Abstract
Background
Several ocular factors have been identified for primary angle-closure glaucoma (PACG), such as a small cornea, elevated intraocular pressure (IOP), shallow anterior chamber, and short axial length (AL). However, the relationship between the severity of PACG and various ocular parameters [IOP, anterior chamber depth, AL, central corneal thickness] is not fully understood.
Methods
A 7-year cross-sectional study. A total of 2254 eyes of 1312 PACG patients (females = 856 [1479 eyes] and males = 456 [775 eyes]) were included. A detailed eye examination was performed. The participants were categorized into gender subgroups followed by subdivision into three different severity groups according to their mean deviation (MD) of the visual fields results as follows: mild (MD ≤ 6 dB), moderate (MD 6–12 dB), and severe (MD > 12 dB) PACG. The associations of ocular biometry with severity of PACG were analyzed using paired Student’s t test, multivariate linear regression, and logistic regression analysis.
Results
There was a significant positive correlation between the MD and AL in the female subgroup (B = 0.663, p = 0.001, 95%CI = 1.070 to 1.255) but not in the male subgroup. Increased AL levels (mild [OR = 1], moderate [OR = 1.047, p = 0.062, 95%CI = 0.947 to 2.462], and severe [OR = 1.274, p < 0.001, 95%CI = 1.114 to 1.457]) were only associated with the severity of PACG in females. Paired Student’s t tests showed that the long AL female eyes have a higher MD value than in the short AL female eyes (mean difference = 3.09, t = 6.846, p < 0.001) in the same subjects, but not in the male subgroup (p = 0.648).
Conclusions
The AL was positively and significantly related to the severity of PACG in female but not male subjects. This finding refers to the PACG pathogenesis and suggests the use of AL assessment in glaucoma monitoring, diagnosis, and progression. This may contribute to further development of personalized strategies in preventive medicine.
Electronic supplementary material
The online version of this article (10.1007/s13167-019-00174-1) contains supplementary material, which is available to authorized users.
Keywords: Glaucoma, Primary angle-closure glaucoma, Ocular parameters, Axial length, Female, Patient stratification, Clinical medicine, Recommendations, Individualized patient profile, Predictive preventive personalized medicine
Introduction
Primary glaucoma is the most frequent cause of irreversible blindness worldwide—with about 7.14 million people (> 40 years old) affected in China [1–3]—and is characterized by the structural damage to the optic nerve head and visual field loss [1, 4]. Here, people with primary angle-closure glaucoma (PACG) have a 2.5-time higher risk of blindness than those with primary open-angle glaucoma [5]. For PACG, it is known that angle closure is a fundamental pathologic process and that elevation of the intraocular pressure (IOP) is a major risk factor for glaucoma, in which a high IOP causes damage to the optic nerve [4, 6]. However, the pathophysiology of PACG is not yet fully understood.
To date, an elevated IOP induced by angle closure is currently the only modifiable risk factor of PACG [2]. Yet, the relationship between IOP and the severity of PACG is not fully understood. The factors for PACG largely relate to the anatomic configuration of the anterior segment of the eye, and several ocular factors have been identified for PACG, such as a small cornea, shallow anterior chamber, thick lens, anterior lens position, and short axial length (AL) [7–12]. However, the relationship between AL and anterior chamber depth (ACD) and the severity of PACG is not fully understood.
The association of AL with angle-closure glaucoma has been documented in several previous studies reporting that angle-closure glaucoma patients have a smaller AL than normal subjects [13]. However, several studies have also shown that individuals with myopia have a higher prevalence of open-angle glaucoma than those without [14, 15]. Moreover, Qiu et al. [16], Xu et al. [17], and Czudowska et al. [18] have reported that high myopia could be a risk factor associated with glaucomatous optic neuropathy; however, these studies could not distinguish between open-angle glaucoma and angle-closure glaucoma. The underlying hypothesis explaining this association is that individuals with axial myopia have weaker scleral support at the optic nerve, which contributes to a greater susceptibility of the optic nerve to glaucomatous damage [16]. As such, we also applied this underlying hypothesis to explain the association between AL elongation and angle-closure glaucoma. However, to the best of our knowledge, only limited data can be found on the association between AL and the severity of angle-closure glaucoma.
Understanding the association between AL and the severity of angle-closure glaucoma may help to explain the pathogenesis of PACG further. The use of AL assessment may lead to better recognition of patients with increased risk of glaucoma. This makes them important and enables them to be personalized, predictive, and preventive [19, 20]. This may ultimately lead to a more personalized treatment. Thus, we conducted a large-sample cross-sectional investigation in Shanghai to measure ocular biometry and investigate the relationships among IOP, AL, and ACD regarding the severity of PACG.
Materials and methods
Patients
This study was conducted in accordance with the Helsinki Declaration and was approved by the committee of the Eye & ENT Hospital of Fudan University, Shanghai, China. Records of all patients, who were investigated in the glaucoma department of the Eye & ENT Hospital of Fudan University from January 2010 to June 2018, were reviewed. Written informed consent was obtained from all patients enrolled in this study in order to use their clinical data. Additionally, all patient information was anonymized and de-identified prior to analysis.
Examination
The clinical data were obtained from PACG patients who underwent a standardized ophthalmic examination conducted by a glaucoma specialist [21]. This examination included the assessment of the anterior chamber angle by gonioscopy (Haag-Streit, Bern, Switzerland), a three-time IOP measurement using Goldmann applanation tonometry (Haag-Streit, Bern, Switzerland), and then averaged; the analysis of the eye’s fundus using a digital retinal camera (TRC-NW200, Topcon); an A-scan ultrasound (A-Scan Pachymeter, Ultrasonic, Exton, PA, USA) to measure the central corneal thickness (CCT), AL, and ACD; and the vertical cup-disk ratio (VCDR) was evaluated by 2 doctors based on the analysis of the fundus photos and then averaged.
Medical examinations, including the assessment of electrocardiogram, X-ray, liver function, infectious diseases, renal function, blood pressure, heart rate, IOP-lowering medication, visual acuity, body temperature, and body mass index (BMI), were performed for all subjects at the Eye & ENT Hospital of Fudan University. The BMI was calculated as weight in kilograms divided by height in meters squared. The subjects’ self-reported drinking ([> 60 g/time/male, > 40 g/time/female] and > 3 times per week and more than 6 months [current or former]) and smoking (> 1 cigarette per day and more than 6 months [current or former]) history was also collected.
Visual field analysis
The glaucoma department of the Eye & ENT Hospital of Fudan University performed perimetry on glaucoma subjects unless the subjects was unable to see light with both eyes open or had an eye infection. The mean deviation (MD) and mean sensitivity of the visual fields were measured with an Octopus automated perimeter. All patients had a minimum of three visual field tests. After taking into account the learning effect of the visual field tests, the results of the first two tests were excluded. Only reliable (a false positive/negative below 15% and a reliability factor below 20%) and compatible visual field results were included. Each patient had a minimum of one reliable visual field test.
Diagnostic and inclusion criteria
A total of 2598 participants were recruited and evaluated based on the following inclusion criteria:
PACG subjects were selected from inpatients who were scheduled for glaucoma surgery (age ≥ 18 years).
PACG was diagnosed based on narrow anterior chamber angles with glaucomatous optic neuropathy and corresponding visual field loss [21]. The visual field loss was determined, including a cluster of three or more non-edge, contiguous points on the pattern deviation plot, not crossing the horizontal meridian with a probability of less than 5% of being present in age-matched controls (one of which was less than 1%); an abnormal standard deviation pattern with a p < 0.05 occurring in the normal population; and fulfilling the test reliability criteria: fixation losses less than 20%, false positives less than 15%, and/or false negatives less than 15%. Additionally, PACG was diagnosed in eyes with narrow angles; elevated IOP (IOP > 21 mmHg); at least 180° of angle-closure obliterating the pigmented segment of the trabecular meshwork, whether synechial or appositional, segmented, or continuous; and in eyes where the degree of peripheral anterior synechiae was too extensive to be managed by laser peripheral iridotomy.
PACG patients with any secondary glaucoma, any previous eye surgery, nanophthalmos (AL < 19 mm), or any other eye disease that could potentially affect visual acuity or the visual fields were excluded.
Both newly diagnosed and referral PACG patients were included.
Overall, based on above criteria, 1286 (lens opacity that influenced the value of visual field measures = 480, secondary glaucoma = 176, history of eye trauma = 63, previous eye surgery = 116, had other eye diseases = 226, and inadequate subject data = 225) subjects were excluded, resulting in a final sample size of 1312 PACG subjects in this study.
Subgroup analysis
PACG has a gender difference, in which women are more likely than men to have the disorder [22, 23]. Indeed, the present study had a larger number of female PACG subjects than male ones (856 vs. 456). Based on this occurrence, the participants were categorized into female and male subgroups. These were further subdivided into three groups based on disease severity according to the MD results of the patients: mild (MD ≤ 6.00 dB), moderate (MD 6–12 dB), and severe (MD > 12 dB) PACG [24–26]. As PACG is difficult to notice during the stage of mild severity, most glaucoma patients in China pay little attention to their minor eye discomfort until the point they subjectively experience vision deterioration. Therefore, most of the glaucoma patients present in the hospital were at the stage of severe PACG.
Statistical analysis
All analyses were performed using the Statistical Package for the Social Sciences software, version 13.0 (SPSS Inc., Chicago, IL, USA). The figures were created using GraphPad Prism 6 software (La Jolla, CA, USA). Data are expressed as the mean ± standard deviation (SD). Furthermore, normality was assessed using the Kolmogorov-Smirnov test. For the categorical variables, a chi-squared test was used to evaluate the differences among the groups. Additionally, an independent Student’s t test and Mann-Whitney U test were used to compare the participants’ characteristics among the groups. A paired Student’s t test was used to compare the MD values between the long and short AL eyes in the same individual. One-way ANOVA was used to compare the levels of the ocular parameters among the three groups. Correlations among the ocular parameters were assessed using Pearson correlation analysis. Multivariate linear regression analyses, adjusted for age, gender, BMI, SDP, SBP, smoking, drinking, IOP-lowering medications, diabetes, and hypertension, were performed to evaluate the association among the ocular parameters. Furthermore, logistic regression analyses, adjusted for age, gender, BMI, SDP, SBP, smoking, drinking, IOP-lowering medications, diabetes, and hypertension, were performed to identify the association between the ocular parameter levels and the severity of PACG (reference, OR of mild PACG group = 1). Finally, a two-sided p value of < 0.05 was considered statistically significant.
Results
Characteristics of the study subjects
A total of 1312 PACG subjects (females = 856 [binoculus = 623, monocular = 233] and males = 456 [binoculus = 319, monocular = 137]) were enrolled in this study according to the inclusion criteria and comprised 2254 eyes (females = 1479, males = 775). The subject’s average age was 63.2 ± 10.8 (range 36–94) years. The general and ocular biometry of the studied eyes is presented in Table 1.
Table 1.
Characteristics of subjects with PACG
| PACG | ||
|---|---|---|
| No./mean ± SD | Range | |
| Age (years) | 63.2 ± 10.8 | 36–94 |
| Male/female | 456/856 | |
| BMI (kg/m2) | 22.81 ± 3.34 | 13.41–46.26 |
| SDP (mmHg) | 75.0 ± 9.4 | 42–106 |
| SBP (mmHg) | 130.1 ± 15.67 | 61–193 |
| Diabetes (yes/no) | 115/1197 | |
| Hypertension (yes/no) | 385/927 | |
| Smoking (yes/no) | 432/880 | |
| Drinking (yes/no) | 368/944 | |
| Binoculus (male/female) | 942 (319/623) | |
| Monocular (male/female) | 370 (137/233) | |
| IOP-lowering medication (yes/no) | 1046/266 | |
| Number of IOP-lowering medication | ||
| 0–2 | 404 | |
| 2–4 | 499 | |
| > 4 | 143 | |
| Visual acuity | 0.46 ± 0.35 | 0.01–1.20 |
| VCDR | 0.58 ± 0.24 | 0.1–1.0 |
| CCT (μm) | 543.4 ± 47.1 | 453–771 |
| ACD (mm) | 1.86 ± 0.45 | 0.68–3.98 |
| AL (mm) | 22.39 ± 1.21 | 19.1–29.60 |
| MD (dB) | 13.42 ± 8.83 | 0.2–28.5 |
| MS (dB) | 13.57 ± 8.39 | 0–28.7 |
| IOP (mmHg) | 21.0 ± 11.8 | 9.0–65.0 |
Data are expressed as mean ± standard deviation (SD)
VCDR vertical cup/disc ratio, CCT central corneal thickness, ACD anterior chamber depth, AL axial length, MD mean deviation values for the visual field, IOP intraocular pressure, BMI body mass index, SBP systolic blood pressure, SDP diastolic blood pressure, PACG primary angle closure glaucoma
Comparison of ocular biometry in subjects with PACG was stratified according to glaucoma severity
Based on the MD, the PACG subjects were categorized into three subgroups of different PACG severity levels: 630 eyes were classified as mild, 598 as moderate, and 1026 as severe. A comparison of the ocular biometry of subjects with PACG is shown in Table 2. The mean IOP, VCDR, and MD were the lowest in the mild PACG group, followed by the moderate and then the severe one (p < 0.001). Similar results were also observed in the male and female subgroups. For the AL, the mean length was lower in the mild (22.25 ± 0.95 mm) and moderate PACG groups (22.25 ± 1.00 mm) compared to the severe one (22.51 ± 1.34 mm) (p < 0.001). Furthermore, in the female subgroup, the mean AL were the lowest (p < 0.001) in the mild PACG group (22.06 ± 0.79 mm), followed by the moderate (22.13 ± 0.95 mm) and severe ones (22.37 ± 1.39 mm). However, the AL were not found significantly different (p = 0.354) in the male subgroup regarding the three severity groups.
Table 2.
Comparison of ocular biometry in subjects with PACG, stratified according to glaucoma severity
| Factors | Mild PACG (n = 630) | Moderate PACG (n = 598) | Severe PACG (n = 1026) | F value | P value |
|---|---|---|---|---|---|
| IOP (mmHg) | 16.4 ± 7.2 | 17.4 ± 9.0 | 24.5 ± 13.1 | 128.625 | < 0.001a,c |
| Male | 16.5 ± 6.9 | 17.3 ± 9.3 | 23.8 ± 13.3 | 39.319 | < 0.001a,c |
| Female | 16.4 ± 7.4 | 17.4 ± 11.7 | 24.9 ± 13.5 | 89.542 | < 0.001a,c |
| VCDR | 0.44 ± 0.16 | 0.49 ± 0.18 | 0.72 ± 0.22 | 14.701 | < 0.001a,b,c |
| Male | 0.44 ± 0.15 | 0.50 ± 0.18 | 0.76 ± 0.22 | 186.020 | < 0.001a,b,c |
| Female | 0.44 ± 0.16 | 0.48 ± 0.18 | 0.69 ± 0.22 | 203.208 | < 0.001a,b,c |
| CCT (mm) | 539.0 ± 41.3 | 545.2 ± 48.2 | 543.9 ± 48.0 | 2.439 | 0.088 |
| Male | 540.6 ± 37.4 | 555.8 ± 62.9 | 546.5 ± 45.5 | 3.328 | 0.037b |
| Female | 538.28 ± 43.2 | 541.8 ± 41.9 | 542.4 ± 49.4 | 0.888 | 0.412 |
| ACD (mm) | 1.86 ± 0.30 | 1.84 ± 0.44 | 1.85 ± 0.48 | 0.214 | 0.807 |
| Male | 1.89 ± 0.34 | 1.91 ± 0.49 | 1.92 ± 0.53 | 0.257 | 0.773 |
| Female | 1.84 ± 0.28 | 1.81 ± 0.42 | 1.80 ± 0.44 | 1.142 | 0.320 |
| AL (mm) | 22.25 ± 0.95 | 22.25 ± 1.00 | 22.51 ± 1.34 | 15.747 | < 0.001a,c |
| Male | 22.62 ± 1.11 | 22.59 ± 1.08 | 22.74 ± 1.22 | 1.040 | 0.354 |
| Female | 22.06 ± 0.79 | 22.13 ± 0.95 | 22.37 ± 1.39 | 9.141 | < 0.001a,c |
Data are expressed as mean ± standard deviation (SD). One-way ANOVA was used. The mean IOP, VCDR, and MD were the lowest in the mild PACG group, followed by the moderate and then the severe one. However, the AL was found significantly different in the female subgroup but not male subgroup regarding the three severity groups
IOP intraocular pressure, VCDR vertical cup-disc ratio, CCT central corneal thickness, AL axial length, ACD anterior chamber depth, MD mean deviation values for the visual field, MS mean sensitivity values for the visual field, PACG primary angle closure glaucoma, n the number of eyes
aP < 0.05 for the difference between mild PACG and severe PACG (one-way ANOVA with the LSD post hoc test)
bP < 0.05 for the difference between mild PACG and moderate PACG (one-way ANOVA with the LSD post hoc test)
cP < 0.05 for the difference between moderate PACG and severe PACG (one-way ANOVA with the LSD post hoc test)
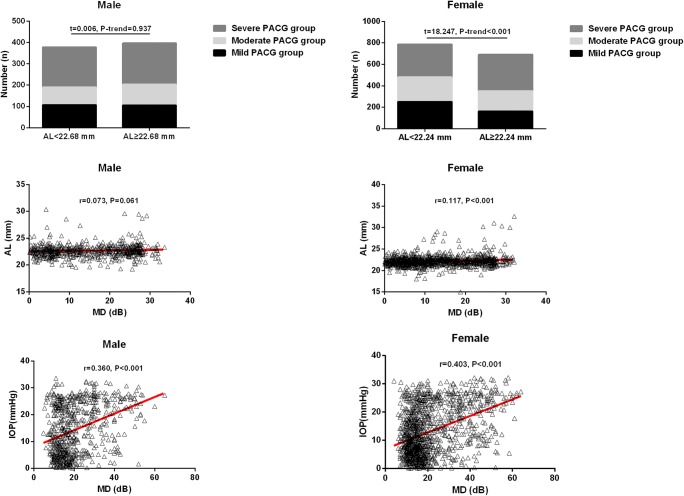
Based on the mean AL (AL = 22.24 mm) in female subjects with PACG, the 1479 female eyes with PACG could be divided into a short (AL < 22.24 mm, n = 787) and a long AL group (AL ≥ 22.24 mm, n = 692). In the short AL group, 253 eyes were categorized as mild, 228 as moderate, and 306 as severe PACG. In the long AL group, 164 subjects were categorized as mild, 189 as moderate, and 339 as severe PACG. The proportion of female eyes with mild, moderate, and severe PACG between the short and long AL groups was significantly different (t = 18.247, p-trend < 0.001) (Fig. 1).
Fig. 1.
The proportion of subjects with different PACG severities in the short and long AL groups. Female: short AL group (AL < 22.24 mm, n = 787) and a long AL group (AL ≥ 22.24 mm, n = 692). Male: short AL group (AL < 22.68 mm, n = 378) and a long AL group (AL ≥ 22.68 mm, n = 397). The proportion of female eyes with mild, moderate, and severe PACG between the short and long AL groups was significantly different (t = 18.247, p-trend < 0.001) but not in male eyes. Scatterplot of individual subject’s measurements of IOP and AL versus MD. Each data point represents one patient. Linear regression is displayed. There was a significant correlation between the MD and AL in the female subgroup but not in the male subgroup, a significant correlation between the MD and IOP in PACG patients. AL axial length, MD mean deviation values for the visual field, IOP intraocular pressure
Based on the mean AL (22.68 mm) in male subjects with PACG, the 775 male eyes with PACG could be divided into a short (AL < 22.68 mm, n = 378) and a long AL group (AL ≥ 22.68 mm, n = 397). In the short AL group, 107 eyes were categorized as mild, 83 as moderate, and 188 as severe PACG. In the long AL group, 106 eyes were categorized as mild, 98 as moderate, and 193 as severe PACG. However, the proportion of male eyes with mild, moderate, and severe PACG between the short and long AL groups was not significantly different (t = 0.006, p-trend = 0.937) (Fig. 1). Receiver operating characteristics curve analysis for AL was performed in predicting the severity of PACG (Table S1).
Comparison of characteristics and ocular parameters between male and female PACG subjects
The male PACG group (1.92 ± 0.47 mm) had a significantly deeper ACD level than the one in the female group (1.82 ± 0.43 mm) (p = 0.011). Furthermore, the male subjects (22.68 ± 1.15 mm) had a statistically significant longer AL compared to the female ones (22.24 ± 1.21 mm) (p < 0.001); see Table 3.
Table 3.
Comparison of characteristics and ocular parameters between male and female PACG subjects
| Male | Female | t value | p value | |
|---|---|---|---|---|
| Age (years) | 62.9 ± 10.6 | 63.4 ± 10.7 | 0.964 | 0.335 |
| BMI (kg/m2) | 23.22 ± 3.02 | 22.57 ± 3.34 | 3.749 | < 0.001 |
| SDP (mmHg) | 75.1 ± 9.2 | 74.9 ± 9.6 | 0.533 | 0.594 |
| SBP (mmHg) | 129.4 ± 14.8 | 130.6 ± 15.8 | 1.727 | 0.084 |
| IOP-lowering medication (yes/no) | 368/88 | 678/178 | 0.412 | 0.521 |
| Diabetes (yes/no) | 42/414 | 73/783 | 0.173 | 0.677 |
| Hypertension (yes/no) | 123/333 | 262/594 | 1.895 | 0.169 |
| Smoking (yes/no) | 268/188 | 164/692 | 211.389 | < 0.001 |
| Drinking (yes/no) | 257/199 | 111/795 | 299.275 | < 0.001 |
| VCDR | 0.61 ± 0.25 | 0.56 ± 0.23 | 4.865 | < 0.001 |
| CCT (μm) | 546.4 ± 46.9 | 541.8 ± 47.2 | 1.949 | 0.051 |
| ACD (mm) | 1.92 ± 0.47 | 1.82 ± 0.43 | 4.610 | < 0.001 |
| AL (mm) | 22.68 ± 1.15 | 22.24 ± 1.21 | 8.096 | < 0.001 |
| MD (dB) | 14.37 ± 9.32 | 12.93 ± 8.54 | 2.964 | 0.003 |
| MS (dB) | 12.58 ± 8.81 | 14.08 ± 8.13 | 3.084 | 0.002 |
| IOP (mmHg) | 21.0 ± 11.2 | 20.9 ± 12.0 | 1.295 | 0.195 |
Data are expressed as mean ± standard deviation (SD). Independent-samples t test, Mann-Whitney test, and chi-square test were used. The male PACG group had a significantly deeper ACD level and longer AL level than the one in the female group
VCDR vertical cup/disc ratio, CCT central corneal thickness, ACD anterior chamber depth, AL axial length, MD mean deviation values for the visual field, IOP intraocular pressure, BMI body mass index, SBP systolic blood pressure, SDP diastolic blood pressure, PACG primary angle closure glaucoma
Multiple linear regression analysis to evaluate associations between MD and IOP, ACD, CCT, and AL in PACG subjects
Pearson correlation analyses were performed to identify the associations between IOP and ocular biometry with the severity of PACG (Fig. 1). In addition to the Pearson analysis, multiple linear regression of the MD levels with the ocular parameters was determined as well; see Table 4. A significant positive correlation was found between the MD and IOP (B = 0.302, p < 0.001, 95%CI = 0.271 to 0.332) and MD and AL (B = 0.665, p < 0.001, 95%CI = 0.326 to 0.998) in the whole PACG group. For instance, in the female subgroup, a significant positive correlation was observed between the MD and IOP (B = 0.295, p < 0.001, 95%CI = 0.260 to 0.330) and the MD and AL (B = 0.663, p = 0.001, 95%CI = 1.070 to 1.225). For the male subgroup, a significant positive correlation was found between the MD and IOP (B = 0.315, p < 0.001, 95%CI = 0.255 to 0.375) as well, but no significant correlation was found between the MD and AL (p = 0.063).
Table 4.
Multiple linear regressions for associations between MD and IOP, ACD, CCT, AL in subjects with PACG
| B | P value | 95%CI | |
|---|---|---|---|
| PACG | |||
| IOP | 0.302 | < 0.001 | 0.271 to 0.332 |
| AL | 0.665 | < 0.001 | 0.326 to 0.998 |
| ACD | − 0.324 | 0.497 | − 1.260 to 0.612 |
| CCT | 0.003 | 0.561 | − 0.006 to 0.012 |
| Male PACG | |||
| IOP | 0.315 | < 0.001 | 0.255 to 0.375 |
| AL | 0.582 | 0.063 | − 0.031 to 1.196 |
| ACD | 0.581 | 0.456 | − 0.948 to 2.109 |
| CCT | 0.001 | 0.918 | − 0.016 to 0.017 |
| Female PACG | |||
| IOP | 0.295 | < 0.001 | 0.260 to 0.330 |
| AL | 0.663 | 0.001 | 1.070 to 1.225 |
| ACD | − 1.008 | 0.096 | − 2.196 to 0.180 |
| CCT | 0.004 | 0.495 | − 0.007 to 0.014 |
Multiple linear regressions for associations between MD and ocular biometry in subjects with PACG after adjusting for age, IOP-lowering medications and gender. A significant positive correlation was found between the MD and IOP in the whole PACG group. However, a significant positive correlation was observed between the MD and AL in the female subgroup but not male subgroup
AL axial length, MD mean deviation values for the visual field, IOP intraocular pressure, 95%CI 95% confidence interval
Logistic regression analysis of the association between IOP, AL, and the severity of PACG
Logistic regression analyses were performed to identify the association between IOP, AL, and the severity of PACG (Table 5). The results showed that an increased IOP and AL in PACG eyes were associated with the overall severity of PACG. Here, the severity of PACG in the female subgroup was associated with an increased IOP and AL, while in the male subgroup, the severity of PACG was associated with an increased IOP only.
Table 5.
Logistic regression analysis of the association between IOP and AL with severity of PACG
| Severity | Factor | B | OR | p value | 95%CI | |
|---|---|---|---|---|---|---|
| PACG | Mild (reference) | 1.0 | ||||
| Moderate | IOP | 0.015 | 1.016 | 0.044 | 1.000 to 1.031 | |
| Severe | IOP | 0.079 | 1.082 | < 0.001 | 1.067 to 1.096 | |
| PACG | Mild (reference) | 1.0 | ||||
| Moderate | AL | 0.067 | 0.998 | 0.981 | 0.875 to 1.139 | |
| Severe | AL | 0.224 | 1.251 | < 0.001 | 1.125 to 1.391 | |
| Female | Mild (reference) | 1.0 | ||||
| Moderate | IOP | 0.016 | 1.016 | 0.080 | 0.998 to 1.034 | |
| Severe | IOP | 0.081 | 1.085 | < 0.001 | 1.067 to 1.103 | |
| Female | Mild (reference) | 1.0 | ||||
| Moderate | AL | 0.110 | 1.116 | 0.042 | 1.031 to 2.339 | |
| Severe | AL | 0.308 | 1.360 | < 0.001 | 1.172to 1.578 | |
| Male | Mild (reference) | 1.0 | ||||
| Moderate | IOP | 0.015 | 1.015 | 0.324 | 0.985 to 1.046 | |
| Severe | IOP | 0.077 | 1.080 | < 0.001 | 1.056 to 1.104 | |
| Male | Mild (reference) | 1.0 | ||||
| Moderate | AL | − 0.032 | 0.969 | 0.769 | 0.784 to 1.198 | |
| Severe | AL | 0.097 | 1.102 | 0.231 | 0.940 to 1.293 |
Logistic regression analysis of the association between ocular biometry and severity of PACG after adjusting for age, IOP-lowering medications and gender. An increased IOP and AL in PACG eyes were associated with the overall severity of PACG. Here, the severity of PACG in the female subgroup was associated with an increased IOP and AL, while in the male subgroup, the severity of PACG was associated with an increased IOP only
VCDR vertical cup/disc ratio, CCT central corneal thickness, ACD anterior chamber depth, AL axial length, MD mean deviation values for the visual field, IOP intraocular pressure, 95%CI 95%confidence interval
Comparing the MD value between eyes with a long and short AL in the same individual via the paired Student’s t test
A total of 942 subjects with binocular PACG (females = 623 and males = 319) were included in this study. Based on the AL in the same PACG subjects, the PACG-affected eyes were divided into a short and a long AL group. Then, paired Student’s t tests were performed to compare the MD value between the eyes with a short and long AL (Table 6). In the same PACG subjects, the eyes with a long AL showed a higher MD value than the ones with a short AL (mean difference = 2.89, t = 7.248, p < 0.001). In the female subgroup, the eyes with a long AL were also found with a higher MD value than in the ones with a short AL (mean difference = 3.09, t = 6.846, p < 0.001). However, similar results were not found in the male subgroup (p = 0.648).
Table 6.
Comparing the MD value between the long AL eyes and short AL eyes in the same individual by paired Student’s t test
| Mean | SD | Paired difference | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | t value | p value | 95%CI | |||
| All (n = 942) | |||||||
| AL-Longer | 22.53 | 1.29 | |||||
| AL-Shorter | 22.20 | 1.01 | 0.33 | 0.72 | 13.849 | < 0.001 | 0.28 to 0.37 |
| MD (AL-Longer) | 14.91 | 9.05 | |||||
| MD (AL-Shorter) | 12.02 | 8.36 | 2.89 | 12.07 | 7.248 | < 0.001 | 2.11 to 3.67 |
| Male (n = 314) | |||||||
| AL-Longer | 22.80 | 1.18 | |||||
| AL-Shorter | 22.53 | 1.12 | 0.28 | 0.37 | 13.160 | < 0.001 | 0.24 to 0.32 |
| MD (AL-Longer) | 14.79 | 9.44 | |||||
| MD (AL-Shorter) | 14.42 | 9.31 | 0.36 | 13.98 | 0.457 | 0.648 | −1.93 to 1.21 |
| Female (n = 628) | |||||||
| AL-Longer | 22.40 | 1.31 | |||||
| AL-Shorter | 22.04 | 0.91 | 0.35 | 0.84 | 10.371 | < 0.001 | 0.29 to 0.42 |
| MD (AL-Longer) | 14.43 | 8,74 | |||||
| MD (AL-Shorter) | 11.34 | 7.96 | 3.09 | 11.14 | 6.846 | < 0.001 | 2.20 to 3.97 |
In the female subgroup, the eyes with a long AL were found with a higher MD value than in the ones with a short AL. However, similar results were not found in the male subgroup
AL axial length, MD mean deviation values for the visual field, SD standard deviation, n the number of patients
Discussion
To our knowledge, this work is the first large-sample study to assess the relationship between ocular biometry and the severity of PACG in China. Multiple linear and logistic regression analyses showed that the IOP was significantly associated with the severity of PACG and that a longer AL was significantly associated with the severity of PACG in female subjects. Interestingly, no relationship was found between the AL and the severity of male PACG—a finding not previously reported. Although a longer AL remains within the normal range, our findings indicate that a long AL is an independent risk factor for the severity of PACG in female subjects.
Several studies [7, 10, 11, 13, 27–29] have shown that the ACD is also a factor in the development of both open-angle glaucoma and angle-closure glaucoma. For example, Chen et al. [11] reported that the ACD tended to decrease from normal to primary angle closure to PACG. They also found a significant association between a decrease in ACD and an increase of PACG risk. Furthermore, Thapa et al. [13] found that the ACD of the occludable angle group was significantly shallower than that of the normal group. In our current study, the Pearson correlation analyses revealed a statistically significant correlation between the ACD and MD in the female subgroup (r = − 0.101, p < 0.001). The multiple linear regression analysis suggested that the ACD level was not significantly associated with the severity of PACG. Overall, our findings are in line with the result of previous studies indicating that ACD is a factor for the development of glaucoma. However, the ACD may not be a primary factor of PACG’s severity but might be—more accurately—a secondary factor instead, which can increase the risk of PACG.
In this study, the mean AL was the smallest in the mild PACG group, followed by the moderate and severe ones. Our results also showed that a longer AL was significantly associated with the severity of PACG in female subjects but not in male subjects. The difference in AL among mild, moderate, and severe PACG was minimal. Marcus et al. [30] found that both low and high myopia cause an increased risk of glaucoma development. The proportion of females with mild, moderate, and severe PACG between the short and long AL groups varied significantly (t = 18.247, p-trend < 0.001) (Fig. 1). Here, limited data are available in the literature regarding the association of AL with PACG severity. Currently, only one previous study has suggested that an increase in AL was significantly related to a slower visual field progression in the inferior hemifield [31]. However, they found that a longer AL was significantly related to faster visual field progression only for the papillomacular bundle area. Additionally, in the same study, eyes with high myopia were also included; the results showed that in severe glaucomatous eyes with long AL, visual field damage could be the result of both glaucomatous damage and myopic change. The mechanism of the development of severe glaucoma in eyes with a longer AL is still unclear, and further study is required.
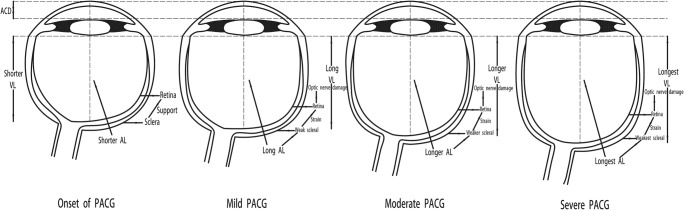
Next, paired Student’s t tests revealed in the female subgroup that eyes with a long AL have a higher MD value than the ones with a short AL (mean difference = 3.09, t = 6.846, p < 0.001). The difference in AL between eyes with a long and short AL in the same subjects was maximal. In general, a gender difference existed among PACG patients; females were more likely than males to have this disorder [22, 23]. Moreover, the present study had a larger number of female than male PACG participants (856 vs. 456). The predominance of the female sex in the longer AL group might suggest that females are more susceptible to the progression of PACG. The mechanisms underlying this phenomenon remain unclear. There are, however, several potential explanations for our findings. First, eyes with longer axial length are more likely to have a non-glaucomatous field loss. Jonas et al. [32] reported that axial length elongation was associated with retinal thinning in the equatorial and pre-equatorial region. Moreover, retinal pigment epithelium cell density decreased with longer axial length [33], and lamina cribrosa thickness got thinner with longer axial length [34]. Therefore, retinal ganglion cell density decreased with longer axial length. There are gender differences in the prevalence of eyes with high myopia in our study cohort, and these respective subjects were not removed from our analysis, which might explain why there was no difference in AL in the male group. Second, as shown in Table 3, females and males had different ocular characteristics. For example, male PACG subjects had a longer AL than female ones (22.68 ± 1.15 mm vs. 22.24 ± 1.21 mm, p < 0.001). Yanagisawa et al. [31] reported that AL elongation was more pronounced in the eyes with no long AL. In other words, AL elongation was more pronounced in the eyes of female than male PACG subjects. The underlying hypothesis explaining this phenomenon is that female PACG subjects with a more pronounced AL elongation have weaker scleral support at the optic nerve, which contributes to a greater susceptibility of the optic nerve resulting in glaucomatous damage (Fig. 2) [35]. In addition, female eyes are anatomically shorter in ACD compared to male ones (1.92 ± 0.47 mm vs. 1.82 ± 0.43 mm, p < 0.001), which might clarify our findings. Elongation of AL, which is related to changes in the structure of the optic disc, was more pronounced in female eyes with a shorter ACD compared to male eyes. Therefore, AL was positively related to the severity of PACG in female but not in male subjects.
Fig. 2.
Increased axial length has weak scleral support at the optic nerve. ACD anterior chamber depth, AL axial length, VL vitreous length, PACG primary angle closure glaucoma. The underlying hypothesis explaining this phenomenon is that PACG subjects with a more pronounced AL elongation have weaker scleral support at the optic nerve, which contributes to a greater susceptibility of the optic nerve resulting in glaucomatous damage
The strengths of our study include the following points: (1) the large sample number of PACG subjects and the detailed and comprehensive clinical examination; (2) the strict inclusion criteria that led to 1286 excluded subjects in this study; (3) the multiple linear regression analyses, logistic regression analyses, and subgroup analyses that were performed to classify the results further; and (4) the paired Student’s t test, which avoids bias caused by using different patients, was used to compare the MD values between the eyes with a long AL and the ones with a short AL in the same individuals.
In addition to the study’s strengths, there were also certain limitations. First, cataracts (lens opacity), which affect the MD value of the visual field, are listed as an exclusion criterion in this study. Nevertheless, lens thickness is also a risk factor for PACG. As this work was a cross-sectional study, lens thickness was not available. Thus, a total of 480 cataract patients were excluded to ensure accurate measurements of the MD value for each subject. However, a significant number of people at higher risk for advanced disease were excluded. Then, the second limitation is that the hospital-based design may not be truly representative of the entire Chinese population. Lastly, PACG subjects were selected from inpatients who were scheduled for glaucoma surgery, which might create a bias toward patients with a higher IOP and more severe glaucoma. The study of AL and its relation to glaucoma severity is extremely challenging. Therefore, a forward-looking, multicenter study with a larger sample size should be conducted to verify our results further.
Conclusions and expert recommendations
This paper conforms with the PPPM concepts presented in the 2012 European Association for Predictive, Preventive and Personalised Medicine (EPMA) white paper and the 2016 EPMA position paper [36, 37], and may play important role to the paradigm shift from reactive medicine to predictive, preventive, and personalized medicine (PPPM). Our findings show that the IOP was significantly associated with the severity of PACG and that AL was positively related to the severity of PACG in female subjects but not in male subjects. Thus, measurement of IOP and AL is a useful tool to predict the severity of PACG which can easily be incorporated in daily ophthalmic practice. In this context, a simple, non-invasive, and reliable PACG severity risk assessment appears to be a plausible approach for early/predictive diagnosis of progression/severity of PACG, which may lead to a more efficient treatment tailored to the patient and prevent progression of glaucoma damage, especially in PACG patients. Targeted predictive longer AL may lead to better recognition of patients with increased risk. This may ultimately lead to a more personalized treatment.
Electronic supplementary material
(DOCX 13 kb)
Funding information
This work was supported by Shanghai Sailing Program (18YF1403500), Shanghai Municipal Commission of Health and Family Planning (20174Y0169), Shanghai Municipal Commission of Health and Family Planning (201840050), The State Key Program of National Natural Science Foundation of China (81430007), The subject of major projects of National Natural Science Foundation of China (81790641), The Shanghai Committee of Science and Technology, China (17410712500), and The top priority of clinical medicine center of Shanghai (2017ZZ01020). Shanghai Science and Technology Committee Foundation grant (19411964600). The sponsor or funding organization had no role in the design or conduct of this research.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All individuals were informed about the purposes of the study and have signed their consent for publishing the data.
Ethical approval
All the patient investigations conformed to the principles outlined in the Declaration of Helsinki and have been performed with the permission EENT2015011 released by the responsible Ethic’s Committee of Eye & ENT Hospital of Fudan University. All the patients were informed about the purposes of the study and have signed their “consent of the patient.” This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shengjie Li, Email: lishengjie6363020@163.com.
Wenjun Cao, Email: wgkjyk@aliyun.com.
References
- 1.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Dai Y, Chen Y, Yu D-Y, Cringle SJ, Chen J, Kong X, Wang X, Jiang C. Primary angle closure glaucoma: what we know and what we don’t know. Prog Retin Eye Res. 2017;57:26–45. doi: 10.1016/j.preteyeres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Karvonen E, Stoor K, Luodonpää M, Hägg P, Kuoppala J, Lintonen T, Ohtonen P, Tuulonen A, Saarela V. Prevalence of glaucoma in the northern Finland birth cohort eye study. Acta Ophthalmol. 2019;97:200–207. doi: 10.1111/aos.13912. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet Lond Engl. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binggeli T, Schoetzau A, Konieczka K. In glaucoma patients, low blood pressure is accompanied by vascular dysregulation. EPMA J. 2018;9:387–391. doi: 10.1007/s13167-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George R, Paul PG, Baskaran M, Ramesh SV, Raju P, Arvind H, McCarty C, Vijaya L. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol. 2003;87:399–402. doi: 10.1136/bjo.87.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan Y-W, Hsieh J-W, Hung P-T. Ocular biometry in acute and chronic angle-closure glaucoma. Ophthalmologica. 2007;221:388–394. doi: 10.1159/000107498. [DOI] [PubMed] [Google Scholar]
- 9.Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998;105:2091–2098. doi: 10.1016/S0161-6420(98)91132-0. [DOI] [PubMed] [Google Scholar]
- 10.Sihota R, Dada T, Gupta R, Lakshminarayan P, Pandey RM. Ultrasound biomicroscopy in the subtypes of primary angle closure glaucoma. J Glaucoma. 2005;14:387–391. doi: 10.1097/01.ijg.0000176934.14229.32. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y-Y, Chen Y-Y, Sheu S-J, Chou P. The biometric study in different stages of primary angle-closure glaucoma. Eye. 2013;27:1070–1076. doi: 10.1038/eye.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan WP, Baasanhu J, Undraa A, Uranchimeg D, Ganzorig S, Johnson GJ. Screening for primary angle closure in Mongolia: a randomised controlled trial to determine whether screening and prophylactic treatment will reduce the incidence of primary angle closure glaucoma in an east Asian population. Br J Ophthalmol. 2003;87:271–274. doi: 10.1136/bjo.87.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thapa SS, Paudyal I, Khanal S, Paudel N, van Rens GHMB. Comparison of axial lengths in occludable angle and angle-closure glaucoma--the Bhaktapur Glaucoma study. Optom Vis. 2011;88:150–154. doi: 10.1097/OPX.0b013e318205e320. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Varma R, Wu S, Torres M, Azen SP, Francis BA, Chopra V, Nguyen BB-T. Los Angeles Latino eye study group baseline risk factors that predict the development of open-angle glaucoma in a population: the Los Angeles Latino eye study. Ophthalmology. 2012;119:2245–2253. doi: 10.1016/j.ophtha.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Iwase A, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Invest Ophthalmol Vis Sci. 2013;54:830–835. doi: 10.1167/iovs.12-11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing eye study. Ophthalmology. 2007;114:216–220. doi: 10.1016/j.ophtha.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Czudowska MA, Ramdas WD, Wolfs RCW, Hofman A, De Jong PTVM, Vingerling JR, Jansonius NM. Incidence of glaucomatous visual field loss: a ten-year follow-up from the Rotterdam study. Ophthalmology. 2010;117:1705–1712. doi: 10.1016/j.ophtha.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15. doi: 10.1186/s13167-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabel BA, Wang J, Cárdenas-Morales L, Faiq M, Heim C. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018;9:133–160. doi: 10.1007/s13167-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Chen Y, Shao M, Tang L, Sun X, Cao W. Association of plasma complement C3 levels with primary angle-closure glaucoma in older women. Invest Ophthalmol Vis Sci. 2017;58:682–689. doi: 10.1167/iovs.16-20675. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains eye study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/S0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 23.Casson RJ, Baker M, Edussuriya K, Senaratne T, Selva D, Sennanayake S. Prevalence and determinants of angle closure in Central Sri Lanka: the Kandy eye study. Ophthalmology. 2009;116:1444–1449. doi: 10.1016/j.ophtha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Shao M, Tang B, Zhang A, Cao W, Sun X. The association between serum uric acid and glaucoma severity in primary angle closure glaucoma: a retrospective case-control study. Oncotarget. 2017;8:2816–2824. doi: 10.18632/oncotarget.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atalay E, Nongpiur ME, Yap SC, Wong TT, Goh D, Husain R, Perera SA, Aung T. Pattern of visual field loss in primary angle-closure glaucoma across different severity levels. Ophthalmology. 2016;123:1957–1964. doi: 10.1016/j.ophtha.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, Belghith A, Manalastas PIC, Medeiros FA, Weinreb RN. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123:2498–2508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim NR, Kim CY, Oh JH, Lee ES. Corneal thickness and anterior chamber depth by Orbscan in normal and primary open-angle glaucoma patients in Korea. J Glaucoma. 2008;17:465–469. doi: 10.1097/IJG.0b013e31815f52f6. [DOI] [PubMed] [Google Scholar]
- 28.Moghimi S, Vahedian Z, Fakhraie G, Ghaffari R, Eslami Y, Jabarvand M, Zarei R, Mohammadi M, Lin S. Ocular biometry in the subtypes of angle closure: an anterior segment optical coherence tomography study. Am J Ophthalmol. 2013;155:664–673, 673.e1. doi: 10.1016/j.ajo.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Adewara BA, Adegbehingbe BO, Onakpoya OH, Ihemedu CG. Relationship between intraocular pressure, anterior chamber depth and lens thickness in primary open-angle glaucoma patients. Int Ophthalmol. 2018;38:541-7. [DOI] [PubMed]
- 30.Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994.e2. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Yanagisawa M, Yamashita T, Matsuura M, Fujino Y, Murata H, Asaoka R. Changes in axial length and progression of visual field damage in glaucoma. Invest Ophthalmol Vis Sci. 2018;59:407–417. doi: 10.1167/iovs.17-22949. [DOI] [PubMed] [Google Scholar]
- 32.Jonas JB, Xu L, Wei WB, Pan Z, Yang H, Holbach L, Panda-Jonas S, Wang YX. Retinal thickness and axial length. Invest Ophthalmol Vis Sci. 2016;57:1791–1797. doi: 10.1167/iovs.15-18529. [DOI] [PubMed] [Google Scholar]
- 33.Bai HX, Mao Y, Shen L, Xu XL, Gao F, Zhang ZB, Li B, Jonas JB. Bruch’s membrane thickness in relationship to axial length. PLoS One. 2017;12:e0182080. doi: 10.1371/journal.pone.0182080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonas JB, Kutscher JN, Panda-Jonas S, Hayreh SS. Lamina cribrosa thickness correlated with posterior scleral thickness and axial length in monkeys. Acta Ophthalmol. 2016;94:e693–e696. doi: 10.1111/aos.13070. [DOI] [PubMed] [Google Scholar]
- 35.Primary Open-Angle Glaucoma PPP (2015) Available online: https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-ppp-2015. Accessed 11 Nov 2018.
- 36.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. [DOI] [PMC free article] [PubMed]
- 37.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)