Abstract
Seventeen new flavone derivatives substituted at the 4′-OH position were designed, synthesized and evaluated for their anticancer and antibacterial activities. Among them, compounds 3, 4, 6f, 6e, 6b, 6c and 6k demonstrated the most potent antiproliferative activities against a human erythroleukemia cell line (HEL) and a prostate cancer cell line (PC3). The results also showed that the IC50 value of compounds 3, 4, 6f, 6e, 6b, 6c and 6k were close to that of the anticancer drug cisplatin (DDP) and lower than that of apigenin. All of the derivatives did not present antibacterial activities. The structure–activity relationships evaluation showed that the configuration of methyl amino acid might affect their biological activities.
Keywords: flavone, amino acid, anticancer activity, antiproliferation
1. Introduction
Cancer is ranked as the main cause of human death and the most important barrier to increasing life expectancy around the world. There were 18.1 million new cases and 9.6 million cancer deaths worldwide in 2018 [1]. Current cancer treatments mainly rely on surgery, chemotherapy, and radiotherapy, which cannot reduce recurrence and metastasis, as well as other limitations and drawbacks, such as severe side-effects, intolerance and increasing resistance. Hence, the need for developing new anticancer agents is growing [2].
Nowadays, phytochemicals have become an important part of anticancer drugs. Actually, over 75% of nonbiological anticancer drugs approved are either natural products or developed based on them [3]. Flavonoids, which are polyphenolic secondary metabolites mainly from plants and fungi, have diverse biological activities—especially anticancer activity through the regulation of different targets. For instance, some research results have shown flavonoids targeting protein kinase (PKC) [4], tankyrase (TNKS) [5], tyrosine kinases [6] and cyclin dependent kinases [7]. Therefore, flavone was chosen as the lead skeleton for further structural modification for discovering new anticancer drug candidates.
As far back as 1986, Zhao et al. [8] studied the structure–activity relationships of flavone and found that 4′-OH was important for inhibitory activity. Golub et al. [9] reported the design, synthesis and characterization of novel flavone 4′-OH derivatives, which showed better anticancer activities than the natural lead compound. Meanwhile, the 4′-OH could form a hydrogen bond with Asp175 and stacking interaction with Phe113, which further enhances their activities [9]. Additionally, Zhou et al. [10] designed a series of flavone derivatives with alkanes substituted in 4′-OH to generate ether.
The lipophilicity and hydrophilicity of an amino acid may have a large influence on the ester–water distribution coefficient of the target molecule, thereby retaining the possibility of a hydrogen bond interaction. Biological activity results showed that flavone binds the methyl amino acid at the 4′-position, which can increase its anticancer activities. Meanwhile, two alkyl amines (propylamine and n-butylamine) and 2-fluoroaniline were chosen to bond to flavone for comparison with methyl amino acid. Based on this analysis, a series of flavone derivatives were designed and synthesized by replacing the 4′-OH with N-amino substituents.
2. Results and Discussion
2.1. Chemistry
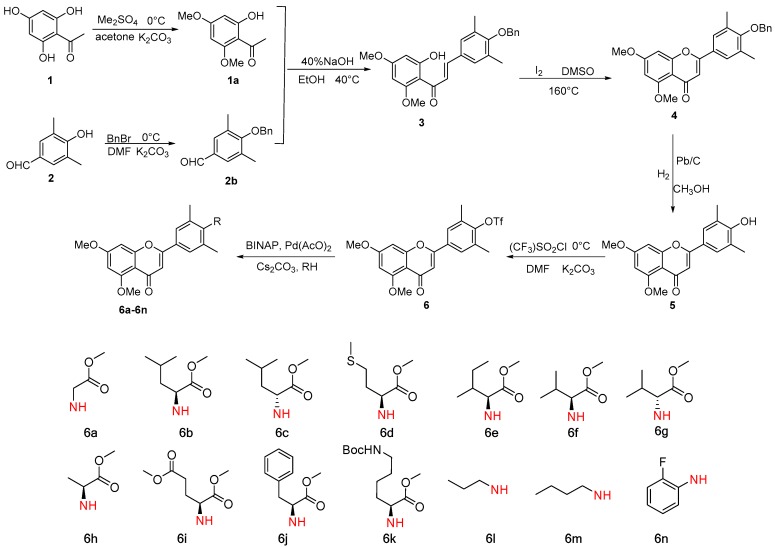
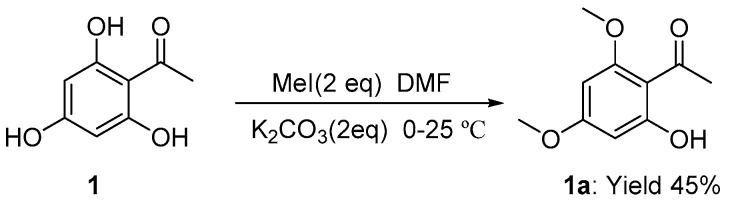
Seventeen new flavone derivatives (3, 4, 6, 6a–6n) were synthesized (Scheme 1). To begin with, commercially available 2,4,6-trihydroxyacetophenone was methylated with MeI in dried DMF (Scheme 2), resulting in the desired product in 45% yield. Then, the synthetic method was changed by using Me2SO4 as the methylation reagent instead of MeI, and only the target product was obtained in 98% yield [11]. The mixture was subsequently filtered and the pure product was obtained after removing the solvent under vacuum distillation (without further purification). By following a method analogous to that described in Reference [12,13], 2b was easily obtained. The intermediate chalcone 3 was achieved via aldol condensation between 1a and 2b at 40 °C with NaOH (aq 40%) as the base [14,15]. The temperature and NaOH concentration were the key issues to this transformation. Chalcone 3 was cyclized with I2 as a catalyst at 160 °C in DMSO to form compound 4 [15]. Compound 5 was synthesized by a known method with Pd/C (10%) as catalyst in MeOH [16]. With excess K2CO3 as a base, compound 6 could be prepared from 5 [17,18]. Compounds 6a–6n were synthesized from various amines and compound 6 by palladium catalyzed N-arylation reactions [17]. In summary, 17 new flavone derivatives (3, 4, 6, 6a–6n) were synthesized via several steps (Scheme 1).
Scheme 1.
Synthesis of flavone derivatives.
Scheme 2.
Synthesis of 1a.
2.2. Anticancer Bioactivity
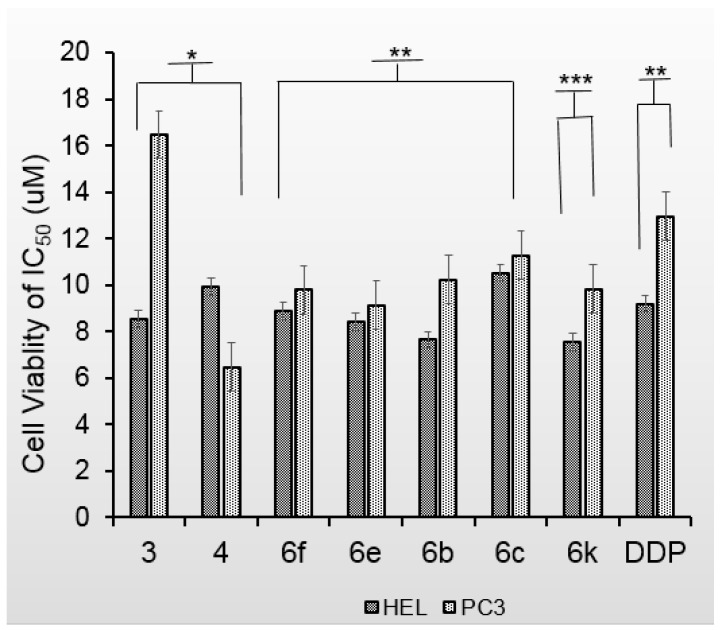
The 17 flavone derivatives were tested against HEL and PC3 cell lines. The IC50 values of the flavone derivatives against the growth of HEL and PC3 cell lines are shown in Table 1 and Figure 1.
Table 1.
IC50 values of the flavone derivatives against the growth of the human erythroleukemia cell line (HEL) and the prostate cancer cell line (PC3).
| Compd | IC50 (μM) | Compd | IC50 (μM) | ||
|---|---|---|---|---|---|
| HEL | PC3 | HEL | PC3 | ||
| 5 | >20 | >20 | 6n | >20 | >20 |
| 6 | >20 | >20 | 6k | 7.563 ± 0.844 | 9.836 ± 0.939 |
| 6a | >20 | >20 | 6b | 7.649 ± 0.837 | 10.242 ± 0.952 |
| 6d | >20 | >20 | 6e | 8.416 ± 0.888 | 9.140 ± 0.904 |
| 6g | >20 | >20 | 3 | 8.547 ± 0.932 | 16.471 ± 0.872 |
| 6h | >20 | >20 | 6f | 8.886 ± 0.872 | 9.795 ± 0.991 |
| 6i | >20 | >20 | 4 | 9.945 ± 0.930 | 6.473 ± 0.811 |
| 6j | >20 | >20 | 6c | 10.526 ± 0.992 | 11.266 ± 0.971 |
| 6l | >20 | >20 | Apigenin | >20 | >20 |
| 6m | >20 | >20 | cisplatin | 8.783 ± 0.818 | 11.873 ± 1.075 |
Figure 1.
Comparison of cell viability for the flavone derivatives against the growth of HEL and PC3 cell lines (* p < 0.01).
From the MTT assay after 48 h of treatment; the values are averaged from at least three independent experiments; variation ±10%.
Both apigenin and the anticancer drug cisplatin (DDP) were chosen as positive controls. As depicted in Table 1, compounds 3, 4, 6f, 6e, 6b, 6c, and 6k exhibited more potent effects against the growth of HEL and PC3 cell lines compared to that of apigenin. The results showed that 6e, 6b and 6k had better anticancer activities than the positive control DDP (Figure 1). Interestingly, the anticancer activities of compounds 3 and 4, which were protected with benzyloxy at 4′-OH, were greater than that of 5. This means it makes sense to modify the structure of flavone at 4′-OH. Four methyl amino acids (methyl l-leucinate, methyl d-leucinate, l-valine methyl ester, and d-valine methyl ester) were chosen to bond to flavone by comparing their activities against the growth of cancer cells. We found the activity of 6f was better than that of 6g, while 6b and 6c (D and L configuration, respectively) had similar IC50 values. These results indicate that the configuration of the methyl amino acid may have an effect on the biological activities.
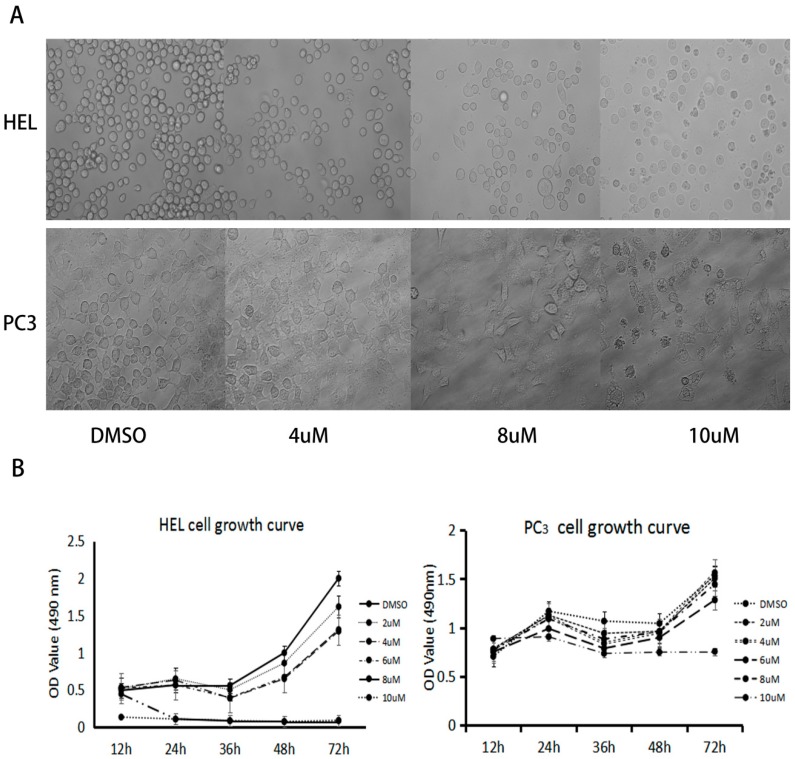
The cell growth curves following treatment by compound 6k are presented in Figure 2. Cell morphology was significantly changed after drug treatment; an increase in concentration and duration of 6k treatment led to an increase in the inhibition rate of cell growth for HEL and PC3 cells. When the time of drug treatment reached 36 h, the OD (absorbance values) value decreased slowly, meaning that the inhibition rate of cell growth increased gradually. This indicated that the biological activities of the flavone derivatives were dependent on time and concentration.
Figure 2.
Inhibition effect of 6k on proliferation of HEL and PC3. (A) Morphological changes of HEL and PC3 cells at different concentrations of 6k after 24 h of drug treatment, and (B) cytotoxic effects of 6k on HEL and PC3 cell proliferation at the indicated times.
Meanwhile, the 17 flavone derivatives were also tested against numerous bacteria species (Bacillus subtilis, Pseudomonas aeruginosa, anthraci, Staphylococcus aureus 6538, Staphylococcus aureus 43300, Staphylococcus aureus 25923, and Escherichia coli). The results showed that the derivatives did not have antibacterial activities.
3. Materials and Methods
3.1. Instruments and Materials
Reagents and solvents were purchased from commercial sources. Solvents were purified according to the guidelines in Purification of Laboratory Chemicals. Column chromatography was performed on silica gel (Huang Hai, 200–300 mesh) using the indicated eluents. A stock solution of 6k (20 µM) was prepared in DMSO (dimethyl sulfoxide) and stored in the refrigerator at −20 °C. MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide or thiazolyl blue tetrazolium bromide) was purchased from Solarbio (Shang Hai, China). Melting points were measured on SGW X-4 apparatus and were not corrected. 1H-NMR and 13C-NMR spectra were recorded on 400 MHz (Varian, Palo Alto, CA, USA) or 600 MHz (Bruker, Karlsruhe, Germany) spectrometers in appropriate solvents using TMS (tetramethylsilane) as the internal standard or the solvent signals as secondary standards. Multiplicities of the NMR signals were designated as s (singlet), d (doublet), t (triplet), q (quartet), br (broad), and m (multiplet). High-resolution mass spectra(HRMS) were obtained using Thermo Fisher QE Focus apparatus(USA).
3.2. Methods of Synthesis
3.2.1. Synthesis of 2,4-Dimethoxy-3-hydroxyacetophenone 1a
After K2CO3 (10.0 mmol, 2.0 eq) was added to a solution of 2,4,6-trihydroxyacetophenone 1 (5.0 mmol, 1.0 eq), the resulting solution was stirred in acetone at room temperature under argon. Then, Me2SO4 (10.0 mmol, 2.0 eq) was added dropwise and the temperature was slowly increased to 40 °C. The reaction was monitored by thin layer chromatography (TLC) after about 4 h. The mixture was then filtered and washed with acetone three times. The organic phase was evaporated to provide 1a (white powder; yield 98%). Other data can be found in Reference [11].
3.2.2. Synthesis of 3,5-Dimethyl-4-benzyloxybenzaldehyde 2b
K2CO3 (12.0 mmol, 2.0 eq) was added to a solution of 3,5-dimethyl-4-hydroxybenzaldehyde 2 (6.0 mmol, 1.0 eq) in DMF (dimethyl formamide) (40 mL), which was stirred at 0 °C. Then, BrBn (6.6 mmol, 1.1 eq) was added dropwise and the mixture was allowed to increase in temperature to room temperature. The end of reaction was monitored by TLC about 4.5 h, then water was added (50 mL). The solution was then extracted with EA (ethyl acetate) (20 mL × 3).The combined organic phase was washed with water (20 mL × 3) and brine (20 mL × 3), and dried over anhydrous Na2SO4 after filtration. The solvent was removed under reduced pressure to get the crude product, which was purified through flash column chromatography to afford compound 2b (white solid; yield 95%). Other data can be found in Reference [13].
3.2.3. Synthesis of 2′,4′-Dimethoxyl-5′-hydroxy-3,5-dimethyl-4-benzyloxychalcone 3
An aqueous solution of 40% NaOH (50 mmol, 10.0 eq) was added to a solution of 1a (5.0 mmol, 1.0 eq) and 2b (5.0 mmol, 1.0 eq) in EtOH (40 mL) at room temperature. After stirring for 4 h at 40 °C, the mixture was adjusted with 1 N HCl to pH 5 and filtered. The precipitate was recrystallized with EtOH to afford 3 (yellow solid; yield 70%). Melting point (mp): 120.6–121.4 °C; Log P: 5.52; 1H-NMR (400 MHz, CDCl3) δ(ppm): 14.40 (s, 1H, OH), 7.82 (d, J = 15.6 Hz, 1H, C=CH), 7.73 (d, J = 15.6 Hz, 1H, C=C–H), 7.50 (d, J = 6.8 Hz, 2H, Ar–H), 7.37–7.45 (m, 3H, Ar–H), 7.30 (s, 2H, Ar–H), 6.12 (d, J = 2.4 Hz, 1H, Ar–H), 5.98 (d, J = 2.4 Hz, 1H, Ar–H), 4.85 (s, 2H, ArCH2), 3.93 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 2.34 (s, 6H, ArCH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 192.6, 168.3, 166.0, 162.4, 157.6, 142.4, 137.2, 131.7, 131.2, 129.2, 128.5, 128.1, 127.8, 126.3, 106.3, 93.7, 91.2, 74.1, 55.9, 55.6, 16.6; HRMS (ESI) calcd. C26H27O5: m/z 419.1853 [M + H]+, found: 419.1850.
3.2.4. Synthesis of 5,7-Dimethoxy- 3′,5′-dimethyl-4′-benzyloxyflavone 4
A mixture of 3 (2.4 mmol, 1.0 eq) and iodine (0.024 mmol, 0.01 eq) in DMSO (30 mL) was refluxed for 4 h, then cooled down to room temperature and poured into water. The crude product was obtained after filtration and recrystallized in EA to get product 4 (white solid; yield 72%). Mp: 208.1–209.2 °C; Log P: 5.4; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.56 (s, 2H, Ar–H), 7.50 (d, J = 4.0 Hz, 2H, Ar–H), 7.43 (t, J = 4.0 Hz, 2H, Ar–H), 7.39 (t, J = 4.0 Hz, 1H, Ar–H), 6.62 (s, 1H, C=CH), 6.58 (d, J = 1.2 Hz, 1H, Ar–H), 6.37 (d, J = 1.2 Hz, 1H, Ar–H), 4.87 (s, 2H, ArCH2), 3.96 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 2.37 (s, 6H, ArCH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.7, 164.0, 160.9, 160.7, 159.9, 158.5, 137.1, 132.0, 128.6, 128.2, 127.9, 127.0, 126.7, 109.2, 108.5, 96.1, 92.8, 74.2, 56.4, 55.8, 16.7; HRMS (ESI) calcd. C26H25O5: m/z 417.1697 [M + H]+, found: 417.1695.
3.2.5. Synthesis of 5,7-Dimethoxy- 3′,5′-dimethyl-4′-hydroxyflavone 5
Compound 4 (2.4 mmol, 1.0 eq) and Pd/C (10%, 0.24 mmol, 0.1 eq) were added to a two-neck flask. The air in the flask was then exchanged by hydrogen after MeOH (50 mL) was added. The mixture was stirred at room temperature for 4 h before it was filtered and washed with heated MeOH (20 mL × 3). The MeOH was removed under reduced pressure to get product 5 (white solid, yield 95%). Mp: 206.3–207.1 °C; Log P: 3.4; 1H-NMR (600 MHz, DMSO-d6) δ(ppm): 9.03 (s, 1H, OH), 7.64 (s, 2H, Ar–H), 6.84 (d, J = 0.4 Hz, 1H, Ar–H), 6.57 (s, 1H, C=CH), 6.48 (d, J = 1.2 Hz, 1H, Ar–H), 3.9 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 2.24 (s, 6H, ArCH3); 13C-NMR (151 MHz, DMSO-d6) δ(ppm): 176.1, 164.0, 160.8, 160.6, 159.6, 157.0, 126.7, 125.2, 121.7, 108.7, 106.6, 96.7, 93.7, 56.5, 56.4, 17.1; HRMS (ESI) calcd. C19H19O5N: m/z 327.1227 [M + H]+, found: 327.1226.
3.2.6. Synthesis of 5,7-Dimethoxy-3′,5′-dimethyl-4′-trifluoromethanesulfonyloxyflavone 6
Compound 5 (3.0 mmol, 1.0 eq), K2CO3 (30.0 mmol, 10.0 eq) and anhydrous DMF (40 mL) were added into a two-neck flask under argon. After the mixture was cooled to 0 °C in an ice bath, trifluoromathanesulfonyl chloride was added dropwise. The reaction mixture was slowly warmed to room temperature and stirring was continued for 6 h before it was filtered and then poured into water (50 mL) and extracted with CH2Cl2 (20 mL × 3). The combined organic layers were washed with brine (20 mL), dried over anhydrous Na2SO4 and concentrated in a vacuum to get a crude product. The crude product was chromatographed on silica gel (EA/PE = 2/1) to get 6 (white solid; yield 60%). Mp: 265.5–266.2 °C; Log P: 4.87; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.60 (s, 2H, Ar–H), 6.62 (s, 1H, C=CH), 6.57 (d, J = 2.4 Hz, 1H, Ar–H), 6.37 (d, J = 1.8 Hz, 1H, Ar–H), 3.95 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 2.47 (s, 6H, CH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.2, 164.2, 160.9, 159.8, 159.0, 148.4, 132.5, 131.2, 127.4, 118.6 (q, J = 320.1 Hz, CF3) 109.7, 109.2, 96.3, 92.8, 56.4, 55.8, 17.4; HRMS (ESI) calcd. C20H18O7F3S: m/z 459.0720 [M + H]+, found: 459.0721.
3.2.7. Synthesis of 6a–6n
Toluene (10 mL) was added to a mixture of palladium acetate (0.02 mmol, 0.1 eq), BINAP((±)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene) (0.04 mmol, 0.2 eq), cesium carbonate (0.4 mmol, 2.0 eq), 6 (0.22 mmol, 2.2 eq) and amine (0.2 mmol, 1.0 eq) under argon. The mixture was stirred at reflux for 12 h. After cooling to room temperature and removing solvents, the mixture was extracted with DCM (dichloromethane) and washed with brine. The organic layer was dried over Na2SO4, followed by purification of the crude product by column chromatography on silica gel (DCM/MeOH = 100/1). This afforded compounds 6a–6n.
6a:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)glycinate: Yellow powder; yield 60%; mp: 134.3–135.7 °C; Log P: 2.82; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.49 (s, 2H, Ar–H), 6.56 (s, 2H, Ar–H and C=CH), 6.35 (s, 1H, Ar–H), 4.26 (s, 1H, NH), 3.94 (s, 5H, OCH3 and COCH2), 3.91 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 2.38 (s, 6H, ArCH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.7, 172.4, 163.8, 161.1, 160.8, 159.9, 148.9, 127.6, 126.8, 123.7, 109.2, 107.4, 96.0, 92.8, 56.4, 55.7, 52.4, 49.3, 19.1; HRMS (ESI) calcd. C22H24O6N: m/z 398.1598 [M + H]+, found: 398.1596.
6b:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-leucinate: Yellow powder; yield 55%; mp: 192.7–193.3 °C; Log P: 4.55; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.55–6.56 (m, 1H, Ar–H and C=CH), 6.34 (d, J = 2.0 Hz, 1H, Ar–H), 6.36 (d, J = 2.0 Hz, 1H, Ar–H), 4.10–4.15 (m, 1H, NHCH), 3.93 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 2.36 (s, 6H, ArCH3), 1.61–1.80 (m, 3H, CH2CH), 0.97 (t, J = 7.2 Hz, 6H, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 177.7, 175.1, 163.7, 161.0, 160.8, 159.8, 147.4, 128.1, 126.8, 123.8, 109.2, 107.4, 95.9, 92.7, 57.7, 56.3, 55.7, 51.9, 43.6, 24.9, 22.8, 22.5, 19.2; HRMS (ESI) calcd. C26H32O6N: m/z 454.2224 [M + H]+, found: 454.2223.
6c:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-d-leucinate: Yellow powder; yield 59%; mp: 166.1–167.4 °C; Log P: 4.55; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.57 (s, 1H, C=CH), 6.56 (d, J = 2.0 Hz, 1H, Ar–H), 6.35 (d, J = 2.0 Hz, 1H, Ar–H), 4.13 (t, J = 6.8 Hz, 1H, NHCH), 3.93 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 2.37 (s, 6H, ArCH3), 1.60–1.77 (m, 3H, CH2CH), 0.98 (t, J = 7.2 Hz, 6H, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 177.7, 175.1, 163.8, 161.1, 160.7, 159.8, 147.4, 128.1, 126.9, 123.8, 109.1, 107.3, 95.9, 92.7, 57.7, 56.3, 55.7, 51.9, 43.6, 24.9, 22.8, 22.5, 19.2; HRMS (ESI) calcd. C26H32O6N: m/z 454.2224 [M + H]+, found: 454.2223.
6d:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-methioninate: Yellow powder; yield 40%; mp: 142.0–143.1 °C; Log P: 4.87; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.49 (s, 2H, Ar–H), 6.56–6.57 (m, 2H, Ar–H and C=CH), 6.36 (d, J = 4.0 Hz, 1H, Ar–H), 4.26 (s, 1H, NH), 3.96–4.09 (m, 1H, NHCH), 3.94 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 2.64 (t, J = 8.0 Hz, 2H, SCH2), 2.39 (s, 6H, ArCH3), 2.09 (s, 3H, SCH3), 2.00–2.12 (m, 2H, CHCH2); 13C-NMR (100 MHz, CDCl3) δ(ppm):177.7, 174.4, 163.8, 161.0, 160.7, 159.8, 147.1, 128.2, 126.8, 123.9, 109.1, 107.4, 95.9, 92.7, 57.8, 56.3, 55.7, 52.1, 30.1, 29.6, 19.2, 15.4; HRMS (ESI) calcd. C25H30O6NS: m/z 472.1788 [M + H]+, found: 472.1791.
6e:Methyl (2S)-2-((4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)amino)-3-methylpentanoate: Yellow powder; yield 45%; mp: 151.3–153.4 °C; Log P: 5.84; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.43 (s, 2H, Ar–H), 6.53 (s, 1H, C=C–H), 6.52 (d, J = 1.8 Hz, 1H, Ar–H), 6.30 (d, J = 2.4 Hz, Ar–H), 4.01–4.05 (m, 2H, NHCH), 3.90 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 2.34 (s, 6H, ArCH3), 1.78–1.85 (m, 1H, CH3CH), 1.68–1.72 (m, 1H, CH3CH2), 1.51-1.59 (m, 1H, CH3CH2), 0.90–0.97 (m, 6H, CHCH3 and CH2CH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.8, 174.2, 163.8, 161.2, 160.8, 159.8, 147.5, 127.8, 127.0, 123.5, 109.2, 107.2,, 96.0, 92.8, 63.2, 56.3, 55.7, 51.7, 39.0, 25.9, 19.3, 15.2, 11.7; HRMS (ESI) calcd. C26H32O6N: m/z 454.2224 [M + H]+, found: 454.2225.
6f:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-valinate: Yellow powder; yield 55%; mp: 172.6–173.3 °C; Log P: 5.42; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.55-6.56 (m, 2H, Ar–H and C=CH), 6.34 (d, J = 2.0 Hz, 1H, Ar–H), 4.03–4.06 (m, 1H, COCH), 3.94 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 2.37 (s, 6H, ArCH3), 2.04–2.12 (m, 1H, (CH3)2CH), 1.13 (d, J = 7.2 Hz, 3H, CH3), 1.01 (d, J = 7.2 Hz, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 177.7, 174.3, 163.7, 161.1, 160.7, 159.8, 147.4, 127.8, 126.9, 123.5, 109.2, 107.3, 95.9, 92.7, 64.5, 56.3, 55.7, 51.7, 32.2, 19.3, 18.9, 18.7; HRMS (ESI) calcd. C25H30O6N: m/z 440.2068 [M + H]+, found: 440.2065.
6g:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-d-valinate: Yellow powder; yield 53%; mp: 166.8–168.4 °C; Log P: 4.2; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.55–6.56 (m, 2H, Ar–H and C=CH), 6.35 (d, J = 2.4 Hz, 1H, Ar–H), 4.03–4.06 (m, 1H, NHCH), 3.94 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 2.37 (s, 6H, ArCH3), 2.04–2.12 (m, 1H, (CH3)2CH), 1.13 (d, J = 7.2 Hz, CH3), 1.01 (d, J = 7.2 Hz, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 177.7, 174.3, 163.7, 161.1, 160.7, 159.8, 147.4, 127.8, 126.9, 123.5, 109.0, 107.3, 95.9, 92.7, 64.5, 56.3, 55.7, 51.7, 32.2, 19.2, 18.9, 18.7; HRMS (ESI) calcd. C25H30O6N: m/z 440.2068 [M + H]+, found: 440.2066.
6h:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-alaninate: Yellow powder; yield 55%; mp: 183.2–184.0 °C; Log P: 3.31; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.49 (s, 2H, Ar–H), 6.58 (s, 1H, C=CH), 6.56 (d, J = 1.8 Hz, 1H, Ar–H), 6.35 (d, J = 2.4 Hz, 1H, Ar–H), 4.15 (dd, J = 6.6, 13.8 Hz, 1H, COCH), 3.94 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 2.37 (s, 6H, ArCH3), 1.42 (d, J = 7.2 Hz, 3H, CH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.8, 175.5, 163.9, 161.2, 160.9, 159.9, 147.4, 128.5, 126.9, 124.0, 109.2, 107.5, 96.0, 92.8, 56.4, 55.8, 54.7, 52.2, 19.9, 19.2; HRMS (ESI) calcd. C23H26O6N: m/z 412.1755 [M + H]+, found: 412.1753.
6i:Dimethyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-glutamate: Yellow powder; yield 10%; mp: 123.3–124.0 °C; Log P: 3.08; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.56 (s, 1H, C=CH), 6.55 (d, J = 2.4 Hz, 1H, Ar–H), 6.35 (d, J = 2.4 Hz, 1H, Ar–H), 4.14 (s, 1H), 3.94 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.69 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 2.49–2.55 (m, 2H, CHCH2), 2.36 (s, 6H, ArCH3), 2.10–2.14 (m, 2H, COCH2); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.8, 174.4, 173.2, 163.9, 161.1, 160.9, 159.9, 147.1, 128.4, 127.0, 124.1, 109.3, 107.6, 96.0, 92.8, 58.3, 56.4, 55.8, 52.2, 51.9, 30.1, 28.8, 19.2; HRMS (ESI) calcd. C26H30O8N: m/z 484.1966 [M + H]+, found: 484.1959.
6j:Methyl (4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-phenylalaninate: Yellow powder; yield 60%; mp: 174.1–175.9 °C; Log P: 4.99; 1H-NMR (400 MHz, CDCl3) δ(ppm): 7.46 (s, 2H, Ar–H), 7.25–7.32 (m, 3H, Ar–H), 7.14 (d, J = 7.2 Hz, Ar–H), 6.57 (s, 1H, C=CH), 6.56 (d, J = 2.4 Hz, 1H, Ar–H), 6.35 (d, J = 2.0 Hz, 1H, Ar–H), 4.34 (t, J = 6.4 Hz, 1H, COCH), 3.93 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.60 (s, 3H, COOCH3), 3.10 (m, 2H, ArCH2), 2.23 (s, 6H, ArCH3); 13C-NMR (100 MHz, CDCl3) δ(ppm): 177.7, 174.1, 163.8, 161.0, 160.7, 159.8, 147.1, 136.1, 129.3, 128.4, 128.1, 127.0, 126.8, 123.7, 109.1, 107.3, 96.0, 92.7, 60.2, 56.3, 55.7, 51.9, 40.0, 19.1; HRMS (ESI) calcd. C29H30O6N: m/z 488.2068 [M+H]+, found: 488.2068.
6k:Methyl N6-(tert-butoxycarbonyl)-N2-(4-(5,7-dimethoxy-4-oxo-4H-chromen-2-yl)-2,6-dimethylphenyl)-l-lysinate: Yellow powder; yield 60%; mp: 92.4–94.4 °C; Log P: 4.45, 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.44 (s, 2H, Ar–H), 6.52–6.53 (m, 2H, Ar–H and C=CH), 6.31 (d, J = 1.8 Hz, 1H, Ar–H), 4.61 (s, 1H, NH), 4.05–4.07 (m, 1H, NHCH), 4.0 (br, 1H, CONH), 3.90 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 3.08–3.10 (m, 2H, CONHCH2), 2.33 (s, 6H, CH3), 1.76–1.79 (m, 2H, COCHCH2), 1.36–1.51 (m, 4H, CH2CH2), 1.40 (s, 9H, C(CH3)3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.7, 174.8, 163.8, 161.1, 160.8, 159.8, 156.0, 147.4, 128.2, 126.9, 123.8, 109.2, 107.4, 96.0, 92.8, 79.1, 59.0, 56.4, 55.7, 52.0, 40.2, 33.7, 29.9, 28.4, 22.8, 19.2; HRMS (ESI) calcd. C31H41O8N2: m/z 569.2857 [M + H]+, found: 569.2856.
6l:2-(3,5-dimethyl-4-(propylamino)phenyl)-5,7-dimethoxy-4H-chromen-4-one: Yellow powder; yield 62%; mp: 151.5–152.6 °C; Log P: 4.11; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.49 (s, 2H, Ar–H), 6.54 (s, 2H, Ar–H and C=CH), 6.32 (d, J = 1.6 Hz, 1H, Ar–H), 3.91 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 3.09 (t, J = 7.2 Hz, 2H, NHCH2), 2.31 (s, 6H, ArCH3), 1.54–1.60 (m, 2H, CH3CH2), 0.96 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.8, 163.8, 161.3, 160.8, 159.9, 149.7, 127.5, 126.8, 122.8, 109.2, 107.1, 96.0, 92.8, 56.4, 55.7, 49.9, 24.4, 19.2, 11.5; HRMS (ESI) calcd. C22H26O4N: m/z 368.1856 [M + H]+, found: 368.1856.
6m:2-(4-(butylamino)-3,5-dimethylphenyl)-5,7-dimethoxy-4H-chromen-4-one: Yellow powder; yield 70%; mp: 143.9–145.2 °C; Log P: 4.53; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.47 (s, 2H, Ar–H), 6.55–6.56 (m, 2H, Ar–H and C=CH), 6.34 (d, J = 2.4 Hz, Ar–H), 3.93 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.14 (t, J = 7.2 Hz, 2H, NHCH2), 2.32 (s, 6H, ArCH3), 1.53–1.58 (m, 2H, CH2), 1.37–1.43 (m, 2H, CH2), 0.94 (t, J = 7.8 Hz, CH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.8, 163.8, 161.3, 160.8, 159.9, 149.8, 127.5, 126.8, 122.9, 109.3, 107.1, 96.0, 92.8, 56.4, 55.7, 47.8, 33.4, 20.2, 19.3, 13.9; HRMS (ESI) calcd. C23H28O4N: m/z 382.2013 [M + H]+, found: 382.2012.
6n:2-(4-((2-fluorophenyl)amino)-3,5-dimethylphenyl)-5,7-dimethoxy-4H-chromen-4-one: Yellow powder; yield 70%; mp: 125.9–130.1 °C; Log P: 5.42; 1H-NMR (600 MHz, CDCl3) δ(ppm): 7.61 (s, 2H, Ar–H), 7.05–7.08 (m, 1H, Ar–H), 6.87 (t, J = 4.2 Hz, 1H), 6.70–6.73 (m, 1H, Ar–H), 6.63 (s, 1H, C=CH), 6.57 (d, J = 2.4 Hz, Ar–H), 6.36 (d, J = 2.4 Hz, Ar–H), 6.23–6.26 (m, 1H, Ar–H), 5.45 (s, 1H, NH), 3.94 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 2.26 (s, 6H, ArCH3); 13C-NMR (151 MHz, CDCl3) δ(ppm): 177.7, 164.1, 160.9, 160.6, 159.9,151.8 (d, J = 293.64 Hz), 140.5, 136.0,133.5 (d, J = 11.02 Hz), 128.5,126.3, 124.4 (d, J = 3.47 Hz), 118.6 (d, J = 6.64 Hz), 115.0 (d, J = 18.42 Hz), 113.9 (d, J = 1.96 Hz), 109.2, 108.6, 96.2, 92.8, 56.4, 55.8, 18.6; HRMS (ESI) calcd. C25H23O4NF: m/z 420.1606 [M + H]+, found: 420.1603.
3.3. Method of Bioactivity Study
3.3.1. Cell Lines and Cell Culture
The human leukemic cell line (HEL) and the prostate cancer cell line (PC3) were obtained from Molecular and Cell Biology Research, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada. All cells were cultured in RPMI medium (high glucose) supplemented with 5% fetal bovine serum (FBS; HyClone, GE Healthcare, Australia) and maintained in a humidified incubator with 5% CO2 at 37 °C. When the growing cells reached approximately 60–80% confluence, they were treated with 6k. DMSO was used as the control.
3.3.2. Cell Viability Assay
The cytotoxicity of 6k on HEL and PC3 was measured by the MTT method [19]. The cells were plated at a density of 1 × 104/well in a 96-well plate and incubated at 37 °C for 24 h. The cells were then treated with the compounds of interest for 48 h. After 20 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) was added to each well, the cells were incubated for 4 h. The cells were then removed from the medium and DMSO (100 mL) was added. The cell viability was detected by measuring the absorbance at 490 nm on a plate reader (Bio Tek, Winooski, VT, USA). All experiments were repeated at least three times.
4. Conclusions
Seventeen new flavone derivatives were synthesized via N-amination in the 4′-position. The in vitro tumor growth inhibitory activities of all of the derivatives were assayed using the human cell lines HEL and PC3. In general, compounds 3, 4, 6f, 6e, 6b, 6c and 6k demonstrated the most potent antiproliferative activities against the HEL and PC3 cell lines. Preliminary structure–activity relationship studies indicated that the flavone bind the methyl amino acid at the 4′-position, thereby increasing the anticancer activity. Our findings suggested that the configuration of the methyl amino acid had some effects on the biological activity of the derivatives. These results provide new insight into developing flavonoid-derived anticancer agents. The antibacterial test showed that all of the derivatives did not possess obvious antibacterial activities.
Author Contributions
C.C. and W.-D.P. conceived and designed the experiments; J.Y. and N.Z. performed the biology experiment and revised paper; J.L. and S.Y. performed part of chemical experiment; J.-R.S. contributed reagents/materials; C.C. and K.L. wrote the paper.
Funding
This work was supported by the Science and Technology Department of Guizhou Province, China (QKHZC[2019]2786; QKHJC[2018]1108; QKHRC[2016]4037 and QKHPTRC[2017]5737; the National Natural Science Foundation of China (81660580, 81702914).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;686:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Weerink L.B., Gant C.M., Leeuwen B.L.V., De Bock G.H., Kouwenhoven E.A., Faneyte I.F. Long-term survival in octogenarians after surgical treatment for colorectal cancer: Prevention of postoperative complications is key. Ann. Surg. Oncol. 2018;25:3874–3882. doi: 10.1245/s10434-018-6766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the Last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L.L., Yuan X.Y., Wang J.B., Feng Y.F., Fei J., Li Z.Y., Bian J.L. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg. Med. Chem. 2019;27:677–685. doi: 10.1016/j.bmc.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Alam S., Khan F. 3D-QSAR, Docking, ADME/Tox studies on Flavone analogs reveal anticancer activity through tankyrase inhibition. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-41984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sedlacek H.H., Czech J., Naik R., Kaur G., Worland P., Losiewicz M., Parker B., Carlson B., Smith A., Sendeerowicz A., et al. Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. Int. J. Oncol. 1996;9:1143–1168. doi: 10.3892/ijo.9.6.1143. [DOI] [PubMed] [Google Scholar]
- 7.Mark C., Dhanapalan N., Robert L.G. Synthesis and evaluation of hydroxylated flavones and related compounds as potential inhibitors of the protein-tyrosine kinase P56lck. J. Nat. Prod. 1991;54:1345–1352. doi: 10.1021/np50077a018. [DOI] [PubMed] [Google Scholar]
- 8.Ferriola P.C., Cody V., Middleton E. Protein kinase C inhibition by plant flavoneoids kinetic mechanisms and structure-activity relationships. Biochem. Pharmacol. 1989;38:1617–1624. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 9.Golub A.G., Bdzhola V.G., Ostrynska O.V., Kyshenia I.V., Sapelkin V.M., Prykhod’ko A.O., Kukharenko O.P., Yarmoluk S.M. Discovery and characterization of synthetic 4′-hydroxyflavones-New CK2 inhibitors from flavone family. Bioorg. Med. Chem. 2013;21:6681–6689. doi: 10.1016/j.bmc.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Brasier A.R., Tian B., Liu Z.Q., Chen H.Y., Rytting E. Inhibitors of Bromodomain-Containing Protein 4(BRD4) Priority Paragraph. WO2018/112037. U.S. Patent. 2018
- 11.Obreque-Balboa J.E., Sun Q., Bernhardt G., Konig B., Buschauer A. Flavonoid derivatives as selective ABCC1 modulators: Synthesis and functional characterization. Eur. J. Med. Chem. 2016;109:124–133. doi: 10.1016/j.ejmech.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Lee S., Kim J.N., Lee H.K., Yoon K.S., Shin K.D., Kwon B.M., Han D.C. Biological evaluation of KRIBB3 analogs as a microtubule polymerization inhibitor. Bioorg. Med. Chem. Lett. 2011;21:977–979. doi: 10.1016/j.bmcl.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Stacko P., Solomek T., Klan P. Electronic-state switching strategy in the photochemical synthesis of indanones from o-methyl phenacyl epoxides. Org. Lett. 2011;13:6556–6559. doi: 10.1021/ol202892r. [DOI] [PubMed] [Google Scholar]
- 14.Chen C., Li X., Hu X.F., Li Y., Yin S.F. Synthesis and Calm Activity of Quinoline and Flavone Derivatives of Helicid. Chin. J. Org. Chem. 2011;31:1878–1883. [Google Scholar]
- 15.Cabrera M., Simoens M., Falchi G., Lavaggi M.L., Piro O.E., Castellano E.E., Vidal A., Azqueta A., Monge A., Cerain A.L., et al. Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure-activity relationships. Bioorg. Med. Chem. 2007;15:3356–3367. doi: 10.1016/j.bmc.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Zaveri N.T. Synthesis of a 3,4,5-Trimethoxybenzoyl Ester Analogue of Epigallocatechin-3-gallate (EGCG): A potential route to the natural product green tea catechin, EGCG. Org. Lett. 2001;3:843–846. doi: 10.1021/ol007000o. [DOI] [PubMed] [Google Scholar]
- 17.Zhu S.F., Wang C., Chen L.J., Liang R.X., Yu Y.F., Jiang H.F. Modular approach for synthesis of vicinal diamines containing axial chiral 1,10-Binaphthyl from 1,2-Diaminoethane byPd-Catalyzed N-Arylation Reactions. Org. Lett. 2011;13:1146–1149. doi: 10.1021/ol103169z. [DOI] [PubMed] [Google Scholar]
- 18.Zou Y.J., Qin L.N., Ren X.F., Lu Y.P., Li Y.X., Zhou J.R. Selective arylation and vinylation at the a position of vinylarenes. Chem. Eur. J. 2013;19:3504–3511. doi: 10.1002/chem.201203646. [DOI] [PubMed] [Google Scholar]
- 19.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]