Highlights
-
•
Immune responses may be detrimental to both the safety and efficacy of gene therapy.
-
•
Design and manufacturing can be tuned to reduce vector immunogenicity.
-
•
Active transgene-specific immune tolerance is desirable in gene therapy.
-
•
Novel targeted immune-modulatory strategies can be explored to improve gene therapy.
Keywords: Gene therapy, Immune responses in gene therapy, Transgene-specific immune tolerance, Immune-modulation strategies in gene therapy
Abstract
Lentiviral vectors (LV) are widely used vehicles for gene transfer and therapy in pre-clinical animal models and clinical trials with promising safety and efficacy results. However, host immune responses against vector- and/or transgene-derived antigens remain a major obstacle to the success and broad applicability of gene therapy. Here we review the innate and adaptive immunological barriers to successful gene therapy, both in the context of ex vivo and in vivo LV gene therapy, mostly concerning systemic LV delivery and discuss possible means to overcome them, including vector design and production and immune modulatory strategies.
1. Introduction
Lentiviral vectors (LVs) are replication-defective hybrid enveloped viral particles made by a minimal set of capsid proteins of the parental virus (in most cases HIV), a surface protein of an unrelated virus (referred to as pseudotype) and a recombinant viral genome, comprising the cis acting elements of the parental virus strictly required for gene transfer purposes, and a transgene expression cassette of choice [1]. The vesicular stomatitis virus surface glycoprotein (VSV.G) is often used to pseudotype LVs, as it confers high stability and wide tropism [2], [3]. LVs are emerging as powerful and versatile gene delivery vehicles, by virtue of i) their ability to efficiently transfer genes into (i.e. transduce) a variety of dividing and non-dividing cell types and stably integrate their genome into the target cell chromatin, ii) their relatively large cargo capacity and iii) the lower prevalence of immunity against vector components in humans compared to that of other virus-derived gene transfer vectors [4], [5], [6], [7]. Currently, LVs are involved in 7% of all the gene therapy clinical trials worldwide and in 19% of those for monogenic diseases (http://www.abedia.com/wiley/index.html – updated April 2018). LVs have been exploited for gene therapy applications both ex vivo, in which target cells are collected from the recipient, genetically modified, and then infused back into the recipient, and in vivo, in which LVs are directly administered into the recipient, either locally, such as in the brain or the eye, or systemically to reach organs such as the liver or the spleen [4]. While ex vivo LV gene therapy with hematopoietic stem and progenitor cells (HSPCs) or T cells is in advanced clinical testing [8], [9], [10], [11], [12], in vivo LV gene therapy is mostly at a pre-clinical stage of development [13], [14], [15].
Immune responses directed towards LVs, transgene product, or both may limit the efficacy and safety of gene therapy [16], [17]. After administration of the vector or vector-transduced cells, a primary immune response against the LV envelope or capsid proteins can occur [18]; in this case, it will likely limit re-administration of the same vector or cell product, but it should not affect the efficacy and safety of the procedure, as LV-derived antigens (Ags) are not maintained in the recipient. Indeed, LV lack viral genes, thus viral proteins are not actively produced by LV transduced cells. On the contrary, immune reactivity against vector components pre-existing to LV administration (such as following exposure to the parental virus) may inactivate the vector, inhibiting transduction, and/or attack transduced cells while still exposing vector-derived Ags, as shown in some studies using adeno-associated virus (AAV) derived vectors [19], [20]. Because humans are not the natural hosts of VSV infection [21], it is highly unlikely to find specific immunity against VSV.G in humans, although non-specific cross reacting anti-VSV.G antibodies (Abs) may be present in humans [22], [23]. HIV-infected individuals may have LV-capsid specific immunity, but its impact on LV gene therapy has not been yet investigated. In a clinical trial, HIV-infected patients have been administered with autologous T cells previously transduced with a LV expressing an anti-HIV antisense RNA [24]. The reported persistence of these LV-modified T cells for several years suggests that a pre-existing anti-HIV immunity, if present, did not affect the transduced T cells, although T cells were infused several days after exposure to LV and expansion in culture, thus they may have been free of LV-derived Ags. Interestingly, these patients received multiple infusions of LV-transduced T cells and the second and third infusions appeared to increase the graft size, again suggesting that anti-LV capsid or anti-VSV.G immune responses were not induced in these patients after the first administration of LV-transduced T cells, probably because the LV-transduced T cells did not carry over LV- or VSV.G-derived Ags.
The expression of transgene after LV gene therapy can induce a primary anti-transgene product immune response, which can involve both the humoral and cellular arms of the immune system. Anti-transgene Abs can neutralize transgene activity and/or decrease its half-life, in case of secreted transgenes, while cytotoxic T lymphocytes (CTLs) directed against transgene-derived Ags may cause elimination of transduced cells. Immunity against transgene-derived Ags may also be pre-existing to the gene therapy, in some cases, such as in patients affected by a monogenic disease and treated with a protein replacement therapy [25]. Anti-transgene product immune responses may be detrimental to both the efficacy and safety of the LV gene therapy and should be carefully monitored and avoided, except in those cases in which anti-transgene product immunity is the scope of the intervention, such as in genetic vaccine or some immunotherapy applications [26].
2. Immune responses to ex vivo LV gene therapy
Ex vivo cell-based LV gene therapy is not exempt by anti-vector or anti-transgene product immune responses. While recipients are not directly exposed to LVs, carry-over of vector-derived Ags on infused cells may induce primary anti-vector immune responses. LV-transduced T cells, as mentioned above, are usually expanded in culture for several days before administration, thus rapidly diluting vector-derived Ags. Therefore, transduced T cells are unlikely to carry-over viral Ags and induce anti-LV immune response by exposing LV-derived epitopes in class-I major histocompatibility complex (MHC-I) at the time of injection [27], [28]. However, it has been reported that some patients receiving LV-modified autologous T cells mounted an immune response against the delivered transgene product, such as the murine portions of chimeric antigen receptors (CARs) designed against tumor-associated Ags, which limited the persistence of the CAR-expressing T cells [28]. Higher intensity lympho-depleting regimens were shown to reduce such immune responses [28], [29] and removal of the murine sequences may also reduce their occurrence.
LV-transduced HSPCs are not cultured for as many days as T cells after transduction, thus they may introduce viral Ags in the recipient and induce anti-LV immune responses. The possible occurrence of such responses remains to be clarified in pre-clinical models or clinical trials, although it is not expected to directly interfere with the efficacy or safety of the gene therapy, as transduced cells will dilute out LV-derived Ags following administration to the recipient. Re-administration of the transduced HSPCs is not required, as LV-modified HSPCs are maintained long-term. Moreover, in order to allow engraftment of transduced HSPCs, patients are exposed to conditioning regimens, which are often immune suppressive and thus reduce the likelihood of immune responses at the time of transplant [10], [11], [30]. On the contrary, some patients affected by monogenic diseases, such as lysosomal storage diseases (LSD), and previously treated by enzyme replacement therapy, may have already mounted cellular and/or humoral immune responses against the therapeutic transgene product before the gene therapy, which may jeopardize the efficacy and safety of the therapy. In this case, careful pre-clinical modeling and the choice of an appropriate lympho-depleting conditioning regimen might be crucial.
3. Immune responses to in vivo LV gene therapy
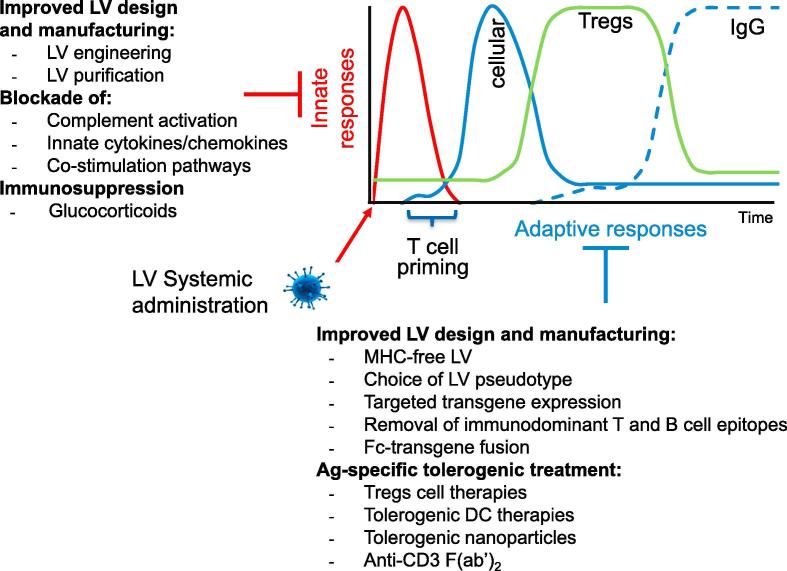
Gene transfer technology has made considerable advances in targeting specific cell types and in the efficiency and regulation of transgene expression; nevertheless, a major obstacle for achieving stable therapeutic efficacy after in vivo LV gene therapy is the development of specific immune responses, which can neutralize activity of the transgene product and lead to the clearance of transgene expressing cells. Local LV delivery facilitates access to target cells and reduces spread of vector- and transgene-derived Ags, limiting immune responses, especially in immune privileged sites, such as the eye or the central nervous system, even if, leaking of vector or transgene-derived Ags into the bloodstream may initiate immune responses [13], [15], [18], [31], [32]. More frequently disease correction requires delivery of a vector to immune-competent organs and robust local or systemic expression of a therapeutic protein. In the latter scenario, the capacity of LV to transduce antigen presenting cells (APCs) [33] inherited from the parental virus play a crucial role in the induction of innate and adaptive immune responses against vector- or transgene-derived Ags, which may limit the efficacy and safety of in vivo LV gene therapy (Fig. 1).
Fig. 1.
Overview of the immune responses to LV systemic administration and possible strategies to modulate them.
3.1. Innate immune responses to LV
The first line of defense against viral infections consists in the innate immune response induced by the complement system, circulating and tissue-resident phagocytes and APCs. VSV.G-pseudotyped LVs have long been shown to be sensitive to complement-mediated inactivation in human serum [22], likely due to the presence of cross-reacting not neutralizing but complement-fixing anti-VSV.G Abs in humans. We have recently shown that complement-mediated LV inactivation is LV-dose dependent, and rapidly saturated at LV concentrations in the serum samples similar to those expected in the circulation shortly after infusion, based on the administered LV doses [14], [34], [35]. This finding might thus explain the successful liver gene transfer obtained in dogs, despite a similar neutralizing activity of LV as found in human sera. Rather than preventing gene transfer to the intended target tissue, complement activation may still have some detrimental consequences, such as triggering innate responses, mediating phagocytosis of LV through complement receptors and thus displacing vector from the intended target, and, possibly, consumption of complement in the circulation. Thus, it may be desirable to prevent such activation at least if large amounts of particles are administered. We have also reported that the lower the content of VSV.G on the LV surface, the more resistant they are to complement-mediated inactivation, confirming the role of VSV.G in determining LV sensitivity to complement [35]. Indeed, we showed that complement-blocking Abs fully preserved LV infectivity in human serum samples. Other groups have provided evidence that alternative LV pseudotypes, co-display of complement-regulatory proteins on LV particles, PEGylated VSV.G or VSV.G mutants generated by directed evolution, can increase the resistance of LVs to complement-mediated inactivation [36], [37], [38], [39].
LV-binding Abs and activated complement components can opsonize LV particles for phagocytosis by liver and spleen macrophages and APCs. These cells are also alert sensors of pathogen-associated molecular patterns (PAMPs), conserved structures of microbial origin, such as the LV RNA, which act as danger signals for the immune system and promote inflammation, APC activation and productive Ag presentations to the adaptive arms of the immune system [40], [41], [42]. Studies by others and ourselves have shown that the LV RNA genome and the reverse transcribed proviral DNA genome trigger the activation of innate immune sensors, in particular plasmacytoid dendritic cells (pDCs), by engaging Toll-like receptor (TLR) 3 and TLR7 induce a type-I interferon (IFN) response [43], [44], [45], [46]. We have reported a transient increase in some pro-inflammatory cytokines in dogs upon portal vein LV administration, indicating LV-mediated activation of innate immunity and subsequent inflammation [14]. We have more recently evaluated the cytokine response to LV administration in mice and observed a rapid transient increase of some pro-inflammatory cytokines, starting a few hours after LV administration (unpublished data). These cytokines may recruit inflammatory cells, interfere with the efficiency of cell transduction, as well as, promote the initiation of the adaptive immune response by activating APCs [44], [46], thus promoting priming of Ag-specific T cell responses [42].
Residual plasmid DNA used to produce LVs by transient transfection of vector components and contaminating lab-grade and, to a lesser extent, purified LV batches may contribute to the activation of innate immune cells, due to its bacterial origin [14]. As the clinical application of LV-based gene therapy continues to expand and eventually progress to commercialization, it is likely that stable LV producer cell lines will be preferred over transient transfection, to meet the scale and standardization requirements and, as a consequence, LV produced by stable cell lines will lack contaminating plasmid DNA [35], [47]. Proteomic analysis of LV batches purified by a two-step chromatography process (anion exchange and size exclusion) revealed the presence of a number of producer-cell derived proteins, besides LV capsid-specific and the VSV.G envelope proteins [48]. Whether these producer-cell proteins are incorporated into virions or associated with debris originating from producer cells remains to be clarified, nonetheless protein aggregates or dying cells debris may represent additional pro-inflammatory stimuli. For these reasons, the LV production/purification process is expected to impact on the innate immune response following LV administration and further optimization of LV manufacturing to reduce additional TLR agonists is desirable, particularly for clinical-grade LV batches dedicated to systemic administration.
Furthermore, to reduce the innate response evoked by LV, a growing number of monoclonal antibodies (mAbs) or small molecular compound are now available to selectively block/inactivate cytokines, receptors, and co-stimulatory molecules, or to deplete/inactivate a specific cell subset. Administration of blocking Abs specific for type-I IFNs receptor (IFNaR1) [49], [50], [51], interleukin (IL)-6 and IL-6 receptor (IL-6R) [52], [53] and IL-1 [54], [55] has been successfully used to modulate immune responses in several settings and may be applied in the context of in vivo LV gene therapy to reduce innate and adaptive immunity to LV-encoded Ags. Our group showed that type-I IFNs inhibit transduction efficiency, specifically within the liver, contribute to immune-mediated clearance of transduced cells, and that LV gene transfer into IFNaR1−/− mice leads to persistent xeno-antigen expression [44]. However, the pleiotropic nature of the above-mentioned mediators needs to be taken into consideration, when interfering with their pathways following LV administration. As an example, IL-6, which is induced by IL-1, IL-2, tumor necrosis factor (TNF-α), and IFNs and released mainly by monocytes, but also by fibroblasts, endothelial, T, and B cells, plays a role in different immune processes. IL-6 is involved both in innate responses as neutrophil activator and potent inducer of terminal macrophage differentiation and in adaptive responses as inducer of final maturation stages of B lymphocytes and T cell growth and CTLs. Although IL-1 and TNF-α induce each other and IL-6 synthesis, the latter terminates this up-regulatory inflammatory cascade, suppressing IL-1 and TNF-α synthesis. Therefore, timing and duration of treatments blocking pleiotropic cytokines of the innate immune system need to be accurately considered to design combined therapy to achieve modulation of adaptive responses after LV gene therapy.
Other drug-based strategies have been already explored to reduce the acute inflammation induced by the activation of the innate response after systemic LV administration. Dexamethasone (DEX, an anti-inflammatory glucocorticoid) is a widely used drug for the treatment of transient and/or chronic inflammatory conditions. DEX and other corticosteroids have been tested in the context of viral vectors-mediated gene transfer, specifically a short DEX regimen administration before/after LV administration resulted in suppression of the innate response mediators and a consequent increase of transduction [45], [56]. However, it remains to be defined whether this approach could also help in the induction of transgene-encoded Ag-specific immune tolerance by LV. How the innate immune response to systemic LV administration changes according to the LV dose, pseudotype, method of production, mouse strain or recipient species remains largely unexplored. Overall, the known interactions of LVs with the innate immune system are multiple and should be taken into careful consideration in designing less immunogenic gene therapies which are more tolerated in the early phases after administration and more likely to induce transgene-encoded Ag-specific immune tolerance.
3.2. Adaptive immune responses to LV
The second line of immune defense consists in the activation of the adaptive immunity, both humoral and cell-mediated, that leads to the generation of specific immune responses directed to vector- and transgene-derived Ags (Fig. 1). The LV-derived viral Ags are expected to induce a robust immune response against them, in both animal models and humans, after systemic LV administration [18]. The immunological memory of this immune response will likely hamper effective subsequent re-administration of the same LV. Changing the LV pseudotype may not be sufficient to evade such an immune response, due to the presence of capsid-derived Ags. However, a detailed characterization of anti-LV immune responses after LV systemic delivery and of their role in LV re-administration remains to be investigated in animal models.
LVs are enveloped viral particles, thus their surface acquires part of the plasma membrane of producer cells, during the process of budding. Since LVs are produced by cells of human origin, most LV membrane proteins should be tolerated by humans. However, the polymorphic MHC-I is a major trigger of allogeneic immune responses [57]. We have recently shown that LVs carry the MHC-I of producer cells on their surface and that human T cells are activated upon co-culture with LV particles-exposed autologous human APCs, suggesting that an allogeneic immune response may occur in vivo after LV administration to humans. To prevent the potential allogeneic immune response raised against the MHC-I inadvertently co-delivered by the LV particles, we have generated LVs devoid of MHC-I, by genetic inactivation of the beta-2 microglobulin gene and subsequent impairment of MHC-I trafficking to the membrane in LV producer cells. The resulting MHC-free LVs maintained full infectivity but showed substantially reduced immunogenicity for human T cells [35].
3.3. Adaptive immune responses to the transgene product
The outcome of the immune response elicited towards transgene-derived Ags following LV administration, ranging between active immunity, ignorance and active immune tolerance, can vary according to a number of different factors, such as the nature of the transgene-product itself, the LV dose, level and pattern of transduction and transgene expression following LV administration, the local and systemic context of immune modulatory cytokines and immune cell types, the genetic background and type of mutation underlying the genetic disease of the recipient. The primary structure of the vector-encoded protein and haplotype of the host are essential elements for the immunogenicity of a given transgene, determining also which and how many epitopes will be presented to T cells. The degree of “novelty” of a certain transgene can have strong impact on the induction of adaptive response. The transgene product will be more likely tolerated as a consequence of central tolerance process, if it shares homology to other self-Ags or it is partially “known” to the recipient, depending on the disease-causing mutation [58]. Another critical aspect is the final localization of the transgene-encoded protein: secreted proteins are more accessible for cells of the immune system and more susceptible to be detected, captured and presented for the induction of adaptive immunity [59].
VSV.G pseudotyping confers to LV the capacity to transduce a wide spectrum of cell types, including macrophages and dendritic cells (DC). Therefore, recipient APCs present transgene-encoded Ag and efficiently prime Ag-specific CD8+ CTLs [60], which, once induced, can target the transgene-expressing cells for killing. This property has been exploited to trigger anti-tumor and anti-pathogen immunity by LV delivery of tumor associated Ags or viral determinants [61], [62]. Following presentation of transgene-derived Ags, B cells can proliferate and differentiate, both into plasma cells (PC) that secrete high-affinity Abs specific for the transgene product, and to memory B cells, ready for a secondary Ag encounter [63]. The development of neutralizing Abs (nAbs) directed to the LV-encoded protein may enhance its clearance through opsonization and/or inactivate its activity. These adaptive T and B cell responses occur when naïve T and B cells recognize their cognate Ag. APCs play a pivotal role in this process since they sample, process, and present Ag epitopes in the context of MHC-I, and MHC-II molecules to CD8+ T cells and CD4+ T helper cells, respectively. Upon Ag recognition, T cell activation and differentiation depend on signal 1, i.e. the strength of interactions of MHC and T cell receptor (TCR), signal 2, i.e. the co-stimulation, and signal 3, i.e. the cytokine milieu at priming [64]. Given that LV particles, as most viruses and viral vectors, may activate innate immune cells, they may aggravate the risk of immune responses towards the transgene product because of induction of inflammatory context at presentation.
The development of CTLs and Abs targeting the transgene product represent one of the major hurdles for long-term in vivo transgene maintenance and the success of the gene therapy. For this reason, in the last decades, gene therapists have dedicated a lot of efforts to design suitable viral vector platforms for in vivo gene transfer able to promote long-term therapeutic levels of the transgene [33], [65], [66], [67], [68] sustained by persistent transgene-product specific immunological tolerance. Immunological tolerance is a process required to maintain unresponsiveness of the immune system towards specific Ags. In nature, several mechanisms are involved in promoting tolerance: passive tolerance, a quiescent immunological state where T cells do not encounter their cognate Ag or where effector T cells are deleted or anergized upon Ag recognition. Conversely, active tolerance is a dynamic process whereby regulatory T cells (FoxP3-expressing (Tregs) and type 1 regulatory, (Tr1) cells) suppress immune responses in an Ag-specific and non-specific manner [69], [70], [71]. The liver, conjugating high synthetic and secretory activity with a tolerogenic microenvironment, represents an optimal target organ for in vivo gene addition, to correct many metabolic or hematologic genetic diseases, which often are caused by monogenic defects of hepatic cells. The liver is one of the largest organs in the body whose primary function is to “filter” and metabolize proteins and toxins in the blood, therefore the liver is a unique immunological site where circulating Ags and immune cells can meet. A distinctive trait of the blood bound for the liver is the elevated concentration of inflammatory bacterial and viral proteins that have been absorbed through the gut mucosa. Ordinarily, the presence of stimulatory signals of this nature induces potent inflammatory responses; however, normally the liver remains immunologically quiescent. The fact that these stimulatory agents do not induce robust, chronic immunity is mostly attributed to the potent tolerogenic mechanisms developed by the liver to suppress chronic inflammatory reactions. The tolerogenic activity of the liver is partly attributed to the composition and diversity of APC and the high levels of regulatory cytokines [72], [73], [74]. Notwithstanding the liver intrinsic pro-tolerogenic features, accumulated evidence by others and us indicated that the pattern of transgene expression in different cell types in vivo is a crucial factor governing the induction of tolerance rather than immunity after in vivo gene therapy and that avoiding transgene expression in APCs favors tolerance induction [5], [17], [43], [75]. Over the years, our group has exploited genetic regulatory elements to allow stable and high levels of transgene expression selectively into hepatocytes, while minimizing off-target expression in APCs [66], [76]. Hepatocyte-restricted expression of coagulation factor IX (FIX) imposed by hepatocyte-specific promoters, such as Enhanced Transthyretin (ET), positive transcriptional regulation, and microRNA-142 target sequences included in the LV, negative post-transcriptional regulation, allowed stable reconstitution of FIX activity in mouse and dog models of hemophilia B, a coagulation disorder due to reduced or absent FIX activity [14], [77]. MicroRNA-142 target sequences incorporated in the vector 3′ untranslated region are meant to minimize off-target expression originating from the ET promoter in hematopoietic-lineage cells, such as liver and spleen APC, thus reducing the likelihood of induction of immunity to the encoded Ag. Further studies in mouse models indicated that stable transgene activity was achieved by induction of active immunological tolerance sustained by the action of Ag-specific Foxp3+ Tregs [78], [79] in naïve, as well as in nAbs-positive hemophilia B mice [80]. Later, the immuno-regulatory properties of the above-described LV platform have been successfully exploited to control the development of autoimmune type 1 diabetes (T1D) in non-obese diabetic (NOD) mice [81]. The Baculovirus GP64 envelope protein, used as LV pseudotype, has been shown to confer tropism restriction against hematopoietic-lineage cells, thus representing an additional layer of regulation to avoid LV transduction of APCs and improve targeting of expression into hepatocytes to further promote or achieve tolerance to transgene product [82], [83]. The correlation between stable transgene expression targeted to hepatocytes and induction of active Ag-specific tolerance that was highlighted by applications of this LV platform is in line with previous evidences obtained by AAV vectors for liver gene therapy, which also showed requirement for hepato-specific expression to achieve long-term transgene expression and tolerance [65], [75]. Besides targeting hepatocytes, alternative combinations of transcriptional and post-transcriptional regulatory elements to support cell type specific expression patterns, also involved in tolerance induction, have been proposed for LV-mediated gene therapy of hemophilia A, a coagulation disorder due to reduced or absent clotting factor VIII (FVIII) activity. Targeting of FVIII expression to liver sinusoidal endothelial cells (LSECs), one of the physiological source of FVIII, by endothelial-specific vascular endothelial cadherin promoter and post-transcriptional regulation achieved by incorporating binding sites for microRNA-122 and microRNA-142 to control off-target expression in hepatocytes and APC, respectively, enabled stable FVIII expression, prevented the formation of nAbs and induced FVIII-specific immune tolerance [84]. Interestingly, a comparable tolerogenic outcome was achieved in some mouse strains when FVIII expression was limited to macrophages and conventional dendritic cells (cDC) in the liver and spleen by using LVs with CD11b promoter and microRNA-126 binding sites, which reduce FVIII expression in pDCs [85]. These data indicate that pDC play a direct role not only in innate response to LV, but also in the induction of anti-FVIII adaptive immune response. Therefore, microRNA-126 mediated post-transcriptional regulation may represent an additional strategy to reduce the immunogenicity of LV-mediated in vivo gene therapy.
4. Immunomodulatory approaches to modulate adaptive immune responses to transgene encoded Ag
Although LVs for in vivo gene transfer were improved in their design to achieve stable transgene expression and tolerance in some experimental settings, we should not consider it as universally applicable due to the multiple and unpredictable obstacles towards clinical application, which may drive the immune balance toward immunity. Combination of factors such as the strength of innate responses triggered by LV components and contaminants, genetic background of the host (MHC I and II molecules), primary structure and final localization of the LV-encoded protein may impact on the immunogenicity of gene therapies. Thus, several possibilities of intervention may be considered in combination with gene therapy to promote Ag-specific tolerance (Fig. 1).
An actual strategy to favor tolerance induction is the modification of the Ag primary structure to reduce its immunogenicity. The identification and modification of immuno-dominant T cell epitopes have been explored to design of less immunogenic FVIII proteins by substitution of one or more anchor residues of MHC peptide-binding in multiple HLA [86]. Similarly, modification of B cell epitopes through substitution of surface-exposed amino acid is a promising approach to interfere with FVIII attachment to Abs and memory B cells [87]. Additionally, the fusion of proteins with the Fc region of immunoglobulins, designed to extend the half-life, resulted also to be immunomodulatory [88], [89]. It has been shown that processing of Fc-Ag leads to presentation of Ag-derived epitopes together with Fc-derived ones, which include epitope sequences, termed Tregitopes able to promote immune tolerance activating and expanding Foxp3+ Tregs [90].
As mentioned above, professional APCs play a pivotal role in determining the fate of immune responses directed to LV-encoded Ags providing co-stimulation and pro-inflammatory signals, therefore modulation/inhibition of APC co-stimulation may represent a potential strategy to favor the induction of Ag-specific tolerance. Cytotoxic T lymphocyte antigen 4 (CTLA-4)Ig is a fusion protein of the extracellular domain of CTLA-4 and IgG1 that binds to both CD80 and CD86 and prevents interaction of B7 proteins with their counterpart CD28 and CTLA-4 expressed on T cells [91]. The humanized version, Abatacept is approved for use in humans to treat Rheumatoid Arthritis and prevent renal transplant rejection. CTLA-4Ig immunoregulation occurs through CD28 pathway blockade that prevents initial T cell activation and appears to be dependent on Treg function and TGF-β [92]. CTLA-4Ig has been successfully administered in mucopolysaccharidosis type-I (MPS-I) cats following in vivo gene therapy with retroviral vectors, to suppress CTL development and stably restore α-l-iduronidase (IDUA) expression [93].
Promising results in the induction of Ag-specific Tregs mediated tolerance in vivo have been obtained by conditioning the APC compartment with biocompatible nanoparticles carrying the Ag and an immune-modulatory drug. Nanoplaticles loaded with Rapamycin, a mTOR inhibitor that induces a tolerogenic DC phenotype capable of inducing Treg differentiation and Ag-specific immune tolerance [94], [95] were co-administered with the Ag in mice to inhibit the development of autoimmunity and in the prevention and abrogation of anti-FVIII neutralizing humoral response in the context of a factor replacement therapy [96], [97]. However, there are contrasting reports regarding the immune functions and inhibition of mTOR in DC, since it has been shown to lead to pro- as well as anti-inflammatory T cell stimulation. In the periphery, DCs sense the environment and once activated rapidly produce pro-inflammatory signals and mTORC1 and mTORC2 activated by TLR ligands participate to these responses, thus Rapamycin inhibiting mTOR leads to immune regulation. Conversely, after maturation and migration to secondary lymphoid organs DC increase the expression of co-stimulatory and inhibitory molecules (such as PDL1) to dampen and eventually terminate T cell activation. Therefore, at this stage of the response mTOR inhibition may enhance Ag presentation, co-stimulation and IL-12 production blocking PD-L1 and IL-10 expression thus intensifying the immune response [98]. Therefore, the timing and route of Rapamycin-loaded nanoparticles administration needs to be carefully considered to design an effective tolerogenic protocol to support in vivo LV gene transfer.
Alternative nanotechnologies have been recently developed to actively regulate undesired responses in vivo. Nanoparticles coated with peptides bound to MHC-II molecules triggers the generation and expansion of Ag-specific Tr1 cells, which drive the differentiation of B regulatory cells in several mouse models [99]. This new technology is potentially applicable to control immune response to the transgene product; however, several limitations can be envisaged including the identification of immune-dominant transgene-derived epitopes and its MHC restriction.
Treg-based cell therapies for tolerance induction have been developed and translated in to the clinic for the treatment of autoimmunity and in transplantation settings [100], [101], [102]. We showed that administration of freshly isolated syngeneic polyclonal not activated Tregs was not able to control the immune response the LV-encoded Ag in immunocompetent mice immunized by in vivo LV administration [103]. Ex-vivo expanded Tregs have been shown to be more suppressive compared to freshly isolated Tregs [104]; adoptive transfer of ex vivo expanded autologous polyclonal Tregs modulated immune responses in gene and protein replacement therapies and the persistence of regulation was due to induction of Ag-specific Tregs in vivo [105]. Therefore, the generation of Ag-specific Tregs ex vivo can be exploited for tolerance induction in combination with in vivo LV gene therapy. In this scenario new strategies are now available either to impose a given Ag-specificity to cells that already are Tregs via CAR or TCR gene transfer technology [106], [107], [108], or to convert Ag-specific conventional T cells in regulatory T cells by overexpressing Foxp3 and IL-10 [109], [110], [111].
The capacity of DCs to promote T cell activation or tolerance is the rationale for DC-based cell therapy to promote immunity for cancer and infectious diseases or tolerance in immune-mediated diseases, including in vivo gene therapy (reviewed in [112]). Our group contributed to the identification of IL-10 as key factor for promoting the differentiation of potent tolerogenic DCs. We set up a protocol to differentiate tolerogenic DCs, named DC-10, from peripheral blood monocytes cultured in the presence of GM-CSF, IL-4, and IL-10. DC-10 expressing high levels of HLA-G and IL-10 are potent inducers of allo-specific Tr1 cells [112], [113]. Thus, it can also be envisaged the application of DC-10-based cell therapy to restore tolerance to a given immunogenic transgene-derived Ag, by converting T effector cells or naïve T cells to Ag-specific Tr1 in vivo.
Alternative tolerogenic approaches compatible with in vivo LV gene transfer can be designed to target directly T effector cell via selective depletion/inactivation and possibly support conversion or de novo induction of Ag-specific Tregs. In this scenario, non-Fc-binding anti-CD3 mAb (F(ab′)2) matches this requirement. This molecule has been shown to be able to efficiently revert T1D in NOD and now reached advanced clinical testing for the treatment of T1D patients. The anti-CD3 tolerogenic capacity develops initially by mediating antigenic down-modulation of the T-cell receptor CD3 complex, preferential induction of apoptosis in activated T-cells and induction of anergy in T cells, while in a second phase, it promotes TGF-β–dependent generation of Tregs [114], [115], [116]. Our group showed that a suboptimal dose of anti-CD3(F(ab′)2) synergizes with LV-mediated hepatic expression of InsulinB 9-23 peptide in reverting T1D and re-stablishing tolerance, where treatment with the LV alone was not sufficient [81]. Therefore, combined therapies can be tailored to be associated to LV gene transfer in vivo to enforce its tolerogenic effect.
5. Conclusions and perspectives
The in vivo induction of Ag-specific T regulatory cells is essential to achieve a stable correction of an undesired immune response and a persistent state of tolerance, after administration of a therapeutic Ag, either by protein replacement therapy or gene therapy, as well as in autoimmunity. Our growing understanding of the complexity of the immune system and ability to steer it towards the tolerance side, will allow us to couple the immune-modulatory features of late-generation gene transfer vectors with targeted immune manipulation by refined drugs and cell therapy strategies, to achieve stable activity of the therapeutic transgene supported by a robust state of immune tolerance and potentially apply these advanced therapeutic interventions to prevent or revert immune mediated disorders.
Author contribution
AA and AC wrote the manuscript. SG and LN critically revised the manuscript.
Conflict of interest
The authors are inventors on pending and issued patents on LV technology, microRNA-regulated LV, tolerogenic DCs filed by the Salk Institute, Cell Genesys, “Fondazione Telethon” and/or “Ospedale San Raffaele”. According to the respective institutional policies, inventors may be entitled to receive some financial benefits from the licensing of such patents.
Acknowledgment
The work in the authors’ laboratories is supported by the Italian Telethon Foundation and Bioverativ sponsored research agreement.
Contributor Information
Andrea Annoni, Email: annoni.andrea@hsr.it.
Alessio Cantore, Email: cantore.alessio@hsr.it.
References
- 1.Kay M.A., Glorioso J.C., Naldini L. Viral vectors for gene therapy : the art of turning infectious. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 2.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 3.Finkelshtein D., Werman A., Novick D., Barak S., Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti-Pierri N., Ng P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum. Mol. Genet. 2011;20:7–13. doi: 10.1093/hmg/ddr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mingozzi F., High K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay M.A. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 8.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L., Mahlaoui N., Kiermer V., Mittelstaedt D., Bellesme C., Lahlou N., Lefrere F., Blanche S., Audit M., Payen E., Leboulch P., l’Homme B., Bougneres P., Von Kalle C., Fischer A., Cavazzana-Calvo M., Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 9.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K., Cavallesco R., Gillet-Legrand B., Caccavelli L., Sgarra R., Maouche-Chrétien L., Bernaudin F., Girot R., Dorazio R., Mulder G.-J., Polack A., Bank A., Soulier J., Larghero J., Kabbara N., Dalle B., Gourmel B., Socie G., Chrétien S., Cartier N., Aubourg P., Fischer A., Cornetta K., Galacteros F., Beuzard Y., Gluckman E., Bushman F., Hacein-Bey-Abina S., Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C., Bosticardo M., Evangelio C., Assanelli A., Casiraghi M., Di Nunzio S., Callegaro L., Benati C., Rizzardi P., Pellin D., Di Serio C., Schmidt M., Von Kalle C., Gardner J., Mehta N., Neduva V., Dow D.J., Galy A., Miniero R., Finocchi A., Metin A., Banerjee P.P., Orange J.S., Galimberti S., Valsecchi M.G., Biffi A., Montini E., Villa A., Ciceri F., Roncarolo M.G., Naldini L. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A., Fiori R., Silvani P., Rancoita P.M.V., Gabaldo M., Benedicenti F., Antonioli G., Assanelli A., Cicalese M.P., del Carro U., Sora M.G.N., Martino S., Quattrini A., Montini E., Di Serio C., Ciceri F., Roncarolo M.G., Aiuti A., Naldini L., Biffi A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 12.Ribeil J.-A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W., Bartolucci P., Weber L., Puy H., Meritet J.-F., Grevent D., Beuzard Y., Chrétien S., Lefebvre T., Ross R.W., Negre O., Veres G., Sandler L., Soni S., de Montalembert M., Blanche S., Leboulch P., Cavazzana M. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 13.Palfi S., Gurruchaga J.M., Scott Ralph G., Lepetit H., Lavisse S., Buttery P.C., Watts C., Miskin J., Kelleher M., Deeley S., Iwamuro H., Lefaucheur J.P., Thiriez C., Fenelon G., Lucas C., Brugières P., Gabriel I., Abhay K., Drouot X., Tani N., Kas A., Ghaleh B., Le Corvoisier P., Dolphin P., Breen D.P., Mason S., Guzman N.V., Mazarakis N.D., Radcliffe P.A., Harrop R., Kingsman S.M., Rascol O., Naylor S., Barker R.A., Hantraye P., Remy P., Cesaro P., Mitrophanous K.A. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 14.Cantore A., Ranzani M., Bartholomae C.C., Volpin M., Della Valle P., Sanvito F., Sergi L.S., Gallina P., Benedicenti F., Bellinger D., Raymer R., Merricks E., Bellintani F., Martin S., Doglioni C., Angelo A.D., Vandendriessche T., Chuah M.K., Schmidt M., Nichols T., Montini E., Naldini L. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa1405. 277ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meneghini V., Lattanzi A., Tiradani L., Bravo G., Morena F., Sanvito F., Calabria A., Bringas J., Fisher-perkins J.M., Dufour J.P., Baker K.C., Doglioni C., Montini E., Bunnell B.A., Bankiewicz K., Martino S., Naldini L., Gritti A. Pervasive supply of therapeutic lysosomal enzymes in the CNS of normal and Krabbe-affected non-human primates by intracerebral lentiviral gene therapy. EMBO Mol. Med. 2016;8:489–510. doi: 10.15252/emmm.201505850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog R.W. Complexity of immune responses to AAV transgene products – example of factor IX. Cell Immunol. 2017 doi: 10.1016/j.cellimm.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abordo-adesida E., Follenzi A., Barcia C., Castro M.G., Naldini L., Lowenstein P.R. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum. Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H., Pierce G.F., Ertl H.C.J., High K.A. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 20.Ertl H.C.J., High K.A. Impact of AAV capsid-specific T-cell responses on design and outcome of clinical gene transfer trials with recombinant adeno-associated viral vectors: an evolving controversy. Hum. Gene Ther. 2017;28:328–337. doi: 10.1089/hum.2016.172. [DOI] [PubMed] [Google Scholar]
- 21.Hastie E., Cataldi M., Marriott I., Grdzelishvili V.Z. Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res. 2013;176:16–32. doi: 10.1016/j.virusres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DePolo N.J., Reed J.D., Sheridan P.L., Townsend K., Sauter S.L., Jolly D.J., Dubensky T.W. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 23.Beebe D.P., Cooper N.R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J. Immunol. 1981;126:1562–1568. [PubMed] [Google Scholar]
- 24.Tebas P., Stein D., Binder-Scholl G., Mukherjee R., Brady T., Rebello T., Humeau L., Kalos M., Papasavvas E., Montaner L.J., Schullery D., Shaheen F., Brennan A.L., Zheng Z., Cotte J., Slepushkin V., Veloso E., Mackley A., Hwang W.T., Aberra F., Zhan J., Boyer J., Collman R.G., Bushman F.D., Levine B.L., June C.H. Antiviral effects of autologous CD4 T cells genetically modified with a conditionally replicating lentiviral vector expressing long antisense to HIV. Blood. 2013;121:1524–1533. doi: 10.1182/blood-2012-07-447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masat E., Laforêt P., De Antonio M., Corre G., Perniconi B., Taouagh N., Mariampillai K., Amelin D., Mauhin W., Hogrel J.-Y., Caillaud C., Ronzitti G., Puzzo F., Kuranda K., Colella P., Mallone R., Benveniste O., Mingozzi F., Bassez G., Bedat-Millet A.L., Behin A., Eymard B., Leonard-Louis S., Stojkovic T., Canal A., Decostre V., Bouhour F., Boyer F., Castaing Y., Chapon F., Cintas P., Durieu I., Echaniz-Laguna A., Feasson L., Furby A., Hamroun D., Ferrer X., Solé G., Froissart R., Piraud M., Germain D., Benistan K., Guffon-Fouilhoux N., Journel H., Labauge P., Lacour A., Levy A., Magot A., Péréon Y., Minot-Myhié M.-C., Nadaj-Pakleza A., Nathier C., Orlikowski D., Pellegrini N., Petiot P., Praline J., Lofaso F., Prigent H., Dutry A., Renard D., Sacconi S., Desnuelle C., Salort-Campana E., Pouget J., Tiffreau V., Vincent D., Zagnoli F. Long-term exposure to Myozyme results in a decrease of anti-drug antibodies in late-onset Pompe disease patients. Sci. Rep. 2016;6:36182. doi: 10.1038/srep36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B., Tai A., Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol. Rev. 2011;239:45–61. doi: 10.1111/j.1600-065X.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter D.L., Hwang W., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V., Ambrose D., Grupp S.A., Chew A., Zheng Z., Milone M.C., Levine B.L., Melenhorst J.J., June C.H. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac5415. 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turtle C.J., Hanafi L.-A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X., Soma L., Wood B., Li D., Heimfeld S., Riddell S.R., Maloney D.G. Immunotherapy of non-Hodgkins lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf8621. 355ra116-355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turtle C.J., Hanafi L., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M., Robinson E., Steevens N.N., Chaney C., Soma L., Chen X., Yeung C., Wood B., Li D., Cao J., Heimfeld S., Jensen M.C., Riddell S.R., Maloney D.G. CD19 CAR – T cells of defined CD4 + : CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016;1:1–16. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S., Benedicenti F., Vallanti G., Biasco L., Leo S., Kabbara N., Zanetti G., Rizzo W.B., Mehta N.A., Cicalese M.P., Casiraghi M., Boelens J.J., Del Carro U., Dow D.J., Schmidt M., Assanelli A., Neduva V., Di Serio C., Stupka E., Gardner J., von Kalle C., Bordignon C., Ciceri F., Rovelli A., Roncarolo M.G., Aiuti A., Sessa M., Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341 doi: 10.1126/science.1233158. 1233158. [DOI] [PubMed] [Google Scholar]
- 31.Maguire C.A., Ramirez S.H., Merkel S.F., Sena-Esteves M., Breakefield X.O. Gene therapy for the nervous system: challenges and new strategies. Neurotherapeutics. 2014;11:817–839. doi: 10.1007/s13311-014-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tardieu M., Zérah M., Husson B., de Bournonville S., Deiva K., Adamsbaum C., Vincent F., Hocquemiller M., Broissand C., Furlan V., Ballabio A., Fraldi A., Crystal R.G., Baugnon T., Roujeau T., Heard J.-M., Danos O. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying Human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type III A disease: results of a phase I/II trial. Hum. Gene Ther. 2014;25:506–516. doi: 10.1089/hum.2013.238. [DOI] [PubMed] [Google Scholar]
- 33.Follenzi A., Battaglia M., Lombardo A., Annoni A., Roncarolo M.G., Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 34.Cantore A., Nair N., Della Valle P., Di Matteo M., Màtrai J., Sanvito F., Brombin C., Di Serio C., D’Angelo A., Chuah M., Naldini L., VandenDriessche T. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120:4517–4520. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 35.Milani M., Annoni A., Bartolaccini S., Biffi M., Russo F., Di Tomaso T., Raimondi A., Lengler J., Holmes M.C., Scheiflinger F., Lombardo A., Cantore A., Naldini L. Genome editing for scalable production of alloantigen-free lentiviral vectors for in vivo gene therapy. EMBO Mol. Med. 2017;9:1558–1573. doi: 10.15252/emmm.201708148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croyle M.A., Callahan S.M., Auricchio A., Schumer G., Linse K.D., Wilson J.M., Lane J., Kobinger G.P., Brunner L.J. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum pegylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schauber-Plewa C., Simmons A., Tuerk M.J., Pacheco C.D., Veres G. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 2005;12:238–245. doi: 10.1038/sj.gt.3302399. [DOI] [PubMed] [Google Scholar]
- 38.Guibinga G.H., Friedmann T. Baculovirus GP64-pseudotyped HIV-based lentivirus vectors are stabilized against complement inactivation by codisplay of decay accelerating factor (DAF) or of a GP64 – DAF fusion protein. Mol. Ther. 2005;11:645–651. doi: 10.1016/j.ymthe.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Hwang B., Schaffer D.V. Engineering a serum-resistant and thermostable vesicular stomatitis virus G glycoprotein for pseudotyping retroviral and lentiviral vectors. Gene Ther. 2013;20:807–815. doi: 10.1038/gt.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lillicrap B.D.B. Dangerous liaisons: The role of “danger” signals in the immune response to gene therapy. Blood. 2002;100:1133–1140. doi: 10.1182/blood-2001-11-0067. [DOI] [PubMed] [Google Scholar]
- 41.Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 42.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Annoni A., Goudy K., Akbarpour M., Naldini L., Roncarolo M.G. Immune responses in liver-directed lentiviral gene therapy. Transl. Res. 2013;161:230–240. doi: 10.1016/j.trsl.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Brown B.D., Sitia G., Annoni A., Hauben E., Sergi L.S., Zingale A., Roncarolo M.G., Guidotti L.G., Naldini L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 45.Agudo J., Ruzo A., Kitur K., Sachidanandam R., Blander J.M., Brown B.D. A TLR and non-TLR mediated innate response to lentiviruses restricts hepatocyte entry and can be ameliorated by pharmacological blockade. Mol. Ther. 2012;1–11 doi: 10.1038/mt.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossetti M., Gregori S., Hauben E., Brown B.D., Sergi L.S., Naldini L., Roncarolo M.-G. HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells. Hum. Gene Ther. 2011;22:177–188. doi: 10.1089/hum.2010.085. [DOI] [PubMed] [Google Scholar]
- 47.Throm R.E., Ouma A.A., Zhou S., Chandrasekaran A., Lockey T., Greene M., De Ravin S.S., Moayeri M., Malech H.L., Sorrentino B.P., Gray J.T. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–5111. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denard J., Rundwasser S., Laroudie N., Gonnet F., Naldini L., Radrizzani M., Galy A., Merten O.W., Danos O., Svinartchouk F. Quantitative proteomic analysis of lentiviral vectors using 2-DE. Proteomics. 2009;9:3666–3676. doi: 10.1002/pmic.200800747. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya A., Hegazy A.N., Deigendesch N., Kosack L., Cupovic J., Kandasamy R.K., Hildebrandt A., Merkler D., Kühl A.A., Vilagos B., Schliehe C., Panse I., Khamina K., Baazim H., Arnold I., Flatz L., Xu H.C., Lang P.A., Aderem A., Takaoka A., Superti-Furga G., Colinge J., Ludewig B., Löhning M., Bergthaler A. Superoxide dismutase 1 protects hepatocytes from type i interferon-driven oxidative damage. Immunity. 2015;43:974–986. doi: 10.1016/j.immuni.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teijaro J.R., Ng C., Lee A.M., Sullivan B.M., Sheehan K.C.F., Welch M., Schreiber R.D., Carlos J., Torre D., Oldstone M.B.A. Persistent LCMV infection is controlled by blockade of type I interferon signaling john. Science. 2013:27–29. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson E.B., Yamada D.H., Elsaesser H., Herskovitz J., Deng J., Cheng G., Aronow B.J., Karp C.L., Brooks D.G. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones S.A., Scheller J., Rose-john S. Science in medicine Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. Cell. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genovese M.C., Fleischmann R., Furst D., Janssen N., Carter J., Dasgupta B., Bryson J., Duncan B., Zhu W., Pitzalis C., Durez P., Kretsos K. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann. Rheum. Dis. 2014;73:1689–1694. doi: 10.1136/annrheumdis-2013-204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinarello C. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3733. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu R.-Z., Xiang D., Xie C., Li J.-J., Hu J.-J., He H.-L., Yuan Y.-S., Gao J., Han W., Yu Y. Protective effect of recombinant human IL-1Ra on CCl 4-induced acute liver injury in mice. World J. Gastroenterol. 2010;16:2771–2779. doi: 10.3748/wjg.v16.i22.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seregin S.S., Appledorn D.M., McBride A.J., Schuldt N.J., Aldhamen Y.A., Voss T., Wei J., Bujold M., Nance W., Godbehere S., Amalfitano A. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiina T., Hosomichi K., Inoko H., Kulski J.K. The HLA genomic loci map: expression, interaction, diversity and disease. J. Hum. Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 58.Richards S.M. Immunologic considerations for enzyme replacement therapy in the treatment of lysosomal storage disorders. Clin. Appl. Immunol. Rev. 2002;2:241–253. [Google Scholar]
- 59.Rowe H.M., Lopes L., Ikeda Y., Bailey R., Barde I., Zenke M., Chain B.M., Collins M.K. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 2006;13:310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Grommé M., Neefjes J. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol. Immunol. 2002;39:181–202. doi: 10.1016/s0161-5890(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 61.Lopes L., Fletcher K., Ikeda Y., Collins M. Lentiviral vector expression of tumour antigens in dendritic cells as an immunotherapeutic strategy. Cancer Immunol. Immunother. 2006;55:1011–1016. doi: 10.1007/s00262-005-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarei S., Abraham S., Arrighi J.-F., Haller O., Calzascia T., Walker P.R., Kündig T.M., Hauser C., Piguet V. Lentiviral transduction of dendritic cells confers protective antiviral immunity in vivo. J. Virol. 2004;78:7843–7845. doi: 10.1128/JVI.78.14.7843-7845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dogan I., Bertocci B., Vilmont V., Delbos F., Mégret J., Storck S., Reynaud C.-A., Weill J.-C. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 64.O’Shea J.J., Paul W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mingozzi F., Liu Y., Dobrzynski E., Kaufhold A., Liu J.H., Wang Y., Arruda V.R., High K.A., Herzog R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown B.D., Venneri M.A., Zingale A., Sergi L.S., Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 67.Vandendriessche T., Thorrez L., Acosta-Sanchez A., Petrus I., Wang L., Ma L., De Waele L., Iwasaki Y., Gillijns V., Wilson J.M., Collen D., Chuah M.K.L. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 68.Brunetti-Pierri N., Liou A., Patel P., Palmer D., Grove N., Finegold M., Piccolo P., Donnachie E., Rice K., Beaudet A., Mullins C., Ng P. Balloon catheter delivery of helper-dependent adenoviral vector results in sustained, therapeutic hFIX expression in rhesus macaques. Mol. Ther. 2012;20:1863–1870. doi: 10.1038/mt.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groux H., Garra A.O., Bigler M., Rouleau M., Antonenko S., De Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;12:230–231. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 70.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 71.Fehervari Z., Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 2004;16:203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Tiegs G., Lohse A.W. Immune tolerance: what is unique about the liver. J. Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Crispe I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 74.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 75.LoDuca P.A., Hoffman B.E., Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown B.D., Gentner B., Cantore A., Colleoni S., Amendola M., Zingale A., Baccarini A., Lazzari G., Galli C., Naldini L. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat. Biotechnol. 2007;25:1457–1467. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- 77.Brown B.D., Cantore A., Annoni A., Sergi L.S., Lombardo A., Della Valle P., D’Angelo A., Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- 78.Annoni A., Brown B.D., Cantore A., Sergi Sergi L., Naldini L., Roncarolo M.-G. In vivo delivery of a microRNA regulated transgene induces antigen-specific regulatory T cells and promotes immunological tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mátrai J., Cantore A., Bartholomae C.C., Annoni A., Wang W., Acosta-Sanchez A., Samara-Kuko E., De Waele L., Ma L., Genovese P., Damo M., Arens A., Goudy K., Nichols T.C., von Kalle C., Marinee M.K., Roncarolo M.G., Schmidt M., Vandendriessche T., Naldini L. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology. 2011;53:1696–1707. doi: 10.1002/hep.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Annoni A., Cantore A., Della Valle P., Goudy K., Akbarpour M., Russo F., Bartolaccini S., D’Angelo A., Roncarolo M.G., Naldini L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol. Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akbarpour M., Goudy K.S., Cantore A., Russo F., Sanvito F., Naldini L., Annoni A., Roncarolo M.G. Insulin B chain 9 – 23 gene transfer to hepatocytes protects from type 1 diabetes by inducing. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa3032. 289ra81. [DOI] [PubMed] [Google Scholar]
- 82.Matsui H., Hegadorn C., Ozelo M., Burnett E., Tuttle A., Labelle A., McCray P.B., Naldini L., Brown B., Hough C., Lillicrap D. A microRNA-regulated and GP64-pseudotyped lentiviral vector mediates stable expression of FVIII in a murine model of Hemophilia A. Mol. Ther. 2011;19:723–730. doi: 10.1038/mt.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Staber J.M., Pollpeter M.J., Anderson C.-G., Burrascano M., Cooney A.L., Sinn P.L., Rutkowski D.T., Raschke W.C., McCray P.B. Long-term correction of hemophilia A mice following lentiviral mediated delivery of an optimized canine factor VIII gene. Gene Ther. 2017;1–7 doi: 10.1038/gt.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merlin S., Cannizzo E.S., Borroni E., Bruscaggin V., Schinco P., Tulalamba W., Chuah M.K., Arruda V.R., VandenDriessche T., Prat M., Valente G., Follenzi A. A Novel platform for immune tolerance induction in hemophilia a mice. Mol. Ther. 2017;25:1815–1830. doi: 10.1016/j.ymthe.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agudo J., Ruzo A., Tung N., Salmon H., Leboeuf M., Hashimoto D., Becker C., Garrett-Sinha L.-A., Baccarini A., Merad M., Brown B.D. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2013;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ettinger R.A., Paz P., James E.A., Gunasekera D., Aswad F., Thompson A.R., Matthews D.C., Pratt K.P. T cells from hemophilia A subjects recognize the same HLA-restricted FVIII epitope with a narrow TCR repertoire. Blood. 2016;128:2043–2054. doi: 10.1182/blood-2015-11-682468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pratt K.P. Engineering less immunogenic and antigenic FVIII proteins. Cell Immunol. 2016 doi: 10.1016/j.cellimm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shapiro A.D., Ragni M.V., Kulkarni R., Oldenberg J., Srivastava A., Quon D.V., Pasi K.J., Hanabusa H., Pabinger I., Mahlangu J., Fogarty P., Lillicrap D., Kulke S., Potts J., Neelakantan S., Nestorov I., Li S., Dumont J.A., Jiang H., Brennan A., Pierce G.F. Recombinant factor VIII Fc fusion protein: extended-interval dosing maintains low bleeding rates and correlates with von Willebrand factor levels. J. Thromb. Haemost. 2014;12:1788–1800. doi: 10.1111/jth.12723. [DOI] [PubMed] [Google Scholar]
- 89.Mahlangu J., Powell J.S., Ragni M.V., Chowdary P., Josephson N.C., Pabinger I., Hanabusa H., Gupta N., Kulkarni R., Fogarty P., Perry D., Shapiro A., Pasi K.J. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Am. Soc. Hematol. 2014;123:317–325. doi: 10.1182/blood-2013-10-529974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Groot A.S., Moise L., Mcmurry J.A., Wambre E., Van Overtvelt L., Moingeon P., Scott D.W., Martin W. Activation of natural regulatory T cells by IgG Fc – derived peptide “Tregitopes” T Reg depletion. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linsley P.S., Nadler S.G. The clinical utility of inhibiting CD28-mediated costimulation. Immunol. Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 92.Deppong C.M., Bricker T.L., Rannals B.D., Van Rooijen N., Hsieh C.-S., Green J.M. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-β. J. Immunol. 2013;191:3082–3089. doi: 10.4049/jimmunol.1300830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ponder K.P., Wang B., Wang P., Ma X., Herati R., Wang B., Cullen K., O’Donnell P., Ellinwood N.M., Traas A., Primeau T.M., Haskins M.E. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol. Ther. 2006;14:5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 94.Turnquist H.R., Raimondi G., Zahorchak A.F., Fischer R.T., Wang Z., Thomson A.W. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 95.Maldonado R.A., LaMothe R.A., Ferrari J.D., Zhang A.-H., Rossi R.J., Kolte P.N., Griset A.P., O’Neil C., Altreuter D.H., Browning E., Johnston L., Farokhzad O.C., Langer R., Scott D.W., von Andrian U.H., Kishimoto T.K. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang A.H., Rossi R.J., Yoon J., Wang H., Scott D.W. Tolerogenic nanoparticles to induce immunologic tolerance: prevention and reversal of FVIII inhibitor formation. Cell. Immunol. 2016;301:74–81. doi: 10.1016/j.cellimm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Tostanoski L.H., Chiu Y.-C., Gammon J.M., Simon T., Andorko J.I., Bromberg J.S., Jewell C.M. Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 2016;16:2940–2952. doi: 10.1016/j.celrep.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sukhbaatar N., Hengstschläger M., Weichhart T. mTOR-mediated regulation of dendritic cell differentiation and function. Trends Immunol. 2016;37:778–789. doi: 10.1016/j.it.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clemente-Casares X., Blanco J., Ambalavanan P., Yamanouchi J., Singha S., Fandos C., Tsai S., Wang J., Garabatos N., Izquierdo C., Agrawal S., Keough M.B., Yong V.W., James E., Moore A., Yang Y., Stratmann T., Serra P., Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 100.Di Ianni M., Falzetti F., Carotti A., Terenzi A., Castellino F., Bonifacio E., Del Papa B., Zei T., Iacucci Ostini R., Cecchini D., Aloisi T., Perruccio K., Ruggeri L., Balucani C., Pierini A., Sportoletti P., Aristei C., Falini B., Reisner Y., Velardi A., Aversa F., Martelli M.F. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 101.Bluestone J.A., Buckner J.H., Fitch M., Gitelman S.E., Gupta S., Hellerstein M.K., Herold K.C., Lares A., Lee M.R., Li K., Liu W., Long S.A., Masiello L.M., Nguyen V., Putnam A.L., Rieck M., Sayre P.H., Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aad4134. 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossetti M., Spreafico R. Regulatory T cell therapy in transplantation and severe autoimmunity. Crit. Rev. Immunol. 2015;35:479–503. doi: 10.1615/CritRevImmunol.2016016222. [DOI] [PubMed] [Google Scholar]
- 103.Annoni A., Battaglia M., Follenzi A., Lombardo A., Sergi-Sergi L., Naldini L., Roncarolo M.G. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4 +CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
- 104.Chai J.-G., Coe D., Chen D., Simpson E., Dyson J., Scott D. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J. Immunol. 2008;180:858–869. doi: 10.4049/jimmunol.180.2.858. [DOI] [PubMed] [Google Scholar]
- 105.Sarkar D., Biswas M., Liao G., Seay H.R., Perrin G.Q., Markusic D.M., Hoffman B.E., Brusko T.M., Terhorst C., Herzog R.W. Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol. Ther. – Methods Clin. Dev. 2014;1:14030. doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skuljec J., Chmielewski M., Happle C., Habener A., Busse M., Abken H., Hansen G. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front. Immunol. 2017;8:1125. doi: 10.3389/fimmu.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Provasi E., Genovese P., Lombardo A., Magnani Z., Liu P.-Q., Reik A., Chu V., Paschon D.E., Zhang L., Kuball J., Camisa B., Bondanza A., Casorati G., Ponzoni M., Ciceri F., Bordignon C., Greenberg P.D., Holmes M.C., Gregory P.D., Naldini L., Bonini C. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hull C.M., Nickolay L.E., Estorninho M., Richardson M.W., Riley J.L., Peakman M., Maher J., Tree T.I.M. Generation of human islet-specific regulatory T cells by TCR gene transfer. J. Autoimmun. 2017;79:63–73. doi: 10.1016/j.jaut.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Passerini L., Mel E.R., Sartirana C., Fousteri G., Bondanza A., Naldini L., Roncarolo M.G., Bacchetta R. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007320. 215ra174. [DOI] [PubMed] [Google Scholar]
- 110.Andolfi G., Fousteri G., Rossetti M., Magnani C.F., Jofra T., Locafaro G., Bondanza A., Gregori S., Roncarolo M.-G. Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4+ T cells. Mol. Ther. 2012;20:1778–1790. doi: 10.1038/mt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Locafaro G., Andolfi G., Russo F., Cesana L., Spinelli A., Camisa B., Ciceri F., Lombardo A., Bondanza A., Roncarolo M.G., Gregori S. IL-10-engineered human CD4+ Tr1 cells eliminate myeloid leukemia in an HLA class I-dependent mechanism. Mol. Ther. 2017;25:1–15. doi: 10.1016/j.ymthe.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amodio G., Annoni A., Gregori S. Dendritic cell immune therapy to break or induce tolerance. Curr. Stem Cell Reports. 2015 [Google Scholar]
- 113.Amodio G., Comi M., Tomasoni D., Gianolini M.E., Rizzo R., Lemaoult J., Roncarolo M.G., Gregori S. Hla-g expression levels influence the tolerogenic activity of human DC-10. Haematologica. 2015;100:548–557. doi: 10.3324/haematol.2014.113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chatenoud L., Bluestone J.A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 115.Chatenoud L., Primo J., Bach J.F. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J. Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 116.Peng B., Ye P., Rawlings D.J., Ochs H.D., Miao C.H. Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy. Blood. 2009;114:4373–4382. doi: 10.1182/blood-2009-05-217315. [DOI] [PMC free article] [PubMed] [Google Scholar]