Abstract
Aim:
Spermidine/spermine N1-acetyltransferase (SSAT-1) regulates cell growth, proliferation and death. Amantadine is converted by SSAT-1 to acetylamantadine (AA). In our earlier studies, although SSAT-1 was activated in patients with cancer, a number of ostensibly healthy adult volunteers had higher than expected AA concentration. This study was therefore undertaken to examine the outlier group.
Materials & methods:
A follow up of urine analysis for AA by liquid chromatography-tandem mass spectrometry as well as clinical assessments and additional blood analyses were conducted.
Results:
In some of the outlier controls, higher than expected AA concentration was linked to increased serum carcinoembryonic antigen. Clinical and radiographic assessments revealed underlying abnormalities in other cases that could represent premalignant conditions. Hematology tests revealed elevations in white blood cells and platelets, which are markers of inflammation.
Conclusion:
High urine concentration of AA could be used as a simple and useful test for screening of cancer in high-risk populations.
Keywords: : amantadine, biomarkers, cancer screening and diagnostics, early detection, SSAT-1
Graphical abstract
Lay abstract
This study describes the potential of a novel noninvasive urine test for screening of cancer using a safe and approved drug, amantadine. We have previously measured high urinary concentration of the acetylated form of amantadine in patients diagnosed with cancer. However, higher than expected acetylated amantadine concentration was also measured in some of the healthy adult volunteers. Subsequent clinical assessments revealed that these healthy individuals could have early clinical signs of cancer. This is a simple test, which may serve as a useful tool for routine screening in populations considered at high risk for cancer.
The burden of cancer worldwide, according to the global cancer incidence, mortality and prevalence report (GLOBOCAN 2018) [1], estimated that in 2018 there were 18.1 million new cancer cases (17.0 million excluding nonmelanoma skin cancer) and 9.6 million cancer deaths (9.5 million excluding nonmelanoma skin cancer). In addition, in both men and women combined, lung cancer was the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths). Other types of cancer with the highest mortality rates are female breast cancer (11.6%), colorectal cancer (9.2%), stomach cancer (8.2%), and liver cancer (8.2%). Of note, lung cancer was the most frequent cancer and the leading cause of cancer death among males, followed by liver and stomach cancer. On the other hand, in women, breast cancer was the most commonly diagnosed cancer as well as the leading cause of cancer death, followed by lung and colorectal cancer. Interestingly, cervical cancer ranks fourth for mortality [1,2]
Early detection and diagnosis of cancer can lead to timely therapeutic/surgical interventions that can increase the chances of survival. Despite intense research and discovery over the years of many candidate biomarkers for cancer screening, only a few have transitioned to routine usage in the clinic [3]. Promising candidates that are in the discovery and developmental mode include circulating tumor cells and cell-free tumor DNA [4,5], miRNAs [6,7], secretome of the gut microbiota [8], exhaled volatile organic compounds, proteomics and metabolomics (liquid biopsy) [9–11]. These face many challenges before routine implementation in the clinical setting [11,12]. In order to introduce a general cancer screening test into practice, the cost and access are important factors that need to be taken into account. Development of a simple, clinically viable, economical, accurate and reproducible screening test would be highly beneficial for the at-risk population.
Polyamines are intimately involved in cell growth, proliferation and cell death [13,14]. Spermidine/spermine N1-acetyltransferase-1 (SSAT-1) is a key enzyme in the polyamine metabolic pathway and is known to be upregulated in cancer [15–17]. Amantadine, which is a US FDA-approved antiviral drug and anti-Parkinson’s medication, is a specific substrate of SSAT-1. Amantadine is acetylated by SSAT-1 to produce acetylamantadine (AA) [18], which is a stable end product excreted in urine [18,19]. We have reported the clinical utility of amantadine to detect elevated SSAT-1 activity by measuring increased concentration of AA in the urine of cancer patients [20,21]. In earlier studies, a proportion of the healthy adult controls were deemed to be ‘outliers’ because of higher than expected AA concentrations in the urine. Accordingly, the present study was undertaken to further investigate the outlier group by: performing a follow-up amantadine test; conducting thorough clinical and hematological assessments; and conducting a follow-up health status questionnaire and accessing electronic medical records of these individuals.
Materials & methods
Regulatory & institutional review board approvals
Ethics approval was obtained from the University of Manitoba Biomedical Research Ethics Board (Ethics File #s: B2012:063 and B2011:073) prior to study implementation. The study protocol was reviewed and approved by Health Canada (File # 9427-B2749-21C): Notice of Authorization was dated 17 July 2012 and was also listed on the NIH Clinicaltrials.gov website (Identifier: NCT02277938). Appropriate Institutional Review Board (Ministry of Health & Family Welfare, the People’s Republic of Bangladesh) approvals were also obtained (Ethics File # NICRH/Ethics/2017/288). Clinical studies were completed under GCP and GLP conditions in accordance with the standards established by the Canadian Tri-Council Policies.
Experimental subjects
Healthy controls (n = 40) were recruited by the National Institute of Cancer Research & Hospital, Department of Medical Oncology, Mohakhali, Dhaka, Bangladesh within the local area as part of a study that was being conducted to investigate urinary AA levels in cancer patients [21]. 20 healthy adult controls were also recruited locally at the Asper Clinical Research Institute, St Boniface Hospital, Winnipeg, Canada. All participants provided a signed informed consent for participation. Inclusion criteria were volunteers aged between 18 and 80 years with no history of liver or kidney disease; exclusion criteria were alcohol consumption within 5 days of amantadine ingestion, previous adverse reaction to amantadine and currently pregnant or lactating females. At time of recruitment and participation in the study, none of the healthy control volunteers reported a diagnosis of cancer. After overnight fasting, participants were requested to provide a complete urine collection on the day of the study prior to ingesting amantadine. They then ingested orally 200 mg (2 × 100 mg) amantadine capsules (Mylan-Amantadine, amantadine hydrochloride, USP). Urine was then collected at 2, 4 and 6 h postamantadine ingestion for analysis as previously described [20,21]. Participants that were deemed as outliers were consented for follow-up after obtaining appropriate approvals from the respective institutional review boards.
Analytical procedures & data cross-validation
Urine was analyzed for AA by established and validated GLP-compliant LC–MS/MS methods using d3-AA as the internal standard for quantitation at Biopharmaceuticals Research Inc. (BC, Canada; Study #: BIM-2015-001). Health Canada authorized Biomark AA assay standard under application no: 229838 on 7 October 2014 (Investigational Testing Authorization). The measurement of urinary concentration of AA was conducted as previously described [20,21], Data were cross-validated as described elsewhere [22,23]. The study staff coded the samples and the technician analyzing the biological samples was blinded to participant information.
Blood analyses & other clinical assessments
Hematological tests, mammogram and ultrasound were conducted using standard procedures at the National Institute of Cancer Research & Hospital, Dhaka, Bangladesh. Serum CEA was measured at Life Labs (ON, Canada) by immunoassay.
Statistical analysis
Microcal Origin 6 software was used for the calculation of mean values and standard error of the mean (SEM). Box plots were constructed using GraphPad Prism 8.1.
Results
Healthy control & cancer patient characteristics
The demographic information of each of the healthy adult participants is shown in Table 1 (Bangladesh site) and Table 2 (Winnipeg site). The mean age (±SEM) of the healthy group at the Bangladesh site (n = 40; 20 male, 20 female) was 52 ± 2 years, whereas the mean age (±SEM) of the healthy group at the Winnipeg site (n = 20; 9 male, 11 female) was 38 ± 3 years. In the Bangladesh cohort, 11/40 (27%) and 2/40 (5%) participants were considered to be overweight (body mass index [BMI] = 25.0–29.9) and obese (BMI ≥30.0), respectively. In the Winnipeg cohort, 10/20 (50%) and 3/20 (15%) were considered to be overweight (BMI = 25.0–29.9) and obese (BMI≥ 30.0), respectively. On the other hand, 6/40 (15%) subjects in the Bangladesh healthy group were deemed to be underweight (BMI <18.5), while only one participant (5%) in the Winnipeg healthy group was determined to be underweight.
Table 1. . Demographic information on healthy adult volunteers recruited locally at Dhaka (Bangladesh site).
| Subject ID | Age (years) | Height (ft; inch) | Weight (lbs) | BMI (kg/m2) | Sex (M/F) | Subject ID | Age (years) | Height (ft; inch) | Weight (lbs) | BMI sex (kg/m2; M/F) |
|---|---|---|---|---|---|---|---|---|---|---|
| H01 | 50 | 5’5” | 132.2 | 22.0 | M | H21 | 54 | 5’1” | 134.5 | 25.4 F |
| H02 | 27 | 5’2” | 119.1 | 21.8 | F | H22 | 51 | 4’11” | 119.1 | 24.1 F |
| H03 | 44 | 4’10” | 141.1 | 29.5 | F | H23 | 55 | 5’0” | 90.4 | 17.7 F |
| H04 | 46 | 5’2” | 156.5 | 28.6 | F | H24 | 58 | 5’6” | 127.7 | 20.6 M |
| H05 | 43 | 5’3” | 154.3 | 27.3 | F | H25 | 56 | 5’6” | 132.3 | 21.4 M |
| H06 | 50 | 5’6” | 110.2 | 17.8 | M | H26 | 57 | 5’5” | 160.9 | 26.8 M |
| H07 | 42 | 5’11” | 178.6 | 24.9 | M | H27 | 52 | 5’2” | 143.3 | 26.2 F |
| H08 | 29 | 5’2” | 121.3 | 22.2 | F | H28 | 42 | 5’1” | 152.1 | 28.7 F |
| H09 | 37 | 5’1” | 121.3 | 22.9 | F | H29 | 52 | 4’11” | 88.2 | 17.8 F |
| H10 | 50 | 5’1” | 108.0 | 20.4 | F | H30 | 67 | 5’1” | 81.6 | 15.4 F |
| H11 | 56 | 4’11” | 194.0 | 39.2 | F | H31 | 58 | 5’5” | 141.1 | 23.5 M |
| H12 | 34 | 5’2” | 114.6 | 21.0 | F | H32 | 50 | 5’4” | 138.9 | 23.8 M |
| H13 | 53 | 5’3” | 123.5 | 21.9 | M | H33 | 55 | 5’7” | 123.5 | 19.3 M |
| H14 | 48 | 5’5” | 143.3 | 23.8 | M | H34 | 58 | 5’8” | 152.1 | 23.1 M |
| H15 | 46 | 5’3” | 132.3 | 22.0 | M | H35 | 59 | 5’5” | 154.3 | 25.7 M |
| H16 | 70 | 4’9” | 121.3 | 26.3 | F | H36 | 55 | 5’6” | 154.3 | 24.9 M |
| H17 | 38 | 5’0” | 138.9 | 27.1 | F | H37 | 62 | 5’8” | 138.9 | 21.1 M |
| H18 | 52 | 4’11” | 154.3 | 31.2 | F | H38 | 55 | 5’7” | 158.7 | 24.9 M |
| H19 | 55 | 5’2” | 83.8 | 15.3 | M | H39 | 63 | 5’5” | 138.9 | 23.1 M |
| H20 | 75 | 4’10” | 88.2 | 18.4 | F | H40 | 61 | 5’4” | 154.3 | 26.5 M |
The BMI is defined according to [46]. BMI scale: <18.5 underweight; 18.5–24.9 normal; 25.0–29.9 overweight; >30.0 obese.
BMI: Body mass index; F: Female; M: Male; ’ = Feet; ” = Inches.
Table 2. . Demographic information on healthy adult volunteers recruited locally at the Asper Clinical Research Institute (Winnipeg site).
| Subject ID | Age (years) | BMI (kg/m2) | Weight (lbs) | BMI (kg/m2) | Sex (M/F) |
|---|---|---|---|---|---|
| BM0001 | 28 | 5’7” | 136.5 | 22.0 | F |
| BM0002 | 24 | 5’5” | 158.6 | 27.2 | F |
| BM0003 | 57 | 5’3” | 164.2 | 29.1 | F |
| BM0004 | 34 | 5’7” | 177.8 | 27.8 | F |
| BM0005 | 49 | 5’3” | 132.0 | 23.4 | F |
| BM0006 | 46 | 5’5” | 180.0 | 30.0 | F |
| BM0007 | 28 | 5’3” | 158.4 | 28.1 | F |
| BM0008 | 49 | 5’3” | 116.5 | 20.6 | F |
| BM0009 | 22 | 5’3” | 144.0 | 25.5 | F |
| BM0010 | 63 | 5’3” | 158.4 | 28.1 | F |
| BM0011 | 25 | 5’7” | 115.7 | 18.1 | F |
| BM0021 | 50 | 5’9” | 200.2 | 29.6 | M |
| BM0022 | 61 | 5’9” | 181.2 | 26.8 | M |
| BM0023 | 51 | 6’0” | 232.4 | 31.5 | M |
| BM0024 | 54 | 6’0” | 203.8 | 27.6 | M |
| BM0025 | 23 | 5’5” | 140.0 | 23.3 | M |
| BM0026 | 43 | 5’5” | 157.0 | 26.1 | M |
| BM0027 | 19 | 5’7” | 141.6 | 22.2 | M |
| BM0028 | 18 | 5’8” | 149.2 | 22.7 | M |
| BM0029 | 18 | 5’1” | 178.4 | 33.7 | M |
The BMI is defined according to [46]. BMI scale: <18.5 underweight; 18.5–24.9 normal; 25.0–29.9 overweight; >30.0 obese.
BMI: Body mass index; F: Female; M: Male; ’ = Feet; ” = Inches.
Delineation of ‘outliers’
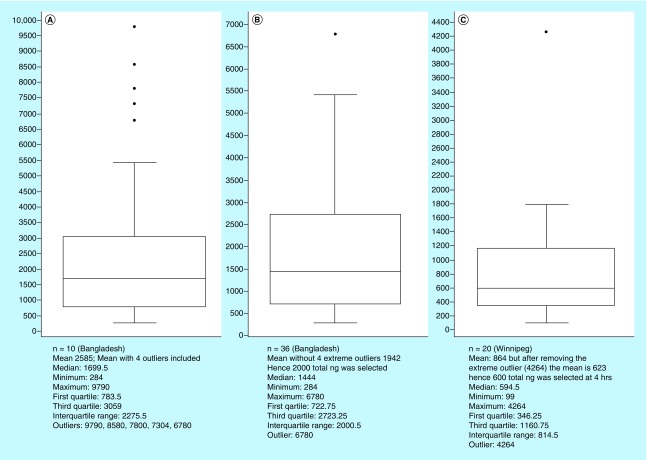
Figure 1 shows the box plots statistical data with minimum, first quartile, median, third quartile and maximum for AA. Total AA mean excretion at 4 h (ng) in Winnipeg volunteers was 600 ng that provided a tighter assessment after extreme outlier were accounted for in isolating outliers. For the Bangladesh cohort, a 2000 ng at 6 h provided a better cutoff point in selection of outlier definition following management of extreme outliers.
Figure 1. . Box plot statistical data for total acetylamantadine amount in healthy volunteers at study sites.
Data are presented for Bangladesh (A & B) and Winnipeg (C) healthy groups. Box plots were constructed to ascertain cutoff points in ostensibly normal individual’s for determination of ‘outliers’ according to the amantadine test.
Urinary concentrations of AA in healthy adult volunteers (Bangladesh site)
Analysis of the urine samples revealed that in the Bangladesh site there, was a higher than expected concentration (≥24.0 ng/ml) or total amount (>2000 ng) of AA at 6 h in 22/40 (50%) of the healthy adult volunteers (Table 3). The serum concentration of CEA was also determined in the 40 healthy adult volunteers. As shown in Table 4, 18/40 (45%) participants exhibited CEA concentration ≥2.5 ng/ml. However, 11/18 (61%) of the participants were considered outliers because of the overlapping between the results of the AA test combined with the CEA concentrations. An attempt to contact these 11 participants for a follow-up was made with seven favorable responders (H03, H07, H11, H12, H17, H18 and H26). The follow-up was conducted at 6 and 9 months after completion of the initial amantadine test. Interestingly, five out of seven (71%) participants showed an increase in the 2 h urinary AA concentration at 6 months (Table 5) as compared with the concentration observed with the initial amantadine test, which decreased at the 9-month follow-up to near initial values.
Table 3. . Urinary acetylamantadine concentration in healthy adult volunteers recruited locally at Dhaka (Bangladesh site).
| Subject ID | 2 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) | 4 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) | 6 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) |
|---|---|---|---|---|---|---|---|---|---|
| H01† | 25.4 | 70 | 1778 | 24.4 | 85 | 2074 | 58.2 | 20 | 1164 |
| H02 | 20.1 | 110 | 2211 | 10.8 | 120 | 1296 | 16.9 | 115 | 1944 |
| H03† | 11.9 | 100 | 1190 | 165 | 100 | 16,500 | 156 | 55 | 8580 |
| H04† | 6.0 | 80 | 478 | 65.3 | 80 | 5224 | 32.1 | 85 | 2729 |
| H05† | 49.5 | 90 | 4455 | 83.3 | 85 | 7081 | 178 | 55 | 9790 |
| H06† | 10.5 | 90 | 945 | 26.2 | 70 | 1834 | 83.2 | 65 | 5408 |
| H07† | 11.0 | 85 | 935 | 29.1 | 90 | 2619 | 66.4 | 60 | 3984 |
| H08† | 24.7 | 55 | 1359 | 37.6 | 80 | 3008 | 34.3 | 90 | 3087 |
| H09† | 42.0 | 100 | 4200 | 42.0 | 70 | 2940 | 40.6 | 100 | 4060 |
| H10† | 11.6 | 75 | 870 | 43.3 | 60 | 2598 | 156 | 50 | 7800 |
| H11† | 3.0 | 130 | 384 | 185 | 35 | 6475 | 45.1 | 60 | 2706 |
| H12† | 31.4 | 70 | 2198 | 132 | 60 | 7920 | 91.3 | 80 | 7304 |
| H13† | 6.4 | 70 | 446 | 53.1 | 65 | 3452 | 113 | 60 | 6780 |
| H14 | 21.1 | 80 | 1688 | 19.4 | 100 | 1940 | 19.4 | 75 | 1455 |
| H15 | 7.7 | 90 | 697 | 10.5 | 70 | 735 | 16.2 | 75 | 1215 |
| H16 | 14.6 | 95 | 1387 | 24.7 | 80 | 1976 | 9.4 | 65 | 614 |
| H17† | 9.8 | 100 | 984 | 60.8 | 100 | 6080 | 27.3 | 90 | 2457 |
| H18† | 22.8 | 100 | 2280 | 28.2 | 90 | 2538 | 25.1 | 90 | 2259 |
| H19 | 5.2 | 120 | 623 | 18.0 | 100 | 1800 | 8.1 | 90 | 725 |
| H20 | 14.9 | 90 | 1341 | 24.0 | 110 | 2640 | 8.0 | 120 | 959 |
| H21 | 9.0 | 75 | 677 | 10.2 | 80 | 816 | 4.7 | 80 | 378 |
| H22 | 6.8 | 60 | 408 | 30.9 | 95 | 2934 | 5.2 | 90 | 466 |
| H23 | 4.3 | 60 | 256 | 7.1 | 60 | 425 | 3.6 | 80 | 284 |
| H24 | 7.5 | 65 | 488 | 14.3 | 100 | 1430 | 9.6 | 75 | 722 |
| H25 | 12.8 | 45 | 576 | 11.6 | 40 | 464 | 2.8 | 110 | 306 |
| H26† | 63.3 | 90 | 5697 | 35.8 | 75 | 2685 | 10.5 | 200 | 2100 |
| H27 | 21.5 | 90 | 1935 | 30.7 | 50 | 1535 | 22.6 | 55 | 1243 |
| H28 | 3.2 | 80 | 254 | 80.6 | 60 | 484 | 12.6 | 115 | 1449 |
| H29 | 1.5 | 110 | 164 | 6.1 | 110 | 673 | 13.7 | 105 | 1439 |
| H30† | 2.9 | 70 | 204 | 13.8 | 55 | 759 | 96 | 30 | 2880 |
| H31† | 3.5 | 120 | 414 | 14.7 | 70 | 1029 | 34.6 | 70 | 2422 |
| H32† | 18.0 | 100 | 1800 | 14.5 | 90 | 1305 | 42.5 | 70 | 2975 |
| H33† | 6.3 | 100 | 626 | 34.6 | 70 | 2422 | 52.6 | 80 | 4208 |
| H34 | 47.2 | 100 | 4720 | 2.8 | 80 | 227 | 7.2 | 75 | 542 |
| H35 | 11.3 | 110 | 1243 | 13.9 | 110 | 1529 | 13.5 | 100 | 1350 |
| H36 | 23.1 | 100 | 2310 | 26.7 | 105 | 2804 | 11.5 | 100 | 1150 |
| H37 | 7.7 | 80 | 617 | 13.7 | 100 | 1370 | 15.2 | 90 | 1368 |
| H38 | 1.6 | 80 | 127 | 8.1 | 75 | 609 | 5.6 | 95 | 535 |
| H39† | 22.0 | 80 | 1760 | 24.2 | 100 | 2420 | 24.0 | 95 | 2280 |
| H40 | 7.4 | 105 | 781 | 19.2 | 85 | 1632 | 11.20 | 85 | 286 |
Identified as an outlier based on total AA amount (ng) at 6 h time point of >2000 ng or concentration of AA >24.0 ng/ml.
AA: Acetylamantadine.
Table 4. . Serum concentrations of carcinoembryonic antigen in healthy adult volunteers recruited locally at Dhaka (Bangladesh site).
| Subject ID | CEA (ng/ml) | Subject ID | CEA (ng/ml) |
|---|---|---|---|
| H01† | 2.7 | H21 | 1.9 |
| H02 | 1.8 | H22 | 1.4 |
| H03† | 4.1 | H23† | 4.5 |
| H04 | 0.5 | H24 | 1.3 |
| H05 | 2.0 | H25† | 4.5 |
| H06† | 3.3 | H26† | 22.1 |
| H07† | 3.8 | H27 | 2.3 |
| H08 | 1.0 | H28 | 1.6 |
| H09 | 1.5 | H29 | 2.0 |
| H10 | 1.7 | H30† | 2.9 |
| H11† | 4.7 | H31 | 1.2 |
| H12† | 3.4 | H32 | 1.9 |
| H13† | 5.4 | H33 | 1.7 |
| H14 | 1.6 | H34† | 2.8 |
| H15 | 2.1 | H35 | 1.8 |
| H16† | 3.9 | H36 | 1.2 |
| H17† | 3.0 | H37† | 2.5 |
| H18† | 6.1 | H38 | 2.4 |
| H19 | 1.5 | H39† | 3.1 |
| H20† | 2.8 | H40 | 2.4 |
Identified as high CEA levels with ≥2.5 ng/ml as the cutoff.
Table 5. . Follow-up amantadine test in the outlier group at Dhaka (Bangladesh site).
| Subject ID | Initial | Follow-up 1 | Follow-up 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 h [AA] (ng/ml) | 4 h [AA] (ng/ml) | 6 h [AA] (ng/ml) | 2 h [AA] (ng/ml) | 4 h [AA] (ng/ml) | 6 h [AA] (ng/ml) | 2 h [AA] (ng/ml) | 4 h [AA] (ng/ml) | 6 h [AA] (ng/ml) | |
| H03 | 11.9 | 165 | 156 | 21.0 | 30.3 | 41.4 | 11.4 | 113 | 30.9 |
| H07 | 11.0 | 29.1 | 66.4 | 28.4 | 60.2 | 64.7 | 1.7 | 33.4 | 19.5 |
| H11 | 3.0 | 185 | 45.1 | 7.1 | 36.3 | 77.2 | 1.5 | 45.2 | 67.3 |
| H12 | 31.4 | 132 | 91.3 | 92.6 | 81.5 | 46.5 | 46.1 | 120 | 54.9 |
| H17 | 9.8 | 60.8 | 27.3 | 39.4 | 71.2 | 42.3 | 6.7 | 25.0 | 48.3 |
| H18 | 22.8 | 28.2 | 25.1 | 17.5 | 74.9 | 103 | 5.9 | 19.7 | 9.3 |
| H26 | 14.9 | 24.0 | 8.0 | 6.8 | 46.9 | 21.5 | 1.8 | 14.3 | 26.4 |
Urinary AA concentrations were determined at 2, 4 and 6 h postamantadine ingestion at first follow-up (6 month) and at second follow-up (9-month) after the initial amantadine test.
AA: Acetylamantadine.
In order to understand the significance of the higher AA concentrations observed at the 6-month follow-up, a clinical assessment was conducted. In the two males in this follow-up cohort (H07 and H26), ultrasound revealed enlarged prostates with normal prostate-specific antigen values. With respect to the clinical features in the female outliers, mammogram revealed fibrotic changes in both breasts (Table 6). In addition, ultrasound also showed the presence of hepatomegaly and fatty changes in the liver in all seven individuals. Hematological assessments showed either elevated white blood cell count (H07 and H12) or elevated platelets (H11) or a platelet count in the upper range of normal (H17).
Table 6. . Clinical features of the healthy adult volunteer outlier group at Dhaka (Bangladesh site).
| Subject ID | Sex (M/F) | Age (years) | Mammogram/ultrasound | Hematology (cells/μl) |
|---|---|---|---|---|
| H03 | F | 44 | Enlarged axillary LN, mild fibrotic change in both breasts; mild hepatomegaly with fatty change; cholelithiasis | Normal |
| H07 | M | 42 | Mildly enlarged prostate (PSA normal); mild fatty change in liver | Elevated WBC (15,000) |
| H11 | F | 56 | Mild fibrotic change in both breasts; mild hepatomegaly with fatty change | Elevated platelets (450,000) |
| H12 | F | 34 | Underlying mass lesion, dense breasts; mild fatty change in liver | Elevated WBC (12,600) |
| H17 | F | 38 | Mild fibrotic change in both breasts; mild fatty change in liver | High platelets (420,000), eosinophilia |
| H18 | F | 52 | Mild fibrotic change in both breasts; mild fatty change in liver | Normal |
| H26 | M | 57 | Upper limit of normal prostate (PSA normal); mild hepatomegaly with fatty change | Normal |
Normal range for WBCs, 4500–11,000 WBC/μl of blood; normal range for platelets 150,000–450,000 platelets per μl of blood.
F: Female; LN: Lymph node; M: Male; WBC: White blood cell.
Urinary concentrations of AA in healthy adult volunteers (Winnipeg site)
We conducted a follow-up with the Winnipeg outliers within the healthy adult group. In this study, 11/20 (55%) participants were considered as outliers, in other words, having a higher than expected urinary concentration (≥6.0 ng/ml) or total amount (>600 ng) of AA at 4 h (Table 7). These individuals were followed-up with a health questionnaire asking if they had experienced any health issue since completion of the study. On the basis of the responses to the questionnaire as well as accessing electronic medical records (CancerCare Manitoba), three of these outliers experienced some medical issue related to cancer, whereas one participant had registered with CancerCare Manitoba, but there was no diagnosis or clinical information available (Table 8).
Table 7. . Urinary acetylamantadine concentration in healthy adult volunteers recruited locally at Asper Clinical Research Institute (Winnipeg site).
| Subject ID | 2 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) | 4 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) | 6 h [AA] (ng/ml) | Urine (ml) | Total AA (ng) |
|---|---|---|---|---|---|---|---|---|---|
| BM0001 | 2.0 | 220 | 429 | 2.2 | 205 | 445 | 3.5 | 130 | 452 |
| BM0002† | 10.5 | 20 | 210 | 10.6 | 40 | 424 | 10.7 | 75 | 803 |
| BM0003† | 1.7 | 180 | 297 | 3.4 | 238 | 809 | 1.4 | 228 | 309 |
| BM0004† | 1.1 | 410 | 442 | 2.8 | 320 | 880 | 4.5 | 130 | 589 |
| BM0005† | 3.8 | 40 | 152 | 3.2 | 190 | 614 | 1.4 | 180 | 252 |
| BM0006† | 1.2 | 85 | 99 | 8.7 | 145 | 1260 | 2.0 | 165 | 333 |
| BM0007† | 3.0 | 165 | 501 | 14.9 | 120 | 1788 | 4.0 | 175 | 707 |
| BM0008 | 1.6 | 190 | 296 | 1.7 | 205 | 344 | 5.0 | 170 | 841 |
| BM0009† | 3.9 | 170 | 660 | 9.2 | 130 | 1191 | 5.8 | 210 | 1210 |
| BM0010 | 1.1 | 7 | 8 | 0.8 | 120 | 99 | 0.4 | 100 | 42 |
| BM0011† | 0.8 | 410 | 314 | 3.2 | 400 | 1288 | 1.7 | 390 | 655 |
| BM0021† | 2.7 | 220 | 603 | 9.3 | 460 | 4264 | 5.3 | 190 | 1005 |
| BM0022 | 2.3 | 90 | 207 | 2.0 | 95 | 194 | 2.1 | 100 | 211 |
| BM0023 | 0.6 | 315 | 201 | 3.3 | 85 | 282 | 2.4 | 155 | 369 |
| BM0024 | 4.4 | 55 | 239 | 4.6 | 90 | 413 | 4.4 | 130 | 569 |
| BM0025 | 1.4 | 50 | 69 | 4.0 | 50 | 198 | 2.3 | 100 | 229 |
| BM0026† | 0.6 | 245 | 142 | 2.6 | 305 | 790 | 3.5 | 75 | 264 |
| BM0027 | 5.2 | 160 | 837 | 4.4 | 80 | 353 | 4.6 | 130 | 593 |
| BM0028† | 3.8 | 220 | 834 | 10.7 | 100 | 1070 | 14.8 | 115 | 1702 |
| BM0029 | 4.6 | 120 | 550 | 5.8 | 100 | 575 | 4.5 | 140 | 631 |
Identified as an outlier based on total AA amount (ng) at 4 h time point of more than 600 ng or concentration of AA more than 6.0 ng/ml.
AA: Acetylamantadine.
Table 8. . Clinical features of the healthy adult volunteer outlier group at the Asper Clinical Research Institute (Winnipeg site).
| Subject ID | Sex (M/F) | Age (years) | Follow-up questionnaire/electronic medical records |
|---|---|---|---|
| BM0021 | M | 50 | No health issue |
| BM0026 | M | 43 | No health issue |
| BM0028 | M | 18 | No health issue |
| BM0002 | F | 24 | No health issue |
| BM0003 | F | 57 | Thyroid nodules (2013) |
| BM0004 | F | 34 | Ovarian cancer stage 1C (2014) |
| BM0005 | F | 49 | Lung nodules NYD (2016) |
| BM0006 | F | 46 | Registered with CCMB, but no diagnosis or information currently available |
| BM0007 | F | 28 | No health issue |
| BM0009 | F | 22 | No health issue |
| BM0011 | F | 25 | No health issue |
CCMB: CancerCare Manitoba; NYD: Stage not yet determined.
Discussion
We have recently reported that human cancer is associated with high urinary concentration of AA [20,21] with receiver-operating characteristic (ROC) for AA demonstrated to be 0.689 (CI: 0.591–0.786, 95%) in lung cancer and 0.717 (CI: 0.577–0.858, 95%) for breast cancer [21]. In the present preliminary study, the initial data from Bangladesh healthy cohort had a high proportion of ‘outliers’ versus normal AA concentration at 6 h time point (<24.0 ng/ml) or total amount of AA (>2000 ng). Initial analysis of outliers demonstrated a large effect on the mean and median, which in turn affected the error (absolute and mean) in our dataset, and large deviations were observed when the error was plotted. Some of the outliers in Bangladesh had urinary AA concentrations that were between two- and six-times higher than the cutoff points, qualifying them as either intermediate or extreme outliers. This important deviation suggested that there could be valuable information to obtain and thus the ‘outliers’ were analyzed separately to try to understand the significance and clinical relevance of the higher AA concentrations. Also, in view of the small outlier sample size in both the Bangladesh versus Winnipeg cohorts it was not possible to conduct sensitivity analyses.
Since there was no apparent measurement or experimental design that would have led to exclusion of the outliers, we commissioned an assessment and follow-up to determine the health and qualification of status over a period of time. Accordingly, a thorough examination of the outliers at 6- and 9-month follow-up that entailed the amantadine test and comprehensive clinical and hematological assessments were conducted at the Bangladesh site. This leads to a follow-up study with the Winnipeg cohort, but this entailed a questionnaire as well as accessing electronic medical records; however, the ‘outliers’ in this cohort were outside of normal AA concentration (<6.0 ng/ml) or total amount of more than 600 ng at the 4 h time point.
The follow-up analysis revealed that the amantadine test is possibly detecting cancer at a very early stage; therefore, it could be useful in screening populations at high risk for cancer. It should be noted that while a further increase in AA concentration in the 6-month follow-up compared with values in the first amantadine test was observed in some of Bangladesh outliers, a reduction in AA concentration at the 9-month follow-up was observed. These values were almost comparable to the AA concentration observed in the initial amantadine test. This biphasic nature of the AA concentrations are suggestive that the increase in AA serves as the trigger in the initiation of the processes involved in cell proliferation and growth, while the subsequent reduction in AA can be seen as an adaptive mechanism.
It should be mentioned that follow-up clinical assessment at the Bangladesh site, seven outliers had developed hepatomegaly and/or fatty liver. Hepatomegaly can occur as a consequence of infection, metabolic disorders, congestive heart failure or hepatic tumor [24–26]. Interestingly, high rates of liver cancer are known to occur in areas with high contamination levels of the carcinogen arsenic, such as in Bangladesh [27–29]. Indeed most of the participants recruited into the study as healthy subjects resided in areas with moderate to severe arsenic contamination [30] (based on recorded area of residence on screening forms [data not shown]). Furthermore, individuals with severe fatty liver (non-alcoholic fatty liver disease) need to be monitored for liver cancer because of the link between fatty liver disease and liver cancer (hepatocellular carcinoma) [31,32]. It is pointed out that the connection between arsenic and cancer has been studied in some regions of Chile, where arsenic contamination is considered to be the primary cause of mortality due to lung cancer that is more than threefold as compared with noncontaminated areas in the same country [33].
While elevated white blood cell count is indicative of an infection, it has also been linked to other inflammations and in particular cancers gastric, lung and blood cancers [34–36]. Two of the outliers (H11 and H17) showed an elevated platelet count and eosinophilia, respectively. Eosinophilia has been linked to a wide variety of non-neoplastic disorders, as well as to neoplastic conditions [37] and an elevation in eosinophils may exert protumor effects [38,39]. On the other hand, inflammation and platelet activation at the site of tissue damage is known to contribute to initiate a cascade of events, which promote tumorigenesis [40]. In fact, platelets release a wide variety of proteins, including growth and angiogenic factors, lipids, and extracellular vesicles rich in genetic material, which can mediate the induction of phenotypic changes in cancer cells, promoting carcinogenesis and metastasis [40].
Although being underweight has been linked to an increase in the risk of adverse health complications, overweight/obesity increases the risk of developing several cancers [41–43]. With respect to our observations, five outliers in the Bangladesh healthy group that had some pathophysiological indication of presymptomatic cancer (Table 6), two of them were classified as obese (H11, H18; BMI >30.0) and two other were deemed as overweight (H17, H26; BMI 25.0–29.9). Similarly, two-third outliers in the Winnipeg healthy group (Table 2) were classified as overweight (BM0003, BM0004). Unfortunately for the one participant that was classified as obese (BM0006) and registered at CancerCare Manitoba, no clinically relevant information was available. Taken together, the amantadine test could be of value for assessing risk of cancer in overweight/obese individuals, a possibility that warrants future large trial in this area.
It should also be mentioned that we used different cutoff values for the basal AA concentration in the healthy adults in the Bangladesh versus Winnipeg cohorts. This may be as a consequence of geographical and environmental factors. Indeed, we have previously observed regional/ethnic differences in AA concentration in healthy adults [44] that may be a reflection of the influence of environmental, socioeconomic and lifestyle factors affecting the basal SSAT-1 activity. However, these factors remain incompletely understood warranting further investigation. In addition, while not recorded in the present study, in subsequent studies, family history of cancer should be documented as it is conceivable that the amantadine test may be detecting presymptomatic cancer in populations that may be genetically predisposed for cancer.
Current standards for determining and verifying the presence of cancer involve computed tomography, MRI and ultrasound in conjunction with molecular and protein biomarkers; however, their use is limited because of the challenges regarding costs, accessibility, exposure to ionizing radiation and levels of false-positive results. In addition, biopsies for cancer diagnosis are invasive, time consuming, distressing, expensive and cannot be performed repeatedly [10,45]. Thus, the simple, noninvasive, no risk, painless and cost-effective amantadine test may become an alternative or supplementary assessment tool to identify and follow malignant disease. Furthermore, with this test there is no need for surgery and the potential to reduce diagnosis needs to be further examined.
Conclusion
Analysis of the ‘outliers’ observed in previous studies using the amantadine test revealed that a large number were at risk or developed cancer, suggesting that the test can predict the occurrence of cancer. Monitoring of urinary AA concentration combined with clinical and hematological characteristics could be established as a useful tool for purposes of screening and follow-up for cancer in high-risk populations.
Future perspective
Biomarkers that detect cancer and monitor response to treatment will be highly beneficial. Our findings in ‘outliers’ support usefulness of amantadine as a screening or surveillance test in populations considered at high risk for developing cancer. It is also possible that this test could be used for monitoring patients after curative surgical or chemoradiation therapy to assess eradication of the tumor. Also, in follow-up, it could possibly detect proliferation of new cancer cells (relapse).
Summary points.
We have previously reported that increased spermidine/spermine N1-acetyltransferase activity is linked to cancer.
In the present study, a higher urinary concentrations of acetylamantadine were measured in some of the healthy adult volunteers at both study sites.
A follow-up of the outliers that entailed hematological assessments, mammogram, ultrasound as well as additional amantadine test was carried out with the Bangladesh group. A health questionnaire and access to electronic medical records was performed with the Winnipeg site outliers.
The follow-up data obtained at both sites are suggestive that the amantadine test may be detecting cancer cases prior to clinical symptoms.
The amantadine test could potentially serve as a novel, simple to use and low-cost early detection test for cancer or for surveillance in populations considered at high risk for cancer.
These possibilities warrant future large-scale studies.
Footnotes
Author contributions
DS Sitar, A Maksymiuk and RB Ahmed designed the study, were involved in reviewing the data analysis, contributed to writing the paper and had primary responsibility for final content as well as procuring financial support for the clinical studies. DS Sitar and A Maksymiuk were also responsible for the development of the overall research plan. A Maksymiuk provided medical oversight for the clinical study at the Canada site. PS Tappia was responsible for the enrollment and informed consent as well as implementation of the study at the Winnipeg site. He also wrote the initial draft of the paper. PS Akhtar provided medical oversight for the clinical study at the Bangladesh site. N Khatun, R Parveen, R Ahmed were responsible for regulatory approvals and study implementation at the Bangladesh site. B Cheng and DS Sitar were involved in the development of the LC–MS/MS assay for detection of acetylated amantadine. B Cheng also analyzed the data and performed the statistical analysis. H Bach and G Huang participated in the data analysis and writing of the manuscript. B Hiebert served as consultant biostatistician for analysis and interpretation of the data. B Ramjiawan provided the expertise for regulatory and institutional review board approvals and contributed to the design of the study. All the authors approved the manuscript.
Financial & competing interests disclosure
RB Ahmed is the President and CEO and B Cheng is the acting CTO of BioMark Diagnostics Inc. A Maksymiuk, DS Sitar, H Bach, PS Tappia and B Ramjiawan are minor shareholders of BioMark Diagnostics Inc. This study was supported, in part, by BioMark Diagnostics Inc. (BC, Canada) and the Maunders-McNeil Foundation (AB, Canada). LC–MS/MS analysis was conducted by Biopharmaceutical Research Inc. (BC, Canada). Infrastructural support was provided by the St Boniface Hospital Foundation and the University of Manitoba. Part of this work was published in abstract form in the 2019 ASCO meeting proceedings (online): https://meetinglibrary.asco.org/record/177214/abstract
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approvals and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Clinical trial data disclosure
The authors certify that this manuscript reports original clinical trial data. The data will not be made publicly available.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.The American Cancer Society. Global cancer facts & figures (2018) www.cancer.org/research/cancer-facts-statistics/global.html
- 2.International Agency for Cancer Research (2014) http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014
- 3.Srivastava A, Creek DJ. Discovery and validation of clinical biomarkers of cancer: a review combining metabolomics and proteomics. Proteomics E1700448 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Gall TMH, Belete S, Khanderia E, Frampton AE, Jiao LR. Circulating tumor cells and cell-free DNA in pancreatic ductal adenocarcinoma. Am. J. Pathol. 189(1), 71–81 (2018). [DOI] [PubMed] [Google Scholar]; • An excellent review summarizing the clinical utility and challenges of blood-based biomarkers for early detection of cancer.
- 5.Marrugo-Ramirez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int. J. Mol. Sci. 19(10), pii: E2877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Guan Z, Cuk K, Brenner H, Zhang Y. Circulating microRNA biomarkers for lung cancer detection in Western populations. Cancer Med. 7(10), 4849–4862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue T, Liang W, Li Y. et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 10(1), 28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megat Mohd Azian PI, Chin SF, Low TY, Hui-Min N, Jamal R. Analysing the secretome of gut microbiota as the next strategy for early detection of colorrectal cancer. Proteomics e1800176 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Peng L, Cantor DI, Huang C, Wang K, Baker MS, Nice EC. Tissue and plasma proteomics for early stage cancer detection. Mol. Omics. 14(6), 405–423 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Jelonek K, Widlak P. Metabolome-based biomarkers: their potential role in early detection of lung cancer. Contemp. Oncol. 22(3), 135–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seijo LM, Peled N, Ajona D. et al. Biomarkers in lung cancer screening: achievements, promises and challenges. J. Thorac. Oncol. pii: S1556-0864(18)33501-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An excellent review on the identification and future challenges of biomarkers for cancer screening.
- 12.Lim M, Kim CJ, Sunkara V, Kim MH, Cho YK. Liquid biopsy in lung cancer: clinical applications of circulating biomarkers (CTCs and ctCNA). Micromachines 9(3), pii E100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegg AE. Functions of polyamines in mammals. J. Biol. Chem. 291(29), 14904–14912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 294(6), E995–E1010 (2008). [DOI] [PubMed] [Google Scholar]; •• A comprehensive review on the spermidine/spermine N1-acetyltransferase (SSAT-1) function.
- 15.Babbar N, Hacker A, Huang Y, Casero RA. Tumor necrosis factor α induces spermidine/spermine N1-acetyltransferase through nuclear factor κB in non-small cell lung cancer cells. J. Biol. Chem. 281(34), 24182–24192 (2006). [DOI] [PubMed] [Google Scholar]; •• A seminal paper on the discovery of SSAT-1 in non-small-cell lung cancer cells.
- 16.Gabrielson E, Tully E, Hacker A. et al. Induction of spermidine/spermine N1-acetyltransferase in breast cancer tissues treated with the polyamine analogue N1, N11-diethylnorspermine. Cancer Chemother. Pharmacol. 54(2), 122–126 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Eickhoff JC, Mehraein-Ghomi F, Church DR, Wilding G, Basu HS. Expression of spermidine/spermine N1-acetyl transferase (SSAT) in human prostate tissues is related to prostate cancer progression and metastasis. Prostate 75(11), 1150–1159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bras AP, Hoff HR, Aoki FY, Sitar DS. Amantadine acetylation may be effected by acetyltransferases other than NAT1 or NAT2. Can. J. Physiol. Pharmacol. 76(7–8), 701–706 (1998). [DOI] [PubMed] [Google Scholar]; • The first demonstration that amantadine undergoes acetylation.
- 19.Bras APM, Jänne J, Porter CW, Sitar DS. Spermidine/spermine N1-acetyltransferase catalyzes amantadine acetylation. Drug Metab. Dispos. 29(5), 676–680 (2001). [PubMed] [Google Scholar]; •• First demonstration that amantadine is a specific substrate for SSAT-1.
- 20.Maksymiuk AW, Sitar DS, Ahmed R. et al. Spermidine/spermine N- acetyltransferase-1 as a diagnostic biomarker in human cancer. Future Sci. OA. 4(10), FSO345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A discovery highlighting the diagnostic importance of acetylated amantadine.
- 21.Maksymiuk AW, Tappia PS, Sitar DS. et al. Use of amantadine as substrate for SSAT-1 activity as a reliable clinical diagnostic assay for breast and lung cancer. Future Sci. OA. 5(2), FSO365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A follow-up paper that validates further the diagnostic importance of acetylated amantadine.
- 22.Hyvönen T, Keinänen TA, Khomutov AR, Khomutov RM, Eloranta TO. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J. Chromatogr. 574(1), 17–21 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Lou G, Zhang M, Minuk GY. Effects of acute ethanol exposure on polyamine and gamma-aminobutyric acid metabolism in the regenerating liver. Alcohol 19(3), 219–227 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Kaczynski J, Hansson G, Wallerstedt S. Clinical features in Hepatocellular carcinoma and the impact of autopsy on diagnosis. A study of 530 cases from a low-endemicity area. Hepatogastroenterology 52(66), 1798–1802 (2005). [PubMed] [Google Scholar]
- 25.Padhan RK, Das P. Shalimar. Primary hepatic lymphoma. Trop. Gastroenterol. 36(1), 14–20 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Teng YC, Neo JC, Wu JC, Chen YF, Kao CH, Tsai TF. Expression of a hepatitis B virus pre-S2 deletion mutant in the liver results in hepatomegaly and hepatocellular carcinoma in mice. J. Pathol. 241(4), 463–474 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol. Sci. 105(1), 24–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An interesting article examining epidemiological and rodent studies on the liver as a target organ for arsenic toxicity.
- 28.Raessler M. The arsenic contamination of drinking and groundwaters in Bangladesh: featuring biogeochemical aspects and implications on public health. Arch. Environ. Contam. Toxicol. 75(1), 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag. Healthc. Policy. 11, 251–261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborti D, Rahman MM, Das B. et al. Groundwater arsenic contamination in Ganga-Meghna-Brahmaputra plain, its health effects and an approach for mitigation. Environ. Earth Sci. 70, 1993–2008 (2013). [Google Scholar]; •• An excellent account of the health consequences of regional arsenic contamination and the approaches undertaken for arsenic safe water consumption.
- 31.Chen K, Ma J, Jia X, Ai W, Ma Z, Pan Q. Advancing the understanding of NAFLD to Hepatocellular carcinoma development: from experimental models to human. Biochim Biophys. Acta. 1871(1), 117–125 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. pii: S2213-8587(18)30154-2 (2018). [DOI] [PubMed] [Google Scholar]
- 33.The Economist. Intelligence Unit Report. https://perspectives.eiu.com/sites/default/files/Cancercontrol,accessandinequalityinLatinAmerica.pdf
- 34.Sun J, Chen X, Gao P. et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta-analysis. Dis. Markers. 2016, 7862469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Y, Wang J, Wang X. et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics 70(7), 524–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 108(3), 804–811 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Larsen RL, Savage NM. How I investigate eosinophilia. Int. J. Lab. Hematol. 41(2), 153–161 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in cancer: favourable or unfavourable? Curr. Med. Chem. 23(7), 650–666 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol. Immunother. (2018) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contursi A, Grande R, Dovizio M, Bruno A, Fullone R, Patrignani P. Platelets in cancer development and diagnosis. Biochem. Soc. Trans. 46(6), 1517–1527 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Sitar DS, Bras AP, Maksymiuk A, Cheng KM, Zhou H. Progress in the development of SSAT1 activity as a biomarker for diagnosis of cancer. Presented at: BIT Life Sciences 2nd World Cancer Congress Proceedings. Beijing, China, 22–25 June 2009. [Google Scholar]; • Interesting study demonstrating ethnic differences in SSAT-1 activity.
- 42.Arnold M, Pandeya N, Byrnes G. et al. Global burden of cancer attributable to high body mass index in 2012: a population-based study. Lancet Oncol. 16(1), 36–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An outstanding population-based study that investigates the relationship between body mass index and occurrence of cancer.
- 43.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology 19(7), 871–881 (2005). [PubMed] [Google Scholar]
- 44.Irigaray P, Newby JA, Lacomme S, Belpomme D. Overweight/obesity and cancer genesis: more than a biological link. Biomed. Pharmacother. 61(10), 665–678 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Shen H. Low-dose CT for lung cancer screening: opportunities and challenges. Front. Med. 12(1), 116–121 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Diabetes Canada. (2019) www.diabetes.ca/en-CA/managing-my-diabetes/tools---resources/body-mass-index-(bmi)-calculator