Abstract
Background: essential oils are well known for their pharmacological effectiveness as well as their repellent, insecticide, and herbicide activities. The emergence of resistant weeds, due to the overuse of synthetic herbicides, makes it necessary to find natural alternatives for weed control. The aim of this study was to evaluate the phytotoxic effects of Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris, three common commercial essential oils, on weeds (Portulaca oleracea, Lolium multiflorum, and Echinochloa crus-galli), food crops (tomato and cucumber), and the invasive species Nicotiana glauca. Methods: to determine herbicidal effects, essential oils were tested at different concentrations (0.125–1 µL/mL). The index of germination and seedling length data were recorded over 14 days. Results: the in vitro assays showed that L. angustifolia with linalool (38.7 ± 0.1%), 1,8-cineole (26.5 ± 0.1%), and camphor (14.2 ± 0.1%) as the main compounds showed the most phytotoxic effects affecting seed germination in weeds and tomato, and the aforementioned invasive species. L. multiflorum was the most sensitive weed, particularly to lavender essential oil, which decreased the growth of its hypocotyl and radicle by 87.8% and 76.7%, respectively, at a dose of 1 µL/mL. Cucumber was the most resistant food crop, with no significant reduction observed in seed germination and hypocotyl growth with E. citriodora and L. angustifolia essential oils. Conclusions: lavender essential oil represents a promising candidate for the development of effective and safe herbicides in the management of L. multiflorum affecting cucumber crops.
Keywords: E. citriodora, L. angustifolia, P. sylvestris, essential oils, GC–MS, phytotoxicity
1. Introduction
The particular characteristics of essential oils—natural mixtures of volatile compounds—provide them with certain pharmacological properties, including their well-known antibacterial, antioxidant, anti-inflammatory, and cancer chemoprotective effects, as well as their repellent, herbicidal, and insecticidal biological activities [1,2,3,4], which have led to valuable applications in human health, food, and cosmetics industries, and in environment and agriculture. Certain essential oils have already demonstrated their influence on both the seed germination and seedling growth of weeds [5,6]. In this regard, origanum (Origanum vulgare L.) essential oil with carvacrol as its main compound has exhibited a significant inhibitory effect against seed germination and seedling growth of common purslane, Italian ryegrass, and barnyardgrass at a range of concentrations (0.125–1 µL/mL), as well as against Sinapsis avensis at 2 µL/mL and also Johnson grass (Sorghum halepense L.) [7,8,9]. P. sylvestris exhibited some inhibition of the early root growth of Cassia occidentalis (L.) Link. [10], and E. citriodrora essential oil affected the development of certain weeds, particularly the seed germination of Amaranthus viridis L. [11]. Among weeds, the following deserve special attention: (i) common purslane (Portulaca oleracea L.), an annual weed commonly affecting cultivated land, protected agriculture, forests, plantations, and orchards, where it competes for resources with many field crops, including cruciferous crops, potato, and tomato, among others [12]; (ii) Italian ryegrass (Lolium multiflorum Lam.), an annual to biennial poaceous species largely spread globally due to its cultivation as a pasture grass [13], which has developed considerable resistance against glyphosate and other synthetic herbicides as an acetolactate synthase (ALS) inhibitor [14,15]; and (iii) barnyardgrass (Echinochloa crus-galli (L.) Beauv.), considered one of the world’s worst weeds, affecting agricultural land and grasslands as well as irrigation channels and wetlands, being, in fact, a very serious weed in rice crops [16]. In addition, other species, such as Nicotiana glauca Graham, native to South America and naturalized in several countries, have a high invasion potential to disturb ecosystems and reduce native biodiversity, growing on roadsides and lakeshores and becoming a problem in relatively dry areas [17,18]. Several studies are necessary to find efficient and sustainable alternatives to synthetic herbicides, whose persistent use has led to the arousal of multiple problems [5], such as the appearance of resistant weeds and toxicity in humans and other living organisms, as well as the persistence of residues in the environment that affect soil, air, the surrounding environment, ground water and crops [19,20,21]. The development of natural herbicides based on essential oils could decrease these negative impacts, mainly by counteracting resistant weeds, since it is difficult to develop resistance using mixtures of natural components with different mechanisms of action. In this sense, agricultural compositions, including oregano essential oil together with others also belonging to the Lamiaceae family such as Lavandula, Mentha, Rosmarinus, and Salvia species, have been elaborated as natural pesticides [22]. Similarly, lemongrass essential oil (Cymbopogon citratus, Poaceae) has been included as a principal ingredient in a natural herbicide invention to control the germination and growth of weeds [23].
On the other hand, it is interesting to demonstrate the selectivity of these eco-friendly active components against weeds and/or invasive species, thereby confirming their harmlessness over food crops. Previous studies demonstrated that winter savory (Satureja montana L.) essential oil is effective in the management of P. oleracea, L. multiflorum, and E. crus-galli, without being pernicious to the food crops maize, rice, and tomato. Similarly, peppermint (Mentha piperita L.) essential oil could be used to control L. multiflorum in rice (Oryza sativa L.) [24]. E. citriodrora essential oil affected seed germination in Amaranthus viridis L., without harming the food crops commonly affected by the weed Triticum aestivum L., Zea mays L., and Raphanus sativus L. [11]. However, this essential oil also produced a cytotoxic effect against food crops such as Lactuca sativa L. [25], and other essential oils, such as wintergreen (Gaultheria procumbens L.) essential oil with methyl salicylate (99.6%) as the main compound, could be employed in the control of the invasive species Cortaderia seollana (Schult. & Schult. f.) Asch. & Graebn, and Nicotiana glauca Graham [26].
The tested essential oils have been selected for their pharmacological or biological properties as well as for their chemical profile. Regarding this, Eucalyptus citriodora L. essential oil showed moderate antioxidant action, potent antimicrobial activity against bacteria and yeasts [27,28], and insect-repellent capacity when included in insect-repellent compositions [29]. Recently, citronellal, the main component of E. citriodora essential oil, has been encapsulated individually in different types of cyclodextrins to maintain its properties for longer [30]. Lavender (Lavandula angustifolia Mill.) essential oil and its main compounds 1,8-cineole and linalool have also shown antimicrobial potential, with synergistic effects with other common antimicrobial agents [31,32,33]. The antibacterial activity of L. angustifolia essential oil can be improved by being embedded with cyclodextrin, because this increases its water solubility and reduces its volatility [34]. The antimicrobial activity of Pinus sylvestris L. essential oil has been also well-established. In addition, P. sylvestris essential oil has shown a higher insect larvicidal potential against Drosophila melanogaster Meigen than other Pinus species, such as P. peuce, P. nigra subsp. nigra and P. musco subsp. musgo [35,36]. The high antimicrobial activity may be due to α- and β-pinene, the major compounds in P. sylvestris essential oil, which have already shown their antibacterial and antifungal potential. Indeed, both pinenes have been combined with commercial antimicrobials resulting in a reduction of their minimum inhibitory concentration and toxicity [37].
Since the phytotoxic effects differ remarkably with the chemical composition and the chemical composition of an essential oil depending on certain intrinsic and extrinsic factors [38] such as the extraction method [39], geographic location [40,41,42], temperature, and drying period, as well as harvesting time [43,44], the aims of this study were: (i) to determine through Gas Chromatography–Mass Spectrometry analysis the chemical composition of commercial Eucalyptus citriodora Hook, Lavandula angustifolia Mill., and Pinus sylvestris L. essential oils; (ii) to test the in vitro phytotoxic activity of these essential oils against the seed germination and seedling growth of the weeds P. oleracea, L. multiflorum and E. crus-galli, to evaluate their herbicidal activity, as well as on food crops such as tomato (Solanum lycopersicum L.) and cucumber (Cucumis sativus L.), to know its harmful effects on crops; and (iii) to test the same against the invasive species Nicotiana glauca Graham, potential reservoir of important viruses, including cucumber mosaic virus and tomato infectious chlorosis virus, which causes economic losses for commercial tomato production.
2. Results and Discussion
2.1. Chemical Composition of E. citriodora, L. angustifolia, and P. sylvestris Essential Oils
Twenty-seven (98.6%), 60 (97.6%) and 38 (99.1%) compounds in commercial E. citriodora, L. angustifolia, and P. sylvestris essential oils, respectively, were identified by Gas Chromatography–Mass Spectrometry analysis. Components were clustered (Table 1) in a homologous series of monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, oxygenated diterpenes, aromatic compounds, and others, and listed according to Kovat’s retention index [45] calculated in GC on an apolar HP-5MS column.
Table 1.
Chemical compositions of commercial E. citriodora, L. angustifolia, and P. sylvestris essential oils.
| RICal | RIRef | Compound |
E. citriodora Relative Area (%) |
L. angustifolia Relative Area (%) |
P. sylvestris Relative Area (%) |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 1.5 ± 0.1 | 7.8 ± 0.1 | 74.4 ± 0.3 | ||
| 924 | 926 | Tricyclene | - | t | 0.1 ± 0.0 |
| 926 | 930 | α-Thujene | t | - | - |
| 939 | 939 | α-Pinene | 0.2 ± 0.0 | 2.5 ± 0.0 | 25.6 ± 0.2 |
| 953 | 954 | Camphene | - | 0.7 ± 0.0 | 6.4 ± 0.1 |
| 977 | 975 | Sabinene | t | 0.3 ± 0.0 | - |
| 985 | 979 | β-Pinene | 0.5 ± 0.0 | 2.4 ± 0.0 | 15.9 ± 0.1 |
| 980 | 987 | 3-p-Menthene | - | - | 0.2 ± 0.0 |
| 998 | 990 | Myrcene | 0.1 ± 0.0 | 0.5 ± 0.0 | 3.5 ± 0.0 |
| 1012 | 1011 | δ-3-Carene | - | - | 0.6 ± 0.0 |
| 1020 | 1017 | α-Terpinene | t | 0.1 ± 0.0 | 2.3 ± 0.0 |
| 1021 | 1024 | p-Cymene | t | 0.5 ± 0.0 | 0.9 ± 0.0 |
| 1028 | 1029 | Limonene | t | - | 18.5 ± 0.2 |
| 1043 | 1037 | cis-Ocimene | - | 0.1 ± 0.1 | - |
| 1053 | 1050 | trans-β-Ocimene | 0.1 ± 0.0 | 0.1 ± 0.0 | - |
| 1056 | 1059 | γ-Terpinene | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| 1090 | 1088 | Terpinolene | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Oxygenated monoterpenes | 94.7 ± 1.2 | 85.5 ± 0.1 | 23.4 ± 0.3 | ||
| 1029 | 1031 | 1,8-Cineole | 0.3 ± 0.0 | 26.5 ± 0.0 | 2.1 ± 0.2 |
| 1051 | 1056 | Bergamal | 0.1 ± 0.0 | - | - |
| 1070 | 1070 | cis-Sabinene Hydrate | - | 0.2 ± 0.0 | - |
| 1076 | 1072 | cis-Linalool Oxide | - | 0.1 ± 0.0 | - |
| 1095 | 1096 | Linalool | 0.1 ± 0.0 | 38.7 ± 0.1 | t |
| 1098 | 1099 | α-Pinene Oxide | - | - | 0.1 ± 0.0 |
| 1104 | 1108 | cis-Rose Oxide | 0.1 ± 0.0 | - | - |
| 1122 | 1125 | trans-Rose Oxide | t | - | - |
| 1129 | Plinol C | - | 0.4 ± 0.1 | - | |
| 1144 | 1146 | Camphor | - | 14.2 ± 0.1 | 0.5 ± 0.0 |
| 1150 | 1149 | Isopulegol | 4.3 ± 1.1 | - | - |
| 1154 | 1153 | Citronellal | 88.0 ± 0.8 | - | - |
| 1158 | 1159 | iso-Isopulegol | 0.5 ± 0.1 | - | - |
| 1159 | 1160 | Isoborneol | - | 0.4 ± 0.0 | - |
| 1168 | 1166 | δ-Terpineol | - | 0.3 ± 0.0 | - |
| 1170 | 1169 | Borneol | - | 1.3 ± 0.0 | - |
| 1179 | 1177 | Terpinen-4-ol | - | 0.3 ± 0.0 | t |
| 1184 | 1182 | p-Cymen-8-ol | - | 0.1 ± 0.0 | - |
| 1187 | 1185 | Cryptone | - | t | - |
| 1188 | 1188 | α-Terpineol | - | 1.6 ± 0.0 | 0.1 ± 0.0 |
| 1196 | 1195 | Myrtenal | - | 0.1 ± 0.0 | - |
| 1197 | 1199 | γ-Terpineol | - | 0.2 ± 0.0 | - |
| 1212 | 1220 | α-Fenchyl Acetate | - | - | 0.1 ± 0.0 |
| 1231 | 1229 | Nerol | - | 0.1 ± 0.0 | - |
| 1256 | 1252 | Piperitone | - | t | - |
| 1258 | 1252 | Geraniol | - | 0.2 ± 0.0 | - |
| 1260 | 1257 | Linalool Acetate | - | 0.5 ± 0.0 | t |
| 1287 | 1288 | Bornyl Acetate | - | 0.1 ± 0.0 | 17.9 ± 0.0 |
| 1311 | 1313 | Citronellic Acid | 0.1 ± 0.0 | - | - |
| 1325 | β-Terpinyl Acetate | - | - | 0.1 ± 0.0 | |
| 1345 | 1349 | α-Terpinyl Acetate | - | - | 2.6 ± 0.0 |
| 1348 | 1352 | Citronellyl Acetate | 1.3 ± 0.1 | - | - |
| 1368 | 1361 | Neryl Acetate | - | 0.2 ± 0.0 | - |
| 1468 | 1468 | Linalool Isovalerate | - | 0.1 ± 0.0 | - |
| 1512 | 1511 | Lavandulyl 2-Methyl Butanoate | - | 0.1 ± 0.0 | - |
| Sesquiterpene hydrocarbons | 2.1 ± 0.2 | 3.3 ± 0.0 | 0.7 ± 0.0 | ||
| 1330 | 1338 | δ-Elemene | - | - | t |
| 1377 | 1376 | α-Copaene | - | t | - |
| 1381 | 1381 | Daucene | - | t | - |
| 1383 | 1388 | β-Bourbonene | - | 0.1 ± 0.0 | - |
| 1385 | 1390 | β-Elemene | - | - | t |
| 1391 | 1391 | 7-epi-Sesquithujene | - | 0.1 ± 0.0 | - |
| 1403 | 1405 | Sesquithujene | - | 0.1 ± 0.0 | - |
| 1407 | 1407 | Longifolene | - | - | 0.1 ± 0.0 |
| 1409 | 1409 | α-Gurjunene | - | 0.1 ± 0.0 | - |
| 1410 | 1411 | α-Cedrene | - | - | 0.1 ± 0.0 |
| 1420 | 1419 | β-Caryophyllene | 2.0 ± 0.2 | 1.8 ± 0.0 | 0.4 ± 0.0 |
| 1427 | 1434 | α-trans-Bergamotene | - | 0.1 ± 0.0 | - |
| 1435 | 1436 | γ-Elemene | - | - | t |
| 1454 | 1454 | α-Humulene | - | 0.1 ± 0.0 | t |
| 1460 | 1456 | trans-β-Farnesene | - | 0.2 ± 0.0 | - |
| 1470 | 1472 | Dauca-5,8-diene | - | t | - |
| 1481 | 1479 | γ-Muurolene | - | 0.3 ± 0.0 | - |
| 1495 | 1500 | Bicyclogermacrene | 0.1 ± 0.0 | - | - |
| 1500 | 1500 | α-Muurolene | - | - | t |
| 1510 | 1505 | β-Bisabolene | - | 0.2 ± 0.0 | - |
| 1514 | 1513 | γ-Cadinene | - | 0.2 ± 0.0 | t |
| 1524 | 1522 | trans-Calamenene | - | t | - |
| 1525 | 1523 | δ-Cadinene | - | t | 0.1 ± 0.0 |
| Germacrene B | - | - | t | ||
| Oxygenated sesquiterpenes | t | 0.3 ± 0.0 | 0.3 ± 0.0 | ||
| 1582 | 1583 | Caryophyllene Oxide | t | 0.2 ± 0.0 | t |
| 1599 | 1600 | Cedrol | - | - | 0.1 ± 0.0 |
| 1641 | 1640 | epi-α-Cadinol | - | 0.1 ± 0.0 | - |
| 1684 | 1685 | α-Bisabolol | - | t | - |
| Oxygenated Diterpenes | - | - | 0.1 ± 0.0 | ||
| 1985 | 1987 | Manool Oxide | - | - | 0.1 ± 0.0 |
| Aromatic compounds | 0.1 ± 0.0 | t | 0.3 ± 0.0 | ||
| 1247 | 1250 | p-Anis Aldehyde | - | - | 0.3 ± 0.0 |
| 1351 | 1359 | Eugenol | 0.1 ± 0.0 | - | - |
| 1434 | 1434 | Coumarin | - | t | - |
| Others | 0.1 ± 0.0 | 0.5 ± 0.1 | - | ||
| 868 | 870 | n-Hexanol | - | t | - |
| 910 | Isobutyl Isobutyrate | 0.1 ± 0.0 | - | - | |
| 983 | 979 | 1-Octen-3-ol | - | t | - |
| 1008 | Isoamyl Isobutyrate | t | - | - | |
| 1194 | 1192 | Hexyl Butanoate | - | 0.1 ± 0.0 | - |
| 1234 | 1332 | Hexyl Tiglate | - | 0.1 ± 0.0 | - |
| 1244 | 1244 | Hexyl Isovalerate | - | 0.3 ± 0.0 | - |
| Total | 98.6 ± 1.2 | 97.6 ± 0.2 | 99.1 ± 0.0 | ||
RICal: retention index relative to C8-C32 n-alkane on HP-5MS column; RIRef: retention index reported in Adams 2007 [45]; t: trace amounts < 0.05. Values are means ± standard deviation of the three samples.
Citronellal was the major component in E. citriodora essential oil (88.0 ± 0.8%), whereas linalool (38.7 ± 0.1%) together with 1,8-cineole (26.5 ± 0.1%) and camphor (14.2 ± 0.1%) were the main components in L. angutifolia essential oil (Table 1). Citronellal was also the main compound in E. citriodora essential oils of different origins and light conditions [46,47], making E. citriodora distinct from other Eucalyptus species, such as E. camaldulensis Dehnh [48].
However, qualitative and quantitative differences in the chemical composition of lavender essential oil have been reported depending on the biological raw material, level of dryness, extraction method, and origin. Previous studies showed that the drying process reduced the concentrations of the principal components in L. angustifolia essential oil obtained from flowers and aerial parts [49]. Similarly, the extraction method employed varied the content of linalool, detected in a much higher content using hydrodistillation in comparison to supercritical CO2 and hexane extraction [50]. Furthermore, L. angustifolia essential oil hydrodistilled from aerial parts coming from Yazd (Iran) had a dissimilar chemical composition to our results, with 1,8-cineole, camphor, and borneol as its main components. This was also dissimilar to a sample from lavender essential oil obtained from the inflorescences of L. angustifolia “Sevtopolis” cultivated in western Romania, which had linalyl acetate (40.7%), linalool (22.5%), caryophyllene (8.9%), and lavandulyl acetate (7.5%) as its principal components [51]. In addition, it has been recently observed that the chemical composition of L. angustifolia essential oil can be modified by the application of gold and silver metals as elicitors, decreasing lower-molecular-weight compounds such as α- and β-pinene, camphene, δ-3-carene, p-cymene, 1,8-cineole, pinocarveol, etc., which are replaced by higher-molecular-weight compounds such as α-cadinol 9-cedranone, cadalene, α-bisabolol, and (E,E)-farnesol, varying the biological properties [52]. Other presentations, such as hydrolates, produced a reduction in volatile compound content and a reduction in antioxidant activity [53].
On the other hand, α-pinene (25.6 ± 0.2%), limonene (18.5 ± 0.2%), and β-pinene (15.9 ± 0.1%) were the main components (Table 1) in P. sylvestris essential oil. The predominance of monoterpene hydrocarbons is a characteristic feature of essential oils obtained from the Pinaceae family, e.g., monoterpene hydrocarbons were the major fraction of P. nigra var. italica essential oil (63.4%), with α-pinene as its most abundant compound (49.0%) [54], as well as in the essential oil obtained from the hydrodistillation of P. armandii, P. nigra and P. halepensis cones with α-pinene, limonene, and β-pinene as principal components [55,56].
The remarkable concentration of limonene in P. sylvestris essential oil may also contribute to the antimicrobial properties. In fact, a limonene emulsion has been effectively stabilized by Ulva fasciata Delile polysaccharide to be applied to food to avoid foodborne pathogen contamination and consequently prolong shelf-life [57,58].
In contrast to our results, sesquiterpene hydrocarbons have been described as one of the most representative phytochemical groups in the Pinus genus together with monoterpene hydrocarbons, with germacrene D or β-caryophyllene being the most characteristic compounds within the group [59]. Thus, essential oils obtained from the needles of other Pinus species such as P. roxburghaii contain large amounts of α-pinene (29.3%) and β-caryophyllene (21.9%), whereas α-pinene (35.4%) and germacrene D (28.1%) were the main components of the P. nigra subsp. nigra essential oil [36,60].
2.2. Seed Germination Inhibition of P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber and N. galuca with E. citriodora, L. angustifolia and P. sylvestris Essential Oils
The in vitro phytotoxic potential of E. citriodora, L. angustifolia, and P. sylvestris essential oils was evaluated against seed germination in weeds (P. oleracea, L. multiflorum, and E. crus-galli), as well as against two Mediterranean food crops (tomato and cucumber), and the invasive species N. glauca, at several doses (0.125, 0.25, 0.50, and 1 µL/mL) (Table 2 and Table 3).
Table 2.
In vitro phytotoxic effect of different doses of E. citriodora, L. angustifolia, and P. sylvestris essential oils on Portulaca oleracea, Lolium multiflorum, Echinochloa crus-galli, tomato, and cucumber seed germination.
| Seed Germination (% ± S.E.) | |||||
|---|---|---|---|---|---|
| * Dose | E. citriodora essential oil | ||||
| P. oleracea | L. multiflorum | E. crus-galli | Tomato | Cucumber | |
| Control | 74.0 ± 4.6 a | 65.0 ± 6.9 a | 69.0 ± 2.9 a | 71.0 ± 2.5 a | 99.0 ± 1.0 a |
| 0.125 | 80.0 ± 2.2 a | 67.0 ± 4.4 a | 74.0 ± 3.7 a | 71.0 ± 4.3 a | 98.0 ± 1.2 a |
| 0.25 | 76.0 ± 2.9 a | 52.0 ± 2.0 a | 72.0 ± 2.6 a | 73.0 ± 3.4 a | 95.0 ± 2.2 a |
| 0.5 | 74.0 ± 4.3 a | 58.0 ± 2.6 a | 61.0 ± 4.6 a | 61.0 ± 3.7 a | 97.0 ± 1.2 a |
| 1 | 81.0 ± 6.2 a | 57.0 ± 7.2 a | 72.0 ± 3.7 a | 25.0 ± 11.3 b | 96.0 ± 1.8 a |
| Dose | L. angustifolia essential oil | ||||
| Control | 74.0 ± 3.7 a | 65.0 ± 6.9 a | 71.0 ± 4.3 a | 71.0 ± 2.5 a | 99.0 ± 1.0 a |
| 0.125 | 69.0 ± 5.3 a | 65.0 ± 3.2 a | 71.0 ± 2.8 a | 73.0 ± 4.4 a | 97.0 ± 1.2 a |
| 0.25 | 67.0 ± 2.0 a | 50.0 ± 2.7 a,b | 72.0 ± 2.6 a | 58.0 ± 4.1 a,b | 98.0 ± 2.0 a |
| 0.5 | 66.0 ± 5.8 a | 36.0 ± 8.4 b,c | 72.0 ± 3.4 a | 41.0 ± 13.2 b,c | 97.0 ± 1.2 a |
| 1 | 69.0 ± 3.7 a | 24.0 ± 7.0 c | 58.0 ± 2.6 b | 22.005.8 c | 94.0 ± 1.9 a |
| Dose | P. sylvestris essential oil | ||||
| Control | 75.0 ± 7.1 a | 67.0 ± 2.0 a | 74.0 ± 3.3 a | 68.0 ± 3.4 a | 100.0 ± 0.0 a |
| 0.125 | 74.0 ± 3.7 a | 65.0 ± 8.8 a | 69.0 ± 7.0 a | 67.0 ± 4.4 a | 94.0 ± 2.9 a,b |
| 0.25 | 71.0 ± 2.9 a | 65.0 ± 5.0 a | 74.0 ± 1.9 a | 67.0 ± 4.1 a | 94.0 ± 1.9 a,b |
| 0.5 | 71.0 ± 1.9 a | 58.0 ± 5.2 a | 74.0 ± 4.6 a | 66.0 ± 3.7 a | 95.0 ± 1.6 a,b |
| 1 | 68.0 ± 2.6 a | 51.0 ± 12.8 a | 75.0 ± 5.0 a | 64.0 ± 3.7 a | 90.0 ± 2.3 b |
Values are the mean percentage of five replications ± standard error, after 14 days of incubation. Means followed by different letters in the same column indicate significant difference at p < 0.05, according to T3 Dunnett and Tukey tests. * Dose: µL/mL.
Table 3.
In vitro phytotoxic effect of different doses of E. citriodora and L. angustifolia essential oils on the seed germination and seedling growth of N. glauca.
| Concentration (µL/mL) | E. citriodora | ||
| Germination | Hypocotyl | Radicle | |
| Control | 91.0 ± 3.3 a | 2.5 ± 0.2 a | 3.1 ± 0.3 a |
| 0.125 | 72.00 ± 6.8 a | 1.4 ± 0.3 b | 2.5 ± 0.4 a |
| 0.25 | 68.0 ± 9.0 a | 1.4 ± 0.3 b | 2.5 ± 0.4 a |
| 0.5 | 67.003.4 a | 1.3 ± 0.2 b | 2.5 ± 0.3 a |
| 1 | 66.0 ± 4.7 b | 0.4 ± 0.1 c | 1.0 ± 0.3 b |
| Concentration (µL/mL) | L. angustifolia | ||
| Germination | Hypocotyl | Radicle | |
| Control | 91.0 ± 3.3 a | 2.5 ± 0.3 a | 3.1 ± 0.3 a |
| 0.125 | 81.0 ± 4.0 a | 2.6 ± 0.4 a | 2.8 ± 0.3 a,b |
| 0.25 | 81.0 ± 2.9 a | 2.6 ± 0.2 a | 2.9 ± 0.3 a,b |
| 0.5 | 78.0 ± 3.7 a | 1.8 ± 0.1 a,b | 2.4 ± 0.2 a,b |
| 1 | 64.0 ± 3.7 b | 1.2 ± 0.2 b | 2.0 ± 0.1 b |
Values are the mean of five replications ± error deviation, after 14 days of incubation. Means followed by different letters in the same column indicate significantly difference at p < 0.05, according to T3 Dunnett and Tukey tests.
Regarding the phytotoxic effects of the selected essential oils against weeds, variability at the intraindividual level was observed in the seed germination percentage, without statistical significance. E. citriodora and P. sylvestris did not cause a significant inhibition of seed germination in either P. oleracea, L. multiflorum, or E. crus-galli at any assayed dose (0.125, 0.25, 0.5, and 1 µL/mL) (Table 2). However, citronellal, the main compound in E. citriodora essential oil analyzed here, showed seed germination inhibition against other weeds including Ageratum conyzoides L., Chenopodium album L., Parthenium hysterophorus L., Malvastrum coromandelianum (L.) Garcke, Cassia occidentalis L., and Philaris minor Retz. at 100 µg/g [61]. In relation to food crops, citronellal was able to inhibit seed germination in L. sativa, reaching 49–15% of the control [62], as well as seed germination in tomato at a percentage of 64.8% at the highest tested dose (1 µL/mL). P. sylvestris with α-pinene (25.6%) as the main compound showed phytotoxic effects in seed germination in cucumber at all applied doses (0.125, 0.25, 0.50, and 1 µL/mL), while another Eucalyptus species (E. tereticornis), which contained principally α-pinene (34.5%), produced selective toxicity against the seed germination of E. crus-galli without affecting the rice crop to the same extent [63].
By contrast, although L. angustifolia essential oil did not exhibit a significant inhibition of seed germination in P. oleracea, it achieved a remarkable reduction of seed germination in both L. multiflorum and E. crus-galli. This fact may be because L. angustifolia essential oil, among the essential oils analyzed here, contains the largest number (27 vs. 12 and 14) of oxygenated compounds, especially oxygenated monoterpenes (1,8-cineole, linalool, camphor, borneol, α-terpineol) that have shown higher herbicidal properties [64].
L. multiflorum showed more susceptibility to the phytotoxic effect of L. angustifolia essential oil, which decreased the percentage of seed germination in a dose-dependent manner, reaching increasing percentages of inhibition of 44.6% and 63.1% at the highest applied doses (0.5 and 1 µL/mL, respectively) (Table 2). The fact that L. multiflorum showed a certain sensitivity to L. angustifolia essential oil could be interesting in the research of essential oils as natural alternatives to synthetic herbicides used against L. multiflorum, which have caused the emergence of resistance in this weed [65,66,67]. In other studies, peppermint (Mentha piperita L.) essential oil caused a total inhibition of seed germination in L. multiflorum at a range of concentrations between 0.125 and 1 µL/mL, and caused inhibition in food crops (maize, rice, and tomato). In our study, L. angustifolia essential oil produced less phytotoxic effects in food crops. The seed germination of tomato was reduced at the highest dose tested, at a percentage of 69.02% (vs. 99.97% with peppermint essential oil) with respect to the control [24], while the seed germination of cucumber was not significantly inhibited at any assayed dose (0.125, 0.25, 0.50, and 1 µL/mL).
Seed germination in E. crus-galli also showed a certain weakness to exposure to L. angustifolia essential oil at the highest tested dose (1 µL/mL), with a percentage of inhibition of 18.3% (Table 2).
Tomato was more sensitive to E. citriodora and L. angustifolia essential oils with similar remarkable reduction at the highest applied dose (1 µL/mL), reaching 64.8 and 69.0% reduction, respectively (Table 2).
In general, cucumber was more resistant than tomato to the phytotoxic effects of the three commercial essential oils applied, without inhibitory effect at any assayed dose (0.125, 0.25, 0.5, and 1 µL/mL) with E. citriodora and L. angustifolia essential oils, and only a low percentage of inhibition (10.00%) at the highest tested dose (1 µL/mL) with P. sylvestris essential oil (Table 2).
In addition, the two essential oils richest in oxygenated monoterpenes, E. citriodora and L. angustifolia (94.7% and 85.5%, respectively), showed similar significant phytotoxic effects against seed germination in the invasive species N. glauca, but with a lower percentage in relation to weeds (27.5% and 29.7%) at the highest tested dose (1 µL/mL) (Table 3). Therefore, the various main compounds of an essential oil can produce similar phytotoxic effects against different species.
2.3. Seedling Growth Inhibition of P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber and N. glauca with E. citriodora, L. angustifolia, and P. sylvestris Essential Oils
The hypocotyl growth of P. oleracea was not significantly reduced by E. citriodora essential oil at any applied dose (0.125, 0.25, 0.5, and 1 µL/mL); however, this essential oil was able to reduce radicle development at the highest assayed doses (0.5 and 1 µL/mL), reaching 36.4% and 43.2% reduction compared to the control, respectively (Figure 1a). The root growth of P. oleracea was more sensitive than shoot growth to citronellal, according to previous studies, due to the mitotic activity of growing root tip cells [61]. However, other mechanisms would have been present with other essential oils because the roots were not significantly affected at doses that produced toxic effects in the hypocotyl. Therefore, the hypocotyl elongation of P. oleracea was remarkably reduced from the lowest applied dose (0.125 µL/mL) of L. angustifolia (Figure 1b) and P. sylvestris (Figure 1c) essential oils with respect to control, reaching a decrease of 30.6% and 39.3%, respectively, at the highest tested dose (1 µL/mL), whereas radicle development was not significantly affected by L. angustifolia essential oil (Figure 1b), yet P. sylvestris essential oil achieved a significant reduction in radicle development (26.0–44.4%) from the lowest to the highest applied dose (0.125–1 µL/mL) (Figure 1c).
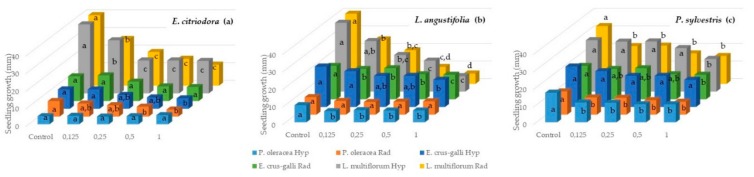
Figure 1.
Phytotoxic effect of E. citriodora (a), L. angustifolia (b), and P. sylvestris (c) essential oils on the seedling growth (hypocotyl and radicle) of P. oleracea, L. multiflorum, and E. crus-galli. Values are mean percentages of five replications, after 14 days of incubation. Doses 0.125–1 µL/mL. Different letters indicate significant difference at p < 0.05, according to T3 Dunnett and Tukey tests.
Regarding the seedling evolution of E. crus-galli after the application of the essential oils, it was observed that L. angustifolia essential oil was the most harmful for E. crus-galli seedling growth, as it decreased its hypocotyl in a high percentage (76.7%) at the highest assayed dose (1 µL/mL), as well as the radicle, in a dose-dependent manner, also reaching a considerable percentage (69.9%) at the highest applied dose (1 µL/mL) (Figure 1b). Although E. citriodora essential oil did not influence radicle elongation at any applied dose (0.125, 0.25, 0.5, and 1 µL/mL), hypocotyl growth was significantly affected at 1 µL/mL, reaching a reduction percentage of 46.1% in comparison to control (Figure 1a). P. sylvestris essential oil was the least phytotoxic essential oil, with no reduction in the hypocotyl development of E. crus-galli at any dose (0.125, 0.25, 0.5, and 1 µL/mL), and a low percentage of radicle elongation reduction (26.5%) at the highest dose (Figure 1c). However, other studies demonstrated that α-pinene exhibited a certain inhibition of the early root growth of other weeds such as Cassia occidentalis (L.) Link., as well as oxidative damage in root tissue [10]. Similarly, the compound β-pinene was shown to be responsible for the disruption of membrane integrity, the enhancement of peroxidation and electrolyte leakage in Phalaris minor and particularly in E. crus-galli [68].
Both the hypocotyl and radicle development of L. multiflorum were significantly inhibited by E. citriodora (Figure 1a) and L. angustifolia (Figure 1b), which caused a strong dose-dependent reduction, reaching 52.3–53.0% and 60.6–75.4% at 0.25-1 µL/mL, and 55.1–77.5 and 80.1–87.8% at 0.5-1 µL/mL, respectively (Figure 1a,b). P. sylvestris essential oil did not significantly affect hypocotyl growth, but it did inhibit the radicle development of L. multiflorum in the range of 0.125 to 1 µL/mL without distinction between doses, reaching 51.67% reduction at the highest dose assayed (Figure 1c). With L. multiflorum, it was corroborated that α-pinene, the main compound of P. sylvestris essential oil analyzed here, affects root development to a greater extent than hypocotyl, as it was also able to inhibit the radicle growth of other weed species such as Amaranthus viridis L., Triticum aestivum L., Pisum sativum L., Cicer arietinum L., and especially C. occidental, which demonstrated solute leakage, lipid peroxidation and the generation of reactive oxygen species upon α-pinene exposure [10].
Regarding the sensitivity of the seedling growth of food crops to essential oils, it was observed that tomato was more susceptible than cucumber to E. citriodora, L. angustifolia, and P. sylvestris essential oils (Table 4, Figure 2 and Figure 3). Both the hypocotyl and radicle development of tomato were significantly reduced in a dose-dependent manner, reaching elevated reduction percentages at the highest applied dose (1 µL/mL) of E. citriodora (89.7 and 79.4%) and L. angustifolia (93.2% and 83.4%) essential oils (Figure 2a,b). L. sativa was another food crop that showed high sensitivity to the application of citronellal [62], and E. citriodora essential oil affected meristematic cells, decreasing the germination and seedling growth of this food crop [25].
Table 4.
In vitro phytotoxic effect of different doses of E. citriodora (EC), L. angustifolia (LA), and P. sylvestris (PS) essential oils on tomato (TO) and cucumber (CU) seedling growth.
| * Dose | Control | 0.125 | 0.25 | 0.5 | 1 | ||
|---|---|---|---|---|---|---|---|
| EC | TO | Hyp | 7.3 ± 1.4 a | 6.8 ± 1.8 a | 5.0 ± 1.2 a,b | 2.7 ± 0.5 a,b | 0.8 ± 0.4 b |
| Rad | 16.7 ± 1.5 a | 14.1 ± 1.7 a | 15.1 ± 1.5 a | 6.3 ± 1.3 b | 3.4 ± 1.7 b | ||
| CU | Hyp | 8.4 ± 0.1 a | 8.3 ± 0.4 a | 8.4 ± 0.2 a | 8.4 ± 0.1 a | 8.5 ± 0.9 a | |
| Rad | 23.1 ± 1.5 a | 20.2 ± 0.5 a | 15.3 ± 0.5 b | 15.1 ± 0.9 b | 13.3 ± 0.4 b | ||
| LA | TO | Hyp | 7.3 ± 1.4 a | 7.2 ± 0.7 a | 4.9 ± 0.9 a,b | 2.1 ± 0.8 b,c | 0.5 ± 0.3 c |
| Rad | 16.7 ± 1.5 a | 16.4 ± 0.9 a | 11.6 ± 1.1 a,b | 7.3 ± 2.1 b,c | 2.8 ± 2.0 c | ||
| CU | Hyp | 8.4 ± 0.1 a | 8.3 ± 1.0 a | 8.1 ± 0.9 a | 8.3 ± 0.9 a | 8.3 ± 0.04 a | |
| Rad | 23.1 ± 1.5 a | 18.9 ± 0.5 b | 17.1 ± 0.5 b,c | 15.6 ± 1.0 b,c | 14.4 ± 0.7 c | ||
| PS | TO | Hyp | 12.6 ± 1.6 a | 3.8 ± 1.2 b | 4.0 ± 0.7 b | 3.2 ± 0.7 b | 3.5 ± 0.3 b |
| Rad | 18.1 ± 1.0 a | 9.4 ± 1.0 b | 10.6 ± 0.5 b | 6.8 ± 1.7 b | 6.7 ± 0.4 b | ||
| CU | Hyp | 8.5 ± 0.9 a | 8.6 ± 0.2 a | 8.4 ± 0.3 a | 8.4 ± 0.8 a | 7.7 ± 0.9 a | |
| Rad | 21.2 ± 1.0 a | 17.8 ± 0.6 a,b | 16.3 ± 1.1 b | 16.5 ± 0.8 b | 15.2 ± 0.8 b | ||
Values are the mean percentage of five replications ± standard error, after 14 days of incubation. Means followed by different letters in the same row indicate significantly difference at p < 0.05, according to T3 Dunnett and Tukey tests. * Dose: µL/mL; Hyp: Hypocotyl (mm); Rad: Radicle (mm).
Figure 2.
Phytotoxic effect of E. citriodora (a), L. angustifolia (b), and P. sylvestris (c) essential oils at 0.125, 0.25, 0.5, and 1 µL/mL on the seedling growth (hypocotyl + radicle) of tomato.
Figure 3.
Phytotoxic effect of E. citriodora (a), L. angustifolia (b), and P. sylvestris (c) essential oils at 0.125, 0.25, 0.5, and 1 µL/mL on the seedling growth (hypocotyl + radicle) of cucumber.
Again, P. sylvestris was the least phytotoxic essential oil, but also showed a significant inhibition of hypocotyl and radicle development, measuring 72.2% and 62.9%, respectively, at the dose of 1 µL/mL (Table 4).
On the other hand, none of the assayed essential oils significantly affected the hypocotyl growth of cucumber (Table 4). However, the radicle development of cucumber was significantly reduced, up to a percentage of 42.4%, 37.8%, and 28.0% at the highest applied doses of E. citriodora, L. angustifolia, and P. sylvestris essential oils (Table 4).
Finally, E. citriodora essential oil showed more phytotoxic effects than L. angustifolia essential oil in both the hypocotyl and radicle elongation of the invasive species N. glauca, reaching percentage reductions of 85.8% and 69.4% versus 51.8% and 37.6%, respectively (Table 3).
3. Materials and Methods
3.1. Essential Oils
Commercial samples of Eucalyptus citriodora Hook (Batch: OF25830; Exp. Date: 02/2022), Lavandula angustifolia Mill. (Batch: 0082842; Exp. Date: 30/11/2020), and Pinus sylvestris L. (Batch: 0065144; Exp. Date: 08/08/2018) essential oils obtained from the hydrodistillation of leaves, flowers, and needles, respectively, were supplied by Pranarôm S.A. (E. citriodora) and Guinama (Valencia, Spain). The essential oils were stored at 4 °C until chemical analysis and phytotoxic assays were carried out.
3.2. Weeds, Food Crops, and Invasive Species Seeds
Mature seeds of the weeds common purslane (Portulaca oleracea L.), Italian ryegrass (Lolium multiflorum Lam.), and barnyardgrass (Echinochloa crus-galli (L.) Beauv.) were purchased from Herbiseed (website: www.herbiseed.com).
Mature seeds of the food crops “Muchamiel” tomato (Solanum lycopersicum L.) and cucumber (Cucumis sativus L.) were obtained from Intersemillas S.A.
Mature seeds of the invasive species tree tobacco (Nicotiana glauca Graham) were supplied by the Botanical Garden of Valencia.
3.3. Gas Chromatography–Mass Spectrometry Analysis
Gas Chromatography–Mass Spectrometry analysis was carried out using a 5977A Agilent mass spectrometer and a gas chromatograph (Agilent 7890B, Valencia, España) apparatus equipped with an Agilent HP-5MS (30 m long and 0.25 mm i.d. with 0.25 µm film thickness) capillary column (95% dimethylpolysiloxane/5% diphenyl). The column temperature program was 60 °C for a duration of 5 min, with 3 °C/min increases up to 180 °C, then 20 °C/min increases up to 280 °C, which was maintained for 10 min. The carrier gas was helium at a flow rate of 1 mL/min. Split-mode injection (ratio 1:30) was employed. Mass spectra were collected over the m/z range 30–650 with an ionizing voltage of 70 eV. The resulting individual compounds were identified by MS and their identity was confirmed by comparison of their Kovat’s retention index, calculated using co-chromatographed standard hydrocarbons relative to C8–C32 n-alkanes and mass spectra with reference samples or with data already available in the NIST 11 mass spectral library and in the literature [45].
3.4. In Vitro Assays: P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber, and N. glauca Seed Germination and Seedling Growth with Essential Oils
Sets of 20 seeds each with five replicates per treatment were homogenously distributed in Petri dishes (9 cm diameter) between two layers of filter paper (Whatman No.1). The lower filter papers were moistened with 4 mL of distilled water and the upper ones with 0 (control), 0.125, 0.250, 0.5, and 1 µL/mL of E. citriodora, L. angustifolia, and P. sylvestris essential oils, homogeneously distributed in the filter paper with a micropipette (Merck®, Valencia, España). Therefore, the seeds were in contact directly with moistened filter papers and indirectly with the vapors of the essential oils. Petri dishes were sealed with parafilm and incubated in an Equitec EGCS 301 3SHR model germination chamber, according to previous assays [69], alternating between 30.0 ± 0.1 °C 16 h of light and 20.0 ± 0.1 °C 8 h of darkness, with and without humidity. To evaluate the herbicidal activity of the essential oils, the number of germinated seeds was counted and compared with that of untreated seedlings. The emergence of the radicle (≥1 mm) was used as an index of germination, and seedling length (hypocotyl and/or radicle) data were recorded after 3, 5, 7, 10, and 14 days in each replicate.
3.5. Statistics
Experiments were performed in vitro with five replicates. Data were subjected to one-way analysis of variance (ANOVA) using SPSS statistics 24 software. Tukey’s post hoc test was used when variances remained homogeneous (Levene’s test) and T3 Dunnett’s post hoc test was employed if not, assuming equal variances. Differences were considered to be significant at p ≤ 0.05.
4. Conclusions
In this study, the potential of E. citriodora, L. angustifolia, and P. sylvestris essential oils as eco-friendly alternatives to synthetic herbicides was investigated. L. angustifolia essential oil, with a high content of the oxygenated monoterpenes linalool (38.7 ± 0.1%), 1,8-cineole (26.5 ± 0.1%), and camphor (14.2 ± 0.1%), affected seed germination and development of L. multiflorum, E. crus-galli, and N. glauca without any significant phytotoxic effect on cucumber seed germination. E. citriodora, with a high content of the oxygenated monoterpene citronellal (88.0 ± 0.8%), showed more phytotoxic effects than L. angustifolia on the control of N. glauca. Lavender essential oil represents an effective pre-emergent treatment for L. multiflorum affecting cucumber crops, and E.citriodora essential oil could be used in both pre- and post-management of the invasive species N. glauca.
Acknowledgments
The authors thank the Central Service for Experimental Research of the University of Valencia (SCSIE) for providing the Gas Chromatography–Mass Spectrometry equipment, and also thank Professor Pilar Soriano Guarinos at the University of Valencia (Botanical Garden) for collecting and providing the N. glauca seeds.
Author Contributions
Conceptualization, M.A.B.; methodology, M.A.B.; software, M.D.I.; formal analysis, M.D.I.; investigation, M.D.I.; resources, M.A.B.; data curation, M.D.I.; writing—original draft preparation, M.D.I.; writing—review and editing, M.D.I. and M.A.B.; visualization, M.A.B.; supervision, M.A.B.; project administration, M.A.B.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehdizadeh L., Moghaddam M. Essential oils: Biological activity and therapeutic potential. In: Grumezescu A.M., Holban A.M., editors. Therapeutic, Probiotic and Unconventional Foods. Elsevier; London, UK: 2018. pp. 167–176. [Google Scholar]
- 3.Morsy N.F.S. Active Ingredients from Aromatic and Medicinal Plants. InTech; London, UK: 2017. Chemical structure, quality indices and bioactivity of essential oil constituents; pp. 175–206. [DOI] [Google Scholar]
- 4.Zuzarte M., Salgueiro L. Essential oils chemistry. In: Sousa D.P., editor. Bioactive Essential Oils and Cancer. Springer; Basel, Switzerland: 2015. pp. 19–28. [DOI] [Google Scholar]
- 5.Amri I., Hamrouni L., Hanana M., Jamoussi B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weeds management. Int. J. Appl. Biol. Pharm. Technol. 2013;4:96–114. [Google Scholar]
- 6.Synowiec A., Kalemba D., Drozdek E., Bocianowski J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017;90:407–419. doi: 10.1007/s10340-016-0759-2. [DOI] [Google Scholar]
- 7.Ibáñez M.D., Blázquez M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017;28:1168–1180. doi: 10.1080/09540105.2017.1332010. [DOI] [Google Scholar]
- 8.Fouad R., Bousta D., El Ouali A., Chahdi F.O., Amri I., Jamoussi B., Greche H. Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (DC) Stapf, Eucalyptus cladocalyx, Origanum vulgare L. and Artemisia absinthium L. cultivated in Morocco. J. Essent. Oil Bearing Plants. 2015;18:112–123. doi: 10.1080/0972060X.2014.901631. [DOI] [Google Scholar]
- 9.Matković A., Marković T., Vrbničanin S., Sarić-Krsmanović M., Božić D. Chemical composition and in vitro herbicidal activity of five essential oils on Johnson grass (Sorghum halepense [L.] Pers.) Lek. Sirovine. 2019;38:44–50. [Google Scholar]
- 10.Singh H.P., Batish D.R., Kaur S., Arora K., Kohli R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006;98:1261–1269. doi: 10.1093/aob/mcl213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batish D., Setia N., Singh H.P., Kohli R. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Prot. 2004;23:1209–1214. doi: 10.1016/j.cropro.2004.05.009. [DOI] [Google Scholar]
- 12.CABI, Invasice Species Compendium Portulaca oleracea (purslane) [(accessed on 24 March 2019)];2018 Available online: https://www.cabi.org/isc/datasheet/43609.
- 13.CABI, Invasice Species Compendium Lolium multiflorum (Italian ryegrass) [(accessed on 24 March 2019)];2018 Available online: https://www.cabi.org/ISC/datasheet/31165.
- 14.Perez-Jones A., Park K.W., Colquhoun J., Mallory-Smith C., Shaner D. Identification of glyphosate-resistant Italian ryegrass (Lolium multiflorum) in Oregon. Weed Sci. 2005;53:775–779. doi: 10.1614/WS-04-200R.1. [DOI] [Google Scholar]
- 15.Tehranchian P., Nandula V.K., Matzrafi M., Jasieniuk M. Multiple herbicide resistance in California Italian ryegrass (Lolium perenne ssp. multiflorum): Characterization of ALS-inhibiting herbicide resistance. Weed Sci. 2019;67:273–280. doi: 10.1017/wsc.2019.1. [DOI] [Google Scholar]
- 16.CABI, Invasice Species Compendium Echinochloa crus-galli (barnyard grass) [(accessed on 24 March 2019)];2018 Available online: https://www.cabi.org/isc/datasheet/20367.
- 17.Thomas J., El-Sheikh M., Alfarhan A., Alatar A., Sivadasan M., Basahi M., Al-Obaid S., Rajakrishnan R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ. 2016;127:53–65. doi: 10.1016/j.jaridenv.2015.10.009. [DOI] [Google Scholar]
- 18.Ayenew A., Faris G., Seifu A., Merawi E., Seboka N., Misganaw M., Bekeke T. Impact and status of invasive alien plant species (IAPS), Nicotiana glauca, in Eastern and Southern Zones of Tigray regional state, Ethiopia. Biodivers. Int. J. 2018;2:351–355. [Google Scholar]
- 19.Upasani R.R., Barla S. Herbicide resistance in weeds it’s management. J. Pharmacogn. Phytochem. 2018:810–815. [Google Scholar]
- 20.Peterson M.A., Collavo A., Ovejero R., Shivrain V., Walsh M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018;74:2246–2259. doi: 10.1002/ps.4821. [DOI] [PubMed] [Google Scholar]
- 21.Qasem J.R. Herbicides applications: Problems and considerations. In: Kortekamp A., editor. Herbicides and Environment. InTech; London, UK: 2011. pp. 643–664. [Google Scholar]
- 22.Lamb R.D., Johnson M.D. Agricultural compositions and applications utilizing essential oils. US 9.949.490 B2. United States Patent. 2018
- 23.Fernandez L., Campbell B., Huang H., Koivunen M., Marrone P.G. Natural herbicide containing lemongrass essential oil. Application US 20090099022 A1. United States Patent. 2019
- 24.Ibáñez M.D., Blázquez M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants. 2018;7:79. doi: 10.3390/plants7040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aragão F.B., Palmieri M.J., Ferreira A., Costa A.V., Queiroz V.T., Pinheiro P.F., Andrade-Vieira L.F. Phytotoxic and cytotoxic effects of eucalyptus essential oil on lettuce (Lactuca sativa L.) Allelopath. J. 2015;35:259–272. [Google Scholar]
- 26.Ibáñez M.D., Blázquez M.A. Tea tree and wintergreen essential oils in the management of the invasive species Cortaderia selloana and Nicotiana glauca. J. Plant Prot. Res. 2019;59:1–10. doi: 10.24425/jppr.2019.129281. [DOI] [Google Scholar]
- 27.Tolba H., Moghrani H., Benelmouffok A., Kellou D., Maachi R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Med. 2015;25:e128–e133. doi: 10.1016/j.mycmed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Tolba H., Moghrani H., Aboun A., Maachi R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition and antimicrobial activities. Nat. Technol. J. 2018;18:19–27. doi: 10.1016/j.mycmed.2015.10.009. [DOI] [Google Scholar]
- 29.Davies J.H., Moses J. Insect repellent composition and method of use. US2019/0037840 A1. US Patent. 2019
- 30.Abril-Sánchez C., Matencio A., Navarro-Orcajada S., García-Carmona F., López-Nicolás J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids. 2019;219:72–78. doi: 10.1016/j.chemphyslip.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 31.De Rapper S., Viljoen A., van Vuuren S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evidence Based Complement. Altern. Med. 2016;2016:1–9. doi: 10.1155/2016/2752739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simsek M., Duman R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res. 2017;9:234–237. doi: 10.4103/0974-8490.210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Özek T., Tabanca N., Demirci F., Wedge D.E., Can Baser K.H. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010;4:180–192. [Google Scholar]
- 34.Yuan C., Wang Y., Liu Y., Cui B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019;130:104–110. doi: 10.1016/j.indcrop.2018.12.067. [DOI] [Google Scholar]
- 35.Chao S.C., Young D.G., Oberg C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000;12:639–649. doi: 10.1080/10412905.2000.9712177. [DOI] [Google Scholar]
- 36.Mitić Z.S., Jovanović B., Jovanović S., Mihajilov-Krstev T., Stojanović-Radić Z.Z., Cvetković V.J., Mitrović T.L., Marin P.D., Zlatković B.K., Stojanović G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2018;111:55–62. doi: 10.1016/j.indcrop.2017.10.004. [DOI] [Google Scholar]
- 37.Da Silva A.C.R., Monteiro P., de Azevedo M.M.B., Costa D.C.M., Alviano C.S., Alviano D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrich M., Barnes J., Prieto J.M., Gibbons S., Williamson E.M. Fundamentals of Pharmacognosy and Phytotherapy. Elsevier; Amsterdam, The Netherlands: 2018. Complementary/alternative or “integrative” therapies involving use of plant substances; pp. 1–345. [Google Scholar]
- 39.Sourmaghi M.H.S., Kiaee G., Golfakhrabadi F., Jamalifar H., Khanavi M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015;52:2452–2457. doi: 10.1007/s13197-014-1286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zekri N., Elazzouzi H., Drioche A., Satrallah A., El Belghiti M.A., Zair T. Effect of geographic locations on chemical composition of M. spicata L. essential oils from Moroccan Middle-Atlas. Der Pharm. Lett. 2016;8:146–150. [Google Scholar]
- 41.Jaramillo-Colorado B., Julio-Torres J., Duarte-Restrepo E., Gonzalez-Coloma A., Julio-Torres L.F. Comparative study of volatile composition and biological activities of essential oil from Colombian Piper marginatum Jacq. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015;14:343–354. [Google Scholar]
- 42.Karousou R., Hanlidou E., Kokkni S. The Sage plants in Greece: Distribution and intraspecific variation. In: Kintzios S.E., editor. Sage. The genus Salvia. Taylor & Francis; London, UK: 2005. pp. 1–281. [Google Scholar]
- 43.Carvalho Filho J.L.S., Blank A.F., Alves P.B., Ehlert P.A.D., Melo A.S., Cavalcanti S.C.H., Arrigoni-Blank M.d.F., Silva-Mann R. Influence of the harvesting time, temperature and drying period on basil (Ocimum basilicum L.) essential oil. Rev. Bras. Farmacogn. 2006;16:24–30. doi: 10.1590/S0102-695X2006000100007. [DOI] [Google Scholar]
- 44.Inan M., Kirpik M., Kaya D.A., Kirici S. Effect of harvest time on essential oil composition of Thymbra spicata L. growing in flora of Adiyaman. Adv. Environ. Biol. 2011;5:356–358. [Google Scholar]
- 45.Adams R.P. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 46.De Almeida L.F.R., Frei F., Mancini E., De Martino L., De Feo V. Phytotoxic activities of Mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degani A.V., Dudai N., Bechar A., Vaknin Y. Shade effects on leaf production and essential oil content and composition of the novel herb Eucalyptus citriodora Hook. J. Essent. Oil Bear. Plants. 2016;19:410–420. doi: 10.1080/0972060X.2014.890080. [DOI] [Google Scholar]
- 48.Ibrahim J.A., Mustapha B., Ogah J.I., Egharevba H.O. Comparative pharmacognostic and chemical analyses of Eucalyptus camaldulensis Dehnh and Eucalyptus citriodora (Hook) J. Chem. Soc. Niger. 2018;43:560–568. [Google Scholar]
- 49.Smigielski K., Prusinowska R., Stobiecka A., Kunicka-Styczyñska A., Gruska R. Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia) J. Essent. Oil Bear. Plants. 2018;21:1303–1314. doi: 10.1080/0972060X.2018.1503068. [DOI] [Google Scholar]
- 50.Danh L.T., Han L.N., Triet N.D.A., Zhao J., Mammucari R., Foster N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioprocess Technol. 2013;6:3481–3489. doi: 10.1007/s11947-012-1026-z. [DOI] [Google Scholar]
- 51.Cãlin J., Miscã C., Gruia A.T., Bujancã G., Stoin D. Lavandula angustifolia “Sevtopolis” essential oil: The chemical composition and antimicrobial properties; Proceedings of the International Multidisciplinary Scientific GeoConference. Surveying Geology & Mining Ecology Management (SGEM); Albena, Bulgaria. 29 June–5 July 2017; pp. 281–286. [Google Scholar]
- 52.Wesołowska A., Jadczak P., Kulpa D., Przewodowski W. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules. 2019;24:606. doi: 10.3390/molecules24030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prusinowska R., Smigielski K., Stobiecka A., Kunicka-Styczyńska A. Hydrolates from lavender (Lavandula angustifolia)–Their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat. Prod. Res. 2016;30:386–393. doi: 10.1080/14786419.2015.1016939. [DOI] [PubMed] [Google Scholar]
- 54.Canale A., Conti F., Mehlhorn H., Nicoletti M., Cianfaglione K., Ciaschetti G., Maggi F., Benelli G., Pavela R., Senthil-Nathan S. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017;66:166–171. doi: 10.1016/j.parint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Yang X., Zhao H.T., Wang J., Meng Q., Zhang H., Yao L., Zhang Y.C., Dong A.J., Ma Y., Wang Z.Y. Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the Southwest region of China. J. Med. Plants Res. 2010;4:1668–1672. [Google Scholar]
- 56.Tümen I., Hafizogen H., Kilic A., Dönmez I.E., Sivrikaya H., Reunamen M. Yield and constituents of essential oils from cones of Pinaceae spp. native growing in Turkey. Molecules. 2010;15:5797–5806. doi: 10.3390/molecules15085797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao P., Ma H., Qiu Q., Jing W. Physical stability of R-(+)-Limonene emulsions stabilized by Ulva fasciata algae polysaccharide. Int. J. Biol. Macromol. 2016;92:926–934. doi: 10.1016/j.ijbiomac.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Shao P., Zhang H., Niu B., Jiang L. Antibacterial activities of R-(+)-Limonene emulsion stabilized by Ulva fasciata polysaccharide for fruit preservation. Int. J. Biol. Macromol. 2018;111:1273–1280. doi: 10.1016/j.ijbiomac.2018.01.126. [DOI] [PubMed] [Google Scholar]
- 59.Ioannou E., Koutsaviti A., Tzakou O., Roussis V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014;13:741–768. doi: 10.1007/s11101-014-9338-4. [DOI] [Google Scholar]
- 60.Zafar I., Fatima A., Khan S., Rehman Z., Mehmud S. GC-MS studies of needles essential oil of Pinus roxburghaii and their antimicrobial activity from Pakistan. Electron. J. Environ. Agric. Food Chem. 2010;9:468–473. [Google Scholar]
- 61.Singh H.P., Batish D.R., Kaur S., Kohli R.K., Arora K. Phytotoxicity of the volatile monoterpene citronellal against some weeds. Z. Naturforsch. 2006;61c:334–340. doi: 10.1515/znc-2006-5-606. [DOI] [PubMed] [Google Scholar]
- 62.Vokou D., Douvli P., Blionis G.J., Halley J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003;29:2281–2301. doi: 10.1023/A:1026274430898. [DOI] [PubMed] [Google Scholar]
- 63.Vishwakarma G.S. Master’s Thesis. Central University of Punjab; Bathinda, India: 2012. Phytotoxic potential of essential oil from leaves of Eucalyptus tereticornis against rice (Oryza sativa) and its weeds, Echinochloa crus-galli and Cyperus rotundus. [Google Scholar]
- 64.Khare P., Srivastava S., Nigam N., Singh A.K., Singh S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019;14:1–18. doi: 10.1016/j.eti.2019.100343. [DOI] [Google Scholar]
- 65.Perez A., Kogan M. Glyphosate-resistant Lolium multiflorum in Chilean orchards. Weed Res. 2003;43:12–19. doi: 10.1046/j.1365-3180.2003.00311.x. [DOI] [Google Scholar]
- 66.Rauch T.A., Thill D.C., Gersdorf S.A., Price W.J. Widespread occurrence of herbicide-resistant Italian Ryegrass (Lolium multiflorum) in Northern Idaho and Eastern Washington. Weed Technol. 2017;24:281–288. doi: 10.1614/WT-D-09-00059.1. [DOI] [Google Scholar]
- 67.Moss S.R., Hull R., Perryman S.A., Cussans J.W. Lolium multiflorum: Aspects of herbicide resistance, agro-ecology and effects on crop yield in wheat crops. Asp. Appl. Biol. 2017;134:151–160. [Google Scholar]
- 68.Chowhan N., Singh H.P., Batish D.R., Kaur S., Ahuja N., Kohli R.K. β -Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma. 2013;250:691–700. doi: 10.1007/s00709-012-0446-y. [DOI] [PubMed] [Google Scholar]
- 69.Blázquez M.A., Carbó E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crops Prod. 2015;76:515–521. doi: 10.1016/j.indcrop.2015.07.019. [DOI] [Google Scholar]