Abstract
Phytochemical investigation of the aerial parts of Pteris cretica led to the isolation and elucidation of nine pterosins, including four new pterosins, creticolacton A (1), 13-hydroxy-2(R),3(R)-pterosin L (2), creticoside A (3), and spelosin 3-O-β-d-glucopyranoside (4), together with five known pterosins 5–9. Their structures were identified mainly on the basis of 1D and 2D NMR spectral data, ESI-MS and literature comparisons. Compounds 1 and 3 were new type of petrosins with a six membered ring between C-14 and C-15. The new compounds were tested in vitro for their cytotoxic activities against four human tumor cell lines (SH-SY5Y, SGC-7901, HCT-116, Lovo). Results showed that compounds 1 and 2 exhibited cytotoxic activity against HCT-116 cells with IC50 value of 22.4 μM and 15.8 μM, respectively.
Keywords: Pteris cretica Linn., pterosins, cytotoxic activity
1. Introduction
The genus Pteris (Pteridaceae) comprises about 300 species, which are mainly distributed over the tropical and temperate zones throughout the world. There are about 66 species of the genus in China, especially in South and Southwest of China [1]. Pteris is commonly known as “Jue-Cai” in Chinese. Most species of this genus such as Pteris multifida, Pteris cretica, and Pteris nervosa are used as traditional Chinese medicines to clear heat, remove dampness and cool blood in medical practice [2]. In recent years, the phytochemical investigation of this genus has resulted in the isolation and identification of sesquiterpenoids (known as pterosins), diterpenes, flavonoids and various quinic acids [3,4,5]. Among of above secondary metabolites, pterosins, illudane-type sesquiterpenoids are most significant characteristic constituents of Pteris. Moreover, pterosins are used as the chemical markers for Pteridaceae [6]. Pterosins can be classified into three categories according to the number of skeletal carbon, such as 13 carbon, 14 carbon and 15 carbon pterosin derivatives [6,7]. Pharmacological investigations showed that pterosins had many bioactivities, such as antitumor, anti-inflammatory, anti-diabetes and anti-tuberculosis properties [8,9,10,11]. However, most studies on pterosins from this genus focused on Pteris multifida [7]. Other species of the Pteris genera probably possess pharmacological activities and bioactive secondary metabolites that are similar to Pteris multifida. As a part of our ongoing research aimed at finding potentially bioactive components from medicinal plants of the genus Pteris [12,13,14], we have carried out a detailed phytochemical investigation of the aerial parts of Pteris cretica Linn. (P. cretica). Herein described are the isolation and structural elucidation of nine pterosins, including four pterosins (compounds 1, 2, 5 and 6), along with five pterosides (compounds 3, 4 and 7–9).
2. Results and Discussion
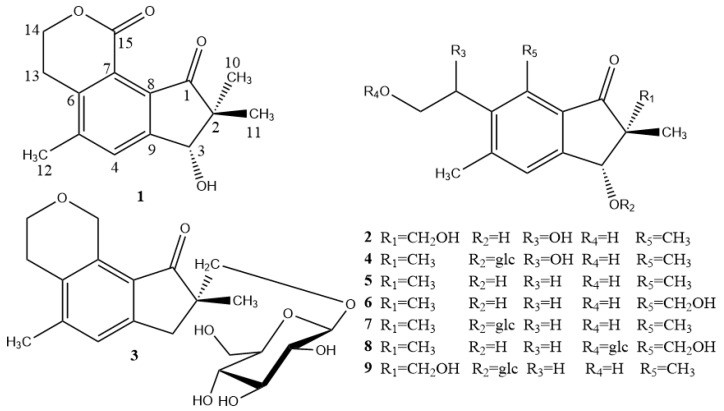
The 70% ethanol extract of P. cretica was extracted with petroleum ether, dichloromethane, EtOAc and n-BuOH, separately. The n-BuOH extraction was repeatedly subjected to thin-layer, normal-phase column and reversed phase semi-preparative column chromatography to afford four new pterosins 1–4, together with five known pterosins, namely pterosin D (5) [15], (3R)-pterosin W (6) [8], (3R)-pterosin D 3-O-β-d-glucopyranoside (7) [16], 3(R)-pteroside W (8) [17], 2R,3R-pterosin L 3-O-β-d-glucopyranoside (9) [18]. The structures of compounds 1–9 are illustrated in Figure 1.
Figure 1.
Structures of compounds 1–9.
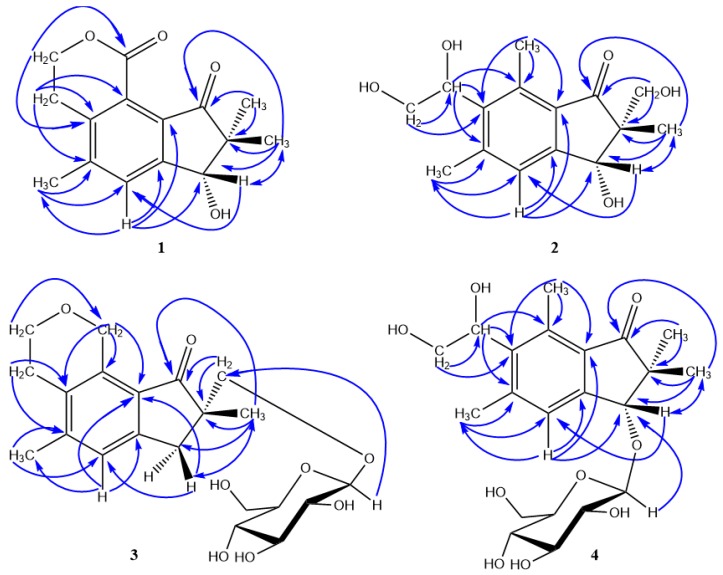
Compound 1 was isolated as colorless amorphous powder (MeOH) and showed a molecular formula of C15H16O4 with eight degrees of unsaturation, as determined by a HRESI-MS peak at m/z 261.1127 [M + H]+ (calcd for C15H17O4, 261.1127) (See Supplementary Materials). The UV spectrum exhibited the characteristic absorptions of pterosin-type sesquiterpenes at 215 (4.03), 258 (3.71), 310 (2.97) nm [15]. In the 1H-NMR spectrum (Table 1), three singlet methyl signals, including two geminal dimethyl groups located at C-2 (δH 1.10 and 1.25, each 3H) and one aromatic methyl group (δH 2.49, 3H), a carbinyl proton at C-3 (δH 4.80, s), an aromatic proton (δH 7.80, s), and two methine groups [δH 3.06 (2H, m, H-13) and 4.55 (1H, m, H-14a), 4.49 (1H, m, H-14b)] were observed. The 13C-NMR displayed 15 carbon resonances (Table 1), including three methyls at δC 20.0 (C-10), δc 20.3 (C-12) and δc 22.6 (C-11), two methylenes at δc 27.0 (C-13) and δc 67.9 (C-14), two methines at δc 77.1 (C-3) and δc 132.4 (C-4), and eight quaternary carbons. Comparison of the NMR data of 1 with those of pterosin D (5) [15], which was isolated at the same time, revealed that they shared a similar structure. The differences between them were that one ester carbonyl group (δc 163.5) replacing the methyl group (C-15) of pterosin D was observed in 1. The proton and carbon chemical shifts of C-14 appeared at the low field region due to the deshielding influence of the oxygenatom suggested 1 possessed a lactone link between positions C-14 and C-15. This was further confirmed by the HMBC spectrum (Figure 2) that showed the correlations from H-14 (δH 4.49, 4.55) to C-15 (δC 163.5). The absolute configuration of 1 was also determined as 3R by the CD spectrum which exhibited a positive cotton effect at 326 nm [19]. Based on these results, compound 1 was identified as creticolactone A, which represents the new type of petrosin possessing a six membered lactone ring between positions C-14 and C-15.
Table 1.
The NMR spectroscopic data of compounds 1 and 2 (MeOH-d4, 1H-NMR 600 MHz, 13C-NMR 150 MHz).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δ C | δH (J in Hz) | δ C | |
| 1 | - | 206.1 | - | 209.7 |
| 2 | - | 53.4 | - | 57.0 |
| 3 | 4.80 (s) | 77.1 | 4.84 (s) | 77.4 |
| 4 | 7.80 (s) | 132.4 | 7.35 (s) | 126.9 |
| 5 | - | 144.4 | - | 146.6 |
| 6 | - | 144.1 | - | 140.4 |
| 7 | - | 123.9 | - | 138.2 |
| 8 | - | 134.0 | - | 133.1 |
| 9 | - | 155.1 | - | 155.6 |
| 10 | 1.10 (s) | 20.0 | 3.73 (d, J = 11.0) | 67.1 |
| 3.68 (d, J = 11.0) | ||||
| 11 | 1.25 (s) | 22.6 | 1.15 (s) | 19.2 |
| 12 | 2.49 (s) | 20.3 | 2.58 (s) | 22.4 |
| 13 | 3.06 (m) | 27.0 | 5.32 (dd, J = 4.9, 8.3) | 72.7 |
| 14 | 4.49 (m) | 67.9 | 3.93 (dd, J = 8.3, 11.4) | 65.6 |
| 4.55 (m) | 3.64 (dd, J = 4.9, 11.4) | |||
| 15 | - | 163.8 | 2.76 (s) | 15.2 |
Figure 2.
The key HMBC (→) and NOESY (↔) of compounds 1–4.
Compound 2 was isolated as colorless amorphous powder (MeOH) and showed a molecular formula of C15H20O5 with six degrees of unsaturation as determined by the HRESI-MS peak at m/z 315.1001 [M + Cl]− (calcd for C15H20O5Cl, 315.0999) (See Supplementary Materials). The UV spectrum exhibited the characteristic absorptions of pterosin-type sesquiterpenes at 222 (4.19), 265 (3.82), 305 (2.78) [15]. In the 1H-NMR spectrum (Table 1), three singlet methyl signals, including one geminal dimethyl group located at C-2 (δH 1.15) and two aromatic methyl groups (δH 2.58 and 2.76), a carbinyl proton at C-3 (δH 4.84) and an aromatic proton (δH 7.35) were observed.
The 13C-NMR displayed 15 carbon resonances, including three methyls at δc 15.2 (C-15), δc 19.2 (C-11) and δc 22.4 (C-12), two oxygenated methylenes at δc 65.6 (C-14) and δc 67.1 (C-10), three methines at δc 72.7 (C-13), 77.4 (C-3) and δc 126.9 (C-4), and eight quaternary carbons. The 1H-NMR and 13C-NMR spectra of compound 2 showed similar features to those of 2(R),3(R)-pterosin L [15]. The difference between the 1H-NMR of 2 and that of 2(R),3(R)-pterosin L was that 2(R),3(R)-pterosin L exhibited an A2X2 coupled system (2H × 2, t, J = 8.0 Hz), but 2 showed characteristic ABX signals [δH 5.32 (1H, dd, J = 4.9, 8.3 Hz, H-13), 3.93 (1H, dd, J = 8.3, 11.4 Hz, H-14a), 3.64 (1H, dd, J = 4.9, 11.4 Hz, H-14b)]. This suggested a 1,2-glycol was located at C-6 of 2. This was also confirmed by the carbon chemical shifts of C-13 (δC 72.7) appearing in the low field region due to the deshielding influence of the oxygen atom. Furthermore, in HMBC spectrum (Figure 2), the methine proton at δH 5.32 (H-13) showed cross-peaks with C-5, C-6 and C-7, which further indicated that the attached hydroxyl group could be assigned to C-13. The cis-configuration of the methyl at C-2 and the H at C-3 in 2 was confirmed by NOESY correlations between H-3 and H-11. The absolute configuration of 2 was determined by its CD spectrum, which exhibited a positive Cotton effect at 329 nm in MeOH, indicating that the oxygenated group at C-3 existed in a pseudoaxial conformation irrespective of the configuration at C-2 [14,19]. Based on these results, compound 2 was assigned as 13-hydroxyl-2(R),3(R)-pterosin L.
Compound 3 was isolated as a colorless amorphous powder (MeOH) and showed a molecular formula of C21H28O8 with eight degrees of unsaturation as determined by a HRESI-MS peak at m/z 431.1672 [M + Na]+ (calcd. for C21H28O8Na, 431.1682) (See Supplementary Materials). The UV spectrum exhibited the characteristic absorptions of pterosin-type sesquiterpenes at 228 (4.28), 270 (3.99), 310 (3.27) nm [15]. The 1H-NMR spectrum (Table 2) showed signals of one singlet methyl (δH 1.14, H-11), one aromatic methyl (δH 2.35, H-12), a methylene group [2.80 (d, J = 17.0 Hz, H-3a), 3.50 (d, J = 17.0 Hz, H-3b)], an aromatic proton [δH 7.22 (s, H-4)], an ethanol grouping located at C-6 [δH 2.74 (2H, t, J = 5.5 Hz, H-13), 3.98 (2H, m, H-14)], and two oxygenated methylene groups [δH 4.10 (1H, d, J = 9.4 Hz, H-10a), 3.48 (1H, d, J = 9.4 Hz, H-10b), 5.08 (2H, d, J = 4.8 Hz, H-15)]. Furthermore, the presence of a β-configuration glucose moiety was evident from the signals assignable to one anomeric proton (δH 4.20, d, J = 7.9 Hz), two oxygenated methine protons [δH 3.78 (dd, J = 1.6, 11.8 Hz) and 3.61 (dd, J = 5.7, 11.8 Hz)], and four overlapping protons (δH 3.03–3.28) [20]. This was further confirmed by GC-MS analysis after acid hydrolysis [21]. The connectivity of the glucose part was identified by HMBC correlations between the H-glc-1 (δH 4.20) and the C-10 (δC 74.86).
Table 2.
The NMR spectroscopic data of compounds 3 and 4 (MeOH-d4, 1H-NMR 600 MHz, 13C-NMR 150 MHz).
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δH (J in Hz) | δ C | δH (J in Hz) | δ C | |
| 1 | - | 211.9 | - | 211.2 |
| 2 | - | 51.2 | - | 52.8 |
| 3 | 2.80 (d, J = 17.0) | 38.5 | 4.84 (s) | 86.0 |
| 3.50 (d, J = 17.0) | ||||
| 4 | 7.22 (s) | 126.9 | 7.52 (s) | 127.8 |
| 5 | - | 146.4 | - | 146.2 |
| 6 | - | 132.5 | - | 140.7 |
| 7 | - | 136.2 | - | 139.1 |
| 8 | - | 130.1 | - | 131.4 |
| 9 | - | 153.9 | - | 152.7 |
| 10 | 4.10 (d, J = 9.4) | 74.86 | 1.08 (s) | 22.3 |
| 3.48 (d, J = 9.4) | ||||
| 11 | 1.14 (s) | 21.8 | 1.28 (s) | 22.8 |
| 12 | 2.35 (s) | 19.9 | 2.57 (s) | 22.0 |
| 13 | 2.74 (t, J = 5.5) | 27.2 | 5.32 (dd, J = 4.9, 8.4) | 72.7 |
| 14 | 3.98 (m) | 65.7 | 3.92 (dd, J = 8.4, 11.5) | 65.5 |
| 3.63 (dd, J = 4.9,11.5) | ||||
| 15 | 5.08 (d, J = 4.8) | 67.5 | 2.74 (s) | 15.2 |
| glc-1 | 4.20 (d, J = 7.9) | 104.7 | 4.57 (d, J = 7.8) | 105.8 |
| glc-2 | 3.21 (m) | 71.5 | 3.36 (m) | 71.7 |
| glc-3 | 3.20 (m) | 77.9 | 3.42 (m) | 78.0 |
| glc-4 | 3.03 (m) | 74.9 | 3.28 (m) | 75.3 |
| glc-5 | 3.28 (m) | 78.1 | 3.27 (m) | 78.2 |
| glc-6 | 3.78 (dd, J = 1.6, 11.8) | 62.8 | 3.85 (dd, J = 2.0, 12.0) | 62.8 |
| 3.61 (dd, J = 5.7, 11.8) | 3.75 (dd, J = 5.4, 12.0) | |||
Compound 3 exhibited 1H- and 13C-NMR data closely resembling to those of rhedynoside A [22]. The difference between NMR spectroscopic data of 3 (Table 2) and those of rhedynoside A was 3 possesses an ether link. The presence of an ether link was assigned to be between positions C-15 and C-14 as their proton and carbon chemical shifts appeared at the low field region due to the deshielding influence of the oxygen atom. This was further confirmed by the HMBC spectrum that showed the correlations from H-15 (δH 5.08) to C-14 (δC 65.6) and from H-14 (δH 3.98) to C-15 (δC 67.5). The absolute configuration of 3 was also determined as 2S by the CD spectrum which exhibited vibronic n–π* transition which concurs with C-2 having 2S configuration [22]. Based on these results, compound 3 was assigned as creticoside A.
Compound 4 was isolated as colorless amorphous powder (MeOH) and showed a molecular formula of C21H30O9 with seven degrees of unsaturation as determined by a HRESI-MS peak at m/z 461.1581 [M + Cl]− (calcd for C21H30O9Cl, 461.1578) and 471.1874 [M + COOH]− (calcd for C22H31O12, 471.1866) (See Supplementary Materials). The UV spectrum exhibited the characteristic absorptions of pterosin-type sesquiterpenes at 219 (4.16), 265 (3.80), 303 (2.88) nm [15]. In the 1H-NMR spectrum (Table 2), four singlet methyl signals, including two geminal dimethyl groups located at C-2 (δH 1.08 and 1.28, each 3H) and two aromatic methyl groups (δH 2.57 and 2.74, each 3H), a carbinyl proton at C-3 (δH 4.84, s), an aromatic proton (δH 7.52, s), and a set of ABX signals [δH 5.32 (dd, J = 4.9, 8.4 Hz), 3.92 (dd, J = 8.4, 11.5 Hz), 3.63 (dd, J = 4.9, 11.5 Hz)] assignable to a 1,2-glycol located at C-6 were observed. Compound 4 exhibited 1H and 13C-NMR data closely resembling to those of spelosin [15]. Comparison of the UV and NMR spectroscopic data of 4 (Table 2) with those of spelosin showed several differences, the main one being the presence of an additional hexose sugar moiety having a signal for an anomeric-H at δH 4.57 (d, J = 7.8 Hz) and remaining sugar proton signals at δH 3.27–3.85. The 13C-NMR spectrum of 4 contained an anomeric carbon signal of a hexose moiety at δH 105.8 and signals for remaining five sugar carbons at δH 62.8–78.2, which were in good agreement with those reported for glucoside compounds [22]. Furthermore, the coupling constant (J = 7.8 Hz) is consistent with a trans 3JH-H, showing that the glucose moiety is in a β-configuration [20]. This was further confirmed by GC-MS analysis after acid hydrolysis [21]. The connectivity of glucose part was identified by HMBC correlations between the H-1″ (δH 4.57) and the C-3 (δC 86.0) (Figure 2). The absolute configuration of 4 was also determined as 3R by the CD spectrum which exhibited a positive Cotton effect at 329 nm [19]. Based on these results, compound 4 was assigned as spelosin 3-O-β-d-glucopyranoside.
Compounds 1–4 were evaluated for cytotoxic activities against four human cell lines (SH-SY5Y (neuroblastoma cell line), SGC-7901 (gastric cancer cell line), HCT-116 (colon cancer cell line) and Lovo (colorectal cancer cell line). Compounds 1–4 were inactive (IC50 > 100 μM) to SH-SY5Y, SGC-7901, and HCT-116 cell lines. Compounds 1 and 2 exhibited cytotoxic activity against HCT-116 cells with IC50 value of 22.4 μM and 15.8 μM, respectively.
3. Experimental Section
3.1. General Information
1D and 2D-NMR spectra were obtained on an AV-600 spectrometer (Bruker, Rheinstetten, Germany) with TMS as internal reference, and methanol-d4 as solvent. Electrospray ionisation (ESI) mass spectra were acquired in the positive ion mode on a LCQ DECAXP instrument (Thermo Finnigan, San Jose, CA, USA) equipped with an ion trap mass analyzer. HRESI-MS were obtained in the positive ion mode on a Waters UPLC Premier Q-TOF system (Waters Inc., Milford, MA, USA). CD spectra were obtained on an Olis DSM 1000 spectrometer (Olis Inc., Augusta, GA, USA). Optical rotations were acquired on Shenguang SGW-1 digital polarimeter (Jingke, Shanghai, China). GC-MS were obtained on a Thermo Finnigan Trace DSQ (TR-5MS column: 60 m × 0.25 mm × 2.5 μm) (ThermoFinnigan, Bremen, Germany). TLC plates were HSGF254 SiO2 from Yantai Jiangyou Silica Gel Development Co., Ltd. (Yantai, China). Column chromatography (CC) silica gel (SiO2; 200–300 mesh; Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), Sephadex LH-20 (GE-Healthcare Bio-Sciences AB, Uppsala, Sweden), ODS (Grace C18, Grace Davison Discovery Sciences, Columbia, MD, USA) were employed as packing materials, semi-preparative HPLC (Grace Prevail C18 column, 5 μm, 10.0 mm I.D × 250 mm, Grace Davison Discovery Sciences, Columbia, MD, USA). All other chemicals were of analytical reagent grade.
3.2. Plant Materials
The aerial parts of P. cretica were collected in Jiangxi China, and identified by Prof. Xiaomei Fu of Jiangxi University of Traditional Chinese Medicine. A voucher specimen (NO. 20161007) was deposited at the Key Laboratory of Modern Preparation of TCM, Jiangxi University of Traditional Chinese Medicine, China.
3.3. Extraction and Isolation
The aerial parts of P. cretica (5 kg) were extracted with 70% (v/v) aqueous ethanol (about 60 L). The 70% EtOH extract was concentrated under reduced pressure to give a residue (803 g), which was then extracted with petroleum ether, dichloromethane, EtOAc and n-BuOH, respectively. The above extracts were concentrated to yield 81 g of petroleum ether fraction, 108 g of dichloromethane fraction, 154 g of EtOAc fraction and 132 g of n-BuOH fraction. The UV analysis of the above fractions monitoring the characteristic absorption bands in the 304 to 205 nm region of pterosin derivatives [15,16], showed that the n-BuOH extract contained more pterosins in comparison with other extracts. Therefore, the n-BuOH extract was subjected to further purification. Part of the dried n-BuOH extract (about 130 g) was subjected to silica gel column chromatography with gradient mixtures of CH2Cl2/ MeOH (from 10:1 to 1:10). Each 200 mL fraction was collected and evaporated, and some fractions was combined after analytical TLC inspection (CHCl3/MeOH 10:1 to 3:1). Finally, 33 fractions (Fr.1–33) were obtained. Fr.8 (1.7 g) was further subjected to silica gel CC (CH2Cl2/MeOH 10:1, 8:1, 4:1). Each 50 mL of eluent was collected and combined after analytical TLC inspection (CHCl3/MeOH 8:1 to 5:1). 12 fractions (Fr.8.1–12) were obtained. Fr.8.4 (256 mg) was further subjected to Sephadex LH-20 column chromatography with MeOH. 15 fractions (Fr.8.4.1–15) were obtained. Fr.8.4.6 (57 mg) was further purified by semi-preparative HPLC (65% MeOH/H2O, 3.0 mL/min) to yield 1 (7.2 mg, tR18.2 min), 5 (9.7 mg, tR14.5 min), 6 (11.1 mg, tR10.5 min). Fr.12 (2.2 g) was further subjected to silica gel CC (CH2Cl2/MeOH 9:1, 7:1, 3:1). Twenty-three fractions (Fr.12.1–23) were obtained. Fr.12.9 (195 mg) was further subjected to Sephadex LH-20 column chromatography with MeOH. Eighteen fractions (Fr.12.9.1–18) were obtained. Fr.12.9.5 (24 mg) was further purified by semi-preparative HPLC (59% MeOH/H2O, 3.0 mL/min) to yield 2 (6.8 mg, tR18.3 min). Fr.12.9.16 (21 mg) was further purified by semi-preparative HPLC (55% MeOH/H2O, 3.0 mL/min) to yield 3 (8.1 mg, tR15.6 min). Fr.18 (1.0 g) was further subjected to silica gel CC (CH2Cl2/MeOH 9:1, 7:1, 3:1). Twenty fractions (Fr.18.1–20) were obtained. Fr.18.6 (195 mg) was further subjected to Sephadex LH-20 column chromatography with MeOH. Eleven fractions (Fr.18.6.1–11) were obtained. Fr.18.6.5 (19 mg) was further purified by semi-preparative HPLC (50% MeOH/H2O, 3.0 mL/min) to yield 4 (7.3 mg, tR17.6 min). Fr.18.6.9 (25 mg) was further purified by semi-preparative HPLC (50% MeOH/H2O, 3.0 mL/min) to yield 7 (10.0 mg, tR15.5 min). Fr.22 (897 mg) was further subjected to Sephadex LH-20 column chromatography with MeOH. Sixteen fractions (Fr.22.1–16) were obtained. Fr.22.7 (68 mg) was further purified by semi-preparative HPLC (45% MeOH/H2O, 3.0 mL/min) to yield 8 (14.3 mg, tR15.3 min). Fr.22.11 (35 mg) was further purified by semi-preparative HPLC (45% MeOH/H2O, 3.0 mL/min) to yield 9 (15.0 mg, tR19.5 min).
3.4. Spectral Data
Creticolactone A (1): Colorless amorphous powder (MeOH). = +51.4° (c =0.032, MeOH). UV (MeOH) λmax (logε) 215 (4.03), 258 (3.71), 310 (2.97) nm; HRESI-MS at m/z 261.1127 [M + H]+ (calcd for C15H17O4, 261.1127); 1H-NMR and 13C-NMR see Table 1.
13-Hydroxy-2(R),3(R)-pterosin L (2): Colorless amorphous powder (MeOH). = +30.0° (c =0.032, MeOH). UV (MeOH) λmax (logε) 222 (4.19), 265 (3.82), 305 (2.78) nm; HRESI-MS at m/z 315.1001 [M + Cl]− (calcd for C15H20O5Cl, 315.0999); 1H-NMR and 13C-NMR see Table 1.
Creticoside A (3): Colorless amorphous powder (MeOH). = −32.0° (c =0.032, MeOH). UV (MeOH) λmax (logε) 228 (4.28), 270 (3.99), 310 (3.27) nm; HRESI-MS at m/z 431.1672 [M + Na]+ (calcd for C21H28O8Na, 431.1682); 1H-NMR and 13C-NMR see Table 2.
Spelosin 3-O-β-d-glucopyranoside (4): Colorless amorphous powder (MeOH). = −17.8° (c =0.032, MeOH). UV (MeOH) λmax (logε) 219 (4.16), 265 (3.80), 303 (2.88) nm; HRESI-MS at m/z 461.1581 [M + Cl]− (calcd for C21H30O9Cl, 461.1578); 1H-NMR and 13C-NMR see Table 2.
3.5. Cytotoxic Activity
The cytotoxicity of the isolated compounds (the purities of compounds 1–4 were 95.5%, 93.0%, 92.5% and 95.6%, respectively) against four human tumor cell lines (SH-SY5Y, SGC-7901, HCT-116, Lovo) was evaluated using the MTT assay performed according to the method described by Liu [23].
3.6. Acid Hydrolysis of 3–4
The procedure of the absolute configuration determination of glucose (compounds 3 and 4) was as previously reported [21].
4. Conclusions
This study describes the successful isolation and identification of nine pterosin-type sesquiterpenoids from the aerial part of P. cretica, including four new pterosins (compounds 1–4). Their chemical structures were elucidated by 1D- and 2D-NMR spectroscopic analysis. creticolactone A (1) and creticoside A (3) belong to a new type of pterosin with a six membered ring between positions C-14 and C-15. To our best knowledge, this is the first report on the occurrence of this new class of pterosins from the genus Pteris, which implies that they might be chemotaxonomic markers for P. cretica. Furthermore, all of the new isolated compounds 1–4 were evaluated for cytotoxic activity. Compounds 1 and 2 exhibited cytotoxic activity against HCT-116 cells with IC50 values of 22.4 μM and 15.8 μM.
Supplementary Materials
The Supplementary Materials are available online.
Author Contributions
The contributions of the respective authors were as follows: J.S. designed the research. J.L. (Jian Lu) and S.C. were responsible for the isolation of the compounds. C.P. and Q.M. were responsible for the bioactivities investigation of new compounds. J.L. (Jianqun Liu) and J.S. were responsible for identification of the isolated compounds and wrote the whole article.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC), grant number 81760703 and 81760721, Natural Science Foundation of Jiangxi Province, grant numbers 20181BAB205077, and the foundation of Health of Jiangxi Province, grant numbers 2016A062.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Editorial Committee of Flora of China of Chinese Academy of Sciences . Flora of China. Science Press; Beijing, China: 1999. pp. 10–15. [Google Scholar]
- 2.Yong L., Yao Z.S. Resource investigation of medicine plants in Pteris genus in Jiangxi. Jiangxi For. Sci. Technol. 1996;6:29–30. [Google Scholar]
- 3.Ouyang D.W., Ni X., Xu H.Y., Chen J., Yang P.M., Kong D.Y. Pterosins from Pteris multifida. Planta Med. 2010;76:1896–1900. doi: 10.1055/s-0030-1249934. [DOI] [PubMed] [Google Scholar]
- 4.Lin L.J., Huang X.B., Lv Z.C. Isolation and identification of flavonoids components from Pteris vittata L. SpringerPlus. 2016;5:1649. doi: 10.1186/s40064-016-3308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harinantenaina L., Matsunami K., Otsuka H. Chemical and biologically active constituents of Pteris multifida. J. Nat. Med. 2008;62:452–455. doi: 10.1007/s11418-008-0265-9. [DOI] [PubMed] [Google Scholar]
- 6.Qin B., Zhu D.Y. Reviews on sesquiterpenoids from spices of Pteridaceae-Structures, physical and chemical properties and spectroscopic characteristics of 1H-inden-1-one sesquiterpenoids. Chem. Res. 2004;15:72–76. [Google Scholar]
- 7.Lu J., Huang Y.Z., Chen S.J., Liu J.Q., Shu J.C. Review on pterosins in ferns. Chin. Tradit. Pat. Med. 2019;42:160–171. [Google Scholar]
- 8.Kim J.W., Kim H.P., Sung S.H. Cytotoxic pterosins from Pteris multifida roots against HCT-116 human colon cancer cells. Bioorg. Med. Chem. Lett. 2017;27:3144–3147. doi: 10.1016/j.bmcl.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Yahara Y., Takemori H., Okada M. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat. Commun. 2016;27:1–12. doi: 10.1038/ncomms10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu F., Huang C., Chen Y. Antidiabetic Effects of Pterosin A, a Small-Molecular-Weight Natural Product on Diabetic Mouse Models. Diabetes. 2013;62:628–638. doi: 10.2337/db12-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J.J., Wang T.C., Yang C.K. New Pterosin sesquiterpenes and antitubercular constituents from Pteris ensiformis. Chem. Biodivers. 2014;45:1903–1909. doi: 10.1002/cbdv.201300072. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Shu J., Zhang R. Two new pterosin dimers from Pteris mutifida Poir. Fitoterapia. 2011;82:1181–1184. doi: 10.1016/j.fitote.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Shu J.C., Pan J.H., Zhang L., Ren X.J., Liu J.Q. Sesquiterpenes from Pteris multifida. Chin. Tradit. Pat. Med. 2011;33:2104–2107. [Google Scholar]
- 14.Shu J., Liu J., Zhong Y., Liu L., Zhang R. Two new pterosin sesquiterpenes from Pteris multifida Poir. Phytochem. Lett. 2012;5:276–279. doi: 10.1016/j.phytol.2012.01.011. [DOI] [Google Scholar]
- 15.Kuraishi T., Murakami T., Taniguchi T., Kobuki Y., Maehashi H., Tanaka N., Saiki Y., Chen C.M. Chemical and chemotaxonomical studies of Ferns. LIV. Pterosin derivatives of the genus Microlepia (Pteridaceae) Chem. Pharm. Bull. 1985;33:2305–2312. doi: 10.1248/cpb.33.2305. [DOI] [Google Scholar]
- 16.Tanaka N., Satake T., Takahashi A., Mochizuki M., Murakami T., Saiki Y., Yang J.Z., Chen C.M. Chemical and chmotaxonomical studies of Ferns. XXXIX. Chemical studies on the constituents of Pteris bella TAGAWA and Pteridium aquilinum subsp. wightianum (WALL) SHICH. Chem. Pharm. Bull. 1982;30:3646–3652. doi: 10.1248/cpb.30.3640. [DOI] [Google Scholar]
- 17.Murakami T., Taguchi S., Nomura Y., Tanaka N., Satake T., Saiki Y., Chen C.M. Weitere Indan-1-on-derivate der gattung Pteris. Chem. Pharm. Bull. 1976;24:1961–1964. doi: 10.1248/cpb.24.1961. [DOI] [Google Scholar]
- 18.Chen Y.H., Chang F.R., Lu M.C., Hsieh P.W., Wu M.J. New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm. Molecules. 2008;13:255–266. doi: 10.3390/molecules13020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroyanagi M., Fukuoka M., Yoshihira K., Natrori S. Circular dichroism and confromations of pterosins, 1-indanone derivatives from Bracken. Chem. Pharm. Bull. 1979;27:731–741. doi: 10.1248/cpb.27.731. [DOI] [Google Scholar]
- 20.Mohamed K.M. Phenylpropanoid glucosides from Chrozophora oblique. Phytochemistry. 2001;58:615–618. doi: 10.1016/S0031-9422(01)00262-X. [DOI] [PubMed] [Google Scholar]
- 21.Xin W.B., Chou G.X., Wang Z.T. Triterpenoids and saponins from leaves of Uncaria hirsute. Helv. Chim. Acta. 2009;92:638–644. doi: 10.1002/hlca.200800312. [DOI] [Google Scholar]
- 22.Mohammad R.H., Nur-E-Alam M., Lahmann M., Parveen I., Tizzard G.J., Coles S.J., Fowler M., Drake A.F., Heyes D., Thoss V. Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome. Phytochemistry. 2016;128:82–94. doi: 10.1016/j.phytochem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Liu X., Liang J., Pan L.L., Chen J.Y., Liu R.H., Zhu G.H., Huang H.L., Shu J.C., Shao F., Liang Y.H., et al. Six new furostanol glycosides from Smilax glauco-china and their cytotoxic activity. J. Asian Nat. Prod. Res. 2017;19:754–765. doi: 10.1080/10286020.2017.1281913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.