Abstract
Campylobacter jejuni is a microaerophilic foodborne pathogen that is sensitive to stress conditions. However, it is not yet understood how this stress-sensitive pathogen may cause a significant number of cases of human gastroenteritis worldwide. In this study, we examined stress tolerance in 70 C. jejuni strains isolated from retail chicken under several stress conditions related to food safety. Compared to oxygen-sensitive (OS) strains of C. jejuni, C. jejuni strains with increased aerotolerance, such as hyper-aerotolerant (HAT) and aerotolerant (AT) strains, were more tolerant to peracetic acid, refrigeration and freeze-thaw stresses. However, the levels of thermotolerance and hyper-osmotolerance were not associated with the aerotolerance level of C. jejuni. The HAT and AT strains of C. jejuni exhibited significantly increased activities of catalase and superoxide dismutase (SOD), compared to the OS strains. Consistently, the HAT and AT strains were highly tolerant to oxidants, such as hydrogen peroxide, cumene hydroperoxide and menadione, compared to the OS strains. The AT and HAT strains that were tolerant to stresses, particularly peracetic acid and refrigeration, predominantly belonged to multilocus sequence typing (MLST) clonal complex (CC)-21. This study shows that oxidative stress resistance plays a role in determining the differential level of aerotolerance in C. jejuni and that AT and HAT strains of C. jejuni are more tolerant to oxidants and low temperatures than OS strains.
Subject terms: Bacteriology, Applied microbiology
Introduction
Campylobacter spp. is one of the primary bacterial causes of gastroenteritis worldwide1. Since campylobacters are isolated from a range of food-producing and companion animals, wildlife, and environmental sources, Campylobacter outbreaks are caused by various sources, such as water, raw milk, and even mud2,3. However, the consumption of contaminated poultry meat is the primary cause of human campylobacteriosis2. Although a number of poultry species carry pathogenic species of Campylobacter (i.e., Campylobacter jejuni and Campylobacter coli)4, chickens are most frequently implicated in human infections5. In the EU, for example, it has been estimated that 50–80% of human campylobacteriosis cases are attributed to chicken5. Consistently, studies show that retail chicken meats are frequently contaminated with Campylobacter. Approximately 61% of retail chicken in the UK is contaminated with Campylobacter6, and 62% of raw chicken legs in Canada7. Due to the high frequency of Campylobacter contamination of chicken, it has been estimated that a two-log reduction of Campylobacter counts on chicken carcasses would decrease the chances of human infection with Campylobacter by 30-fold8. To decrease Campylobacter contamination of poultry carcasses, various intervention methods have been employed in poultry processing, including physical treatment with hot water and steam, chilling and freezing of carcasses, and chemical decontamination9. Among the antimicrobial agents available for poultry carcasses10, peroxyacetic acid (PAA) is widely used by the poultry industry in many countries because the antimicrobial activity of PAA is highly effective11, and it decomposes to acetic acid, oxygen, and water without causing toxicity and environmental risks12. Given that multiple methods used to reduce Campylobacter loads on poultry carcasses, Campylobacter should overcome these stress conditions prior to the initiation of foodborne infection in humans.
Since stress tolerance contributes to bacterial survival under harsh environmental conditions, the stress tolerance of foodborne pathogens plays a significant role in food safety13. Compared to most other enteric pathogens (e.g. Salmonella and enterohemorrhagic Escherichia coli) that efficiently adapt to and survive under stress conditions14, C. jejuni is known to be highly sensitive to environmental stress primarily due to the lack of several stress tolerance genes that are commonly found in other enteric pathogenic bacteria. For instance, C. jejuni is microaerophilic and sensitive to atmospheric oxygen, whereas E. coli and Salmonella grow both aerobically and anaerobically. Additionally, C. jejuni lacks cold stress proteins15 and RpoS16,17, a key stress response regulator and in E. coli and Salmonella18,19. It is not clearly understood how C. jejuni may survive under stress conditions present in food processing, transportation, preservation, and cooking, and is increasingly responsible for gastroenteritis despite its less-conserved stress tolerance mechanisms compared to other foodborne pathogens. Our recent studies showed that certain strains of Campylobacter, both C. jejuni and Campylobacter coli, are highly tolerant to aerobic stress and that such aerotolerant Campylobacter is highly prevalent on retail raw chicken20,21. Interestingly, C. coli strains are more aerotolerant than C. jejuni strains21. Furthermore, clinical strains of C. jejuni with tolerance to environmental stress form genetically unique clusters and are more frequently involved in human infections in Canada compared to oxygen-sensitive C. jejuni22. Although the previous study performed an extensive analysis using 121 clinical strains of C. jejuni, the stress tolerance of C. jejuni strains isolated from retail poultry has not been investigated although retail chicken meat is the major reservoir transmitting C. jejuni to humans. To fill this important knowledge gap, we examined the tolerance of C. jejuni strains isolated from retail chicken meats to five different stress conditions (i.e., disinfectant, refrigeration, freeze-thaw, heat, and high salt concentrations), which C. jejuni may encounter during foodborne transmission to humans.
Results
Disinfectant resistance in C. jejuni isolates from retail chicken
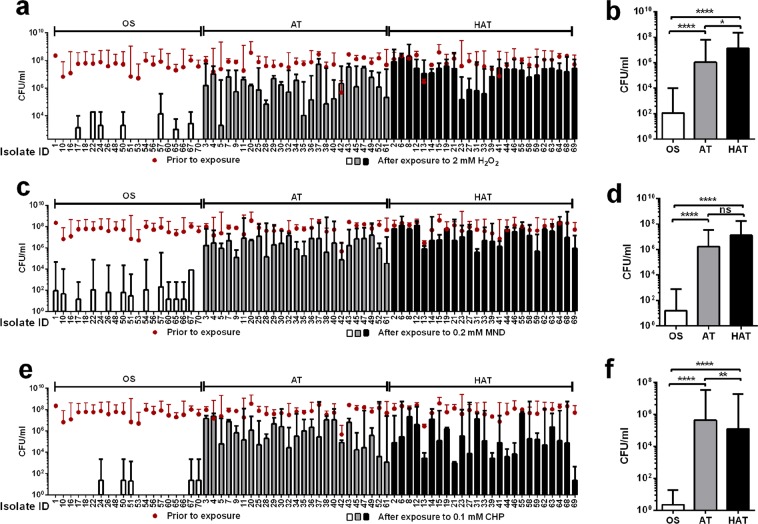
Since chicken carcasses are usually treated with disinfectants during processing, we measured the tolerance of 70 C. jejuni strains to PAA, a common disinfectant used by the poultry industry. The PAA exposure significantly reduced the CFU levels of C. jejuni strains. More significant reduction (ca. 3.7 log CFU/g) was observed in the OS strains compared to the AT and HAT strains (ca. 2.1 and 2.5 log CFU/g, respectively) (Fig. 1). Although most of the tested strains of C. jejuni exhibited a certain level of PAA tolerance, the results showed that AT and HAT strains of C. jejuni were more tolerant to PAA than OS strains.
Figure 1.
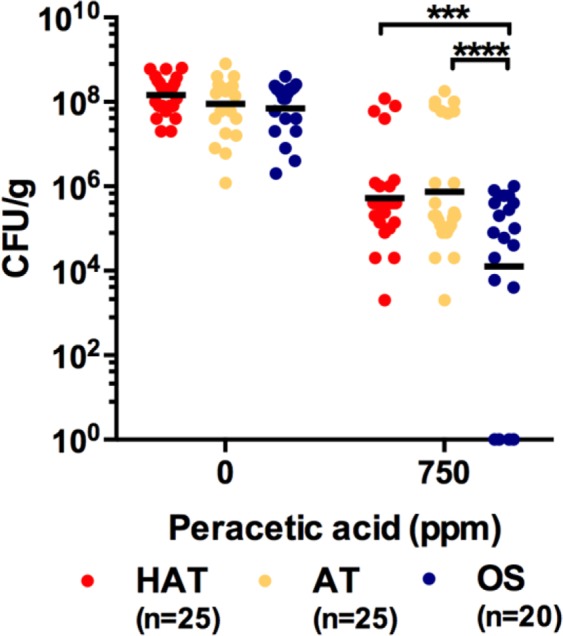
Tolerance to peracetic acid (PAA) in 70 C. jejuni isolates from retail chicken. Solid black bar indicates the mean CFU. The experiment was repeated three times and produced similar results. Two-way ANOVA was used to compare the results in the different aerotolerance groups. ***P < 0.005, ****P < 0.0001.
Increased capability of ROS detoxification in AT and HAT strains of C. jejuni
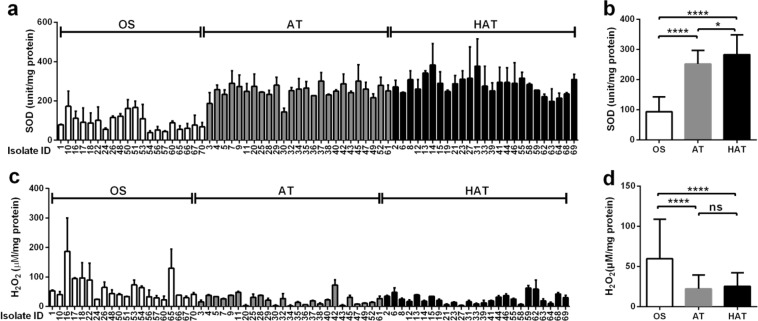
Since oxidative stress defense plays an important role in aerotolerance by detoxifying ROS23,24 and AT and HAT strains of C. jejuni were highly resistant to PAA (Fig. 1), an organic peroxide, we hypothesized that AT and HAT strains of C. jejuni might be more capable of detoxifying ROS than OS strains. To examine this hypothesis, we measured the activities of superoxide dismutase (SOD) and catalase, two important oxidative stress defense enzymes detoxifying the superoxide anion and H2O2, respectively. Interestingly, the SOD activity was significantly higher in the AT and HAT strains than the OS strains (Fig. 2a,b). The catalase activity was determined by measuring the intracellular level of H2O2 in C. jejuni; thus, a lower H2O2 level indicates a higher catalase activity. The AT and HAT strains of C. jejuni accumulated less H2O2 than the OS strains (Fig. 2c,d), suggesting that the catalase activity is higher in the AT and HAT strains than the OS strains. These findings clearly demonstrated that AT and HAT strains of C. jejuni are more resistant to oxidative stress than OS strains; this may contribute to the enhanced PAA tolerance of AT and HAT strains.
Figure 2.
Superoxide dismutase (SOD) and catalase activities in C. jejuni isolates from retail chicken. SOD activities in 70 strains of C. jejuni (a) and the average of SOD activities in OS, AT, and HAT strains (b). Catalase activities in 70 strains of C. jejuni (c) and the average of catalase activities in OS, AT, and HAT strains (d). The results show the means and standard deviations of a single experiment with triplicate samples, and the experiment was repeated three times. Two-way ANOVA was used for statistical analysis. ns: not significant, *P < 0.05, ****P < 0.0001.
The 70 strains of C. jejuni were exposed to three different oxidants, including H2O2, cumene hydroperoxide (CHP; an organic peroxide), and menadione (MND; a superoxide generator). Despite variations depending on the strain and the oxidant (Fig. 3a,c,e), the viability reduction was significant in the OS strains compared to the AT and HAT strains (Fig. 3b,d,f). The results of the oxidative stress defense enzyme assays (Fig. 2) and the susceptibility tests (Fig. 3) consistently showed that HAT and AT strains of C. jejuni were highly tolerant to oxidative stress.
Figure 3.
Tolerance to oxidants in 70 strains of C. jejuni from retail chicken. Viability after 1 h exposure to hydrogen peroxide (H2O2) in 70 strains (a) and the means of CFU in OS, AT, and HAT strains (b), menadione (MND) in 70 strains (c) and the means of CFU in OS, AT, and HAT strains (d), and cumene hydroperoxide (CHP) in 70 strains (e) and the means of CFU in OS, AT, and HAT strains (f). The results show the means and standard deviations of the viability (CFU/ml) in a single experiment with triplicate samples. The CFU level of the inoculum is indicated with a dot graph in red. The experiment was repeated three times. Statistical significance was carried out with two-way ANOVA. ns: not significant, *P < 0.05, **P < 0.01, ****P < 0.0001.
Tolerance to refrigeration, freeze-thaw, and heat in C. jejuni strains from retail chicken
After packaging at processing plants, chicken carcasses are refrigerated or frozen for distribution and preservation. Thus, refrigeration and freezing are harsh stress conditions that C. jejuni encounters in the chicken supply system. In addition, C. jejuni will be exposed to high temperatures during cooking. The OS strains exhibited a significant CFU reduction at a refrigeration temperature. Storing at 4 °C for seven days reduced bacterial counts by approximately 4.9, 5.1, and 7.4 CFU/ml in the HAT, AT, and OS strains of C. jejuni, respectively (Fig. 4a). Similarly, the OS strains of C. jejuni were more sensitive to freeze-thaw stress that the AT and HAT strains. More than half (ca. 52%) of HAT and AT strains survived at −20 °C for seven days; however, only two OS strains were detected after seven days (Fig. 4b). Unlike the results of the refrigeration and freeze-thaw tolerance tests, the aerotolerance level was not associated with thermotolerance as only one HAT strain and one AT strain survived after exposure to 70 °C for 30 sec (Fig. 4c). Based on the findings, C. jejuni strains from retail chicken exhibited different levels of tolerance to cold and heat stresses, and AT and HAT C. jejuni were highly tolerant to refrigeration and freeze-thaw stresses, but not to heat stress.
Figure 4.
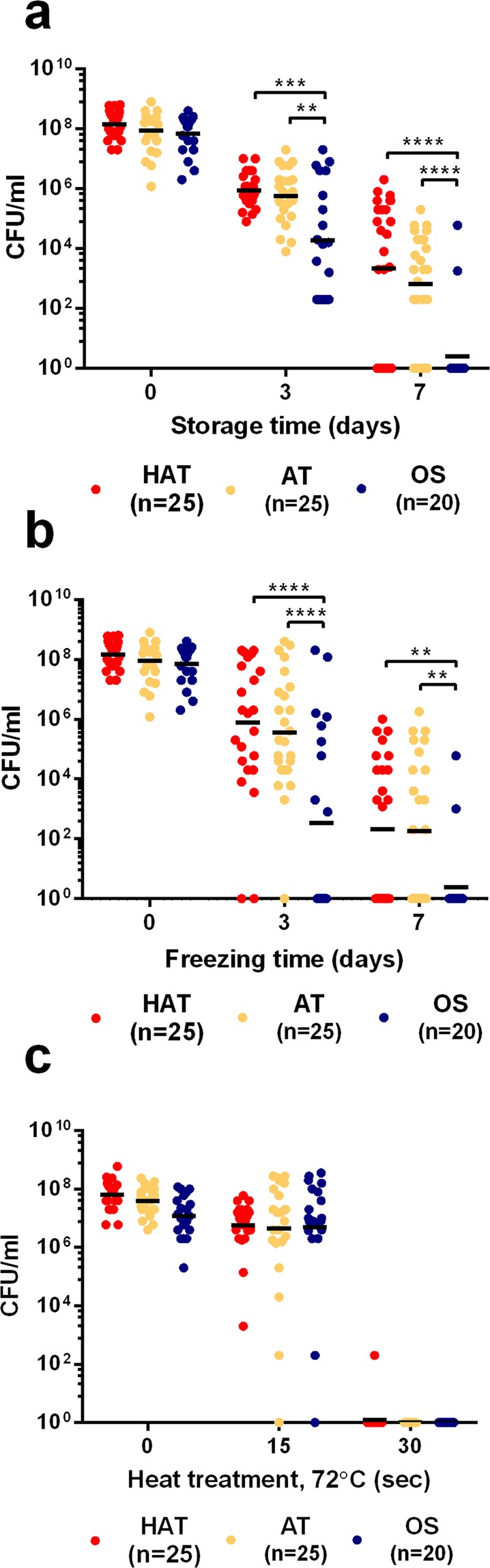
Tolerance to refrigeration (a), freezing (b), and heat (c) stresses in 70 strains of C. jejuni from retail chicken. The results are representative of three independent experiments. Similar results were observed in all three experiments. Solid black bars show the mean CFU. Statistical significance was determined using two-way ANOVA. **P < 0.01, ***P < 0.005, ****P < 0.0001.
Tolerance to hyperosmotic stress
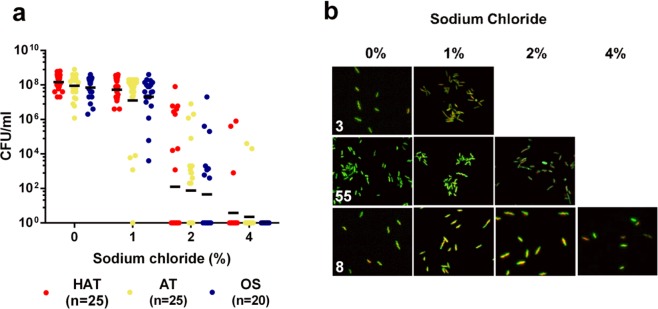
Hyperosmotic stress is a stress condition for Campylobacter in food preservation and cooking, such as marination. When exposed to different concentrations of NaCl (1%, 2%, and 4%), all of the tested strains except for one AT strain survived at 1% NaCl, and approximately 36% of HAT, 48% of AT, and 40% of OS strains survived at 2% NaCl with a wide range of CFU levels (Fig. 5a). Whereas five AT and HAT strains survived at 4% NaCl, none of the OS strains survived; however, the difference was not statistically significant (Fig. 5a). Based on the results of fluorescence microscopic analysis, C. jejuni exhibited heterogeneous morphology with mixed populations of helical rod, elongated, and coccoid cells depending on the strain (Fig. 5b).
Figure 5.
Osmotolerance in 70 strains of C. jejuni from retail chicken and morphological changes under osmotic stress. (a) The viability of 70 strains of C. jejuni under different NaCl concentrations (1, 2, and 4%). Solid horizontal lines indicate the mean values. (b) Morphological changes in C. jejuni strains under osmotic stress. C. jejuni was stained with SYTO9 and propidium iodine, and the strain numbers are indicated in the figure. The experiments were repeated three times. Two-way ANOVA was used for statistical analysis.
MLST sequence types of stress-tolerant C. jejuni strains
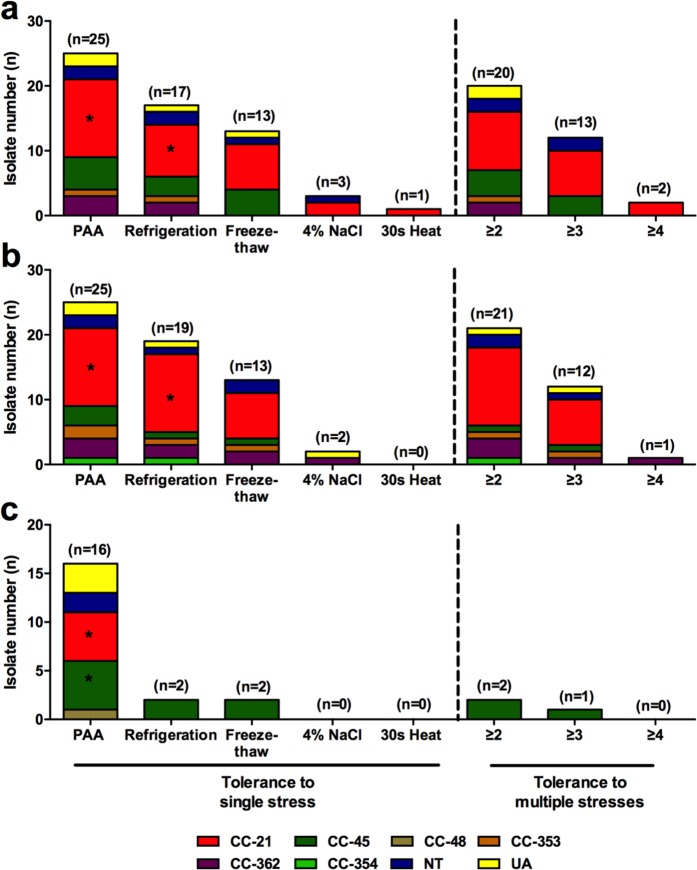
The MLST sequence types of the 70 strains of C. jejuni from raw chicken have been reported in our previous study20. CC-21 was predominant in AT and HAT strains that were tolerant to stress conditions, particularly PAA and refrigeration (Fig. 6a,b). Among the OS strains tolerant to PAA, CCs-21 and 45 were dominant with a statistical significance (Fig. 6c). Commonly, CC-21 is the primary MLST sequence type in stress-tolerant C. jejuni strains from raw chicken.
Figure 6.
MLST clonal complexes of HAT (a), AT (b), and OS (c) strains of C. jejuni tolerant to stress conditions. Statistical significance was determined using two-way ANOVA. *P < 0.01.
Discussion
Most foodborne pathogenic bacteria of public health concern usually originate from animals25. Since poultry is the major reservoir for Campylobacter, poultry carcasses are likely to be contaminated by Campylobacter during processing, particularly defeathering and evisceration26. To maintain food quality by reducing spoilage and pathogenic bacteria on poultry carcasses, various intervention methods are used by the poultry industry, including low storage temperature, marination, modified atmospheric gas packaging, and antimicrobial disinfectants27.
PAA is a disinfectant widely used to decontaminate poultry carcasses in process water for washing, rinsing, and chilling due to its strong antimicrobial efficacy28,29. Interestingly, AT and HAT strains of C. jejuni exhibited enhanced tolerance to PAA compared to OS strains (Fig. 1). This may be attributed to the increased oxidative stress defense in AT and HAT strains (Fig. 2) as PAA is a mixture of H2O2 and acetic acid. Whereas most other bacteria harbor redundant copies of genes encoding ROS-detoxification enzymes, such as KatA, SodB and AhpC, C. jejuni possesses only a single gene copy encoding the enzymes. Although AhpC is the major enzyme contributing to aerotolerance in C. jejuni23, the other two enzymes (i.e., KatA and SodB) also affect the viability of C. jejuni under aerobic conditions24. Presumably, the increased aerotolerance would be associated with the augmented activities of oxidtative stress defense enzymes, which also may increase the capability of decomposing PAA. This is also supported by the augmented survivality of AT and HAT strains after exposure to various types of oxidants (Fig. 3).
Interestingly, AT and HAT strains of C. jejuni were more tolerant to refrigeration and freezing temperatures compared to OS strains (Fig. 4a,b). Most bacterial species, such as E. coli, Salmonella, and Bacillus, produce cold shock proteins upon a temperature downshift30–32. However, C. jejuni does not possess genes encoding cold shock proteins15, which suggests that C. jejuni may have other tolerance mechanisms to respond to cold shocks. Studies thus far have shown oxidative stress defense, particularly SodB, plays an important role in the cold stress tolerance of Campylobacter. A knockout mutation of sodB increases the sensitivity of C. jejuni to both superoxide and peroxide stress33,34. The level of sodB expression in C. jejuni increases by exposure to cold-shock35, and a sodB mutation makes Campylobacter more susceptible to freeze-thaw stress than the wild type36,37. However, the survival of a sodB mutant is comparable to that of the wild type in the absence of oxygen36, indicating that oxidative stress impacts C. jejuni’s ability to survive under freeze-thaw conditions. Notably, the SOD activities were determined to be significantly higher in AT and HAT strains than OS strains (Fig. 2a,b), and the AT and HAT strains were more tolerant to MND, a superoxide generator (Fig. 3c,d). Presumably, the elevated levels of SOD activity may possibly contribute to the enhanced survival of AT and HAT strains under refrigeration and freeze-thaw stress conditions. The association of cold stress with oxidative stress defense has also been reported in some other bacteria. For instance, exposure of Pseudomonas fluorescens MTCC 667, an isolate from Antarctic soil, to low temperature (4 °C) increases the production of ROS and elevates the activity of SOD38. Although molecular mechanisms still remain to be explained, our findings and the studies done by others consistently suggest oxidative stress defense may affect C. jejuni tolerance to cold and freezing stresses. C. jejuni strains from retail chicken were relatively sensitive to heat stress (Fig. 3c), compared to human clinical strains of C. jejuni in our previous study22. The reason for the different levels of thermotolerance between chicken isolates and clinical isolates of C. jejuni remains unknown.
C. jejuni is sensitive to hyperosmotic stress39 and is easily inactivated at >2% NaCl40. Thus, high (1.5% ~ 3%) salt concentrations in marinated poultry meat41 would be another stress to C. jejuni during foodborne transmission. NaCl is a general food preservative and inhibits the growth of foodborne pathogens in foods42. Cameron et al. reported that exposure to 1% NaCl modestly upregulates oxidative stress genes, such as katA and sodB, in C. jejuni, suggesting oxidative stress defense may affect osmotic stress response43. However, in the current study, the level of hyper-osmotolerance was not related to aerotolerance, although the results showed the level of hyper-osmotolerance is highly variable depending on the strain (Fig. 5). In C. jejuni, capsular polysaccharides (CPS) are involved in hyper-osmotolerance as mutations in the genes involved in genes encoding CPS, such as kpsM, kpsS, and kpsC, significantly (ca. 100-fold) increase C. jejuni susceptibility to 1% NaCl43. To maintain the intracellular turgor pressure properly under hyper-osmotic stress, bacteria generally accumulate solutes by increasing the uptake of K+ and synthesizing osmolytes, such as trehalose and glutamate44. Although molecular mechanisms for osmotolerance have not yet been elucidated in C. jejuni, it has been reported that highly-frequent spontaneous variations in housekeeping genes related to purine biosynthesis, such as purF and apt, is associated with C. jejuni response to hyperosmotic stress45.
This study demonstrated that C. jejuni strains isolated from retail raw chicken were tolerant to several different stress conditions that may affect the survival of C. jejuni in chicken and that aerotolerance is significantly related to C. jejuni tolerance to PAA and refrigeration and freezing temperatures. Further comparative genomics analysis as follow-up studies will help us elucidate the molecular mechanisms underlying stress tolerance in C. jejuni.
Methods
Bacterial strains and culture conditions
Seventy strains of C. jejuni were isolated from retail chicken meat in our previous study29. The strains were routinely grown on Muller-Hinton (MH) media at 42 °C under microaerobic condition (5% O2, 10% CO2, 85% N2).
Stress tolerance testing of C. jejuni
Stress tolerance testing was performed as described in our previous study with slight modifications22. C. jejuni strains were grown on MH agar at 42 °C for overnight under microaerobic condition. The strains were harvested in MH broth and resuspended in fresh MH broth to an optical density at 600 nm (OD600) of 0.1 prior to testing.
Tolerance to PAA: Raw chicken skin (0.3 g) was prepared with a sterilized razor, and each piece of chicken skin was inoculated C. jejuni suspension (approximately 108 CFU). The chicken skin spiked with C. jejuni suspension was incubated at 4 °C for 1 h under microaerobic conditions and dipped in 750 ppm PAA solution for 15 sec and immediately washed in ultra-pure water. The chicken skin was transferred into a 15 ml tube with 2 ml fresh MH broth and vortexed for 2 min. Bacterial count was determined with a serial dilution and cultivation on Preston Campylobacter-selective agar. The experiment was repeated at least three times.
Tolerance to refrigeration and freeze-thaw: C. jejuni suspensions were placed into a 96-well plate and incubated at 4 °C for 3 and 7 days for refrigeration stress and also incubated at −20 °C for 3 and 7 days for freeze-thaw stress. After 3 and 7 days, the incubated C. jejuni suspensions were serially diluted and spread on MH agar for enumeration.
Osmotolerance test: The suspension of C. jejuni was diluted 10 times and spotted on MH agar supplemented with 1, 2 and 4% sodium chloride. The culture plates were incubated at 42 °C overnight.
Catalase and SOD activity test
C. jejuni strains were inoculated on MH agar and harvested with 1X PBS buffer (pH 7.4). The C. jejuni suspension was washed with 1X PBS buffer twice and disrupted with a sonicator (Bioruptor Sonication System, Diagenode, USA). After centrifugation at 15,000 rpm for 10 min, the supernatant was used for the assays.
Catalase assay: Catalase assay was performed according to the manufacturer’s protocol (Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit, Invitrogen). Catalase activities were determined by measuring the intracellular concentration of H2O2. The supernatant was placed into a 96-well plate and added the working solution (10 mM Amplex Red reagent, 10 U/mL HPR stock solution and reaction buffer). The mixture was incubated at room temperature for 30 min, and fluorescence at ex/em 530/590 nm. H2O2 concentration was determined by comparing with a standard curve prepared with known H2O2 concentrations and normalized to protein concentrations determine with a Bradford assay.
SOD assay: The SOD activity was carried out according to the guidelines of the manufacturer (SOD assay kit, Sigma Aldrich). The supernatant was placed onto a 96 well plate and mixed with the SOD assay mixture (WST working solution, enzyme solution and SOD solution), and the plate was incubated at 37 °C for 20 min. The absorbance at 450 nm was measured with a microplate reader, and the SOD activity was calculated with a SOD standard with normalization to protein concentrations that were determined with a Bradford assay.
Determination of susceptibility to oxidants
Overnight cultures of C. jejuni were harvested from MH agar and diluted with fresh MH broth to an optical density 600 of 0.1. The diluted C. jejuni suspensions were exposed to oxidants, including 2 mM of hydrogen peroxide (H2O2), 0.2 mM menadione (MND), and 0.1 mM cumene hydroperoxide (CHP), for 1 h. After washing with fresh MH broth twice, the suspension was serially diluted and spread on MH agar.
Fluorescence microscope analysis
The morphological changes observed with a fluorescence microscope with SYTO9 and propidium iodine staining. C. jejuni strains were inoculated on MH agar at 42 °C for overnight and harvested with MH broth. The bacterial suspension was serially diluted with fresh MH broth to an OD 600 of 0.07. The C. jejuni suspension was supplemented with sodium chloride to final concentrations of 1, 2 and 4% and incubated at 42 °C for 5 h with shaking (200 rpm) under microaerobic conditions. After washing with 1X PBS buffer twice, C. jejuni was fixed with 4% paraformaldehyde for 1 h at room temperature. The cells were centrifuged at 8,000 × g for 5 min and washed with 1X PBS buffer twice. After staining with SYTO9 and propidium iodine for 20 min at room temperature and washing twice with 1X PBS buffer, C. jejuni was observed with a fluorescence microscope (Carl Zeiss, Germany).
Statistical analysis
Statistical analysis of the data from stress tolerance tests and catalase and SOD assays was performed with two-way ANOVA (GraphPad Prism Ver. 7, GraphPad Software, USA). Chi-square distribution was performed by SPSS ver. 21 (SPSS Inc., IBM, USA).
Acknowledgments
This study was supported by a research grant (2016P001R) from the Alberta Agriculture and Forestry and the Leaders Opportunity Fund from the Canada Foundation for Innovation (CFI).
Author Contributions
E.O. and B.J. designed the study; E.O. and K.J.A. performed experiments; E.O. and B.J. analyzed the data; E.O. and B.J. wrote the manuscript; E.O. and L.M.M. critically reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirk MD, et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Stuart TL, et al. Campylobacteriosis outbreak associated with ingestion of mud during a mountain bike race. Epidemiol Infect. 2010;138:1695–1703. doi: 10.1017/S095026881000049X. [DOI] [PubMed] [Google Scholar]
- 4.Sahin O, et al. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9:2105. doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 6.Little CL, Richardson JF, Owen RJ, de Pinna E, Threlfall EJ. Prevalence, characterisation and antimicrobial resistance of Campylobacter and Salmonella in raw poultrymeat in the UK, 2003–2005. Int J Environ Health Res. 2008;18:403–414. doi: 10.1080/09603120802100220. [DOI] [PubMed] [Google Scholar]
- 7.Bohaychuk VM, et al. Occurrence of pathogens in raw and ready-to-eat meat and poultry products collected from the retail marketplace in Edmonton, Alberta, Canada. J Food Prot. 2006;69:2176–2182. doi: 10.4315/0362-028X-69.9.2176. [DOI] [PubMed] [Google Scholar]
- 8.Rosenquist, H., Nielsen, N. L., Sommer, H. M., Norrung, B. & Christensen, B. B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol83, 87–103, doi:S0168160502003173 [pii] (2003). [DOI] [PubMed]
- 9.Umaraw P, Prajapati A, Verma AK, Pathak V, Singh VP. Control of Campylobacter in poultry industry from farm to poultry processing unit: a review. Crit Rev Food Sci Nutr. 2017;57:659–665. doi: 10.1080/10408398.2014.935847. [DOI] [PubMed] [Google Scholar]
- 10.Oyarzabal OA. Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J Food Prot. 2005;68:1752–1760. doi: 10.4315/0362-028X-68.8.1752. [DOI] [PubMed] [Google Scholar]
- 11.Nagel GM, Bauermeister LJ, Bratcher CL, Singh M, McKee SR. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int J Food Microbiol. 2013;165:281–286. doi: 10.1016/j.ijfoodmicro.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Z, Ni Y, VanHeiningen ARP. Kinetics of peracetic acid decomposition 1. Spontaneous decomposition at typical pulp bleaching conditions. Can J Chem Eng. 1997;75:37–41. doi: 10.1002/cjce.5450750108. [DOI] [Google Scholar]
- 13.Begley M, Hill C. Stress adaptation in foodborne pathogens. Annu Rev Food Sci Technol. 2015;6:191–210. doi: 10.1146/annurev-food-030713-092350. [DOI] [PubMed] [Google Scholar]
- 14.Spector MP, Kenyon WJ. Resistance and survival strategies of Salmonella enterica to environmental stresses. Food Res Int. 2012;45:455–481. doi: 10.1016/j.foodres.2011.06.056. [DOI] [Google Scholar]
- 15.Hazeleger WC, Wouters JA, Rombouts FM, Abee T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol. 1998;64:3917–3922. doi: 10.1128/aem.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkhill J, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 17.Kelly AF, Park SF, Bovill R, Mackey BM. Survival of Campylobacter jejuni during stationary phase: evidence for the absence of a phenotypic stationary-phase response. Appl Environ Microbiol. 2001;67:2248–2254. doi: 10.1128/Aem.67.5.2248-2254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landini P, Egli T, Wolf J, Lacour S. SigmaS, a major player in the response to environmental stresses in Escherichia coli: role, regulation and mechanisms of promoter recognition. Environ Microbiol Rep. 2014;6:1–13. doi: 10.1111/1758-2229.12112. [DOI] [PubMed] [Google Scholar]
- 19.Dodd CE, Aldsworth TG. The importance of RpoS in the survival of bacteria through food processing. Int J Food Microbiol. 2002;74:189–194. doi: 10.1016/S0168-1605(01)00679-1. [DOI] [PubMed] [Google Scholar]
- 20.Oh E, McMullen L, Jeon B. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front Microbiol. 2015;6:1263. doi: 10.3389/fmicb.2015.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karki AB, Marasini D, Oakey CK, Mar K, Fakhr MK. Campylobacter coli from retail liver and meat products is more aerotolerant than Campylobacter jejuni. Front Microbiol. 2018;9:2951. doi: 10.3389/fmicb.2018.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh E, et al. Frequent implication of multistress-tolerant Campylobacter jejuni in human infections. Emerg Infect Dis. 2018;24:1037–1044. doi: 10.3201/eid2406.171587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baillon ML, van Vliet AH, Ketley JM, Constantinidou C, Penn CW. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh E, McMullen L, Jeon B. Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter jejuni under aerobic conditions. Front Microbiol. 2015;6:295. doi: 10.3389/fmicb.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swartz MN. Human diseases caused by foodborne pathogens of animal origin. Clin Infect Dis. 2002;34(Suppl 3):S111–122. doi: 10.1086/340248. [DOI] [PubMed] [Google Scholar]
- 26.Guerin MT, et al. The change in prevalence of Campylobacter on chicken carcasses during processing: a systematic review. Poult Sci. 2010;89:1070–1084. doi: 10.3382/ps.2009-00213. [DOI] [PubMed] [Google Scholar]
- 27.Rouger Amélie, Tresse Odile, Zagorec Monique. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms. 2017;5(3):50. doi: 10.3390/microorganisms5030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, et al. Efficacy of various antimicrobials on reduction of Salmonella and Campylobacter and quality attributes of ground chicken obtained from poultry parts treated in a postchill decontamination tank. J Food Prot. 2014;77:1882–1888. doi: 10.4315/0362-028X.JFP-14-114. [DOI] [PubMed] [Google Scholar]
- 29.Bauermeister LJ, Bowers JW, Townsend JC, McKee SR. The microbial and quality properties of poultry carcasses treated with peracetic acid as an antimicrobial treatment. Poult Sci. 2008;87:2390–2398. doi: 10.3382/ps.2008-00087. [DOI] [PubMed] [Google Scholar]
- 30.Etchegaray JP, Inouye M. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J Bacteriol. 1999;181:1827–1830. doi: 10.1128/jb.181.6.1827-1830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton AJ, Hak KM, Steffan RJ, Foster JW, Bej AK. Adaptive response to cold temperatures and characterization of cspA in Salmonella typhimurium LT2. Antonie Van Leeuwenhoek. 2000;77:13–20. doi: 10.1023/A:1002055719798. [DOI] [PubMed] [Google Scholar]
- 32.Horn G, Hofweber R, Kremer W, Kalbitzer HR. Structure and function of bacterial cold shock proteins. Cell Mol Life Sci. 2007;64:1457–1470. doi: 10.1007/s00018-007-6388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flint A, et al. Phenotypic screening of a targeted mutant library reveals Campylobacter jejuni defenses against oxidative stress. Infect Immun. 2014;82:2266–2275. doi: 10.1128/IAI.01528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palyada K, et al. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC genomics. 2009;10:481. doi: 10.1186/1471-2164-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stintzi A, Whitworth L. Investigation of the Campylobacter jejuni cold-shock response by global transcript profiling. Genome Lett. 2003;2:18–27. [Google Scholar]
- 36.Stead D, Park SF. Roles of Fe superoxide dismutase and catalase in resistance of Campylobacter coli to freeze-thaw stress. Appl Environ Microbiol. 2000;66:3110–3112. doi: 10.1128/AEM.66.7.3110-3112.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garénaux A, et al. Role of oxidative stress in C. jejuni inactivation during freeze-thaw treatment. Curr Microbiol. 2009;58:134–138. doi: 10.1007/s00284-008-9289-3. [DOI] [PubMed] [Google Scholar]
- 38.Chattopadhyay MK, et al. Increase in oxidative stress at low temperature in an antarctic bacterium. Curr Microbiol. 2011;62:544–546. doi: 10.1007/s00284-010-9742-y. [DOI] [PubMed] [Google Scholar]
- 39.Park SF. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int J Food Microbiol. 2002;74:177–188. doi: 10.1016/S0168-1605(01)00678-X. [DOI] [PubMed] [Google Scholar]
- 40.Doyle MP, Roman DJ. Response of Campylobacter jejuni to sodium chloride. Appl Environ Microbiol. 1982;43:561–565. doi: 10.1128/aem.43.3.561-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarado C, Mckee S. Marination to improve functional properties and safety of poultry meat. J Appl Poult Res. 2007;16:113–120. doi: 10.1093/japr/16.1.113. [DOI] [Google Scholar]
- 42.Doyle ME, Glass KA. Sodium reduction and its effect on food safety, food quality, and human health. Compr Rev Food Sci Food Saf. 2010;9:44–56. doi: 10.1111/j.1541-4337.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- 43.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. Hyperosmotic stress response of Campylobacter jejuni. J Bacteriol. 2012;194:6116–6130. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood JM. Bacterial responses to osmotic challenges. J Gen Physiol. 2015;145:381–388. doi: 10.1085/jgp.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron A, et al. High-frequency variation of purine biosynthesis genes is a mechanism of success in Campylobacter jejuni. mBio. 2015;6:e00612–00615. doi: 10.1128/mBio.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]