Abstract
Dithiocarbamates represent a class of compounds that were evaluated in different biomedical applications because of their chemical versatility. For this reason, several pharmacological activities have already been attributed to these compounds, such as antiparasitic, antiviral, antifungal activities, among others. Therefore, compounds that are based on dithiocarbamates have been evaluated in different in vivo and in vitro models as potential new antimicrobials. Thus, the purpose of this review is to present the possibilities of using dithiocarbamate compounds as potential new antitrypanosomatids-drugs, which could be used for the pharmacological control of Chagas disease, leishmaniasis, and African trypanosomiasis.
Keywords: dithiocarbamates, DETC, antiparasitic activity, Trypanosomatids, Chagas disease, leishmaniasis, African trypanosomiasis
1. Introduction
The year in which the dithiocarbamic acids were discovered is unknown, but the first report of its use was in the year 1850 by Debus [1]. Dithiocarbamic acids are a class of chemical compounds that are used in a variety of pharmacological applications, such as antiparasitic, antifungal, antiviral, and antitumor activities. Structurally, dithiocarbamates (R2CNS-2) consist of a broad class of 1,1-dithioligand monoacids, in which they can be easily synthesized from a wide variety of compounds [2,3,4,5,6,7].
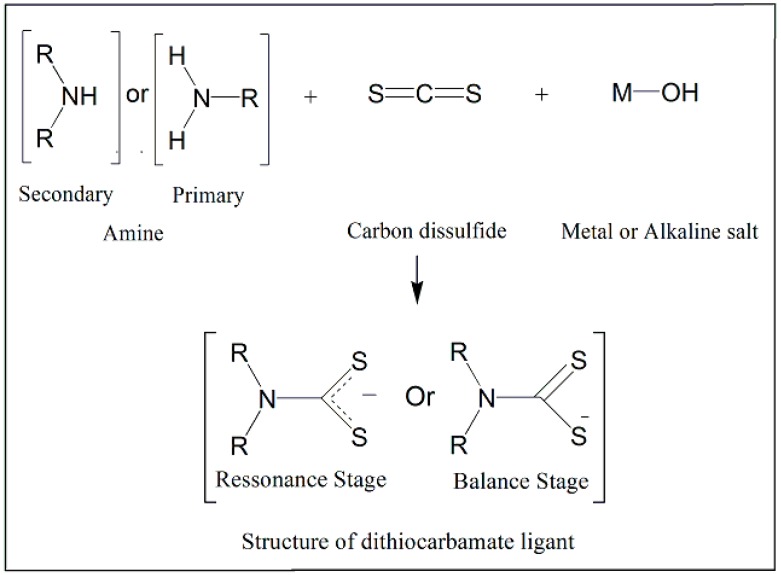
The dithiocarbamate synthesis has a large variety, depending on the final product. The traditional method is based on the reaction of a primary or secondary amine with carbon disulfide in the presence of metal or alkaline salt, due to low stability of these acids (Figure 1). In this process, the type of amine interferes with the substituents formation differing in mono or diacyl dithiocarbamic, and the type of structure can vary between cyclic or linear [8,9,10,11].
Figure 1.
Schematic representation of the chemical synthesis of dithiocarbamates-based compounds.
The structures that are produced may have two polarization states. The resonance stage is decentralized alternating between the two sulfurs in the SCS extremity. This decentralization provokes an approximation between both sulfurs with carbon reducing their facility to bind to another molecule due to system present a biggest stability. The other is the balanced stage, in which the polarization of electron occurs in one of the sulfurs SCS extremity, forming a double bond between C=S in another extremity. Because of this, the C-S extremity will have a negative charge and a bigger distance from the carbon, facilitating the capacity of interaction with other structures, including metals [9,10].
The type of chemical bond that formed among SCS extremity and others molecules may vary, according to the structural characteristics of the compounds, which may be of the monodentate type (a bond between the structure and the grouping), of the bidentate type (two symmetrical linkages between the structure and the grouping) or of the anisobidentate type (two asymmetrical distances). The SCS interaction shows an increase in bond strength, depending on the size of the metallic centre/chemical group and the alignment [10].
The ability of these structures to interact with metals gives an important biological value because a lot of proteins, molecules, and enzymes, which are present in their metallic compositions centres, are essential for these structures. Dithiocarbamates can be acting like chelators of metals, destabilizing the structure of these molecules and inhibiting their action [11]. Furthermore, as revised by Bond and Martin, the interaction with some metals is related to oxidative processes that are important mechanisms in the elimination of microorganisms [12].
Not only does the SCS extremity have a biological function, but also the amine present in this structure can interact with different substituents modulating responses and direct for specific targets. Furthermore, it acts producing reactive oxygen species (ROS), conferring an important biological activity for the dithiocarbamates [13,14].
Even if the general characteristics of the main chain are similar, each chemical group will have its own particular structure. Thus, small changes in structure, such as spatial changes of a certain segment, can generate changes in the function and behaviour of the formed derivatives, allowing for greater versatility for the biological application of new synthesized compounds [15].
The evaluation of compounds based on dithiocarbamates as potential new antimicrobials has been developed since the last century. In this scenario, several new molecules have been tested based on the principle of the basic structure and the derivation of these compounds, in order to show the different dithiocarbamate derivatives as antiprotozoal, anthelmintic, and antiviral agents. Therefore, compounds that are based on dithiocarbamates have been evaluated in different in vivo and in vitro models as potential new antimicrobials. Thus, the purpose of this review is to present the main uses of dithiocarbamate compounds as potential antitrypanosomatids-drugs, mainly for the therapeutic control of protozoa that are responsible for important human infectious diseases, such as Chagas disease, leishmaniasis, and African trypanosomiasis.
2. Main Chemical Groups Used in the Production of Dithiocarbamate Derivatives
Various synthetic compounds that are based on dithiocarbamates are produced because of their ability to form complexes with metals, as well as the ability to add different substituents to the final molecule structure. This attribute confers the classification of the dithiocarbamates based on the different chemical substituents attached to the molecule [15]. In this context, the main groups of chemical complexes based on dithiocarbamates are: (i) group 13 (III-A), which is generally formed by the use of halides with a parent of group 1 (I-A) or ammonium dithiocarbamate salt; (ii) group 14 (IV-A), which is the group of carbon compounds, esters of dithiocarbamic acid, which are also classified as organic compounds, which present applications that are directed towards the pharmaceutical field, agrochemicals, intermediates of organic synthesis, for drug and pro-drug formation, among others; (iii) group 15 (5-A), which is a well-studied group and demonstrates a wide variety of structures that can be employed in pharmacology, these compounds tend to act as an asymmetric chelator, in which a short binding between the metal and the sulfur atom; and, (iv) group 16 (VI-A) are complexes that interact with sulphates, resulting in organic compounds, such as thiurams, which can act as accelerators in vulcanization processes in the manufacture of compounds [16]. However, the main characteristic of group 16 is the change in the coordinates of metallic atoms. The interaction between metal and dithiocarbamate structure alters its spatial geometry and plane coordination relative to other molecular interaction models [17,18].
Several chemical compounds that belong to each of the chemical groups mentioned above might vary according to different characteristics, which may vary among chemical substituent in size, chain type (organic or inorganic), spatial geometry, types of binders, and functions, molecular targets. These compounds may act directly or indirectly on a variety of protozoa due to this chemical diversity [18,19] and are, therefore, evaluated as potential antiparasitic, as discussed below.
3. Pharmacological Evaluation of Dithiocarbamate as Potential Antitrypanosomatids-Drugs
3.1. Evaluation of Dithiocarbamate as Potential Anti-Trypanosoma Cruzi Drugs
Trypanosoma cruzi is a flagellate protozoan of the Trypanosomatidae family, which are responsible for Chagas disease, a neglected tropical disease that affects eight million people worldwide and causes more than 10,000 deaths annually. It is an endemic disease in several Latin American countries and is currently dispersed throughout the world due to migratory flows of populations [20,21].
The natural mechanism of transmission of Chagas disease occurs through vector transmission during blood repayment of previously infected triatomine insects. Other forms of transmission occur during blood transfusion, vertical transmission, organ transplants, and infected blood contacts [22,23]. Currently, the pharmacological treatment of Chagas disease consists of two main antiparasitic drugs, benznidazole and nifurtimox, drugs that are recognized by the World Health Organization for the treatment of infected individuals. These drugs have limitations on their use, mainly due to their high toxicity, and low or no pharmacological efficacy during the chronic treatment of Chagas disease [24].
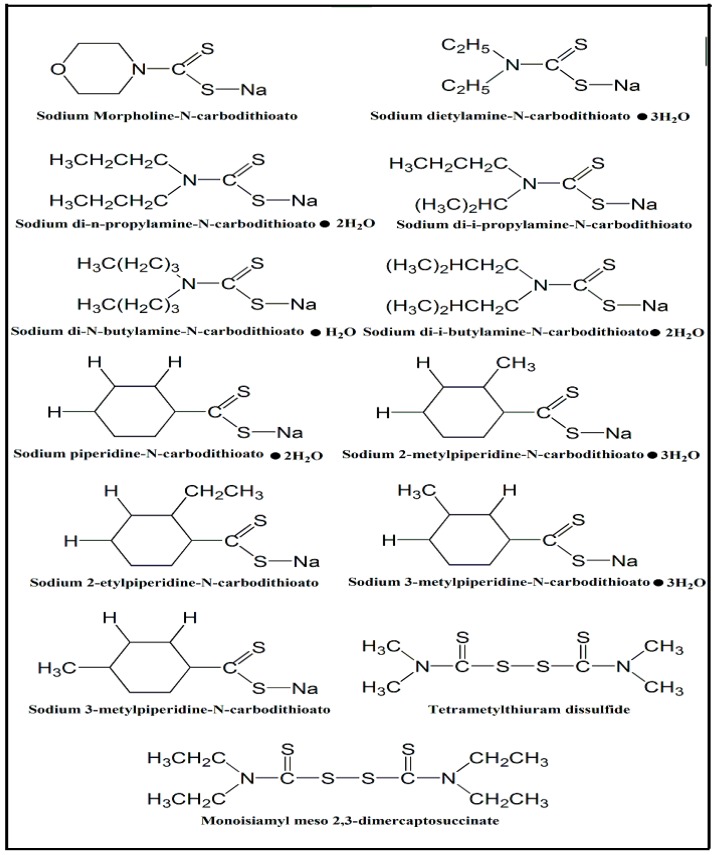
Several studies have demonstrated the ability of these compounds to inhibit the enzyme superoxide dismutase, an important enzyme in the oxidative system of many protozoa, due to the ability of dithiocarbamates to access intracellular compartments [25]. In this context, several compounds that are based on dithiocarbamates were synthesized (Figure 2). These compounds showed antiparasitic activity against T. cruzi when compared to the traditional benznidazole drug. The antiparasitic activity of dithiocarbamates is probably due to their ability to chelate metals, such as zinc, iron, and copper [26]. This biological activity in chelating metals allows for acting in other enzymes of the oxidation metabolism of protozoa, in addition to the enzyme superoxide dismutase, such as the carbonic anhydrase enzyme, which corresponds to another group of metalloproteinases with important function in parasite biology. These enzymes mainly participate in the redox metabolism of the parasite. Dithiocarbamate compounds may act on the metal centre of these enzymes by a chelation mechanism. Consequently, the parasite loses the ability to repair oxidative damage during parasitism in the host [27,28].
Figure 2.
Compounds based on dithiocarbamate used as potential anti-Trypanosoma cruzi drugs. The figure represents the different structures of dithiocarbamates that showed antiparasitic activity. These compounds showed antiparasitic activity at a concentration of 5 µM during 72 h of treatment against the T. cruzi parasite. Increased production of reactive oxygen species seems to be the probable mechanism of action for these compounds to exhibit antiparasitic activity [26].
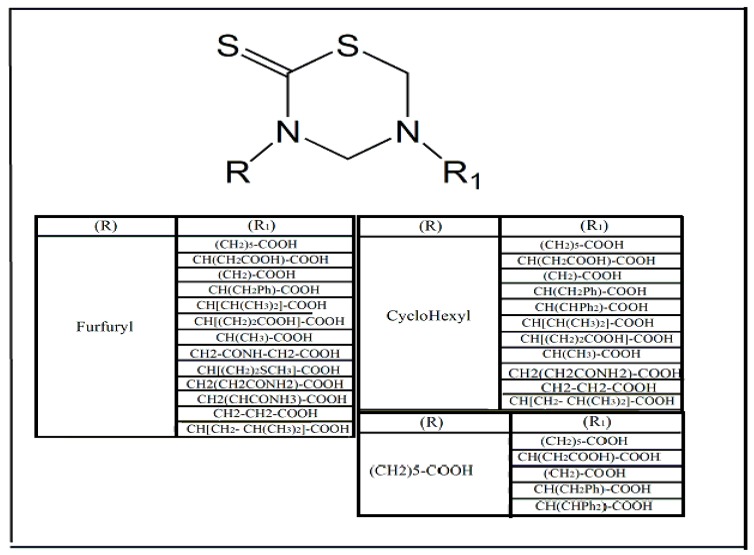
The exploration of new pharmacological targets for the development of new antiparasitic drugs is indispensable for the rational design of more specificity and low toxicity drugs [29]. Cysteine protease enzymes constitute a potential pharmacological target of protozoa that are responsible human infectious diseases. This class of enzymes chemically acts on thiol-imidazole conversion, which is an important process for parasite cell survival [30]. Compounds that are derived from thiadiazine have been shown to be enzymatic cysteine protease inhibitors of Trichomonas vaginalis and amastigote T. cruzi [30,31]. In this context, compounds that are derived from thiadiazine linked to dithiocarbamates were synthesized, resulting in the synthesis and characterization of 29 compounds with antiparasitic activity against T. cruzi (Figure 3). In addition, some of these compounds showed superior antiparasitic activity than nifurtimox, one of the therapeutic options during the pharmacological treatment of Chagas disease. The pharmacological activity that is attributed to these compounds is due to their ability to increase the production of reactive oxygen species, causing oxidative damage in the parasite and, consequently the loss of its viability [32].
Figure 3.
Thiadiazine-based synthetic compounds bound to dithiocarbamates with activity against Trypanosoma cruzi. The different substituents, represented by R1 and R2, may be attached to circular structures formed by thiadiazine and dithiocarbamate. The biological activity of these molecules appears to be related to their ability to inhibit the cysteine protease enzyme and increase oxidative damage in T. cruzi [31].
3.2. Evaluation of Dithiocarbamate as Potential Anti-Leishmania Drugs
Leishmania sp. is a genus of flagellate protozoa that belongs to the family Trypanosomatids, which are etiological agents of zoonosis known as leishmaniasis, a neglected tropical disease that mainly affects populations of social and economic vulnerability. Leishmaniasis is a vector disease that can be transmitted by more than 20 species of Leishmania sp. Leishmaniasis is classified into two main clinical forms, visceral leishmaniasis and cutaneous leishmaniasis [33,34,35]. These are protozoa that present a complex life cycle, consisting of a phase in the invertebrate host of the genus Phlebotomus and another stage in the mammalian hosts (man, dog, wild animals, etc.) [36,37,38].
Pentavalent antimonials and amphotericin B are the major classes of compounds that are used in the pharmacological treatment of leishmaniasis. However, these drugs present high toxicity, which can cause hepatic, pancreatic, and renal impairment, consequently compromising patients’ quality of life during treatment [39,40]. Additionally, these drugs are responsible for causing oxidative damage in cells of humans due to their pharmacological action [41,42,43].
Previous studies with the chemical element osmium (III) have shown an expressive biological activity inhibiting various synthesis processes, such as DNA, RNA, and proteins, which are important for cell survival [44,45]. Due to this property, the metal ion osmium III was used by binding to dinitroimidazole and nitroimidazole, which were complexed to dithiocarbamate chains to increase antiparasitic activity against the protozoan Leishmania donovani (an etiological agent of leishmaniasis) and T. cruzi. In addition, this system presented a moderate cellular toxicity, as well as inhibited the action of several oxidative and energetic metabolism enzymes, such as succinate dehydrogenase, malate dehydrogenase, and pyruvate kinase of promastigotes L. donovani and epimastigote T. cruzi [46].
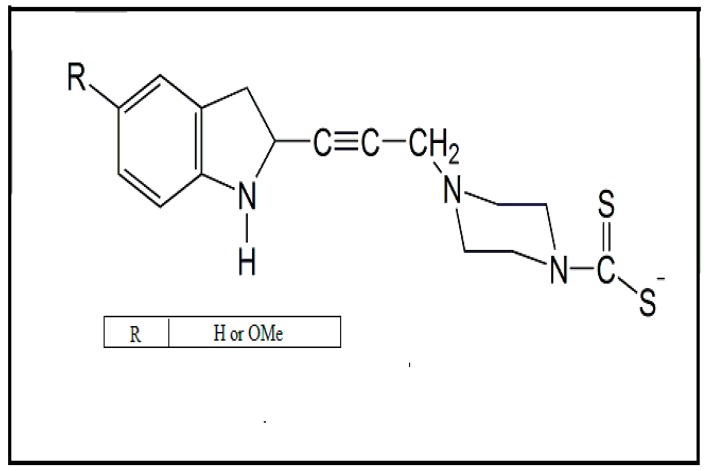
In other scenarios, analogous compounds of tryptophan have the ability to inhibit piroredoxin and the mitochondrial membrane complex II, or mitochondrial membrane complex FAD (Flavin and Adenine Dinucleotide), thereby triggering mitochondrial damage in T. brucei [47]. A series of dithiocarbamate-linked analogs produced compounds that showed antiparasitic efficacy at 30 μM against L. donovani and T. cruzi parasites. Moreover, these compounds, chemical structures, as represented in Figure 4, did not present cellular cytotoxicity in in vitro tests [48].
Figure 4.
Chemical compounds synthesized from tryptophan with dithiocarbamates used as antiparasitic drugs against Leishmania donovani and Trypanosoma cruzi. In this figure is possible that different substituents (R: H or OMe) interact with the structure formed for tryptophan-dithiocarbamate. These structures are more efficient in inducing mitochondrial damage and consequently parasite death [47].
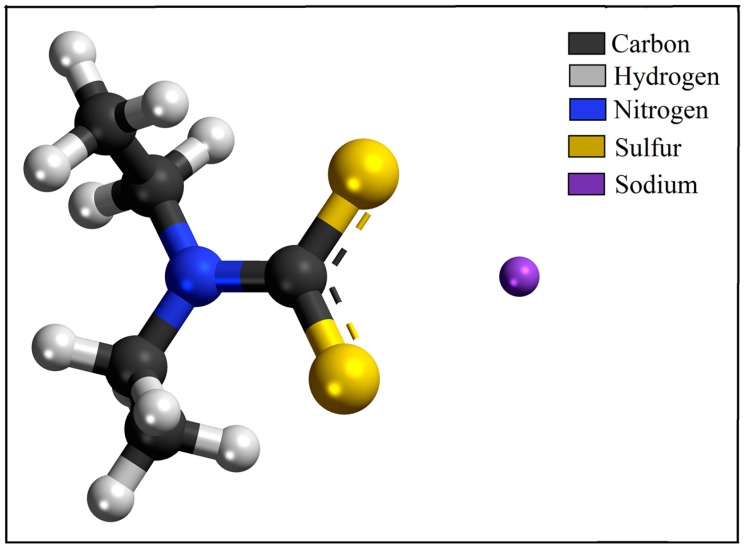
Another approach to the antiparasitic activity of the compounds that are based on diethyldithiocarbamate was demonstrated using the sodium diethyldithiocarbamate compound (DETC—Figure 5) against the different forms of L. amazonensis and L. braziliensis, responsible for visceral leishmaniasis and cutaneous leishmaniasis, respectively.
Figure 5.
Sodium diethyldithiocarbamate (DETC) three-dimensional (3-D) chemical structure used as a potential anti-Leishmania sp. drug. DETC acts as a metal chelator and therefore inactivates enzymes essential for parasite survival [49,50].
Using several in vitro and in vivo models, the antiparasitic activity of DETC at low concentrations (1 mM and 2 mM) was demonstrated for the in vitro system in macrophages cells and for the in vivo system in murine models, respectively. DETC showed anti-Leishmania activity in both experimental models against the amastigote forms of L. amazonensis [49]. In this context, DETC was used to treat cutaneous lesion caused by L. braziliensis, where it was possible to observe a significant reduction in the overall parasitic load, as well as a decrease in the harmful cellular process that is triggered during infection [50]. In addition, DETC showed inhibitory activity for superoxide dismutase (SOD), which is an important enzyme that is involved in the redox metabolism of many protozoa of medical importance. This inhibitory activity is probably due to the interaction of the SCSNa end of the DETC molecule with the metal centre of the SOD enzyme, chelating the ferric centre, which is important for the catalytic activity [51].
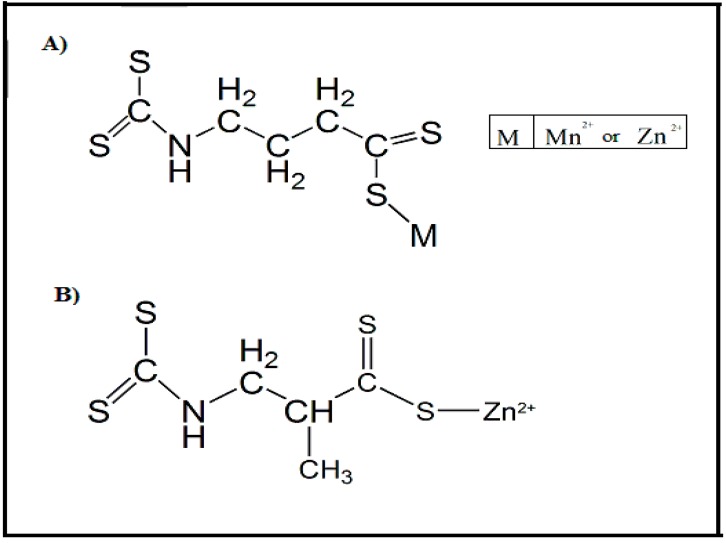
Various new compounds have been synthesized as potential anti-Leishmania drugs due to the versatility of the compounds based on dithiocarbamate. Compounds synthesized in metallic association with dithiocarbamates, named Maneb, Zineb and Propineb, were used against L. donovani [52]. The concentrations of 0.32 μM, 0.28 μM, and 0.14 μM were sufficient for eliminating 50% of the parasites. In addition, these compounds were found to have the ability to inhibit various isoforms of the carbonic anhydrase enzymes, an important component for parasite survival. Regarding the compounds shown in Figure 6, the bond between the dithiocarbamate chain and the metal is weak and easily dissociated. When this binding is broken, these free metals will interfere in oxidative processes and, therefore, stimulate the production of radical oxygen species (ROS) and intensify the response on parasite elimination [53,54,55].
Figure 6.
Synthetic dithiocarbamate derivatives bound to metal centres used as potential anti-Leishmania sp. drugs [52]. Two distinct chemical structures (A and B) that when interacting with metals (Mn2+ or Zn2+) exhibit enhanced antiparasitic activity against L. donovani parasites.
3.3. Evaluation of Dithiocarbamate as Potential Anti-Trypanosoma Brucei Drugs
The protozoan Trypanosoma brucei is the etiological agent of African trypanosomiasis, also known as sleeping sickness. This protozoan is naturally transmitted through the bite of tsetse fly of the genus Glossina sp. Human African Trypanosomiasis (HAT) mainly affects 36 countries in sub-Saharan Africa, accounting for human infection with 100% mortality if not pharmacologically treated. The HAT is transmitted by two main subspecies of T. brucei: T. b. gambiense and T. b. rhodesiense, which are responsible for chronic and acute infections, respectively. Over 70 million people are in risk areas and situations in the context of HAT [56]. HAT therapy is extremely limited, because it has high toxicity and limited pharmacological efficacy. The main drugs that are used are melarsoprol, nifurtimox, eflornithine, and suramin [57].
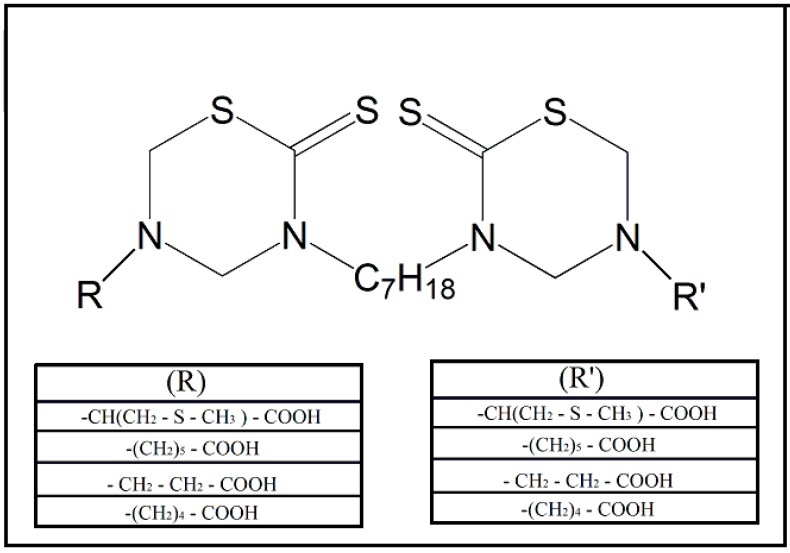
In the context of African trypanosomiasis, thiadiazine-based compounds, such as the tetrahydro-2H-1,3,5-thiadiazine-2-thione (THTT) derivatives, appear to be compounds with potential for use against T. brucei. This class of compounds stands out due to its inhibitory activity on the enzymes cysteine protease [58,59], along with the presence of the chemical group isothiocyanates [29]. This inhibitory activity occurs in a protic medium that is capable of cleaving the ring present in the THTT molecule [60]. On the other hand, due to the high solubility in lipophilic medium, its action in the enzymatic hydrolysis of several enzymes, present in some microorganisms, seems to be facilitated [61]. Various compounds that are based on BIS-THTT were synthesized due to these characteristics [62]. These compounds, as represented in the Figure 7, showed antiparasitic activity against T. b. rhodesiense, and a satisfactory selectivity index.
Figure 7.
Synthetic dithiocarbamate derivatives bound to thiadiazone used as potential anti-Trypanosoma brucei rhodesiense drugs. The basic structures may receive different chemical substituents (R and R‘) that could influence the antiparasitic activity of the new synthesized compounds [62].
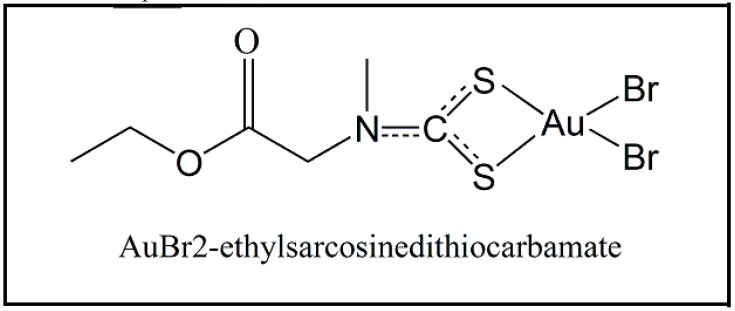
Since 2010, a new class of gold-bound compounds has been shown to have different applications as antiparasitic drugs. These compounds appear to have an inhibitory action against thioredoxin reductase and thioredoxin-glutathione reductase enzymes, transcription factors, and some proteases that are present in various parasites [63,64]. In this context, gold-bound dithiocarbamate compounds, for example AuL12 (AuBr2-ethylsarcosinedithiocarbamate (EDTS)), as represented in the Figure 8, showed antiparasitic activity against T. b. brucei and had an IC50 of 2.06 μM [65]. In addition, this compound was also evaluated against different protozoan species, such as T. cruzi, L. infantum, T. b. rhodesiense, and Plasmodium falciparum (the etiological agent of malaria), and this compound showed a good parasite elimination performance in all cases, presented IC50 1.69, 2.83, 0.51, and 1.97, respectively. Therefore, since these enzymes are part of the antioxidative mechanism of the parasites, their inhibition process, by the action of gold metal, makes the parasites more susceptible to oxidative damages. However, the amine end of the dithiocarbamates is reactive and it stimulates the production of reactive oxygen species, thereby inducing the death of the parasites [65].
Figure 8.
Synthetic dithiocarbamate derivatives bound to gold used as potential antiparasitic drugs against Trypanosoma brucei, Leishmania infantum, Trypanosoma cruzi and Plasmodium falciparum. The main biological activity attributed to this structure is related to its ability to induce oxidative stress and consequently parasite death [65].
4. Final Considerations and Expectations
Several pharmacological applications of dithiocarbamate compounds have been popularized scientifically in recent decades. Due to the chemical and structural flexibility of these compounds, their use has been widely investigated as anti-carcinogenic agents [66,67,68,69,70,71,72,73,74,75,76,77], antibacterial [78,79], antiviral [80,81], antifungal [82,83], among others. In this review, we have presented and discussed the different applications of these compounds as potential antiparasitic compounds, more specifically as antitrypanosomatids-drugs against Chagas disease, leishmaniasis, and African trypanosomiasis. It is also important to note that some of these compounds have already been validated for their specific pharmacological targets in in vitro models [84,85,86,87,88,89,90,91,92,93].
Finally, dithiocarbamates are an important chemical compound that are to be considered in the rational development of antiparasitic drugs, especially in the context of neglected tropical diseases, an urgent need public health in many developing countries. Other important issues need to be resolved for the use of dithiocarbamates as potential new drugs, especially toxicological studies, definition of pharmacological targets, the parameters of chemical structure and biological activity, among others.
Acknowledgments
J.W.d.F.O. and W.M.T.Q.d.M. thanks the financial support provided by Capes/Brazil through postgraduate and postdoctoral scholarships, respectively. M.S.S. thanks the financial support provided by Global Health and Tropical Medicine, Lisbon—Portugal (GHTM-UID/multi/04413/2013). H.A.O.R. is CNPq fellowship honored researcher. We are also grateful to Paulo Fanado for editing this manuscript.
Author Contributions
All authors participated actively in the writing and discussion of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was funded by Global Health and Tropical Medicine—Grant number IHTM-UID/multi/04413/2013, FCT-Portugal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Debus H. Ueber die verbindungen der sulfocarbaminsäure. Justus Liebigs Ann. Chem. 1850;73:26–34. doi: 10.1002/jlac.18500730103. [DOI] [Google Scholar]
- 2.Topping R.J., Jones M.M. Optimal dithiocarbamate structure for immunomodulator action. Med. Hypotheses. 1988;27:55–57. doi: 10.1016/0306-9877(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 3.Gucchait A., Joardar N., Parida P.K., Roy P., Mukherjee N., Dutta A., Yesuvadian R., SinhaBabu S.P., Jana K., Misra A.K. Development of novel anti-filarial agents using carbamo (dithioperoxo) thioate derivatives. Eur. J. Med. Chem. 2018;143:598–610. doi: 10.1016/j.ejmech.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Buac D., Schmitt S., Ventro G., Rani Kona F., Ping Dou Q. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini Rev. Med. Chem. 2012;12:1193–1201. doi: 10.2174/138955712802762040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang H., Chen D., Cui Q.C., Dou Q.P. Sodium diethyldithiocarbamate, an AIDS progression inhibitor and a copper-binding compound, has proteasome-inhibitory and apoptosis-inducing activities in cancer cells. Int. J. Mol. Med. 2007;19:809–816. doi: 10.3892/ijmm.19.5.809. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Cortes S., Domingo C., García-Ramos J.V., Aznárez J.A. Surface-enhanced vibrational study (SEIR and SERS) of dithiocarbamate pesticides on gold films. Langmuir. 2001;17:1157–1162. doi: 10.1021/la001269z. [DOI] [PubMed] [Google Scholar]
- 7.Meco G., Bonifati V., Vanacore N., Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand. J. Work Environ. Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- 8.Bonamico M., Dessy G., Mugnoli A., Vaciago A., Zambonelli L. Structural studies of metal dithiocarbamates. I. The crystal and molecular structure of the α-form of nickel diethyldithiocarbamate. Acta Crystallogr. 1965;19:619–626. doi: 10.1107/S0365110X65003985. [DOI] [Google Scholar]
- 9.Bonamico M., Dessy G., Mugnoli A., Vaciago A., Zambonelli L. Structural studies of metal dithiocarbamates. II. The crystal and molecular structure of copper diethyldithiocarbamate. Acta Crystallogr. 1965;19:886–897. doi: 10.1107/S0365110X65004619. [DOI] [Google Scholar]
- 10.Bonamico M., Mazzone G., Vaciago A., Zambonelli L. Structural studies of metal dithiocarbamates. III. The crystal and molecular structure of zinc diethyldithiocarbamate. Acta Crystallogr. 1965;19:898–909. doi: 10.1107/S0365110X65004620. [DOI] [Google Scholar]
- 11.Hogarth G. Transition metal dithiocarbamates: 1978–2003. In: Kenneth D.K., editor. Progress in Inorganic Chemistry. Volume 53. John Wiley & Sons; Hoboken, NJ, USA: 2005. pp. 71–561. [Google Scholar]
- 12.Bond A.M., Martin R.L. Electrochemistry and redox behaviour of transition metal dithiocarbamates. Coord. Chem. Rev. 1984;54:23–98. doi: 10.1016/0010-8545(84)85017-1. [DOI] [Google Scholar]
- 13.Coucouvanis D. The chemistry of the dithioacid and 1, 1-dithiolate complexes. Prog. Inorg. Chem. 1970;11:233–371. [Google Scholar]
- 14.De Koning P.D., McAndrew D., Moore R., Moses I.B., Boyles D.C., Kissick K., Stanchina C.L., Cuthbertson T., Kamatani A., Rahman L., et al. Fit-for-purpose development of the enabling route to crizotinib (PF-02341066) Org. Proc. Res. Dev. 2011;15:1018–1026. doi: 10.1021/op200131n. [DOI] [Google Scholar]
- 15.Corredor C., Tomasella F.P., Young J. Drug interactions with potential rubber closure extractables: Identification of thiol–disulfide exchange reaction products of captopril and thiurams. J. Chromatogr. A. 2009;1216:43–48. doi: 10.1016/j.chroma.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Heard P.J. Main group dithiocarbamate complexes. In: Kenneth D.K., editor. Progress in Inorganic Chemistry. Volume 53. John Wiley & Sons; Hoboken, NJ, USA: 2005. pp. 1–69. [Google Scholar]
- 17.Chaturvedi D., ZaidI S. The role of Organic Dithiocarbamates in Drug Discovery Research. Res. Rev. J. Chem. 2016;5:10–12. [Google Scholar]
- 18.Kanchi S., Singh P., Bisetty K. Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem. 2014;7:11–25. doi: 10.1016/j.arabjc.2013.04.026. [DOI] [Google Scholar]
- 19.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 20.Piacenza L., Alvarez M.N., Peluffo G., Radi R. Fighting the oxidative assault: The Trypanosoma cruzi journey to infection. Cur. Opin. Microbiol. 2009;12:415–421. doi: 10.1016/j.mib.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Milei J., Guerri-Guttenberg R.A., Grana D.R., Storino R. Prognostic impact of Chagas disease in the United States. Am. Heart J. 2009;157:22–29. doi: 10.1016/j.ahj.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Villela M.M., Rodrigues V.L.C.C., Casanova C., Dias J.C.P. Analysis on the food source of Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) and its present importance as a vector for Trypanosoma cruzi, in the State of Minas Gerais. Rev. Soc. Bras. Med. Trop. 2010;43:125–128. doi: 10.1590/S0037-86822010000200004. [DOI] [PubMed] [Google Scholar]
- 23.Castro J.A., Demecca M.M., Bartel L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 24.Giulivi C., Turrens J.F., Boveris A. Chemiluminescence enhancement by trypanocidal drugs and by inhibitors of antioxidant enzymes in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1988;30:243–251. doi: 10.1016/0166-6851(88)90093-X. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues R.R., Elane J., Ecarter C., Jbogitsh B., Ksingh P., Jzimmerman L., Jmolenda J., Mjones M. Chelating agent inhibition of Trypanosoma cruzi epimastigotes in vitro. J. Inorg. Biochem. 1995;60:277–288. doi: 10.1016/0162-0134(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 26.Winum J., Supuran C.T. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2015;30:321–324. doi: 10.3109/14756366.2014.913587. [DOI] [PubMed] [Google Scholar]
- 27.Pan P., Vermelho A.B., Scozzafava A., Parkkila S., Capasso C., Supuran C.T. Anion inhibition studies of the α-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorganic. Med. Chem. 2013;21:4472–4476. doi: 10.1016/j.bmc.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 28.Verma S., Dixit R., Pandey K.C. Cysteine proteases: Modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016;7:107. doi: 10.3389/fphar.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North M.J., Mottram J.C., Coombs G.H. Cysteine proteinases of parasitic protozoa. Parasitol. Today. 1990;6:270–275. doi: 10.1016/0169-4758(90)90189-B. [DOI] [PubMed] [Google Scholar]
- 30.Atienza J., Martínez Díaz R.A., Gómez Barrio A., Escario J.A., Herrero A., Ochoa C., Rodríguez J. Activity assays of thiadiazine derivatives on Trichomonas vaginalis and amastigote forms of Trypanosoma cruzi. Chemotherapy. 1992;38:441–446. doi: 10.1159/000239040. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa C., Pérez E., Roland P., Suárez M., Ochoab E., Rodríguez H., Barrio A.G., Susana M., Nogal J.J., Martínez R.A. Synthesis and antiprotozoan properties of new 3, 5—Disubstituted -tetrahydro-2H-1, 3, 5-thiadiazine-2-thione derivatives. Arzneimittelforschung. 1999;49:764–769. doi: 10.1055/s-0031-1300499. [DOI] [PubMed] [Google Scholar]
- 32.Herrero A., Ochoa C., Atienza J., Antonio Escario J., Gómez-Barrio A., Martínez Fernández A.R. Synthesis and Antiprotozoal Properties of 1, 2, 6-Thiadiazine 1, 1-Dioxide Derivatives. Arch. Pharm. 1992;325:509–514. doi: 10.1002/ardp.19923250811. [DOI] [PubMed] [Google Scholar]
- 33.Houweling T.A., Karim-Kos H.E., Kulik M.C., Stolk W.A., Haagsma J.A., Lenk E.J., Richardus J.H., de Vlas S.J. Socioeconomic inequalities in neglected tropical diseases: A systematic review. PLoS Negl. Trop. Dis. 2016;10:e0004546. doi: 10.1371/journal.pntd.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uliana S.R., Trinconi C.T., Coelho A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology. 2018;145:464–480. doi: 10.1017/S0031182016002523. [DOI] [PubMed] [Google Scholar]
- 35.Alvar J., Aparicio P., Aseffa A., Den Boer M., Cañavate C., Dedet J.P., Gradoni L., Ter Horst R., López-Vélez R., Moreno J. The relationship between leishmaniasis and AIDS: The second 10 years. Clin. Microbiol. Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lestinova T., Rohousova I., Sima M., de Oliveira C.I., Volf P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017;11:e0005600. doi: 10.1371/journal.pntd.0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Leishmaniases: Epidemiological Report of the Americas. N° 7. World Health Organization; Genève, Switzerland: 2019. [Google Scholar]
- 38.No J.H. Visceral leishmaniasis: Revisiting current treatments and approaches for future discoveries. Acta Trop. 2016;155:113–123. doi: 10.1016/j.actatropica.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Sundar S., Singh A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology. 2018;145:481–489. doi: 10.1017/S0031182017002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laniado-Laborín R., Cabrales-Vargas M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009;26:223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson S.R., Bot C., Kelly J.M., Hall B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011;11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 42.Kato K.C., Morais-Teixeira E., Reis P.G., Silva-Barcellos N.M., Salaün P., Campos P.P., Dias Corrêa-Junior J., Rabello A., Demicheli C., Frézard F. Hepatotoxicity of pentavalent antimonial drug: Possible role of residual Sb (III) and protective effect of ascorbic acid. Antimicrob. Agents Chemother. 2014;58:481–488. doi: 10.1128/AAC.01499-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brajtburg J., Elberg S., Schwartz D.R., Vertut-Croquin A., Schlessinger D., Kobayashi G.S., Medoff G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob. Agents Chemother. 1985;27:172–176. doi: 10.1128/AAC.27.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukášová E., Vojtísková M., Jelen F., Sticzay T., Palecek E. Osmiuminduced alteration in DNA-structure. Gen. Physiol. Biophys. 1984;3:75–191. [PubMed] [Google Scholar]
- 45.Rodriguez M., Bard A.J. Electrochemical studies of the interaction of metal chelates with DNA. 4. Voltammetric and electrogenerated chemiluminescent studies of the interaction of tris (2, 2’-bipyridine) osmium (II) with DNA. Anal. Chem. 1990;62:2658–2662. doi: 10.1021/ac00223a002. [DOI] [PubMed] [Google Scholar]
- 46.Castilla J.J., Mesa-Valle C.M., Sanchez-Moreno M., Arnedo T., Rosales M.J., Mascaro C., Craciunescu D., Osuna A. In vitro activity and biochemical effectiveness of new organometallic complexes of osmium (III) against Leishmania donovani and Trypanosoma cruzi. Arzneimittel-Forschung. 1996;46:990–996. [PubMed] [Google Scholar]
- 47.Markham A., Hooper M., Fairlamb A.H., Berger B.J., Mung’ong’o S.G. Activity of Novel Tryptophan Analogs against Mammalian and Trypanosomal Monoamine Oxidases. East Cent. Afr. J. Pharm. Sci. 2003;6:43–49. [Google Scholar]
- 48.Mugoyela V.K., Hooper M., Fairlamb A.H., Mung’ong’o S.G., Croft S. Activity of Novel Tryptophan Analogs against Trypanosoma cruzi and Leishmania donovani. East Cent. Afr. J. Pharm. Sci. 2008;11 doi: 10.4314/ecajps.v11i3.46283. [DOI] [Google Scholar]
- 49.Khouri R., Novais F., Santana G., de Oliveira C.I., Vannier dos Santos M.A., Barral A., Barral-Netto M., Van Weyenbergh J. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: A promising therapeutic alternative in Leishmaniasis. PLoS ONE. 2010;5:e14394. doi: 10.1371/journal.pone.0014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celes F.S., Trovatti E., Khouri R., van Weyenbergh J., Ribeiro S.J.L., Borges V.M., Barud H.S., de Oliveira C.I. DETC-based bacterial cellulose bio-curatives for topical treatment of cutaneous leishmaniasis. Sci. Rep. 2016;6:38330. doi: 10.1038/srep38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosh S., Goswami S., Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem. J. 2003;369:447–452. doi: 10.1042/bj20021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal D.S., Mondal D.K., Datta R. Identification of metal dithiocarbamates as a novel class of antileishmanial agents. Antimicrob. Agents Chemother. 2015;59:2144–2152. doi: 10.1128/AAC.05146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vermelho A.B., Capaci G.R., Rodrigues I.A., Cardoso V.S., Mazotto A.M., Supuran C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorganic Med. Chem. 2017;25:1543–1555. doi: 10.1016/j.bmc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 54.Nocentini A., Cadoni R., Dumy P., Supuran C.T., Winum J.Y. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J. Enzym. Inhib. Med. Chem. 2018;33:286–289. doi: 10.1080/14756366.2017.1414808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Da Silva Cardoso V., Vermelho A.B., Ricci Junior E., Almeida Rodrigues I., Mazotto A.M., Supuran C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzym. Inhib. Med. Chem. 2018;33:850–857. doi: 10.1080/14756366.2018.1463221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett M.P. The elimination of human African trypanosomiasis is in sight: Report from the third WHO stakeholders meeting on elimination of gambiense human African trypanosomiasis. PLoS Negl. Trop. Dis. 2018;12:e0006925. doi: 10.1371/journal.pntd.0006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wéry M. Drug used in the treatment of sleeping sickness (human African trypanosomiasis: HAT) Int. J. Antimicrob. Agents. 1994;4:227–238. doi: 10.1016/0924-8579(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 58.Rieche A., Hilgetag G., Martini A., Nejedly O., Schlegel J. New compounds with bactericidal and fungicidal activity and which inhibit the growth of viruses. I. 2-thiotetrahydro-1, 3, 5-thiadiazine (“carbothialdine”) and dithiocarbamic acid salts. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1960;293:957–967. doi: 10.1002/ardp.19602931102. [DOI] [PubMed] [Google Scholar]
- 59.Goksoyr J. Chemical and fungicidal reactions of 3, 5-dimethyltetrahydro-1, 3, 5-thiadiazine-2-thione (3, 5-D). A comparison with sodium N-methyl dithiocarbamate and methyl isothiocyanate. Acta Chem. Scand. 1964;18:1341–1352. doi: 10.3891/acta.chem.scand.18-1341. [DOI] [Google Scholar]
- 60.El-Shorbagi A. Model for delivery of amines through incorporation into a tetrahydro-2H-1, 3, 5-thiadiazine-2-thione structure. Eur. J. Med. Chem. 1994;29:11–15. doi: 10.1016/0223-5234(94)90120-1. [DOI] [Google Scholar]
- 61.Aboul-Fadl T., El-Shorbagi A. New prodrug approach for amino acids and amino-acid-like drugs. Eur. J. Med. Chem. 1996;31:165–169. doi: 10.1016/0223-5234(96)80450-8. [DOI] [Google Scholar]
- 62.Bermello J.C., Piñeiro R.P., Fidalgo L.M., Cabrera H.R., Navarro M.S. Thiadiazine derivatives as antiprotozoal new drugs. Open Med. Chem. J. 2011;5:51. doi: 10.2174/1874104501105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Micale N., Cinellu M.A., Maiore L., Sannella A.R., Severini C., Schirmeister T., Gabbiani C., Messori L. Selected gold compounds cause pronounced inhibition of Falcipain 2 and effectively block P. falciparum growth in vitro. J. Inorg. Biochem. 2011;105:1576–1579. doi: 10.1016/j.jinorgbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Madeira J.M., Gibson D.L., Kean W.F., Klegeris A. The biological activity of auranofin: Implications for novel treatment of diseases. Inflammopharmacology. 2012;20:297–306. doi: 10.1007/s10787-012-0149-1. [DOI] [PubMed] [Google Scholar]
- 65.Massai L., Messori L., Micale N., Schirmeister T., Maes L., Fregona D., Cinellu M.A., Gabbiani C. Gold compounds as cysteine protease inhibitors: Perspectives for pharmaceutical application as antiparasitic agents. BioMetals. 2017;30:313–320. doi: 10.1007/s10534-017-0007-0. [DOI] [PubMed] [Google Scholar]
- 66.Cilibrasi V., Tsang K., Morellia M., Solfa F., Wiggins H.L., Jones A.T., Westwell A.D. Synthesis of substituted carbamo (dithioperoxo) thioates as potential BCA2-inhibitory anticancer agents. Tetrahedron Lett. 2015;56:2583–2585. doi: 10.1016/j.tetlet.2015.03.132. [DOI] [Google Scholar]
- 67.Song S., Abdelmohsen K., Zhang Y., Becker K.G., Gorospe M., Bernier M. Impact of pyrrolidine dithiocarbamate and interleukin-6 on mammalian target of rapamycin complex 1 regulation and global protein translation. J. Pharm. Exp. Ther. 2011;339:905–913. doi: 10.1124/jpet.111.185678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amir M.K., Hayat F., Khan S.Z., Hogarth G., Kondratyuk T., Pezzutode J.M., Tahir M.N. Monofunctional platinum (II) dithiocarbamate complexes: Synthesis, characterization and anticancer activity. RSC Adv. 2016;6:110517–110524. doi: 10.1039/C6RA19469A. [DOI] [Google Scholar]
- 69.Altaf M., Monim-ul-Mehboob M., Kawde A.-N., Corona G., Larcher R., Ogasawara M., Casagrande N., Celegato M., Borghese C., Siddik Z.H., et al. New bipyridine gold (III) dithiocarbamate-containing complexes exerted a potent anticancer activity against cisplatin-resistant cancer cells independent of p53 status. Oncotarget. 2017;8:490. doi: 10.18632/oncotarget.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Aarag B.Y., Kasai T., Zahran M.A., Zakhary N.I., Shigehiro T., Sekhar S.C., Agwa H.S., Mizutani A., Murakami H., Kakuta H., et al. In vitro anti-proliferative and anti-angiogenic activities of thalidomide dithiocarbamate analogs. Int. Immunopharmacol. 2014;21:283–292. doi: 10.1016/j.intimp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Ding P.-P., Gao M., Mao B.-B., Cao S.-L., Liu C.-H., Yang C.-R., Li Z.-F., Liao J., Zhao H., Li Z., et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur. J. Med. Chem. 2016;108:364–373. doi: 10.1016/j.ejmech.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 72.Ali I., Wani W.A., Saleem K., Hseih M.-F. Design and synthesis of thalidomide based dithiocarbamate Cu (II), Ni (II) and Ru (III) complexes as anticancer agents. Polyhedron. 2013;56:134–143. doi: 10.1016/j.poly.2013.03.056. [DOI] [Google Scholar]
- 73.Anthwal ASingh K., Rawat M.S.M., Kumar A., Aggarwal B.B., Rawat D.S. C5-curcuminoid-dithiocarbamate based molecular hybrids: Synthesis and anti-inflammatory and anti-cancer activity evaluation. RSC Adv. 2014;4:28756–28764. doi: 10.1039/C4RA03655G. [DOI] [Google Scholar]
- 74.Li Z., Zhang J., Jin Z., Zhang W.Z.Y. Synthesis and biodistribution of novel 99m Tc labeled 4-nitroimidazole dithiocarbamate complexes as potential agents to target tumor hypoxia. Med. Chem. Commun. 2015;6:1143–1148. doi: 10.1039/C5MD00042D. [DOI] [Google Scholar]
- 75.Mansouri-Torshizi H., Saeidifar M., Divsalar A., Saboury A.A. Interaction studies between a 1, 10-phenanthroline adduct of palladium (II) dithiocarbamate anti-tumor complex and calf thymus DNA. A synthesis spectral and in-vitro study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010;77:312–318. doi: 10.1016/j.saa.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y.C., Duan Y.C., Ma J.L., Xu R.M., Zi X., Lv W.L., Wang M.M., Ye X.W., Zhu S., Mobley D., et al. Triazole–dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013;56:8543–8560. doi: 10.1021/jm401002r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keter F.K., Guzei I.A., Nell M., Zyl W.E., Darkwa J. Phosphinogold (I) dithiocarbamate complexes: Effect of the nature of phosphine ligand on anticancer properties. Inorg. Chem. 2014;53:2058–2067. doi: 10.1021/ic4025926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takii T., Horita Y., Kuroishi R., Chiba T., Mori M., Hasegawa T., Ito T., Tagami T., Ozeki T., Ito S., et al. The potential therapeutic usage of dithiocarbamate sugar derivatives for multi-drug resistant tuberculosis. In: Cardona P.J., editor. Understanding Tuberculosis-New Approaches to Fighting Against Drug Resistance. IntechOpen; Rijeka, Croatia: 2012. pp. 263–272. [Google Scholar]
- 79.Horita Y., Takii T., Kuroishi R., Chiba T., Ogawa K., Kremer L., Sato Y., Lee Y., Hasegawa T., Onozaki K. Synthesis and evaluation of anti-tubercular activity of new dithiocarbamate sugar derivatives. Bioorganic Med. Chem. Lett. 2011;21:899–903. doi: 10.1016/j.bmcl.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 80.Wiesener N., Zimmer C., Jarasch-Althof N., Wutzler P., Henke A. Therapy of experimental influenza virus infection with pyrrolidine dithiocarbamate. Med. Microbiol. Immunol. 2011;200:115–126. doi: 10.1007/s00430-010-0182-x. [DOI] [PubMed] [Google Scholar]
- 81.Qiu M., Chen Y., Cheng L., Chu Y., Song H.Y., Wu Z.W. Pyrrolidine dithiocarbamate inhibits herpes simplex virus 1 and 2 replication, and its activity may be mediated through dysregulation of the ubiquitin-proteasome system. J. Virol. 2013;87:8675–8686. doi: 10.1128/JVI.00869-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y., Li S. Facile synthesis and antifungal activity of dithiocarbamate derivatives bearing an amide moiety. J. Serb. Chem. Soc. 2015;80:1367–1374. doi: 10.2298/JSC150114047L. [DOI] [Google Scholar]
- 83.Ferreira I.P., de Lima G.M., Paniago E.B., Takahashi J.A., Pinheiro C.B. Synthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni (II), Pd (II) and Pt (II) Inorg. Chem. Acta. 2014;423:443–449. doi: 10.1016/j.ica.2014.09.002. [DOI] [Google Scholar]
- 84.Zhang J., Zhang S., Guo H., Wang X. Synthesis and biological evaluation of a novel 99mTc (CO) 3 complex of ciprofloxacin dithiocarbamate as a potential agent to target infection. Bioorganic Med. Chem. Lett. 2010;20:3781–3784. doi: 10.1016/j.bmcl.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 85.Yang C.R., Peng B., Cao S.L., Ren T.T., Jiang W., Wang F.C., Li Y.S., Wang G., Li Z., Xu S., et al. Synthesis, cytotoxic evaluation and target identification of thieno [2, 3-d] pyrimidine derivatives with a dithiocarbamate side chain at C2 position. Eur. J. Med. Chem. 2018;154:324–340. doi: 10.1016/j.ejmech.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 86.Liao J., Zhao H., Zhou L. Theoretical study on the bifunctional substitution reactions between gold (III) dithiocarbamate derivative Au (DMDT) Cl2 (DMDT = N,N-dimethyldithiocarbamate) and target molecules. Comput. Theor. Chem. 2014;1048:84–94. doi: 10.1016/j.comptc.2014.08.027. [DOI] [Google Scholar]
- 87.Fu D., Zhang S.Y., Liu Y.C., Zhang L., Liu J.J., Song J., Zhao R.H., Li F., Sun H.H., Liu H.M., et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate–chalcone derivates. Bioorganic Med. Chem. Lett. 2016;26:3918–3922. doi: 10.1016/j.bmcl.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Yurttaş L., Özkay Y., Demirci F., Göğer G., Yıldırım Ş.U., Mohsen U.A., Öztürk Ö.Y., Kaplancıklı Z.A. Synthesis, anticandidal activity, and cytotoxicity of some thiazole derivatives with dithiocarbamate side chains. Turk. J. Chem. 2014;38:815–824. doi: 10.3906/kim-1312-62. [DOI] [Google Scholar]
- 89.Yurttaş L., Yusuf Ö., Murat D., Gülhan T., Ahmet Ö., Zerrin C., Kaan K., Asım K.Z. Synthesis and antimicrobial activity evaluation of new dithiocarbamate derivatives bearing thiazole/benzothiazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2016;191:1166–1173. doi: 10.1080/10426507.2016.1150277. [DOI] [Google Scholar]
- 90.Duan Y.-C., Zheng Y.-C., Li X.-C., Wang M.-M., Ye X.-W., Guan Y.-Y., Liu G.-Z., Zheng J.-X., Liu H.-M. Design, synthesis and antiproliferative activity studies of novel 1, 2, 3-triazole–dithiocarbamate–urea hybrids. Eur. J. Med. Chem. 2013;64:99–110. doi: 10.1016/j.ejmech.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 91.Behar M., Barken D., Werner S.L., Hoffmann A. The dynamics of signaling as a pharmacological target. Cell. 2013;155:448–461. doi: 10.1016/j.cell.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Innocenti A., Scozzafava A., Supuran C.T. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII, and XIV with less investigated anions including trithiocarbonate and dithiocarbamate. Bioorganic. Med. Chem. Lett. 2010;20:1548–1550. doi: 10.1016/j.bmcl.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 93.Al-Jaroudi S.S., Altaf M., Seliman A.A., Yadav S., Arjmand F., Alhoshani A., Korashy H.M., Ahmad S., Isab A.A. Synthesis, characterization, in vitro cytotoxicity and DNA interaction study of phosphanegold (I) complexes with dithiocarbamate ligands. Inorg. Chem. Acta. 2017;464:37–48. doi: 10.1016/j.ica.2017.04.040. [DOI] [Google Scholar]