shown that probiotics can increase intestinal IgA excretion [40, 41]. However, the E. faecium supplementation did not result in overt upregulation of sIgA, both 3 and 7 days post-infection, which was similar to a previous study using the same challenging pathogen [1].
Bacterial translocation, defined as intestinal bacteria moving from the lumen to the mesentery or other parenteral organs, occurs frequently in damaged intestine [42]. Therefore, bacterial translocation is a useful indicator of the integrity of the intestinal structure [43]. In the present study, E. faecium supplementation markedly decreased the number of E. coli colonies in the liver of broiler chickens challenged with E. coli O78 3 and 7 days post-infection. The results manifested that E. faecium had the ability to maintain the intestinal integrity and limit the high permeability of the intestine caused by E. coli infection.
Conclusions
Dietary E. faecium supplementation improved the growth performance and reduced the death rate of broiler chickens by enhancing the humoral immune response, modulating inflammatory cytokine secretion, enhancing TJ proteins’ expression, and maintaining the intestinal barrier against E. coli O78 infection. The beneficial effects of E. faecium may be partially associated with its effects on intestinal integrity and system humoral immunity. More studies are needed to further explore the potential mechanism of E. faecium in intestinal immunity.
Acknowledgments
We especially thank Zhong Wang, State key laboratory of animal nutrition, College of animal science and technology, China Agricultural University, Beijing, for his effort in the experimental design and data analysis.
Funding
This study was supported by the China Agricultural Research System Poultry-Related Science and Technology Innovation Team of Peking (CARS-PSTP).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional guidelines for the care and use of animals adhered to the Animal Care and Use Committee of China Agricultural University (permit number: 20121209–1).
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent is not applicable in this study.
Contributor Information
Zhong Wang, Phone: +86 62732337, Email: wangzh@cau.edu.cn.
Zhaofei Xia, Phone: +86 62733781, Email: zhaofeixiacau@126.com.
References
- 1.Cao G, Zeng X, Chen A, Zhou L, Zhang L, Xiao Y, Yang C. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- 2.Sandford EE, Orr M, Balfanz E, Bowerman N, Li X, Zhou H, Johnson TJ, Kariyawasam S, Liu P, Nolan LK, Lamont SJ. Spleen transcriptome response to infection with avian pathogenic Escherichia coli in broiler chickens. BMC Genomics. 2011;12:469. doi: 10.1186/1471-2164-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma V, Jakhar KK, Dahiya S. Immuno-pathological studies on broiler chicken experimentally infected with Escherichia coli and supplemented with neem (Azadirachta indica) leaf extract. Vet World. 2016;9:735–741. doi: 10.14202/vetworld.2016.735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop Sao Paulo. 2014;5:341–346. doi: 10.1590/S0036-46652014000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Zhang L, Zhan X, Zeng X, Zhou L, Cao G, Chen A, Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samli HE, Dezcan S, Koc F, Ozduven ML, Okur AA, Senkoylu N. Effects of Enterococcus faecium supplementation and floor type on performance, morphology of erythrocytes and intestinal microbiota in broiler chickens. Br Poult Sci. 2010;51:564–568. doi: 10.1080/00071668.2010.507241. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Guo YM, Guo SS, Tan J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol. 2013;97:6477–6488. doi: 10.1007/s00253-013-4970-2. [DOI] [PubMed] [Google Scholar]
- 8.Levkut M, Revajova V, Laukova A, Sevcikova Z, Spisakova V, Faixova Z, Levkutova M, Strompfova V, Pistl J, Levkut M. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis. Res Vet Sci. 2012;93:195–201. doi: 10.1016/j.rvsc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Capcarova M, Chmelnicna L, Kolessarova A, Massanyi P, Kovacik J. Effects of Enterococcus faecium M 74 strain on selected blood and production parameters of laying hens. Br Poult Sci. 2010;51:614–620. doi: 10.1080/00071668.2010.513961. [DOI] [PubMed] [Google Scholar]
- 10.Jin LZ, Marquardt RR, Zhao X. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl Environ Microbiol. 2000;66:4200–4204. doi: 10.1128/AEM.66.10.4200-4204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galeano JAC, Herrera AL, Suescun JP. The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Rev Fac Nal Agr. 2015;69:7803–7811. doi: 10.15446/rfna.v69n1.54748. [DOI] [Google Scholar]
- 12.Siepert B, Reinhardt N, Kreuzer S, Bondzio A, Twardziok S, Brockmann G, Nockler K, Szabo L, Janczyk P, Pieper R, Tedin K. Enterococcus faecium NCIMB 10415 supplementation affects intestinal immune-associated gene expression in post-weaning piglets. Vet Immunol Immunopathol. 2014;157:65–77. doi: 10.1016/j.vetimm.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Kuritza LN, Loureco MC, Miglino L, Pickler L, Kraieski AL, Santin E. Effects of Enterococcus faecium on diet in the dynamics of CD4+ and CD8+ cell infiltration in the intestinal mucosa of broilers challenged with Salmonella Minnesota. Int J Poult Sci. 2013;12:523–528. doi: 10.3923/ijps.2013.523.528. [DOI] [Google Scholar]
- 14.Salim HM, Kang HK, Akter N, Kim DW, Kim JH, Kim MJ, Na JC, Jong HB, Choi HC, Suh OS, Kim WK. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci. 2013;92:2084–2090. doi: 10.3382/ps.2012-02947. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Li W, Liu W, Zou L, Yan C, Lu K. T4-like phage Bp7, a potential antimicrobial agent for controlling drug-resistant Escherichia coli in chickens. Appl Environ Microbiol. 2013;79:5559–5565. doi: 10.1128/AEM.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan J, Liu S, Guo YM, Applegate TJ, Eicher SD. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br J Nutr. 2014;111:1394–1404. doi: 10.1017/S0007114513003863. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Ingale SL, Kim YW, Kim KH, Sen S, Ryu MH, Lohakare JD, Kwon IK, Chae BJ. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J Anim Physiol Anim Nutr. 2012;96:618–626. doi: 10.1111/j.1439-0396.2011.01187.x. [DOI] [PubMed] [Google Scholar]
- 18.Dkhil MA, Delic D, Al-Quraishy S. Goblet cells and mucin related gene expression in mice infected with Eimeria papillata. Sci World J. 2013;2013:1–6. doi: 10.1155/2013/439865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Guo S, Gao J, Guo Y, Du E, Lv Z, Zhang B. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J Nutr Biochem. 2015;26:173–183. doi: 10.1016/j.jnutbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gadde UD, Oh S, Lee Y, Davis E, Zimmerman N, Rehberger T, Lillehoj HS. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res Vet Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Lee SH, Gadde UD, Oh ST, Lee SJ, Lilllehoj HS. Dietary Allium hookeri reduces inflammatory response and increases expression of intestinal tight junction proteins in LPS-induced young broiler chicken. Res Vet Sci. 2013;112:149–155. doi: 10.1016/j.rvsc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes. 2010;1:148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson MEV, Holmén-Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc Natl Acad Sci U S A. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KG, Hunt JL. On the use of spleen mass as a measure of avian immune system strength. Oecologia. 2004;138:28–31. doi: 10.1007/s00442-003-1409-y. [DOI] [PubMed] [Google Scholar]
- 26.Redmond SB, Tell RM, Coble D, Mueller C, Palic D, Andreasen CB, Lamont SJ. Differential splenic cytokine responses to dietary immune modulation by diverse chicken lines. Poult Sci. 2010;89:1635–1641. doi: 10.3382/ps.2010-00846. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Nii T, Isobe N, Yoshimura Y. Expression of toll-like receptors and effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokine in the testis and epididymis of roosters. Poult Sci. 2012;91:1997–2003. doi: 10.3382/ps.2012-02236. [DOI] [PubMed] [Google Scholar]
- 28.Netea MG, van der Graaf C, Van der Meer JWM, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75:749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 29.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Keestra AM, Zoete MR, Bouwman LI, Vaezirad MM, Putten PM. Unique features of chicken toll-like receptors. Dev Comp Immunol. 2013;41:316–323. doi: 10.1016/j.dci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Keestra AM, Putten JPM. Unique properties of the chicken TLR4/MD-2 complex: selective lipopolysaccharide activation of the MyD88-dependent pathway. J Immunol. 2008;181:4354–4362. doi: 10.4049/jimmunol.181.6.4354. [DOI] [PubMed] [Google Scholar]
- 32.Munyaka PM, Tactacan G, Jing M, House JD, Rodriguez-Lecompte JC. Immunomodulation in young laying hens by dietary folic acid and acute immune responses after challenge with Escherichia coli lipopolysaccharide. Poult Sci. 2012;91:2454–2463. doi: 10.3382/ps.2012-02381. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LM, Zhu M, Li M, Du Y, Duan S, Huang Y, Lu Y, Zhang J, Wang T, Fu F. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget. 2017;8:55384–55393. doi: 10.18632/oncotarget.19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodes L, Khan A, Paul A, Coussa-Charley M, Marinescu D, Tomaro-Duchesneau C, Shao W, Kahouli I, Prakash S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: an in vitro study using a human colonic microbiota model. J Microbiol Biotechnol. 2013;23:518–526. doi: 10.4014/jmb.1205.05018. [DOI] [PubMed] [Google Scholar]
- 35.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Sci. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 37.Wang ZY, Burwinkal M, Chai W, Lange E, Blohm U, Breithaupt A, Hoffmann B, Twardzio S, Rieger J, Janczyk P, Pieper R, Osterrieder N. Dietary Enterococcus faecium NCIMB 10415 and zinc oxide stimulate immune reactions to trivalent influenza vaccination in pigs but do not affect virological response upon challenge infection. PLoS One. 2014;9:e87007. doi: 10.1371/journal.pone.0087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreuzer S, Janczyk P, Assmus J, Schmidt MF, Brockmann GA, Nockler K. No beneficial effects evident for Enterococcus faecium NCIMB 10415 in weaned pigs infected with Salmonella enterica serovar Typhimurium DT104. Appl Environ Microbiol. 2012;78:4816–4825. doi: 10.1128/AEM.00395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shini S, Kaiser P, Shini A, Bryden WL. Differential alterations in ultrastructural morphology of chicken heterophils and lymphocytes induced by corticosterone and lipopolysaccharide. Vet Immunol Immunopathol. 2008;122:83–93. doi: 10.1016/j.vetimm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B, Chambers JR, Sharif S. Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol. 2006;13:975–980. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharek-Tedin L, Filter M, Taras D, Wrede P, Schmidt MF. Influence of an Enterococcus faecium probiotic on the development of Peyer's patches B cells in piglets. Arch Anim Nutr. 2009;63:343–355. doi: 10.1080/17450390903052771. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez E, Casafont F, Guerra A, de Benito I, Pons-Romero F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Rev Esp Enferm Dig. 2005;97:805–814. doi: 10.4321/s1130-01082005001100005. [DOI] [PubMed] [Google Scholar]
- 43.Yajima M, Nakayama M, Hatano S, Yamazaki K, Aoyama Y, Yajima T, Kuwata T. Bacterial translocation in neonatal rats: the relation between intestinal flora, translocated bacteria, and influence of milk. J Pediatr Gastroenterol Nutr. 2011;33:592–601. doi: 10.1097/00005176-200111000-00015. [DOI] [PubMed] [Google Scholar]