Abstract
During hibernation, mammalian cells are exposed to severe environmental stressors such as low temperature, lowered O2 supply, and glucose deficiency. The cellular metabolic rate is markedly reduced for adapting to these conditions. AMP-activated protein kinase (AMPK) senses the cellular energy status and regulates metabolism. Therefore, we examined AMPK signaling in several brain regions and peripheral tissues in hibernating chipmunk. Eukaryotic elongation factor 2 (eEF2) is a downstream target of AMPK. Phosphorylation of eEF2, indicating its inactivation, is enhanced in the cerebral cortex of hibernating chipmunks. The study indicated that the sequential regulation of AMPK-mammalian target of rapamycin complex 1-eEF2 signaling was altered and protein synthesis ability was reduced in the cerebral cortex of hibernating chipmunks.
Subject terms: Neurochemistry, Molecular neuroscience, Animal physiology
Introduction
Hibernation is an adaptive strategy of some mammals to survive the severe cold and food scarcity in winter. It is a drastic circannual phenotypic alteration in such mammals. In chipmunks, the body temperature falls to nearly 0 °C and heartbeat reduces dramatically during hibernation1. In general, the cells of hibernators can tolerate low temperature and low O2 supply as well as energy deficiency, presumably reducing the metabolic rate of glucose, lipids, and proteins.
It has been proposed that the circannual hibernation of chipmunks is regulated by hibernating proteins (HPs) produced in the liver and worked in the brain during hibernation1,2, which suggests that hibernation is triggered in the brain. It is widely accepted that hibernation is associated with downregulation of metabolism and ensuing energy insufficiency, at least in the brain, although it is unclear whether these metabolic changes are the cause or the result of hibernation3,4. Therefore, this study aimed to analyze and compare the energy-sensing and anabolic signaling in the brains, and peripheral tissues of hibernating chipmunks.
AMP-activated protein kinase (AMPK), a heterotrimeric serine/threonine protein kinase, comprising a catalytic α subunit and regulatory β and γ subunits, senses the cellular energy status by detecting the AMP/ATP ratio and regulates metabolism5. AMPK is activated when ATP levels fall in situation such as nutrient insufficiency and hypoxia. AMPK activity is coupled with phosphorylation of Thr172 residue of AMPKα subunit5–7. When activated by reduced cellular ATP levels, AMPK induces energy-generating catabolic pathways, such as autophagy, and inhibits energy-consuming anabolic pathways, such as fatty acid synthesis, gluconeogenesis, and protein synthesis, by suppressing mammalian target of rapamycin (mTOR) complex 1 (mTORC1)8. Our study also focuses on eukaryotic elongation factor 2 (eEF2), because it lies downstream of AMPK-mTORC19. eEF2 is a GTPase that translocates peptidyl-tRNA from the A-site to P-site of the ribosome, thereby controlling translation elongation. Upon phosphorylation by eEF2 kinase (eEF2K) at Thr56, eEF2 is unable to bind a ribosome and is thus inactivated10. eEF2K activity is regulated either negatively or positively by multiple kinases targeting different phosphorylation sites. For example, p70S6 kinase, a substrate of mTORC1, phosphorylates eEF2K Ser366 and inhibits its activity11. In contrast, AMPK phosphorylates eEF2K at Ser39812, and Ser491/49213 and enhances its activity, leading to eEF2 inactivation. Altered AMPK activity14,15, mTOR signaling16, eEF2 phosphorylation17,18, and protein synthesis17,18 have been reported in several tissues of several species during hibernation. However, changes of AMPK activity in the brain have been less focused. In squirrels, no change of AMPK activity in the brain was reported15. In addition, there has been no systematic analysis of these changes in the brain during hibernation in chipmunks. Considering that eEF2 activity is a major regulator of protein synthesis in neurons19, and protein synthesis is a major consumer of cellular energy, we analyzed sequential AMPK-mTORC1-eEF2 signaling and protein synthesis ability in the brain and peripheral tissues of active and hibernating chipmunks.
Results
Active and hibernation cycle
The surface body temperature of each animal was monitored over a year. Figure 1a shows the typical active and hibernation cycle. The cycle was almost circannual, however, this could be owing to the constant dark condition and low temperature (4 °C), the cycles of some chipmunks displayed free-running (Supplemental Fig. 1).
Figure 1.
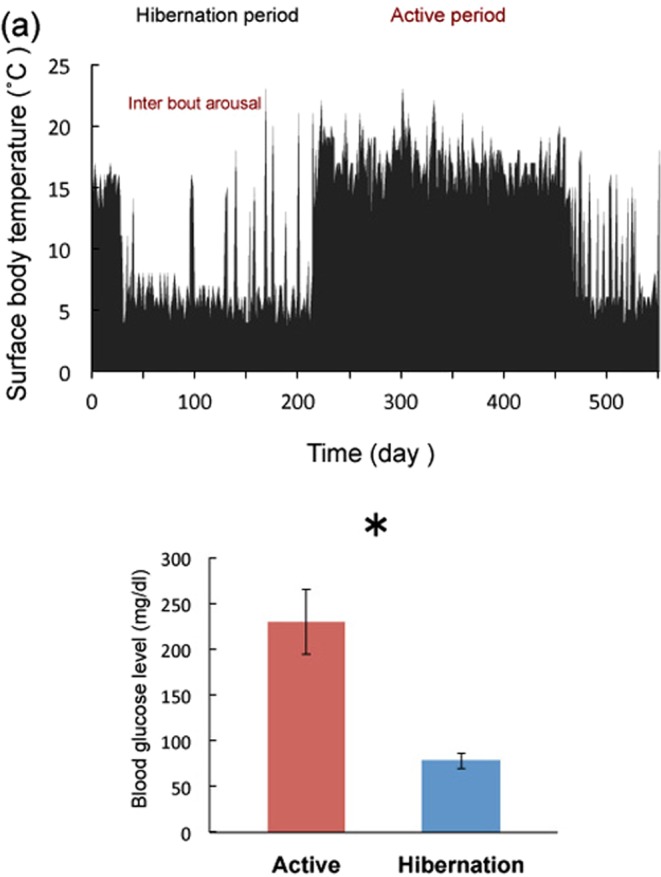
Surface body temperature and blood glucose levels. (a) Changes in the surface body temperature as monitored with infrared irradiation thermometer during active and hibernation. The examples of interbout arousals are indicated by arrows. (b) Blood glucose levels during each condition. Bars represent mean ± SE (n = 5). *p < 0.05 (Student’s t-test).
Blood glucose level
Tissue samples were collected during active and hibernation periods based on the surface body temperature (Fig. 1a). Interbout arousal periods was avoided for sampling. Glucose is a primary regulator of AMPK; therefore, blood glucose level was measured during both active and hibernation periods (Fig. 1b). As expected, blood glucose was reduced during hibernation compared with the active period. Blood glucose levels were found to be high compared with those in rats or mice (approximately 130 mg/dl). This finding may not be applicable to chipmunks in general and could be attributed to the feeding conditions (ad libitum) or genetic traits of the group of chipmunks sampled in this study. Chipmunks are not laboratory animals; our samples were obtained from the wild, therefore, their genetic background may vary. We observed a difference between euthermic and hibernating chipmunks in this study.
AMPK and acetyl-CoA carboxylase (ACC) phosphorylation
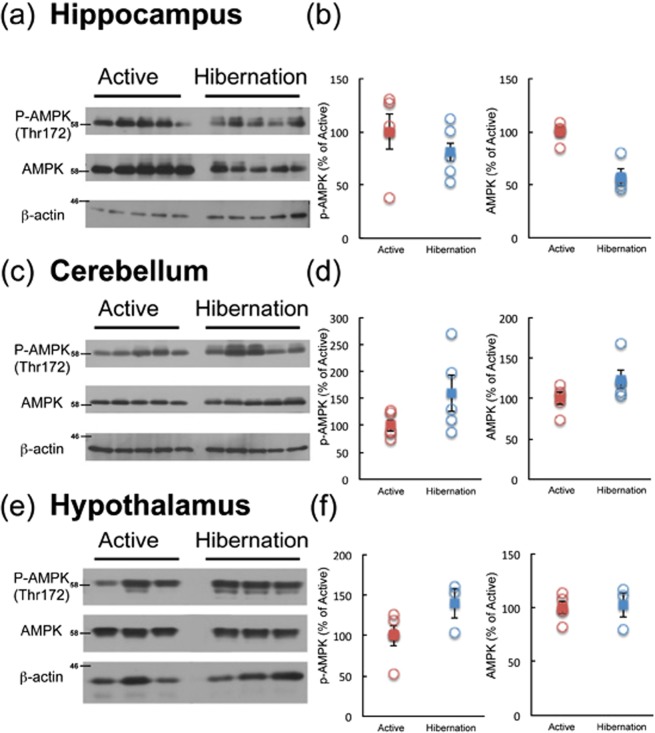
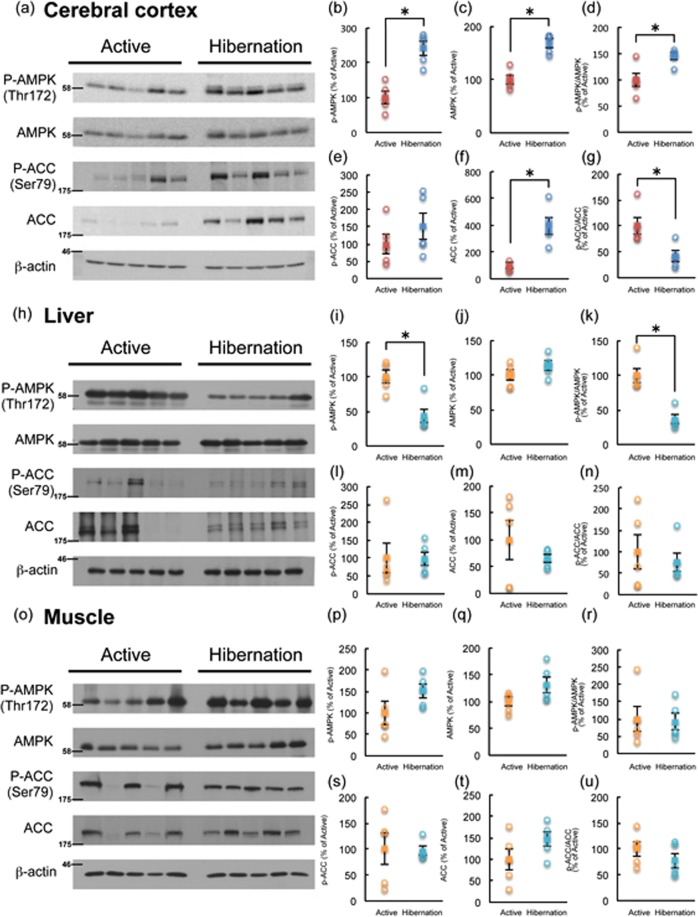
Phosphorylation of AMPKα (Thr172), reflecting its activation, was examined in multiple brain regions of active and hibernating chipmunks by Western blotting. Among the brain regions tested, AMPKα phosphorylation was significantly enhanced during hibernation only in the cerebral cortex, but not in the hippocampus, cerebellum, or hypothalamus (Figs 2, 3). Accordingly, phosphorylation of ACC tended to increase in the cerebral cortex during hibernation compared with the active period (Fig. 3). Because ACC is a direct substrate of AMPK, increase of phospho-ACC indicates the increased net activity of AMPK in the cerebral cortex. In contrast, AMPKα phosphorylation was somewhat reduced in the liver and unchanged in the skeletal muscles of hibernating chipmunks (Fig. 3), despite low blood glucose levels. These results indicate that AMPK is activated only in the cerebral cortex during hibernation.
Figure 2.
Phosphorylation of AMPKα in the hippocampus (a,b), cerebellum (c,d), and hypothalamus (e,f) of chipmunks during active and hibernation periods. All the samples were applied to the same gel and blotted to a single membrane as displayed. Panels b, d, and f show quantitation of the blots by Image J. Circles represent each band. Squares represent mean ± SE (n = 5 or 3).
Figure 3.
Phosphorylation of AMPKα and ACC in the cerebral cortex (a–g), liver (h–n), and skeletal muscle (o–u) of chipmunks during active and hibernation periods as revealed by Western blotting. All the samples were applied to the same gel and blotted to single membrane as displayed. Each band was quantified by Image J. (b,i,p); P-AMPKα, (c,j,q); AMPKα, (d,k,r); ratio of P-AMPKα/AMPKα, (e,l,s); P-ACC, (f,m,t); ACC, (g,n,u); ratio of P-ACC/ACC. Circles represent each band. Squares represent mean ± SE (n = 5). *p < 0.05 (Wilcoxon rank-sum test (g,i,k,l,n) or Student’s t-test (all the rest)).
Downstream signaling of AMPK
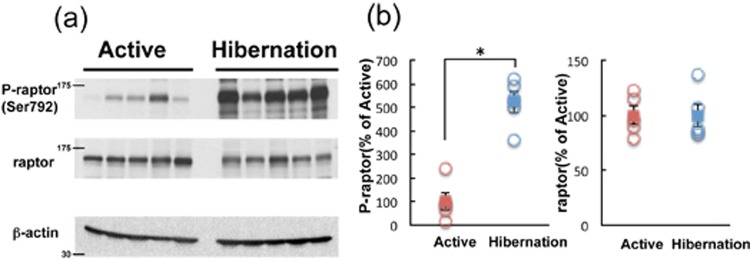
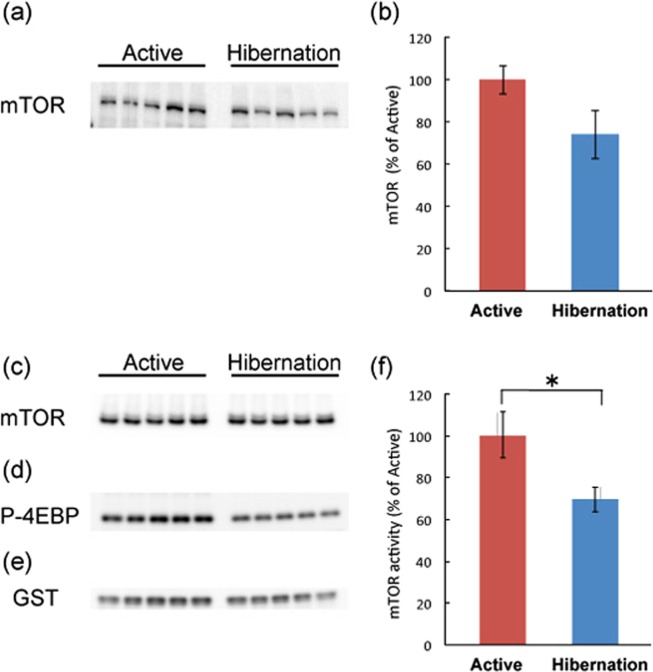
We then examined the signaling status of mTORC1, a major downstream target of AMPK, in the cerebral cortex of active and hibernating chipmunks. Raptor is an indispensable component of mTORC120,21 and is phosphorylated at Ser792 by AMPK22, which suppresses mTORC1 activity. Phosphorylation of raptor was increased in the cerebral cortex of hibernating chipmunks (Fig. 4), suggesting reduced activity of mTORC1; therefore, mTORC1 kinase activity was directly measured. Therefore, equal amounts of mTOR were immunoprecipitated (Fig. 5c) from cerebral cortical tissues of each period and incubated with recombinant GST-4EBP, as a substrate, with ATP, followed by Western blotting with an anti-phosph-4EBP(Thr37/46) (Fig. 5d). As shown in Fig. 5f, mTORC1 activity was downregulated in the cerebral cortex of hibernating chipmunks. In addition, total mTOR protein levels are lower in the cortex of hibernating chipmunks, although it is not significant (Fig. 5a,b). Cumulatively, net mTORC1 activity in the cortex of hibernating chipmunks is lower than that in the cortex of active animals.
Figure 4.
Phosphorylation of raptor in cerebral cortex of chipmunks during active and hibernation periods (a,b). All the samples were applied to the same gel and blotted to a single membrane as displayed (a). Panel b shows quantitation of the blots by Image J. Circles represent each band. Squares represent mean ± SE (n = 5). p < 0.05 (Student’s t-test).
Figure 5.
Levels of mTOR in the cerebral cortex of chipmunks during active and hibernation periods. All the samples were applied to the same gel and blotted to single membrane as displayed (a). Panel b shows quantitation of the blots by Image J. Bars represent mean ± SE. (n = 5) Kinase activity of mTORC1 during active and hibernation periods is shown in f. Panels c shows immunoprecipitated mTOR. Panel d shows phosphorylated GST-4EBP after in vitro phosphorylation. Panel e shows GST, as the control of total GST-4EBP in the reaction mixture. All the samples were applied to the same gel and blotted to single membrane as displayed (c–e). Kinase activity (f) is calculated by (GST-P-4EBP (d)/GST (e))/mTOR (c). Bars represent mean ± SE (n = 5). *p < 0.05 (Student’s t-test).
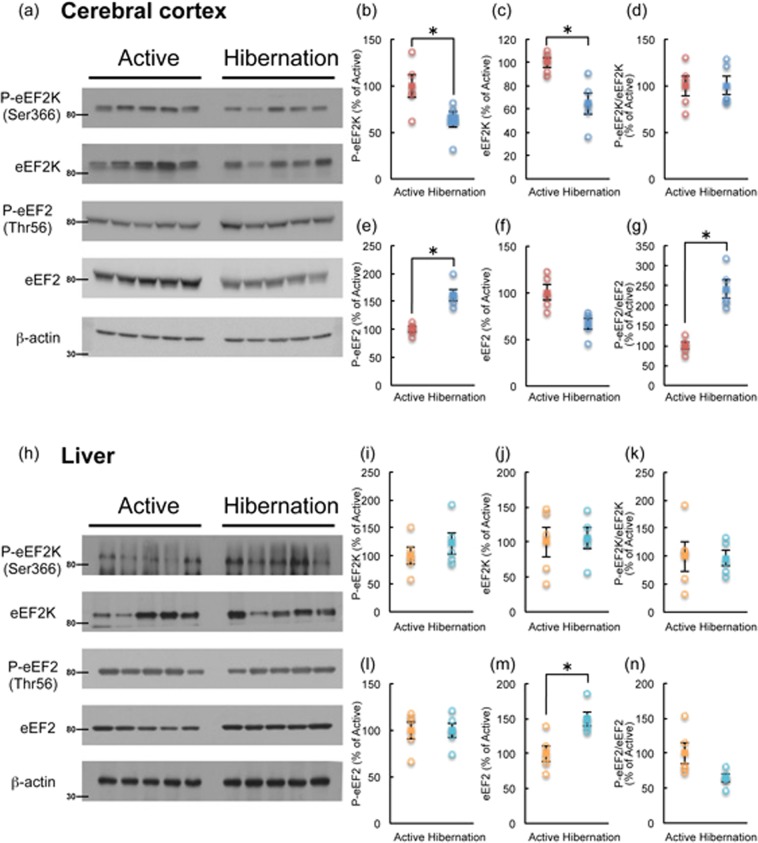
eEF2 activity is suppressed by eEF2K-mediated phosphorylation at Thr56, which in turn reduces translation elongation efficiency10. eEF2K activity is downregulated by phosphorylation at Ser366 by mTORC1 signaling; therefore, reduced eEF2K phosphorylation at Ser366 would allow eEF2 phosphorylation (inactivation) and thus reduce protein synthesis11. eEF2K phosphorylation at Ser366 was decreased in the cerebral cortex of hibernating chipmunks (Fig. 6a,b). This finding is consistent with the reduced mTORC1 activity and suggests increased activity of eEF2K, in line with the upregulation of eEF2 phosphorylation at Thr56 (Fig. 6a,e). Conversely, these changes were not observed in the liver (Fig. 6h,i,l). The results suggest that translation elongation and ensuing total protein synthesis are downregulated in the cerebral cortex, but not in the liver, of hibernating chipmunks.
Figure 6.
Phosphorylation of eEF2K and eEF2 in the cerebral cortex (a–g) and liver (h–n) of chipmunks during active and hibernation periods. All samples were applied to the same gel and blotted to a single membrane as displayed. Each band was quantified by Image J. (b,i); P-eEF2K, (c,j); eEF2K, (d,k); ratio of P-eEF2K/eEF2K, (e,l); P-eEF2, (f,m); eEF2, (g,n); ratio of P-eEF2/eEF2. Each circle represents the each band. Squares represent mean ± SE (n = 5) *p < 0.05 (Student’s t-test).
Rate of protein synthesis in active and hibernating tissues
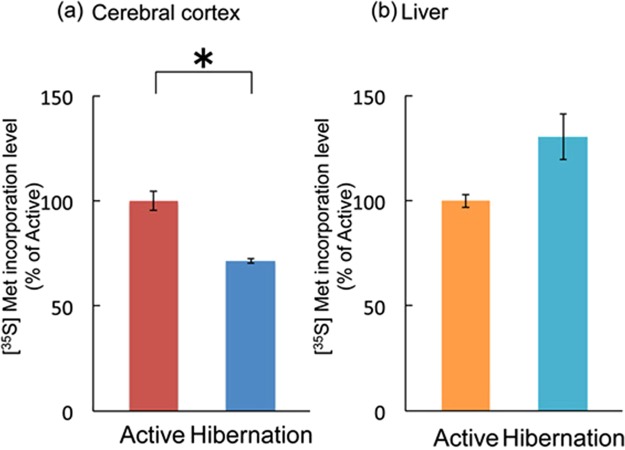
Protein synthesis capacity was examined in the cerebral cortical and liver tissues during the active and hibernation periods by in vitro [35S]methionine incorporation as previously reported19. In accordance with the decrease in mTORC1 activity and inactivation of eEF2 (phosphorylation), protein synthesis rate was lower in the cerebral cortex of hibernating chipmunks than in that of active chipmunks, whereas liver protein synthesis rate did not differ between the active and hibernation periods (Fig. 7).
Figure 7.
Comparison of protein synthesis capacity in vitro as measured by [35S]methionine incorporation using chipmunks between cerebral cortex (a) and liver (b) during active and hibernation periods. Bars represent mean ± SD (n = 5). *p < 0.05 (Student’s t-test).
Discussion
In this study, we analyzed the signaling pathways underlying the altered metabolic and protein synthesis rates in the cerebral cortex of hibernating chipmunks. During hibernation, the cerebral cortex exhibited (1) an increase of AMPKα phosphorylation (indicating elevated AMPK activity), (2) suppression of mTORC1 activity accompanied with raptor phosphorylation, (3) phosphorylation (inactivation) of eEF2, resulting in (4) reduced ability of protein synthesis as evidenced by lower in vitro [35 S]methionine incorporation.
Among the brain regions tested, significant AMPK activation was observed only in the cerebral cortex of hibernating chipmunks. However, no changes in the liver and skeletal muscle were observed during either active or hibernation period. The lack of increase in AMPK in the liver and skeletal muscles is in accordance with previous results from studies of the ground squirrel15. Liver and skeletal muscles synthesize and store glycogen; therefore, AMPK activity may not quickly respond to falling blood glucose levels. Instead, AMPK activity may change depending on the period of torpor and fasting. Indeed, AMPKα phosphorylation in the liver and skeletal muscles of rats is not altered in a short-term fasting23. Further, long-term caloric restriction rather decreased phospho-AMPKα levels in rat liver24. In contrast to peripheral tissues, phosphorylation status of AMPKα and ACC was increased in the cortex of hibernating chipmunks, a result not observed in the whole brain of thirteen striped-ground squirrels15. The increase in AMPK activity observed in the cerebral cortex of ground squirrels may have been obscured by a lack of change in the rest of the brain. In the hypothalamus of golden mantled ground squirrels, phosphorylation of ACC, but not AMPKα, was increased14, which is in partial accordance with our finding of unaltered AMPKα phosphorylation in the hypothalamus of chipmunks. In the brain, glycogen stores and fatty acid beta-oxidation are limited and almost all energy is derived from blood glucose. Therefore, the reduction in blood glucose levels may rapidly affect AMPK activity in the cerebral cortex, but not in energy-storing peripheral organs. However, this does not explain why phosphorylation of AMPKα in other brain regions was unaltered. Further studies are required to reveal possible differences in the regulation of AMPKα phosphorylation status and its activity among brain regions.
Along with AMPK activation, eEF2 phosphorylation (inactivation) was observed in the cerebral cortex, but not in the liver. AMPK is known to phosphorylate eEF2K directly12,13 and to activate indirectly through the suppression of the mTORC1 pathway11. In this study, both total and phosphorylated eEF2K at Ser366 were reduced in the cortex of hibernating chipmunks. Thus, direct phosphorylation of eEF2K by AMPK may contribute to the activation of eEF2K, thus inhibiting eEF2 activity.
Hallenbeck’s group has reported eEF2 phosphorylation, prolonged ribosomal transit time that indicates slowing elongation rate, and reduced protein synthesis in the brains of hibernating ground squirrels17,18. Therefore, eEF2 inactivation in the brain may be a common mechanisms for reducing protein synthesis in hibernating rodents. It has also been reported the Decreased activity of protein phosphatase 2A, the dephosphorylation enzyme for eEF2 has also been reported in the brain of hibernating ground squirrels18. It may also occur in thecerebral cortex of hibernating chipmunks.
In addition to the cellular energy status, cell type-specific Ca2+ dynamics may influence AMPKα and eEF2 phosphorylation. AMPKα is phosphorylated by calcium-calmodulin-dependent protein kinase kinase (CaMKK)25–27 and eEF2 is phosphorylated by eEF2K, (also known as calcium/calmodulin dependent protein kinase III)28. The activity of both kinases is Ca2+-dependent. Intracellular Ca2+ dynamics are known to be altered in cardiac muscle cells during hibernation29, suggesting that cell type-specific changes in Ca2+ mobilization lead to differential activity of AMPK and eEF2 among tissues. Indeed, cold-stress-induced eEF2K activation via Ca2+ upregulation was previously reported30.
Another possible explanation for the cerebral cortex-specific activation of AMPK is the direct involvement of the hibernation protein complex (HPc) comprising HP20, HP25, and HP272. These HPs have collagen-like domains in the N-terminal region and form a triple-helix31. Structurally, HPc is predicted to belong to the C1q protein family31,32. Adiponectin and CTRPs, other members of this family, are reported to induce AMPK activation33,34. Because HPc is suggested to act in the brain1, HPc in the cortex may directly activate AMPK.
Although the phosphorylation levels of the molecules tested were enhanced in the cortex during hibernation, this is not a general phenomenon (for instance, due to massive loss of phosphatase activity). In fact, phosphorylation of Akt was decreased in the cerebral cortex (Supplemental Fig. 2), possibly due to the downregulation of mTORC2 and/or low levels of neural activity.
As previously reported18, protein synthesis ability in the cortical tissue is downregulated as revealed by in vitro assay at 37 °C. This finding reflects the status of translation machinery, in other words, “readiness for protein synthesis”. This suggests that the function of translation machinery in the hibernating cortex is suppressed. Under colder condition during hibernation, actual protein synthesis in vivo may be further downregulated. In fact, protein synthesis as revealed by [14C]leucine incorporation was markedly decreased in the liver and heart, (in addition to the brain) of hibernating squirrels in situ at 7 °C body temperature18.
The remaining unsolved question regards the relationship between circannual (hibernation) and circadian rhythms. The changes in each signaling molecule and its phosphorylation status may have been modified by circadian rhythms because the animals in the current study were housed in constant darkness and thus their rhythms may have free-run. Indeed, circadian change in AMPK activity has been previously reported in rats35. Unfortunately, we have no data in this study; however it is an important point in hibernation studies.
Based on the present study, we suggest that the increased activity of AMPK and decreased protein synthesis may contribute to the low-temperature resistance of brain cells during hibernation. Future studies are required to assess the involvement of hibernation proteins in these metabolic changes and low-temperature resistance.
Materials and Methods
Materials
Anti-AMPKα, anti-phospho-AMPKα (Thr172), anti-ACC, anti-phospho-ACC (Ser79), anti-eEF2K, anti-phospho-eEF2K(Ser366), anti-Akt, anti-phospho-Akt(Ser473), anti-p44/42MAPK, and anti-phosopho-p44/42MAPK (Thr202/Tyr204) were purchased from Cell Signaling Technology. Anti-mTOR was obtained from Immuno-Biological Laboratories. Anti-eEF2 and anti-phospho-eEF2 antibodies were prepared as described previously36. Validation of antibody specificity to chipmunk proteins is shown in Supplemental Fig. 4. [35S]methionine and Protein G sepharose were purchased from Perkin Elmer and GE Healthcare, respectively.
Antibody validation
The target molecules analyzed in this study are well conserved among species, and tge amino acid sequences and phosphorylation sites of AMPKα1 in mouse, rat, rabbit, human and squirrel are shown in Supplemental Fig. 3. To assess the specificity of the antibodies (anti-AMPKα, anti-P-AMPKα, anti-eEF2 and anti-P-eEF2), freshly prepared brain lysate was applied to SDS-PAGE and transferred and blotted in full size (from top to bottom). Antigen absorption was also performed. cDNAs of rat AMPKα37,38 fused with GST and rat eEF219 tagged with Flag were transfected to HEK293T cells and starved with serum to increase phosphorylated forms. GST-AMPKα and Flag eEF2 were pull-down with Glutathione-sepharose and anti-DYKDDDDK(Flag)-agarose, respectively. Western blots of the antigens were shown in Supplemental Fig. 4. Antigens bound to resins were incubated with each antibody. As shown in Supplemental Fig. 4, each antibody recognized a single band of similar molecular weight (rat or human AMPKα and eEF2 both total and phosphorylated forms). Signals were completely abolished when using antigen-absorbed antibodies (Supplemental Fig. 4).
Animals
All the animal experiments were conducted in compliance with the protocol which was reviewed by the Institutional Animal Care and Use Committee and approved by the President of Niigata University (Permit Number: Niigata Univ. Res.258-1). Protocols were performed in accordance with the Guiding Principles for Care and Use of Laboratory Animals (NIH, USA). Male chipmunks (Tamias sibiricus) less than one year of age were purchased from Arcland Sakamoto Co. Ltd. The chipmunks were housed individually at 23 °C under 12h-light/dark cycle conditions and provided a standard rat diet and water ad libitum. The animals were maintained for at least six months to acclimate to laboratory conditions. After checking general health conditions, they were transferred to the experimental condition of 4 °C and constant darkness. Periodical changes in the body temperature were monitored with an infrared irradiation thermometer once a day more than one year. Only individuals that exhibited periodical changes (Fig. 1a as an example) were used for the study. There are certain populations of chipmunks that do not exhibit temperature change and these individuals were excluded from this study. The onset, duration and termination of hibernation were recognized by monitoring the surface body temperature1. Animals (five chipmunks of each group (active: surface body temperature >18 °C and hibernation: surface body temperature <6 °C) were deeply anesthetized with carbon dioxide and sacrificed by decapitation and tissues were collected at 13:00–15:00 h from October to November. Tissue and blood samples were collected, immediately frozen in liquid nitrogen, and stored at −80 °C until further analysis.
Measurement of blood glucose level
The blood glucose levels during each period were measured using self-measurement instrument (Freestyle freedom, Nipro). After thawing the cryopreserved samples on ice, 0.3 μl blood was used for measuring the blood glucose levels.
Tissue extracts, electrophoresis, and Western blotting
Tissue extracts, electrophoresis, and Western blotting were performed as previously reported19. Briefly, frozen tissue samples were weighted and homogenized in ten volumes of lysis buffer (62.5 mM Tris-HCl (pH6.8), 2% SDS, Complete protease inhibitor cocktail(Roche Applied Science Ltd.), and PhosStop(Roche Applied Science Ltd.)). After centrifugation (15,000 rpm × 60 min), supernatants were collected. The protein concentration of each sample was determined by Micro BCA (PIERCE Ltd.). Equal amounts of protein (50 μg/lane for indicated molecules and 10 μg/lane for β-actin) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to plolyvinylidene fluoride membranes. The membranes were cropped around the appropriate molecular size to save the amount of antibodies. The membranes were blocked in TNT (150 mM NaCl, 10 nM Tris-HCl (pH 7.4), and 0.05% tween-20) containing 10% BSA and then incubated with the indicated primary antibody overnight. After washing with TNT, blotted membranes were incubated with horseradish-peroxidase (HRP)-conjugated anti-rabbit IgG (1:10000 dilution; Dako cytomation) or HRP-conjugated anti-mouse IgG (1:10000 dilution; Jackson immune research Inc.) for 1 h. After washing, peroxidase activity was detected by chemiluminescence reagents (Western Lightning, PerkinElmer Life Science) and visualized on X-ray film (Fujifilm Medical Co., Ltd). The quantity of protein expressed was quantified by “Image J” software for Macintosh OS X and standardized by β-actin. β-actin blot was performed to validate the protein concentration. It is impossible to reprobe with anti-β-actin in the same blots, for example AMPK because the amount of total applied protein is too much for β-actin blot. The actin signal is saturated and is not applicable for quantification. If the protein amount is adjusted to actin level, other molecules cannot be detected. All blots presented in this study are from the same samples so that actin blots are the same in all the Western blot experiments. In some cases, brightness and/or intensity of the gels were manipulated to clarify the signals. The manipulation covered the entire area of the blots to ensure that the changes were made equally to each band.
mTOR immunoprecipitation and in vitro kinase assay
The kinase activity of mTOR in tissue samples was measured as in previous reports39,40. Tissue samples were homogenized in 20 volumes of lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM, NaCl, 1 mM EDTA, 5 mM EGTA, 20 mM glycerophosphate, 1 mM dithiothreitol, 1 mM protease inhibitor cocktail (Complete, Roche), phosphatase inhibitor cocktail (PhosStop, Roche)) and centrifuged for 30 min at 4 °C. The protein concentration of the supernatant was determined and 800 μg of each sample was used for immunoprecipitation. Samples were pre-absorbed with Protein G Sepharose for 60 min at 4 °C, centrifuged for 3 min at 4 °C, then mixed with mouse anti-mTOR antibody (1 μg) and incubated overnight at 4 °C. To measure kinase activity more accurately, saturation amount of lysates and a small amount of antibody was used to precipitate same amount of mTOR from active and hibernating chipmunks. Protein G sepharose was added to the samples and incubated for 2 h at 4 °C. After washing, immunoprecipitates were mixed with kination buffer including recombinant GST 4E-BP and ATP, and incubated for 30 min at 30 °C. The reaction was stopped by adding SDS sample buffer and boiling for 5 min at 95 °C. In the same membrane, mTOR and P-4EBP was immunoblotted. After that, membrane was reprobed with anti-GST antibody. mTOR kinase activity was calculated by the signal intensity of (P-4EBP/GST)/mTOR.
Metabolic labeling by [35S]methionine
Protein synthesis was measured by [35S]methionine incorporation19. Samples of brain and liver were gently homogenized with 10 volumes of DMEM and protein concentration was determined. Each sample (50 μg) was incubated with 10 µCi of [35S]methionine for 30 min at 37 °C. Samples were then lysed in equal volumes of 1 M NaOH containing casein as carrier, and incubated for 30 min at 42 °C. An equal volume of 20% ice-cold trichloroacetic acid was added to each sample, and the mixture was incubated for 1 h at 4 °C. After centrifugation, free methionine and methionine incorporated into protein were estimated by determining the radioactivity of supernatants and the pellet, respectively. Protein synthesis was estimated based on the ratio of methionine in the pellet to total methionine.
Statistical analysis
All values are presented as mean ± SE. Differences between groups were statistically determined by Student’s t-test when data were normally distributed or Wilcoxon rank-sum test when data were not normally distributed. Analysis were performed by the statistical software “R” (version 3.1.2). p < 0.05 were considered statistically significant.
Supplementary information
Acknowledgements
This study was supported by research grants from the Japan Society for the Promotion of Science (Grant-in-Aid for Challenging Exploratory Research (16K15060) to TS and NT).
Author Contributions
N.T., H.N. and T.S. designed the study and interpreted the data. S.Y., T.K. and N.T. performed the experiments. S.Y. prepared the figures. N.T. wrote the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48172-7.
References
- 1.Kondo N, et al. Circannual control of hibernation by HP complex in the brain. Cell. 2006;125:161–72. doi: 10.1016/j.cell.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Kondo N, Kondo J. Identification of novel blood proteins specific for mammalian hibernation. J Biol Chem. 1992;267:473–478. [PubMed] [Google Scholar]
- 3.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 4.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 5.Hardie DG. The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 6.Kemp B.E., Stapleton D., Campbell D.J., Chen Z.-P., Murthy S., Walter M., Gupta A., Adams J.J., Katsis F., van Denderen B., Jennings I.G., Iseli T., Michell B.J., Witters L.A. AMP-activated protein kinase, super metabolic regulator. Biochemical Society Transactions. 2003;31(1):162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 7.Witters LA, Kemp BE, Means AR. Chutes and Ladders: the search for protein kinases that act on AMPK. Trends Biochem. Sci. 2006;31:13–16. doi: 10.1016/j.tibs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 9.Horman S, et al. Activation of AMP activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002;12:1419–1423. doi: 10.1016/S0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 10.Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, et al. Regulation of elongation factor 2 kinase by p90 (RSK1) and p70, S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 13.Johanns M, et al. Direct and indirect activation of eukaryotic elongation factor 2 kinase by AMP-activated protein kinase. Cell Signal. 2017;36:212–221. doi: 10.1016/j.cellsig.2017.05.010.. [DOI] [PubMed] [Google Scholar]
- 14.Healy JE, Gearhart CN, Bateman JL, Handa RJ, Florant GL. AMPK and ACC change with fasting and physiological condition in euthermic and hibernating golden-mantled ground squirrels (Callospermophilus lateralis) Comp Biochem Physiol A Mol Integr Physiol. 2011;159:322–331. doi: 10.1016/j.cbpa.2011.03.026.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horman S, Hussain N, Dilworth SM, Storey KB, Rider MH. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:374–382. doi: 10.1016/j.cbpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Wu CW, Storey KB. Regulation of the mTOR signaling network in hibernating thirteen-lined ground squirrels. J Exp Biol. 2012;215(Pt 10):1720–1727. doi: 10.1242/jeb.066225. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, et al. Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry. 2001;40:11565–11570. doi: 10.1021/bi010649w. [DOI] [PubMed] [Google Scholar]
- 18.Frerichs KU, et al. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA. 1998;95:14511–14516. doi: 10.1073/pnas.95.24.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takei N, et al. Brain-derived neurotrophic factor enhances the basal rate of protein synthesis by increasing active eukaryotic elongation factor 2 levels and promoting translation elongation in cortical neurons. J. Biol. Chem. 2009;284:26340–26348. doi: 10.1074/jbc.M109.023010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara K, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 22.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajita K, et al. Effect of fasting on PPARgamma and AMPK activity in adipocytes. Diabetes Res Clin Pract. 2008;81:144–149. doi: 10.1016/j.diabres.2008.05.003.. [DOI] [PubMed] [Google Scholar]
- 24.To K, et al. Down-regulation of AMP-activated protein kinase by calorie restriction in rat liver. Exp Gerontol. 2007;42:1063–1071. doi: 10.1016/j.exger.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Hurley RL, et al. The Ca2+/calmodulin-dependen kinase kinases are AMP-activated protein kinase kinase. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 27.Woods A, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated proteinkinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987;262:17299–172303. [PubMed] [Google Scholar]
- 29.Kondo N, Shibata S. Calcium source for excitation-contraction coupling in myocardium of nonhibernating and hibernating chipmunks. Science. 1984;225:641–643. doi: 10.1126/science.6740332. [DOI] [PubMed] [Google Scholar]
- 30.Knight JR, et al. Eukaryotic elongation factor 2 kinase regulates the cold stress response by slowing translation elongation. Biochem J. 2015;465:227–238. doi: 10.1042/BJ20141014. [DOI] [PubMed] [Google Scholar]
- 31.Takamatsu N, Ohba K, Kondo J, Kondo N, Shiba T. Hibernation-associated gene regulation of plasma proteins with a collagen-like domain in mammalian hibernators. Mol Cell Biol. 1993;13:1516–1521. doi: 10.1128/MCB.13.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishore U, et al. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 2004;25:551–561. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 34.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation of AMP-activatedprotein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem. 2011;286:15652–15665. doi: 10.1074/jbc.M110.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203:615–623. doi: 10.1111/j.1432-1033.1992.tb16591.x. [DOI] [PubMed] [Google Scholar]
- 36.Inamura N, Nawa H, Takei N. Enhancement of translation elongation in neurons by brain-derived neurotrophic factor: implications for mammalian target of rapamycin signaling. J Neurochem. 2005;95:1438–1445. doi: 10.1111/j.1471-4159.2005.03466.x. [DOI] [PubMed] [Google Scholar]
- 37.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J. Biol. Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 38.Ishizuka Y, et al. AMP-activated protein kinase (AMPK) counteracts brain-derived neurotrophic factor (BDNF)-induced mammalian target of rapamycin complex 1 (mTORC1) signaling in neurons. J. Neurochem. 2013;127:66–77. doi: 10.1111/jnc.12362. [DOI] [PubMed] [Google Scholar]
- 39.Qi S, Mizuno M, Yonezawa K, Nawa H, Takei N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci Res. 2010;68:88–93. doi: 10.1016/j.neures.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Ohne Y, et al. Isolation of hyperactive mutants of mammalian target of rapamycin. J Biol Chem. 2008;283:31861–31870. doi: 10.1074/jbc.M801546200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).