Abstract
Background
Understanding sexual networks involving acute human immunodeficiency virus (HIV)-1 infections (AHI) may lead to prevention opportunities to mitigate high rates of onward transmission. We evaluated HIV-1 phylogenetic and behavioral characteristics among persons with AHI and their referred partners.
Methods
Between 2012 and 2014, 46 persons with AHI in Malawi participated in a combined behavioral and biomedical intervention. Participants referred sexual partners by passive referral. Demographics and sexual behaviors were collected through interviews and HIV-1 genetic relationships were assessed with phylogenetics.
Results
Among 45 AHI participants with HIV-1 sequences, none was phylogenetically-linked with another AHI index. There were 19 (42%) AHI participants who referred a single partner that returned for testing. Most partners (n = 17) were HIV-infected, with 15 (88%) presenting with an established infection. There were 14 index-partner pairs that had sequences available; 13 (93%) pairs were phylogenetically-linked dyads. The AHI index was female in 7/13 (54%) dyads. Age-disparate relationships among dyads were common (≥5-year age difference in 67% of dyads), including 3/6 dyads involving a male index and a younger woman. Index participants with a referred partner were more likely to report no casual partners and to be living with their current partner than participants not in dyads.
Conclusions
Passive-partner referral successfully identified partners with genetically-similar HIV infections—the likely source of infection—but only 40% of index cases referred partners who presented for HIV-1 testing. Future work evaluating assisted partner notification may help reach susceptible partners or more people with untreated HIV-1 infections connected to acute transmission.
Clinical Trials Registration
Keywords: molecular epidemiology, HIV-1, phylogeny: transmission, Malawi, partner notification
Persons diagnosed with acute human immunodeficiency virus (HIV)-1 infection in Malawi had low recruitment of sexual partners through passive referrals. Nearly all successfully-recruited partners had established HIV-1 infections, and 93% of these had a phylogenetically-linked virus to the acute index.
The detection of people with acute human immunodeficiency virus (HIV)-1 infection (AHI) offers an important opportunity for intervention. In Malawi, an estimated 38% of heterosexual transmissions result from onward spread during AHI [1], and many other studies support the critical role of AHI in the spread of HIV-1 [2]. Such high onward spread of HIV by people with AHI could compromise community-wide “treatment as prevention” [3] and HIV-1 eradication efforts. These HIV-1 prevention efforts may be facilitated by a detailed understanding of local HIV-1 transmission dynamics and networks, through combinations of phylogenetic analyses and behavioral data, including contact networks [4]. Phylogenetic analyses of persons diagnosed during AHI and their sexual partners provide opportunities for insight into transmission patterns at the leading edge of the epidemic. Such opportunities include assessing the effect of interventions [5, 6] and tailoring HIV-1 testing and notification strategies based on knowledge of the transmission network. Effective partner notification strategies are important to identify HIV-uninfected persons who are at high risk of HIV-1 acquisition, as well as persons with HIV who are unaware of their infection [7], such that they can be linked to antiretroviral therapy to decrease onward transmission [8]. However, partner services to facilitate notification and testing for partners of persons with newly-diagnosed HIV-1 are limited in sub-Saharan Africa [9].
Between 2012 and 2014, persons with AHI in Malawi participated in a pilot clinical trial to assess the effect of a combined behavioral and biomedical intervention in reducing onward transmission [10]. Sexual partners were recruited for HIV-1 testing by passive referral from index cases. In this report, we evaluate the extent to which index cases with AHI are connected to their partners and each other, using phylogenetic analysis of viral sequences, and we examine the behavioral, demographic, and phylogenetic characteristics of referred sexual partners. The results highlight potential opportunities important for HIV-1 prevention.
METHODS
Study Setting
The Malawi Methods and Packages in Prevention Programming acute intervention study (ClinicalTrials.gov #NCT01450189) enrolled 46 persons with AHI, detected using a pooled HIV-1 RNA polymerase chain reation algorithm, from 2 HIV testing and counseling centers and 2 sexually transmitted infection (STI) clinics in Lilongwe, Malawi. Screening occurred between June 2012 and January 2014. Index participants were randomized to standard HIV-1 counseling, a behavioral intervention, or a behavioral intervention plus antiretroviral therapy. Detailed methods have been reported previously [11]. In brief, AHI was defined as a positive HIV-1 RNA (ie, detectable virus) and negative or discordant HIV-1 antibody results. Per Malawi HIV testing algorithms, antibody testing included serial rapid testing using Alere Determine HIV-1/2 (Alere) and Uni-Gold Recombigen HIV-1/2 (Trinity Biotech), with a tiebreaker in the event of discordant results. Persons with 2 positive results were considered HIV-seropositive. This study was approved by the Institutional Review Board at the University of North Carolina and the National Health Sciences Research Committee of Malawi.
Passive Partner Notification
Index participants were encouraged to refer all sexual partners in the 3 months prior to diagnosis for HIV-1 testing using referral cards. Partners who were 18 years of age or older and reported to the clinic with their card were offered enrollment in the study. After providing written informed consent, enrolled partners completed a baseline questionnaire and underwent serial HIV antibody (Ab) testing. Partners found to have an established (Ab-positive) HIV-1 infection were asked to submit a blood specimen for HIV-1 sequencing and were dismissed from further study participation, following referral to appropriate HIV care. Partners testing Ab-negative or Ab-discordant were screened for AHI. If found to be acutely infected, partners were provided with post-test counseling and asked to provide a blood specimen for HIV-1 sequencing. All HIV-seronegative partners completed additional HIV-1 testing at quarterly study visits designed to align with the study visits for the index participant. Follow-up for partners concluded upon study completion by the referring index participant. Behavioral and demographic information were collected from index and partner participants at each visit using audio, computer-assisted self-interview software. Small financial incentives were provided to index participants and referred partners for participation in the study (up to $5 per session); however, there were no financial incentives provided to index cases specifically for referrals.
Human Immunodeficiency Virus–1 Sequences and Phylogenetic Analyses
Samples were collected for HIV-1 sequencing at baseline (index cases and partners) and follow-up visits (index cases only). Partial pol sequences were amplified from plasma-derived HIV RNA (Qiagen Viral RNA kit) in 2 fragments, as previously described [12]. Approximately the first 200 amino acids of the reverse transcriptase (RT) gene and the entire integrase gene (index cases only) were sequenced. Major drug resistance mutations were evaluated using the Stanford HIV Drug Resistance Database (hivdb.stanford.edu). Reference RT sequences were selected, using a Basic Local Alignment Search Tool (BLAST) search to identify the 10 most closely-related sequences to each study sequence in the Los Alamos National Laboratory HIV database (http://www.hiv.lanl.gov). Sequences were aligned using MUltiple Sequence Comparison by Log-Expectation (MUSCLE) [13] and edited manually with stripping of gapped positions. A maximum-likelihood phylogenetic tree was constructed with all study and reference RT sequences in Randomized Axelerated Maximum Likelihood (RAxML) [14], with the general time-reversible model of nucleotide substitution and gamma distribution of invariant sites. Bootstrapping of 1000 replicates was performed to assess statistical support of clades. Clades involving sequences from ≥2 individuals were assessed for phylogenetic linkage based on a short genetic distance (ie, maximum pairwise distance <0.015 nucleotide substitutions per site) and high bootstrap nodal support (ie, >70%) [15]. Associations between phylogenetic linkage and demographic/behavioral variables among index cases were assessed with Fisher’s exact test.
RESULTS
Study Population
Index participants with acute HIV-1 infection were predominately male (27/46, 59%), with a median age of 25 years (interquartile range [IQR] 22–32). Nearly all (45/46, 98%) index participants had at least 1 sequence suitable for the phylogenetic analysis, and were thus included in our analyses. Of these 45 index cases, 19 (42%) referred a single sexual partner that reported for HIV testing and enrolled in the study. In total, however, these 45 index cases named 136 unique sexual partners, so the 19 successfully-enrolled partners represented only 14% of all named partners.
Index participants who referred a partner that presented for HIV-1 testing were similar by most demographic features to those who did not have a partner return for testing (Table 1). However, index participants who had a partner return were more likely to name a steady/main partner (78.9% vs 40.0%; P = .01), report no casual partners (57.9% vs 16.0%; P = .02), and be married or living with their main/steady partner (89.5% vs 61.5%; P = .05), compared to index participants who did not have a partner return.
Table 1.
Demographics and Sexual Risk Behaviors
| Characteristic | Index Participant (n = 45) | P Value | |||
|---|---|---|---|---|---|
| No Partner Returned (n = 26) | Partner Returned (n = 19) | ||||
| n | % | n | % | ||
| Age, median years (IQR) | 25 | (22–32) | 28 | (23–31) | .54 |
| Gender | .52 | ||||
| Male | 16 | 61.5 | 11 | 57.9 | |
| Female | 10 | 38.5 | 8 | 42.1 | |
| Married or living with a steady partner | .05 | ||||
| Yes | 16 | 61.5 | 17 | 89.5 | |
| No | 10 | 38.5 | 2 | 10.5 | |
| Education | .64 | ||||
| ≤ Primary completed | 14 | 53.8 | 12 | 63.2 | |
| Some secondary | 6 | 23.1 | 5 | 26.3 | |
| ≥ Secondary completed | 6 | 23.1 | 2 | 10.5 | |
| Employment status | |||||
| Employed | 19 | 73.1 | 15 | 78.9 | .74 |
| Not employed | 7 | 26.9 | 4 | 21.1 | |
| Total partners, last 3 months | |||||
| ≤ 1 | 13 | 52.0 | 11 | 57.9 | .77 |
| >1 | 12 | 48.0 | 8 | 42.1 | |
| Named partners, last 3 months | |||||
| Total, median (IQR) | 2 | (1–3) | 1 | (1–2) | .37 |
| Steady/main partner | |||||
| 0 | 15 | 60.0 | 4 | 21.1 | .01 |
| 1 | 6 | 24.0 | 13 | 68.4 | |
| 2–3 | 4 | 16.0 | 2 | 10.5 | |
| Casual partner | |||||
| 0 | 4 | 16.0 | 11 | 57.9 | .02 |
| ≥1 (range 1–4) | 21 | 84.0 | 8 | 41.2 | |
Data were reported at baseline among index participants diagnosed during acute HIV-1 infection who had a partner return for HIV testing by passive referral, compared to those who did not have a partner return.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
Partners presented for HIV-1 testing a median of 9.5 days (IQR 4–29) after their referring index partner enrolled in the study. Of the 18 referred partners with demographic information available, most were women (61%, 11/18; Table 2). Among the 19 referred partners, 15 (79%) had an established HIV-1 infection (HIV-1 antibodies) at presentation, 2 (10.5%) seroconverted during follow-up, and 2 (10.5%) remained HIV-seronegative for the duration of their follow-up (~3.5 months each). Of the 2 who seroconverted during follow-up, 1 male partner had AHI at the time of presentation and 1 female partner was initially Ab- and RNA-negative and subsequently seroconverted by her first follow-up visit (5.5 months after initial presentation). Of the 18 referred partners with questionnaire data available, nearly all were married or living with a steady partner (17/18, 94%) and most reported only 1 sexual partner in the last 3 months (15/18, 83%; Table 2) Given the nature of the questionnaire, sexual behaviors reported by partners could not explicitly be identified as having occurred with the referring participant.
Table 2.
Demographics and Sexual Risk Behaviors Among Referred Partners of Persons with Acute HIV Infections
| Characteristic | Overall (n = 18)a |
|---|---|
| Median (IQR) | |
| Age, years | 26.5 (22–32) |
| Number of sexual acts, last month | 8.0 (2–10) |
| n (%) | |
| Gender | |
| Male | 7 (38.9) |
| Female | 11 (61.1) |
| Married or living with a steady partner | |
| Yes | 17 (94.4) |
| No | 1 (5.6) |
| Education | |
| ≤ Primary completed | 14 (77.8) |
| Some secondary | 1 (5.6) |
| ≥ Secondary completed | 3 (16.7) |
| Employment status | |
| Employed | 11 (61.1) |
| Not employed | 7 (38.9) |
| Total partners, last 3 months | |
| ≤1 | 15 (83.3) |
| >1 | 3 (16.7) |
| Total partners, last monthb | |
| ≤1 | 15 (88.2) |
| >1 | 2 (11.8) |
| Any unprotected sex in the last monthb | |
| Yes | 13 (76.5) |
| No | 4 (23.5) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aThe analysis is based on 18 partners, with full demographic information.
bn = 17; 1 referred partner was missing data on sexual behaviors in the last month.
Phylogenetic analysis was conducted on all participants with sequences. Sequencing was successful for 45/46 participants with AHI and 14/17 (82%) of the partners who were or became HIV-positive. For the 3/17 HIV-positive partners for whom sequences could not be obtained, 2 had HIV-1 RNA <50 copies/mL and were likely on treatment; the remaining partner seroconverted after baseline sampling. Each of the 14 partners with sequences was referred to the clinic by a unique index case, yielding 14 index-partner pairs.
Phylogenetic Analysis
The maximum-likelihood tree included sequences from 45 index cases (n = 44 baseline and 18 follow-up sequences), 14 partner baseline samples, and 345 unique reference sequences. We discarded 2 RT sequences from analysis due to suspected mislabeling (each from an index case with an RT sequence available at baseline and follow-up time points). Complementary phylogenetic analysis of integrase sequences from multiple time points, including these index cases, showed only intra-individual clustering; thus, mislabeling was suspected rather than multi-variant infection (Supplementary Figure 1). Most (94%) of the 59 participants with sequences had sequences with subtype C; 2 were subtype A. Viral diversity among index participants was high (mean pairwise genetic distance >6%), demonstrating a lack of connection of index cases. Index sequences were interspersed throughout the tree, which included reference sequences sampled throughout southern Africa (Figure 1).
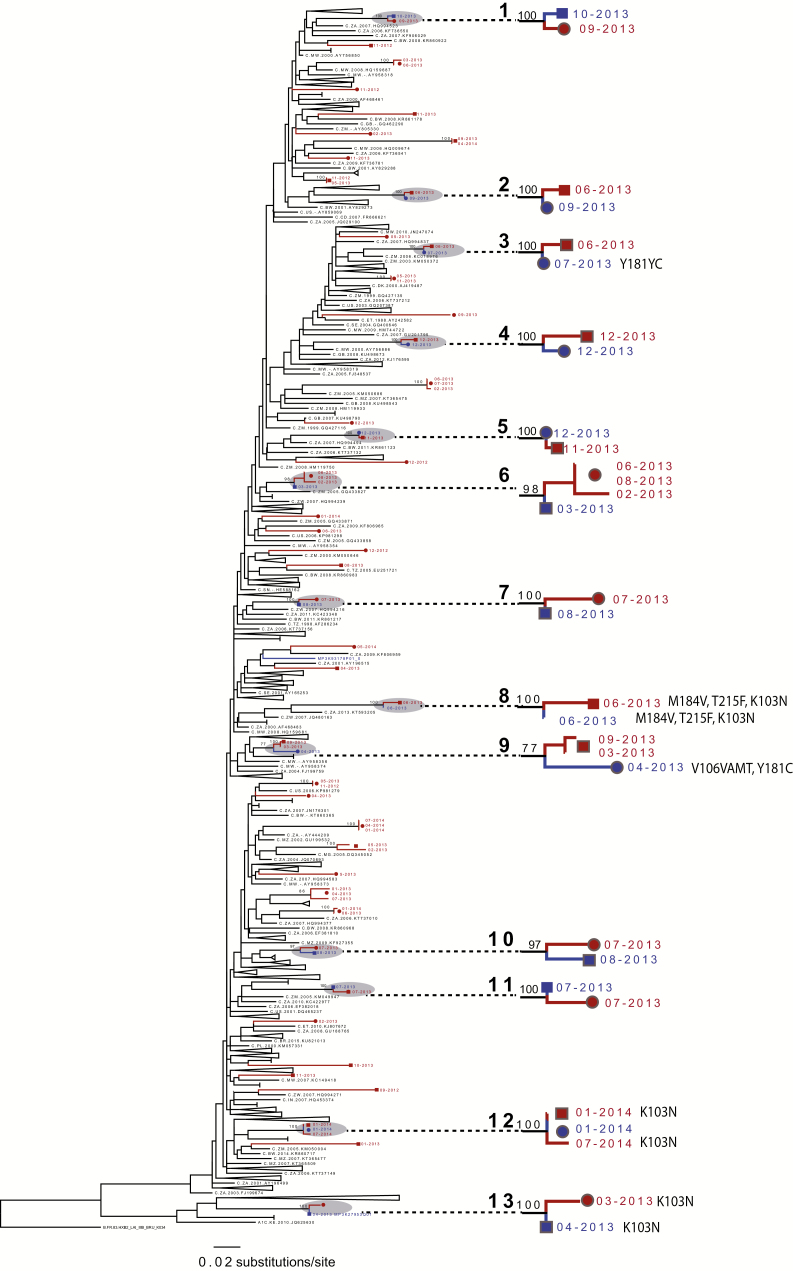
Figure 1.
Maximum-likelihood phylogenetic tree, constructed with HIV-1 partial pol sequences from 59 study subjects (76 sequences) and 335 reference sequences, identified through a BLAST search of the LANL HIV database. Reference tips are labeled with LANL accession numbers. Clades involving only LANL references are collapsed for display purposes. Study sequences are labeled in black (acute index cases) and blue (partners). Individual persons are shown with shapes at tips: the circles are men and squares are women. The numbers at the tips are dates of sampling (month, year). Dyads are labeled as 1–13. Bootstrap support is labeled at clades involving study sequences. Abbreviations: HIV, human immunodeficiency virus; LANL, Los Alamos National Laboratory.
Among the 14 index-partner pairs with sequences available for both partners, 13 (93%) were in highly-related, phylogenetically-linked dyads (<0.015 nucleotide substitutions per site); all dyads involved index-referred partner pairs (Figure 1; Table 3). Only 1 referred partner among all the 14 index-partner pairs with sequences was not phylogenetically linked with the index case. All phylogenetically-linked participants were in male-female dyads; 7 (54%) of the 13 linked dyads had female and 6 (46%) had male index cases (Table 3). There was 1 acute-acute partner pair (Dyad #5) detected. None of the 45 index AHI participants were phylogenetically-linked with 1 another.
Table 3.
Characteristics of 14 Index-partner Pairs with HIV-1 Infections and an HIV-1 pol Sequence Available
| Pair ID | Participant | Gender | Age | Married | HIV-1 RNA (Copies/mL) |
Days to First Seqa | Genetic Distanceb | Major Drug Resistance Mutation |
|---|---|---|---|---|---|---|---|---|
| 1 | Index | M | 41 | 0 | >10 000 000 | 7 | 0.004 | … |
| 1 | Partner | F | 27 | 1 | 281 895 | 15 | … | |
| 2 | Index | F | 35 | 0 | >10 000 000 | 7 | 0.003 | … |
| 2 | Partner | M | 32 | 1 | 201 030 | 91 | … | |
| 3 | Index | F | 19 | 1 | 2 538 529 | 5 | 0.003 | … |
| 3 | Partner | M | 25 | 1 | 298 907 | 14 | … | |
| 4 | Index | F | 23 | 1 | 14 975 | 12 | 0.007 | … |
| 4 | Partner | M | 27 | 1 | 94 271 | 12 | … | |
| 5 | Index | F | 38 | 1 | 168 106 | 12 | 0.001 | … |
| 5 | Partner | M | 49 | 1 | >10 000 000 | 17 | … | |
| 6 | Index | M | 27 | 1 | >10 000 000 | 4 | 0.004 | … |
| 6 | Partner | F | 20 | 1 | 20 076 | 7 | … | |
| 7 | Index | M | 23 | 1 | 2 141 468 | 7 | 0.005 | … |
| 7 | Partner | F | 27 | 1 | 192 750 | 17 | … | |
| 8 | Index | F | 25 | 0 | >10 000 000 | 10 | 0.007 | M184V, T215F, K103N |
| 8 | Partner | NA | NA | NA | 131 763 | 25 | M184V, T215F, K103N | |
| 9 | Index | F | 19 | 1 | 1 485 143 | 10 | 0.012 | … |
| 9 | Partner | M | 21 | 1 | 2982 | 18 | V106VAMT,Y181YC | |
| 10 | Index | M | 30 | 1 | 951 731 | 6 | 0.011 | … |
| 10 | Partner | F | 36 | 1 | 12 296 | 17 | … | |
| 11 | Index | M | 23 | 1 | 208 792 | 8 | 0.004 | … |
| 11 | Partner | F | 34 | 0 | NA | 11 | … | |
| 12 | Index | F | 25 | 1 | 109 739 | 5 | 0.001 | K103N |
| 12 | Partner | M | 34 | 1 | 110 615 | 11 | … | |
| 13 | Index | M | 29 | 1 | 3 517 217 | 15 | 0.003 | K103N |
| 13 | Partner | F | 21 | 1 | 389 | 34 | K103N | |
| 14 | Index | F | 21 | 1 | 491 952 | 6 | 0.042 | … |
| 14 | Partner | M | 21 | 1 | 108 970 | 9 | … |
Abbreviations: HIV, human immunodeficiency virus; NA, not available.
aDays from diagnosis (index cases) or enrollment (partners) to HIV-1 sequences sampling.
bPairwise genetic distance between HIV-1 partial pol sequences (substitutions per site), calculated using the general time-reversible model of nucleotide substitution.
Altogether, the analysis shows a high degree of genetic linkage (putative transmission partnerships) between index cases and their referred partners, indicating shared transmission. Among all index cases (n = 45), gender and age were not associated with membership in a phylogenetically-linked dyad. However, index cases identified in a dyad (whether male or female index) were more likely to report no casual partners in the prior month at their initial, visit compared to index cases with an unrecognized source of infection (45% vs 10%; P = .009), and were more likely to be living with their current partner (92% vs 59%; P = .04). In 2 dyads, both the index and partner shared identical, major drug resistance mutations (K103N in Dyad #13 and M184V, T215F, and K103N in Dyad #8) indicating transmitted mutations (Figure 1; Table 3).
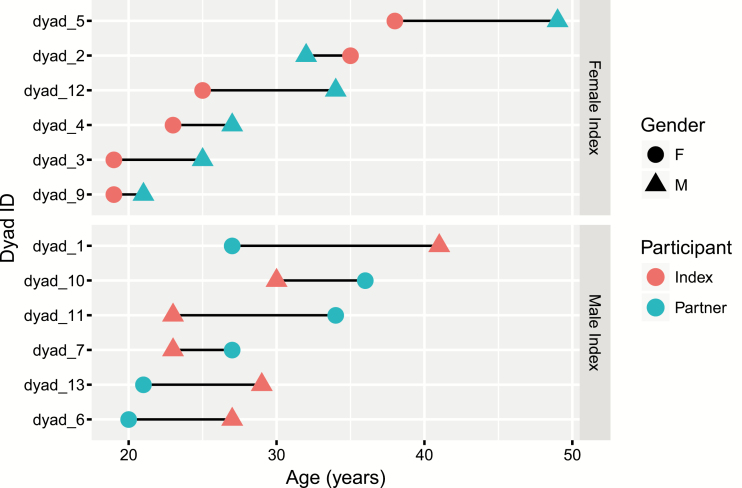
Age-disparate relationships were common among the phylogenetically-linked dyads. Among the 12/13 linked dyads with complete demographic information, 8 (67%) had a greater than 5 years age difference between the index case and the partner, with 6 of these involving younger women and older men (Figure 2). Among 6 partner dyads with female AHI index participants, 3 involved age-disparate partnerships with older men. Conversely, in 6 dyads with a male with AHI, 3 involved younger women.
Figure 2.
Relationship between age at enrollment for index and referred partners in 12 phylogenetically linked dyads. Dyads are categorized by female index (top panel) versus male index (bottom panel), diagnosed during acute HIV-1 infection. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
People with acute HIV-1 infection play a critical role in the spread of HIV-1 [2]. We have previously developed a variety of strategies to detect people with acute HIV-1 infection in Malawi [11, 16–20] and the United States [21–23]. In the current study, we evaluated the potential HIV-1 phylogenetic relationships among participants diagnosed during acute HIV infection and their sexual partners, by examining viral sequences.
The importance of HIV-1 transmission in early HIV-1 infection is a critical question in the era of “treatment as prevention.” We have previously identified geographically- and phylogenetically-linked clusters of people with acute HIV-1 infection in North Carolina [24]. In contrast, in Lilongwe, we noted no special relationship between 45 participants with AHI, although all were detected in the same city and the same clinic over 19 months. The absence of close phylogenetic linkages in this population may be due to the relatively small number of acute infections identified through presentation for concomitant STI symptoms, rather than through a comprehensive, region-wide AHI surveillance program. We are continuing with a larger study in Malawi (ClinicalTrials.gov #NCT02467439) that considers the potential of such clusters and looks more closely at the location and venues of subjects with AHI.
Acutely-infected index participants were asked to refer their sexual partners using passive referral, where the index person is expected to inform and direct their partner to the clinic. With passive referral, nearly 40% of index participants successfully referred a single partner that presented for HIV-1 testing; however, fewer than 15% of all named partners presented for evaluation. Most of the presenting partners had a chronic HIV-1 infection that was phylogenetically-linked to the acutely-infected index, suggesting that these partners may have been the source of the index’s acute infection. Thus, although passive referral was inefficient as a means to reach most partners, the partners that presented were important in the transmission chains.
We have previously shown that active partner notification using either a contract or provider referral identifies twice as many partners as passive referral, and is cost-effective in this setting [7, 25]. Active partner referral strategies, including referral with incentives or provider-assisted partner notification, may also be more efficient in quickly diagnosing and treating HIV-infected partners of persons with AHI [26]. Furthermore, active referral may improve the timely identification of HIV-uninfected partners engaged in serodiscordant partnerships [9], facilitating the early initiation of preexposure prophylaxis.
We observed a high proportion of phylogenetically-linked, stable partnerships among the index-partner dyads. These observations lead to 2 key questions: first, why now? What was the contributing factor(s) for transmission from these largely-stable partnerships at this moment in time? Given that most indexes presented to STI clinics, a concomitant STI seems like a plausible contributing co-factor. Second, would active forms of partner referral lead to additional phylogenetically-linked partnerships and greater connectedness between partnerships? Given the greater effectiveness of active referral, additional linked partnerships would likely be identified, but whether more linkages between dyads would be identified through active partner services is unclear. In our study, while index participants who did not have a partner return through passive referral reported more casual than steady partners, over half were living with steady partners. Thus, active referral may be high-yield in identifying transmission sources, as well as high-risk HIV-1–negative partners.
We were able to evaluate 19 index-partner pairs. Earlier work demonstrated that partners in longer relationships were more likely to present for evaluation [7]. Accordingly, nearly all partners we evaluated were married and were thought to be in steady partnerships with the referring index participant. Among this group, 93% of HIV-infected pairs with available sequences had a close phylogenetic linkage. The percentage of linkages between AHI cases and partners in this study was higher than reported in longitudinal studies of serodiscordant couples [5, 6], where up to a third of new HIV-1 acquisition events have been ascribed to an unrelated sexual partner.
We found that many phylogenetically-linked dyads involved younger women, both as a partner (putative donor) and acute index (putative recipient). Such age discrepancies in sexual partnering has been proposed in similar settings as an important driver of transmission [27], though findings have been inconclusive in other studies [28]. Nonetheless, interventions addressing age-disparate partnering need to be further studied and addressed.
Among index cases who successfully referred partners in our study, the majority of subjects appeared to have acquired HIV-1 from a partner with an established infection, although we do not know the time of the partner’s infection acquisition or whether the partner was unaware of his/her infection. Regardless of the duration of a relationship, transmission of HIV-1 from a person who has not initiated antiretroviral therapy remains a constant and substantial risk. Our phylogenic analysis is based on RT consensus sequences and, thus, we cannot delineate transmission directionality or estimate the duration of HIV infection among partners. We observed 1 acute-acute transmission event. This is consistent with an elevated risk of infection during AHI. We observed identical drug resistance mutations in 2 dyads. Recently, non-adherence and drug resistance were found to be associated with ongoing HIV-1 transmission among discordant couples in Zambia and Rwanda, which underscores the need for strategies to promote adherence with increasing antiretroviral availability [29].
The HIV-1 incidence has decreased in many countries, which is generally ascribed to the detection and treatment of HIV-1 infection, as emphasized by the Population-Based HIV Impact Assessment Projects [30]. However, people with acute HIV-1 infections, which are demonstrably more contagious, represent a major challenge in HIV-1 detection and management. Furthermore, given the potential for onward transmission, identifying the partners of people with acute HIV-1 infections is critically important. In the current study, many partners of acutely-infected people were not reached with passive referral. Nevertheless, among the sub-group of partners presenting through passive referral, all but 1 or 2 were likely the source of infection and required HIV care. We anticipate that strategies of intensified AHI detection and partner referral will lead to improvements in HIV-1 prevention approaches.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01-AI083059 to W. C. M. and A. E. P., T32 AI102623 to K. B. R., F30-MH09873 and T32-GM008719 to S. E. R., T32 AI070114 to D. K. P., K08AI112432 to A. M. D., and P30-AI50410).
Acknowledgements. The authors thank study participants and the staff at the Lighthouse Trust and University of North Carolina Project Malawi.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. M. S. C. received payments for travel from Merck and Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 2011; 378:256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med 2011; 364:1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen MS, Dye C, Fraser C, Miller WC, Powers KA, Williams BG. HIV treatment as prevention: debate and commentary–will early infection compromise treatment-as-prevention strategies? PLOS Med 2012; 9:e1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dennis AM, Herbeck JT, Brown AL, et al. Phylogenetic studies of transmission dynamics in generalized HIV epidemics: an essential tool where the burden is greatest? J Acquir Immune Defic Syndr 2014; 67:181–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eshleman SH, Hudelson SE, Redd AD, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network 052 trial. J Infect Dis 2011; 204:1918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell MS, Mullins JI, Hughes JP, et al. ; Partners in Prevention HSV/HIV Transmission Study Team. Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLOS One 2011; 6:e16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown LB, Miller WC, Kamanga GC, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr 2011; 56:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosseinipour MC, Rosenberg NE. HIV partner notification: possible and essential. Sex Transm Dis 2013; 40:915–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc 2017; 20:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutstein SE, Pettifor AE, Phiri S, et al. Incorporating acute HIV screening into routine HIV testing at sexually transmitted infection clinics, and HIV testing and counseling centers in Lilongwe, Malawi. J Acquir Immune Defic Syndr 2016; 71:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farr SL, Nelson JAE, Ng’ombe TJ, et al. Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr 2010; 54:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006; 22:2688–90. [DOI] [PubMed] [Google Scholar]
- 15. Hassan AS, Pybus OG, Sanders EJ, Albert J, Esbjörnsson J. Defining HIV-1 transmission clusters based on sequence data. AIDS 2017; 31:1211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg NE, Kamanga G, Phiri S, et al. Detection of acute HIV infection: a field evaluation of the determine® HIV-1/2 Ag/Ab combo test. J Infect Dis 2012; 205:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pettifor A, MacPhail C, Corneli A, et al. ; NIAID Center for HIV/AIDS Vaccine Immunology. Continued high risk sexual behavior following diagnosis with acute HIV infection in South Africa and Malawi: implications for prevention. AIDS Behav 2011; 15:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powers KA, Miller WC, Pilcher CD, et al. ; Malawi UNC Project Acute HIV Study Team. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS 2007; 21:2237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiscus SA, Pilcher CD, Miller WC, et al. ; Malawi-University of North Carolina Project Acute HIV Infection Study Team. Rapid, real-time detection of acute HIV infection in patients in Africa. J Infect Dis 2007; 195:416–24. [DOI] [PubMed] [Google Scholar]
- 20. Rucinski KB, Rutstein SE, Powers KA, et al. Sustained sexual behavior change after acute HIV diagnosis in Malawi. Sex Transm Dis 2018; 45:741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med 2005; 352:1873–83. [DOI] [PubMed] [Google Scholar]
- 22. Kuruc JD, Cope AB, Sampson LA, et al. Ten years of screening and testing for acute HIV infection in North Carolina. J Acquir Immune Defic Syndr 2016; 71:111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller WC, Leone PA, McCoy S, Nguyen TQ, Williams DE, Pilcher CD. Targeted testing for acute HIV infection in North Carolina. AIDS 2009; 23:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dennis AM, Pasquale DK, Billock R, et al. Integration of contact tracing and phylogenetics in an investigation of acute HIV infection. Sex Transm Dis 2018; 45:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rutstein SE, Brown LB, Biddle AK, et al. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan 2014; 29:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalal S, Johnson C, Fonner V, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS 2017; 31:1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Oliveira T, Kharsany AB, Gräf T, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2017; 4:e41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maughan-Brown B, George G, Beckett S, et al. HIV risk among adolescent girls and young women in age-disparate partnerships: evidence from KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2018; 78:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodson E, Goldberg A, Michelo C, et al. HIV transmission in discordant couples in Africa in the context of antiretroviral therapy availability. AIDS 2018; 32:1613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown KM, Bright LM. Teaching caring and competence: Student transformation during an older adult focused service-learning course. Nurse Educ Pract 2017; 27:29–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.