Abstract
Background
Tuberculosis (TB) prevalence is high among Tibetan refugees in India, with almost half of cases occurring in congregate facilities, including schools. A comprehensive program of TB case finding and treatment of TB infection (TBI) was undertaken in schools for Tibetan refugee children.
Methods
Schoolchildren and staff in Tibetan schools in Himachal Pradesh, India, were screened for TB with an algorithm using symptoms, chest radiography, molecular diagnostics, and tuberculin skin testing. Individuals with active TB were treated and those with TBI were offered isoniazid-rifampicin preventive therapy for 3 months.
Results
From April 2017 to March 2018, we screened 5391 schoolchildren (median age, 13 years) and 786 staff in 11 Tibetan schools. Forty-six TB cases, including 1 with multidrug resistance, were found in schoolchildren, for a prevalence of 853 per 100 000. Extensively drug-resistant TB was diagnosed in 1 staff member. The majority of cases (66%) were subclinical. TBI was detected in 930 of 5234 (18%) schoolchildren and 334 of 634 (53%) staff who completed testing. Children in boarding schools had a higher prevalence of TBI than children in day schools (915/5020 [18%] vs 15/371 [4%]; P < .01). Preventive therapy was provided to 799 of 888 (90%) schoolchildren and 101 of 332 (30%) staff with TBI; 857 (95%) people successfully completed therapy.
Conclusions
TB prevalence is extremely high among Tibetan schoolchildren. Effective active case finding and a high uptake and completion of preventive therapy for children were achieved. With leadership and community mobilization, TB control is implementable on a population level.
Keywords: tuberculosis, pediatrics, Tibet, case finding, preventive therapy
We conducted school-based screening for active and latent tuberculosis in children in Tibetan schools in India. A high prevalence of active disease (853/100 000) and latent infection (19%) was found. Tolerance and compliance with 3 months of isoniazid-rifampicin preventive therapy were excellent.
Approximately 1 million children develop tuberculosis (TB) and 250 000 die of the disease annually [1–3]. Tibetan refugees in India, mostly settled in the northern and southern states of Himachal Pradesh, Uttrakhand, and Karnataka, have very high TB rates, with a large proportion occurring in children [4–7]. Residence in congregate living facilities and a combination of socioeconomic, biomedical, and political challenges over several decades contribute to the high TB burden in the Tibetan refugee population. TB infection rates of 65%–98% have been reported for Tibetan immigrants in the United States and Canada [8–10]. A population-wide active TB case finding campaign between 2011 and 2013 identified a case prevalence of 394 per 100 000 among Tibetan schoolchildren in India [5]. Because of the high burden of TB in Tibetan refugee children, we undertook a TB case finding and preventive therapy program in Tibetan schools in northern India. We present here the findings from the first year of the initiative in which schoolchildren and staff residing in Tibetan schools in India were screened and treated for TB disease and infection on a population level using a community-based approach.
METHODS
Study Setting and Population
The Zero TB Kids project is a collaboration between the Delek Hospital, Dharamsala; the Johns Hopkins University School of Medicine; the University of Wisconsin–Madison; and the Central Tibetan Administration Department of Health (CTA-DoH) and Department of Education (CTA-DoE). Spiritual and political leaders of the Tibetan exile community endorsed the project prior to its launch [11]. Between April 2017 and March 2018, Zero TB Kids conducted on-site screening for active and latent TB among children and staff residing in 7 boarding and 4 Tibetan day schools in the state of Himachal Pradesh, India. The boarding schools are home to the children, many of whom are from poor families living in Tibetan settlements across India and Nepal. Children from prekindergarten to grade 12 are cared for by housemothers in the school dormitories and hostels. All students and staff at each school were eligible for TB symptom screening and testing for latent TB infection (TBI), which were performed at the school. Anti-TB treatment was provided at a CTA-DoH treatment facility. The study was ruled exempt by the Johns Hopkins Medicine Institutional Review Board as a public health initiative and approved by the CTA-DoH, CTA-DoE, and each school administration. Identifying information of participants was maintained by the Department of Health and anonymized data were provided to Johns Hopkins University personnel. Consent was obtained from parents by the Department of Health and the school before treatment was provided to children.
Project Design and Procedures
Screening for Active TB
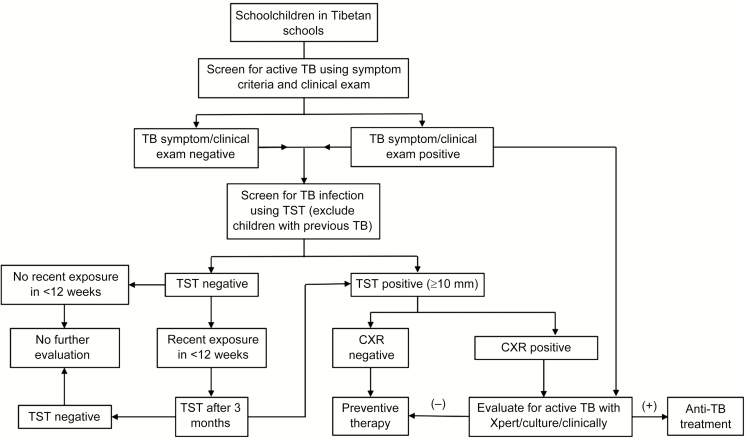
The algorithm followed for screening for active and latent TB and for providing preventive therapy is shown in Figure 1. Basic demographic information was provided by the school administration. TB exposure history in the current and previous school years at school was obtained through review of school health records. Additional information on index or source TB cases in the schools was obtained from the DoH treatment facility. Children and staff members were considered contacts of a TB case if they shared living space or a classroom at the time of the latter’s diagnosis. Each schoolchild and staff member was interviewed by project staff using a standardized questionnaire. Information was collected on TB-related symptoms including cough, fever, night sweats, weight loss, and fatigue as well as past TB history or exposure, other health conditions, and receipt of concomitant medications. Each participant was examined by a project medical officer. Individuals with cough ≥2 weeks or other symptoms underwent further evaluation with a chest radiograph (CXR) and/or testing of respiratory secretions with the Xpert MTB/RIF IV assay (Cepheid, Sunnyvale, California). Children with TB symptoms who were not able to produce sputum had a gastric aspiration performed at the school infirmary for Xpert testing. Sputum or lavage fluid from all Xpert-positive individuals underwent culture using the Mycobacterial Growth Indicator Tube system (Becton Dickinson, Sparks, Maryland) and drug susceptibility testing at the P. D. Hinduja National Laboratory in Mumbai. Second-line drug susceptibility testing was performed for isolates with rifampicin resistance by Xpert. Because this was a schoolwide TB screening program, contacts of recent cases were automatically evaluated for active TB under the study algorithm. For cases presenting passively afterward, contact evaluation was carried out, but results are not yet available.
Figure 1.
Study flowchart. Abbreviations: CXR, chest radiograph; TB, tuberculosis; TST, tuberculin skin test.
Screening for Latent TB and Preventive Therapy
TBI was diagnosed by tuberculin skin testing (TST) using 5 TU of purified protein derivative RT23 (Span Diagnostics, Surat, India). Induration measuring ≥10 mm after 2–3 days was considered positive.
Preventive therapy with 3 months of daily isoniazid and rifampicin (3HR) was provided to TST-positive individuals after ruling out active TB with CXR and, if warranted, an Xpert test. Children and staff with exposure only to drug-resistant TB cases were not provided preventive therapy but underwent routine monitoring according to World Health Organization (WHO) guidelines. People with mixed exposure to both drug-susceptible and drug-resistant TB cases were provided preventive therapy with 3HR. Individuals with a diagnosis of chronic hepatitis B virus (HBV) infection had liver enzyme tests and abdominal ultrasonography performed before being provided preventive therapy. Those with elevated bilirubin levels or liver enzymes (alanine aminotransferase or aspartate aminotransferase) greater than twice the upper limit of normal or with ultrasound findings of liver parenchymal disease were not provided preventive therapy. Preventive therapy medications packed and labeled with recipients’ name in separate boxes were distributed to housemothers who supervised therapy every morning for children. Parents provided supervision for children who were day scholars. Staff members self-administered preventive therapy. Adherence was further ensured through a treatment card that housemothers and staff checked after each dose. The project staff conducted monthly follow-up for preventive therapy recipients at each school.
Side effects experienced by participants were recorded on treatment cards developed for the initiative. Hepatotoxicity from isoniazid or rifampicin was determined by the clinician based on development of (1) clinical symptoms of nausea, vomiting, loss of appetite, tiredness, upper abdominal pain, or jaundice; (2) elevated serum aminotransferases (≥5 times upper limit of normal); and (3) subsidence of symptoms/laboratory values following discontinuation of drug [12]. Preventive therapy was temporarily withheld in the event of hepatoxicity or other adverse drug reactions as determined by the clinician and restarted after subsidence of symptoms and laboratory values. Preventive therapy regimen was modified to 4 months of rifampicin where isoniazid was implicated, or to 6 months of isoniazid where rifampicin was implicated. Therapy modification or premature termination was carried out by the clinician in consultation with the school nurse, housemothers, individuals, and parents. Project staff communicated via phone with school nurse and housemothers once every 2 weeks to discuss outstanding issues related to preventive therapy implementation.
Data Collection and Statistical Analysis
Data were processed and analyzed using Stata version 13.1 software (StataCorp, College Station, Texas). Descriptive, univariate, and multivariable logistic regression analyses adjusting for age and sex were carried out to assess the risk of TB infection and disease for schoolchildren.
RESULTS
Study Population and TB Exposure History
Between April and October 2017, 5391 schoolchildren and 786 staff in 11 Tibetan schools (7 boarding schools [n = 5726] and 4 day schools [n = 451]) were screened for TBI and disease. The median age of students was 13 years (interquartile range [IQR], 10–16 years) and 50% were female (Table 1). The majority of the population (95%) had received Bacille Calmette-Guérin (BCG) vaccination. Previous TB treatment was reported by 135 (3%) students and 139 (18%) staff members; 6 individuals with previous therapy (2%) had been treated for multidrug-resistant TB (MDR-TB). Thirty percent (n = 1601) of schoolchildren reported close contact with a TB case in the past 2 years, including 108 (2%) who were exposed to MDR-TB. Twenty-six percent of schoolchildren had exposure to a TB case in school and 3% had exposure to a TB case at home. Eighty-eight (2%) schoolchildren and 40 (5%) staff reported having chronic HBV infection.
Table 1.
Characteristics of Students and Staff Screened for Latent and Active Tuberculosis Under the Zero TB Kids Project at Tibetan Schools in Himachal Pradesh State, India, 2017–2018
| Characteristics | All Participants (N = 6177) | Students (n = 5391) | Staff (n = 786) |
|---|---|---|---|
| Age, y, median (IQR) | 14 (10–17) | 13 (10–16) | 40 (33–48) |
| Sex | |||
| Female | 3172 (51.4) | 2700 (50.1) | 472 (60.0) |
| Male | 3005 (48.7) | 2691 (49.9) | 314 (40.0) |
| Occupation | |||
| Student | 5391 (87.3) | 5391 (100) | … |
| Teacher | 393 (6.4) | … | 393 (6.4) |
| Housemother | 107 (1.7) | … | 107 (1.7) |
| Office staff | 81 (1.3) | … | 81 (1.3) |
| Cook | 41 (0.7) | … | 41 (0.7) |
| Other staff | 164 (2.7) | … | 164 (2.7) |
| Place of birth | |||
| India | 4946 (80.1) | 4414 (81.9) | 532 (67.7) |
| Tibet | 776 (12.6) | 531 (9.9) | 245 (31.2) |
| Nepal | 422 (6.8) | 414 (7.7) | 8 (1.0) |
| Bhutan | 25 (0.4) | 24 (0.5) | 1 (0.1) |
| Other | 8 (0.1) | 8 (0.2) | 0 (0.0) |
| Ethnicity | |||
| Tibetan | 5790 (93.8) | 5020 (93.1) | 770 (98.0) |
| Indian | 94 (1.5) | 80 (1.5) | 14 (1.8) |
| Himalayan origina | 292 (4.7) | 290 (5.4) | 2 (0.3) |
| Weight, kg, median (IQR) | 45.5 (32–56) | 42.6 (31–53) | 65 (57–74) |
| History of previous TB treatment | |||
| Previously treated for TB | 274 (4.4) | 135 (2.5) | 139 (17.7) |
| Previously treated for MDR-TB | 6/275 (2.2) | 3/135 (2.2) | 3/139 (2.2) |
| Exposure to TB case in past 2 y | 1685 (27.3) | 1601 (29.7) | 84 (10.7) |
| Exposure to drug-susceptible TB | 1286 (20.8) | 1229 (22.8) | 57 (7.3) |
| Exposure to MDR-TBb | 108 (1.8) | 105 (2.0) | 3 (0.4) |
| Drug susceptibility status unknown | 291 (4.7) | 267 (5.0) | 24 (3.1) |
| Received BCG vaccinec | |||
| Yes | 5855 (94.8) | 5159 (95.7) | 696 (88.6) |
| No | 309 (5) | 224 (4.2) | 85 (10.8) |
| Unknown | 13 (0.2) | 8 (0.2) | 5 (0.6) |
| Coexisting medical conditions | |||
| Asthma | 24 (0.4) | 15 (0.3) | 9 (1.2) |
| Seizure disorder | 23 (0.4) | 22 (0.4) | 1 (1.1) |
| Chronic hepatitis B | 128 (2.1) | 88 (1.6) | 40 (5.1) |
| Hypertension | 40 (0.7) | 0 (0) | 40 (5.1) |
| Diabetes mellitus | 8 (0.1) | 1 (0.0) | 7 (0.9) |
| Acid peptic disease | 36 (0.6) | 20 (0.4) | 16 (2.0) |
| Other conditions | 17 (0.3) | 13 (0.2) | 4 (0.5) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BCG, Bacille Calmette-Guérin; IQR, interquartile range; MDR-TB, multidrug-resistant tuberculosis; TB, tuberculosis.
aIncludes non-Tibetan students from the Ladakh, Kinnaur, and Lahaul areas of India and Nepal.
bHistory of MDR-TB in last 5 years. School nurse/parent contacted for confirmation when a child responded positive to contact with drug-resistant TB. Pleural TB successfully treated with first-line antituberculosis therapy with no drug susceptibility test result was considered drug susceptible.
cBCG vaccine status (left arm scar) checked only for people born in Tibet. Persons born outside Tibet were assumed to have received BCG at birth under their national immunization program.
Active TB
There were 47 TB cases in total, of which 46 were detected in 5391 schoolchildren for a prevalence of 853 per 100 000 (Table 2). One child had MDR-TB. The median age of the children with TB was 16 years (IQR, 14–17 years; Table 3). Among staff members (n = 786), 1 case was detected (prevalence, 127/100 000), which was extensively drug resistant (XDR) with resistance to isoniazid, rifampicin, ofloxacin, and kanamycin. Another staff member had disease with nontuberculous mycobacteria. All cases were detected in the 7 boarding schools. The TB prevalence in schoolchildren at the 7 boarding schools was 916 per 100 000 (range 371–3205 per 100 000). The prevalence in children aged <15 years was 307 per 100 000. Of the 47 TB cases (46 schoolchildren and 1 staff member), 43 were pulmonary and 4 were extrapulmonary (1 lymph node, 1 hip joint, and 2 pleural TB). Of the 43 pulmonary cases, 41 (95%) were Xpert positive, of which 6 (15%) were acid-fast bacilli smear positive and 14 (34%) were culture positive (Supplementary Table 1). Gastric aspirates constituted 63% (n = 26) of Xpert-positive specimens. Cough in the previous 2 weeks was commonly reported (15% [n = 825]), but 29 of 44 children (66%) with active lung or pleural TB did not have cough. The positive predictive value for cough was 2% in this setting. On multivariable analyses, increasing age, exposure history, multiple exposures, exposure in both classroom and dormitory, cough >2 weeks, and fever in the last 2 weeks had statistically significant associations with TB disease among schoolchildren (Table 3). Among TST positives, those with a recent TB exposure had higher risk of disease (odds ratio [OR], 2.6; P = .002). The majority of TB cases (n = 42/47 [90%]) were detected after CXR examination following TST positivity.
Table 2.
Prevalence of Active Tuberculosis (TB) Disease Among Students and Staff Screened for Active TB in Various Tibetans Schools in Himachal Pradesh, India, 2017–2018
| School | No. of Students Screened | No. of Staff Screenedc | No. of TB Cases in Students | TB Disease Prevalence in Students (per 100 000) |
|---|---|---|---|---|
| School Aa | 523 | 58 | 10 | 1952 |
| School Ba | 809 | 116 | 7 | 865 |
| School Ca | 1311 | 253 | 6 | 458 |
| School Da | 1149 | 128 | 9 | 783 |
| School Ea | 812 | 85 | 3 | 369 |
| School Fa | 161 | 26 | 5 | 3106 |
| School Ga | 255 | 40 | 6 | 2353 |
| School Hb | 28 | 9 | 0 | 0 |
| School Ib | 47 | 10 | 0 | 0 |
| School Jb | 111 | 9 | 0 | 0 |
| School Kb | 185 | 52 | 0 | 0 |
| Overall | 5391 | 786 | 46 | 853 |
Abbreviation: TB, tuberculosis.
aBoarding schools, where all TB cases were detected for a prevalence of 916 per 100 000 (46/5020) students.
bDay schools.
cOnly 2 staff members were diagnosed with active disease: 1 with extensively drug-resistant TB and 1 with nontuberculous mycobacterial disease.
Table 3.
Relationship Between Baseline/Clinical Characteristics and Risk of Tuberculosis Disease Among Schoolchildren in Various Tibetan Schools in Himachal Pradesh, India, 2017
| Characteristic | Schoolchildren Detected With TB Disease (n = 46) | Schoolchildren Without TB Disease (n = 5345) |
TB Risk (Univariate Analysis) OR (95% CI) |
P Value | TB Risk (Multivariable Analysis) ORa (95% CI) |
P Value |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 16 (14–17) | 13 (10–15) | 1.2 (1.1–1.4) | < .001 | 1.2 (1.1–1.4) | < .001 |
| Sex | ||||||
| Female | 24 (52.2) | 2676 (50.1) | Referent | .776 | Referent | .795 |
| Male | 22 (47.8) | 2669 (49.9) | 0.92 (.5–1.6) | … | 0.93 (.5–1.7) | … |
| Previous TB history | 1 (2.2) | 134 (2.5) | 0.88 (.1–6.3) | .886 | 0.45 (.06–3.4) | .438 |
| No previous TB history | 45 (97.8) | 5211 (97.5) | Referent | … | Referent | … |
| Recent exposure (<2 y) to a TB caseb | ||||||
| Yes | 30 (65.2) | 1571 (29.4) | 4.5 (2.4–8.3) | .002 | 2.8 (1.5–5.5) | .002 |
| No | 16 (34.8) | 3771 (70.6) | Referent | … | Referent | … |
| TST+, recently exposed | 28 (65.1) | 426 (40.8) | 2.9 (1.5–5.6) | .001 | 2.6 (1.3–5.2) | .006 |
| TST+, not recently exposed | 15 (34.9) | 618 (59.2) | Referent | … | Referent | … |
| Multiple TB exposure | ||||||
| Exposed to 1 case | 9 (30.0) | 988 (62.9) | Referent | .001 | Referent | .003 |
| Exposed to ≥2 cases | 21 (70.0) | 583 (37.1) | 4.0 (1.8–8.7) | … | 3.5 (1.5–8.0) | … |
| Exposure setting in school | ||||||
| Classroom | 10 (35.7) | 605 (43.8) | Referent | .888 | Referent | .993 |
| Dormitory/hostel | 9 (34.2) | 510 (36.9) | 1.1 (.4–2.6) | .030 | 1.0 (.4–2.5) | .063 |
| Classroom and dormitory/hostel | 9 (32.1) | 198 (14.3) | 2.8 (1.1–6.9) | … | 2.4 (.95–6.2) | … |
| Cough in last 2 wk | ||||||
| Yes | 17 (37.0) | 808 (15.1) | 3.3 (1.8–6.0) | < .001 | 2.8 (1.5–5.1) | .001 |
| No | 29 (63.0) | 4537 (84.9) | Referent | … | Referent | … |
| Fever in last 2 wk | ||||||
| Yes | 5 (10.9) | 171 (3.2) | 3.7 (1.4–9.5) | .007 | 3.4 (1.3–8.7) | .012 |
| No | 41 (89.1) | 5175 (96.8) | Referent | … | Referent | … |
| Night-sweats in last 2 wk | ||||||
| Yes | 5 (10.9) | 201 (3.8) | 3.1 (1.2–8.0) | .018 | 2.0 (.8–5.3) | .142 |
| No | 41 (89.1) | 5144 (96.2) | Referent | … | Referent | … |
| Weight loss in last 1 mo | ||||||
| Yes | 12 (26.1) | 847 (15.9) | 1.9 (.96–3.6) | .063 | 1.3 (.6–2.5) | .503 |
| No | 34 (73.9) | 4498 (84.2) | Referent | … | Referent | … |
| Increased tiredness in last 2 wk | ||||||
| Yes | 13 (28.3) | 586 (11.0) | 3.2 (1.7–6.1) | < .001 | 1.9 (.97–3.8) | .063 |
| No | 33 (71.7) | 4759 (89.0) | Referent | … | Referent | … |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval; IQR, interquartile range; OR, odds ratio; TB, tuberculosis; TST, tuberculin skin test.
aAdjusted for age and sex.
bSchoolchildren were considered exposed to Mycobacterium tuberculosis if they were sharing classroom or residential quarters with another student at the time of the latter’s TB diagnosis. Students diagnosed with lung or pleural TB were considered index TB cases.
TB Infection
A total of 5234 schoolchildren and 634 staff members were screened for TBI. The prevalence of TBI was 18% (930/5234) for schoolchildren and 53% (334/634) for staff members (Table 4). TST was not performed for 135 schoolchildren and 139 staff members who had active TB previously and for 22 schoolchildren and 13 staff for other reasons including refusals. The median induration of reactive TSTs was 16 mm (IQR, 13–19 mm) for schoolchildren, 18 mm (IQR, 13–20 mm) for staff, and 18 mm (IQR, 14–20 mm) for persons diagnosed with active TB. TST conversion was assessed after 12 weeks for 250 schoolchildren in 2 schools who were newly exposed and TST negative at the time of screening. Thirty-two (13%) schoolchildren converted to TST positive and 1 TB case was diagnosed among converters. TBI was higher in the boarding schools than the day schools: 19% vs 4% (P < 0.001) for schoolchildren and 54% vs 42% (P = .078) for staff. Increasing age, male sex, TB exposure history, exposure to ≥2 cases, and exposure in dormitory were significantly associated with an increased risk of TBI (Table 5). Risk of TBI for staff members was described in Supplementary Table 2. Distribution of TBI and disease by age categories and sex is presented in Supplementary Figure 1.
Table 4.
Prevalence of Tuberculosis Infection Among Students and Staff in Various Tibetan Schools in Himachal Pradesh, India, 2017
| School | No. of Students Screened for TB Infection | No. of Staff Screened for TB Infection | TB Infection Prevalence in Students, No. (%) |
TB Infection Prevalence in Staff, No. (%) |
|---|---|---|---|---|
| School Aa | 513 | 50 | 140 (27) | 11 (22) |
| School Ba | 779 | 198 | 188 (24) | 64/198 (65) |
| School Ca | 1281 | 208 | 183 (14) | 109/208 (52) |
| School Da | 1106 | 82 | 208 (19) | 49/82 (60) |
| School Ea | 795 | 77 | 97 (12) | 42//77 (55) |
| School Fa | 157 | 22 | 47 (30) | 15/22 (68) |
| School Ga | 234 | 31 | 52 (22) | 16/31 (52) |
| School Hb | 28 | 8 | 1 (4) | 1/8 (13) |
| School Ib | 47 | 9 | 4 (9) | 5/9 (56) |
| School Jb | 111 | 8 | 3 (2.7) | 2/8 (25) |
| School Kb | 183 | 41 | 7 (4) | 20/41 (49) |
| Overall | 5234 | 734 | 930/5234 (18) | 334/634 (53) |
Abbreviation: TB, tuberculosis.
aBoarding school.
bDay school.
Table 5.
Relationship Between Various Baseline/Clinical Characteristics and Risk of Mycobacterium tuberculosis Infection Among Schoolchildren in Various Tibetan Schools in Himachal Pradesh, India, 2017
| Characteristics of Schoolchildren | Schoolchildren With TB Infection, n/N (%) |
Risk of TB Infection (Univariate Analysis), OR (95% CI) |
P Value | Risk of TB Infection (Multivariable Analysis), OR (95% CI) |
P Value |
|---|---|---|---|---|---|
| Age range, y | |||||
| ≥5–9 | 77/1121 (6.9) | Referent | … | Referent | … |
| 10–14 | 365/2398 (15.2) | 2.4 (1.9–3.2) | < .001 | 2.4 (1.9–3.1) | < .001 |
| 15–19 | 458/1621 (28.3) | 5.3 (4.1–6.9) | < .001 | 5.4 (4.2–7.0) | < .001 |
| 20–24 | 30/92 (32.6) | 6.6 (4.0–10.8) | < .001 | 6.5 (4.0–10.7) | < .001 |
| Sex | |||||
| Female | 414/2625 (15.8) | Referent | … | Referent | … |
| Male | 516/2609 (19.8) | 1.3 (1.1–1.5) | < .001 | 1.4 | < .001 |
| Body weight, kg | |||||
| ≤24 | 44/595 (7.4) | Referent | … | Referent | … |
| 25–39 | 201/1751 (11.5) | 1.6 (1.2–2.3) | .005 | 1.1 (.8–1.6) | .540 |
| 40–54 | 386/1874 (20.6) | 3.2 (2.3–4.5) | < .001 | 1.5 (.97–2.2) | .071 |
| 55–69 | 261/877 (29.8) | 5.3 (3.8–7.5) | < .001 | 1.9 (1.2–2.9) | .006 |
| ≥70 | 38/136 (27.9) | 4.9 (3.0–7.9) | < .001 | 1.6 (.9–2.8) | .113 |
| Recent exposure (<2 y) to a TB casea | |||||
| Yes | 433/1430 (30.3) | 2.9 (2.5–3.4) | < .001 | 2.0 (1.7–2.4) | < .001 |
| No | 497/3802 (13.1) | Referent | … | Referent | … |
| Exposure setting in school | |||||
| Classroom | 146/578 (25.3) | Referent | … | Referent | … |
| Dormitory/hostel | 168/497 (33.8) | 1.5 (1.2–2.0) | .002 | 1.4 (1.1–1.9) | .010 |
| Both dormitory/hostel & classroom | 76/197 (38.6) | 1.9 (1.3–2.6) | < .001 | 1.7 (1.2–2.4) | .005 |
| Other unspecified setting | 17/68 (25) | 1.0 (.6–1.8) | .963 | 0.9 (.5–1.7) | .831 |
| Exposure to TB case at homeb | |||||
| No | 873/5078 (17.2) | Referent | < .001 | Referent | < .001 |
| Yes | 57/156 (36.5) | 2.8 (2.0–3.9) | … | 2.3 (1.6–3.2) | … |
| Multiple exposures | |||||
| No history of exposure | 498/3807 (13.1) | Referent | … | Referent | … |
| Contact with 1 case | 216/871 (24.8) | 2.2 (1.8–2.6) | < .001 | 1.7 (1.4–2.1) | < .001 |
| Contact with ≥2 cases | 216/556 (38.9) | 4.2 (3.5–5.1) | < .001 | 2.7 (2.2–3.4) | < .001 |
| BCG vaccine status | |||||
| No | 46/214 (21.5) | Referent | … | Referent | … |
| Yes | 880/5012 (17.6) | 0.8 (.6–1.1) | .141 | 1.4 (.96–1.9) | .078 |
Abbreviations: BCG, Bacille Calmette-Guérin; CI, confidence interval; OR, odds ratio; TB, tuberculosis.
aSchoolchildren were considered to be exposed to Mycobacterium tuberculosis if they were sharing a classroom or residential quarters with another student at the time of the latter’s TB diagnosis. Students diagnosed with lung or pleural TB were considered index TB cases.
bExposure at home was identified in the schoolchildren by asking them during the screening process whether anyone in their family had TB while they were at home in the last 2 years. Exposure at home was assessed for children in sixth grade or higher.
TB Preventive Therapy and Outcomes
Of 930 schoolchildren and 334 staff with TBI, 888 and 332, respectively, were eligible to receive preventive therapy after excluding active disease (42 TST-positive active TB disease in schoolchildren and 2 TST-positive active disease in staff). Preventive therapy was provided to 90% (799/888) of schoolchildren and 30% (101/332) of staff with latent TBI. Thirty-three children and 45 staff members had contraindications to preventive therapy, including 24 with chronic HBV infection. Of those eligible, preventive therapy was declined by 56 (6%) schoolchildren and 186 (56%) staff. Nine people with exclusive MDR-TB contact were not provided preventive therapy. Two hundred twenty-seven (25%) people developed at least 1 treatment side effect, almost all of which were attributed to isoniazid. Toxicity was more common in adolescents aged 10–18 years (36%) and less common for children aged 5–9 years (14%). Fatigue, drowsiness, and gastrointestinal complaints were reported by 8%–10% of schoolchildren and 5%–13% of staff receiving preventive therapy. Eleven schoolchildren (1.4%) and 1 staff member (1.0%) experienced hepatoxicity that was attributed to isoniazid, and elevated liver enzymes and symptoms regressed after the drug was discontinued. Preventive therapy was permanently stopped for 2 people with hepatotoxicity; 10 others went on to successfully complete treatment. Twenty of 45 (44%) individuals with latent TB and HBV coinfection received preventive therapy with isoniazid and rifampicin, and 1 developed hepatoxicity requiring discontinuation of treatment. Preventive therapy was modified to 4 months of rifampicin alone for 7 (0.8%) people, isoniazid preventive therapy for 6 months for 1 person (0.1%), and prematurely terminated for 5 (0.6%) people due to drug toxicity. Of the 900 people who were provided preventive therapy, 857 (95%) successfully completed therapy, 5 (0.6%) prematurely stopped preventive therapy, 7 (0.8%) were lost to follow-up, and therapy was ongoing for 30 (3.4%) people (Supplementary Table 3).
DISCUSSION
We detected a high prevalence of TB disease (853/100 000) and infection (18%) among Tibetan schoolchildren in India and a high prevalence of TBI in adult staff (53%). The majority of the cases were subclinical (66%), including 1 MDR-TB case and 1 XDR-TB case detected in a child and a staff member, respectively. One-fifth of the schoolchildren investigated for TB were Xpert positive, nearly twice of what was reported (11%) for children in a recent systematic review [13]. The infection prevalence observed for Tibetan adults (53%) was comparable to the rate observed for Tibetan refugees in New York City (65%) in 1995–1999 but lower than the prevalence of 96%–98% observed for Tibetan refugees in Minnesota and Toronto in 1992–1994 and 1998–2000, respectively [8–10].
The proportion of new TB disease detected among schoolchildren in Tibetan boarding schools during the 1-year study period (916/100 000) is >4 times that of India’s incidence (211/100 000 in 2016) [2], and higher than the incidence observed in high-HIV-prevalence settings such as South Africa (781/100 000 in 2016) [2]. HIV infection is uncommon in the Tibetan community [5, 7]. The proportion of new TB cases detected in Tibetan children aged <15 years (307/100 000) was 5–8 times higher than the incidence calculated for children aged <15 years for India (63/100 000), China (41/100 000), and globally (54/100 000) based on data from the WHO [2]. The heterogeneity of TB prevalence across various Tibetan boarding schools (371–3201 per 100 000) could be due to many factors, including recent TB cases in the school, overcrowding and ventilation in the dormitories, and healthcare facilities and healthcare access for the school. The low smear positivity is likely due to the disease being paucibacillary in the majority of the cases and Xpert’s higher sensitivity than smears. Culture sensitivity was probably compromised by the need to transport specimens from schools to a referral laboratory.
Nearly one-third (26%) of students reported exposure to someone with active TB in the previous 2 years at school, suggesting that over several years the majority of the school population would be contacts, especially as children enroll in the boarding schools at an early age. We observed a higher risk (OR, 3.0; P = .002) of TB disease among recently exposed schoolchildren. While 26% contacts were reported to have occurred within the school, the contact history in household (3%) in the previous 2 years for schoolchildren was also substantial and higher than the estimated global rate; a modeling study estimated that 15 million children had household TB exposure in 2010—that is, approximately 0.8% of the global child population [14]. Our findings of higher risk of infection in boarding schools (19%) than day schools (5%) and dormitories than classrooms (OR, 1.4; P = .01) suggest that the living facilities, including their ventilation and time spent in them, play important roles in TB transmission and acquisition. The TB infection rate we observed for schoolchildren aged 5–20 years (18%) was higher than the rate (11%) observed for children aged 5–20 years in rural China [15] and the estimated rate of 3.5% (67 million of 1.9 billion children) for children aged <15 years globally [14]. Among TST-positive schoolchildren, we were able to confirm established knowledge that those who are recently exposed have higher risk of disease progression compared with those not recently exposed (OR, 2.6; P = .006) [16-18]. We observed high TST conversion (13%) for children recently exposed to TB, underscoring the need for regular screening. Although 95% of schoolchildren received BCG vaccine, the majority (~90%) received it at birth and are now older than 5 years; as such, false-positive TST reactions are less of a concern [19].
We ensured the community’s acceptance of large-scale case finding and implementation of preventive therapy [20, 21] by sensitizing and mobilizing the community about the Zero TB Kids program months in advance through (1) news channels and newspapers regularly accessed by the community; (2) Zero TB Kids social media campaign; (3) advocacy and support by community leaders and care providers; and (4) engagement of community members in planning and making decisions. We ensured active involvement of school nurses, housemothers, and parents, the key care providers, by organizing a special meeting at each school between them, the school administrator, and the project staff to facilitate open discussions on ensuring adherence and improving care of children detected with active TB. The lower uptake of preventive therapy among staff (31%) compared to schoolchildren (86%) may have been due to the program’s emphasis on children, as well as the older age and a lower perception of risk of staff and the absence of standard guidelines for treating older contacts of cases in global TB guidelines.
Despite a high prevalence of HBV infection, the rate of hepatotoxicity in our study population (1%) was similar to rates reported for other populations. Because 98% of the side effects were attributed to isoniazid, a preventive therapy regimen of 4 months of rifampicin or once-weekly isoniazid-rifapentine could improve acceptance and tolerance [22–27]. The majority of children with seizure disorder (88%) could not receive preventive therapy due to unclear guidelines and physician hesitancy in the context of drug–drug interactions with antiepileptic agents. Our community-based approach ensured excellent adherence to preventive therapy (>98%), which has been low in most preventive therapy initiatives [20–22, 28].
TB preventive therapy is grossly underimplemented in high-burden countries. Globally, only 13% of children aged <5 years eligible for preventive therapy in 2016 received it, while in India, a mere 1.9% of eligible children aged <5 years received preventive therapy [2]. Global guidelines for TB preventive therapy did not exist for older children (aged >5 years), adolescents, and adults in high-burden countries until early 2018, despite a proven benefit across age groups [29–32]. TB control efforts and policies targeting children and adolescents will be essential to the achievement of the 90-90-90 targets of the Global Plan to End TB, as well as the WHO’s End TB Strategy to end the global TB epidemic by 2035 [33]. New guidelines from the WHO issued in 2018 now clarify that household contacts of people with pulmonary TB should be offered preventive therapy [31]. Our initiative is the first of its kind to include population-level implementation of preventive therapy in a multipronged strategy to control and eliminate TB in an at-risk population in India. With this effort, we have demonstrated that TBI screening and preventive therapy can be successfully implemented on a large scale in high-TB-burden settings and have identified important challenges in the process. This initiative also highlights the importance and feasibility of community mobilization in efforts to control TB in vulnerable populations, including children and refugees.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are thankful to the community leaders, parents, school administration and staff, and all of the schoolchildren for their overwhelming support and dedication to this project.
Disclaimer. Study sponsors played no role in the design of the study; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the paper for publication.
Financial support. This work was supported by the Tibet Fund; Friends of the Delek Hospital, the Pittsfield Anti-Tuberculosis Association, the Johns Hopkins University Alliance for a Healthier World, and private donations.
Potential conflicts of interest. R. E. C. reports consulting fees from Merck, Otsuka, and Janssen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383:1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO global tuberculosis report 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 3. Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhatia S, Dranyi T, Rowley D. Tuberculosis among Tibetan refugees in India. Soc Sci Med 2002; 54:423–32. [DOI] [PubMed] [Google Scholar]
- 5. Dierberg KL, Dorjee K, Salvo F, et al. Improved detection of tuberculosis and multidrug-resistant tuberculosis among Tibetan refugees, India. Emerg Infect Dis 2016; 22:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson LJ, Naik Y, Tsering K, Cegielski JP. Population-based risk factors for tuberculosis and adverse outcomes among Tibetan refugees in India, 1994-1996. Int J Tuberc Lung Dis 2005; 9:1018–26. [PubMed] [Google Scholar]
- 7. Salvo F, Dorjee K, Dierberg K, et al. Survey of tuberculosis drug resistance among Tibetan refugees in India. Int J Tuberc Lung Dis 2014; 18:655–62. [DOI] [PubMed] [Google Scholar]
- 8. Lee YA, Munsiff SS, Li J, Driver CR, Mathema B, Kreiswirth BN. Rising number of tuberculosis cases among Tibetans in New York City. J Immigr Health 2001; 3:173–80. [DOI] [PubMed] [Google Scholar]
- 9. Marras TK, Wilson J, Wang EE, Avendano M, Yang JW. Tuberculosis among Tibetan refugee claimants in Toronto: 1998 to 2000. Chest 2003; 124:915–21. [DOI] [PubMed] [Google Scholar]
- 10. Truong DH, Hedemark LL, Mickman JK, Mosher LB, Dietrich SE, Lowry PW. Tuberculosis among Tibetan immigrants from India and Nepal in Minnesota, 1992-1995. JAMA 1997; 277:735–8. [PubMed] [Google Scholar]
- 11.Tibetan Delek Hospital. Endorsement of Zero TB in Kids by His Holiness the Dalai Lama 2017. Available at: https://www.youtube.com/watch?v=EkS0AqDY26U Accessed 19 June 2018.
- 12. Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA 1999; 281:1014–8. [DOI] [PubMed] [Google Scholar]
- 13. Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014; 2:e453–9. [DOI] [PubMed] [Google Scholar]
- 15. Gao L, Lu W, Bai L, et al. LATENTTB-NSTM Study Team. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15:310–9. [DOI] [PubMed] [Google Scholar]
- 16. Lee RS, Proulx JF, Menzies D, Behr MA. Progression to tuberculosis disease increases with multiple exposures. Eur Respir J 2016; 48:1682–9. [DOI] [PubMed] [Google Scholar]
- 17. Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:278–85. [PubMed] [Google Scholar]
- 18. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 19. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10:1192–204. [PubMed] [Google Scholar]
- 20. Horsburgh CR Jr, Goldberg S, Bethel J, et al. Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010; 137:401–9. [DOI] [PubMed] [Google Scholar]
- 21. Rutherford ME, Hill PC, Triasih R, Sinfield R, van Crevel R, Graham SM. Preventive therapy in children exposed to Mycobacterium tuberculosis: problems and solutions. Trop Med Int Health 2012; 17:1264–73. [DOI] [PubMed] [Google Scholar]
- 22. Spyridis NP, Spyridis PG, Gelesme A, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis 2007; 45:715–22. [DOI] [PubMed] [Google Scholar]
- 23. Villarino ME, Scott NA, Weis SE, et al. International Maternal Pediatric and Adolescents AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr 2015; 169:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterling TR, Villarino ME, Borisov AS, et al. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 25. Aspler A, Long R, Trajman A, et al. Impact of treatment completion, intolerance and adverse events on health system costs in a randomised trial of 4 months rifampin or 9 months isoniazid for latent TB. Thorax 2010; 65:582–7. [DOI] [PubMed] [Google Scholar]
- 26. Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011; 365:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Page KR, Sifakis F, Montes de Oca R, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med 2006; 166:1863–70. [DOI] [PubMed] [Google Scholar]
- 28. van Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis 2006; 10:13–8. [PubMed] [Google Scholar]
- 29. Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis 2014; 14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Comstock GW, Baum C, Snider DE Jr. Isoniazid prophylaxis among Alaskan Eskimos: a final report of the Bethel isoniazid studies. Am Rev Respir Dis 1979; 119:827–30. [DOI] [PubMed] [Google Scholar]
- 31. Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015; 386:2344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO, 2018. ISBN 978-92-4-155023-9. [PubMed] [Google Scholar]
- 33.World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO, 2015. Available from https://www.who.int/tb/post2015_strategy/en/. Accessed 28 November 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.