Abstract
Head and neck squamous cell carcinoma (HNSCC) develops in the mucosal lining of the upper aerodigestive tract, principally as a result of exposure to carcinogens present in tobacco products and alcohol, with oncogenic papillomaviruses also being recognized as etiological agents in a limited proportion of cases. As such, there is considerable scope for prevention of disease development and progression. However, despite multimodal approaches to treatment, tumor recurrence and metastatic disease are common problems, and clinical outcome is unsatisfactory. As our understanding of the genetics and biochemical aberrations in HNSCC has improved, so the development and use of molecularly targeted drugs to combat the disease have come to the fore. In this article, we review molecular mechanisms that alter signal transduction downstream of the epidermal growth factor receptor (EGFR) as well as those that perturb orderly cell cycle progression, such as p53 mutation, cyclin overexpression, and loss of cyclin-dependent kinase inhibitor function. We outline some of the tactics that have been employed to combat the altered biochemistry. These include blockade of the EGFR using humanized monoclonal antibodies such as cetuximab and small molecule tyrosine kinase inhibitors (TKIs) such as erlotinib/gefitinib and subsequent generations of TKIs, restoration of p53 function using MIRA compounds, and inhibition of cyclin-dependent kinase and aurora kinase activity using drugs such as palbociclib and alisertib. Knowledge of the underlying molecular mechanisms may be utilizable in order to predict disease behavior and tailor therapeutic interventions in a more personalized approach to improve clinical response. Use of liquid biopsy, omics platforms, and salivary diagnostics hold promise in this regard.
Keywords: EGFR, Signal transduction, Cell cycle, p53, Molecular targets, Predictive preventive personalized medicine, PPPM
Introduction
Head and neck cancer is a heterogenous group of tumors, both clinically and biologically, with high morbidity and mortality rates. Its very nature, therefore, mandates the development of biomarkers that can be used to predict disease development (primary prevention), or that are indicative of disease course or therapeutic response, allowing subsequent institution of personalized approaches to management. Almost 90% of head and neck cancers are squamous cell carcinomas (HNSCC) that arise from the mucosal surfaces of the upper aerodigestive tract [1]. Carcinogen exposure (primarily tobacco and alcohol), which mostly cause genomic damage, and infection with high-risk human papillomaviruses (HPV) are considered as the major etiological factors of HNSCC. The standard therapies for these tumors include surgery, radiation, chemotherapy, or combinations thereof, although combination therapy is associated with greater toxicity and no clear increment in overall survival (OS) [2, 3]. Despite multimodal therapeutic approaches, a significant proportion of HNSCC patients develops recurrence and metastatic disease [2]. For this reason, molecularly targeted drugs have become very important additions to the therapeutic toolkit, in an effort to improve clinical outcomes. In this article, rather than provide an exhaustive review of all current and developing therapeutic approaches, we will focus on molecular mechanisms that deregulate growth factor signaling and cell cycle progression and outline some of the tactics that have been employed by way of personalized therapies to combat the altered biochemistry in HNSCC as pertinent to these processes.
EGFR/PI3K/AKT/mTOR pathway
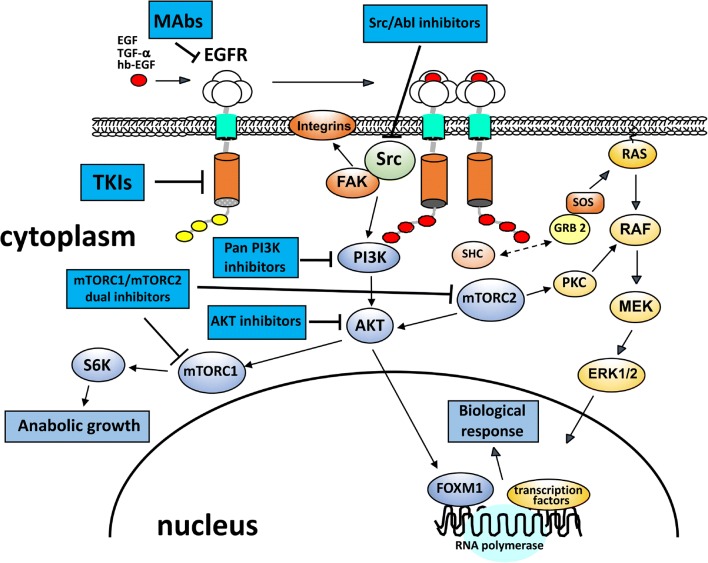
A wide range of candidate biomarkers may be useful in predicting the development of head and neck squamous cell carcinoma (HNSCC), and aberrantly functioning pathway components can be considered as feasible targetable options for therapy. One of the most frequently altered signaling pathways in HNSCC is the epidermal growth factor receptor (EGFR)/phosphoinositide 3-kinase (PI3K)/AKT/mTOR cascade [4] (Fig. 1). EGFR is a transmembrane protein tyrosine kinase and the prototype member of the ERBB family, and it serves as a receptor for a variety of polypeptide ligands such as epidermal growth factor (EGF), transforming growth factor alpha (TGFα), heparin-binding EGF-like growth factor (HBEGF), amphiregulin (AREG), epiregulin (EREG), betacellulin (BTC), and neuregulins (NRGs). EGFR activation leads to phosphorylation and activation of downstream signaling mediators such as the PI3K/AKT pathway, MEK-ERK pathway, phospholipase C signaling, or signal transducers and activators of transcription (STATs), promoting proliferation, survival, angiogenesis, invasion, and adhesion [5–8]. It is reported that EGFR is overexpressed in more than 90% of HNSCC and contributes to tumor cell invasion and metastasis [9]. Moreover, it is markedly increased in cells that develop from dysplasia to SCC and frequently indicates a poor clinical outcome [10]. One reason for EGFR overexpression in cell lines and primary head and neck tumors is chromosomal amplification [11], and amplification with overexpression of EGFR has also been detected in around 60% of larynx tumors, again correlated with poor prognosis [12]. In addition, mutations in EGFR can lead to overexpression of this receptor. Four mutations in the tyrosine kinase domain of EGFR were identified in HNSCC, in exon 18 (resulting in G719R and V726M substitutions), exon 19 (L730V), and exon 21 (G824D) [13]. Moreover, mutations in the same domain have been detected in non-small cell lung cancer (NSCLC), in exon 18 (G719S), exon 19 (in-frame deletions), and exon 21 (L858R) [14–17]. However, mutations that activate the EGFR kinase activity are relatively rare in HNSCC [18]. Src, the cellular homolog of the Rous sarcoma virus oncoprotein, is a non-receptor tyrosine kinase that is involved in modulating cell signaling downstream of multiple receptors, including EGFR family members, and it participates in the regulation of cell proliferation, migration, adhesion, and apoptosis [19]. Activation of Src-dependent pathways has been reported in many human cancers including lung, colon, and head and neck [20]. Consistent with its role in regulation of proliferation and motility, substrates for Src kinase activity include focal adhesion kinase (FAK), PI3K, STAT3 [21], and EPS8 (EGFR pathway substrate 8) [22]. Src kinase activity also potentiates EGFR signaling, with Src-dependent EGFR phosphorylation and complex formation between Src and EGFR [23]. Thus, Src activity might promote resistance to EGFR-targeted personalized therapies, either through independent activation or association with other receptors. Therefore, determination of Src kinase activity may be key to predicting the potential for a positive clinical response with targeted therapeutics (Table 1).
Fig. 1.
Therapies targeting EGFR signaling pathways. Upon ligand binding, receptor tyrosine kinases (RTKs), such as EGFR, are activated and autophosphorylated. This creates docking sites for the adaptor proteins, GRB2 and SOS1, which recruit RAS and PI3K, leading to the activation of major signaling pathways that include RAS/ERK and PI3K/AKT. These pathways are involved in regulating multiple biological functions, such as cell proliferation, invasion, adhesion, and survival. Two main strategies are available for EGFR kinase inhibition: a monoclonal antibodies (mAbs) that act on the extracellular domain of the receptor, blocking EGFR ligand binding; and b small-molecule tyrosine kinase inhibitors (TKIs) that compete with ATP for binding to the kinase domain of the receptor. Several inhibitors targeting PI3K/AKT signaling and Src kinase are indicated
Table1.
Targeted therapeutics in HNSCC
| Pathway/mechanism | Target molecule | Targeted therapeutic | Clinical effects in HNSCC | References |
|---|---|---|---|---|
| EGFR/PI3K/AKT/mTOR signaling | EGFR | Cetuximab | Active and well-tolerated in platinum-resistant RM-HNSCC; improved response in combination with cisplatin; no improvement when added to radiation/cisplatin; enhanced locoregional control in combination with radiotherapy compared to RT alone; well-tolerated in combination with bevacizumab or pembrolizumab; robust response and enhanced PFS when combined with palbociclib in platinum-resistant HNSCC | [24–32] |
| EGFR/PI3K/AKT/mTOR signaling | EGFR | Erlotinib, gefitinib | No significant benefit from erlotinib + everolimus in platinum-resistant HNSCC; no improvement in OS with gefitinib compared with methotrexate | [33, 34] |
| EGFR/PI3K/AKT/mTOR signaling | EGFR | Afatinib | Anti-tumor effects similar to cetuximab; sustained benefit with sequential afatinib/cetuximab therapy; modest increase in PFS compared with methotrexate | [35, 36] |
| EGFR/PI3K/AKT/mTOR signaling | EGFR | Osimertinib (AZD9291) | Resistance due to PIK3CA mutation (lung cancer); effect in HNSCC unknown | [37, 38] |
| EGFR/PI3K/AKT/mTOR signaling | PI3K | buparlisib (BKM120) | Modest increase in PFS in combination with paclitaxel; may be useful second-line therapy in platinum-refractory RM-HNSCC | [39, 40] |
| EGFR/PI3K/AKT/mTOR signaling | PI3K | Copanlisib | Clinical effect in HNSCC unknown | [41, 42] |
| EGFR/PI3K/AKT/mTOR signaling | mTOR | Rapamycin (sirolimus) everolimus temsirolimus | No significant benefit from erlotinib + everolimus in platinum-resistant HNSCC; partial response in 25% of patients when temsirolimus combined with cetuximab and bevacizumab; PFS 8.15 months for everolimus combined with cetuximab and carboplatin | [33, 43, 44] |
| EGFR/PI3K/AKT/mTOR signaling | Src | Dasatinib (BMS-354825) | No significant activity in advanced HNSCC; no effect alone, and no additive effect in combination with erlotinib | [45–47] |
| EGFR/PI3K/AKT/mTOR signaling | Src | Saracatinib (AZD-0530) | No efficacy as a single agent in RM-HNSCC | [48] |
| Cell cycle | Aurora kinases |
ENMD2076 AZD1152 AMG900 MLN9237 |
MLN9237 (alisertib) well-tolerated, partial response in HNSCC | [49–55] |
| Cell cycle | CDKs | Dinaciclib | No complete or partial anti-tumor responses observed | [56, 57] |
| Cell cycle | CDKs (G1-specific) | Palbociclib | Robust response and enhanced PFS when combined with cetuximab in platinum-resistant HNSCC | [58] |
| Cell cycle/DNA damage checkpoint | Mutant p53 | Styrylquinazoline (CP-31398) | Clinical effect in HNSCC unknown | [59–61] |
| Cell cycle/DNA damage checkpoint | Mutant p53 | MIRA compounds | Clinical effect in HNSCC unknown | [62–65] |
| Cell cycle/DNA damage checkpoint | Mutant p53 | p53 peptide vaccine | 88% DFS at 2 years; good safety | [66] |
| Cell cycle/DNA damage checkpoint | MDM2/p53 | RITA | Clinical effect in HNSCC unknown | [67, 68] |
| G2/M checkpoint | Protein Kinase C | UCN-01 | Clinical effect in HNSCC unknown | [62] |
| G2/M checkpoint | Polo-like Kinase | BI-2536 | Clinical effect in HNSCC unknown | [62] |
Selected agents that target the pathways discussed in this article are listed
DFS disease-free survival, OS overall survival, PFS progression-free survival, RM-HNSCC recurrent-metastatic head and neck squamous cell carcinoma, RT radiotherapy. Additional information on targeted therapeutics under clinical investigation for HNSCC can be found at https://clinicaltrials.gov. Many ongoing and even completed trials lack reported data
The PI3K/AKT signaling pathway is of particular importance in HNSCC, and it is frequently activated in this disease. Overexpression or mutation of the mediators of this pathway can lead to increased cellular growth, survival, and invasion, thus contributing to tumor progression and metastasis [69]. These mutational events have been reported to be associated with advanced stage HNSCC [70] and, of these, PIK3CA is the most commonly mutated component of this pathway, both in HPV-positive and -negative disease (56% and 34%, respectively) [71]. In particular, it is frequently related to increased tumor growth in advanced stage disease [72]. The hotspot mutation sites in the PIK3CA gene, encoding the alpha catalytic subunit of PI3K, include H1047R/L in the kinase domain (exon 20) and E545K/G and E542K in the helical domain (exon 9) [73, 74]. In addition to oncogenic activation of pathway components, the failure to restrain signal transduction is also of critical importance in tumorigenesis. The tumor suppressor PTEN is a dual-specificity phosphatase that acts as a negative regulator of the PI3K pathway, controlling cell growth and survival. Deletions or somatic mutations of the PTEN gene are common in different tumor types, including breast [75], thyroid [76], prostate [77], lung [78], melanoma [79], and lymphoma [80]. PTEN mutations have been reported in up to 10% of HNSCC, implying that this is a minor contribution to PI3K pathway activation [81], so perhaps of limited value as an indicator of pathway deregulation in this disease. Other important players in this pathway are the serine-threonine protein kinases of the AKT family, of which there are three isoforms (AKT1–3). AKT activation is regulated by 3-phosphoinositide-dependent protein kinase-1 (PDK1), leading to enhanced cell survival and proliferation, mediated through phosphorylation of various substrates such as FOXO1, glycogen synthase kinase 3 beta (GSK3β), BAD, and IKKα [82, 83]. It is reported that the genes encoding AKT family members display copy number variations, increased expression, and missense mutations in human cancers, including HNSCC [83–85]. E17K activating mutations of AKT1 have been found in a number of solid tumors [74, 84, 86]. However, the E17K mutation has not been reported so far in HNSCC [86, 87] and it may be that PI3K mutation is a more frequent mechanism of activation.
Targeting EGFR/PI3K/AKT/mTOR signaling for personalized treatment of HNSCC
A variety of strategies to block the function of EGFR have been developed as personalized approaches to inhibit tumor growth and metastasis, and some are currently in clinical use or undergoing investigation. These can be broadly divided into monoclonal antibodies (mAbs) directed at the extracellular domain of the receptor and small molecule inhibitors of the receptor’s kinase activity. Cetuximab, a humanized mouse anti-EGFR IgG1 monoclonal antibody, has been widely studied, both in patients with locoregionally advanced (LA) disease and in those with recurrent and/or metastatic (RM) HNSCC. In an open-label multicenter phase II study, the efficacy and safety of cetuximab as a monotherapy for patients with advanced platinum-refractory HNSCC were evaluated [24]. Cetuximab alone provided a 13% response rate over a 6-week treatment cycle, a disease control rate of 46%, and overall good tolerance of the agent. In other studies, addition of cetuximab to cisplatin chemotherapy was found to improve the response rate significantly for RM-HNSCC patients who had not received any prior chemotherapy. However, progression-free survival (PFS) and overall survival (OS) did not differ significantly among the groups [25]. A recent study investigated the safety of a combined regimen of cetuximab and an immune checkpoint inhibitor, pembrolizumab [26]. Initial reports indicate good tolerance of the combination, although clinical efficacy remains to be tested.
Small molecule tyrosine kinase inhibitors (TKIs), which bind to the cytoplasmic region of EGFR, have been tested extensively in HNSCC treatment protocols. First-generation compounds, such as erlotinib and gefitinib, have a minimal clinical impact on HNSCC when used alone. Combination therapy, in which erlotinib and the mTOR inhibitor, everolimus, were used concurrently, resulted in a 3% response rate after 4 weeks, with median overall survival a little over 10 months [33]. Afatinib, a second-generation TKI that acts as an irreversible ERBB-family blocker, showed efficacy in a phase II study with RM-HNSCC patients following failed platinum-based therapy [35], while another study showed that afatinib can produce improvements in PFS in patients with recurrent or metastatic HNSCC following platinum-based therapy [36]. Although promising results have been observed using reversible TKIs in preclinical and clinical studies, and OS has been improved, their efficacy as a monotherapy remains unsatisfactory [34, 88], likely due to the development of resistance as a result of upregulation of alternative intracellular pathways. Third-generation TKIs have been developed in an attempt to overcome some of the limitations of earlier agents. Osimertinib (AZD9291) is active against a number of EGFR mutants (including the T790M resistance mutation) and has shown some promise in the treatment of lung cancer [37], although resistance even to this has now been documented as a result of additional EGFR mutations and activation of other pathways. Perhaps of particular relevance to HNSCC is the role of PIK3CA mutation as a means of osimertinib resistance [38]. As yet, no data are available on its clinical activity against HNSCC.
Agents that target Src, such as dasatinib (BMS-354825) and saracatinib (AZD-0530), have been evaluated in preclinical studies and have also been tested for clinical utility in HNSCC. Both compounds are dual inhibitors of Src and Abl tyrosine kinases; however, saracatinib is more active against Src than Abl compared to dasatinib [89, 90]. In preclinical models, dasatinib was found to inhibit migration and invasion and induce apoptosis in NSCLC and HNSCC [45]. However, in phase II studies for patients with recurrent or metastatic HNSCC, both dasatinib and saracatinib as monotherapies failed to elicit any significant clinical response [46, 48]. Moreover, the combination of dasatinib with erlotinib in patients with operable stage II-IVa HNSCC did not add any therapeutic benefit compared to erlotinib therapy alone [47].
As PI3K signaling is hyperactivated in many types of cancers and implicated in tumor progression, this pathway is considered as an attractive target for treatment of HNSCC [91]. Multiple drugs have been designed to target the PI3K/AKT/mTOR axis, making it the most targeted pathway for human cancer treatment. Of these drugs, buparlisib (BKM120) is an oral PI3K inhibitor that inhibits the activity of all four p110 isoforms of class I PI3K [39]. In a recent randomized phase II trial, addition of buparlisib to paclitaxel improved PFS of HNSCC patients, although there was no significant improvement in OS [40]. Another PI3K inhibitor, copanlisib, is a highly selective and potent intravenous agent with an IC50 against p110α and p110δ isoforms in the sub-nanomolar range [41]. Phase I trials of copanlisib in patients with advanced solid tumors demonstrated good patient tolerance and some evidence of disease control [42]. Other drugs have been developed to target mTOR, including sirolimus (rapamycin) and its analogs. In clinical trials, targeting of mTOR with temsirolimus together with bevacizumab and cetuximab was effective in achieving partial responses in two of eight patients with HNSCC, although concerns with toxicity were noted [43].
Cell cycle mediators
Control of cell division is one of the important cellular processes that is deregulated in HNSCC, leading to markedly enhanced cell growth. One of the important cell cycle mediators is forkhead box M1 (FOXM1), a transcription factor downstream of the EGFR/PI3K/AKT cascade (Fig. 1), and which functions as a master regulator of the cell cycle by influencing the transitions from G1 to S-phase and G2 to M [92]. FOXM1 also controls cell survival, apoptosis, migration, and angiogenesis [93]. It has been reported previously that FOXM1 is involved in HNSCC initiation and progression [94, 95]. Furthermore, FOXM1 overexpression contributes to tumorigenesis by inducing expansion of the epithelial stem/progenitor cell compartment [96]. Therefore, FOXM1 could be a good therapeutic target for HNSCC. Although transcription factors have previously been considered “undruggable”, strategies are being developed to circumvent this [97]. Moreover, aurora kinases A and B (AURKA, AURKB) are two cell cycle regulators under the control of FOXM1 [98, 99]. These kinases control the structure and function of the cytoskeleton and chromosomes, and the interactions between these at the kinetochore. It is reported that AURKA is overexpressed in up to 90% of HNSCC and contributes to tumor progression and metastatic spread [100–102]. EGFR signaling induces AURKA overexpression by increasing efficiency of AURKA translation, and by increasing its transcription through cooperative interaction with STAT5 and binding to the AURKA promoter [103, 104]. Thus, given the frequency of deregulated EGFR signaling in HNSCC, AURKA could be an attractive option for targeted therapy.
G1-phase regulators are also altered in HNSCC by a number of different mechanisms, including viral oncoprotein-induced degradation, mutation, deletion, and amplification [105]. Cyclin D1 is encoded by the CCND1 gene, located on human chromosome 11q13. Complexes consisting of cyclin D1 with CDK4 or CDK6 drive cell cycle progression through the G1 phase by phosphorylation of multiple substrates (Fig. 2), the most well-studied being the retinoblastoma protein (pRB) [106]. Abnormalities in cyclin D1 expression may result from genomic inversion [107], translocation [108], gene amplification [109], or upregulated transcriptional activity, and it has been reported that the chromosomal region where CCND1 is located shows gain or amplification in patients with HPV-negative HNSCC [110]. Overexpression of cyclin D1 leads to enhanced cell growth and tumorigenesis [111] and is associated with recurrence [112], lymph node metastasis [113], and poor prognosis [114]. Alterations in negative regulators of CDKs—cyclin-dependent kinase inhibitors—are discussed below in the context of tumor suppression.
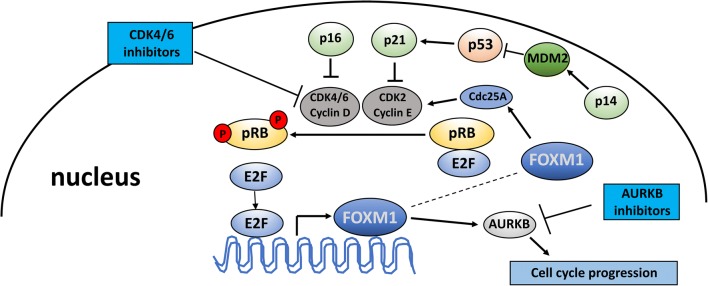
Fig. 2.
Therapies targeting the cell cycle machinery. p16 (INK4A) and p14 (ARF) are both transcribed from the CDKN2A gene. p16 regulates cell-cycle progression by blocking CDK4/6 activity. p14 inhibits MDM2-mediated p53 degradation, thus inducing p21 expression, which then blocks CDK2/cyclin E. CDK4/6–cyclin D complexes can directly regulate the transcription factor FOXM1 that modulates expression of a range of genes such as cyclin B, survivin, cdc25b phosphatase, and AURKB that are important for the G2–M transition. AURKB inhibitors block the activity of AURKB, while CDK4/6 inhibitors specifically target CDK4/6 activity, slowing or arresting cell cycle progression. CDK, cyclin-dependent kinase; FOXM1, forkhead box protein M1; RB, retinoblastoma protein; E2F, E2F transcription factor; AURKB, Aurora kinase B; p, phosphorylated
Targeting cell cycle kinases for personalized HNSCC therapy
As important cell cycle regulators, aurora kinases are credible targets in multiple malignancies, including HNSCC [101, 115–118], and several aurora kinase inhibitors have been developed. In preclinical evaluation studies, the aurora kinase A and B inhibitors ENMD2076 and AZD1152, and pan-aurora agents such as AMG900, were found to induce growth arrest and apoptosis [49–51]. Other compounds have progressed towards clinical trials, such as MLN8237 (alisertib), an aurora-A-specific inhibitor [52], which was shown to be well-tolerated in combination with docetaxel. This was further confirmed in a single-agent phase I study [53]. In a phase I/II study, the use of MLN8237 as a single agent was evaluated in multiple solid tumors including HNSCC and it was found to produce a partial response in three out of twenty patients [54]. However, in a phase II study, only 9% of HNSCC patients showed a partial response, with overall serious side effects noted in 43% of the study cohort [55]. Such a low success rate would seem to reinforce the need to stratify patient cohorts for personalized intervention, with greater emphasis on targeting patients whose tumors show elevated kinase activity and, thus, may sustain a more robust clinical response. In addition to aurora kinase inhibitors, multiple CDK inhibitors have been developed or are currently under development. Dinaciclib is a multi-CDK inhibitor that inhibits the activities of CDK1, CDK2, CDK5, and CDK9. This compound advanced into phase III clinical trials for the treatment of refractory chronic lymphocytic leukemia [119] although the interaction of dinaciclib with bromodomains could limit its use [120]. The limitations of broad-range CDK inhibitors have focused efforts toward the development of compounds that selectively block smaller subsets of CDKs. Of these, palbociclib is a highly specific inhibitor of G1-specific kinases CDK4 and CDK6, having no activity against 39 other protein kinases representative of most of the primary protein kinase families [121, 122]. A phase II trial showed the efficacy of the combination of palbociclib and cetuximab in treating platinum-resistant recurrent/metastatic HNSCC patients [58], with an anti-tumor response in 35% of patients. PFS of 6.4 months and OS of 12.1 months were indicative of a robust response for this combination. To date, results from preclinical and clinical studies indicate that monotherapy might not be the best application of anti-CDK therapeutics [56, 123].
Tumor suppressors
Related to cell cycle control, discussed above, almost 70% of HNSCCs show loss of heterozygosity (LOH) at chromosome 9p21, where the tumor suppressor genes CDKN2A (p16/INK4A/MTS1) and CDKN2B (p15/INK4B) are located [124–126]. The protein product of the CDKN2A gene, p16, is a negative regulator of G1 CDKs, therefore acting as an inhibitor of G1 progression [127]. It has been reported previously that p16 is overexpressed and has been used as a biomarker for, HPV-positive HNSCCs and cervical cancers [128, 129], thus providing some indication of clinical outcome as HPV-positive tumors tend to respond better to chemoradiotherapy [27]. However, approximately 90% of HNSCC exhibit loss of p16 function, frequently as a result of homozygous deletion [130], intragenic mutation [131], or promoter methylation [132]. Indeed, CDKN2A promoter hypermethylation has been reported in around 60–66% of oral squamous cell carcinomas and oral leukoplakias [133, 134]. There is a relationship between CDKN2A methylation and tobacco use, but not with the use of alcohol [135], with longer exposure and heavier use increasing the chance of promoter hypermethylation. Additionally, up to 15% of smokers show hypermethylation of the CDKN2A promoter in the absence of cancer or premalignant lesions [135]. It is not known if the methylation status is predictive for future disease development, although it is likely that modification of this exposure (i.e., smoking cessation) may prevent or limit the chance of malignant change. The related CDK inhibitor, p15, also represses G1 CDK function and is a mediator of the anti-proliferative effects of transforming growth factor β (TGF-β) [136]. Inactivation of p15 by promoter hypermethylation or homozygous deletion has been observed in many human malignancies, including HNSCC, suggesting that its down-regulation may also be important during neoplastic transformation [137, 138] and may underpin, at least in part, the loss of TGF-β responsiveness seen in some HNSCCs [139–141].
In addition to these important cell cycle regulators, the p53 tumor suppressor protein (Fig. 3) has pivotal functions in restraining cell cycle progression and triggering apoptosis in response to genotoxic stress, as well as inhibiting aerobic glycolysis and promoting mitochondrial respiration through multiple mechanisms [142]. It has been shown previously that p53 negatively regulates expression of the FOXM1 transcription factor and can bind directly to, and inactivate, AURKA [143, 144], in addition to other well-established mechanisms that include upregulation of the cell cycle inhibitors p21 (WAF1/Cip!) and p15. The gene encoding p53 is frequently mutated in multiple cancer types and impacts up to 80% of HNSCC cases [145–147]. Many p53 mutations in HNSCC are missense mutations, occurring within the central region of the p53 DNA-binding domain, resulting in amino acid substitutions at codons 175, 245, 248, 249, 273, and 282, which are recognized as hotspot mutation sites in HNSCC [148]. These missense mutations lead to expression of a full-length aberrant p53 protein with a prolonged half-life, which can act in a dominant-negative manner to inhibit wild-type p53 activity [148]. However, in most cases co-expression of aberrant and wild-type p53 is not found, as the most frequent mode of loss is deletion of one allele and mutation of the second. Thus, many aberrant p53 proteins act as gain-of-function (GOF) dominant-transforming oncoproteins that can promote tumorigenesis, metastasis, and drug resistance [149], in contrast to the anti-tumorigenic function of the wild-type protein.
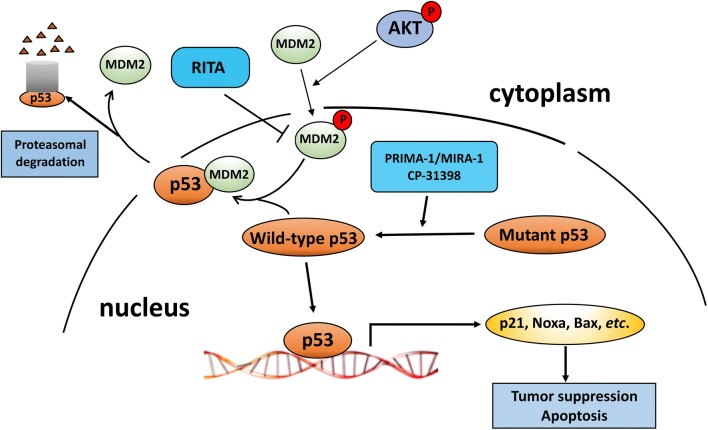
Fig. 3.
Therapies targeting p53 functions. p53 is stabilized and accumulates upon environmental stresses such as DNA damage or oncogene activation, resulting in cell cycle arrest or cell death. AKT activation through the PI3K pathway can phosphorylate cytoplasmic MDM2, resulting in its nuclear import. Nuclear MDM2 ubiquitinates p53 and targets it for proteasomal degradation in the cytoplasm. RITA binds to p53 and interferes with the interaction of MDM2 and p53, enabling activation of p53. Agents such as PRIMA-1 restore the ability of mutant p53 to bind to the p53 transcriptional response elements of target genes and CP-31398 stabilizes the DNA binding domain of mutant and wild-type p53
Targeting p53 for HNSCC treatment
Different therapeutic approaches have been developed to target mutant p53, through depletion of mutant p53 to restoration the wild-type p53 transcriptional activity, as well as inhibition of downstream targets involved in mediating mutant p53 GOF. Inherent to such approaches is a need to identify P53 sequence alterations in order to tailor treatment options for each patient. CP-31398 (Styrylquinazoline) is an example of a therapeutic that restores wild-type p53 conformation and inhibits growth of cancer cells containing GOF p53 mutants [59–61]. In addition to p53-dependent anticancer effects, it also induces p53-independent cell death through generation of free radicals [62, 63, 150]. Other examples of agents that aim to refold mutant p53 into an active conformation include the MIRA compounds (MIRA-1/NSC19630 and analogs), which also produce an increase in expression of p53 downstream target genes such as p21 and inhibit cancer cell proliferation [62–65]. Moreover, a small molecule, RITA (reactivation of p53 and induction of tumor cell apoptosis), blocks the interaction between MDM2 and p53, increasing nuclear p53 levels [67] and enhancing cisplatin-induced growth inhibition and apoptosis of HNSCC cells [68]. However, while this may be attractive and a useful approach for tumors that retain wild-type p53, its applicability in HNSCC therapeutics is likely limited. In a phase I clinical study, adjuvant dendritic cell-based vaccination against p53 has been shown to improve disease-free survival for HNSCC patients [66]. Furthermore, treatment with G2/M checkpoint blocking agents, such as the protein kinase C inhibitor UCN-01 and the polo-like kinase (PLK) inhibitor BI-2536, abrogates the G2/M checkpoint induced by DNA damage, resulting in a much-improved cytotoxic response in cancer cells containing mutant p53 [62]. Thus, identification of P53 mutations in tumors may be a useful means of predicting which lesions are more likely to respond to G2/M checkpoint inactivators.
Combining therapeutics for treatment of HNSCC
Despite advanced knowledge of the role of molecular and genetic alterations in the pathogenesis and clinical course of HNSCC, these tumors remain one of the most significant causes of mortality from malignant disease worldwide. Early stage lesions have a high chance of cure with local treatment alone, but for patients who present with more advanced disease, the risk of recurrence or distant metastatic spread is greater than 50% [151]. The complexity of disease is magnified by the heterogeneity of organs involved and their diverse functions; therefore, a multimodal approach is required when considering therapy. Standard therapeutic modalities for patients with HNSCC are non-selective, cause damage to normal tissue, and may be associated with systemic toxicity through the use of a variety of cytotoxic drugs (for example, platinum agents and 5-fluorouracil). As discussed above, new molecular targets have been identified in HNSCC as playing key roles in tumor proliferation and metastasis; however, the ability to target many of these for clinical benefit in HNSCC remains elusive. The first target approved for molecular-based therapy in HNSCC was EGFR, and several other targeted therapies are under development or in clinical trials. Over a decade ago, a survival benefit was shown in patients with locally advanced HNSCC treated with the anti-EGFR antibody cetuximab combined with radiotherapy [28]. Moreover, in phase III randomized trials, the combination of cetuximab with cisplatin in recurrent or metastatic HNSCC significantly improved the overall response rate when compared to cisplatin alone [25]. However, despite some improvement in treatment results obtained by combining cetuximab with chemoradiotherapy for patients with locally advanced HNSCC, PFS and OS were not significantly improved, particularly in patients with stage III and IV HNSCC [25, 27]. A major issue is the development of resistance mechanisms that enable the tumor cells to subvert the effects of a particular targeted therapy. As an example, primary resistance to EGFR tyrosine kinase inhibitor therapy in lung cancer patients is reportedly due to amplification of MET, the gene encoding another tyrosine kinase, in this case the receptor for hepatocyte growth factor (HGF) [152]. Thus, dual targeting may provide a more effective strategy for optimizing cancer treatment and improving clinical benefits compared to monotherapies. For example, sunitinib, a small molecule kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR) and the stem cell factor receptor (KIT), demonstrated poor activity in the treatment of HNSCC as a monotherapy [153, 154]. However, the combination of cetuximab with sunitinib causes a reduction in tumor cell proliferation in a preclinical head and neck cancer model [29], while sunitinib plus irradiation has been tested in patients with oligometastatic disease from a number of solid tumors, including HNSCC, and has shown a degree of efficacy [30]. The CDK inhibitor, palbociclib, was tested in phase I trials in combination with cetuximab for treatment of HNSCC, and positive tumor responses were observed in cetuximab-resistant disease [31]. Adding cetuximab together with the VEGFR inhibitor bevacizumab produced a response rate of 16% and a disease control rate of 73% [32]. Initial (phase I) studies [44] of carboplatin, together with cetuximab and the mTOR inhibitor everolimus, indicated some positive outcome. In contrast, treatment with everolimus plus erlotinib resulted in no significant benefit in patients with platinum-resistant tumors [33]. Hence, while some multi-targeted approaches may provide improved clinical outcomes, other combination therapies face challenges in terms of clinical response and toxic side effects [155].
Conclusions and expert recommendations
With the advent of cutting edge technologies that give us the ability to interrogate cancers and potentially premalignant lesions on multiple levels, there are now significant opportunities available to identify biomarkers that could help predict whether a particular lesion in the oral cavity is likely to develop into overt malignancy. Development of oral cancer typically progresses through a series of dysplastic lesions of increasing severity. To a certain extent, these may be reversible when the microenvironmental conditions are changed (for example, if the patient stops smoking—in other words, altering modifiable risk factors). However, it is not known, at a molecular level, what determines if a dysplasia is reversible or not. Furthermore, although oral leukoplakias are examples of potentially premalignant lesions, the transformation rate is low and most of these do not develop into cancer. Thus, if a predictive molecular signature were available to discriminate between those lesions likely to progress and those having a more benign course, there would be considerable benefit in terms of cost saving, clinical time for follow-up, and patient reassurance. In this regard, the use of “omics” approaches [156] to compare the genomic, transcriptomic, proteomic, and metabolomic profiles of potentially premalignant lesions that have developed into malignant disease with those that have reverted is now possible to aid primary prevention, given the sensitivity of the methodologies and their application to archival clinical specimens. Secondary prevention can be carried out using similar approaches, in which the propensity of an established tumor to metastasize, or to respond adequately to a specific therapy, is based on its expression profile, or metabolic signature, and so forth. Although these analyses need to be comprehensive and applied to large sample numbers, investment of time and resources is likely to be worthwhile, as personalized regimens for patient care will be possible in terms of determining which potenially premalignant lesions will progress and, as such, require early intervention and/or follow-up; whether malignant lesions are likely to metastasize and, therefore, may require a more radical therapeutic approach; and also determining the treatment regimen likely to provide the best clinical outcome. In fact, some progress has been made with the use of salivary metabolites that discriminate between persistent suspicious oral mucosal lesions and cancer and/or dysplasia [157]. Indeed, the use of liquid biopsy approaches and, in particular, the utility of saliva, salivary exosomes, and salivary extracellular RNA are all under intense investigation and development [158–161]. A recent study [162] reported that saliva is enriched for tumor DNA from patients with oral cavity tumors and this was present and detectable following surgical excision of the primary lesion prior to clinical recurrence, indicating the potential utility of this approach in terms of preventing relapse. Of particular relevance to this review article, mutations in PIK3CA, CDKN2A, and P53 were identified using this approach.
Within the oral cavity, SCC may arise at a number of sites, such as floor of mouth, lateral border of tongue, retromolar trigone, and other areas. Whereas HPV is primarily associated with oropharyngeal cancers and a better clinical outcome, the dysregulated networks that are responsible for driving malignancy at particular oral sites (in non-HPV-related tumors) remain largely unexplored. It may be that, at the molecular level, there are no differences. However, in-depth analysis using the omics platforms would help to address this question and may afford clues as to why disease development may be more common at some sites than others, or why tumors at particular sites may be associated with a worse prognosis. The study by Wang et al. [162] indicated that tumor cell DNA was detecable in saliva from patients with lesions that had arisen at different locations; thus, this approach is likely to be applicable to determining site-dependent differences in tumor development. Pathways identified as being deregulated could then be targeted with appropriate therapeutics.
Our enhanced understanding of the mechanisms that underpin HNSCC development, progression, and response as a result of comprehensive genomic analyses [163, 164] has already informed new, rational, personalizable therapeutic approaches with the potential to make a tangible difference in clinical outcome for HNSCC patients, both in longevity and quality. For example, a recent phase III study identified patient cohorts that could derive increased benefit from afatinib therapy, based on a number of biomarkers present or absent in their tumors [165]. While targeted monotherapies, though attractive in themselves, are frequently associated with development of resistant phenotypes and/or lack of clinical response, their use in multimodal approaches holds some significant promise for the future. However, the lack of a robust clinical response to targeted therapies in many cases remains a major roadblock, in spite of the new drugs that are available based upon our understanding of the genetics, biology, and biochemistry related to tumor development and progression. There is a pressing need to obtain and employ this basic information for each patient, in order to predict how a tumor will respond upon exposure to a specific agent and, hence, truly personalize HNSCC cancer therapy for each patient and achieve a positive outcome. Thus, stratification of patients based on genetic and biochemical parameters would seem to be crucial. In this regard, next-generation sequencing of patients’ tumors is undertaken at a number of centers to identify putative targets, guide personalized therapeutic approaches, and to predict disease outcome. Moreover, knowledge gained from previous trials will enable us to determine what additional mutations may arise as the result of implementing the initial treatment modality, and to block multiple downstream pathways and thus prevent treatment relapse and tumor recurrence, major clinical concerns in HNSCC. However, it is important to realize that mutiple contributors influence what may be termed the personalized phenome [166], including metabolomic and proteomic components that are modified by both extrinsic and instrinsic environmental factors, as well as being under genomic and epigenetic control, and that these must also be taken into consideration when developing personalized medicine strategies.
Funding
R01-DE024381.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13(1):35–46. doi: 10.1007/s11864-011-0176-y. [DOI] [PubMed] [Google Scholar]
- 3.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3305–3313. doi: 10.1200/JCO.2015.62.0963. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 5.Rubin Grandis J, Zeng Q, Drenning SD. Epidermal growth factor receptor--mediated stat3 signaling blocks apoptosis in head and neck cancer. Laryngoscope. 2000;110(5 Pt 1):868–874. doi: 10.1097/00005537-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest. 1998;102(7):1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Gupta K, Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994;124(4):547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92(8):1341–8. doi: 10.1038/sj.bjc.6602510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, Rimm D, Burtness BA. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11(16):5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 11.Gollin SM. Chromosomal alterations in squamous cell carcinomas of the head and neck: window to the biology of disease. Head Neck. 2001;23(3):238–253. doi: 10.1002/1097-0347(200103)23:3<238::aid-hed1025>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Miyaguchi M, Olofsson J, Hellquist HB. Expression of epidermal growth factor receptor in laryngeal dysplasia and carcinoma. Acta Otolaryngol. 1990;110(3–4):309–313. doi: 10.3109/00016489009122553. [DOI] [PubMed] [Google Scholar]
- 13.Nagalakshmi K, Jamil K, Pingali U, Reddy MV, Attili SSV. Epidermal growth factor receptor (EGFR) mutations as biomarker for head and neck squamous cell carcinomas (HNSCC) Biomarkers. 2014;19(3):198–206. doi: 10.3109/1354750X.2014.895852. [DOI] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. [DOI] [PubMed]
- 16.Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Shibata T, Sakiyama T, Yoshida T, Tamura T. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(28):6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 17.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Soung YH, Kim SY, Nam HK, Park WS, Nam SW, Kim MS, Sun DI, Lee YS, Jang JJ, Lee JY, Yoo NJ, Lee SH. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(8):2879–2882. doi: 10.1158/1078-0432.CCR-04-2029. [DOI] [PubMed] [Google Scholar]
- 19.Frame MC. Newest findings on the oldest oncogene; how activated src does it. J Cell Sci. 2004;117(Pt 7):989–998. doi: 10.1242/jcs.01111. [DOI] [PubMed] [Google Scholar]
- 20.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22(4):337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 21.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham DL, Creese AJ, Auciello G, Sweet SMM, Tatar T, Rappoport JZ, Grant MM, Heath JK. Novel binding partners and differentially regulated phosphorylation sites clarify Eps8 as a multi-functional adaptor. PLoS One. 2013;8(4):e61513. doi: 10.1371/journal.pone.0061513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92(15):6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 25.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology Group Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an eastern cooperative oncology group study. J Clin Oncol. 2005;23(34):8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 26.Sacco AG, Messer K, Natsuhara A, Chen R, Wong DJL, Wordenet FP, et al. An open-label, non-randomized, multi-arm, phase II trial evaluating pembrolizumab combined with cetuximab in patients with recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): Results of the interim safety analysis. J Clin Oncol. 2018;36(15_suppl):6037. [Google Scholar]
- 27.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, el-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 29.Bozec A, Sudaka A, Toussan N, Fischel JL, Etienne-Grimaldi MC, Milano G. Combination of sunitinib, cetuximab and irradiation in an orthotopic head and neck cancer model. Ann Oncol. 2009;20(10):1703–1707. doi: 10.1093/annonc/mdp070. [DOI] [PubMed] [Google Scholar]
- 30.Tong CC, Ko EC, Sung MW, Cesaretti JA, Stock RG, Packer SH, et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PLoS One. 2012;7(6):e36979. doi: 10.1371/journal.pone.0036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel L, Ley J, Wildes TM, Schaffer A, Robinson A, Chun SE, Lee W, Lewis J, Jr, Trinkaus K, Adkins D. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;58:41–48. doi: 10.1016/j.oraloncology.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argiris A, Kotsakis AP, Hoang T, Worden FP, Savvides P, Gibson MK, Gyanchandani R, Blumenschein GR, Jr, Chen HX, Grandis JR, Harari PM, Kies MS, Kim S. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24(1):220–225. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massarelli E, Lin H, Ginsberg LE, Tran HT, Lee JJ, Canales JR, Williams MD, Blumenschein GR, Jr, Lu C, Heymach JV, Kies MS, Papadimitrakopoulou V. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2015;26(7):1476–1480. doi: 10.1093/annonc/mdv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27(11):1864–71. [DOI] [PubMed]
- 35.Seiwert TY, Fayette J, Cupissol D, del Campo JM, Clement PM, Hitt R, Degardin M, Zhang W, Blackman A, Ehrnrooth E, Cohen EEW. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25(9):1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 37.Lamb YN, Scott LJ. Osimertinib: areview in T790M-positive advanced non-small cell lung Cancer. Target Oncol. 2017;12(4):555–562. doi: 10.1007/s11523-017-0519-0. [DOI] [PubMed] [Google Scholar]
- 38.Le X, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to Osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24(24):6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chene P, Shoemaker K, de Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, Garcia-Echeverria C, Sellers WR, Voliva CF. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11(2):317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 40.Soulieres D, Faivre S, Mesía R, Remenár É, Li SH, Karpenko A, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017;18(3):323–35. [DOI] [PubMed]
- 41.Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, Fracasso PR, Wilkie DP, Hentemann M, Wilhelm SM, Scott WJ, Mumberg D, Ziegelbauer K. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 42.Doi T, Fuse N, Yoshino T, Kojima T, Bando H, Miyamoto H, Kaneko M, Osada M, Ohtsu A. A phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother Pharmacol. 2017;79(1):89–98. doi: 10.1007/s00280-016-3198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Kambrick S, Fu S, Naing A, Subbiah V, Blumenschein GR, et al. Advanced malignancies treated with a combination of the VEGF inhibitor bevacizumab, anti-EGFR antibody cetuximab, and the mTOR inhibitor temsirolimus. Oncotarget. 2016;7(17):23227–38. [DOI] [PMC free article] [PubMed]
- 44.Saba NF, Hurwitz SJ, Magliocca K, Kim S, Owonikoko TK, Harvey D, Ramalingam SS, Chen Z, Rogerio J, Mendel J, Kono SA, Lewis C, Chen AY, Higgins K, el-Deiry M, Wadsworth T, Beitler JJ, Shin DM, Sun SY, Khuri FR. Phase 1 and pharmacokinetic study of everolimus in combination with cetuximab and carboplatin for recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer. 2014;120(24):3940–3951. doi: 10.1002/cncr.28965. [DOI] [PubMed] [Google Scholar]
- 45.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11(19 Pt 1):6924–32. [DOI] [PubMed]
- 46.Brooks HD, Glisson BS, Bekele BN, Johnson FM, Ginsberg LE, el-Naggar A, Culotta KS, Takebe N, Wright J, Tran HT, Papadimitrakopoulou VA. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011;117(10):2112–2119. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauman JE, Duvvuri U, Gooding WE, Rath TJ, Gross ND, Song J, et al. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight. 2017;2(6):e90449. [DOI] [PMC free article] [PubMed]
- 48.Fury MG, Baxi S, Shen R, Kelly KW, Lipson BL, Carlson D, Stambuk H, Haque S, Pfister DG. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) Anticancer Res. 2011;31(1):249–253. [PMC free article] [PubMed] [Google Scholar]
- 49.Fletcher GC, Brokx RD, Denny TA, Hembrough TA, Plum SM, Fogler WE, Sidor CF, Bray MR. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther. 2011;10(1):126–137. doi: 10.1158/1535-7163.MCT-10-0574. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Koeffler HP, Taguchi H, Yokoyama A. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110(6):2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 51.Payton M, Cheung HK, Ninniri MSS, Marinaccio C, Wayne WC, Hanestad K, Crispino JD, Juan G, Coxon A. Dual targeting of Aurora kinases with AMG 900 exhibits potent preclinical activity against acute myeloid leukemia with distinct post-mitotic outcomes. Mol Cancer Ther. 2018;17(12):2575–2585. doi: 10.1158/1535-7163.MCT-18-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graff JN, Higano CS, Hahn NM, Taylor MH, Zhang B, Zhou X, Venkatakrishnan K, Leonard EJ, Sarantopoulos J. Open-label, multicenter, phase 1 study of alisertib (MLN8237), an aurora a kinase inhibitor, with docetaxel in patients with solid tumors. Cancer. 2016;122(16):2524–2533. doi: 10.1002/cncr.30073. [DOI] [PubMed] [Google Scholar]
- 53.Falchook G, Kurzrock R, Gouw L, Hong D, McGregor KA, Zhou X, Shi H, Fingert H, Sharma S. Investigational Aurora a kinase inhibitor alisertib (MLN8237) as an enteric-coated tablet formulation in non-hematologic malignancies: phase 1 dose-escalation study. Investig New Drugs. 2014;32(6):1181–1187. doi: 10.1007/s10637-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee P, Alvarez RH, Melichar B, Adenis A, Bennouna J, Schusterbauer C, et al. Phase I/II study of the investigational aurora A kinase (AAK) inhibitor MLN8237 (alisertib) in patients (pts) with non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast cancer (BrC), head/neck cancer (H&N), and gastroesophageal (GE) adenocarcinoma: Preliminary phase II results. J Clin Oncol. 2012;30(15_suppl):3010.
- 55.Melichar B, Adenis A, Lockhart AC, Bennouna J, Dees EC, Kayaleh O, Obermannova R, DeMichele A, Zatloukal P, Zhang B, Ullmann CD, Schusterbauer C. Safety and activity of alisertib, an investigational aurora kinase a inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16(4):395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 56.Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Jou YM, Zhang D, Statkevich P, Yao SL, Zhu Y, Zhou H, Small K, Bannerji R, Edelman MJ. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer. 2014;83(2):219–223. doi: 10.1016/j.lungcan.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Mita MM, Mita AC, Moseley JL, Poon J, Small KA, Jou YM, Kirschmeier P, Zhang D, Zhu Y, Statkevich P, Sankhala KK, Sarantopoulos J, Cleary JM, Chirieac LR, Rodig SJ, Bannerji R, Shapiro GI. Phase 1 safety, pharmacokinetic and pharmacodynamic study of the cyclin-dependent kinase inhibitor dinaciclib administered every three weeks in patients with advanced malignancies. Br J Cancer. 2017;117:1258–1268. doi: 10.1038/bjc.2017.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adkins D, Oppelt PJ, Ley JC, Trinkaus K, Neupane PC, Saccoet AG, et al. Multicenter phase II trial of palbociclib, a selective cyclin dependent kinase (CDK) 4/6 inhibitor, and cetuximab in platinum-resistant HPV unrelated (−) recurrent/metastatic head and neck squamous cell carcinoma (RM HNSCC). J Clin Oncol. 2018;36(15_suppl):6008.
- 59.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22(51):8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- 60.Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117(12):3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286(5449):2507–2510. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 62.Parrales A, Iwakuma T. Targeting oncogenic mutant p53 for Cancer therapy. Front Oncol. 2015;5:288. doi: 10.3389/fonc.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80(5):724–730. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, et al. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem. 2005;280(34):30384–91. [DOI] [PubMed]
- 65.Saha MN, Chen Y, Chen MH, Chen G, Chang H. Small molecule MIRA-1 induces in vitro and in vivo anti-myeloma activity and synergizes with current anti-myeloma agents. Br J Cancer. 2014;110(9):2224–2231. doi: 10.1038/bjc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuler PJ, Harasymczuk M, Visus C, DeLeo A, Trivedi S, Lei Y, Argiris A, Gooding W, Butterfield LH, Whiteside TL, Ferris RL. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res. 2014;20(9):2433–2444. doi: 10.1158/1078-0432.CCR-13-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LGGC, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 68.Roh JL, Ko JH, Moon SJ, Ryu CH, Choi JY, Koch WM. The p53-reactivating small-molecule RITA enhances cisplatin-induced cytotoxicity and apoptosis in head and neck cancer. Cancer Lett. 2012;325(1):35–41. doi: 10.1016/j.canlet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg Å, Parsons R. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 70.Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3(7):761–9. [DOI] [PMC free article] [PubMed]
- 71.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, Brown C, Pugh TJ, Stojanov P, Cho J, Lawrence MS, Getz G, Brägelmann J, DeBoer R, Weichselbaum RR, Langerman A, Portugal L, Blair E, Stenson K, Lingen MW, Cohen EEW, Vokes EE, White KP, Hammerman PS. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isaacsson Velho PH, Castro G, Jr, Chung CH. Targeting the PI3K Pathway in Head and Neck Squamous Cell Carcinoma. Am Soc Clin Oncol Educ Book. 2015;35:123–128. doi: 10.14694/EdBook_AM.2015.35.123. [DOI] [PubMed] [Google Scholar]
- 73.Qiu W, Schönleben F, Li X, Ho DJ, Close LG, Manolidis S, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(5):1441–1446. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du L, Shen J, Weems A, Lu SL. Role of phosphatidylinositol-3-kinase pathway in head and neck squamous cell carcinoma. J Oncol. 2012;2012:450179. doi: 10.1155/2012/450179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, Skoog L, Stal O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13(12):3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 76.Hou P, Ji M, Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. 2008;113(9):2440–2447. doi: 10.1002/cncr.23869. [DOI] [PubMed] [Google Scholar]
- 77.Bedolla R, Prihoda TJ, Kreisberg JI, Malik SN, Krishnegowda NK, Troyer DA, Ghosh PM. Determining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activation. Clin Cancer Res. 2007;13(13):3860–3867. doi: 10.1158/1078-0432.CCR-07-0091. [DOI] [PubMed] [Google Scholar]
- 78.Lu HY, Qin J, Han N, Lei L, Xie F, Li C. EGFR, KRAS, BRAF, PTEN, and PIK3CA mutation in plasma of small cell lung cancer patients. Onco Targets Ther. 2018;11:2217–26. [DOI] [PMC free article] [PubMed]
- 79.Mikhail M, Velazquez E, Shapiro R, Berman R, Pavlick A, Sorhaindo L, Spira J, Mir C, Panageas KS, Polsky D, Osman I. PTEN expression in melanoma: relationship with patient survival, Bcl-2 expression, and proliferation. Clin Cancer Res. 2005;11(14):5153–5157. doi: 10.1158/1078-0432.CCR-05-0397. [DOI] [PubMed] [Google Scholar]
- 80.Dal Col J, Zancai P, Terrin L, Guidoboni M, Ponzoni M, Pavan A, Spina M, Bergamin S, Rizzo S, Tirelli U, de Rossi A, Doglioni C, Dolcetti R. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111(10):5142–5151. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 81.Shao X, Tandon R, Samara G, Kanki H, Yano H, Close LG, Parsons R, Sato T. Mutational analysis of the PTEN gene in head and neck squamous cell carcinoma. Int J Cancer. 1998;77(5):684–688. doi: 10.1002/(sici)1097-0215(19980831)77:5<684::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 82.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. [DOI] [PubMed]
- 83.Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ, Bando H, el-Khoueiry AB, Pérez-Fidalgo JA, Mita A, Schellens JHM, Chang MT, Reichel JB, Bouvier N, Selcuklu SD, Soumerai TE, Torrisi J, Erinjeri JP, Ambrose H, Barrett JC, Dougherty B, Foxley A, Lindemann JPO, McEwen R, Pass M, Schiavon G, Berger MF, Chandarlapaty S, Solit DB, Banerji U, Baselga J, Taylor BS. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol. 2017;35(20):2251–2259. doi: 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bleeker FE, Felicioni L, Buttitta F, Lamba S, Cardone L, Rodolfo M, Scarpa A, Leenstra S, Frattini M, Barbareschi M, Grammastro MD, Sciarrotta MG, Zanon C, Marchetti A, Bardelli A. AKT1(E17K) in human solid tumours. Oncogene. 2008;27(42):5648–5650. doi: 10.1038/onc.2008.170. [DOI] [PubMed] [Google Scholar]
- 85.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, Zhao M, Ortega Alves MV, Chang K, Drummond J, Cortez E, Xie TX, Zhang D, Chung W, Issa JPJ, Zweidler-McKay PA, Wu X, el-Naggar AK, Weinstein JN, Wang J, Muzny DM, Gibbs RA, Wheeler DA, Myers JN, Frederick MJ. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3(7):770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eom HS, Kim MS, Hur SY, Yoo NJ, Lee SH. Absence of oncogenic AKT1 E17K mutation in prostate, esophageal, laryngeal and urothelial carcinomas, hepatoblastomas, gastrointestinal stromal tumors and malignant meningiomas. Acta Oncol. 2009;48(7):1084–1085. doi: 10.1080/02841860902878152. [DOI] [PubMed] [Google Scholar]
- 87.Kim MS, Jeong EG, Yoo NJ, Lee SH. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. Br J Cancer. 2008;98(9):1533–1535. doi: 10.1038/sj.bjc.6604212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Souza JA, Davis DW, Zhang Y, Khattri A, Seiwert TY, Aktolga S, Wong SJ, Kozloff MF, Nattam S, Lingen MW, Kunnavakkam R, Stenson KM, Blair EA, Bozeman J, Dancey JE, Vokes EE, Cohen EEW. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18(8):2336–2343. doi: 10.1158/1078-0432.CCR-11-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manley PW, Cowan-Jacob SW, Mestan J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochim Biophys Acta. 2005;1754(1–2):3–13. doi: 10.1016/j.bbapap.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 90.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LAM, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 91.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. doi: 10.1016/B978-0-12-407173-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 93.Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013;12(3):245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, Sugden D, Thurlow JK, Cheong SC, Teo SH, Wan H, Waseem A, Parkinson EK, Fortune F, Teh MT. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS One. 2009;4(3):e4849. doi: 10.1371/journal.pone.0004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teh MT, Gemenetzidis E, Patel D, Tariq R, Nadir A, Bahta AW, Waseem A, Hutchison IL. FOXM1 induces a global methylation signature that mimics the cancer epigenome in head and neck squamous cell carcinoma. PLoS One. 2012;7(3):e34329. doi: 10.1371/journal.pone.0034329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gemenetzidis E, Elena-Costea D, Parkinson EK, Waseem A, Wan H, Teh MT. Induction of human epithelial stem/progenitor expansion by FOXM1. Cancer Res. 2010;70(22):9515–9526. doi: 10.1158/0008-5472.CAN-10-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lambert M, Jambon S, Depauw S, David-Cordonnier MH. Targeting Transcription Factors for Cancer Treatment. Molecules. 2018;23(6). 10.3390/molecules23061479. [DOI] [PMC free article] [PubMed]
- 98.Yang N, Wang C, Wang Z, Zona S, Lin SX, Wang X, Yan M, Zheng FM, Li SS, Xu B, Bella L, Yong JS, Lam EWF, Liu Q. FOXM1 recruits nuclear Aurora kinase a to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene. 2017;36(24):3428–3440. doi: 10.1038/onc.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25(24):10875–10894. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoellein A, Pickhard A, von Keitz F, Schoeffmann S, Piontek G, Rudelius M, Baumgart A, Wagenpfeil S, Peschel C, Dechow T, Bier H, Keller U. Aurora kinase inhibition overcomes cetuximab resistance in squamous cell cancer of the head and neck. Oncotarget. 2011;2(8):599–609. doi: 10.18632/oncotarget.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reiter R, Gais P, Jütting U, Steuer-Vogt MK, Pickhard A, Bink K, Rauser S, Lassmann S, Höfler H, Werner M, Walch A. Aurora kinase a messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12(17):5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- 102.Kelly KR, Ecsedy J, Mahalingam D, Nawrocki ST, Padmanabhan S, Giles FJ et al. Targeting aurora kinases in cancer treatment. Curr Drug Targets. 2011;12(14):2067–78. [DOI] [PubMed]
- 103.Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, Chuang YH, Lai CH, Chang WC. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-a gene expression. Nucleic Acids Res. 2008;36(13):4337–4351. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai CH, Tseng JT, Lee YC, Chen YJ, Lee JC, Lin BW, Huang TC, Liu YW, Leu TH, Liu YW, Chen YP, Chang WC, Hung LY. Translational up-regulation of Aurora-a in EGFR-overexpressed cancer. J Cell Mol Med. 2010;14(6b):1520–1531. doi: 10.1111/j.1582-4934.2009.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miracca EC, Kowalski LP, Nagai MA. High prevalence of p16 genetic alterations in head and neck tumours. Br J Cancer. 1999;81(4):677–683. doi: 10.1038/sj.bjc.6690747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenberg CL, Kim HG, Shows TB, Kronenberg HM, Arnold A. Rearrangement and overexpression of D11S287E, a candidate oncogene on chromosome 11q13 in benign parathyroid tumors. Oncogene. 1991;6(3):449–453. [PubMed] [Google Scholar]
- 108.Akiyama N, Tsuruta H, Sasaki H, Sakamoto H, Hamaguchi M, Ohmura Y, Seto M, Ueda R, Hirai H, Yazaki Y. Messenger RNA levels of five genes located at chromosome 11q13 in B-cell tumors with chromosome translocation t(11;14)(q13;q32) Cancer Res. 1994;54(2):377–379. [PubMed] [Google Scholar]
- 109.Lammie GA, Fantl V, Smith R, Schuuring E, Brookes S, Michalides R, Dickson C, Arnold A, Peters G. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991;6(3):439–444. [PubMed] [Google Scholar]
- 110.Smeets SJ, Braakhuis BJM, Abbas S, Snijders PJF, Ylstra B, van de Wiel MA, Meijer GA, Leemans CR, Brakenhoff RH. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25(17):2558–2564. doi: 10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 111.Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace AM, Infante AS, Doi S, Santella RM, Weinstein IB. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993;8(12):3447–3457. [PubMed] [Google Scholar]
- 112.Michalides R, van Veelen N, Hart A, Loftus B, Wientjens E, Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995;55(5):975–978. [PubMed] [Google Scholar]
- 113.Fracchiolla NS, Pruneri G, Pignataro L, Carboni N, Capaccio P, Boletini A, Buffa R, Neri A. Molecular and immunohistochemical analysis of the bcl-1/cyclin D1 gene in laryngeal squamous cell carcinomas: correlation of protein expression with lymph node metastases and advanced clinical stage. Cancer. 1997;79(6):1114–1121. [PubMed] [Google Scholar]
- 114.Meredith SD, Levine PA, Burns JA, Gaffey MJ, Boyd JC, Weiss LM, Erickson NL, Williams ME. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch Otolaryngol Head Neck Surg. 1995;121(7):790–794. doi: 10.1001/archotol.1995.01890070076016. [DOI] [PubMed] [Google Scholar]
- 115.Tatsuka M, Sato S, Kitajima S, Suto S, Kawai H, Miyauchi M, Ogawa I, Maeda M, Ota T, Takata T. Overexpression of Aurora-a potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 2005;24(6):1122–1127. doi: 10.1038/sj.onc.1208293. [DOI] [PubMed] [Google Scholar]
- 116.Chen CH, Chang AYW, Li SH, Tsai HT, Shiu LY, Su LJ, Wang WL, Chiu TJ, Luo SD, Huang TL, Chien CY. Suppression of Aurora-A-FLJ10540 signaling axis prohibits the malignant state of head and neck cancer. Mol Cancer. 2015;14:83. doi: 10.1186/s12943-015-0348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pan C, Yan M, Yao J, Xu J, Long Z, Huang H, Liu Q. Aurora kinase small molecule inhibitor destroys mitotic spindle, suppresses cell growth, and induces apoptosis in oral squamous cancer cells. Oral Oncol. 2008;44(7):639–645. doi: 10.1016/j.oraloncology.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 118.Pannone G, Hindi SAH, Santoro A, Sanguedolce F, Rubini C, Cincione RI, de Maria S, Tortorella S, Rocchetti R, Cagiano S, Pedicillo C, Serpico R, Muzio LL, Bufo P. Aurora B expression as a prognostic indicator and possible therapeutic target in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2011;24(1):79–88. doi: 10.1177/039463201102400110. [DOI] [PubMed] [Google Scholar]
- 119.Flynn J, Jones J, Johnson AJ, Andritsos L, Maddocks K, Jaglowski S, Hessler J, Grever MR, Im E, Zhou H, Zhu Y, Zhang D, Small K, Bannerji R, Byrd JC. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29(7):1524–1529. doi: 10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martin MP, Olesen SH, Georg GI, Schönbrunn E. Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chem Biol. 2013;8(11):2360–2365. doi: 10.1021/cb4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 122.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9(8):576–596. [PubMed] [Google Scholar]
- 123.Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K, Bannerji R, Shapiro CL. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14(3):169–176. doi: 10.1016/j.clbc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 124.Garcia Martinez J, Pérez-Escuredo J, García-Carracedo D, Alonso-Guervós M, Suárez-Nieto C, Llorente-Pendás JL, et al. Analysis of microsatellite instability in laryngeal squamous cell carcinoma. Acta Otorrinolaringol Esp. 2012;63(2):79–84. [DOI] [PubMed]
- 125.van der Riet P, Nawroz H, Hruban RH, Corio R, Tokino K, Koch W, Sidransky D. Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer Res. 1994;54(5):1156–1158. [PubMed] [Google Scholar]
- 126.Kamb A, Gruis N, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian S, Stockert E, Day R, Johnson B, Skolnick M. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 127.Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50(25):5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lesnikova I, Lidang M, Hamilton-Dutoit S, Koch J. p16 as a diagnostic marker of cervical neoplasia: a tissue microarray study of 796 archival specimens. Diagn Pathol. 2009;4:22. doi: 10.1186/1746-1596-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A Review of HPV-Related Head and Neck Cancer. J Clin Med. 2018;7(9). 10.3390/jcm7090241. [DOI] [PMC free article] [PubMed]
- 130.Mori T, Miura K, Aoki T, Nishihira T, Mori S, Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994;54(13):3396–3397. [PubMed] [Google Scholar]
- 131.Yeudall WA, Crawford RY, Ensley J, Robbins K. MTS1/CDK4I is altered in cell lines derived from primary and metastatic oral squamous cell carcinoma. Carcinogenesis. 1994;15(12):2683–2686. doi: 10.1093/carcin/15.12.2683. [DOI] [PubMed] [Google Scholar]
- 132.Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, Bartek J, Sidransky D. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56(16):3630–3633. [PubMed] [Google Scholar]
- 133.El-Naggar AK, Lai S, Clayman G, Lee JK, Luna MA, Goepfert H et al. Methylation, a major mechanism of p16/CDKN2 gene inactivation in head and neck squamous carcinoma. Am J Pathol. 1997;151(6):1767–74. [PMC free article] [PubMed]
- 134.Asokan GS, Jeelani S, Gnanasundaram N. Promoter hypermethylation profile of tumour suppressor genes in oral leukoplakia and oral squamous cell carcinoma. J Clin Diagn Res. 2014;8(10):Zc09–Zc12. doi: 10.7860/JCDR/2014/9251.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diez-Perez R, Campo-Trapero J, Cano-Sánchez J, López-Durán M, Gonzalez-Moles MA, Bascones-Ilundain J, et al. Methylation in oral cancer and pre-cancerous lesions (review). Oncol Rep. 2011;25(5):1203–9. [DOI] [PubMed]
- 136.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 137.Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56(4):722–727. [PubMed] [Google Scholar]
- 138.Drexler HG. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia. 1998;12(6):845–859. doi: 10.1038/sj.leu.2401043. [DOI] [PubMed] [Google Scholar]
- 139.Reiss M, Munoz-Antonia T, Cowan JM, Wilkins PC, Zhou ZL, Vellucci VF. Resistance of human squamous carcinoma cells to transforming growth factor beta 1 is a recessive trait. Proc Natl Acad Sci U S A. 1993;90(13):6280–6284. doi: 10.1073/pnas.90.13.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prime SS, Matthews JB, Patel V, Game SM, Donnelly M, Stone A, Paterson IC, Sandy JR, Yeudall WA. TGF-beta receptor regulation mediates the response to exogenous ligand but is independent of the degree of cellular differentiation in human oral keratinocytes. Int J Cancer. 1994;56(3):406–412. doi: 10.1002/ijc.2910560320. [DOI] [PubMed] [Google Scholar]
- 141.Edmiston JS, Yeudall WA, Chung TD, Lebman DA. Inability of transforming growth factor-beta to cause SnoN degradation leads to resistance to transforming growth factor-beta-induced growth arrest in esophageal cancer cells. Cancer Res. 2005;65(11):4782–4788. doi: 10.1158/0008-5472.CAN-04-4354. [DOI] [PubMed] [Google Scholar]
- 142.Lane D, Levine A. p53 research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol. 2010;2(12):a000893. doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28(48):4295–4305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 2002;21(17):4491–9. [DOI] [PMC free article] [PubMed]
- 145.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, Westra W, Sidransky D, Koch WM. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Houten VM, Tabor MP, van den Brekel MWM, Alain Kummer J, Denkers F, Dijkstra J, Leemans R, van der Waal I, Snow GB, Brakenhoff RH. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J Pathol. 2002;198(4):476–486. doi: 10.1002/path.1242. [DOI] [PubMed] [Google Scholar]
- 147.Balz V, Scheckenbach K, Götte K, Bockmühl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63(6):1188–1191. [PubMed] [Google Scholar]
- 148.Zhou G, Liu Z, Myers JN. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem. 2016;117(12):2682–2692. doi: 10.1002/jcb.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rippin TM, Bykov VJN, Freund SMV, Selivanova G, Wiman KG, Fersht AR. Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene. 2002;21(14):2119–2129. doi: 10.1038/sj.onc.1205362. [DOI] [PubMed] [Google Scholar]
- 151.Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, Mittal BB, Pelzer H, Fung BB, Witt ME, Wenig B, Portugal L, Weichselbaum RW, Vokes EE. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179–1186. doi: 10.1093/annonc/mdh308. [DOI] [PubMed] [Google Scholar]
- 152.Cappuzzo F, Jänne PA, Skokan M, Finocchiaro G, Rossi E, Ligorio C, Zucali PA, Terracciano L, Toschi L, Roncalli M, Destro A, Incarbone M, Alloisio M, Santoro A, Varella-Garcia M. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol. 2009;20(2):298–304. doi: 10.1093/annonc/mdn635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Machiels JP, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010;28(1):21–28. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 154.Choong NW, Kozloff M, Taber D, Hu HS, Wade J, Ivy P, Karrison TG, Dekker A, Vokes EE, Cohen EEW. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Investig New Drugs. 2010;28(5):677–683. doi: 10.1007/s10637-009-9296-7. [DOI] [PubMed] [Google Scholar]
- 155.Bauman JE, Arias-Pulido H, Lee SJ, Fekrazad MH, Ozawa H, Fertig E, Howard J, Bishop J, Wang H, Olson GT, Spafford MJ, Jones DV, Chung CH. A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol. 2013;49(5):461–467. doi: 10.1016/j.oraloncology.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9(1):77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishikawa S, Wong DTW, Sugimoto M, Gleber-Netto FO, Li F, Tu M, et al. Identification of salivary metabolites for oral squamous cell carcinoma and oral epithelial dysplasia screening from persistent suspicious oral mucosal lesions. Clin Oral Investig. 2018. [DOI] [PubMed]
- 158.Aro K, Wei F, Wong DT, Tu M. Saliva Liquid Biopsy for Point-of-Care Applications. Front Public Health. 2017;5(77). 10.3389/fpubh.2017.00077.. [DOI] [PMC free article] [PubMed]
- 159.Cheng J, Nonaka T, Wong DTW. Salivary exosomes as Nanocarriers for Cancer biomarker delivery. Materials. 2019;12(4):654. doi: 10.3390/ma12040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li F, Kaczor-Urbanowicz KE, Sun J, Majem B, Lo HC, Kim Y, Koyano K, Rao SL, Kang SY, Kim SM, Kim KM, Kim S, Chia D, Elashoff D, Grogan TR, Xiao X, Wong DTW. Characterization of human salivary extracellular RNA by next-generation sequencing. Clin Chem. 2018;64(7):1085–1095. doi: 10.1373/clinchem.2017.285072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nonaka T, Wong DTW. Liquid biopsy in head and neck Cancer: promises and challenges. J Dent Res. 2018;97(6):701–708. doi: 10.1177/0022034518762071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7(293):293ra104. [DOI] [PMC free article] [PubMed]
- 163.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, Zhang N, el-Naggar AK, Jasser SA, Weinstein JN, Trevino L, Drummond JA, Muzny DM, Wu Y, Wood LD, Hruban RH, Westra WH, Koch WM, Califano JA, Gibbs RA, Sidransky D, Vogelstein B, Velculescu VE, Papadopoulos N, Wheeler DA, Kinzler KW, Myers JN. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cohen EEW, Licitra LF, Burtness B, Fayette J, Gauler T, Clement PM, Grau JJ, del Campo JM, Mailliez A, Haddad RI, Vermorken JB, Tahara M, Guigay J, Geoffrois L, Merlano MC, Dupuis N, Krämer N, Cong XJ, Gibson N, Solca F, Ehrnrooth E, Machiels JPH. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol. 2017;28(10):2526–2532. doi: 10.1093/annonc/mdx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhan X, Long Y, Lu M. Exploration of variations in proteome and metabolome for predictive diagnostics and personalized treatment algorithms: innovative approach and examples for potential clinical application. J Proteome. 2018;188:30–40. doi: 10.1016/j.jprot.2017.08.020. [DOI] [PubMed] [Google Scholar]