Abstract
Metal-catalyzed β-C-H functionalization of saturated carbonyls via dehydrogenative desaturation proved to be a powerful tool for simplifying synthesis of valuable β-substituted carbonyls. Here, we report a copper-catalyzed dehydrogenative γ-C(sp3)-H amination of saturated ketones that initiates the three-component coupling of saturated ketones, amines and N-substituted maleimides to construct polysubstituted anilines. The protocol presented herein enables both linear and α-branched butanones to couple a wide spectrum of amines and various N-substituted maleimides to produce diverse tetra- or penta-substituted anilines in fair-to-excellent yields with good functional group tolerance. The mechanism studies support that this ketone dehydrogenative γ-C(sp3)-H amination was triggered by the ketone α,β-dehydrogenation desaturation that activates the adjacent γ-C(sp3)-H bond towards functionalization. This α,β-dehydrogenation desaturation-triggered cascade sequence opens up a new avenue to the remote C(sp3)-H functionalization of saturated ketones and has the potential to enable the rapid syntheses of complex compounds from simple starting materials.
Subject terms: Homogeneous catalysis, Catalytic mechanisms, Synthetic chemistry methodology
Functionalising ketones at the γ-position is not a trivial synthetic task. Here, the authors developed a copper-catalyzed dehydrogenative γ-amination of saturated ketones, which can then participate in a 3-component coupling reaction to construct polysubstituted anilines.
Introduction
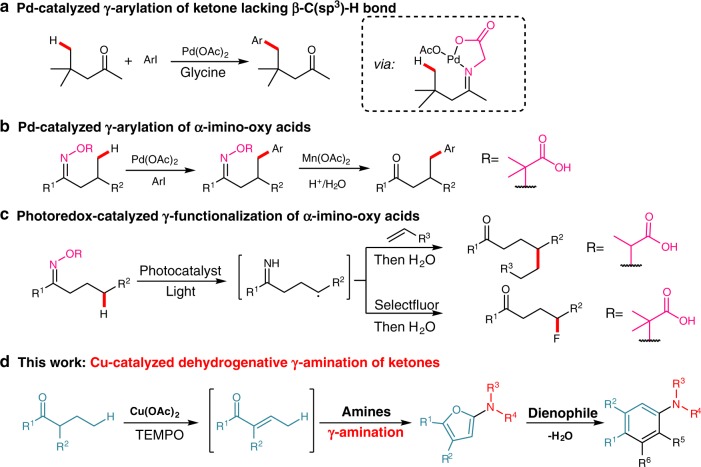
Ketones, a large class of versatile and readily available substrates, conventionally participate in reactions with their electrophilic ipso- carbons or nucleophilic α-carbons1. Recently, the β-functionalization reactions of saturated ketones have been achieved by employing directing group-assisted Pd-catalyzed C(sp3)–H activation methods2–6, or the strategies of merging photoredox catalysis with organocatalysis7,8, or metal-catalyzed ketone α,β-dehydrogenation desaturation/the resultant enone coupling cascade sequence9–18. Despite these advances in the development of the approaches to reactions at β-carbons of ketones, for the direct γ-C(sp3)–H functionalization of saturated ketone, only a few of examples have been reported to date2,19–21. The first example is the Pd-catalyzed γ-arylation reaction of the ketone lacking any β-C(sp3)–H bond using glycine as a transient directing group (Fig. 1a), which was limited to only a single ketone substrate2. The other two examples for ketone γ-C(sp3)–H functionalization both involved the use of α-imino-oxy acids prepared from the condensation of ketones with α-aminoxy acids as starting materials: one was the auxiliary-assisted Pd-catalyzed γ-C(sp3)–H arylation reaction of α-imino-oxy acid with aryl iodides that afforded γ-arylated ketones after Mn-catalyzed removal of α-imino-oxy acid auxiliary (Fig. 1b)19; the other was the photoredox-catalyzed cross-coupling between α-imino-oxy acid and radical trapping reagents that furnished, after treatment of the reaction products with water, the γ-functionalized ketones as final products (Fig. 1c)20,21. Notably, the metal-catalyzed remote C(sp3)–H functionalization, which is similar to ketone γ-C(sp3)–H functionalizaion in terms of the distance between the reaction position and functional group, has been accessible to a handful of carboxylic acid derivatives containing specific auxiliary directing groups22–27. These reactions of carboxylic acid derivatives disclosed that their appropriate auxiliary directing groups were essential for remote C(sp3)–H functionalization. However, the relatively low reactivity of ketones severely limits the scope of the directing groups that are pre-installed or in situ installed to saturated ketone frameworks, and therefore poses a great challenge to the development of transient directing group strategy for metal-catalyzed γ-C(sp3)–H functionalization of simple ketones. The metal-catalyzed methods for direct γ-functionalization of saturated ketones without the need for pre-installation of auxiliary directing group to ketone framework is highly desired, given that such methods would expand the reactivity patterns of ketones, streamline syntheses of value-added γ-functionalized ketones.
Fig. 1.
γ-C(sp3)–H functionalization of ketones. a Pd-catalyzed γ-arylation reaction of the ketone using glycine as a transient directing group. b Auxiliary-assisted Pd-catalyzed γ-C(sp3)–H arylation reaction of α-imino-oxy acid. c Photoredox-catalyzed γ-C(sp3)–H functionalization of α-imino-oxy acid with radical trapping reagents. d Our work proposed that α,β-desaturation initiated γ-functionalization of ketones
Metal-catalyzed dehydrogenative β-C(sp3)–H functionalization reactions via dehydrogenative desaturation28,29 were also amenable to esters30–34, lactams35, and other substrates36–48, demonstrating the ability of this type of reaction to rapidly construct complex molecule from simple substrates. Such reactions will continue to flourish in the future in view of the recent advance in the development of catalytic methods for generation of unsaturated compounds via dehydrogenation39–49 as well as the versatility of unsaturated compounds as synthetic intermediates. Recently, we have discovered a Cu-catalyzed successive dehydrogenation of aliphatic ketones to furnish dienketones or polyenketones50. This successive dehydrogenation sequence started with α,β-dehydrogenation desaturation to form enone intermediates, and therefore implicated that the carbonyl group activated γ-C(sp3)–H bond of enone through α,β-carbon–carbon double bond, as occurred in organocatalyzed γ-functionalization of enals51–54 and β-functionalization of alkyl aldehydes55–57 in which organocatalyst moieties covalently linked to substrates through formyl group exerted an effect on the remote C–H bonds through carbon–carbon double bonds. Inspired by our previous findings, we envisioned the feasibility of the α,β-desaturation initiated γ-functionalization of aliphatic ketones with nucleophilic amines that was expected to take place through α,β-desaturation of ketone to enone intermediate, subsequent oxidation of γ-C(sp3)–H bond of enone to form electrophilic species, final capture of the resulting electrophilic species with amine to form C–N bond at the position γ to carbonyl group. This targeted dehydrogenative ketone γ-amination is challenging to access because the electron-withdrawing carbonyl disfavors the oxidation of enone γ-C(sp3)–H bond to generate electrophilic species58, mechanistically contrasting with the previously reported secondary amine-catalyzed γ-functionalization of enal that is, in principle, the dehydrogenative cross-coupling of enal with electrophilic reagent via deprotonation of γ-C(sp3)–H in the iminum intermediate of enal to form nucleophilic species51,52,54,59,60.
Herein, we report a Cu-catalyzed dehydrogenative γ-amination reaction of saturated ketone that initiates a 2-amino furan formation/[4 + 2] cycloaddition of resultant 2-amino furan with N-substituted maleimide cascade sequence toward construction of polysubstituted anilines (Fig. 1d). The mechanism studies reveals that this Cu-catalyzed γ-amination reaction is triggered by ketone α,β-dehydrogenative desaturation, which activates the γ-C(sp3)–H bond of the resultant enone intermediate toward amination. Our findings demonstrate that the α,β-dehydrogenative desaturation triggered ketone γ-functionalization is viable even using nucleophilic amines as coupling partners. Since these polysubstituted anilines are readily converted to diverse valuable compounds61,62, this operationally simple, efficient method for the syntheses of polysubstituted anilines from simple substrates will attract the attentions from chemists working in a variety of research fields.
Results
Discovery and development of reaction
To check our hypothesis, we initially investigated the reaction of 1-phenyl-1-butanone (1a) (2 equiv.) with diisopropylamine (2a) in toluene at 120 °C with 10 mol% Cu(OAc)2/10 mol% 2,2′-bipyridine (bpy) as a catalyst and 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO)63,64 (3 equiv.) as an oxidant, and gratifyingly found that the expected ketone γ-amination reaction did occur, but afforded 2-diisopropylamino-5-phenylfuran (6a) as a final product. Due to partial decomposition of electron-rich 2-amino furan during the work-up stage, 2-amino-5-phenylfuran was isolated in only moderate yield (about 45%). Thus, we attempted to use N-substituted maleimide as a dienophile to capture the reactive 2-amino furan generated from ketone γ-amination and obtain polysubstituted aniline as a final product65 since polysubstituted anilines are highly useful compounds and generally accessed via multistep synthetic sequences66. To our delight, once introducing N-ethyl maleimide as the third component to the reaction system, three-component cross-coupling occurred to furnish tetra-substituted aniline in a 80% isolated yield (Table 1, entry 1), providing a step- and atom-economic approach to synthesis of polysubstituted aniline from simple starting materials. Optimization studies for this three-component cross-coupling reaction (Table 1) revealed that acids as an additive affected the reaction outcome. Totally, 0.2 equiv. of ortho-nitro-benzoic acid slightly increased the yield to 85% (Table 1, entry 2), while weaker carboxylic acids led to decrease in yield (Table 1, entries 5 and 6). Although acetic acid had a negative effect on the reaction, a solution of p-TsOH in acetic acid benefited the reaction. The beneficial effect of strong acid p-TsOH relied on its amount (Table 1, entry 8 versus entries 7 and 10). Totally, 0.1 equiv. of p-TsOH gave the best result (Table 1, entry 8). p-TsOH monohydrate was inferior to a solution of p-TsOH in acetic acid (Table 1, entry 9 versus entry 8), implicating the detriment of water to the reaction. As revealed by the control experiments, the reaction gave the desired product in the diminished yields in the absence of Cu catalyst (Table 1, entries 11–13), while the reaction was almost shut down on removing both Cu catalyst and TsOH (Table 1, entry 14). The results of control experiments indicated that both Cu catalyst and TsOH facilitated the targeted reaction. A solvent was also observed to affect the reaction outcome (Table 1, entry 2 versus entries 3 and 4).
Table 1.
Selected results of reaction optimizationa
|
| |||
|---|---|---|---|
| Entry | Additive (equiv.) | Solvent | Yield(%)b |
| 1 | – | PhCH3 | 80 |
| 2 | o-nitro-benzoic acid (0.2) | PhCH3 | 85 |
| 3 | o-nitro-benzoic acid (0.2) | o-DCB | 67 |
| 4 | o–nitro-benzoic acid (0.2) | CH3CN | 63 |
| 5 | benzoic acid (0.2) | PhCH3 | 53 |
| 6 | acetic acid (0.2) | PhCH3 | 56 |
| 7 | p-TsOH (0.05) | PhCH3 | 87 |
| 8 | p-TsOH (0.1) | PhCH3 | 96 |
| 9 | p-TsOH•H2O (0.1) | PhCH3 | 83 |
| 10 | p-TsOH (0.2) | PhCH3 | 91 |
| 11c | p-TsOH•H2O (0.08) | PhCH3 | 30 |
| 12c | p-TsOH (0.1) | PhCH3 | 33 |
| 13c | p-TsOH (0.2) | PhCH3 | 21 |
| 14c | – | PhCH3 | trace |
aReaction conditions: 1a (2 equiv.), 2a (0.4 mmol), 3a (1.5 equiv.), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), additive, solvent (1.5 mL), N2, 120 °C for 48 h
bIsolated yields
cWithout Cu(OAc)2/bpy
Substrate scope of ketones
With the optimized reaction conditions in hand, we explored the substrate scope with respect to ketones. As shown in Fig. 2, 1-aryl-1-butanones bearing diverse substituents on their phenyl rings smoothly underwent reactions to give the corresponding polysubstituted anilines in excellent yields (4a–4g) with the exception of cyano-substituted ketone (4e) that probaly coordinated to Cu catalyst through cyano group to intervene with Cu-catalysis. α-Substituted 1-aryl-1-butanones (4h–m), such as butanones containing β-diketone (4j, 4k), β-keto-ester (4i, 4l, 4m) moieties, participated in the reaction to furnish penta-substituted anilines in moderate-to-excellent yields. Among these substrates, α-acetyl-substuted 1-phenyl-1-butanone gave two regioisomers with near 1: 1.6 ratio (4k). 1-Heteroaryl-1-butanones proved to be suitable substrates for this reaction, as exemplified by 1-(furan-2-yl)butan-1-one (4n) and 1-(thiophen-2-yl)butan-1-one (4o). Both α-substituent-lacking and α-substituent-containing dialkyl ketones also underwent the polysubstituted aniline formation reaction occurring exclusively on the propyl moiety (4p–w). 1-Phenylhexan-3-one gave styrenyl-substitued target product as a result of over-dehydrogenation (4s). In spite of a lot of attempt, β-substituted ketone did not work for this reaction presumably due to the difficulty in α,β-dehydrogenation desaturation. The established reaction conditions were also amenable to the three-component coupling reaction of but-2-enal, as shown by 2-phenylbut-2-enal (4x), which provided a complement to the existing method for enal γ-functionalization51–54.
Fig. 2.
Scope of ketonesa. aReaction conditions: 1 (2 equiv.), 2a (0.4 mmol), 3a (1.5 equiv.), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), p-TsOH (10 mol%), PhCH3 (1.5 mL), N2, 120 °C for 48 h. Isolated yields
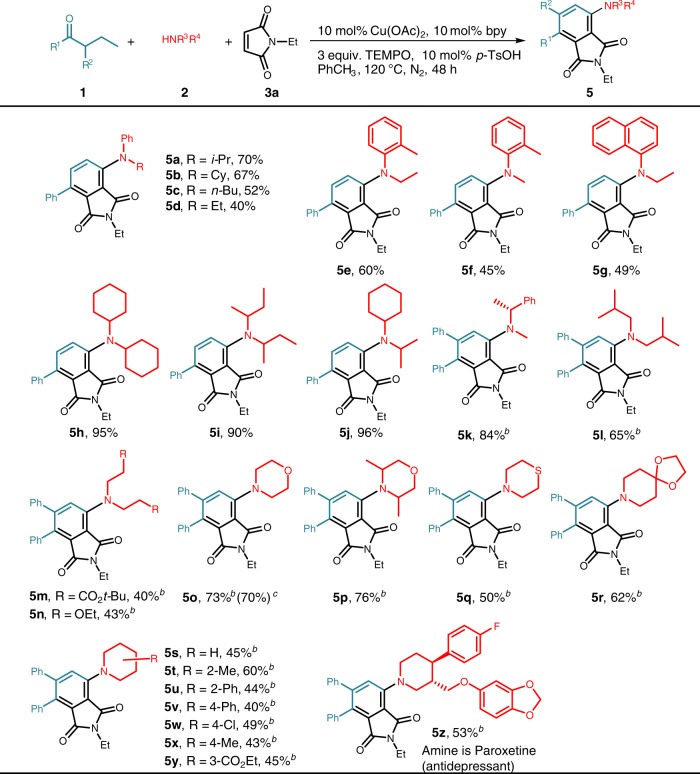
Substrate scope of secondary amines
Figure 3 demonstrates that the Cu-catalyzed three-component coupling reaction was compatible with a broad range of secondary amines using 1-phenyl-1- butanone or α-phenyl-substituted 1-phenyl-1- butanone as a coupling partner. N-alkyl anilines furnished the corresponding products in good yields (5a–g). Besides, acyclic dialkyl amines afforded products in fair-to-excellent yields (5h–5n). Notably, an array of alicyclic amines with varying substitutents and substitution patterns, including methyl (5t and 5x), phenyl (5u and 5v), chloro (5w), ester (5y) groups were well tolerated in this reaction. Thiomorpholine (5q) was also suitable substrate and provided synthetically useful yields. To demonstrate the practicability of our method, the gram-scale reaction of 1h (1.61 g, 7.14 mmol) was conducted to afford the corresponding product (5o) in 70% yield (2.06 g). The three-component coupling reaction was also capable of modifying paroxetine containing the alicyclic amine moiety (5z), illustrating its potential in practical utilities.
Fig. 3.
Scope of secondary aminesa. aReaction conditions: 1 (2 equiv.), 2 (0.4 mmol), 3a (1.5 equiv.), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), p-TsOH (10 mol%), PhCH3 (1.5 mL), N2, 120 °C for 48 h. Isolated yields. bReaction conditions: 1 (0.4 mmol), 2 (0.4 mmol), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), CsOAc (20 mol%), PhCH3 (1.5 mL), N2, 120 °C for 38 h; then 3a (1.5 equiv.) is added for another 10 h. cReaction was performed on 7.14 mmol scale
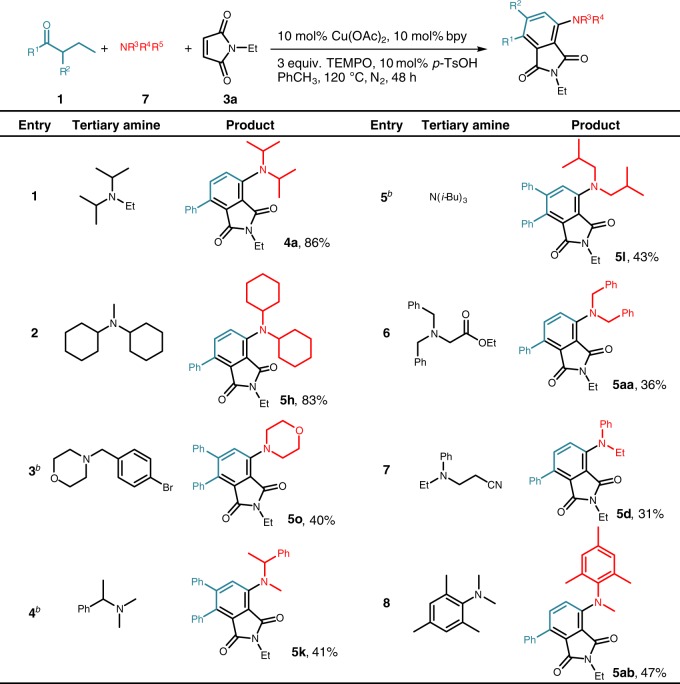
Substrate scope of tertiary amines
Interestingly, tertiary amines could participate in this three-component coupling reaction via cleavage of C–N bond67,68, thus play the similar role to secondary amines (Fig. 4). Although the exact reason for the cleavage of C–N bond remains to be clarified, among the investigated tertiary amines, the C–N bond cleavage occurred preferentially either at more sterically accessible carbon atoms (entries 1, 2, 4, and 8) or at carbon atoms adjacent to substituents capable of stabilizing carbon radicals (entries 3 and 6). For example, diisopropyl ethylamine gave excellent yield (entry 1) via cleavage of C–N bond of less sterically hindered ethyl group while bulkier triisobutyl amines gave moderate yield (entry 5).
Fig. 4.
Scope of tertiary aminesa. aReaction conditions: 1 (0.4 mmol), 7 (2 equiv.), 3a (1.5 equiv.), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), p-TsOH (10 mol%), PhCH3 (1.5 mL), N2, 120 °C for 48 h. Isolated yields. bReaction conditions: 1 (0.4 mmol), 2 (2 equiv.), Cu(OAc)2 (10 mol%), bpy (10 mol%), TEMPO (3 equiv.), CsOAc (20 mol%), PhCH3 (1.5 mL), N2, 120 °C for 38 h; Then 3a (1.5 equiv.) is added for another 10 h
In addition, this three-component coupling reaction was compatible with the variation of substituent on nitrogen atom of maleimide. A series of N-substituents of maleimide, such as benzyl, aryl and alkyl groups, were tolerable for the reaction (See Supplementary Methods for details).
Mechanistic studies
To gain an insight into this Cu-catalyzed dehydrogenative γ-amination of saturated ketone, we performed mechanistic investigations. The reaction of 1-phenyl-1-butanone (1a) with TEMPO under standard conditions was observed to produce 2,2,6,6-tetramethyl-1-(5-phenylfuran-2-yl) piperidine (6b) as a result of in situ generation of 2,2,6,6-tetramethyl piperidine from TEMPO (eq. 1, Fig. 5), which prevented us from identifing the intermediates from oxidation of saturated ketone by TEMPO. Despite this, on reducing reaction temperature to 110 °C, 1-phenylbut-2-en-1-one (6c) and 4-oxo-4-phenylbut-2-enal (6d) were identified along with formation of 6b in the reaction of 1a with TEMPO (eq. 2, Fig. 5). Both enone 6c and γ-keto enal 6d were observed to react with diisopropyl amine under standard conditions to produce 2-diisopropylamino-5-phenyl-furan (6a) (eqs. 3 and 4, Fig. 5). Identification of 6c and 6d in the reaction of 1a with TEMPO, in combination with their conversion to 2-diisopropylamino furan (6a), suggested that both 6c and 6d could be intermediates in the reaction of saturated 1-phenyl-1-butanone with diisopropyl amine to generate 2-amino-furan. Moreover, the reaction of enone 6c with TEMPO under milder conditions (110 °C) was observed to generate γ-keto enal 6d and 2-amino furan 6b (eq. 5, Fig. 5). Generation of 6d from the reaction of 6c with TEMPO illustrated that enone 6c was likely the precursor to γ-keto enal 6d. In light of our previous findings that γ-TEMPO substituted enone was found in the reaction of enone with TEMPO50 and that α-C(sp3)–H of imine was oxidized by TEMPO to C=O bond via α-TEMPO substituted imine intermediate69, conversion of enone 6c to γ-keto enal 6d likely stemmed from the γ-oxygenation of enone 6c with TEMPO, in which 2,2,6,6-tetramethyl piperidine released from TEMPO was incorporated into 6b.
Fig. 5.
Preliminary studies of mechanism. a The control experiments show that the cascade sequence may proceed through initial ketone α,β-dehydrogenation desaturation that activates the adjacent γ-C(sp3)–H bond. b The control experiments show that Cu catalyst is the main contributor to the ketone α,β-dehydrogenation desaturation step and p-TsOH catalyze the conversions of enone intermediates. c The proposed reaction pathway to generate polysubstituted anilines
In the optimization studies, control experiments showed that p-TsOH could facilitate the reaction, but was less effective than Cu catalyst alone. In the reaction of 1-phenyl-1-butanone (1a) with diisopropylamine to form 6a (eq. 6, Fig. 5), the simultaneous use of Cu catalyst and p-TsOH gave 45% yield while 16% yield was obtained in the absence of Cu catalyst, which is consistent with the results obtained in optimization studies. In contrast, in the reactions of enone 6c with diisopropylamine (eq. 3, Fig. 5) or with diisopropylamine and N-ethyl maleimide (eq. 7, Fig. 5), p-TsOH alone gave the comparable yields to those obtained with the simultaneous use of Cu catalyst and p-TsOH. These results implicated that Cu catalyst was more active than p-TsOH in the catalysis of ketone α,β-dehydrogenation desaturation step and that p-TsOH as a catalyst was effective in the conversions of enone intermediates.
On the basis of the above investigations, a cascade sequence was proposed for the three-component coupling reaction (Fig. 5c). The cascade sequence may proceed through initial α,β-dehydrogenation desaturation to form α,β-enone, γ-C(sp3)-H oxidation of resultant α,β-enone to a γ-keto enal intermediate, amine–aldehyde condensation between γ-keto enal with secondary amine to form 2-amino furan via intramolecular cyclization of iminium species, and final cycloaddition of 2-amino furan to N-substituted maleimide to afford polysubstituted aniline after dehydration aromatization.
Discussion
In summary, a Cu-catalyzed three-component coupling of saturated ketones, amines and N-substituted maleimides via dehydrogenative γ-C(sp3)–H amination of saturated ketones has been developed for syntheses of polysubstituted anilines. The dehydrogenative γ-C(sp3)–H amination of saturated ketones was triggered by the ketone α,β-dehydrogenation desaturation that activates the adjacent γ-C(sp3)–H bond. This α,β-dehydrogenation desaturation triggered sequence opens up a new avenue to the remote C(sp3)–H functionalization of saturated ketones, and has the potential to enable the rapid syntheses of complex compounds from readily available saturated ketones in a single operation. Efforts to develop other dehydrogenative γ-C(sp3)–H functionalization reactions28,29,70 of ketones through clarifying reaction mechanism are underway in our group.
Methods
General procedure for ketones with secondary amines reaction
In a nitrogen-filled glovebox, a 25 mL Schlenk tube equipped with a stir bar was charged with Cu(OAc)2 (7.26 mg, 0.04 mmol, 10 mol%), 2,2′-bipyridine (6.25 mg, 0.04 mmol, 10 mol%), N-substituted maleimide (0.6 mmol, 1.5 equiv.) and TEMPO (187.50 mg, 1.2 mmol, 3.0 equiv.). The tube was fitted with a rubber septum and moved out of the glove box. Then amine (0.4 mmol), ketone (0.8 mmol), p-Toluenesulfonic acid (6.88 mg, 0.04 mmol, 10 mol%, 12 wt% solution in pure acetic acid), and toluene (1.5 mL) were added in turn to the Schlenk tube through the rubber septum using syringes, and the septum was replaced with a Teflon screwcap under nitrogen flow. The reaction mixture was allowed to stir for 48 h at 120 °C. After completion of the reaction, the reaction mixture was cooled to room temperature. Then the reaction mixture was diluted with ethyl acetate (10 mL), followed by filtration through a pad of silica gel with several washings. Then the filtrate was concentrated under reduced pressure, and purified by flash column chromatography on silica gel to provide the corresponding product.
General procedure for ketones with tertiary amines reaction
In a nitrogen-filled glovebox, a 25 mL Schlenk tube equipped with a stir bar was charged with Cu(OAc)2 (7.26 mg, 0.04 mmol, 10 mol%), 2,2′-bipyridine (6.25 mg, 0.04 mmol, 10 mol%), TEMPO (187.50 mg, 1.2 mmol, 3 equiv), and N-substitutedmaleimide (75.08 mg, 0.6 mmol, 1.5 equiv). The tube was fitted with a rubber septum and moved out of the glove box. Then ketone (0.4 mmol), tertiary amine (0.8 mmol, 2.0 equiv.), p-Toluenesulfonic acid (6.88 mg, 0.04 mmol, 10 mol%, 12 wt% solution in pure acetic acid) and toluene (1.5 mL) were added in turn to the Schlenk tube through the rubber septum using syringes, and then the septum was replaced with a Teflon screwcap under nitrogen flow. The reaction mixture was stirred at 120 °C for 48 h. Upon cooling to room temperature, the reaction mixture was diluted with 10 mL of ethyl acetate, followed by filtration through a pad of silica gel with several washings. The filtrate was concentrated under reduced pressure, and then purified by flash column chromatography on silica gel to provide the corresponding product.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0206801), National Natural Science Foundation of China Grants (21431008, u1505242, and 21702205), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000 and XDB10040304) and the Key Research Program of the Chinese Academy of Sciences (ZDRW-CN-2016-1).
Author contributions
R.H. and W.S. conceived the project. R.H. performed the experiments and analyzed the data. F.-J.C. and M.Z. discussed the results. X.Z. characterized X-ray structures of two compounds. W.S. prepared the paper.
Data availability
All data generated and analyzed during this study are included in this article and its Supplementary Information files, and are also available from the authors on reasonable request. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1905935 (4a), 1905936 (6b), and can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11624-9.
References
- 1.Otera J. Modern Carbonyl Chemistry. New York, NY: John Wiley & Sons; 2008. [Google Scholar]
- 2.Zhang F-L, Hong K, Li T-J, Park H, Yu J-Q. Functionalization of C(sp3)-H bonds using a transient directing group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong K, Park H, Yu J-Q. Methylene C(sp3)-H arylation of aliphatic ketones using a transient directing group. ACS Catal. 2017;7:6938–6941. doi: 10.1021/acscatal.7b02905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai LV, Hull KL, Sanford MS. Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds. J. Am. Chem. Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 5.Kang T, Kim Y, Lee D, Wang Z, Chang S. Iridium-catalyzed intermolecular amidation of sp3 C-H bonds: late-stage functionalization of an unactivated methyl group. J. Am. Chem. Soc. 2014;136:4141–4144. doi: 10.1021/ja501014b. [DOI] [PubMed] [Google Scholar]
- 6.Gao P, et al. Iridium(III)-catalyzed direct arylation of C-H bonds with diaryliodonium salts. J. Am. Chem. Soc. 2015;137:12231–12240. doi: 10.1021/jacs.5b06758. [DOI] [PubMed] [Google Scholar]
- 7.Petronijević FR, Nappi M, MacMillan DWC. Direct β-functionalization of cyclic ketones with aryl ketones via the merger of photoredox and organocatalysis. J. Am. Chem. Soc. 2013;135:18323–18326. doi: 10.1021/ja410478a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirnot DM, Rankic TA, Martin DBC, MacMillan DWC. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science. 2013;339:1593–1596. doi: 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terao Y, et al. Multiple arylation of alkyl aryl ketones and α,β-unsaturated carbonyl compounds via palladium catalysis. Tetrahedron. 2001;57:5967–5974. doi: 10.1016/S0040-4020(01)00555-5. [DOI] [Google Scholar]
- 10.Ueno S, Shimizu R, Kuwano R. Nickel-catalyzed formation of a carbon-nitrogen bond at the β position of saturated ketones. Angew. Chem. Int. Ed. 2009;48:4543–4545. doi: 10.1002/anie.200900892. [DOI] [PubMed] [Google Scholar]
- 11.Shang Y, et al. Pd-catalyzed C-H olefination of (hetero)arenes by using saturated ketones as an olefin source. Angew. Chem. Int. Ed. 2013;52:1299–1303. doi: 10.1002/anie.201208627. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Dong G. Catalytic C-C bond forming transformations via direct β-C-H functionalization of carbonyl compounds. Tetrahedron Lett. 2014;55:5869–5889. doi: 10.1016/j.tetlet.2014.09.005. [DOI] [Google Scholar]
- 13.Huang Z, Dong G. Catalytic direct β-arylation of simple ketones with aryl iodides. J. Am. Chem. Soc. 2013;135:17747–17750. doi: 10.1021/ja410389a. [DOI] [PubMed] [Google Scholar]
- 14.Jie X, Shang Y, Zhang X, Su W. Cu-catalyzed sequential dehydrogenation-conjugate addition for β-functionalization of saturated ketones: scope and mechanism. J. Am. Chem. Soc. 2016;138:5623–5633. doi: 10.1021/jacs.6b01337. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Huang D, Zhao Y, Newhouse TR. Allyl-palladium-catalyzed ketone dehydrogenation enables telescoping with enone α,β-vicinal difunctionalization. Angew. Chem. Int. Ed. 2017;56:8258–8262. doi: 10.1002/anie.201704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Yang X, Dai X-J, Li C-J. Palladium-catalyzed direct β-C-H arylation of ketones with arylboronic acids in water. Adv. Synth. Catal. 2017;359:2402–2406. doi: 10.1002/adsc.201700277. [DOI] [Google Scholar]
- 17.Wang C, Dong G. Direct β-alkylation of ketones and aldehydes via Pd-catalyzed redox cascade. J. Am. Chem. Soc. 2018;140:6057–6061. doi: 10.1021/jacs.8b03530. [DOI] [PubMed] [Google Scholar]
- 18.Li H, et al. Rh/Cu-catalyzed ketone β-functionalization by merging ketone dehydrogenation and carboxyl-directed C-H alkylation. ACS Catal. 2018;8:4777–4782. doi: 10.1021/acscatal.8b00923. [DOI] [Google Scholar]
- 19.Zhu R-Y, Li Z-Q, Park HS, Senanayake CH, Yu J-Q. Ligand-enabled γ-C(sp3)-H activation of ketones. J. Am. Chem. Soc. 2018;140:3564–3568. doi: 10.1021/jacs.8b01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dauncey EM, Morcillo SP, Douglas JJ, Sheikh NS, Leonori D. Photoinduced remote functionalisations by iminyl radical promoted C-C and C-H bond cleavage cascades. Angew. Chem. Int. Ed. 2018;57:744–748. doi: 10.1002/anie.201710790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Studer A. α-aminoxy-acid-auxiliary-enabled intermolecular radical γ-C(sp3)-H functionalization of ketones. Angew. Chem. Int. Ed. 2018;57:1692–1696. doi: 10.1002/anie.201712066. [DOI] [PubMed] [Google Scholar]
- 22.He G, Zhao YS, Zhang SY, Lu CX, Chen G. Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C(sp3)-H and C(sp2)-H bonds at γ and δ positions. J. Am. Chem. Soc. 2012;134:3–6. doi: 10.1021/ja210660g. [DOI] [PubMed] [Google Scholar]
- 23.Nadres ET, Daugulis O. Heterocycle synthesis via direct C-H/N-H coupling. J. Am. Chem. Soc. 2012;134:7–10. doi: 10.1021/ja210959p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He G, Zhang S-Y, Nack WA, Li Q, Chen G. Use of a readily removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated γ C(sp3)-H bonds. Angew. Chem. Int. Ed. 2013;52:11124–11128. doi: 10.1002/anie.201305615. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, et al. Easily accessible auxiliary for palladium-catalyzed intramolecular amination of C(sp2)-H and C(sp3)-H bonds at δ- and ε-positions. Angew. Chem. Int. Ed. 2014;53:9884–9888. doi: 10.1002/anie.201404854. [DOI] [PubMed] [Google Scholar]
- 26.Topczewski JJ, Cabrera PJ, Saper NI, Sanford MS. Palladium-catalysed transannular C-H functionalization of alicyclic amines. Nature. 2016;531:220–224. doi: 10.1038/nature16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Zhu R-Y, Xiao K-J, Yu J-Q. Ligand-enabled arylation of γ-C-H bonds. Angew. Chem. Int. Ed. 2016;55:4317–4321. doi: 10.1002/anie.201512020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobereiner GE, Crabtree RH. Dehydrogenation as a substrate-activating strategy in homogeneous transition-metal catalysis. Chem. Rev. 2010;110:681–703. doi: 10.1021/cr900202j. [DOI] [PubMed] [Google Scholar]
- 29.Choi J, MacArthur AHR, Brookhart M, Goldman AS. Dehydrogenation and related reactions catalyzed by iridium pincer complexes. Chem. Rev. 2011;111:1761–1779. doi: 10.1021/cr1003503. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen M, Lee S, Liu X, Wolkowski JP, Hartwig JF. Efficient synthesis of α-aryl esters by room-temperature palladium-catalyzed coupling of aryl halides with ester enolates. J. Am. Chem. Soc. 2002;124:12557–12565. doi: 10.1021/ja027643u. [DOI] [PubMed] [Google Scholar]
- 31.Renaudat A, et al. Palladium-catalyzed β arylation of carboxylic esters. Angew. Chem. Int. Ed. 2010;49:7261–7265. doi: 10.1002/anie.201003544. [DOI] [PubMed] [Google Scholar]
- 32.Larini P, et al. On the mechanism of the palladium-catalyzed β-arylation of ester enolates. Chem. Eur. J. 2012;18:1932–1944. doi: 10.1002/chem.201103153. [DOI] [PubMed] [Google Scholar]
- 33.Leskinen MV, Yip K-T, Valkonen A, Pihko PM. Palladium-catalyzed dehydrogenative β’-functionalization of β-keto esters with indoles at room temperature. J. Am. Chem. Soc. 2012;134:5750–5753. doi: 10.1021/ja300684r. [DOI] [PubMed] [Google Scholar]
- 34.Nimje RY, Leskinen MV, Pihko PM. A three-component palladium-catalyzed oxidative C-C coupling reaction: a domino process in two dimensions. Angew. Chem. Int. Ed. 2013;52:4818–4822. doi: 10.1002/anie.201300833. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Liu F, Dong G. Direct palladium-catalyzed β-arylation of lactams. Angew. Chem. Int. Ed. 2018;130:3877–3881. doi: 10.1002/ange.201800958. [DOI] [PubMed] [Google Scholar]
- 36.Stang EM, White MC. Molecular complexity via C-H activation: a dehydrogenative Diels-Alder reaction. J. Am. Chem. Soc. 2011;133:14892–14895. doi: 10.1021/ja2059704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman AS, et al. Catalytic alkane metathesis by tandem alkane dehydrogenation-olefin metathesis. Science. 2006;312:257–261. doi: 10.1126/science.1123787. [DOI] [PubMed] [Google Scholar]
- 38.Jia X, Huang Z. Conversion of alkanes to linear alkylsilanes using an iridium-iron-catalysed tandem dehydrogenation-isomerization-hydrosilylation. Nat. Chem. 2016;8:157–161. doi: 10.1038/nchem.2417. [DOI] [PubMed] [Google Scholar]
- 39.Muzart Jacques. One-Pot Syntheses of α,β-Unsaturated Carbonyl Compounds through Palladium-Mediated Dehydrogenation of Ketones, Aldehydes, Esters, Lactones and Amides. European Journal of Organic Chemistry. 2010;2010(20):3779–3790. doi: 10.1002/ejoc.201000278. [DOI] [Google Scholar]
- 40.Stahl SS, Diao T. Oxidation adjacent to C X bonds by dehydrogenation. Compr. Org. Synth. 2014;7:178–212. doi: 10.1016/B978-0-08-097742-3.00707-2. [DOI] [Google Scholar]
- 41.Izawa Y, Pun D, Stahl SS. Palladium-catalyzed aerobic dehydrogenation of substituted cyclohexanones to phenols. Science. 2011;333:209–213. doi: 10.1126/science.1204183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahuja R, et al. Catalytic dehydroaromatization of n-alkanes by pincer-ligated iridium complexes. Nat. Chem. 2011;3:167–171. doi: 10.1038/nchem.946. [DOI] [PubMed] [Google Scholar]
- 43.Bigi MA, Reed SA, White MC. Diverting non-haem iron catalysed aliphatic C-H hydroxylations towards desaturations. Nat. Chem. 2011;3:216–222. doi: 10.1038/nchem.967. [DOI] [PubMed] [Google Scholar]
- 44.Gao W, He Z, Qian Y, Zhao J, Huang Y. General palladium-catalyzed aerobic dehydrogenation to generate double bonds. Chem. Sci. 2012;3:883–886. doi: 10.1039/C1SC00661D. [DOI] [Google Scholar]
- 45.Chen Y, Romaire JP, Newhouse TR. Palladium-catalyzed α,β-dehydrogenation of esters and nitriles. J. Am. Chem. Soc. 2015;137:5875–5878. doi: 10.1021/jacs.5b02243. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Turlik A, Newhouse TR. Amide α,β-dehydrogenation using allyl-palladium catalysis and a hindered monodentate anilide. J. Am. Chem. Soc. 2016;138:1166–1169. doi: 10.1021/jacs.5b12924. [DOI] [PubMed] [Google Scholar]
- 47.Chen M, Dong G. Direct catalytic desaturation of lactams enabled by soft enolization. J. Am. Chem. Soc. 2017;139:7757–7760. doi: 10.1021/jacs.7b04722. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, He Z, Zhang L, Huang Y. Iridium-catalyzed aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids: activation of both α-and β-C-H bonds through an allyl-iridium intermediate. J. Am. Chem. Soc. 2018;140:735–740. doi: 10.1021/jacs.7b11351. [DOI] [PubMed] [Google Scholar]
- 49.Teskey CJ, Adler P, GonÅalves CR, Maulide N. Chemoselective α,β-dehydrogenation of saturated amides. Angew. Chem. Int. Ed. 2019;58:447–451. doi: 10.1002/anie.201808794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shang Y, Jie X, Jonnada K, Zafar SN, Su W. Dehydrogenative desaturation-relay via formation of multicenter-stabilized radical intermediates. Nat. Commun. 2017;8:2273. doi: 10.1038/s41467-017-02381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertelsen S, Marigo M, Brandes S, Dinér P, Jørgensen KA. Dienamine catalysis: organocatalytic asymmetric γ-amination of α,β-unsaturated aldehydes. J. Am. Chem. Soc. 2006;128:12973–12980. doi: 10.1021/ja064637f. [DOI] [PubMed] [Google Scholar]
- 52.Bergonzini G, Vera S, Melchiorre P. Cooperative organocatalysis for the asymmetric γ alkylation of α-branched enals. Angew. Chem. Int. Ed. 2010;49:9685–9688. doi: 10.1002/anie.201004761. [DOI] [PubMed] [Google Scholar]
- 53.Mo J, Chen X, Chi YR. Oxidative γ-addition of enals to trifluoromethyl ketones: enantioselectivity control via lewis acid/N-heterocyclic carbene cooperative catalysis. J. Am. Chem. Soc. 2012;134:8810–8813. doi: 10.1021/ja303618z. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X-L, et al. Enantioselective functionalization of inactive sp3 C-H bonds remote to functional group by metal/organo cooperative catalysis. Org. Lett. 2015;17:5120–5123. doi: 10.1021/acs.orglett.5b02653. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi Y, Itoh T, Ishikawa H. Oxidative and enantioselective cross-coupling of aldehydes and nitromethane catalyzed by diphenylprolinol silyl ether. Angew. Chem. Int. Ed. 2011;50:3920–3924. doi: 10.1002/anie.201006885. [DOI] [PubMed] [Google Scholar]
- 56.Zhang S-L, et al. Organocatalytic enantioselective β-functionalization of aldehydes by oxidation of enamines and their application in cascade reactions. Nat. Commun. 2011;2:211. doi: 10.1038/ncomms1214. [DOI] [PubMed] [Google Scholar]
- 57.Fu Z, Xu J, Zhu T, Leong WWY, Chi RY. β-carbon activation of saturated carboxylic esters through N-heterocyclic carbene organocatalysis. Nat. Chem. 2013;5:835–839. doi: 10.1038/nchem.1710. [DOI] [PubMed] [Google Scholar]
- 58.Næsborg L, Corti V, Leth LA, Poulsen PH, Jørgensen KA. Catalytic asymmetric oxidative γ-coupling of α,β-unsaturated aldehydes with air as the terminal oxidant. Angew. Chem. Int. Ed. 2018;130:1622–1626. doi: 10.1002/ange.201711944. [DOI] [PubMed] [Google Scholar]
- 59.Terao Y, Satoh T, Miura M, Nomura M. Regioselective arylation on the γ-position of α,β-unsaturated carbonyl compounds with aryl bromides by palladium catalysis. Tetrahedron Lett. 1998;39:6203–6206. doi: 10.1016/S0040-4039(98)01275-1. [DOI] [Google Scholar]
- 60.Chen X, Liu X, Mohr JT. Direct regioselective γ-amination of enones. Org. Lett. 2016;18:716–719. doi: 10.1021/acs.orglett.5b03689. [DOI] [PubMed] [Google Scholar]
- 61.Hatoum F, et al. Photodecarboxylative addition of carboxylates to phthalimides: a concise access to biologically active 3-(alkyl and aryl)methylene-1H-isoindolin-1-ones. Tetrahedron Lett. 2012;53:5573–5577. doi: 10.1016/j.tetlet.2012.07.142. [DOI] [Google Scholar]
- 62.Das S, et al. Selective catalytic monoreduction of phthalimides and imidazolidine-2,4-diones. Angew. Chem. Int. Ed. 2011;50:9180–9184. doi: 10.1002/anie.201104226. [DOI] [PubMed] [Google Scholar]
- 63.Tebben L, Studer A. Nitroxides: applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. 2011;50:5034–5068. doi: 10.1002/anie.201002547. [DOI] [PubMed] [Google Scholar]
- 64.Ryland BL, McCann SD, Brunold TC, Stahl SS. Mechanism of alcohol oxidation mediated by copper (II) and nitroxyl radicals. J. Am. Chem. Soc. 2014;136:12166–12173. doi: 10.1021/ja5070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padwa A, Dimitroff M, Waterson AG, Wu T. Diels-Alder reaction of 2-amino-substituted furans as a method for preparing substituted Anilines. J. Org. Chem. 1997;62:4088–4096. doi: 10.1021/jo9702599. [DOI] [Google Scholar]
- 66.Weissermel K, Arpe HJ. Industrial Organic Chemistry. Weinheim: Wiley-VCH; 1997. pp. 374–377. [Google Scholar]
- 67.Ouyang K, Hao W, Zhang W-X, Xi Z. Transition-metal-catalyzed cleavage of C-N single bonds. Chem. Rev. 2015;115:12045–12090. doi: 10.1021/acs.chemrev.5b00386. [DOI] [PubMed] [Google Scholar]
- 68.Gao B, Huang H. Palladium-catalyzed hydroaminocarbonylation of alkynes with tertiary amines via C-N bond cleavage. Org. Lett. 2017;19:6260–6263. doi: 10.1021/acs.orglett.7b03331. [DOI] [PubMed] [Google Scholar]
- 69.Jie X, et al. Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO. Nat. Commun. 2018;9:5002. doi: 10.1038/s41467-018-07534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Girard SA, Knauber T, Li C-J. The cross-dehydrogenative coupling of C(sp3)-H bonds: a versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated and analyzed during this study are included in this article and its Supplementary Information files, and are also available from the authors on reasonable request. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 1905935 (4a), 1905936 (6b), and can be obtained free of charge from the CCDC via http://www.ccdc.cam.ac.uk/data_request/cif.