Abstract
Prediction of benefit from adjuvant chemotherapy following resection of early breast cancer and, as a result, proper selection of candidates remains an elusive goal since the relative magnitude of benefit is the same regardless of the presence of clinicopathologic factors. Multiple studies, including randomized trials, establish the role of certain gene expression signatures in node-negative disease since they predict the risk of breast cancer relapse being so low that adjuvant chemotherapy can be omitted. In contrast, more limited data are available in higher risk, node-positive breast cancer patients, making the exclusion of adjuvant chemotherapy potentially hazardous. ‘Prospective–retrospective’ studies and limited prospective data show that several signatures, namely Oncotype Dx, MammaPrint, Prosigna, EndoPredict and Breast Cancer Index, select with different levels of success node-positive patients at very low risk for distant recurrence despite not receiving chemotherapy, although the long-term follow-up is still awaited. Pending, however the publication of the results from ongoing randomized studies which enroll patients with node-positive disease, major caution is warranted. Improper use and misinterpretation of these transcriptomic profiles can lead to undertreatment and exposure of patients to unnecessary risks resulting in increased breast cancer mortality for patients with axillary node-positive disease. With this review we critically discuss the available data on gene expression signatures that are used in clinical practice and offer practical recommendations regarding the management of patients with ER-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive breast cancer.
Keywords: adjuvant chemotherapy, breast cancer, gene expression signature, node-positive, Oncotype Dx
Key Message
Gene expression signatures may select with various levels of success patients with node-negative breast cancer at sufficiently low risk for relapse that adjuvant chemotherapy will not meaningfully improve outcomes; however, data in node-positive disease are scarce and caution is needed in order to avoid undertreating patients and exposing them to unnecessary risks.
Introduction
Substantial advances on the understanding of the underlying complexity and heterogeneity of breast cancer (BC) biology and clonal architecture [1–3] have allowed for the development, validation and regulatory approval of transcriptomic gene expression profiles (GEPs) that aim to select patients that could be spared from adjuvant chemotherapy [4]. Several of these GEPs, described in this review, are recommended for clinical use by the American Society of Clinical Oncology (ASCO) [5, 6], although not in combination due to discrepancies in patient classification. GEP impact on patterns of usage of adjuvant chemotherapy has been shown prospectively, with published studies reporting a 20%–35% reduction in chemotherapy administration with the use of Recurrence Score (RS) determined by the Oncotype Dx assay [7–9]. These results are mirrored by retrospective, real-world data which demonstrate a marked decline in adjuvant chemotherapy use especially in patients with node-negative disease. Notably, chemotherapy use in patients with node-positive disease has also decreased, despite the fact that the assay is not universally reimbursed for this indication [10, 11]. The latter is of particular interest, taking into account the relative paucity of available data regarding node-positive BC. As a result, pending the publication of ongoing randomized trials regarding node-positive disease, there is a real risk for undertreatment associated by extrapolation of the available GEP. In this article, we aim to critically discuss the available data from published clinical trials, with an emphasis on node-positive BC.
Benefit from adjuvant chemotherapy
Data regarding the effect of chemotherapy on patient outcomes following resection of early BC are derived from the Early Breast Cancer Trialists’ Collaboration Group (EBCTCG) meta-analysis of 100 000 individual patients that were randomized in 123 trials [12]. The postoperative administration of an anthracycline, when compared with no chemotherapy, improves the absolute relapse free survival (RFS) at 10 years by 8% and breast cancer specific survival (BCSS) by 6.5%, which correspond to a relative risk reduction of ∼25%. Importantly, the proportional risk reduction for BCSS is the same regardless of nodal spread, estrogen receptor (ER) status, age and used endocrine therapy. Further adding a taxane to an anthracycline compared with anthracyclines alone results in a 4.6% absolute improvement in RFS and 2.8% in BCSS at 8 years, a relative reduction of 15%. Again, the relative improvement is independent of clinicopathologic factors. Interestingly and in contrast with the effect of anthracyclines which concerns mainly the first 5 years following BC diagnosis, the relative risk reduction in all end points with the addition of a taxane was similar between years 0–4 and years 5+.
Additional improvements in outcomes are conferred by the administration of every 2 weeks or dose-dense chemotherapy, as proposed by the Norton–Simon hypothesis and evaluated in a number of trials [13]. At 10 years, the absolute decrease in the risks for disease recurrence and for death due to BC was 4.3% and 2.8% in favor of dose-dense schedules respectively, a relative reduction of ∼15% compared with the same treatment administered every 3 weeks [14]. The absolute benefit in ER-negative disease was 3.7% and in ER-positive disease 3.1%. The absolute risk is higher for patients with ER-negative disease so the decrease in recurrence and death is greater than for ER-positive disease though the proportional risk reduction is the same. These results confirm that dose-dense therapy should be considered as the standard of care for high-risk patients with node-positive disease and clearly do not support a generalized recommendation for de-escalation by omitting standard adjuvant chemotherapy.
Biomarkers in BC: prognostic, predictive or both?
In order to adequately evaluate the performance of a biomarker (clinical, protein-based or gene-based) one needs to grasp the difference between prediction and prognostication. The former implies benefit from administered treatment; in the presence of a positive predictive marker, the patient’s outcome improves when treated with a specific therapy. The latter implies disease behavior; in the presence of a positive prognostic marker, the patient’s outcome improves regardless of administered therapy.
The majority of evaluated biomarkers at the adjuvant setting in BC carry prognostic information: they select patients at very low absolute risk for recurrence, so that the presumed 1%–2% risk for death or serious long-term adverse events caused by chemotherapy is not justified. Assuming a stable benefit from chemotherapy at ∼30% reduction in recurrences and a 10% risk for recurrence at 10 years that a patient with very early disease has, the absolute risk reduction is 3%—only a small difference from the toxicity threshold. In contrast, for a patient with locoregionally advanced disease and an estimated 40% risk for recurrence at 10 years [15], the net benefit from chemotherapy would be 12%, significantly higher than the anticipated serious adverse events. Thus, chemotherapy administration is justified and the recommendation becomes even clearer when considering dose-dense chemotherapy and the further relative risk reduction it confers compared with standard interval treatment.
These observations however presume a constant reduction in BC recurrence of ∼30% using standard every 3 weeks regimens. This hold true, at least regarding clinicopathologic factors [12]. Some evidence however indicates that the risk of relapse of Luminal A tumors might be less affected by adjuvant chemotherapy [16–18]. For example, in the Danish Breast Cancer Group (DBCG) 77B trial patients were randomized to no adjuvant therapy, levamisole, cyclophosphamide (C) or CMF (C, methotrexate, 5-fluorouracil); endocrine therapy, which could have diluted the effect of chemotherapy, was not administered. Luminal A patients, as determined by immunohistochemistry, did not derive any benefit from cyclophosphamide-based chemotherapy [19]. How these results translate to patients treated with contemporary, anthracycline and taxane-containing regimens are unclear. Similarly, GEP such as RS may distinguish patients with node-negative disease that seem to benefit less from adjuvant chemotherapy [hazard ratio (HR)=0.95, 95% CI 0.75–1.22 for RS = 11–15 in the TailorX trial and HR = 1.19, 95% CI 0.40–3.49 for RS < 18 in the NSABP-B20 trial) [20, 21].
Prediction and prognostication in early BC according to clinical factors
As clearly shown in the EBCTCG meta-analyses, traditional clinicopathologic factors such as tumor size, nodal stage, age and ER status are not predictive for benefit from adjuvant chemotherapy. Even within the ER-positive subgroup, the relative effect is same regardless of age and tumor grade, with a relative risk reduction for death due to BC of ∼20% in the anthracycline versus no chemotherapy comparison [12]. In addition, although clearly prognostic, these routine factors alone may be inadequate to identify patients with sufficiently low absolute risk for recurrence so as the use of adjuvant chemotherapy is not justified, especially if the patients have node-positive disease. In another EBCTCG analysis of long-term data from 63 000 women treated with 5 years of endocrine therapy, distant recurrences occurred at a steady rate with the cumulative risk never reaching a plateau. Patients with node-negative disease had a 22% 20-year risk for distant recurrence (one-third had received chemotherapy) and patients with node-positive disease 31% (two thirds had received chemotherapy; however, chemotherapy allocation was not randomized and its effect cannot be evaluated). Even patients with T1N0 disease had a 13% 20-year risk for metastasis [22]. Although these results can be generalized regarding the long-term disease behavior of ER-positive, human epidermal growth factor receptor 2 (HER2)-negative BC, the risks may be overestimated due to the considerable recent therapeutic advances, as acknowledged by the authors of the study.
Prospective randomized data of gene expression signatures as prognostic and predictive factors in early BC, regardless of nodal status
Oncotype Dx
Oncotype Dx (Genomic Health Inc., Redwood City, CA) is a GEP which consists of 16 genes and 5 reference genes, whose expression is used in order to calculate RS [23]. RS has been validated in ‘prospective–retrospective’ [20, 24], prospective non-randomized [25] and prospective randomized studies [21, 26]. In the TailorX trial, 10 273 women with ER-positive, HER2-negative, node-negative BC were enrolled. Those with low risk disease (RS ≤ 10, n = 1626) did not receive chemotherapy and had a 9-year disease-free survival (DFS) of 84%. Patients with intermediate risk disease (RS = 11–25, n = 6711) were randomized to chemotherapy versus no chemotherapy. Although there were no differences in the entire group in terms of DFS, subgroup analyses revealed that patients younger than 50 years had the following improvements in outcomes with chemotherapy and according to RS: RS = 11–15, 3.5% in DFS and 0.8% in distant disease free survival (DDFS); RS = 16–20, 9% in DFS and 1.6% in DDFS; RS = 21–25, 6.3% in DFS and 6.5% in DDFS [21, 26]. The relative contribution of chemotherapy-induced amenorrhea, if any, cannot be assessed since such information was not collected but may contribute to the benefit.
The results of the TailorX trial imply that the clinical utility of RS is somewhat limited since, as discussed above, patients younger than 50 and at intermediate risk of recurrence (at least with RS = 16–25) seem to benefit from the administration of chemotherapy. In addition, the identification of patients belonging to the low RS group that do not need chemotherapy may be feasible without the use of genomic assays as shown in a Swedish population-based registry study [27], therefore negating the necessity for Oncotype Dx testing.
MammaPrint
MammaPrint (Agendia Inc., Amsterdam, The Netherlands) is a 70-gene GEP that divides patients in low- and high-risk for BC recurrence [28] and has been validated in retrospective [29–31], prospective non-randomized [32] and randomized studies [33]. In the latter trial, 6693 women with early BC were enrolled; ∼21% were node-positive. Patients were randomized to utilize the discordant clinical or genomic risk profile to allocate chemotherapy or not. The trial met its primary end point, since the lower boundary for DDFS at 5 years in the test population (high clinical and low genomic risk) was 92.5%, exceeding the prespecified threshold of 92%. With the use of MammaPrint, 46.2% of high clinical risk patients would be spared from chemotherapy, at the cost of an increased risk for distant recurrence by 1.5% in the intention-to-treat population (HR = 0.78, 95% CI 0.50–1.21) and 2.1% in the per-protocol analysis (HR = 0.65, 95% CI 0.38–1.10). Consistent trends in favor of chemotherapy were noted in discordant subgroups including a 2.5% absolute decrease in distant metastases in the node-negative, high clinical/low genomic risk group (HR = 0.69, 95% CI 0.39–1.21). The trial was not powered to exclude benefit from chemotherapy and also needs longer follow-up especially for patients with node-positive ER-positive disease [33].
Use of gene expression signatures in node-positive BC
Results from published prospective and ‘prospective–retrospective’ studies that have evaluated the prognostic and predictive power of the GEP in node-positive populations are discussed below and summarized in Table 1 [15, 33–38]. It should be noted that only one of these studies has a direct comparison of no chemotherapy to chemotherapy in the node-positive group [15].
Table 1.
Prospective and ‘prospective–retrospective’ studies on the use of gene expression signatures in node-positive breast cancer: distant metastasis-free survival events in low risk groups not treated with chemotherapy
| Trial [references] | N (total node-positive) | Definition of low risk | N (low risk) | Distant recurrence rate in low risk group | Comparison with chemotherapy |
|---|---|---|---|---|---|
| Oncotype Dx | |||||
| SWOG S8814 [15] | 367 | RS < 18 | 146 (40%) | 40% any disease recurrence at 10 years | HR = 1.02 (95% CI 0.54–1.93) |
| TransATAC [34] | 306 | RS < 18 | 160 (52%) | 17% at 9 years | NA |
| PlanB [35] | 905 | RS < 12 | 170 (19%) | 5.6% any disease recurrence at 5 years | NA |
| MammaPrint | |||||
| MINDACT [33] | 1404 | MammaPrint Index > 0.0 | 737 (high clinical, low genomic risk) | 4.4% at 5 years | HR = 0.88 (95% CI 0.42–1.82) |
| Prosigna | |||||
| ABCSG-8 [36] | 382 | ROR < 16 | 15 (143 intermediate) | 0% (6.4% intermediate) at 10 years | NA |
| TransATAC and ABCSG-8 [37] | 557 | ROR < 27 | 137 (24.6%) | 3.3% (years 5–10) | NA |
| EndoPredict | |||||
| TransATAC [38] | 183 | EPclin < 3.3 | 43 (23.5%) | 5.6% at 10 years | NA |
| Breast Cancer Index | |||||
| TransATAC [38] | 183 | BCI < 5.0825 | 95 (51.9%) | 15.5% at 10 years | NA |
BCI, Breast Cancer Index; EPclin, EndoPredict; HR, hazard ratio; ROR, risk of recurrence; RS, Recurrence Score.
The distribution of RS scores has been shown to be similar regardless of nodal status, therefore implying that a subset of node-positive patients in the continuum might not benefit from adjuvant chemotherapy [39]. In the SWOG-8814 trial, the use of anthracycline-containing chemotherapy did not improve DFS in the 146 patients with RS < 18 (HR = 1.02, 95% CI 0.54–1.93). The improvement in DFS was not statistically significant in the intermediate risk group as well (RS = 18–30; HR = 0.72, 95% CI 0.39–1.31, P = 0.48), although the sample size was small (103 patients). Similar results were reported for other end points (BCSS, overall survival) [15]. These results, despite the lack of power, represent the only randomized evidence on a GEP predicting the lack of benefit from adjuvant chemotherapy in node-positive BC. Similarly, large retrospective analyses show excellent BCSS among patients with limited node involvement and RS < 18 [40, 41]. For example, a SEER data analysis of 3919 patients with 1–3 involved nodes and RS < 18 showed 5-year BCSS rates of 92.8%, 95.1%, 97.1%, 99.4% and 98.9% for patients with ≥4, 3, 2, 1 involved node and micrometastasis, respectively. It should be noted however that the follow-up is short, 18%–59% of patients were treated with chemotherapy and chemotherapy is commonly underreported to SEER [40]. Further support for RS is given by the reported long-term results from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial [34] and prospectively from the planB trial [25, 35]; however, the relatively short follow-up of 5 years and low number of patients in the latter preclude any robust conclusions. The ongoing RxPONDER trial (Clinicaltrials.gov identifier NCT01272037) enrolls patients with ER-positive, HER2-negative BC with 1–3 positive lymph nodes and a RS ≤ 25, which are randomized to receive chemotherapy or not; the trial has completed accrual and the results are eagerly awaited. Pending their publication, no specific recommendations can be made. Notably, the inclusion criteria of RxPONDER seem to be utilized in clinical practice in order to exclude patients from chemotherapy before the publication of the results of the trial [42].
Limited support for the use of MammaPrint in node-positive disease is offered by the MINDACT trial [33]. In the node-positive subgroup of the primary study population of high clinical/low genomic risk patients, the absolute benefit from chemotherapy in terms of DDFS was 0.7% (HR = 0.88, 95% CI 0.42–1.82). As stated in the ASCO recommendations, this trial cannot exclude benefit from chemotherapy due to lack of power [6].
Prosigna (NanoString Technologies Inc., Seattle, WA) is an assay that can determine the tumor’s intrinsic subtype by using the 50-gene predictor analysis of microarray 50 (PAM50) gene signature and tumor size and therefore calculate a risk of recurrence (ROR) score [43]. ‘Prospective–retrospective’ data show that ROR can predict the long-term risk of distant recurrence in both node-negative and node-positive patients [36, 37, 44, 45]. Importantly, the prognostic capacity of ROR seems to outperform that of RS, adding long-term prognostic information in node-positive patients and identifying more precise low- and high-risk groups [38, 44]. Prosigna is being assessed in the ongoing OPTIMA (Optimal Personalized Treatment of early breast cancer usIng Multi-parameter Analysis; registration number ISRCTN42400492) randomized trial which will inform on the management of clinically high-risk patients (ER-positive, HER2-negative BC with 1–9 positive nodes or tumor size >30 mm). Participants are randomized to either chemotherapy or to experimental biomarker-driven therapy (chemoendocrine therapy for patients with ROR > 25 and endocrine therapy alone for those with ROR ≤ 25).
EndoPredict (EP, Myriad Genetics Inc., Salt Lake City, UT) is a 12-gene prognostic assay [46] that can be combined with clinical factors (tumor size and nodal status) to form EPclin, a tool which integrates both genomic and anatomical prognostication. Both EP and EPclin identify patients at very low risk for late distant recurrence following adjuvant endocrine therapy [47], as well as patients with node-positive disease treated with [48] or without chemotherapy. This GEP also provides the most prognostic value for late distant recurrence compared with all tested prognostic signatures in the transATAC analysis [38, 49].
Finally, Breast Cancer Index (BCI) comprises two distinct GEPs. The first component is the HOXB13 (homeobox B13) to interleukin 17B receptor (IL17BR) ratio, which predicts shorter DFS, resistance to tamoxifen therapy [50, 51] and can identify patients who benefit from extended letrozole adjuvant therapy [52, 53]. The second component of BCI is the 5-gene molecular grade index and the full GEP has been shown to robustly identify node-negative patients with very low risk for distant recurrence [38, 52, 54–56].
Synthesis of the available data
Available ‘prospective–retrospective’ and population-based data indicate that GEP provide additional prognostic information when added to clinical factors [38, 57]. These data, in addition to the two published randomized trials whose shortcomings are well-known [58], support the role of GEP in therapy selection at least for node-negative patients; however, the interpretation of older trials where sub-optimal according to contemporary standard chemotherapy was administered has a number of caveats. In addition, contemporary clinical trials imply that the 1% treatment-related mortality and severe morbidity might be exaggerated: in two trials of dose-dense adjuvant chemotherapy enrolling 4108 patients, only one toxic death occurred and 7 hematologic malignancies (of which 5 in the dose-dense groups) were diagnosed after 5.3–7.0 years of follow-up [59, 60]. As a result, the necessary threshold that needs to be exceeded in order to classify an absolute risk reduction as clinically meaningful, which is a function of the treatment effect and treatment-related mortality, might be lower than previously thought. In contrast, advances in adjuvant endocrine therapy such as extended treatment and ovarian ablation in combination with tamoxifen or exemestane in premenopausal patients [61] improve the efficacy of chemotherapy-free adjuvant strategies and may increase the number of potential candidates without excess risk. The latter is of particular interest, since the absence of chemotherapy-induced ovarian suppression [62] should be mitigated by the administration of optimal endocrine therapy. This however should also be weighed against the potential adverse events, their impact on patients’ quality of life and overall modest treatment compliance [63].
Choosing the ‘right’ GEP can be a challenge and even more so in node-positive BC. While more data—including the only two randomized trials—are available on RS and 70-gene assay compared with other tools, limited head-to-head comparisons indicate that ROR and EP/EPclin might better select patients at very low risk for distant recurrence, despite the presence of nodal spread and lack of adjuvant chemotherapy. Of particular concern is the fact that the comparative analysis by Sestak et al. demonstrated the limited long-term prognostic information that RS adds to clinical factors, further underscoring the need for long-term, mature data from the TailorX and RxPONDER trials in order to properly utilize RS in routine clinical practice [38].
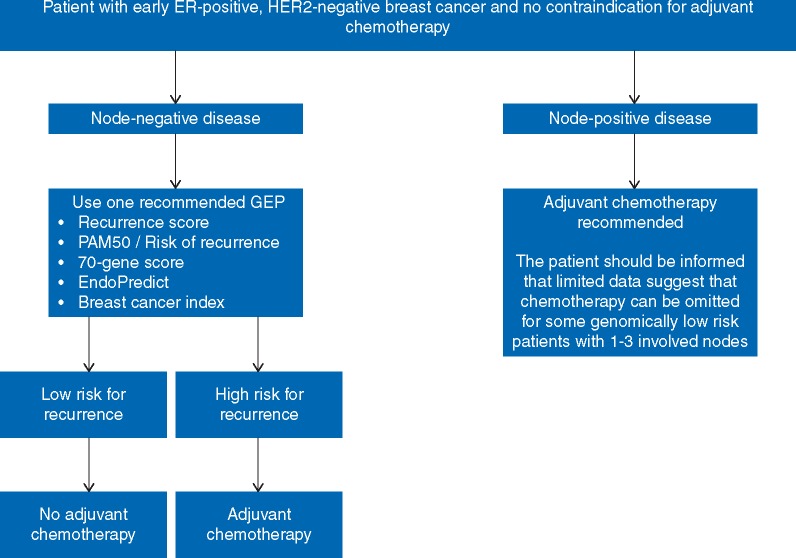
Definitive and proper recommendations regarding the clinical use of GEP among patients with node-positive disease can only be given when the results of randomized trials are available (Figure 1). Until then, any of three strategies can be applied [64]: treating everyone with chemotherapy, potentially overtreating some causing excess toxicity in an effort to minimize risk for recurrence; applying any one GEP to all patients with 1–3 positive lymph nodes, regardless of nodal burden and the presence of extracapsular invasion, and extrapolating the cut-offs from the available evidence, therefore sparing patients from chemotherapy but potentially exposing some to undertreatment; finally, employing a hybrid approach with GEP testing in those with low nodal burden (for example, only one positive lymph node with no extracapsular invasion, or selecting only patients with micrometastases in a single lymph node), in an effort to minimize both over- and undertreatment; however, no data support the latter and the definition of ‘low nodal burden’ is rather arbitrary. A frank discussion with the patient is highly encouraged in order to reach an informed and shared decision regarding the optimal management strategy. Until high quality data are available however, we recommend against the generalized usage of GEP in all patients with node-positive disease and patients should be informed that present standard of care still includes chemotherapy for most.
Figure 1.
Approach to the clinical use of gene expression signatures following resection of early breast cancer. ER, estrogen receptor; GEP, gene expression profile; HER2, human epidermal growth factor receptor 2; PAM50, predictor analysis of microarray 50.
Discussion
Conclusion
Whether the use of GEP demonstrates improved clinical utility among node-positive patients compared with (or, added to) anatomic grouping is clearly an unresolved issue. Caution is warranted, since overinterpreting the scant evidence that is currently available can expose women to unnecessarily increased risks for disease recurrence, incurable metastatic disease and, ultimately, death due to BC. Ongoing and future biomarker-driven studies that integrate clinical, pathologic, genomic and transcriptomic components will help optimize the science of adjuvant BC therapy.
Acknowledgements
AM is supported by the Stockholm Region (clinical postdoctoral appointment); TF is recipient of the Senior Clinical Investigator Award from the Swedish Cancer Society (grant number CAN 2017/1043); JB research group receives funding from the Stockholm Region, the Swedish Cancer Society, the funds at Radiumhemmet, the Swedish Research Council, the Knut and Alice Wallenberg fund (no grant number applicable).
Disclosure
AM has no conflicts of interest to disclose. TF: institutional grants from Roche and Pfizer and personal fees from Novartis, Pfizer, Roche and UpToDate; SS: in the past 24 months (as of 31 January 2019), SS reports personal fees for consulting/advisory services from Athenex, Daiichi-Sankyo, Eli Lilly & Company, Genentech/Roche, Genomic Health, Inivata, Novartis, Pfizer, Pieris Pharmaceuticals and Tocagen; non-financial support (i.e. travel and accommodations) from Athenex, Caris Life Sciences, Daiichi-Sankyo, Eli Lilly & Company, Genentech/Roche, Inivata, NanoString Technologies and Pieris Pharmaceuticals; research support to institution from Genentech/Roche; and other support from AstraZeneca (member, independent data monitoring committee). JB receives research funding from Merck paid to Karolinska Institutet and from Amgen, Bayer, Pfizer, Roche and Sanofi-Aventis paid to Karolinska University Hospital. No personal payments. Payment from UpToDate for a chapter in breast cancer prediction paid to Asklepios Medicine HB.
References
- 1.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perou CM, Sorlie T, Eisen MB. et al. Molecular portraits of human breast tumours. Nature 2000; 406(6797): 747–752. [DOI] [PubMed] [Google Scholar]
- 3. Sorlie T, Perou CM, Tibshirani R. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98(19): 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sotiriou C, Pusztai L.. Gene-expression signatures in breast cancer. N Engl J Med 2009; 360(8): 790–800. [DOI] [PubMed] [Google Scholar]
- 5. Harris LN, Ismaila N, McShane LM. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016; 34(10): 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krop I, Ismaila N, Andre F. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 2017; 35(24): 2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albanell J, Gonzalez A, Ruiz-Borrego M. et al. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol 2012; 23(3): 625–631. [DOI] [PubMed] [Google Scholar]
- 8. Eiermann W, Rezai M, Kummel S. et al. The 21-gene Recurrence Score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol 2013; 24(3): 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo SS, Mumby PB, Norton J. et al. Prospective multicenter study of the impact of the 21-gene Recurrence Score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 2010; 28(10): 1671–1676. [DOI] [PubMed] [Google Scholar]
- 10. Park SJ, Lee MH, Kong SY. et al. Use of adjuvant chemotherapy in hormone receptor-positive breast cancer patients with or without the 21-gene expression assay. Breast Cancer Res Treat 2018; 170(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 11. Loncaster J, Armstrong A, Howell S. et al. Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol 2017; 43(5): 931–937. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists' Collaborative Group, Peto R, Davies C. et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100, 000 women in 123 randomised trials. Lancet 2012; 379: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matikas A, Foukakis T, Bergh J.. Dose intense, dose dense and tailored dose adjuvant chemotherapy for early breast cancer: an evolution of concepts. Acta Oncol 2017; 56(9): 1143–1151. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists' Collaborative Group. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 2019; 393(10179): 1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albain KS, Barlow WE, Shak S. et al. Prognostic and predictive value of the 21-gene Recurrence Score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11(1): 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohnstad HO, Borgen E, Falk RS. et al. Prognostic value of PAM50 and risk of Recurrence Score in patients with early-stage breast cancer with long-term follow-up. Breast Cancer Res 2017; 19: 120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen MB, Laenkholm AV, Nielsen TO. et al. The Prosigna gene expression assay and responsiveness to adjuvant cyclophosphamide-based chemotherapy in premenopausal high-risk patients with breast cancer. Breast Cancer Res 2018; 20: 79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindstrom LS, Yau C, Czene K. et al. Intratumor heterogeneity of the estrogen receptor and the long-term risk of fatal breast cancer. J Natl Cancer Inst 2018; 110(7): 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen TO, Jensen MB, Burugu S. et al. High-risk premenopausal luminal a breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res 2017; 23(4): 946–953. [DOI] [PubMed] [Google Scholar]
- 20. Geyer CE Jr, Tang G, Mamounas EP. et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer 2018; 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sparano JA, Gray RJ, Makower DF. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018; 379(2): 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan H, Gray R, Braybrooke J. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017; 377(19): 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paik S, Shak S, Tang G. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351(27): 2817–2826. [DOI] [PubMed] [Google Scholar]
- 24. Paik S, Tang G, Shak S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24(23): 3726–3734. [DOI] [PubMed] [Google Scholar]
- 25. Gluz O, Nitz UA, Christgen M. et al. West German study group phase III PlanB trial: first prospective outcome data for the 21-gene Recurrence Score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 2016; 34(20): 2341–2349. [DOI] [PubMed] [Google Scholar]
- 26. Sparano JA, Gray RJ, Makower DF. et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015; 373(21): 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foukakis T, Falato C, Bergh J.. A 21-gene expression assay in breast cancer. N Engl J Med 2016; 374(14): 1386–1387. [DOI] [PubMed] [Google Scholar]
- 28. van 't Veer LJ, Dai H, van de Vijver MJ. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–536. [DOI] [PubMed] [Google Scholar]
- 29. Buyse M, Loi S, van't Veer L. et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 2006; 98(17): 1183–1192. [DOI] [PubMed] [Google Scholar]
- 30. van de Vijver MJ, He YD, van't Veer LJ. et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347(25): 1999–2009. [DOI] [PubMed] [Google Scholar]
- 31. Mook S, Schmidt MK, Viale G. et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat 2009; 116(2): 295–302. [DOI] [PubMed] [Google Scholar]
- 32. Drukker CA, Bueno-de-Mesquita JM, Retel VP. et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer 2013; 133(4): 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardoso F, van't Veer LJ, Bogaerts J. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016; 375(8): 717–729. [DOI] [PubMed] [Google Scholar]
- 34. Dowsett M, Cuzick J, Wale C. et al. Prediction of risk of distant recurrence using the 21-gene Recurrence Score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010; 28(11): 1829–1834. [DOI] [PubMed] [Google Scholar]
- 35. Nitz U, Gluz O, Christgen M. et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 2017; 165(3): 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gnant M, Filipits M, Greil R. et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of Recurrence Score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014; 25(2): 339–345. [DOI] [PubMed] [Google Scholar]
- 37. Sestak I, Cuzick J, Dowsett M. et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of Recurrence Score. J Clin Oncol 2015; 33(8): 916–922. [DOI] [PubMed] [Google Scholar]
- 38. Sestak I, Buus R, Cuzick J. et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4(4): 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bello DM, Russell C, McCullough D. et al. Lymph node status in breast cancer does not predict tumor biology. Ann Surg Oncol 2018; 25(10): 2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roberts MC, Miller DP, Shak S, Petkov VI.. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Cancer Res Treat 2017; 163(2): 303–310. [DOI] [PubMed] [Google Scholar]
- 41. Petkov VI, Miller DP, Howlader N. et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer 2016; 2: 16017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jasem J, Fisher CM, Amini A. et al. The 21-gene Recurrence Score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Canc Netw 2017; 15(4): 494–503. [DOI] [PubMed] [Google Scholar]
- 43. Parker JS, Mullins M, Cheang MC. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009; 27(8): 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dowsett M, Sestak I, Lopez-Knowles E. et al. Comparison of PAM50 risk of Recurrence Score with Oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013; 31(22): 2783–2790. [DOI] [PubMed] [Google Scholar]
- 45. Lænkholm A-V, Jensen M-B, Eriksen JO. et al. PAM50 risk of Recurrence Score predicts 10-year distant recurrence in a comprehensive Danish cohort of postmenopausal women allocated to 5 years of endocrine therapy for hormone receptor-positive early breast cancer. J Clin Oncol 2018; 36(8): 735–740. [DOI] [PubMed] [Google Scholar]
- 46. Filipits M, Rudas M, Jakesz R. et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011; 17(18): 6012–6020. [DOI] [PubMed] [Google Scholar]
- 47. Dubsky P, Brase JC, Jakesz R. et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br J Cancer 2013; 109(12): 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin M, Brase JC, Calvo L. et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res 2014; 16: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buus R, Sestak I, Kronenwett R. et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016; 108(11). doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jansen MP, Sieuwerts AM, Look MP. et al. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: a retrospective study. J Clin Oncol 2007; 25(6): 662–668. [DOI] [PubMed] [Google Scholar]
- 51. Ma XJ, Wang Z, Ryan PD. et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 2004; 5(6): 607–616. [DOI] [PubMed] [Google Scholar]
- 52. Sgroi DC, Carney E, Zarrella E. et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013; 105(14): 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sanft T, Aktas B, Schroeder B. et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015; 154(3): 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y, Schnabel CA, Schroeder BE. et al. Breast Cancer Index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013; 19(15): 4196–4205. [DOI] [PubMed] [Google Scholar]
- 55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T. et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016; 18: 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma XJ, Salunga R, Dahiya S. et al. A five-gene molecular grade index and HOXB13: iL17BR are complementary prognostic factors in early stage breast cancer. Clin Cancer Res 2008; 14(9): 2601–2608. [DOI] [PubMed] [Google Scholar]
- 57. Lundberg A, Lindstrom LS, Harrell JC. et al. Gene expression signatures and immunohistochemical subtypes add prognostic value to each other in breast cancer cohorts. Clin Cancer Res 2017; 23(24): 7512–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dowsett M, Turner N.. Estimating risk of recurrence for early breast cancer: integrating clinical and genomic risk. J Clin Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 59. Foukakis T, von Minckwitz G, Bengtsson NO. et al. Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: a randomized clinical trial. JAMA 2016; 316(18): 1888–1896. [DOI] [PubMed] [Google Scholar]
- 60. Del Mastro L, De Placido S, Bruzzi P. et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 2015; 385(9980): 1863–1872. [DOI] [PubMed] [Google Scholar]
- 61. Francis PA, Pagani O, Fleming GF. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018; 379(2): 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Swain SM, Jeong JH, Geyer CE Jr. et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010; 362(22): 2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murphy CC, Bartholomew LK, Carpentier MY. et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012; 134(2): 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Foukakis T, Bergh J, Prognostic and predictive factors in early, nonmetastatic breast cancer. Topic 782 Version 52.0 In Post T. (ed), UpToDate. Waltham, MA: UpToDate Inc; https://www.uptodate.com/contents/prognostic-and-predictive-factors-in-early-non-metastatic-breast-cancer?search=prognosis%20breast%20cancer&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2 (10 December 2018, date last accessed). [Google Scholar]