Abstract
BACKGROUND
Myocardial bridge (MB) will compress the mural coronary artery (MCA) during the systole and cause myocardial ischemia. In the diagnosis of coronary heart disease (CHD), because the structure of MB is difficult to be observed by coronary angiography (CAG), the clinical study of the influence of MB on CHD is lacking. With the advancement of computed tomography coronary angiography technology, detailed observations of the MB anatomy have realized.
AIM
To explore the main influencing factors of MB-related CHD and to find potential indicators for predicting MB-related CHD.
METHODS
A total of 1718 patients with suspected CHD due to the symptoms of myocardial ischemia were enrolled as subjects. Patients diagnosed with CHD were included in a CHD group, and patients with no significant abnormalities were included in a control group. In the CHD group, patients were divided into an MB-CHD subgroup if MB-related CHD was found. In the control group, patients were divided into a simple MB subgroup if MB was found. The patient's clinical data and MB-related indicators, including the branch of MB, MB type (superficial/deep type), MB length, MB thickness, systolic and diastolic compression of the MCA, and MCA systolic stenosis rate were recorded and compared. Logistic regression analysis was used to explore the independent influencing factors of MD-related CHD. ROC curve was used to analyze the diagnostic efficacy of potential indicators for MB-related CHD.
RESULTS
There were 1060 cases in the CHD group and 658 cases in the control group, and there were 236 cases in the MB-CHD subgroup and 52 cases in the simple MB subgroup. Multivariate logistic regression analysis showed that the combined MB had a significant effect on the occurrence of CHD (P < 0.05). MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate had significant effects on the occurrence of MB-related CHD (P < 0.05). The area under the curve (AUC) of the combination of these influencing factors for the diagnosis of MB-related CHD was 0.959, which was significantly higher than the AUCs of the four indicators separately (P < 0.05). The sensitivity was 97.06% and the specificity was 87.63%.
CONCLUSION
MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis are independent influencing factors for MB-related CHD. The combination of these factors has potential diagnostic value for MB-related CHD.
Keywords: Myocardial bridge, Coronary heart disease, Mural coronary artery, Computed tomography coronary angiography, Independent influencing factor
Core tip: With the deep understanding of myocardial bridge (MB), it is considered to be the reason of such complications as acute myocardial infarction, ventricular tachycardia, syncope, and sudden cardiac death. Currently, how MB affects coronary heart disease remains unclear. Computed tomography coronary angiography can directly measure MB-related coronary heart disease (CHD), which was used to explore the relationship between MB and CHD. In this study, MB thickness, systolic compression, diastolic compression, and mural coronary artery systolic stenosis were proved to be the independent influencing factors for MB-related CHD. The combination of these factors has potential diagnostic value for MB-related CHD.
INTRODUCTION
Myocardial fiber bundle covering the mural coronary artery (MCA) is called myocardial bridge (MB)[1-3]. With the development of computed tomography coronary angiography (CTA), studies have reported that MB can compress the MCA during systole, causing myocardial ischemia such as angina pectoris and myocardial infarction[4-7]. It is called MB-related coronary heart disease (CHD)[8]. Because the low detection rate of MB and the inconspicuous myocardial ischemia symptoms in most patients with MB, and common risk factors for CHD including age, dyslipidemia, hypertension, smoking, diabetes, obesity, and family history are numerous[9-12], clinicians pay insufficient attention to MB and the studies on MB-related CHD are few. Currently, although coronary angiography (CAG) cannot specifically observe the detailed anatomical structure of MB, CTA has been developed to directly measure MB-related indicators in detail[13-16]. Therefore, the present study utilized CTA to assess the association between MB and CHD, in order to explore the main influencing factors of MB-related CHD and to find potential indicators for predicting MB-related CHD.
MATERIALS AND METHODS
Study participants
This study was approved by the Ethics Committee of the Capital Medical University Affiliated to Beijing Anzhen Hospital, and written informed consent was obtained from all patients. Study participants were recruited from the department of cardiology, Beijing Anzhen Hospital, Capital Medical University due to the symptoms of myocardial ischemia from December 2016 to June 2018. All patients were diagnosed utilizing CAG or CTA. The inclusion criteria were: (1) Patients with myocardial ischemia symptoms such as angina pectoris and ST-T segment changes; and (2) Glomerular filtration rate (GFR) between 30 and 60 mL/min. The exclusion criteria were: (1) Patients with other heart diseases besides CHD; (2) Patients with poor physical condition and multiple organ failure; (3) Patients allergic to contrast agent; and (4) Patients with coagulopathy. Of all the subjects, if the MCA was found in the CAG, CTA would be performed to observe MB. Patients diagnosed with CHD were divided into a CHD group, and patients with no significant abnormalities were divided into a control group. In the CHD group, patients were divided into an MB-CHD subgroup if MB-related CHD was found. In the control group, patients were divided into a simple MB subgroup if MB was found.
Clinical data recording
The family history of CHD, hypertension, diabetes, hyperlipidemia, smoking, ECG ST-segment changes, lipoprotein(a) [Lp(a)], homocysteine (HCY), plasminogen activator inhibitor-1 (PAI-1), and fibrinogen conditions of subjects were recorded in this study. The diagnosis of hypertension was based on the 2017 American Diabetes Diagnostic Criteria[17]: Grade 1 hypertension: systolic blood pressure 130-139 mmHg, diastolic blood pressure 80-89 mmHg; Grade 2 hypertension: ≥ 140/90 mmHg. The diagnosis of diabetes was based on the 2017 American Diabetes Association diagnostic criteria[18]: Fasting blood glucose ≥ 7.0 mmol/L; random blood glucose ≥ 11.1 mmol/L and patients with diabetic symptoms; 2 h postprandial blood glucose ≥ 11.1 mmol/L in the glucose tolerance test. Patients with atypical symptoms needed to repeat the test to determine the presence of diabetes according to the above criteria. The diagnosis of hyperlipidemia was based on: serum total cholesterol (TC) > 5.2 mmol/L; triglyceride (TG) > 1.7 mmol/L; serum low-density lipoprotein cholesterol (LDL-C) > 3.64 mmol/L[19].
CAG examination
Selective CAG (Siemens Artis Zee floor) was performed in this study. The left coronary angiography was routinely performed in a spider position (left front oblique 45° + foot position 30°), left shoulder position (left front oblique 45° + head position 30°), head position (positive head position 30°), right shoulder position (right front oblique 30° + head position 30°), and liver position (right front oblique 30° + foot position 30°). The right CAG was performed with left front oblique 45° and right front oblique 30°[20].
CTA examination
The scanning was performed using TOSHIBA AQUILION ONE TSX-301A. The ECG was placed on the patient's chest before scanning. ECG-gated scanning was used to scan from the bottom of the heart (marked by tracheal carina) to the whole heart. For enhanced scanning, a double-tube high-pressure syringe was used and the bolus tracking technique was adopted. The contrast agent iohexol 350 was injected through the middle of the elbow vein. The injection speed was 5 mL/s, and the total amount was 60-80 mL. Subsequently, 50 mL of physiological saline was injected at the same rate. After the images were acquired, the resulting data were delivered to the workstation for further analysis. In the section along the longitudinal axis of the coronary artery, if a certain coronary artery was distributed in a certain myocardium, it can be diagnosed as an MB. The branch of the MB was observed and recorded. MB type (superficial/deep type), MB length, MB thickness, MCA end-systolic diameter, and end-diastolic diameter were measured. The MCA systolic stenosis rate was calculated as (MCA end-diastolic diameter - MCA end-systolic diameter)/MCA end-diastolic diameter. The diameter method was used to estimate the systolic and diastolic compression of the MCA.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0; Chicago, IL, USA). The numerical data are expressed as the mean ± SD and the comparison between the two groups was compared using the t-test. The categorical variables are expressed as number and percentage and the comparison between the two groups was performed by the chi-square test. Logistic regression was used for multivariate analysis to screen out the potential independent influencing factors of MB-related CHD. ROC curve was used to analyze the diagnostic efficacy of potential indicators for MB-related CHD. Statistical significance was defined as 2-tailed P < 0.05 for all tests.
RESULTS
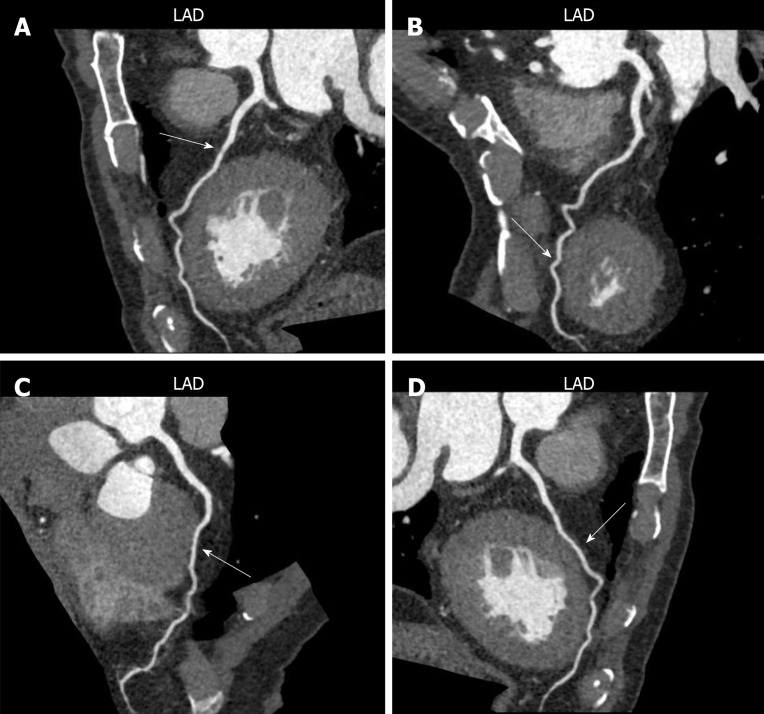
Totally 1718 patients who underwent CTA or CAG were enrolled. Of all the participants, 1060 patients diagnosed with CHD were included in the CHD group, and the remaining 658 patients were included in the control group. There were 288 MB patients found in the present study. Totally 236 patients diagnosed with MB-related CHD were divided in the MB-CHD subgroup. It included 121 males and 115 females, and the average age was 60.25 ± 15.34 years. The remaining 52 patients diagnosed with simple MB were divided into the simple MB subgroup. It included 32 males and 20 females, and the average age was 52.65 ± 12.86 years. The lesions of 288 MB were all located in the left anterior descending wall coronary artery, as shown in Figure 1.
Figure 1.
Computed tomography coronary angiography images of myocardial bridge lesions. Myocardial bridge (MB) was marked by the arrow in figures. A and B: MB in the left anterior descending coronary artery; C: MB in the anterior descending coronary artery; D: MB in the right anterior descending coronary artery.
Analysis of risk factors for CHD
Clinical characteristics of the CHD group and the control group are shown in Table 1. The ratio of gender, family history of CHD, abnormal TC, HDL-C, TG, Lp(a), HCY, PAI-1, and fibrinogen were comparable in the two groups (P > 0.05). The average age, percentage of patients with abnormal hypertension, diabetes, smoking, ECG ST-segment changes, LDL-C, and combined MB in the CHD group were significantly higher than those in the control group (P < 0.05). Multivariate logistic regression analysis indicated that the occurrence of CHD was influenced remarkably by hypertension, ECG ST-segment changes, diabetes, LDL-C, and combined MB (P < 0.05) except for age and smoking (P > 0.05). The further analysis revealed that factors affecting CHD ranked based on OR values as follows: hypertension, diabetes, MB, LDL-C, and ECG ST-segment changes (Table 2).
Table 1.
Comparison of clinical characteristics between the coronary heart disease group and the control group, n (%)
| Characteristic | CHD group (n =1060) | Control group (n = 658) | t/X2 value | P-value |
| Age | 60.25 ± 13.25 | 52.65 ± 10.86 | 12.360 | 0.000 |
| Gender (Male/Female) | 592/468 | 388/270 | 1.610 | 0.204 |
| Family history of CHD | 215 (20.3) | 113 (17.2) | 2.542 | 0.111 |
| Hypertension | 707 (66.7) | 352 (53.5) | 29.929 | 0.000 |
| Diabetes | 424 (40.0) | 102 (15.5) | 114.702 | 0.000 |
| Smoking | 286 (27.0) | 122 (18.5) | 15.970 | 0.000 |
| ST-segment changes | 456 (43.0) | 72 (10.9) | 196.224 | 0.000 |
| Abnormal TC | 445 (42.0) | 264 (40.1) | 0.579 | 0.447 |
| Abnormal LDL-C | 583 (55.0) | 260 (39.5) | 38.960 | 0.000 |
| Abnormal HDL-C | 456 (43.0) | 270 (41.0) | 0.656 | 0.418 |
| Abnormal TG | 472 (44.5) | 289 (43.9) | 0.061 | 0.805 |
| Abnormal Lp(a) | 311 (29.3) | 182 (27.7) | 0.560 | 0.454 |
| Abnormal HCY | 225 (21.2) | 115 (17.5) | 3.595 | 0.058 |
| Abnormal PAY-1 | 178 (16.8) | 99 (15.0) | 0.916 | 0.339 |
| Abnormal fibrinogen | 157 (14.8) | 87 (13.2) | 0.842 | 0.359 |
| Combined MB | 235 (22.2) | 53 (8.1) | 57.969 | 0.000 |
CHD: Coronary heart disease; MB: Myocardial bridge; TC: Total cholesterol; TG: Triglyceride; LDL-C: Low-density lipoprotein cholesterol; Lp(a): Lipoprotein(a); HCY: Homocysteine; PAI-1: Plasminogen activator inhibitor-1.
Table 2.
Multivariate logistic regression analysis factors affecting the development of coronary heart disease
| Risk factor | B | SE | Wald | P | OR |
95%CI |
|
| Lower limit | Upper limit | ||||||
| Age | 0.622 | 0.127 | 3.902 | 0.048 | 1.863 | 1.452 | 2.390 |
| Hypertension (Yes = 1, No = 0) | 1.831 | 0.227 | 9.824 | 0.001 | 6.238 | 3.998 | 9.734 |
| Diabetes (Yes = 1, No = 0) | 1.450 | 0.127 | 6.205 | 0.012 | 4.263 | 3.324 | 5.468 |
| Smoking (Yes = 1, No = 0) | 0.257 | 0.228 | 0.245 | 0.089 | 1.293 | 0.827 | 2.022 |
| ST-segment changes (Yes = 1, No = 0) | 0.638 | 0.311 | 5.079 | 0.023 | 1.892 | 1.028 | 3.481 |
| LDL-C (Abnormal = 1, Normal = 0) | 0.790 | 0.384 | 4.357 | 0.036 | 2.204 | 1.038 | 4.678 |
| MB (with = 1, without = 0) | 1.260 | 0.012 | 10.262 | 0.000 | 3.524 | 3.442 | 3.608 |
LDL-C: Low-density lipoprotein cholesterol; MB: Myocardial bridge.
Comparison of MB-related parameters between the MB-CHD subgroup and simple MB subgroup
The results of the comparisons between the MB-CHD subgroup and the simple MB subgroup showed that the difference of the MB length between the two groups was similar (P > 0.05). The proportion of deep MB type, MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate in the MB-CHD subgroup were significantly higher than those in the simple MB subgroup (P < 0.05, Table 3).
Table 3.
Comparison of myocardial bridge parameters between the myocardial bridge-coronary heart disease subgroup and the simple myocardial bridge subgroup
| Parameter |
Group |
t/X2 value | P-value | |
| MB-CHD subgroup (n = 236) | Simple MB subgroup (n = 52) | |||
| MB type (Superficial /Deep) | 125/111 | 38/14 | 7.016 | 0.008 |
| Systolic compression (%) | 50.28 ± 11.24 | 39.86 ± 12.08 | 7.326 | 0 |
| Diastolic compression (%) | 34.25 ± 10.20 | 24.74 ± 10.75 | 7.424 | 0 |
| MCA systolic stenosis rate (%) | 44.35 ± 9.43 | 32.92 ± 6.60 | 10.865 | 0 |
MB: Myocardial bridge; MCA: Mural coronary artery; CHD: Coronary heart disease.
Multivariate analysis of independent influencing factors of MB-related CHD
MB type, thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate were included in the logistic regression analysis. The results showed that MB type had no effect on the occurrence of MB-related CHD (P > 0.05). MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate had obvious effects on the occurrence of MB-related CHD (P < 0.05). The further study revealed that the factors influencing the occurrence of MB-related CHD were as follows in descending order: diastolic compression, MB thickness, MCA systolic stenosis rate, and systolic compression (Table 4).
Table 4.
Multivariate analysis of independent influencing factors of myocardial bridge-related coronary heart disease
| B | SE | Wald | P | OR |
95.0%CI |
||
| Lower limit | Upper limit | ||||||
| MB type (Deep = 1, Superficial = 0) | 0.459 | 0.316 | 2.858 | 0.072 | 1.582 | 0.852 | 2.939 |
| MB thickness | 1.001 | 0.274 | 10.420 | 0.000 | 2.722 | 1.591 | 4.657 |
| Systolic compression | 0.514 | 0.162 | 12.341 | 0.000 | 1.672 | 1.217 | 2.297 |
| Diastolic compression | 1.266 | 0.347 | 18.226 | 0.000 | 3.548 | 1.797 | 7.004 |
| MCA systolic stenosis rate | 0.602 | 0.242 | 9.854 | 0.001 | 1.825 | 1.136 | 2.933 |
MB: Myocardial bridge; MCA: Mural coronary artery.
Effectiveness analysis of MB-related parameters in diagnosing MB-related CHD
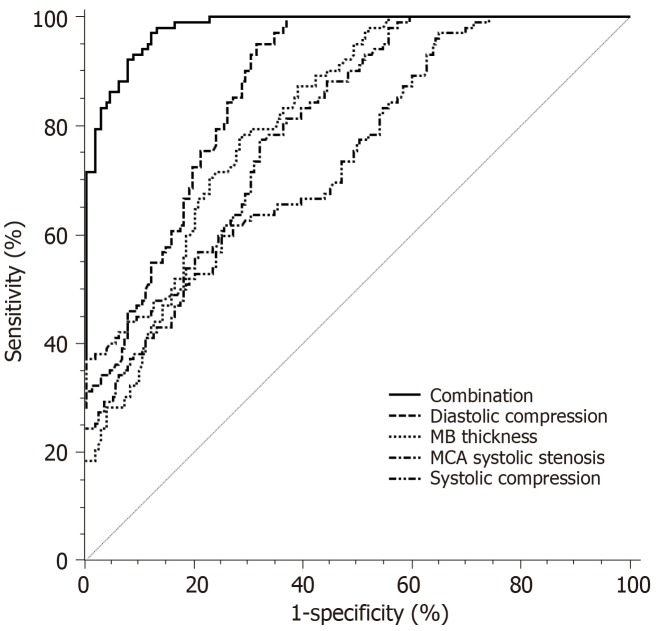
The results of ROC curve analysis for the diagnosis of MB-related CHD by MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate are shown in Table 5 and Figure 2.The area under the curve (AUC) of diastolic compression was the highest, and its sensitivity reached 95.10%, followed by MB thickness, MCA systolic stenosis rate, and systolic compression. However, the AUC of each indicator did not exceed 0.9. Based on the logistic regression model, we established a combined diagnosis of four indicators, and found that the AUC of the combined diagnosis was 0.959, which was significantly higher than the AUCs of the four indicators separately (P < 0.05). The sensitivity was 97.06% and the specificity was 87.63%.
Table 5.
Receiver operating characteristic curve analysis of myocardial bridge indexes for diagnosis of coronary heart disease
| AUC | 95%CI | Cut-off point | Sensitivity | Specificity | |
| MB thickness | 0.814a | 0.764 - 0.857 | 21.54 mm | 78.43% | 70.97% |
| Systolic compression | 0.755a | 0.701 - 0.803 | 45.04% | 37.25% | 99.46% |
| Diastolic compression | 0.870a | 0.826 - 0.907 | 48.65% | 95.10% | 68.28% |
| MCA systolic stenosis rate | 0.795a | 0.744 - 0.840 | 43.45% | 77.45% | 67.74% |
| Combination | 0.959 | 0.925 - 0.972 | 0.275 | 97.06% | 87.63% |
P< 0.05 vs Combination. MB: Myocardial bridge; MCA: Mural coronary artery.
Figure 2.
Efficacy of myocardial bridge related indicators and their combination in the diagnosis of myocardial bridge-related coronary heart disease. MB: Myocardial bridge; MCA: Mural coronary artery.
Logistic regression analysis of the effect of the combination of four factors on MB-related CHD
Since the combination of four indicators was the best indicator for the diagnosis of MB-related CHD in this study. We used logistic regression to analyze its effect on MB-related CHD using its optimal diagnostic point. The results showed that when the combination was > 0.275, the risk of the occurrence of MB-related CHD was 15.963 times higher than that when the combination was ≤ 0.275 (Table 6).
Table 6.
Logistic regression analysis of the effect of the combination of the four indicators on myocardial bridge-related coronary heart disease
| B | SE | Wald | P | OR |
95.0%CI |
||
| Lower limit | Upper limit | ||||||
| Combination ( > 0.275 = 1, < 0.275 = 0) | 2.770 | 0.226 | 9.752 | 0.001 | 15.953 | 10.244 | 24.844 |
DISCUSSION
Studies have reported that some patients with sudden cardiac death did not die from myocardial infarction caused by coronary atherosclerosis, but related to MB[8,21-24]. MB may compress coronary arteries during the systolic period, which can cause angina pectoris, myocardial infarction, left ventricular dysfunction, paroxysmal atrioventricular block, myocardial ischemia during exertion, sudden cardiac death, etc[25]. With the rapid development of imaging diagnostic technology, the detection rate of myocardial ischemia caused by MB has increased recently[26,27]. CTA has been widely used in MB detection, and it has been proved to non-invasively measure the length and thickness of MB, the systolic and diastolic pressure on MCA, and other MB anatomical features[28,29]. Therefore, in the present study, 288 patients with MB were screened from 1718 patients with suspected CHD. The detailed analyses of MB were performed by CTA in order to explore the effect of MB on CHD.
This study first compared the general data of CHD patients and patients without CHD. In addition to age, hypertension, diabetes, smoking, ECG ST segment changes, and LDL-C, which known as CHD influencing factors, the number of patients with combined MB in the CHD group was significantly higher than that in the control group. Further multivariate analysis showed that combined MB was an independent factor affecting CHD. Furthermore, its influencing effect was second only to the effect of diabetes on CHD. This finding indicates that patients with MB are more likely to suffer with CHD. It is indeed necessary to observe MB in detail and take action to prevent CHD.
In order to fully analyze the MB using CTA, we analyzed various anatomical features of MB including MB type (superficial/depth), MB length, thickness, systolic compression, and diastolic compression. The results showed that the proportion of deep MB, MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate in the MB-CHD subgroup were significantly greater than those in the simple MB subgroup. To further explore the effects of MB anatomical features on CHD, we performed a multivariate regression analysis using a logistic regression model. The results showed that MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis were four independent influencing factors on the occurrence of MB-related CHD. The diastolic compression and MB thickness had the greatest influence on MB-related CHD, suggesting that thicker MB will compress MCA during the diastolic phase, affecting MCA perfusion and leading to coronary ischemia.
To better analyze the clinical value of the above four MB anatomical features in the diagnosis of MB-related CHD. The MB thickness, systolic compression, diastolic compression, MCA systolic stenosis rate, and their combination were included in the ROC curve analysis. The results showed that the AUC of the diastolic compression was the largest when the four indicators were used for diagnosis separately, which was 0.870. The AUC of the other three anatomical features were not high. Although the specificity of the systolic compression was close to 100%, the sensitivity was only 37.25%. It cannot be used as a reliable indicator for the diagnosis of CHD. Similarly, although the sensitivity of diastolic compression was very high, reaching 95.1%, the specificity was only 68.28%. The AUC of combined diagnosis increased to 0.959, suggesting that the accuracy of the combination of the four features for diagnosing MB-related CHD was the best. Logistic regression showed that when the probability distribution of the combination of the four MB anatomical features was > 0.275, the risk of occurrence of MB-related CHD was 15.963 times higher than that when the value was ≤ 0.275. It is suggested that the combination of the four MB anatomical features has potential diagnostic value for MB-related CHD.
The results of this study are unable to be used as clinical criteria currently due to its limited sample size and single-center research. We suggest that a multi-center and large-scale study should be performed to obtain effective indicators for MB-related CHD, in order to improve patients’ life quality.
In conclusion, this study found that MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis were independent risk factors for MB-related CHD. The combination of the four MB anatomical features has potential diagnostic value for MB-related CHD. Clinical attention should be paid to MB, and the diagnostic value of MB anatomical features for MB-related CHD should be further explored.
ARTICLE HIGHLIGHTS
Research background
More and more reports indicate that myocardial bridge (MB) can compress the mural coronary artery during cardiac contraction, causing myocardial ischemia such as angina pectoris and myocardial infarction. It is clinically called MB-related coronary heart disease (CHD). Therefore, the relationship between MB and CHD has an important influence on the diagnosis of MB-related CHD. Currently, only when the MB reaches a certain thickness, it can be found in coronary angiography (CAG), which results in a high missing diagnosis rate.
Research motivation
Currently, CAG cannot specifically observe MB-related details. Computed tomography coronary artery (CTA) has become an important means of non-invasive diagnosis of CHD. Studies have shown that CTA can directly show the anatomical relationship between the coronary artery and myocardium. Therefore, this study used CTA to assess the association between MB and coronary atherosclerosis, in order to explore the effect of MB on patients with CHD and improve the detection rate of MB-related CHD.
Research objectives
In this study, CTA was used to observe the anatomy of MB and analyzed the effects of MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis rate on CHD. The purpose of this study was to evaluate the relationship between MB and coronary atherosclerosis by CTA.
Research methods
CHD patients who underwent CTA or CAG were defined as the CHD group and the control group, respectively. CHD patients with combined MB were defined as the MB-CHD subgroup. Patients with simple MB were defined as the simple MB subgroup. The anatomical features of patients with MB were analyzed by multi-factor logistic regression. The ROC curve was used to analyze the diagnostic efficacy of the potential indicators for MB-related CHD.
Research results
MB thickness, systolic compression, diastolic compression, and MCA systolic stenosis had significant effects on the incidence of MB-related CHD (P < 0.05). The areas under the ROC curves for the four indicators in diagnosing CHD were 0.814, 0.755, 0.870, and 0.795, respectively. The efficacy of diastolic compression in the diagnosis of CHD was the highest. When the degree of MB diastolic compression was > 48.68%, the risk of CHD was 15.953 times than that when the value was ≤ 48.68% (P < 0.05).
Research conclusions
MB length, MB thickness, systolic and diastolic compression of MCA have significant effects on the occurrence of MB-related CHD.
Research perspectives
CTA is a non-invasive economic examination that can directly display the anatomical relationship between the coronary arteries and myocardium. The combination of the four MB anatomical features has potential diagnostic value for MB-related CHD. However, the results of this study cannot be regarded as the clinical criteria because of the limited sample size. We suggest that further multi-center study should be performed to obtain the effective indicators for MB-related CHD, in order to provide a reference for early diagnosis of MB.
Footnotes
Institutional review board statement: The study was approved by the Ethics Committee of Beijing Anzhen Hospital Affiliated to Capital Medical University.
Informed consent statement: All patients gave informed consent.
Conflict-of-interest statement: The authors declare that they have no competing interests.
STROBE statement: The authors have read the STROBE Statement, and the manuscript was prepared and revised according to the STROBE Statement.
Manuscript source: Unsolicited manuscript
Peer-review started: March 28, 2019
First decision: May 31, 2019
Article in press: July 3, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Orbell JH, Ryan EM S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Wu YXJ
Contributor Information
Dong-Hui Zhao, Department of Cardiology, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing 100029, China. zhaodonghui3486@126.com.
Qian Fan, Department of Cardiology, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing 100029, China.
Jun-Xia Ning, Department of Cardiology, The First People's Hospital of Pingyuan County, Dezhou 253100, Shandong Province, China.
Xin Wang, Department of Cardiology, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing 100029, China.
Jia-Yu Tian, Department of Cardiology, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing 100029, China.
References
- 1.Cerrato E, Barbero U, D'Ascenzo F, Taha S, Biondi-Zoccai G, Omedè P, Bianco M, Echavarria-Pinto M, Escaned J, Gaita F, Varbella F. What is the optimal treatment for symptomatic patients with isolated coronary myocardial bridge? A systematic review and pooled analysis. J Cardiovasc Med (Hagerstown) 2017;18:758–770. doi: 10.2459/JCM.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 2.An JW, Zhao XB, Zhao HL, Zhou W. [Correlation of Coronary Artery Tortuosity Caused by Myocardial Bridge with Coronary Atherosclerosis] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40:151–157. doi: 10.3881/j.issn.1000-503X.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Pourhoseini S, Bakhtiari M, Babaee A, Ostovan MA, Eftekhar-Vaghefi SH, Ostovan N, Dehghani P. Increased risk of coronary perforation during percutaneous intervention of myocardial bridge: What histopathology says. J Cardiovasc Thorac Res. 2017;9:108–112. doi: 10.15171/jcvtr.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding SJ, Huang RC, Jia CF, Zhong L, An P, Wang ZQ, Zhu H, Wu BL, Zhou XC. [The relationship between myocardial bridge in mural coronary artery segment and coronary atherosclerosis] Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44:873–878. doi: 10.3760/cma.j.issn.0253-3758.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Saito Y, Kitahara H, Shoji T, Tokimasa S, Nakayama T, Sugimoto K, Fujimoto Y, Kobayashi Y. Relation between severity of myocardial bridge and vasospasm. Int J Cardiol. 2017;248:34–38. doi: 10.1016/j.ijcard.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Yu M, Zhang Y, Li Y, Li M, Li W, Zhang J. Assessment of Myocardial Bridge by Cardiac CT: Intracoronary Transluminal Attenuation Gradient Derived from Diastolic Phase Predicts Systolic Compression. Korean J Radiol. 2017;18:655–663. doi: 10.3348/kjr.2017.18.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimiya K, Matsumoto Y, Wang H, Piao Z, Ohyama K, Uzuka H, Hao K, Tsuburaya R, Takahashi J, Ito K, Shimokawa H. Absence of adventitial vasa vasorum formation at the coronary segment with myocardial bridge - An optical coherence tomography study. Int J Cardiol. 2018;250:275–277. doi: 10.1016/j.ijcard.2017.09.211. [DOI] [PubMed] [Google Scholar]
- 8.Rogers IS, Tremmel JA, Schnittger I. Myocardial bridges: Overview of diagnosis and management. Congenit Heart Dis. 2017;12:619–623. doi: 10.1111/chd.12499. [DOI] [PubMed] [Google Scholar]
- 9.Cademartiri F, Nistri S, Tarantini G, Maffei E. Management of coronary artery disease with cardiac CT beyond gatekeeping. Heart. 2017;103:975–976. doi: 10.1136/heartjnl-2016-310473. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow DI, Humphries SE. Genetics of CHD in 2016: Common and rare genetic variants and risk of CHD. Nat Rev Cardiol. 2017;14:73–74. doi: 10.1038/nrcardio.2016.209. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Zhu B, Zhuo L, He MY, Xu Y, Wang TT, Cai QQ, Hu B, Xu JC, Zhang WH. [Risk factors for congenital heart disease in Chinese neonates: a Meta analysis] Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:754–758. doi: 10.7499/j.issn.1008-8830.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver JM, Gallego P, Gonzalez AE, Garcia-Hamilton D, Avila P, Yotti R, Ferreira I, Fernandez-Aviles F. Risk factors for excess mortality in adults with congenital heart diseases. Eur Heart J. 2017;38:1233–1241. doi: 10.1093/eurheartj/ehw590. [DOI] [PubMed] [Google Scholar]
- 13.Aksoy F, Baş HA, Altınbaş A. Nonsymptomatic myocardial bridge causing systolic total narrowing of circumflex artery. J Saudi Heart Assoc. 2018;30:153–156. doi: 10.1016/j.jsha.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, He Y, Xie LJ, Yang MX, Yang ZG, Guo YK. Myocardial bridging in left main coronary artery. Coron Artery Dis. 2018;29:274–275. doi: 10.1097/MCA.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 15.Boyd JH, Pargaonkar VS, Scoville DH, Rogers IS, Kimura T, Tanaka S, Yamada R, Fischbein MP, Tremmel JA, Mitchell RS, Schnittger I. Surgical Unroofing of Hemodynamically Significant Left Anterior Descending Myocardial Bridges. Ann Thorac Surg. 2017;103:1443–1450. doi: 10.1016/j.athoracsur.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Han R, Sun K, Lu B, Zhao R, Li K, Yang X. Diagnostic accuracy of coronary CT angiography combined with dual-energy myocardial perfusion imaging for detection of myocardial infarction. Exp Ther Med. 2017;14:207–213. doi: 10.3892/etm.2017.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannidis JPA. Diagnosis and Treatment of Hypertension in the 2017 ACC/AHA Guidelines and in the Real World. JAMA. 2018;319:115–116. doi: 10.1001/jama.2017.19672. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien SH, Vesely SK, Schwarz EB. Response to Comment on O'Brien et al. Hormonal Contraception and Risk of Thromboembolism in Women With Diabetes. Diabetes Care 2017;40:233-238. Diabetes Care. 2017;40:e62. doi: 10.2337/dci17-0004. [DOI] [PubMed] [Google Scholar]
- 20.Aikawa Y, Kataoka Y, Kanaya T, Amaki M, Tahara Y, Asaumi Y, Kanzaki H, Noguchi T, Fujita T, Kobayashi J, Yasuda S. Procedural challenge of coronary catheterization for ST-segment elevation myocardial infarction in patient who underwent transcatheter aortic valve replacement using the CoreValveTM. Cardiovasc Diagn Ther. 2018;8:190–195. doi: 10.21037/cdt.2018.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocovski L, Lee JD, Parpia S, Fernandes J, Nair V. Association of Waist-Hip Ratio to Sudden Cardiac Death and Severe Coronary Atherosclerosis in Medicolegal Autopsies. Am J Forensic Med Pathol. 2017;38:226–228. doi: 10.1097/PAF.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 22.Finocchiaro G, Papadakis M, Sharma S, Sheppard M. Sudden Cardiac Death. Eur Heart J. 2017;38:1280–1282. doi: 10.1093/eurheartj/ehx194. [DOI] [PubMed] [Google Scholar]
- 23.Basso C, Aguilera B, Banner J, Cohle S, d'Amati G, de Gouveia RH, di Gioia C, Fabre A, Gallagher PJ, Leone O, Lucena J, Mitrofanova L, Molina P, Parsons S, Rizzo S, Sheppard MN, Mier MPS, Kim Suvarna S, Thiene G, van der Wal A, Vink A, Michaud K Association for European Cardiovascular Pathology. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471:691–705. doi: 10.1007/s00428-017-2221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal H, Sexson-Tejtel SK, Qureshi AM, Alam M, Masand P, Fraser CD, Jr, Molossi S, Mery CM. Aborted Sudden Cardiac Death After Unroofing of Anomalous Left Coronary Artery. Ann Thorac Surg. 2017;104:e265–e267. doi: 10.1016/j.athoracsur.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, Jang WY, Kim W, Choi JY, Park EJ, Na JO, Choi CU, Lim HE, Kim EJ, Park CG, Seo HS, Oh DJ, Rha SW. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. 2018;270:8–12. doi: 10.1016/j.atherosclerosis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Wu NQ, Evora M, Lam UP, Ip MF, Li JJ. Acute myocardial infarction caused by myocardial bridging alone confirmed by using intravascular ultrasonography. Chronic Dis Transl Med. 2017;3:260–262. doi: 10.1016/j.cdtm.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikuchi S, Okada K, Hibi K, Maejima N, Matsuzawa Y, Konishi M, Kimura Y, Kosuge M, Iwahashi N, Ebina T, Tamura K, Kimura K. Myocardial Infarction Caused by Accelerated Plaque Formation Related to Myocardial Bridge in a Young Man. Can J Cardiol. 2018;34:1687.e13–1687.e15. doi: 10.1016/j.cjca.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Yu M, Zhang J, Li M, Lu Z, Wei M. Non-invasive imaging of myocardial bridge by coronary computed tomography angiography: the value of transluminal attenuation gradient to predict significant dynamic compression. Eur Radiol. 2017;27:1971–1979. doi: 10.1007/s00330-016-4544-7. [DOI] [PubMed] [Google Scholar]
- 29.McElwee SK, Velasco A, Doppalapudi H. Mechanisms of sudden cardiac death. J Nucl Cardiol. 2016;23:1368–1379. doi: 10.1007/s12350-016-0600-6. [DOI] [PubMed] [Google Scholar]