Abstract
Background
Normotensive pregnancy may develop into preeclampsia (PE) and other adverse pregnancy complications (APCs), for which the causes are still unknown. Suboptimal health status (SHS), a physical state between health and disease, might contribute to the development and progression of PE. By integration of a routine health measure in this Ghanaian Suboptimal Health Cohort Study, we explored the usefulness of a 25-question item SHS questionnaire (SHSQ-25) for early screening and prediction of normotensive pregnant women (NTN-PW) likely to develop PE.
Methods
We assessed the overall health status among a cohort of 593 NTN-PW at baseline (10–20 weeks gestation) and followed them at 21–31 weeks until 32–42 weeks. After an average of 20 weeks follow-up, 498 participants returned and were included in the final analysis. Hematobiochemical, clinical and sociodemographic data were obtained.
Results
Of the 498 participants, 49.8% (248/498) had ‘high SHS’ at baseline (61.7% (153/248) later developed PE) and 38.3% (95/248) were NTN-PW, whereas 50.2% (250/498) had ‘optimal health’ (17.6% (44/250) later developed PE) and 82.4% (206/250) were NTN-PW. At baseline, high SHS score yielded a significantly (p < 0.05) increased adjusted odds ratio, a wider area under the curve (AUC) and a higher sensitivity and specificity for the prediction of PE (3.67; 0.898; 91.9% and 87.8%), PE coexisting with intrauterine growth restriction (2.86, 0.838; 91.5% and 75.9%), stillbirth (2.52; 0.783; 96.6% and 60.0%), hemolysis elevated liver enzymes and low platelet count (HELLP) syndrome (2.08; 0.800; 97.2% and 63.8%), acute kidney injury (2.20; 0.825; 95.3% and 70.0%) and dyslipidaemia (2.80; 0.8205; 95.7% and 68.4%) at 32–42 weeks gestation.
Conclusions
High SHS score is associated with increased incidence of PE; hence, SHSQ-25 can be used independently as a risk stratification tool for adverse pregnancy outcomes thereby creating an opportunity for predictive, preventive and personalized medicine.
Keywords: Suboptimal health status, Preeclampsia, Pregnancy complications, Patient stratification, Primary healthcare, Risk assessment, Population screening, Education, Predictive preventive personalized medicine
Introduction
Given the advances in research and technology, one would expect that pregnancy and childbirth should be safe without mortalities. To date, however, this expectation has largely been a mirage [1]. An estimation from the United Nations Maternal Mortality Estimation Inter-Agency Group and the current World Health Organization (WHO) shows that the regional maternal mortality rate was estimated at 546 deaths per 100,000 livebirths in sub-Saharan Africa (SSA) [1]. One of the main causes behind these disturbing estimates is preeclampsia (PE).
Preeclampsia (PE) (ICD-10-014) is a disorder of pregnancy characterized by a combination of measurable proteinuria and hypertension after 20 weeks of gestation, in pregnant women who were previously normotensive [2]. PE is associated with multi-organ dysfunction and other adverse pregnancy complications (APCs) such as stillbirth, intrauterine growth restriction (IUGR), fetal distress and death, abruptio placenta and HELLP syndrome [3, 4]. PE afflicts about 5 to 8% of all pregnancies worldwide [1] and is responsible for up to 4% of all maternal morbidities and mortalities in sub-Saharan Africa (SSA) [5, 6].
Despite its positive association with maternal morbidities and mortalities, the etiology of PE is not fully understood. The unclear pathogenesis of PE is now a dilemma for clinicians and researchers working to develop appropriate therapeutic and diagnostic measures, aside from delivery of the placenta and the baby under intensive care which remain the major protective measures for PE [2]. The stressful demands of pregnancy may cause pregnant mothers to present with poor health complaints and this has led to an unexpected onset of PE and delayed therapeutic intervention among normotensive pregnant women (NTN-PW) who were previously devoid of a diagnosable condition [7]. Since an early detection coupled with appropriate therapeutic intervention is important in preventing the clinical manifestation of diseases, there is the need for clinicians to shift from the perspective of delayed intervention approach to predictive, preventive and personalized medicine (PPPM) [8–10]. A paradigm shift from reactive to PPPM would allow screening of patients at the preclinical or suboptimal stage prior to the onset of a disease [11]. PPPM has over the past few years adopted environmental, traditional and behavioral factors to solving public health conditions, and this approach has impacted significantly on the prevention and treatment of chronic diseases [11]. The perspective of PPPM if integrated in maternal and neonatal health screening may inform early detection of PE onset and improve diagnosis, prevention, and therapeutics. There is an urgent need to screen and identify normotensive pregnant women who may be experiencing poor health prior to the onset of PE.
In recent public health studies, a search for an inexpensive, reduced turnaround time and a non-invasive health screening measure has yielded a 25-question item of suboptimal health status questionnaire (SHSQ-25) [12]. SHSQ-25 represents a new PPPM, which can be used in both health care and field/community settings to identify individuals who complain of poor health without a diagnosable condition [12, 13]. Over the past few years, SHSQ-25 has made a significant impact in the field of PPPM and has been used to explain the concept of suboptimal health status (SHS), which is defined as the overall physical state between health and disease [12–14]. The SHSQ-25 expresses the overall health of an individual from five domains, including fatigue, cardiovascular, digestive, immune and mental health [12, 13]. SHS represents a subclinical reversible stage of chronic disease and is typified by health complaints, low energy and general weakness within a period of 3 months [14, 15]. The SHSQ-25 has been used in our previous studies as a potential risk stratification measure for cardiovascular and other chronic diseases in different populations including Asia [16–18] and Africa [19]. Also, SHSQ-25 was used along with the endothelial dysfunction (ED) index to predict the onset of cardiovascular disease in European population [20]. Furthermore, SHS was found to be associated with telomere length [21], psychosocial stress, plasma cortisol and mRNA expression of glucocorticoid receptor a/b in lymphocytes in a Chinese population [22].
Although SHS has been associated with blood pressure disorders, no study to date has explored the usefulness of SHSQ-25 in pregnancy and childbirth. While previous studies extensively explored SHS from the perspective of PPPM in several chronic conditions, its relevance as a predictive measure of PE onset has not been reported. Thus, we examined the potential of the subjective tool, SHSQ-25 along with clinical biochemical measures for prediction and early identification of suboptimal health in normotensive pregnant women likely to develop PE coexisting with and without other adverse pregnancy complications (APCs). The findings of this study are expected to increase our knowledge of the pathogenesis of PE and create a window of opportunity for predictive, preventive and personalized medicine (PPPM) specific measures such as risk assessment, screening programmes and targeted prevention [8–10].
Methods
Study design/study participants
This prospective cohort study included 593 normotensive pregnant women (NTN-PW) aged from 18 to 45 years who had no history of a clinically diagnosed disease during the previous 3 months and were visiting the antenatal clinic at the Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana, from June 2017 to December 2018. Pregnant women were initially contacted through a letter of introduction and were invited via a phone call for an interview as well as clinical and biochemical evaluation. Both nulliparous and multiparous pregnant women with a singleton pregnancy were contacted. After written informed consent and ethical consideration, all participants were physically examined and assessed by a qualified consultant obstetrician/gynecologist before inclusion in the study. Exclusion criteria were women with a twin pregnancy, those below 18 years, advanced maternal age (> 45 years), previous history of preeclampsia, gestational diabetes, gestational hypertension, obesity, hyperlipidemia, cancers, smoking, alcoholism, sexually transmitted infections, sickle cell anemia, cerebrovascular conditions and cardiovascular conditions of any form.
Baseline assessment of SHS
At baseline (10–20 weeks gestation (average gestation of 17 weeks)), the overall health status of 593 NTN-PW was measured using a validated SHSQ-25. The SHSQ-25 is made up of five domains, namely, fatigue, cardiovascular system, digestive system, immune system and mental health (Fig. 1). These questions were explained to each participant in the native Ghanaian language and their responses were translated into English by the consultant obstetrician/gynecologist with 100% accuracy. Based on how often each pregnant woman had experienced a particular health complaint in the last 3 months, they were asked to rate a health statement on a 5-point Likert scale: never or almost never (1), occasionally (2), often (3), very often (4) and always (5). These scores were recoded as 0–4 followed by a summation of the codes for the 25 answered items. The median of the total score was recorded as the cut-off point and values ≥ the cut-off represented ‘high SHS’ (poor health) and those ˂ the cut-off indicates ‘optimal health’ [13–15]. In this study, a median score ≥ 19 depicted high SHS and < 19 depicted optimal health status (OHS). A Cronbach’s alpha coefficient value of 0.95 was found after testing the reliability of SHSQ-25. SHS was measured for all participants at baseline (10–20 weeks) only.
Fig. 1.

SHS assessment based on five domains. Figure produced with permission from Wang et al. [13]
Sociodemographic, obstetric and clinical assessment
Information regarding sociodemographic characteristics such as age, highest level of education attained, occupational history and household income as well as obstetric data such as parity, gravidity, gestational age, contraceptive use, family history of hypertension and previous pregnancy complications were obtained via the antenatal folder and the participant’s record in the hospital database. Blood pressure (BP) in mmHg was measured by trained personnel and midwives using mercury sphygmomanometers (Accoson, England) and a stethoscope in accordance with recommendations by the National High Blood Pressure Education Program (NHBEP) working group (2000). The procedure was performed twice at a 5- to 10-min interval for each participant after the first measurement. The average values of the two measurements were recorded as the BP. Participants were classified as normotensive pregnant women if their pregnancy was without measurable proteinuria and blood pressure was ≤ 120/80 mmHg on two occasions at least 4 h apart. Weight and height were read and recorded to the nearest 0.1 kg and 0.1 cm, respectively. Briefly, pregnant women were made to stand on a weighing scale (Seca 762 Mechanical Personal Scale, Hamburg, Germany) and against the stadiometer (Seca 213 Portable Height Measuring Rod Stadiometer, Hamburg, Germany) without their shoes, belongings or any extraneous weight. The body mass index (BMI) was calculated with the formula (weight/height2) and written in kg/m2 units. Pre-gestation BMI was recorded. Proteinuria was measured using a urine reagent dipstick (a semi-quantitative colour scale on the URIT 2VPG Medical electronic Co., Ltd. China). The absence of proteinuria was recorded as ‘negative’. The presence of protein in urine was recorded as ≥ 0.3 g/L on microalbuminuria or ≥ 1+ on dipstick urinalysis. For each pregnant woman, the BP, BMI and proteinuria were measured at three-time points (10–20 weeks, 21–31 weeks and 32–42 weeks) and data recorded.
Follow-up and the events of preeclampsia
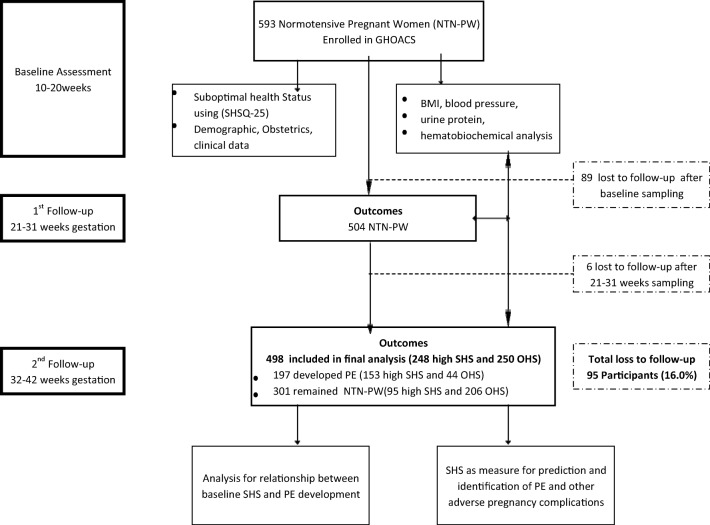
After an average period of 20 weeks follow-up from baseline until birth, 498 out of 593 participants returned for delivery and were included in the final assessment. By the time of delivery, 301 had normal blood pressure without proteinuria (i.e. NTN-PW) and were classified as control, whereas 197 developed PE and were classified as cases. Of the 498 participants, 248 of them had ‘high SHS’ at baseline (153 later developed PE and 95 were NTN-PW) whereas 250 had ‘optimal health’ at baseline (44 developed PE and 206 were NTN-PW). Of the initial 593 participants, 95 women comprising 89 and 6 participants were lost to follow-up and thus did not partake in the first (21–31 weeks gestation) and second (32–42 weeks gestation) follow-ups, respectively (Fig. 2). The reasons for these losses were unwillingness to continue (n = 32), relocation (n = 48), spontaneous abortion (n = 4) and self-induced abortion (n = 11).
Fig. 2.
Flowchart of study participants. GHOACS, Ghanaian Suboptimal Health Status Cohort Study; OHS, optimal health status; SHS, suboptimal health status; PE, preeclampsia
Participants were physically examined and diagnosed in addition to the examination of selection criteria by a qualified consultant Obstetrician/Gynecologist. Preeclampsia (ICD-10-CM-014) was defined as the presence of proteinuria (≥ 1+ or 0.3 g/L) and hypertension (≥ 140/90 mmHg) on two occasions at least 4 h apart detected after the 20th week of gestation in pregnant women who were previously normotensive. Alternatively, high blood pressure combined with multi-systemic manifestations such as HELLP syndrome, renal insufficiency, pulmonary edema and visual or cerebral disturbances supported the diagnosis of PE even in the absence of proteinuria [23].
Biochemical evaluation
Fasting venous blood samples were collected in the morning hours (8 am to 11 am) from each pregnant woman into vacutainer® tubes. Plasma and serum were separated into two cryovials and stored at − 80 °C (Thermo Scientific™ Freezers, USA) until analysis. Biochemical measures including fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), total protein (TP), albumin (ALB), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), urea, creatinine (Cr), uric acid (UA), sodium (Na), potassium (K), chloride (Cl−), magnesium (Mg) and calcium (Ca) were measured using an automatic chemistry analyser (Roche Diagnostics, COBAS INTEGRA 400 Plus, USA). Hemoglobin and red blood cell distribution width (RDW) were analyzed using the Mindray Haematology Analyzer BC 2800. These hematobiochemical determinations were performed at three-time points (10–20 weeks, 21–31 weeks and 32–42 weeks) for each participant.
Diagnostic criteria for adverse pregnancy complications
IUGR, stillbirth, HELLP syndrome and acute kidney injury (AKI) were diagnosed by a qualified consultant obstetrician/gynaecologist following the criteria of ICD-10-036.5990 [24], ICD-10-Z37.1 [24], ICD-10-014.2 [25] and ICD-10-N17 [26], respectively. Dyslipidaemia was classified based on the National Cholesterol Education Programme Adult Treatment Panel III (NCEP-ATP III) criteria as reduced HDL-c < 1.29 mmol/L or specific treatment for this lipid abnormality, raised TG ≥ 1.7 mmol/L or specific treatment for this lipid abnormality, TC > 6.2 mmol/L and LDL-c > 3.37 mmol/L [27].
Statistical analysis
Data was analyzed using SPSS version 24 (IBM Corp, NY, USA), XLSTAT Premium version 2018.1 and R version 3.4.3 (R core Team 2017). The normality of the data was performed using the Kolmogorov-Smirnov test. Data was presented as mean ± SD for parametric continuous variables, median (interquartile ranges) for non-parametric continuous variables and frequency (percentages) for categorical variables. A Chi-square test was used to test the association between the proportions of variables among high SHS and optimal health status NTN-PW. Mean comparison between three independent variables was performed using one-way ANOVA followed by Bonferroni post hoc multiple comparison test. Median comparison between three independent variables was performed using Kruskal-Wallis one-way ANOVA followed by Bonferroni post hoc multiple comparison test. Since neither the SHS nor PE incidence data meet the assumptions for Pearson’s correlation, Spearman rho correlation was used to test the association between the individual SHS-specific domains scores and the incidence of PE. Benjamini-Hochberg correction was performed to adjust for false discovery rates. A multivariate logistic regression model was performed to test the association between SHS and PE with and without adverse pregnancy complications and adjusted odds ratios (aOR) were recorded. A receiver operating characteristic (ROC) curve and area under the curve (AUC) were generated to evaluate the diagnostic performance of the model. P < 0.05 was considered statistically significant.
Results
A total of 593 normotensive pregnant women (NTN-PW) were assessed at baseline. Of these, 498 of them returned and were included in the final analysis. At baseline, a higher proportion of NTN-PW had completed secondary education (41.8%), were married (83.5%), were Akan (81.7%) by ethnicity, had an informal occupation (66.1%), had low basic monthly income (38.4%), were nulliparous (36.9%), were primigravida (46.8%) and had no history of family hypertension (77.9%), spontaneous abortion (72.1%) and caesarean section (80.1%). When the 498 NTN-PW were stratified into high SHS and optimal health status (OHS) from baseline, NTN-PW with high SHS had a significantly increased percentage history of spontaneous abortion (36.7% vs. 19.2%; p = 0.0001), nulliparity (41.1% vs. 32.8%; p = 0.0202) and primigravidity (62.9% vs. 30.8%; p < 0.0001) compared to those with optimal health. None of the NTN-PW had proteinuria at baseline. There was a statistically significant difference between the mean level of SBP among NTN-PW with high SHS compared to those with optimal health (116.0 vs. 113.2; p = 0.0036). Meanwhile, there was no statistically significant difference between the mean age, gestational age, DBP, pre-gestational and gestational BMI among high SHS NTN-PW compared to those with optimal health (p > 0.05) (Table 1).
Table 1.
Baseline (10–20 weeks gestation) sociodemographic characteristics of normotensive pregnant women stratified by high SHS and optimal health status (OHS)
| Characteristics | Total (N = 498) | High SHS (N = 248) | OHS (N = 250) | Statistics | p value |
|---|---|---|---|---|---|
| Highest level of education | 1.794, 3 | 0.6163 | |||
| Unschooled | 3 (0.4) | 1 (0.4) | 2 (0.8) | ||
| Primary | 168 (33.7) | 82 (33.1) | 86 (34.4) | ||
| Secondary | 208 (41.8) | 110 (44.4) | 98 (39.2) | ||
| Tertiary | 119 (23.9) | 55 (22.2) | 64 (25.6) | ||
| Marital status | 0.207, 2 | 0.9018 | |||
| Never married | 78 (15.7) | 37 (14.9) | 41 (16.4) | ||
| Married | 416 (83.5) | 209 (84.3) | 207 (82.8) | ||
| De-facto | 4 (0.8) | 2 (0.8) | 2 (0.8) | ||
| Ethnicity | 2.768, 3 | 0.4288 | |||
| Akan | 407 (81.7) | 196 (79.0) | 211 (84.4) | ||
| Ga-Adangbe | 9 (1.8) | 6 (2.4) | 3 (1.2) | ||
| Mole Dagbani | 75 (15.1) | 42 (16.9) | 33 (13.2) | ||
| Ewe | 7 (1.4) | 4 (1.6) | 3 (1.2) | ||
| Occupation | 0.199, 2 | 0.3687 | |||
| Unemployed | 47 (9.4) | 28 (11.3) | 19 (7.6) | ||
| Formal | 122 (24.5) | 59 (23.8) | 63 (25.2) | ||
| Informal | 329 (66.1) | 161 (64.9) | 168 (67.2) | ||
| Basic income (GH₡) | 2.777, 3 | 0.4273 | |||
| None | 47 (9.4) | 28 (11.3) | 19 (7.6) | ||
| Low (< 500.0) | 191 (38.4) | 92 (37.1) | 99 (39.6) | ||
| Middle (500.0–1000.0) | 170 (34.1) | 87 (35.1) | 83 (33.2) | ||
| High (> 1000.0) | 90 (18.1) | 41 (16.5) | 49 (19.6) | ||
| Parity | 7.706, 2 | 0.0212 | |||
| Nulliparous | 184 (36.9) | 102 (41.1) | 82 (32.8) | ||
| Primiparous | 135 (27.1) | 54 (21.7) | 81 (32.4) | ||
| Multiparous | 179 (36.0) | 92 (37.1) | 87 (34.8) | ||
| Gravidity | 51.54, 1 | < 0.0001 | |||
| Primigravida | 233 (46.8) | 156 (62.9) | 77 (30.8) | ||
| Multigravida | 265 (53.2) | 92 (37.1) | 173 (69.2) | ||
| FH of hypertension | 0.230, 1 | 0.6314 | |||
| Yes | 110 (22.1) | 57 (23.0) | 53 (21.2) | ||
| No | 388 (77.9) | 191 (77.0) | 197 (78.8) | ||
| History of spontaneous abortion | 5.083, 1 | 0.0001 | |||
| Yes | 139 (27.9) | 91 (36.7) | 48 (19.2) | ||
| No | 359 (72.1) | 157 (63.3) | 202 (80.8) | ||
| Previous CS | 0.085, 1 | 0.7701 | |||
| Yes | 99 (19.9) | 48 (19.4) | 51 (20.4) | ||
| No | 399 (80.1) | 200 (80.6) | 199 (79.6) | ||
| Dipstick proteinuria (< 0.3 g/g/24 h) | 498 (100.0) | 248 (100.0) | 250 (100.0) | 1.0000 | |
| Age (years) | 29.64 ± 5.98 | 29.42 ± 5.92 | 29.60 ± 6.08 | 0.667 | 0.5049 |
| Gestational age (weeks) | 16.98 ± 2.01 | 16.97 ± 2.08 | 17.04 ± 1.98 | 0.060 | 0.9586 |
| SBP (mmHg) | 114.7 ± 10.57 | 116.0 ± 11.00 | 113 .2 ± 10.01 | 2.703 | 0.0036 |
| DBP (mmHg) | 72.58 ± 9.26 | 73.0 ± 8.78 | 71.8 ± 8.42 | 1.618 | 0.1341 |
| Pre-gestational BMI (Kg/m2) | 27.04 ± 4.83 | 26.86 ± 4.74 | 27.07 ± 4.92 | 0.405 | 0.6887 |
| Gestational BMI (Kg/m2) | 27.33 ± 4.81 | 27.32 ± 4.74 | 27.2 ± 4.92 | 0.298 | 0.7658 |
Values are presented as mean ± SD, frequency (percentage). p values in italics indicate statistically significant difference in comparison between high SHS and OHS
CS caesarean section, GH₡ Ghana cedi, SBP systolic blood pressure, DBP diastolic blood pressure, OHS optimal health status
A total of the 498 participants completed the study. Of the 498 participants, 248 of them representing 49.8% (248/498) had ‘high SHS’ at baseline (10–20 weeks gestation). Of the 248 high SHS participants, 61.7% (153/248) later developed PE, whereas 38.3% (95/248) were NTN-PW. Conversely, 250 of the 498 participants representing 50.2% (250/498) had ‘optimal health’ at baseline. Of the 250 optimal health participants, 17.6% (44/250) later developed PE whereas 82.4% (206/250) were NTN-PW. When the high SHS and optimal health participants were stratified into pregnant women who later developed PE (PWLD-PE) and NTN-PW at baseline, high SHS PWLD-PE had significantly increased SBP (118.3 vs. 112.4; p < 0.0001), DBP (75.05 vs. 69.91; p = 0.0002), ALT (14.3 vs. 10.7; p < 0.0001), ALP (97 vs. 70; p = 0.0002), urea (4.34 vs. 3.55; p = 0.0011), creatinine (65.61 vs. 56.92; p < 0.0001), uric acid (326.0 vs. 286.0; p = 0.0034) and triglyceride (1.56 vs. 1.21; p = 0.0003) but significantly lower levels of serum Mg (0.88 vs. 0.96; p = 0.0082) and albumin-adjusted calcium (2.03 vs. 2.20; p = 0.0004) compared to high SHS NTN-PW. Optimal health PWLD-PE had significantly higher ALP (95 vs. 91.5; p = 0.0006), UA (304.9 vs. 275.3; p = 0.0039) and TG (1.44 vs. 1.16; p = 0.0031) compared to optimal health NTN-PW (Table 2).
Table 2.
Baseline (10–20 weeks gestation) clinical and hematobiochemical profile among high SHS and OHS normotensive pregnant women who later developed preeclampsia (PWLD-PE) compared to NTN-PW who did not develop PE
| Parameter | High SHS at 10–20 weeks (baseline) | OHS at 10–20 weeks (baseline) | ||||
|---|---|---|---|---|---|---|
| PWLD-PE (N = 153) | NTN-PW (N = 95) | p value | PWLD-PE (N = 44) | NTN-PW (N = 206) | p value | |
| Age (years) | 28.86 ± 6.08 | 30.24 ± 5.11 | 0.0664 | 28.07 ± 7.21 | 30.03 ± 5.76 | 0.0531 |
| Gestational age (weeks) | 16.93 ± 1.95 | 17.04 ± 2.26 | 0.9768 | 17.11 ± 1.76 | 17.02 ± 1.98 | 0.9933 |
| SBP (mmHg) | 118.3 ± 10.84 | 112.4 ± 10.52 | < 0.0001 | 114.0 ± 10.95 | 115.1 ± 9.54 | 0.9641 |
| DBP (mmHg) | 75.05 ± 9.49 | 69.91 ± 8.93 | 0.0002 | 72.70 ± 9.45 | 71.61 ± 9.29 | 0.8932 |
| Pre-gestational BMI (kg/m2) | 26.95 ± 4.68 | 27.12 ± 4.44 | 0.7712 | 26.64 ± 5.04 | 27.15 ± 5.01 | 0.9186 |
| Gestational BMI (kg/m2) | 27.45 ± 4.64 | 27.11 ± 4.39 | 0.9475 | 27.01 ± 5.10 | 27.25 ± 5.00 | 0.9910 |
| Mg (mmol/L) | 0.88 ± 0.24 | 0.96 ± 0.22 | 0.0082 | 0.94 ± 0.12 | 0.99 ± 0.13 | 0.2867 |
| Adjusted Ca (mmol/L) | 2.03 ± 0.37 | 2.20 ± 0.31 | 0.0004 | 2.13 ± 0.44 | 2.26 ± 0.31 | 0.0573 |
| Na (mmol/L) | 136.4 ± 2.0 | 136.2 ± 1.96 | 0.9652 | 136.5 ± 2.17 | 136.2 ± 2.01 | 0.9441 |
| K (mmol/L) | 4.18 ± 0.41 | 4.20 ± 0.48 | 0.9605 | 4.12 ± 0.35 | 4.17 ± 0.31 | 0.8641 |
| Cl− (mmol/L) | 105.6 ± 2.19 | 105.5 ± 2.42 | 0.9987 | 105.8 ± 2.40 | 105.6 ± 2.33 | 0.9394 |
| LDH (IU/L) | 187 (138.5–198.0) | 168 (139–196) | 0.6822 | 192 (147.5–198) | 187 (139–196) | 0.3045 |
| AST (IU/L) | 17.2 (14.30–27.1) | 16.1 (13.8–29.4) | 0.9324 | 15.7 (13.7–19.3) | 15.2 (13.6–19.30) | 0.9918 |
| ALT (IU/L) | 14.3 (10.7–28.4) | 10.7 (10.2–15.3) | < 0.0001 | 12.6 (10.2–17.3) | 11.3 (10.3–14.6) | 0.9997 |
| ALP (IU/L) | 97 (77.3–105) | 70 (56.3–100) | 0.0002 | 95 (90.8–111.8) | 91.5 (65–105) | 0.0006 |
| GGT (IU/L) | 10.9 (10.1–15.1) | 11.3 (10.1–15.4) | 0.9991 | 10.3 (9.51–12.2) | 10.3 (9.8–12.2) | 0.9999 |
| Total protein (g/L) | 68.08 ± 2.21 | 67.76 ± 2.20 | 0.6943 | 67.74 ± 2.46 | 67.97 ± 2.23 | 0.9283 |
| Albumin (g/L) | 37.0 ± 1.26 | 36.81 ± 1.26 | 0.6741 | 36.84 ± 1.38 | 36.91 ± 1.29 | 0.9870 |
| Urea (mmol/L) | 4.34 ± 2.08 | 3.55 ± 1.36 | 0.0011 | 3.58 ± 1.55 | 3.61 ± 1.33 | 0.9996 |
| Creatinine (μmol/L) | 65.61 ± 16.49 | 56.92 ± 10.75 | < 0.0001 | 63.84 ± 11.22 | 59.47 ± 11.0 | 0.1753 |
| UA (μmol/L) | 326.0 ± 39.77 | 286.0 ± 44.8 | 0.0034 | 304.9 ± 38.21 | 275.3 ± 48.98 | 0.0039 |
| Hb (g/dL) | 10.92 ± 0.62 | 11.57 ± 0.63 | 0.0573 | 11.74 ± 0.56 | 11.70 ± 0.57 | 0.9705 |
| RDW-CV (%) | 13.70 ± 1.34 | 13.67 ± 1.31 | 0.9989 | 13.56 ± 1.34 | 13.64 ± 1.24 | 0.9802 |
| PLT (X109/L) | 284.5 ± 85.3 | 300.4 ± 85.56 | 0.5006 | 292.3 ± 88.78 | 301.7 ± 89.18 | 0.9154 |
| FBG (mmol/L) | 4.85 ± 0.76 | 4.930 ± 0.79 | 0.0854 | 5.21 ± 0.76 | 5.08 ± 0.71 | 0.6785 |
| TC (mmol/L) | 4.76 ± 1.30 | 4.70 ± 1.15 | 0.9801 | 4.63 ± 1.18 | 4.65 ± 1.11 | 0.9997 |
| TG (mmol/L) | 1.56 ± 0.91 | 1.21 ± 0.48 | 0.0003 | 1.44 ± 0.94 | 1.16 ± 0.41 | 0.0031 |
| HDL-c (mmol/L) | 1.38 ± 0.31 | 1.46 ± 0.31 | 0.1062 | 1.50 ± 0.27 | 1.48 ± 0.35 | 0.9753 |
| LDL-c (mmol/L) | 2.86 ± 1.19 | 2.93 ± 1.00 | 0.9709 | 2.73 ± 1.02 | 2.79 ± 0.98 | 0.9801 |
Values are presented as mean ± SD; median (IQR). p values in italics indicate statisitically significant difference between PWLD-PE and NTN-PW
PWLD-PE pregnant women who later developed PE
As depicted in Table 3, when the high SHS were stratified into PE and NTN-PW at 32–42 weeks gestation, there were significantly (p < 0.0001) elevated levels of SBP, DBP, Na, LDH, AST, ALT, ALP, GGT, urea, creatinine, UA and TG among PE women compared to NTN-PW, who previously had high SHS at baseline. Conversely, PE women were at an increased risk of preterm delivery as depicted by a significantly lower gestational age than NTN-PW who previously had high SHS at baseline. Additionally, levels of Mg, Ca, total protein, albumin and platelet (PLT) count were significantly reduced among PE women compared to NTN-PW, who previously had high SHS at baseline sampling.
Table 3.
Clinical and hematobiochemical profile at 32–42 weeks of gestation (birth) among high SHS and OHS participants who developed PE compared to NTN-PW
| Parameter | High SHS | OHS | ||||
|---|---|---|---|---|---|---|
| PE (N = 153) | NTN-PW (N = 95) | p value | PE (N = 44) | NTN-PW (N = 206) | p value | |
| Age (years) | 28.94 ± 6.10 | 30.28 ± 5.09 | 0.1718 | 28.37 ± 7.33 | 30.46 ± 5.82 | 0.1480 |
| Gestational age (weeks) | 33.99 ± 2.43 | 38.12 ± 1.52 | < 0.0001 | 35.0 ± 2.25‡ | 37.98 ± 1.52 | < 0.0001 |
| SBP (mmHg) | 172.7 ± 16.76 | 119.8 ± 8.74 | < 0.0001 | 160.2 ± 12.29‡ | 115.1 ± 9.54† | < 0.0001 |
| DBP (mmHg) | 109.9 ± 10.05 | 76.34 ± 9.29 | < 0.0001 | 109.2 ± 7.89 | 74.78 ± 8.69 | < 0.0001 |
| Pre-gestational BMI (kg/m2) | 26.95 ± 4.68 | 27.12 ± 4.44 | 0.7712 | 26.64 ± 5.04 | 27.15 ± 5.01 | 0.9186 |
| Gestational BMI (kg/m2) | 28.37 ± 4.58 | 27.99 ± 4.35 | 0.5230 | 28.17 ± 4.85 | 27.98 ± 4.92 | 0.9948 |
| Mg (mmol/L) | 0.57 ± 0.21 | 0.99 ± 0.24 | < 0.0001 | 0.69 ± 0.19‡ | 1.11 ± 0.16† | < 0.0001 |
| Adjusted Ca (mmol/L) | 1.74 ± 0.37 | 2.25 ± 0.41 | < 0.0001 | 1.97 ± 0.23‡ | 2.49 ± 0.31† | < 0.0001 |
| Na (mmol/L) | 145.7 ± 3.18 | 141.2 ± 1.96 | < 0.0001 | 143.0 ± 3.66 | 143.2 ± 2.01 | 0.0682 |
| K (mmol/L) | 3.56 ± 0.32 | 3.58 ± 0.35 | 0.8041 | 3.60 ± 0.44 | 3.66 ± 0.38 | 0.8787 |
| Cl-(mmol/L) | 110.6 ± 2.19 | 110.5 ± 2.42 | 0.8767 | 110.9 ± 2.29 | 110.6 ± 2.33 | 0.8471 |
| LDH (IU/L) | 264 (227–330.0) | 175 (146.0–203.0) | < 0.0001 | 238 (223.5–301.8)‡ | 174.4 (146.0–203.0) | < 0.0001 |
| AST (IU/L) | 31.9 (25.6–47.4) | 23.5 (19.9–25.7) | < 0.0001 | 29.1 (24.9–38.58)‡ | 22.5 (20.35–25.70) | < 0.0001 |
| ALT (IU/L) | 52.0 (39.8–72.4) | 31.1 (24.0–39.2) | < 0.0001 | 40.3 (37.8–70.0)‡ | 30.5 (23.8–39.2) | < 0.0001 |
| ALP (IU/L) | 383 (335–423) | 238 (195–275) | < 0.0001 | 344 (263.8–382) | 235 (203–253) | < 0.0001 |
| GGT (IU/L) | 20.4 (17.9–47.3) | 18.8 (17.6–22.9) | < 0.0001 | 19.3 (17.2–35.6) | 17.8 (17.3–19.7) | < 0.0001 |
| Total protein (g/L) | 57.90 ± 3.07 | 62.71 ± 2.20 | < 0.0001 | 60.51 ± 2.98‡ | 62.97 ± 2.23 | < 0.0001 |
| Albumin (g/L) | 30.93 ± 1.73 | 33.91 ± 1.26 | < 0.0001 | 32.73 ± 1.69‡ | 34.01 ± 1.25 | < 0.0001 |
| Urea (mmol/L) | 9.17 ± 2.46 | 5.67 ± 1.27 | < 0.0001 | 8.94 ± 2.10 | 5.42 ± 1.33 | < 0.0001 |
| Creatinine (μmol/L) | 107.8 ± 43.43 | 68.45 ± 11.21 | < 0.0001 | 102.0 ± 15.17 | 66.6 ± 11.0 | < 0.0001 |
| UA (μmol/L) | 413.5 ± 73.9 | 314.0 ± 37.3 | < 0.0001 | 398.8 ± 72.66 | 301.6 ± 43.91 | < 0.0001 |
| Hb (g/dL) | 11.02 ± 0.63 | 10.97 ± 0.62 | 0.5212 | 11.14 ± 0.56 | 11.10 ± 0.57 | 0.9705 |
| RDW-CV (%) | 16.40 ± 1.34 | 16.37 ± 1.31 | 0.8868 | 16.26 ± 1.34 | 16.34 ± 1.24 | 0.9802 |
| PLT (X109/L) | 247.7 ± 90.6 | 292.4 ± 85.56 | 0.0007 | 268.5 ± 86.01 | 293.2 ± 88.76 | 0.3354 |
| FBG (mmol/L) | 5.58 ± 0.68 | 5.50 ± 0.70 | 0.6198 | 5.67 ± 0.59 | 5.65 ± 0.71 | 0.9981 |
| TC (mmol/L) | 5.64 ± 1.29 | 5.33 ± 0.97 | 0.1330 | 5.63 ± 1.16 | 5.23 ± 0.94 | 0.1236 |
| TG (mmol/L) | 1.84 ± 0.96 | 1.42 ± 0.52 | < 0.0001 | 1.78 ± 0.79 | 1.35 ± 0.41 | 0.0010 |
| HDL-C (mmol/L) | 1.11 ± 0.29 | 1.18 ± 0.35 | 0.3284 | 1.14 ± 0.27 | 1.19 ± 0.35 | 0.7798 |
| LDL-C (mmol/L) | 3.59 ± 1.12 | 3.57 ± 0.98 | 0.9984 | 3.49 ± 1.16 | 3.39 ± 0.87 | 0.9218 |
| Birthweight (kg) | 1.97 ± 0.01 | 2.83 ± 0.01 | < 0.0001 | 2.69 ± 0.01 | 2.87 ± 0.01 | 0.0812 |
Values are presented as mean ± SD; median (IQR). p values in italics indicate statistically significant difference between PE women and NTN-PW
Mg magnesium, Ca calcium, Na sodium, K potassium, Cl− chloride, LDH lactate dehydrogenase, UA uric acid, RDW red blood cell distribution width
†Significant difference compared to high SHS NTN-PW
‡Significant difference compared to high SHS PE
At 32–42 weeks gestation when participants who previously had optimal health were stratified into PE and NTN-PW, there were significantly (p < 0.0001) elevated levels of SBP, DBP, LDH, AST, ALT, ALP, GGT, urea, creatinine, UA and TG but a reduced GA, Mg, Ca, total protein, albumin, platelet (PLT) count and baby birthweight in PE compared to the NTN-PW.
Furthermore, when PE women who previously had high SHS were compared to PE women who previously had optimal health, there were statistically significantly (p < 0.05) lower GA, Mg, Ca, total protein and albumin levels, but elevated levels of SBP, LDH, AST, ALT, ALP. Also, when NTN-PW who previously had high SHS were compared to NTN-PW who previously had optimal health at 32–42 weeks gestation, there were statistically significantly (p < 0.05) lower levels of serum Mg and Ca, but elevated levels of SBP (Table 3).
On exploring the association between the incidence of PE and the individual SHS domain score (Table 4), there was a significant positive correlation between PE incidence and fatigue (r = 0.300; p = 0.0038), cardiovascular system (r = 0.291; p = 0.0174), digestive system (r = 0.287; p = 0.0291), immune system (r = 0.342; p = 0.0010), mental health status (r = 0.442; p < 0.0001) and the overall SHS score (r = 0.509; p < 0.0001) (Table 4).
Table 4.
Association between the individual and overall SHS domain scores among participants at baseline (10–20 weeks gestation) and PE incidence at 32–42 weeks gestation (n = 498)
| SHS domains | PE incidence | |
|---|---|---|
| r | p value | |
| Fatigue | 0.300 | 0.0038 |
| Cardiovascular system | 0.291 | 0.0174 |
| Digestive system | 0.287 | 0.0291 |
| Immune system | 0.342 | 0.0010 |
| Mental health status | 0.442 | < 0.0001 |
| Overall SHS | 0.509 | < 0.0001 |
r = Spearman rho correlation coefficient. P values were adjusted for the false discovery rate using Benjamini-Hochberg correction. p value < 0.05 indicates statistically significant association
The univariate logistic regression analysis explained that high BP, low Mg, low Ca, low Hb, low HDL, high LDH, high AST levels, high creatinine and high TG levels yielded a significantly increased odds ratio for predicting high SHS among NTN-PW at baseline who later developed PE. After adjusting for confounding factors on multivariate analysis, the association remained significant and the odds ratios were only slightly attenuated if at all. Overall, high BP (aOR = 2.84, 95% CI (1.94–5.40), p = 0.0314), low Mg (aOR = 2.99, 95% CI (1.29–6.20), p = 0.0038) and low Ca (aOR = 4.20, 95% CI (1.57–5.63), p < 0.0001), high TG (aOR = 2.08, 95% CI (1.12–4.27), p = 0.0151) and low HDL-c (aOR = 2.30, 95% CI (1.20–6.83), p = 0.03071) were significant independent risk factors associated with baseline high SHS PWLD-PE (Table 5).
Table 5.
Univariate and multivariate logistic regression model of baseline clinical and hematobiochemical profile for risk stratification of high SHS among pregnant women who later developed PE (PWLD-PE)
| Characteristics | Model 1 | Model 2 | ||
|---|---|---|---|---|
| cOR (95% CI) | p value | aOR (95% CI) | p value | |
| BP (mmHg) | ||||
| Optimal | 1.00 | 1.00 | ||
| High normal | 2.96 (2.39–4.85) | < 0.0001 | 2.84(1.94–5.40) | 0.0314 |
| Mg (mmol/L) | ||||
| Low | 3.47 (3.16–7.15) | < 0.0001 | 2.99 (1.29–6.20) | 0.0038 |
| Normal | 1.00 | 1.00 | ||
| Adj. Ca (mmol/L) | ||||
| Low | 4.19 (1.19–5.03) | < 0.0001 | 4.20 (1.57–5.63) | < 0.0001 |
| Normal | 1.00 | 1.00 | ||
| LDH (IU/L) | ||||
| High | 2.75 (0.60–5.07) | 0.0818 | 1.94 (0.76–4.98) | 0.2104 |
| Normal | 1.00 | 1.00 | ||
| AST (IU/L) | ||||
| High | 1.82 (0.68–8.14) | 0.0518 | 1.10 (0.37–24.2) | 0.4613 |
| Normal | 1.00 | 1.00 | ||
| ALP (IU/L) | ||||
| High | 1.08 (0.78–1.93) | 0.8054 | 0.78 (0.39–1.57) | 0.5944 |
| Normal | 1.00 | 1.00 | ||
| Urea (IU/L) | ||||
| High | 1.03 (0.73–10.51) | 0.0910 | 1.22 (0.41–16.55) | 0.4729 |
| Normal | 1.00 | 1.00 | ||
| Creatinine (IU/L) | ||||
| High | 1.15 (0.55–7.04) | 0.258 | 1.39 (1.04–6.33) | 0.1449 |
| Normal | 1.00 | 1.00 | ||
| Uric Acid (μmol/L) | ||||
| High | 1.18 (0.41–3.88) | 0.8531 | 0.48(0.15–1.53) | 0.3138 |
| Normal | 1.00 | 1.00 | ||
| Hb (g/dL) | ||||
| Anemia | 1.58 (1.01–2.62) | 0.0597 | 1.81 (0.74–4.38) | 0.2276 |
| Non-anemia | 1.00 | 1.00 | ||
| FBG (mmol/L) | ||||
| High normal | 1.85 (0.81–3.85) | 0.1068 | 0.84 (0.31–2.28) | 0.7942 |
| Normal | 1.00 | 1.00 | ||
| TC (mmol/L) | ||||
| High | 1.30 (0.94–2.03) | 0.2750 | 1.82 (0.81–4.10) | 0.1884 |
| Normal | 1.00 | 1.00 | ||
| TG (mmol/L) | ||||
| High | 2.14 (1.08–4.79) | 0.0206 | 2.08 (1.12–4.27) | 0.0151 |
| Normal | 1.00 | 1.00 | ||
| HDL (mmol/L) | ||||
| Low | 2.44 (1.15–7.05) | 0.0418 | 2.30 (1.20–6.83) | 0.0307 |
| Normal | 1.00 | 1.00 | ||
| LDL (mmol/L) | ||||
| High | 1.38 (0.689–2.67) | 0.0890 | 1.23 (0.50–3.05) | 0.8252 |
| Normal | 1.00 | 1.00 | ||
Model 2 adjusted for maternal age, parity, gravidity, family history of hypertension, maternal blood pressure, history of spontaneous abortion and pre-gestational BMI. p values in italics indicate statistically significant odds ratio
cOR crude odds ratio, aOR adjusted odds ratio, CI confidence interval, 1.00 reference category
To explore the usefulness of SHS in predicting PE and other APCs, we performed a multivariate logistic regression model and used the cut-off to generate sensitivity, specificity and area under the ROC curve. Using high SHS alone as a screening measure yielded significantly increased odds, a wider area under the ROC curve (AUC) and a high sensitivity and specificity for identifying PE (aOR = 3.67, AUC = 0.8987, 91.9% and 87.8%), PE coexisting with IUGR (aOR = 2.86, AUC = 0.8378, 91.5% and 75.9%) and stillbirth (aOR = 2.52, AUC = 0.7832, 96.6% and 60.0%) compared to its combination with Mg and Ca (Table 6).
Table 6.
Predictive performance of baseline high SHS score with serum Mg and Ca levels for prediction and diagnosis of PWLD-PE and PE-coexisting IUGR and stillbirth at 32–42 weeks gestation
| Baseline SHS | Model 1 | Model 2 | p value | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | LR+ | AUC |
|---|---|---|---|---|---|---|---|---|---|
| cOR (95% CI) | aOR (95% CI) | ||||||||
| Overall PE | |||||||||
| High SHS | 3.51 (2.18–9.41)* | 3.67 (2.73–8.32) | < 0.0001 | 91.9 (87.6–94.8) | 87.8 (83.2–91.3) | 87.1 | 92.4 | 7.55 | 0.8987 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 3.00 (2.51–7.33)* | 2.58 (1.15–5.95) | 0.0381 | 66.5 (59.6–72.7) | 90.9 (86.3–94.4) | 52.4 | 81.9 | 2.67 | 0.6212 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 2.82 (2.06–8.41)* | 2.22 (1.51–6.72) | 0.0461 | 70.6 (61.1–78.9) | 93.9 (90.4–99.7) | 58.3 | 82.9 | 2.71 | 0.7559 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
| PE+ IUGR | |||||||||
| High SHS | 3.19 (2.01–8.87)* | 2.86 (1.62–8.87) | < 0.0001 | 91.5 (86.6–94.8) | 75.9 (70.8–80.4) | 70.1 | 93.6 | 3.81 | 0.8378 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 1.04 (0.57–7.35) | 1.37 (0.92–6.09) | 0.0934 | 22.2 (10.1–39.2) | 80.3 (79.2–93.7) | 33.3 | 82.6 | 2.06 | 0.5211 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 2.33 (2.26–8.07)* | 2.08 (1.68–8.32) | 0.0328 | 62.5 (48.6–75.1) | 89.2 (83.2–89.5) | 46.7 | 83.2 | 2.25 | 0.6462 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
| PE + stillbirth | |||||||||
| High SHS | 2.61 (2.60–9.00)* | 2.52 (2.34–10.12) | < 0.0001 | 96.6 (89.8–99.1) | 60.0 (55.3–64.7) | 33.9 | 99.8 | 2.41 | 0.7832 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 1.87 (1.61–9.38)* | 1.91 (1.53–11.92) | 0.0430 | 37.5 (15.2–64.6) | 90.8 (86.1–94.3) | 23.1 | 95.2 | 4.06 | 0.5805 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 2.35 (1.85–10.56)* | 2.67 (2.40–9.74) | < 0.0001 | 72.7 (49.8–89.3) | 86.2 (84.6–89.9) | 62.8 | 96.3 | 2.82 | 0.7203 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
Model 1: unadjusted odds ratio. Model 2: model adjusted for maternal age, parity, gravidity, family history of hypertension, maternal blood pressure, history of spontaneous abortion, pre-gestational BMI, low HDL and high TG. p values in italics indicate statistically signficant adjusted odds ratio
cOR crude odds ratio, aOR adjusted odds ratio, CI confidence interval, 1.00 reference category, HSHS high SHS, OHS optimal health status, Mg magnesium, Ca albumin-adjusted calcium, IUGR intrauterine growth restriction
*Significant crude odds ratio (p < 0.05)
Also using high SHS alone as a screening measure yielded better predictive and diagnostic accuracies for identifying PE coexisting with HELLP syndrome (aOR = 2.08, AUC = 0.8009, 97.2% and 63.8%), AKI (aOR = 2.2, AUC = 0.8246, 95.3% and 70.0%) and dyslipidaemia (aOR = 2.80, AUC = 0.8205, 95.7% and 68.4%) compared to its combination with Mg and Ca (Table 7).
Table 7.
Predictive performance of baseline high SHS score with serum Mg and Ca levels for prediction and diagnosis of PE coexisting with HELLP syndrome, acute kidney injury (AKI) and dyslipidaemia at 32–42 weeks gestation
| Baseline SHS | Model 1 | Model 2 | p value | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | LR+ | AUC |
|---|---|---|---|---|---|---|---|---|---|
| cOR (95% CI) | aOR (95% CI) | ||||||||
| PE+ HELLP syndrome | |||||||||
| High SHS | 2.47 (1.88–9.25)* | 2.08 (1.95–6.83) | 0.0001 | 97.2 (91.5–99.4) | 63.8 (58.1–67.6) | 41.5 | 98.8 | 2.62 | 0.8009 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 1.59 (1.09–6.32)* | 1.97 (1.30–8.14) | 0.0225 | 66.5 (59.6–72.7) | 90.9 (86.3–94.3) | 13.1 | 97.6 | 4.11 | 0.6437 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 1.75 (1.31–7.04)* | 2.05 (1.39–5.36) | 0.0013 | 74.3 (44.9–92.2) | 78.3 (62.7–73.5) | 16.7 | 97.5 | 2.80 | 0.7310 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
| PE + AKI | |||||||||
| High SHS | 2.15 (1.33–5.31)* | 2.20 (1.58–6.03) | 0.0051 | 95.3 (90.4–97.8) | 70.0 (64.6–74.2) | 57.3 | 97.1 | 3.14 | 0.8246 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 1.61 (1.53–8.47)* | 1.84 (1.36–7.58) | 0.0330 | 31.0 (15.3–50.8) | 91.1 (86.6–94.5) | 31.0 | 91.1 | 3.49 | 0.5023 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 2.08 (1.74–4.82)* | 2.13 (1.50–5.10) | 0.0018 | 72.6 (58.3–84.1) | 73.8 (67.3–79.6) | 40.2 | 91.7 | 2.77 | 0.7630 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
| PE + dyslipidaemia | |||||||||
| High SHS | 2.77 (1.80–9.07)* | 2.80 (2.30–10.35) | 0.0004 | 95.7 (90.7–98.2) | 68.4 (63.3–72.9) | 54.5 | 97.6 | 3.02 | 0.8205 |
| OHS | 1.00 | 1.00 | |||||||
| High SHS + low Mg | 1.29 (1.02–4.16) | 1.18 (0.80–7.20) | 0.0599 | 50.0 (6.7–93.2) | 90.8 (86.2–94.3) | 9.1 | 99.0 | 5.45 | 0.6321 |
| OHS + normal Mg | 1.00 | 1.00 | |||||||
| High SHS + low Ca | 1.64 (1.00–7.13) | 1.52 (0.79–9.06) | 0.1244 | 60.0 (14.7–94.7) | 73.7 (67.2–79.5) | 5.1 | 98.7 | 2.28 | 0.6594 |
| OHS + normal Ca | 1.00 | 1.00 | |||||||
Model 1: unadjusted odds ratio. Model 2: model adjusted for maternal age, parity, gravidity, family history of hypertension, maternal blood pressure, history of spontaneous abortion, pre-gestational BMI, low HDL and high TG. p values in italics indicate statistically signficant adjusted odds ratio
cOR crude odds ratio, aOR adjusted odds ratio, CI confidence interval, 1.00 reference category, HSHS high SHS, OHS optimal health status, Mg magnesium, Ca albumin-adjusted calcium, HELLP hemolysis elevated liver enzymes and low platelet count
*Significant crude odds ratio (p < 0.05)
Meanwhile, a novel combination of SHS, Mg and Ca levels yielded a fair discriminating power, sensitivity and specificity for identifying PE coexisting with APCs. Particularly, a combination of high SHS and low Ca levels yielded a better predictive power and diagnostic performance compared to the combination of high SHS and low Mg. Overall, SHS is an independent predictive and screening measure for PE and its associated APCs (Tables 6 and 7).
As shown in Fig. 3, high SHS yielded a significantly high discriminating power (area under the ROC curve) for identifying all PE women (AUC = 0.7832; p < 0.0001) (Fig. 3a), PE coexisting with IUGR (AUC = 0.8378; p < 0.0001) (Fig. 3b), stillbirth (AUC = 0.8205; p < 0.0001) (Fig. 3c), HELLP syndrome (AUC = 0.8009; p < 0.0001) (Fig. 3d), AKI (AUC = 0.8378; p < 0.0001) (Fig. 3e) and dyslipidemia (AUC = 0.8987; p < 0.0001) (Fig. 3f).
Fig. 3.
a–f Area under the ROC curve (AUC) of baseline high SHS score for prediction of PE and other adverse pregnancy complications (APCs)
Discussion
For the first time in a Ghanaian Suboptimal Health Cohort Study (GHOACS), the present study determined the usefulness of SHSQ-25 for prediction and early identification of normotensive pregnant women (NTN-PW) likely to develop PE and other adverse pregnancy complications (APCs).
One major finding of the present study indicated that 61.7% of high SHS NTN-PW developed PE compared to 17.6% for optimal health NTN-PW (Tables 2 and 3). At baseline (10–20 weeks), normotensive pregnant women who had high SHS were at approximately 4-fold increased odds of developing PE after adjusting for maternal age, gestational age, parity, gravidity, family history of hypertension, maternal BP, history of spontaneous abortion, pre-gestational BMI, high TG and low HDL. This signifies that the association between SHS and PE is independent of these confounders. Moreover, high SHS at baseline (10–20 weeks gestation) yielded a sensitivity of 91.9%, a specificity of 87.8% and an area under the ROC curve (AUC)/discriminating power of 89.9% (Fig. 3a), indicating the power of high SHS for predicting the onset of PE. The ability of high SHS at baseline to predict the onset of PE signifies that SHSQ-25 may be an important measure in predictive, preventive and personalized medicine (PPPM).
In the present study, the link between SHS and PE onset was further supported by a significant relationship between the individual SHS-specific domains and the incidence of PE. Particularly, the incidence of PE increased with increasing SHS-specific domains score for fatigue, cardiovascular complaints, digestive system disorder, immune health disorder and mental health complaints (Table 4). The probable reason(s) for this relationship between SHS and the onset of PE are not currently understood. PE which is a multi-systemic and multi-organ syndrome, however, may share a common biological and/or physiological pathway to SHS. Particularly, SHS is associated with hypertension and other cardiovascular-related disorders [17, 18, 20] which links to PE. SHSQ-25 evaluates the general health status via five specific domains: fatigue, cardiovascular system, digestive system, immune system and mental health. Although the exact etiology of PE is still unknown, previous studies have linked PE onset to immune [28, 29] and cardiovascular disease [30, 31]. Additionally, the clinical symptoms of PE have also been associated with digestive disorders such as hyperemesis gravidarum (severe vomiting), nausea and constipation [32], fatigue [32] and mental health [33]. All these symptoms are significant components of SHS and thus uncover the hidden link between SHS and the onset of PE, although we are the first to study the factors together. The probable explanation for the association between PE and the five SHS-specific domains may be due to the common symptoms, biological and/or physiological pathway both share [28–33]. Hence, integration of SHSQ-25 as a SHS screening tool in antenatal care will generate a new approach with potential for early identification of normotensive pregnant women likely to develop PE, thereby creating a window of opportunity for PPPM. Here, PPPM intervention will promote adequate patient surveillance, risk stratification, optimal diagnosis, prediction of adverse drug to drug interactions and early disease identification [8–10].
Previous case-control studies by Ephraim et al. [34] among a Ghanaian population and Guo et al. [35] among a Chinese population [34, 35] have observed several serum biochemical changes including reduced levels of magnesium and calcium in preeclamptic pregnancies at the point of diagnosis, although these imbalances are not commonly reported in early normotensive pregnancies prior to the onset of PE. However, at baseline (10–20 weeks gestation), in the present longitudinal cohort study, high SHS NTN-PW who later developed PE at 32–42 weeks gestation had significantly reduced levels of magnesium and calcium compared to optimal health NTN-PW (Table 2). High SHS NTN-PW had low serum magnesium and calcium levels with 3- and 4-fold increased odds respectively, compared to those with optimal health status (Table 5). This relationship is novel. This novel finding signifies that SHS can represent a tool for PPPM by predicting early risk of low dietary magnesium and calcium intake. A cross-sectional study in a Ghanaian population observed significantly low Mg and Ca levels among NTN-PW compared to non-pregnant women [36]. The low calcium levels at baseline may have led to hypertension by stimulating the increased release of renin and parathyroid hormone, which in turn increases intracellular calcium in smooth muscle, culminating in vasoconstriction [34]. The observed low magnesium levels may also be due to an increased clearance by the renal system, reduced dietary intake, hemodilution caused by an expansion of the extracellular space and high consumption of minerals by the growing foetus [34, 35]. From the PPPM perspective, SHS can be used to predict an early signs of calcium and magnesium malnutrition and also inform therapeutic options needed for high SHS NTN-PW likely to develop PE.
Preeclampsia is a multifactorial syndrome, indicating that it can coexist with other adverse pregnancy complications (APCs) such as intrauterine growth restriction (IUGR), stillbirth, hemolysis elevated liver enzymes and low platelet count (HELLP) syndrome [3, 37], dyslipidaemia [38, 39] and acute kidney injury (AKI) [40]. These reports agree with the findings of the present study, although the identification of these APCs are mostly delayed and diagnosis occurs late in gestation which highly support earlier PPPM approaches. For this purpose, we performed a predictive model using baseline high SHS scores alone as well as an algorithm of high SHS score, low magnesium and/or calcium and generated an area under ROC curve (AUC)/discriminating power, sensitivity and specificity to predict and identify the risk of PE coexisting with other APCs. Our findings indicated that using high SHS score alone can yield a better predictive odds ratio, sensitivity and specificity than its combination with low magnesium and calcium levels. Conversely, a combination of SHS with Mg and Ca levels yielded a significantly high specificity but low sensitivity compared to using SHS alone (Tables 6 and 7). This confirms our findings that SHS is an independent predictor of PE and other APCs that supports the paradigm shift of clinical medicine from delayed medical intervention to PPPM.
Particularly in the present study, high SHS NTN-PW at baseline were at 3-fold increased odds of developing PE coexisting with IUGR at 32–42 weeks gestation. This occurred at 83.8% (Fig. 3b) discriminating power, a sensitivity of 91.5% and a specificity of 75.9% (Table 6). The occurrence of IUGR may be due to endothelial dysfunction [41]. Our previous cross-sectional study among an adult European population found an association between SHS and endothelial dysfunction, and thus, the relationship between SHS and IUGR may possibly be due to endothelial dysfunction [20]. Endothelial dysfunction may be caused by placental hypoxia, oxidative stress and nitric oxide deficiency originating from poor extravillous trophoblast invasion and incomplete maternal artery remodeling [42]. Another factor that may explain PE coexisting with IUGR is maternal psychosocial stress [7, 43, 44], previously shown by a cross-sectional study among a Chinese population that found an association between SHS and psychosocial stress [22] potentially associated with fatigue which is one of the SHS-specific domains.
Another novel finding of the present study was a significant association between SHS and PE coexisting with stillbirth. Normotensive pregnant women who had high SHS at baseline (10–20 weeks gestation) were 2.5 times more likely to develop PE coexisting with stillbirth during birth (32–42 weeks gestation). At a 2.5-fold predictive odds ratio for high SHS, a sensitivity of 96.6%, a specificity of 60.0% (Table 6) and a discriminating power of 78.3% (Fig. 3c) was observed. Preeclampsia is known to complicate the development of stillbirth and the underlying cause has been linked to placental insufficiency and incomplete maternal artery remodeling [3, 42, 45]. Thus, SHSQ-25 suggests a non-invasive subjective measure for the identification of stillbirth strongly emphasizes the advantage of PPPM to prevent this fatal outcome.
SHS and PE-coexisting with HELLP syndrome in this study is another novel finding. In the present study, HELLP syndrome identified by hemolysis (high levels of LDH), elevated liver enzymes (ALP, AST, ALT and GGT) and low platelet count was observed at a higher rate in PE women compared to NTN-PW. Pregnant women who had high SHS at baseline (10–20 weeks) were 2.08 times more likely to develop PE-coexisting HELLP syndrome at 32–42 weeks (Table 7). This syndrome, which is characterized by microangiopathic anemia, thrombocytopenia and periportal hepatic necrosis [25], is a known cause of eclampsia-associated mental health problems in pregnancy [33]. Our findings indicated that high SHS can identify PE-coexisting HELLP syndrome at 97.2% sensitivity, 63.8% specificity and a discriminating power (AUC) (Fig. 3d) of 80.1%. Hepatic involvement in PE may be explained by precipitation of fibrin within the portal and periportal areas of the liver lobule and hepatic arterial vasospasm resulting in hepatic cell necrosis and lobular ischemia [25]. The link between SHS and PE-coexisting HELLP syndrome could be also related to the mental health phenomenon they both share, though this mechanism is speculative. Early detection of mental health complaints using SHSQ-25 in pregnancy will inform clinicians of the likelihood of HELLP syndrome development.
As acute kidney injury (AKI) is not commonly associated with normotensive pregnancy, but can occur in pregnancies and is potentially associated with severe PE, HELLP syndrome [26, 40], intrauterine fetal death and stillbirth [46], it may explain the reason for the PE coexisting with AKI. In the present study, AKI was diagnosed based on either a sudden increase in serum creatinine ≥ 88.4 μmol/Lor oligoanuria or the need for dialysis [46]. Our present study found that increased creatinine levels were associated with high SHS women who developed PE compared to normotensive pregnant women. Using high SHS score at baseline as a predictive measure, a discriminating power of 82.5% (Fig. 3e), a sensitivity of 95.3%, a specificity of 70.0% and a predictive odds ratio of 2.2 were generated to identify the risk of PE coexisting with AKI. Since high SHS score was associated with high creatinine levels, which is the hallmark of AKI, early detection of abnormal creatinine will inform clinicians of the likelihood of AKI in pregnancy and thus supporting the integration and use of SHSQ-25 as a potential SHS measure in antenatal care.
In a previous prospective cohort study among NTN-PW in a Turkish population, early dyslipidemia (10–20 weeks) was found to be a significant risk factor for PE [39]. This agrees with the present study findings, as a low HDL-c and high TG at baseline (10–20 weeks gestation) were associated with high SHS NTN-PW who later developed PE. Thus, hypertriglyceridemia [47] is ideal for early PE detection and management. Using high SHS score as an independent measure a high predictive odds ratio (2.8), discriminating power of 82.1% (Fig. 3f) and diagnostic performance (sensitivity of 95.7% and specificity of 68.4%) was observed for the prediction of PE coexisting with dyslipidemia (Table 7). An association between SHS and cardiovascular and/or cardiometabolic risk has been established in our previous studies [18, 20]. Although pregnancy-induced hyperlipidemia may be physiologic, dyslipidemia may predispose the mother to atherosclerosis and directly contributes to cardiovascular disease (CVD) [27, 47]. The development of dyslipidemia may be associated with systemic inflammation originating from N-glycosylation-induced changes in immunoglobulin G (IgG) structure and function [48]. Since, dyslipidemia remains one of the predisposing factors PE [30], early identification of women at risk of dyslipidemia would be an opportunity for selective monitoring and management. This supports the need to integrate SHSQ-25 in antenatal care as a dynamic screening tool for PE complicated by dyslipidemia. Since SHS correlates with cardiovascular index like, dyslipidamia it will generate an early risk stratification for PE participants at risk of cardiovascular disease as well as promote an opportunity for personalized medicine.
To our knowledge, this is the first study integrating SHS as a screening tool in pregnancy and childbirth and the largest prospective cohort study in a Ghanaian population. Nevertheless, there were some limitations. First, the recruitment of participants in this present study was single-hospital centered, in the sense that only one teaching hospital was involved; thus, there was a possibility of ethnic bias as most of the participants were Akan’s and few were distributed across other ethnic groups. Second, this study could not recruit baseline participants at early but rather late first trimester of pregnancy; hence, we could not perform SHS evaluation at 21–31 weeks gestation to see the changes in health status over time in relation to the risk of PE. The findings of this study, however, are novel and, thus, further studies are needed in another population to establish the observed association. The relationship between SHS and PE as well as PE coexisting with other APCs, although not well-understood, may have an interconnection with placenta-derived factors (angiogenic growth mediators) and oxidative stress, which are key factors contributing to the pathogenesis of PE. Our next study will address this by evaluating an association between SHS, angiogenic growth mediators and oxidative stress among NTN-PW in this on-going Ghanaian Suboptimal Health Cohort Study (GHOACS).
Conclusion
In summary, a higher percentage of high SHS NTN-PW at baseline is more likely to develop PE. The incidence of PE increased with increasing SHS-specific domain scores. This association was supported by a significantly deranged hematobiochemical profile, an increased adjusted odds ratio, a wider area under the ROC curve and a better sensitivity and specificity in favour of high SHS NTN-PW compared to optimal health participants. Overall, high SHS in early pregnancy is an independent risk factor of PE as well as PE coexisting with IUGR, stillbirth, HELLP syndrome, AKI and dyslipidemia.
Integration of SHSQ-25 as a screening tool in both early antenatal care and follow-up of pregnant women will allow early detection of adverse pregnancy complications while creating an opportunity for PPPM policies such as screening programs, education, risk assessment and targeted prevention. The idea of SHS (suboptimal health status) profile—highly correlated with biochemical/physiological risk factors—implies that we can ‘feel’ the internal pathologies—though usually perceived as ‘subjective’, is very important for self-education/responsibility, suggesting a potential for ‘subjective’ PPPM approach. Hence, SHSQ-25 can be used as an alternative health pre-screening measure in clinical laboratory-limited communities, field and community health centres in sub-Saharan African countries in emergency situations.
Acknowledgements
We wish to thank the biomedical staff of the Department of Biochemistry and Serology, and midwives of the Department of Obstetrics and Gynaecology of the Komfo Anokye Teaching Hospital, Ghana, for their support during the participant’s recruitment and biological sample processing. We also thank the research assistants of the Department of Molecular Medicine, Kwame Nkrumah University of Science and Technology for their support during the biological sample analysis. We finally thank the American Association of Clinical Chemistry (AACC) Academy Research Fellows for selecting our abstract coined from the present study entitled, ‘Algorithm of Suboptimal Health Status, Serum Magnesium and Calcium Levels as a Novel Approach for Prediction and Identification of Pregnant Women Likely to Develop Preeclampsia and Adverse Perinatal Complications in a Ghanaian Population’ for Scientific Excellence in Maternal and Foetal Medicine and AACC Academy’s Distinguished Abstract Award at the 71st AACC Scientific Annual Meeting, Anaheim, CA.
Abbreviations
- SHS
suboptimal health status
- OHS
optimal health status
- SHSQ-25
25-question-based suboptimal health status questionnaire
- GHOACS
Ghanaian Suboptimal Health Cohort Study
- PE
preeclampsia
- APCs
adverse pregnancy complications
- PPPM
preventive, predictive and personalized medicine
- IUGR
intrauterine growth restriction
- HELLP
hemolysis elevated liver enzymes and low platelet count
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- Mg
magnesium
- Ca
calcium
- Na
sodium
- K
potassium
- Cl
chloride
- LDH
lactate dehydrogenase
- UA
uric acid
- RDW
red cell distribution width
- FBG
fasting blood glucose
- TG
triglyceride
- TC
total cholesterol
- HDL-c
high-density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma glutamyl transferase
- TP
total protein
- ALB
albumin
- ALP
alkaline phosphatase
- aOR
adjusted odds ratio
- CI
confidence interval
- ROC
receiver’s operating characteristics
- AUC
area under the ROC curve
Authors’ contribution
EOA, PR, DC and WW conceived the study. EOA and CAT performed the investigation and collected the data. EOA performed the statistical analysis. EOA, PR, DC, EA, YW and WW wrote the paper. All authors read and approved the final manuscript.
Funding information
This work was supported by the Australia-China International Collaborative Grant (NHMRC-APP1112767-NSFC81561120) and Edith Cowan University (ECU)-Collaborative Enhancement Scheme Round 1 (G1003363). Enoch Odame Anto was supported by ECU-International Postgraduate Research Scholarship.
Compliance and ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
Not applicable.
Ethical approval and consent to participate
Approval for this study was obtained from the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Science (SMS) /KNUST and Komfo Anokye Teaching Hospital (KATH) (CHRPE/AP/146/17) and the Human Research Ethics Committee of Edith Cowan University (ECU) (17509). This study was conducted in accordance with the guidelines of the Helsinki Declaration. Written informed consent in the form of a signature and fingerprint was obtained from all participants and legally authorized representatives after the protocol of the study was explained to them in plain English language and native Ghanaian language where appropriate.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet (London, England) 2016;387(10017):462–474. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–13. 10.2215/cjn.12081115. [DOI] [PMC free article] [PubMed]
- 3.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37(4):386–397. doi: 10.1016/j.semnephrol.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Sumankuuro J, Crockett J, Wang S. Maternal health care initiatives: causes of morbidities and mortalities in two rural districts of Upper West Region, Ghana. PLoS One. 2017;12(8):e0183644. doi: 10.1371/journal.pone.0183644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adua E, Frimpong K, Li X, Wang W. Emerging issues in public health: a perspective on Ghana’s healthcare expenditure, policies and outcomes. EPMA J. 2017;8(3):197–206. doi: 10.1007/s13167-017-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandesh P, Bruce H, Yadav B, Sharma P. Psychosocial stress during pregnancy and its relation to fetal outcome: a study from Patan Hospital, Lalitpur, Nepal. J Instit Med. 2018;41(2):73–79. [Google Scholar]
- 8.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golubnitschaja O, Kinkorova J, Costigliola V. Predictive, preventive and personalised medicine as the hardcore of ‘Horizon 2020’: EPMA position paper. EPMA J. 2014;5(1):6. doi: 10.1186/1878-5085-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemke HU, Golubnitschaja O. Towards personal health care with model-guided medicine: long-term PPPM-related strategies and realisation opportunities within ‘Horizon 2020’. EPMA J. 2014;5(1):8. doi: 10.1186/1878-5085-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golubnitschaja O. Time for new guidelines in advanced diabetes care: paradigm change from delayed interventional approach to predictive, preventive & personalized medicine. EPMA J. 2010;1(1):3–12. doi: 10.1007/s13167-010-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Y-X, Liu Y-Q, Li M, Hu P-F, Guo A-M, Yang X-H, Qiu JJ, Yang SS, Shen J, Zhang LP, Wang W. Development and evaluation of a questionnaire for measuring suboptimal health status in urban Chinese. J Epidemiol. 2009;19(6):333–341. doi: 10.2188/jea.JE20080086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Russell A, Yan Y. Traditional Chinese medicine and new concepts of predictive, preventive and personalized medicine in diagnosis and treatment of suboptimal health. EPMA J. 2014;5(1):4. doi: 10.1186/1878-5085-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Ge S, Yan Y, Wang A, Zhao Z, Yu X, Qiu J, Alzain MA, Wang H, Fang H, Gao Q, Song M, Zhang J, Zhou Y, Wang W. China suboptimal health cohort study: rationale, design and baseline characteristics. J Transl Med. 2016;14(1):291. doi: 10.1186/s12967-016-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Yan Y. Suboptimal health: a new health dimension for translational medicine. Clin Transl Med. 2012;1(1):28. doi: 10.1186/2001-1326-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge S, Xu X, Zhang J, Hou H, Wang H, Liu D, Zhang X, Song M, Li D, Zhou Y, Wang Y, Wang W. Suboptimal health status as an independent risk factor for type 2 diabetes mellitus in a community-based cohort: the China suboptimal health cohort study. EPMA J. 2019;10(1):65–72. doi: 10.1007/s13167-019-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan YX, Dong J, Liu YQ, Yang XH, Li M, Shia G, et al. Association of suboptimal health status and cardiovascular risk factors in urban Chinese workers. J Urban Health. 2012;89(2):329–38. 10.1007/s11524-011-9636-8. [DOI] [PMC free article] [PubMed]
- 18.Wang Y, Liu X, Qiu J, Wang H, Liu D, Zhao Z, Song M, Song Q, Wang X, Zhou Y, Wang W. Association between ideal cardiovascular health metrics and suboptimal health status in Chinese population. Sci Rep. 2017;7(1):14975. doi: 10.1038/s41598-017-15101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adua E, Roberts P, Wang W. Incorporation of suboptimal health status as a potential risk assessment for type II diabetes mellitus: a case-control study in a Ghanaian population. EPMA J. 2017;8(4):345–355. doi: 10.1007/s13167-017-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kupaev V, Borisov O, Marutina E, Yan YX, Wang W. Integration of suboptimal health status and endothelial dysfunction as a new aspect for risk evaluation of cardiovascular disease. EPMA J. 2016;7(1):19. doi: 10.1186/s13167-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzain MA, Asweto CO, Zhang J, Fang H, Zhao Z, Guo X, et al. Telomere length and accelerated biological aging in the China suboptimal health cohort: a case-control study. Omics. 2017;21(6):333–9. 10.1089/omi.2017.0050. [DOI] [PubMed]
- 22.Yan YX, Dong J, Liu YQ, Zhang J, Song MS, He Y, et al. Association of suboptimal health status with psychosocial stress, plasma cortisol and mRNA expression of glucocorticoid receptor alpha/beta in lymphocyte. Stress (Amsterdam, Netherlands) 2015;18(1):29–34. doi: 10.3109/10253890.2014.999233. [DOI] [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 24.Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R, W Shand A, Lavin T, Pereira G. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG : Int J Obstet Gynaecol. 2018;125(2):183–192. doi: 10.1111/1471-0528.14906. [DOI] [PubMed] [Google Scholar]
- 25.Alese MO, Moodley J, Naicker T. Preeclampsia and HELLP syndrome, the role of the liver. J Matern Fetal Neonatal Med. 2019:1–7. 10.1080/14767058.2019.1572737. [DOI] [PubMed]
- 26.Rao S, Jim B. Acute kidney injury in pregnancy: the changing landscape for the 21st century. Kidney Int Rep. 2018;3(2):247–257. doi: 10.1016/j.ekir.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasioudis D, Doulaveris G, Kanninen TT. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. 2019;71(2):155–162. doi: 10.23736/S0026-4784.18.04330-7. [DOI] [PubMed] [Google Scholar]
- 28.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94(2):247–257. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 29.LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, Wallace K. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2016;311(1):R1–R9. doi: 10.1152/ajpregu.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol. 2017;29(6):383–389. doi: 10.1097/GCO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 31.Perry H, Khalil A, Thilaganathan B. Preeclampsia and the cardiovascular system: an update. Trends Cardiovasc Med. 2018;28(8):505–513. doi: 10.1016/j.tcm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Carter W, Bick D, Mackintosh N, Sandall J. A narrative synthesis of factors that affect women speaking up about early warning signs and symptoms of pre-eclampsia and responses of healthcare staff. BMC Pregnancy Childbirth. 2017;17(1):63. 10.1186/s12884-017-1245-4. [DOI] [PMC free article] [PubMed]
- 33.Delahaije D, Dirksen C, Peeters L, Smits L. PP105. Mental health problems following preeclampsia or HELLP syndrome: do we have a case? A systematic review. Pregnancy Hypertens. 2012;2(3):296. doi: 10.1016/j.preghy.2012.04.216. [DOI] [PubMed] [Google Scholar]
- 34.Ephraim RK, Osakunor DN, Denkyira SW, Eshun H, Amoah S, Anto EO. Serum calcium and magnesium levels in women presenting with pre-eclampsia and pregnancy-induced hypertension: a case-control study in the Cape Coast metropolis, Ghana. BMC Pregnancy Childbirth. 2014;14:390. doi: 10.1186/s12884-014-0390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo X, Xu L, Huang J, Zhao M. Case-control study on serum calcium and magnesium levels in women presenting with preeclampsia. BMC Pregnancy Childbirth. 2017;20(14):390. doi: 10.1186/s12884-014-0390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djagbletey R, Darkwa EO, de Graft-Johnson PK, DAY S, Essuman R, Aryee G, et al. serum calcium and magnesium levels in normal Ghanaian pregnant women: a comparative cross-sectional study. Open Access Macedonian J Med Sci. 2018;6(11):2006–11. doi: 10.3889/oamjms.2018.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anto EO, Owiredu WKBA, Sakyi SA, Turpin CA, Ephraim RKD, Fondjo LA, et al. Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: a prospective cohort study in Ghana. PLoS One. 2018;13(7):e0200581. doi: 10.1371/journal.pone.0200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ephraim R, Doe P, Amoah S, Antoh E. Lipid profile and high maternal body mass index is associated with preeclampsia: a case-control study of the Cape Coast Metropolis. Ann Med Health Sci Res. 2014;4(5):746–750. doi: 10.4103/2141-9248.141542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demirci O, Tugrul AS, Dolgun N, Sozen H, Eren S. Serum lipids level assessed in early pregnancy and risk of pre-eclampsia. J Obstet Gynaecol Res. 2011;37(10):1427–1432. doi: 10.1111/j.1447-0756.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- 40.Tangren JS, Wan M, Adnan WAH, Powe CE, Ecker J, Bramham K, et al. Risk of Preeclampsia and Pregnancy Complications in Women With a History of Acute Kidney Injury. Hypertension. 2018;72(2):451–9. 10.1161/hypertensionaha.118.11161. [DOI] [PMC free article] [PubMed]
- 41.O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017;7(1):5887. doi: 10.1038/s41598-017-06178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anto EO, Roberts P, Turpin CA, Wang W. Oxidative stress as a key signaling pathway in placental angiogenesis changes in preeclampsia: updates in pathogenesis, novel biomarkers and therapeutics. Curr Pharmacogenomics Pers Med (Formerly Current Pharmacogenomics) 2018;16(3):167–181. doi: 10.2174/1875692117666181207120011. [DOI] [Google Scholar]
- 43.Rondo PH, Ferreira RF, Nogueira F, Ribeiro MC, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr. 2003;57(2):266–272. doi: 10.1038/sj.ejcn.1601526. [DOI] [PubMed] [Google Scholar]
- 44.Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006;1092:138–147. doi: 10.1196/annals.1365.012. [DOI] [PubMed] [Google Scholar]
- 45.Gibbins KJ, Silver RM, Pinar H, Reddy UM, Parker CB, Thorsten V, Willinger M, Dudley DJ, Bukowski R, Saade GR, Koch MA, Conway D, Hogue CJ, Stoll BJ, Goldenberg RL. Stillbirth, hypertensive disorders of pregnancy, and placental pathology. Placenta. 2016;43:61–68. doi: 10.1016/j.placenta.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakash J, Ganiger VC, Prakash S, Iqbal M, Kar DP, Singh U, Verma A. Acute kidney injury in pregnancy with special reference to pregnancy-specific disorders: a hospital based study (2014-2016) J Nephrol. 2018;31(1):79–85. doi: 10.1007/s40620-017-0466-y. [DOI] [PubMed] [Google Scholar]
- 47.Gallos ID, Sivakumar K, Kilby MD, Coomarasamy A, Thangaratinam S, Vatish M. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. BJOG. 2013;120(11):1321–32. 10.1111/1471-0528.12375. [DOI] [PubMed]
- 48.Liu D, Chu X, Wang H, Dong J, Ge SQ, Zhao ZY, Peng HL, Sun M, Wu LJ, Song MS, Guo XH, Meng Q, Wang YX, Lauc G, Wang W. The changes of immunoglobulin G N-glycosylation in blood lipids and dyslipidaemia. J Transl Med. 2018;16(1):235. doi: 10.1186/s12967-018-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]