Abstract
Osteosarcoma and Ewing sarcoma are the most common malignant primary bone tumors mainly occurring in children, adolescents and young adults. Current standard therapy includes multidrug chemotherapy and/or radiation specifically for Ewing sarcoma, associated with tumor resection. However, patient survival has not evolved for the past decade and remains closely related to the response of tumor cells to chemotherapy, reaching around 75% at 5 years for patients with localized forms of osteosarcoma or Ewing sarcoma but less than 30% in metastatic diseases and patients resistant to initial chemotherapy. Despite Ewing sarcoma being characterized by specific EWSR1-ETS gene fusions resulting in oncogenic transcription factors, currently, no targeted therapy could be implemented. It seems even more difficult to develop a targeted therapeutic strategy in osteosarcoma which is characterized by high complexity and heterogeneity in genomic alterations. Nevertheless, the common point between these different bone tumors is their ability to deregulate bone homeostasis and remodeling and divert them to their benefit. Therefore, targeting different actors of the bone tumor microenvironment has been hypothesized to develop new therapeutic strategies. In this context, it is well known that the Wnt/β-catenin signaling pathway plays a key role in cancer development, including osteosarcoma and Ewing sarcoma as well as in bone remodeling. Moreover, recent studies highlight the implication of the Wnt/β-catenin pathway in angiogenesis and immuno-surveillance, two key mechanisms involved in metastatic dissemination. This review focuses on the role played by this signaling pathway in the development of primary bone tumors and the modulation of their specific microenvironment.
Keywords: Wnt/β-catenin, bone sarcoma, bone tumor microenvironment
1. Primary Bone Tumors: Osteosarcoma and Ewing Sarcoma
Osteosarcoma and Ewing sarcoma are the most common primary bone tumors mainly occurring in children, adolescents and young adults. Current standard therapy includes multidrug chemotherapy and/or radiation for Ewing sarcoma, associated with tumor resection. However, the 5-year survival rates have not been improved during the last decades reaching 70–80% for patients with localized forms, but less than 30% in metastatic diseases and patients resistant to initial chemotherapy [1,2,3,4,5].
Osteosarcoma that mainly occurs at the ends of long bones is not associated with any clinical signs except severe pain or spontaneous fracture. Osteosarcoma does not express specific oncogenic markers but exhibits a large number and variety of genetic alterations. Ewing sarcoma is a tumor composed of small undifferentiated round cells that can appear mainly in bones but also in soft tissues in 15% of cases. In contrast to osteosarcoma, Ewing sarcoma is characterized by a chromosomal translocation between the EWSR1 and FLI1 genes in 90% of cases, or by the fusion of EWSR1 with other transcription factors of the E26 Transformation-Specific (ETS) gene family in 10% of cases [6,7,8].
Despite progress in understanding the biology of osteosarcoma and Ewing sarcoma, no targeted therapy could be currently implemented to improve patient survival. Nevertheless, the common point between these two pediatric bone tumors is their ability to deregulate bone homeostasis and remodeling to divert them to their benefit. Therefore, new therapeutic research is moving towards targeting different actors of the bone microenvironment.
2. Tumor Microenvironment: Crucial Role in Bone Sarcoma Tumor Growth and Metastatic Progression
It is well established that the bone tumor microenvironment (TME) plays a major role during tumor initiation, progression, and metastatic processes. This very complex and dynamic environment includes different cell types such as osteoblasts, osteoclasts, stromal cells, mesenchymal stem cells, immune or endothelial cells and non-cellular components such as the extracellular matrix compounds [9]. These cells are able to communicate with each other in order to influence their behavior [10]. This microenvironment is able to support bone sarcoma primary development, resistance to chemotherapy [11] and metastatic spread of tumor cells [12,13,14].
The crucial role played by the TME on sarcoma development has been recently highlighted in several studies. As an example, a modification of the transcriptome has been observed in cell lines established from synovial sarcomas compared to initial tumors or directly engrafted tumors in mice, demonstrating the impact of TME on tumor behavior, particularly through interactions with the immune system and stromal cells [15]. The bone TME is also able to affect the behavior and the fate of tumor cells and particularly, their ability to migrate and invade new tissues. Indeed, the development of sarcoma in orthotopic models of xenograft was associated with the establishment of metastases whereas the subcutaneous model did not allow to observe any metastatic spread [16]. These enhanced invasive and migratory capacities have been confirmed in a rat model of prostate carcinoma and related to the acidification of the bone tumor microenvironment [17]. Similarly, Chattopadhyay et al. have observed that solid sarcoma developed differentially when implanted into two different anatomical sites, related to a greater neovascularization and the presence of tumor-associated fibroblasts and tumor-associated adipocytes, which could support sarcoma tumor growth [18].
2.1. Hijacking of the Bone Tumor Microenvironment by Bone Sarcoma Cells
One of the main hallmarks of osteosarcoma and Ewing sarcoma is their ability to disrupt bone remodeling in favor of an exacerbated osteoclast-mediated bone resorption, leading to the release of pro-tumoral factors initially embedded in the bone matrix [19,20,21]. It thus establishes a vicious cycle between bone sarcoma proliferation and bone resorption, leading to investigations about the beneficial effect of anti-resorptive agents on bone sarcoma progression. Thus zoledronate, an N-containing bisphosphonate able to inhibit osteoclasts proliferation and activity, reduces osteosarcoma and Ewing sarcoma primary and metastatic development in corresponding pre-clinical models, disrupting the vicious cycle between tumor growth and osteoclast-mediated bone resorption [22,23,24,25].
Human Mesenchymal Stem Cells (hMSCs) play also a major role in the communication between tumor cells and their microenvironment, as highlighted by recent studies, including increased proliferation and migration of tumor cells. On one hand, bone marrow MSCs promote migration and invasion through direct secretion of several cytokines such as C-X-C motif chemokine ligand 12 (CXCL12) that binds to C-X-C motif chemokine receptor 4 (CXCR4) or CXCR7 on osteosarcoma associated with lower overall patient survival [26,27,28]. Interleukin-6, secreted by MSCs, contributes to promote metastatic dissemination of osteosarcoma [29,30]. On the other hand, MSCs release exosomes that promote tumor growth and metastases [31]. Other types of stem cells such as the stromal adipose-derived mesenchymal stem cells stimulate tumor growth and invasion of osteosarcoma cells, by activating the signal transducer and activator of transcription 3 (STAT3) signaling pathway, resulting in an increase of the expression of matrix metalloproteinase (MMP) 2 and MMP9 [32].
Bone sarcoma is able to modulate the recruitment, expansion and differentiation of immune cells to establish an immunosuppressive microenvironment, allowing tumor development and metastatic dissemination [33]. Regarding innate immunity, bone sarcomas are mainly invaded by TAMs that are initially divided into two categories: anti-tumor M1-polarized macrophages and tumor-promoting M2-polarized macrophages [34]. Macrophages massively invade osteosarcoma, this infiltration being associated with a good prognosis in osteosarcoma patients [35,36]. However, the implication of tumor-promoting M2-polarized macrophages infiltrates in osteosarcoma progression remains controversial. Indeed, M2-type macrophage infiltrates in the tumor have been correlated with a poor prognosis in some studies [36,37]. Moreover, on one hand, an imbalance in favor of M1 macrophages has been observed in patients without metastases [37]. On the other hand, M2-polarized macrophages have been associated with increased tumor growth and metastases in preclinical models of osteosarcoma [38]. However, a recent study based on the most important cohort of osteosarcoma patients from the OS2006 clinical trial has demonstrated that the presence of CD163+ M2-polarized macrophages is crucial for the inhibition of osteosarcoma progression [35]. In contrast, macrophages do not seem to play such a role in Ewing sarcoma development and patient survival [39,40]. Moreover, M2-polarized macrophages contribute to the exhaustion of T lymphocytes infiltrating the tumor, reducing their proliferation and secretion of pro-inflammatory cytokines [41]. These results may be associated with the existing metabolic competition between sarcoma cells and infiltrating T lymphocytes. Indeed, excessive consumption of glucose from the microenvironment by tumor cells restricts the consumption of T cells, which show decreased glycolysis and under-production of interferon γ (IFNγ). T cells respond less to stimuli, allowing the tumor to escape from immune control. Thus, metabolic competition between tumor cells and T cells influences tumor progression [42].
Besides cellular components, the physical properties of the TME can influence the behavior of cancer cells. Indeed, mechanical (composition of the extracellular matrix) and chemical stress (hypoxia) increase the motility and capacity of sarcoma cells to metastasize, through increased expression of procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2), a collagen cross-linker [43]. Hypoxic microenvironment also leads to increased interleukin-6 expression in osteosarcoma cells, which contributes to increased lung metastases [44]. Finally, Ewing sarcoma in response to Wnt3a stimulation can modify the acellular TME by increasing the secretion of proteins involved in the composition of the extracellular matrix [45].
2.2. Bone Sarcoma Microenvironment as a Prognostic Marker or Therapeutic Target
Several studies have highlighted the importance of analyzing TME in order to predict patient outcomes. For example, Volchenboum et al. have demonstrated the importance of studying stromal cells in contact with the Ewing sarcoma, to predict patients at high risk of relapse, thanks to a gene set enrichment analysis conducted on human Ewing sarcomas samples by the Children’s Oncology Group (COG) [46]. A strong expression of C-C motif ligand (CCL) 12 produced by infiltrating immune cells is correlated with an increased ratio of CD8+/CD4+ infiltrating T lymphocytes, leading to a better response to chemotherapy and overall patient survival [47]. In osteosarcoma, tissue micro-arrays analyses showed that the immune infiltrate of primary tumors was enriched with anti-tumor M1-type macrophages for the localized forms (CD68+ iNOS+, inducible nitric oxide synthase), as compared to metastatic diseases [37]. In addition, metastatic tumors have a higher vascular density than localized tumors, supporting that greater angiogenesis may partly explain metastatic spread.
Osteoclasts and immune cells are the main potential therapeutic targets for osteosarcoma and Ewing sarcoma. Based on preclinical data, the clinical trial OS2006 (NCT00470223, available online: https://clinicaltrials.gov/ct2/show/NCT00470223) has been developed to evaluate the combination of zoledronate with current chemotherapy and surgery in osteosarcoma patients. Unfortunately, the results do not recommend the use of zoledronate as no improvement in event-free survival was observed, zoledronate showing a trend to increase the risk of failure in osteosarcoma patients [48]. Several therapeutic approaches targeting infiltrating immune system are currently in clinical development. For example, some of osteosarcoma and Ewing sarcoma express programmed cell death ligand (PDL)-1 that binds to programmed cell death (PD)-1 on infiltrating T cells, inhibiting their cytotoxic activities and contributing to the local immunosuppression and consequently to the tumor progression [49]. Based on pre-clinical data demonstrating the beneficial effect of PD-1/PDL-1 blockade [50] and correlations between PDL-1 expression and clinical outcomes [51,52], clinical trials evaluating the effects of anti-PD1 antibodies are in progress in osteosarcoma and Ewing sarcoma (Pembrolizumab, NCT02301039 (available online: https://clinicaltrials.gov/ct2/show/NCT02301039) and Nivolumab, NCT02304458 (available online: https://clinicaltrials.gov/ct2/show/NCT02304458)).
3. The Canonical Wnt/β-Catenin Signaling Pathway
The Wnt signaling pathway is essential during development and is involved in many cellular processes, such as proliferation, migration, polarization and cellular differentiation. This signaling pathway is therefore finely regulated and mutations or deregulation of the Wnt signaling pathway are often associated with tumor development, chemoresistance, or relapse [53,54,55]. Three Wnt signaling pathways have been described so far: the canonical Wnt pathway or Wnt/β-catenin pathway on which we will focus, and the non-canonical Wnt/PCP (Planar Cell Polarity) and Wnt/Ca2+ pathways which are not detailed in this review.
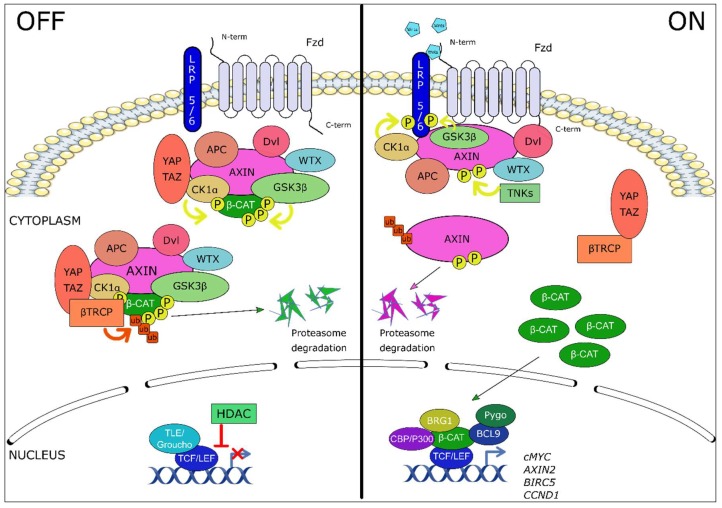
Wnt ligands are a family of 19 secreted glycoproteins, some of which can bind to one of the 10 receptors of the Frizzled family (Fzd), or to a co-receptor lipoprotein receptor-related protein (LRP)-5 or LRP-6, receptor tyrosine-kinase-like orphan receptor (ROR), related to tyrosine kinase (Ryk), to activate cell signaling. In the absence of Wnt ligands, the Wnt/β-catenin pathway is inactive, β-catenin is sequestered by a protein complex for degradation. This complex is composed of scaffold proteins, dishevelled (Dvl), adenomatous polyposis coli (APC) and Axin1/2 and Wilms tumor gene on X chromosome (WTX) protein [56], and two kinases, casein kinase 1 alpha (CK1α) and glycogen synthase kinase 3 beta (GSK3β) which sequentially phosphorylate β-catenin on Serine 45 (CK1α), and on serine 33 and 37 and threonine 41 (GSK3β). Yes-Associated protein/transcriptional co-activator with a PDZ-binding domain (YAP/TAZ) proteins, part of the complex, then recruit beta-transducin-repeat-containing protein (β-TrCP) an ubiquitin ligase responsible for the ubiquitination of β-catenin [57,58,59,60,61]. β-Catenin is then degraded by the proteasome pathway, and therefore does not play its role as a co-transcription factor. In addition, the transcriptional proteins of the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) family interact with the transcriptional repressors groucho/transducin-like enhancer of split (TLE), recruiting histone deacetylases (HDACs) responsible for repressing transcription [62] (Figure 1, left panel).
Figure 1.
The canonical Wnt/β-catenin signaling pathway. Left panel: in the absence of Wnt ligand, β-catenin is sequestered by a protein complex composed of dishevelled (Dvl), adenomatous polyposis coli (APC), Axin1/2, Wilms tumor gene on X chromosome protein (WTX) and two kinases responsible for the phosphorylation of β-catenin, CK1α (casein kinase 1 alpha) and GSK3β (glycogen synthase kinase 3 beta). Then, YAP/TAZ (yes-associated protein/transcriptional co-Activator with a PDZ-binding domain) proteins recruit β-TrCP (beta-transducin-repeat-containing protein), a ubiquitin ligase responsible for the ubiquitination of β-catenin and its degradation by the proteasome pathway. In the nucleus, the transcriptional proteins of the TCF/LEF family (T-cell factor/lymphoid enhancer-binding factor) interact with the transcriptional repressors groucho/TLE (transducin-like cnhancer of split), recruiting histone deacetylases (HDACs) responsible for repressing transcription. Right panel: Binding of the Wnt ligands to the frizzled (Fzd) receptor and low-density-lipoprotein-related protein 5/6 (LRP5/6) co-receptor complex induces the recruitment of the scaffold protein Dvl to Fzd and leads to LRP5/6 phosphorylation (P) by CK1α and GSK3β kinases. The β-catenin destruction complex is then trapped to the membrane through Axin/Fzd interaction, leading to its inactivation. In parallel, Axin proteins are degraded following poly-ADP-ribosylation by tankyrases (TNKS). Newly synthesized β-catenin accumulates in the cytoplasm and translocates into the nucleus where it interacts with the transcription factors of the TCF/LEF family and with histones modifying co-activators p300 or CREB binding protein (CBP), B cell CLL/lymphoma 9 (BCL-9), brahma-related gene 1 (BRG1), and pygopus. These transcription complexes activate the transcription of target genes such as cMYC, AXIN2, BIRC5 or CCND1.
The binding of a Wnt ligand (i.e., Wnt1, Wnt3a) to an Fzd receptor and its co-receptor LRP5/6 activates the canonical Wnt pathway, inducing the recruitment of Dvl on Fzd receptor and its activation. CK1α and GSK3β kinases then phosphorylate the LRP5/6 co-receptor, allowing the recruitment of Axin on LRP5/6 and the inactivation of the β-catenin degradation complex which is trapped to the membrane. Neosynthesized β-catenin is no longer degraded and accumulates in the cytoplasm [63], before being translocated into the nucleus. It interacts with the transcription factors of the TCF/LEF family and with histones modifying co-activators p300 or cAMP-response element binding protein (CREB) binding protein (CBP), B cell CLL/lymphoma 9 (BCL-9), brahma-related gene 1 (BRG1), and pygopus [64,65]. These complexes activate the transcription of target genes such as AXIN2, LEF1, cMYC, CCND1, BIRC5 (Figure 1, right panel).
Numerous molecules are implicated in the regulation of Wnt ligands and the canonical Wnt pathway activation. These include secreted extracellular inhibitors: dickkopf (DKK), secreted-Fzd-related proteins (SFRPs), Wnt inhibitory factor 1 (WIF1), sclerostin (SOST), cerberus, insulin-like growth factor binding protein 4 (IGFBP4). DKK and SOST proteins are competitive receptor and co-receptor antagonists that prevent signaling activation by Wnt ligands. The WIF1, SFRPs (domain Fzd-like C-terminal) and cerberus proteins are able to bind to Wnt ligands preventing their interaction with receptors and co-receptors [65,66].
The canonical Wnt signaling pathway is involved in many cancers, notably in gastrointestinal cancers (colorectal carcinoma, hepatocellular carcinoma, gastric cancer, cholangiocarcinoma, pancreatic ductal adenocarcinoma), hematopoietic cancers (acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, chronic lymphoblastic leukemia, lymphoma, multiple myeloma), breast cancer, melanoma, and brain tumors such as glioblastoma [67,68]. Its involvement in the tumorigenesis of many cancerous diseases makes it an interesting target for cancer treatment. That is why recent reviews of the literature have described the latest advances in targeting this signaling pathway [55,69,70,71,72,73]. Some Wnt inhibitor molecules are in clinical trials, most of them in Phase I [74,75], although the ubiquitous nature of this signaling pathway and its involvement in various major physiological processes raise the question of the side effects of this strategy [76].
4. Key Role of Wnt/β-Catenin Signaling Pathway in Osteosarcoma
The most recent researches on Wnt/β-catenin implication in osteosarcoma deal with metastatic dissemination and recurrence that are associated with poor prognosis for patients. Thus, following a brief description of the implication of the canonical Wnt/β-catenin signaling pathway in osteosarcoma development, this part will focus on these recent studies.
Canonical Wnt/β-catenin signaling pathway has been involved in the development of osteosarcoma but current knowledge remains unclear because of the high complexity of this pathway. Many studies support an aberrant activation of the canonical Wnt signaling pathway in osteosarcoma cells which seems to play a crucial role in tumorigenicity as well as metastatic dissemination [77,78]. As an example, two recent analyses on patient samples described a high β-catenin level in osteosarcoma tissues compared to adjacent healthy tissues associated with poor prognosis and lung metastatic dissemination [79,80] (Figure 2a). However, other studies highlighted that inactivation of the Wnt/β-catenin pathway plays a key role in osteosarcoma development [81,82]. In particular, frequent deletions of genes involved in the Wnt signaling pathway genes have been described in osteosarcoma patients [83]. In this context, Shimozaki et al. showed that pharmacological inhibition or depletion of GSK3β by siRNA decreases osteosarcoma cell proliferation despite an increased nuclear translocation of β-catenin and expression of its target genes supporting the tumor suppressor role of β-catenin [84]. However, to our knowledge, no additional recent study seems to support this second hypothesis.
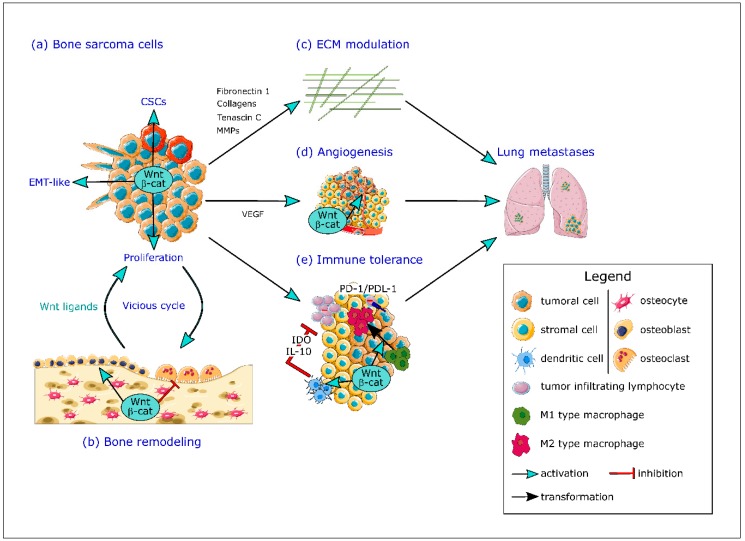
Figure 2.
The crucial role of Wnt/β-catenin signaling pathway in multiple steps of bone sarcoma progression and metastatic dissemination. (a) The canonical Wnt/β-catenin signaling pathway is able to enhance bone sarcoma cells proliferation, to induce an epithelial-mesenchymal transition (EMT)-like through secretion of fibulin-3 and to promote the acquisition of stem cells properties of bone sarcoma cancer stem cells (CSCs). (b) The Wnt/β-catenin pathway participates to the hijacking of the bone microenvironment by the bone sarcoma cells, leading to the establishment of a vicious cycle between bone remodeling and tumor cells proliferation associated with the release of pro-tumoral factors including Wnt ligands from the bone matrix. (c) The Wnt/β-catenin pathway promotes the modulation of the extracellular matrix (ECM), increasing secretion of extracellular matrix components such as tenascin C, fibronectin 1 or collagens and stimulates the ECM degradation by upregulation of proteolytic enzymes such as MMPs. (d) The Wnt/β-catenin pathway induces an over-expression of vascular endothelial growth factor (VEGF), the most important pro-angiogenic factor and is also able to modulate endothelial cell migration, leading to an increase in tumor-associated angiogenesis. (e) The Wnt/β-catenin signaling pathway participates to the establishment of an immune tolerance in the TME, enhancing pro-tumoral M2 macrophages polarization and inhibiting cytotoxic T cell infiltration and functions and inducing resistance to anti-PD1 or anti-PDL-1 therapy. The activation of the Wnt/β-catenin signaling pathway in dendritic cells leads to up-regulation of interleukin-10 (IL-10) and indoleamine 2,3-dioxygenase 1 (IDO) secretion leading to an inhibition of tumor-infiltrating lymphocytes (TILs) cytotoxic properties. By targeting both osteosarcoma and Ewing sarcoma cells and the bone TME, the Wnt/β-catenin signaling pathway participates to the disease progression and the establishment of lung metastases.
A key issue of osteosarcoma metastasis is the epithelial-mesenchymal transition (EMT)-like multi-step process, based on a transformation from an epithelial to a mesenchymal phenotype, promoting the invasive capacities of cancer cells. EMT is characterized by repression of key components of the intercellular junctions such as E-cadherin associated with an increase in mesenchymal markers including N-cadherin, vimentin and fibronectin. This modulation of gene expression is regulated by different transcription factors such as Snail-1, Snail-2, ZEB-1, ZEB-2 or Twist [85]. Despite the fact that osteosarcoma derives from cells of mesenchymal origin, an overexpression of EMT transcription factors such as Snails, ZEBs or Twist has been associated to increased invasive properties of osteosarcoma cells promoting the formation of metastases [86,87,88,89]. Transforming growth factor beta (TGFβ) seems to be the main inducer of EMT in tumor cells [90], but cooperates with other signaling pathways including the Wnt/β-catenin pathway to induce complete EMT [91]. Recent studies focusing on molecules implicated in EMT in osteosarcoma highlighted the implication of β-catenin in this biological process. Thus, Tian et al. have recently demonstrated that bone morphogenetic protein (BMP)-2 promotes EMT of osteosarcoma cells associated with enhanced motility and invasiveness through the Wnt/β-catenin signaling pathway [92]. Similarly, Fibulin-3, an extracellular glycoprotein, enhances EMT of osteosarcoma cells, associated with Wnt/β-catenin pathway activation [93] (Figure 2a). Some other molecules implicated in osteosarcoma development and metastasis, such as ubiquitin-specific protease 7 and N-terminal truncated form of carboxypeptidase seem to exert their effects through induction of EMT in osteosarcoma cells by activating the Wnt/β-catenin signaling pathway [94,95]. These numerous recent studies demonstrate the interest to target Wnt/β-catenin pathway in order to inhibit EMT in osteosarcoma cells.
EMT can also promote tumor cells to develop stem cell characteristics and consequently actively participate in the cancer stem cell (CSC) phenotype acquisition [96]. CSCs (also known as tumor-initiating cells or TIC) constitute a subpopulation of tumor cells with properties to self-renew and are responsible for tumor initiation and progression and, thanks to their chemotherapy resistance, seem to drive recurrences and metastases. CSCs are characterized by persistent activation of highly conserved signal transduction pathways including notch, hedgehog and Wnt pathways [97] and some evidence support the presence of CSCs in osteosarcoma and their contribution to metastatic chemotherapy-resistant properties [98,99]. Martin-Neves et al. have described a constitutive activation of the Wnt/β-catenin signaling in osteosarcoma CSCs rather than in parental osteosarcoma cells [100]. More recently, Liu et al., highlighted that β-catenin positively regulates osteosarcoma stem cells properties in vitro [79] (Figure 2a). These results support evidence that a therapy against the Wnt/β-catenin pathway in osteosarcoma could allow to target CSCs and potentially improve treatment for metastatic and chemotherapy-resistant patients.
New insights about the role of long non-coding RNA (lncRNA) and micro RNA (miR) in osteosarcoma progression have also recently emerged. On one hand, the overexpression of a number of miR has been demonstrated in osteosarcoma cells leading to increased osteosarcoma growth and metastasis. Most of these miRs downregulate antagonists of the Wnt/β-catenin signaling pathway. As an example, miR-552-5p is overexpressed in osteosarcoma tissues and cell lines compared to healthy tissue and osteoblasts respectively. Knockdown of this miR decreases osteosarcoma proliferation and metastasis by directly inhibiting WIF1 [101]. On the other hand, some miRs that are able to restrain the expression of key intermediates or regulators of the Wnt/β-catenin signaling pathway have been described to be downregulated, causing an aberrant activation of this pathway in osteosarcoma cells. Very recent studies described a decrease in miR-377, miR-873, miR-758 and miR-885-5p in osteosarcoma tissues and/or cell lines, leading to increased primary tumor and metastatic development in association with activated Wnt/β-catenin signaling pathway [102,103,104,105]. Other studies deal with lncRNA such as lncRNA AWPPH which levels are increased in osteosarcoma tissues compared to healthy counterparts and promote tumor proliferation and metastasis through activation of the Wnt/β-catenin pathway [106].
In addition to β-catenin, other modulators of the canonical Wnt signaling pathway, in particular, Wnt antagonists, are affected during osteosarcoma primary and metastatic development, but have already been described in several reviews and will not be developed here [107,108].
5. Involvement of Wnt/β-Catenin Signaling Pathway in Ewing Sarcoma
The involvement of the Wnt signaling pathway in Ewing sarcoma is less described than in osteosarcoma and seems to be controversial. First, no recurrent mutations in the Wnt/β-catenin signaling pathway have been reported in Ewing sarcoma but the activation of this pathway has been demonstrated in aggressive sarcoma, the bone microenvironment being the source of Wnt ligands. Moreover, this activation can be potentiated through the R-spondin (RSPO)/leucine rich repeat containing G protein-coupled receptor 5 (LGR5)/β-catenin axis. Indeed, LGR5 is highly expressed in aggressive Ewing sarcoma and CSCs and the binding of RSPO-2 on LGR5 was able to potentiate Wnt3a-induced canonical Wnt signaling. However, it seems that an important variability in Wnt responsiveness exists among Ewing sarcoma cells based on the differential expression of LGR5 and the availability of Wnt and RSPO ligands in the tumor microenvironment [109]. The proliferation of Ewing sarcoma cells is not affected by the Wnt/β-catenin pathway activation [110]. However, this signaling pathway appears to play a crucial role in the cellular motility of Ewing sarcoma cells [111]. Thus, the activation of the Wnt/β-catenin signaling pathway in Ewing sarcoma cells induces, on one hand, the formation of neurite outgrowth and, on the other hand, changes in transcriptional profile, leading to the acquisition of a more aggressive metastatic phenotype by tumor cells [109,112]. To go further, molecular mechanisms have been explored. Surprisingly, the activation of the Wnt/β-catenin pathway partially antagonizes the transcriptional function of the fusion protein EWS/FLI1 (found in the majority of Ewing sarcomas), resulting in an up regulation of metastasis-promoting genes that are normally repressed by EWS/FLI1 and consequently, the acquisition of a more migratory phenotype. Inversely, some studies have shown that EWS/FLI1 inhibits β-catenin dependent transcription through a direct interaction between EWS/FLI1 and the transcription factor LEF1, inhibiting the interaction between β-catenin and LEF1 in Ewing sarcoma-cell lines [113]. To sum up, an inverse relationship between the expression of EWS/FLI1 and TCF/LEF target genes is observed in Ewing sarcoma cells [109,113].
The analysis of the secretome of Wnt3a-activated Ewing sarcoma cells has revealed an increased secretion of proteins implicated in the composition and the structure of the extracellular matrix. In details, Wnt3a-activated cells present an upregulation of the signaling pathways involved in the communication between the tumor and its microenvironment, such as integrin linked kinase, focal adhesion kinase, cadherin angiogenesis, TGFβ and matrix metalloproteinase pathways [45].
Finally, the effects of WNT974, a Porcupine protein inhibitor involved in the palmitoylation of Wnt ligands, an indispensable process for Wnt secretion has been investigated in Ewing sarcoma. The WNT974-mediated inhibition of endogenous Wnt ligands secreted by tumor cells decreases the migration of Ewing sarcoma cells in vitro, downregulating the expression of genes involved in cell migration. In addition, WNT974 appears to reduce the development of metastatic disease in vivo in a murine subcutaneous xenograft model and to delay the time of first lung metastasis establishment. WNT974 inhibits Wnt signaling pathways (canonical and non-canonical) and cell migration and dissemination, therefore demonstrated the role of the Wnt signaling pathway during Ewing sarcoma metastatic spread [114].
6. Wnt/β-Catenin Pathway and the Bone Tumor Microenvironment
As described in the first part of the review, the bone TME is a complex and dynamic environment composed of both cellular and non-cellular components. This TME which includes bone, vascular and immune “niches” plays a key role in initiation, primary development and metastasis of osteosarcoma and Ewing sarcoma [115]. This part will focus on the implication of the canonical Wnt/β-catenin signaling pathway in these three “niches” of the bone TME.
6.1. Wnt/β-Catenin Pathway and Bone Remodeling
Bone remodeling results from a balance between osteoclasts-mediated bone resorption and osteoblasts-promoted bone formation. Osteocytes, trapped in the bone matrix, also contribute to the regulation of osteoblast and osteoclast functions during bone remodeling. In this context, the Wnt/β-catenin signaling pathway is able to modulate both osteoblast and osteoclast differentiation and activity. Indeed, osteoblasts and their precursors, osteocytes and osteoclasts are able to secrete various Wnt ligands which participate in the control of bone remodeling [116,117,118]. Briefly, the Wnt/β-catenin signaling pathway directly enhances osteoblast differentiation and bone formation whereas indirectly represses osteoclast differentiation and bone resorption through the production of osteoprotegerin by osteoblasts and osteocytes. Nevertheless, osteoclasts are also affected by Wnt ligands given that β-catenin activation enhances the proliferation of osteoclast precursors but inhibits osteoclastogenesis at a later stage [66,116,119].
The early-stage development of osteosarcoma and Ewing sarcoma initiates a dysregulation of the bone remodeling in favor of an exacerbated bone resorption, leading to the release of pro-tumorigenic factors initially trapped into the bone tissue (Figure 2b). Nevertheless, the implication of the Wnt/β-catenin signaling pathway in tumor cell-induced bone osteolysis has mainly been described in bone metastasis rather than in bone sarcoma. Interestingly, in breast cancer bone metastases, the Wnt/β-catenin signaling pathway seems to be active inside the tumor with overexpression of canonical Wnt ligands (Wnt2 and Wnt8b), maintaining tumor cell proliferation. Conversely, Wnt2 and Wnt8b are downregulated at the tumor/bone interface while Wnt antagonists WIF1 and SFRP4 are overexpressed and could be responsible, at least in part, of suppression of osteoblast proliferation and enhanced bone destruction by osteoclasts [120]. Moreover, the DKK1 overexpression has been described in osteolytic breast cancer and could promote osteoclastogenesis and bone resorption at the site of metastasis [121]. To our knowledge, no studies compare the activation of the canonical Wnt signaling pathway between the bone sarcoma core and the bone/tumor interface.
An important step in the metastatic process of bone sarcoma is the degradation of the extracellular matrix related to upregulation of proteolytic enzymes such as MMPs. Several studies have highlighted that the invasive capacities of osteosarcoma cells are correlated with MMPs expression, particularly MMP-2 and MMP-9 [122]. Moreover, MMP-2 and/or MMP-9 expression can be associated with the development of metastasis, poor prognosis and resistance to chemotherapy in osteosarcoma patients [123,124,125]. It is also well established that MMP2 and MMP9 are expressed in Ewing sarcoma and closely associated with Ewing sarcoma invasion and metastasis [126,127]. β-catenin gene silencing by siRNA reduced invasion and motility of the U2OS human osteosarcoma cell line associated with inhibition of membrane type 1 metalloproteinase (MT1-MMP) expression. On one hand, MT1-MMP directly degrades extracellular matrix components such as type II collagen or fibronectin and, on the other hand, activates by cleavage other members of the MMP family such as MMP-2. Moreover, a dominant-negative soluble LPR5 reduces the expression of MMP-2 and MMP-14, consistent with a decrease in invasive properties of the human SaOS2 osteosarcoma cell line [128]. Thus, these results suggest that β-catenin inhibition may decrease osteosarcoma invasion through downregulation of MMPs expression and activation [129,130,131]. In Ewing sarcoma, the implication of the Wnt/β-catenin signaling pathway does not seem to be predominant for the expression and activation of MMPs. However, in Ewing sarcoma, Wnt/β-catenin activation-induced expression of tenascin C, a secreted extracellular matrix protein that plays a key role during metastasis establishment in different types of cancer [132]. High level of tenascin C contributes to the Ewing sarcoma metastatic phenotype, modulating tumor microenvironment [109]. Activation of the canonical Wnt pathway also leads to increased secretion of the extracellular matrix components such as fibronectin or collagens, that alter the biochemical and structural properties of the bone TME and can modulate the metastatic process of Ewing sarcoma [45] (Figure 2c).
6.2. Wnt/β-Catenin Pathway, Angiogenesis and Hypoxia
Angiogenesis is a complex biological process that leads to new blood vessel development and plays a key role in primary tumor progression and metastatic potential of several cancers including bone sarcoma. Stages of angiogenesis include the proliferation and migration of endothelial cells to form a vessel sprout and then the recruitment of pericytes or smooth muscle cells to produce a mature and functional blood vessel [133]. This process is necessary for tumor growth allowing the supply of nutrients and metastatic dissemination, providing an intravasation site for cancer cells [133,134]. Vascular endothelial growth factor (VEGF) is the most important pro-angiogenic factor involved in the formation of new functional vessels in tumors through recruitment and differentiation of endothelial progenitor cells [135].
Osteosarcoma patients with high VEGF expression levels exhibit both lower disease-free survival and lower overall survival [136]. Similarly, a genetic amplification of the VEGFA gene was detected in osteosarcoma and significantly associated with high microvessels density and low patient disease-free survival [137]. Several studies have also highlighted the importance of VEGF in angiogenesis of Ewing sarcoma. Indeed, high levels of VEGF have been detected in the serum of Ewing sarcoma patients and correlated with increased microvascular density and poor patient outcomes [135,138,139]. In particular, Ewing sarcoma cells produce several VEGF isoforms including soluble spliced isoform of VEGFA, VEGF-165, which promotes the recruitment of bone marrow derived cells. These cells can differentiate into both tumor-associated pericytes and endothelial cells, contributing to the new vasculature of Ewing sarcoma [140,141].
Regarding the role of the Wnt/β-catenin signaling pathway in angiogenesis, transcriptional regulation of VEGF by the β-catenin/TCF complex has been demonstrated, involving TCF binding sites in the VEGF gene promoter. Moreover, defects in APC, resulting in constitutively active β-catenin, induce the over-expression of VEGF [142,143]. Furthermore, the SFRPs proteins are a family of soluble Wnt inhibitors that act either through direct binding to Wnt proteins and preventing its interaction with Fzd receptors or through direct interaction with Fzd receptors [144]. First, endothelial cells seem to have a sequential response to SFRP-1 including an initial inhibition of angiogenesis by antagonizing the canonical Wnt signaling pathway followed by an activation of the non-canonical Wnt/PCP signaling pathway that promotes angiogenesis [145,146]. Second, a study has demonstrated that SFRP-4 induces a decrease in endothelial cell migration correlated with a reduction in tumor-associated angiogenesis in a mouse model of ovarian cancer [147]. All these studies highlighted the role of the canonical Wnt/β-catenin signaling pathway in promoting angiogenesis (Figure 2d).
Only a few studies deal with the implication of the canonical Wnt/β-catenin signaling pathway in bone sarcoma-associated angiogenesis. Nevertheless, a study has described that naked cuticle homolog 2 (NKD2), a negative regulator of Wnt signaling, downregulates the expression of genes involved in the vasculature development of osteosarcoma [148], suggesting a pro-angiogenic role of the Wnt/β-catenin pathway in osteosarcoma. To our knowledge, the implication of this pathway on Ewing sarcoma-associated angiogenesis has not been described yet.
It is well recognized that hypoxia is an important regulator of angiogenesis during tumor development [149]. A major regulator of hypoxia is the transcription factor hypoxia inducible factor 1 (HIF-1), composed of the oxygen regulated HIF-1α subunit and the constitutively expressed HIF-1β. Under the hypoxic TME, HIF-1 directly activates the transcription of pro-angiogenic genes including VEGF to promote angiogenesis [150,151] as demonstrated in osteosarcoma and Ewing sarcoma [152]. Thus, HIF-1α silencing by siRNA reduces VEGF production by osteosarcoma cells in vitro and inhibits angiogenesis in a mouse model of osteosarcoma [153].
Besides its regulation of angiogenesis, hypoxia has been directly correlated to bone sarcoma progression, metastasis and patient prognosis. Thus, hypoxia enhances the expression and the transcriptional function of the fusion protein EWS/FLI1 in a HIF-1α dependent-manner in Ewing sarcoma cells and consequently increases their invasiveness [154], HIF-1α is also able to promote osteosarcoma cell proliferation and migration through activation of several signaling pathway including Akt and STAT3 signaling pathways [155,156]. Moreover, the expression of HIF-1α has been correlated to the development of lung metastasis associated with poorer survival in osteosarcoma patients [157,158,159].
The implication of the Wnt/β-catenin signaling pathway in hypoxia has mainly been reported in carcinoma. As an example, hypoxia promotes β-catenin nuclear localization related to a more invasive phenotype in colorectal adenocarcinoma cells [160]. Moreover, β-catenin directly interacts with HIF-1α to promote HIF-1 mediated transcription allowing an enhanced survival and adaptation to hypoxia of colon cancer cells [161]. Although the canonical Wnt/β-catenin signaling pathway seems to play a crucial role in hypoxia, only a few studies deal with its implication in the hypoxic TME of bone sarcoma. The most relevant one has described that inhibitors of the Wnt signaling pathway re-sensitizes osteosarcoma cells to doxorubicin under hypoxic conditions [162].
6.3. Wnt/β-Catenin Pathway and the Immune System
The immune microenvironment of bone sarcomas is highly heterogeneous composed of effectors of the innate immunity such as natural killer (NK) cells, dendritic cells (DC) and tumor-associated macrophages (TAMs) and cytotoxic T cells which drive adaptive immunity [33,35]. Nevertheless, immune and inflammatory infiltrates remain lower in Ewing sarcoma, limiting the development of immunotherapy [163].
Regarding innate immunity, bone sarcomas are mainly invaded by TAMs which have been associated with a good prognosis in osteosarcoma patients. However, as described above, it remains difficult to conclude about the role of M2-polarized macrophages in osteosarcoma progression, probably because of the difficulty to clearly identify the different TAMs subtypes in tumors and by the fact that, in contrast to the binary M1/M2 subtype, TAMs correspond to several distinct populations that often share features of both subtypes [164]. In Ewing sarcoma, a first study has initially described a correlation between macrophages infiltrate and poor prognosis but a second one did not observe any correlation between CD68+ macrophages and survival [39,40]. The canonical Wnt/β-catenin signaling pathway seems to drive the polarization of macrophages in cancers. Indeed, hepatic tumor cells produce Wnt ligands that enhance M2 macrophages polarization through the Wnt/β-catenin signaling pathway, resulting in increased primary and metastatic tumor development [165] (Figure 2e).
T lymphocytes in cancer, also known as tumor-infiltrating lymphocytes (TIL), correspond to the second most important immune cells in osteosarcoma [33,166]. TILs had higher cytotoxic capacities against tumor cells than peripheral lymphocytes but immunomodulatory molecules present in the TME prevent their expansion and activation. In particular, ligands of the B7 family are usually expressed by tumor cells in order to modulate T lymphocytes activities [167]. Osteosarcoma cells express HERV-H LTR-associating 2 (HHLA2), PDL-1 and B7-H3, three members of the B7 proteins that contribute to local immunosuppression. HHLA2 expression has been associated with metastatic properties of osteosarcoma cells and poor survival of osteosarcoma patients [168,169]. The binding of PDL-1, expressed by osteosarcoma cells to PD-1 on TILs induces the inhibition of their cytotoxic activities, contributing to local immunosuppression and consequently to tumor development. On the contrary, Ewing sarcoma does not constitutively express PDL-1 although its expression could be induced under inflammatory conditions [170]. Thus, only one case of the response of Ewing sarcoma to the blockade of PD-1/PDL-1 interaction has been described [171]. Finally, osteosarcoma cells also express B7-H3 which is correlated with tumor aggressiveness and metastasis [172,173]. In this context, Wnt/β-catenin pathway activation in the TME seems to inhibit T cell infiltration and functions in several cancers [174,175]. Moreover, the active Wnt/β-catenin signaling pathway has been associated with T cell exclusion leading to resistance to anti-PDL-1 therapy in human melanoma [176] (Figure 2e). Similarly, β-catenin activation enhances immune evasion and induces resistance to anti-PD1 therapy in hepatocellular carcinoma [177].
Another important point is that cross regulations between TAMs and TILs play a key role in osteosarcoma development. Thus, M2-polarized macrophages promote the suppression of TILs in osteosarcoma and, conversely, anti-PD1 therapy targeting TILs is able to stimulate a transition from M2 macrophages to M1 macrophages, leading to a decrease in lung metastases [41,178].
Finally, it seems important to note that, besides TAMs and TILs, the Wnt/β-catenin pathway is able to modulate the proliferation, recruitment, and/or functions of the different effectors of the immune system and to regulate multiple aspects of the immune cells/tumor crosstalk. As an example, Wnt ligands secreted by tumor cells can activate the Wnt/β-catenin signaling pathway in DC, leading to up regulated interleukin-10 and indoleamine 2,3-dioxygenase 1 secretion. This results in an increase in regulatory T cells inside the tumor and consequently an inhibition of TILs cytotoxic properties [179].
7. Conclusions
The canonical Wnt/β-catenin signaling pathway plays a crucial role during the different steps of bone sarcoma growth and the metastatic process, although its involvement has been more studied in osteosarcoma than in Ewing sarcoma. This pathway seems to promote bone sarcoma development through both a direct effect on bone sarcoma cells and an indirect modulation of the bone TME cell activities. Thus, aberrant activation of the canonical Wnt signaling pathway in osteosarcoma cells seems to be highly involved in cell proliferation, induction of an EMT-like process, acquisition of stem cells properties of osteosarcoma CSCs as well as metastatic dissemination. The Wnt/β-catenin pathway activation does not affect the proliferation of Ewing sarcoma cells but appears to play a role in the cellular motility of these cells. The Wnt/β-catenin pathway also participates to the hijacking of the bone TME by the bone sarcoma cells, promoting modulation of the ECM to allow the tumor cells invasiveness and inducing immune tolerance and angiogenesis in the TME. This results in an increase in metastatic dissemination.
The crucial role of the canonical Wnt/β-catenin signaling pathway is not restricted to bone sarcoma. That is why different therapeutic strategies targeting the Wnt/β-catenin signaling pathway in cancers have been developed. Corresponding clinical trials have been recently described in details in two reviews [67,174]. Briefly, they include inhibitors of porcupin, the enzyme responsible for palmitoylation of Wnt ligands, a necessary process to allow their extracellular secretion (LGK974 or WNT974), anti-Fzd antibodies (OMP18R5) or inhibitors of the β-catenin transcriptional complex (PRI724), mainly evaluated in patients with solid tumors.
The complexity of the Wnt signaling offers multiple targets to reduce β-catenin activation in tumor cells. However, better knowledge about the role of Wnt/β-catenin in each tumor cell type, in particular, osteosarcoma and Ewing sarcoma, is necessary to safely target this pathway. It is also necessary to focus on crosstalks between the Wnt/β-catenin and other signaling pathways during bone sarcoma growth and metastatic processes.
Acknowledgments
The figures were produced with the support of Servier Medical Art (www.servier.com).
Abbreviations
| APC | Adenomatous Polyposis Coli |
| BCL-9 | B cell CLL/lymphoma 9 |
| β-TrCP | beta-Transducin-repeat-Containing Protein |
| BRG1 | Brahma-related gene 1 |
| CBP | CREB (cAMP-Response Element Binding protein) binding protein |
| CCL | C-C motif ligand |
| CK1α | Casein Kinase 1 alpha |
| CSC | Cancer Stem Cell |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| CXCR | C-X-C Motif Chemokine Receptor |
| DC | Dendritic cell |
| DKK | Dickkopf |
| Dvl | Dishevelled |
| EMT | Epithelial-Mesenchymal Transition |
| ETS | E26 Transformation-Specific |
| Fzd | Frizzled |
| GSK3β | Glycogen synthase kinase 3 beta |
| HDAC | histone deacetylase |
| HHLA2 | HERV-H LTR-associating 2 |
| HIF-1 | Hypoxia Inducible Factor 1 |
| IFNγ | Interferon γ |
| IGFBP4 | Insulin-like Growth Factor Binding Protein 4 |
| iNOS | inducible Nitric Oxide Synthase |
| LEF | Lymphoid Enhancer-binding Factor |
| LGR5 | Leucine Rich Repeat Containing G Protein-Coupled Receptor 5 |
| lncRNA | long non-coding RNA |
| LRP | Lipoprotein Receptor-related Protein |
| miR | miRNA |
| MMP | Matrix Metalloproteinase |
| MSC | Mesenchymal Stem Cell |
| MT1-MMP | Membrane type 1 metalloprotease |
| NK | Natural killer |
| NKD2 | Naked cuticle homolog 2 |
| PCP | Planar Cell Polarity |
| PD-1 | Programmed cell Death 1 |
| PDL-1 | Programmed cell Death Ligand 1 |
| PLOD2 | Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 2 |
| ROR | Receptor tyrosine-kinase-like Orphan Receptor |
| RSPO | R-spondin |
| Ryk | Related to tyrosine kinase |
| SFRP | Secreted-Fzd-Related Protein |
| SOST | Sclerostin |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAF | Tumor Associated Fibroblast |
| TAM | Tumor Associated Macrophage |
| TAZ | Transcriptional co-Activator with a PDZ-binding domain |
| TCF | T-Cell Factor |
| TGFβ | Transforming Growth Factor beta |
| TLE | Transducin-Like Enhancer of split |
| TIL | Tumor Infiltrating Lymphocyte |
| TME | Tumor MicroEnvironment |
| VEGF | Vascular Endothelial Growth Factor |
| WIF1 | Wnt Inhibitory Factor 1 |
| WTX | Wilms tumor gene on X chromosome |
| YAP | Yes-Associated Protein |
Author Contributions
All authors have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ladenstein R., Pötschger U., Le Deley M.C., Whelan J., Paulussen M., Oberlin O., van den Berg H., Dirksen U., Hjorth L., Michon J., et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J. Clin. Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L., Troisi R.J., Savage S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages, and elderly persons. Int. J. Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Galindo C., Navid F., Liu T., Billups C.A., Rao B.N., Krasin M.J. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann. Oncol. 2008;19:814–820. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 5.Strauss S.J., Whelan J.S. Current questions in bone sarcomas. Curr. Opin. Oncol. 2018;30:252–259. doi: 10.1097/CCO.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 6.Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 7.Delattre O., Zucman J., Melot T., Garau X.S., Zucker J.M., Lenoir G.M., Ambros P.F., Sheer D., Turc-Carel C., Triche T.J. The Ewing family of tumors—A subgroup of small-round-cell tumors defined by specific chimeric transcripts. N. Engl. J. Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg J.P., de Alava E., Ladanyi M., Wexler L.H., Kovar H., Paulussen M., Zoubek A., Dockhorn-Dworniczak B., Juergens H., Wunder J.S., et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J. Clin. Oncol. 1999;17:1809–1814. doi: 10.1200/JCO.1999.17.6.1809. [DOI] [PubMed] [Google Scholar]
- 9.Lu P., Weaver V.M., Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D., Coussens L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Crenn V., Biteau K., Amiaud J., Dumars C., Guiho R., Vidal L., Nail L.-R.L., Heymann D., Moreau A., Gouin F., et al. Bone microenvironment has an influence on the histological response of osteosarcoma to chemotherapy: Retrospective analysis and preclinical modeling. Am. J. Cancer Res. 2017;7:2333–2349. [PMC free article] [PubMed] [Google Scholar]
- 12.Alfranca A., Martinez-Cruzado L., Tornin J., Abarrategi A., Amaral T., de Alava E., Menendez P., Garcia-Castro J., Rodriguez R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015;72:3097–3113. doi: 10.1007/s00018-015-1918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H., Barrott J.J., Cable M.G., Monument M.J., Lerman D.M., Smith-Fry K., Nollner D., Jones K.B. The Impact of Microenvironment on the Synovial Sarcoma Transcriptome. Cancer Microenviron. 2017;10:1–7. doi: 10.1007/s12307-017-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein S.D., Hayashi M., Albert C.M., Jackson K.W., Loeb D.M. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin. Exp. Metastasis. 2015;32:703–715. doi: 10.1007/s10585-015-9738-x. [DOI] [PubMed] [Google Scholar]
- 17.Riemann A., Schneider B., Gündel D., Stock C., Gekle M., Thews O. Acidosis Promotes Metastasis Formation by Enhancing Tumor Cell Motility. Adv. Exp. Med. Biol. 2016;876:215–220. doi: 10.1007/978-1-4939-3023-4_27. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay S., Chaklader M., Chatterjee R., Law A., Law S. Differential expression of mitotic regulators and tumor microenvironment influences the regional growth pattern of solid sarcoma along the cranio-caudal axis. Exp. Cell Res. 2016;340:91–101. doi: 10.1016/j.yexcr.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Lamoureux F., Richard P., Wittrant Y., Battaglia S., Pilet P., Trichet V., Blanchard F., Gouin F., Pitard B., Heymann D., et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: Blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 20.Picarda G., Matous E., Amiaud J., Charrier C., Lamoureux F., Heymann M.-F., Tirode F., Pitard B., Trichet V., Heymann D., et al. Osteoprotegerin inhibits bone resorption and prevents tumor development in a xenogenic model of Ewing’s sarcoma by inhibiting RANKL. J. Bone Oncol. 2013;2:95–104. doi: 10.1016/j.jbo.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor R., Knowles H.J., Athanasou N.A. Ewing sarcoma cells express RANKL and support osteoclastogenesis. J. Pathol. 2011;225:195–202. doi: 10.1002/path.2869. [DOI] [PubMed] [Google Scholar]
- 22.Dass C.R., Choong P.F.M. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol. Cancer Ther. 2007;6:3263–3270. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- 23.Heymann D., Ory B., Blanchard F., Heymann M.-F., Coipeau P., Charrier C., Couillaud S., Thiery J.P., Gouin F., Redini F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Odri G.A., Dumoucel S., Picarda G., Battaglia S., Lamoureux F., Corradini N., Rousseau J., Tirode F., Laud K., Delattre O., et al. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing’s sarcoma patients. Cancer Res. 2010;70:7610–7619. doi: 10.1158/0008-5472.CAN-09-4272. [DOI] [PubMed] [Google Scholar]
- 25.Ory B., Heymann M.-F., Kamijo A., Gouin F., Heymann D., Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104:2522–2529. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 26.Han Y., Wu C., Wang J., Liu N. CXCR7 maintains osteosarcoma invasion after CXCR4 suppression in bone marrow microenvironment. Tumour Biol. 2017;39:101042831770163. doi: 10.1177/1010428317701631. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.-J., Dai Y.-L., Zhang W.-B., Li S.-J., Tu C.-Q. Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: A meta-analysis. Clin. Exp. Med. 2017;17:59–69. doi: 10.1007/s10238-015-0405-y. [DOI] [PubMed] [Google Scholar]
- 28.Perissinotto E., Cavalloni G., Leone F., Fonsato V., Mitola S., Grignani G., Surrenti N., Sangiolo D., Bussolino F., Piacibello W., et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin. Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 29.Cortini M., Massa A., Avnet S., Bonuccelli G., Baldini N. Tumor-Activated Mesenchymal Stromal Cells Promote Osteosarcoma Stemness and Migratory Potential via IL-6 Secretion. PLoS ONE. 2016;11:e0166500. doi: 10.1371/journal.pone.0166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Ma Q., Liu T., Guan G., Zhang K., Chen J., Jia N., Yan S., Chen G., Liu S., et al. Interleukin-6 suppression reduces tumour self-seeding by circulating tumour cells in a human osteosarcoma nude mouse model. Oncotarget. 2016;7:446–458. doi: 10.18632/oncotarget.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi J., Zhou Y., Jiao Z., Wang X., Zhao Y., Li Y., Chen H., Yang L., Zhu H., Li Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell. Physiol. Biochem. 2017;42:2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Chu Y., Yue B., Ma X., Zhang G., Xiang H., Liu Y., Wang T., Wu X., Chen B. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget. 2017;8:23803–23816. doi: 10.18632/oncotarget.15866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heymann M.-F., Lézot F., Heymann D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2017 doi: 10.1016/j.cellimm.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Noy R., Pollard J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Brouchet A., Illac C., Gilhodes J., Bouvier C., Aubert S., Guinebretiere J.-M., Marie B., Larousserie F., Entz-Werlé N., de Pinieux G., et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology. 2017;6:e1331193. doi: 10.1080/2162402X.2017.1331193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buddingh E.P., Kuijjer M.L., Duim R.A.J., Bürger H., Agelopoulos K., Myklebost O., Serra M., Mertens F., Hogendoorn P.C.W., Lankester A.C., et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011;17:2110–2119. doi: 10.1158/1078-0432.CCR-10-2047. [DOI] [PubMed] [Google Scholar]
- 37.Dumars C., Ngyuen J.-M., Gaultier A., Lanel R., Corradini N., Gouin F., Heymann D., Heymann M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. 2016;7:78343–78354. doi: 10.18632/oncotarget.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q., Xian M., Xiang S., Xiang D., Shao X., Wang J., Cao J., Yang X., Yang B., Ying M., et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017;5:547–559. doi: 10.1158/2326-6066.CIR-16-0259. [DOI] [PubMed] [Google Scholar]
- 39.Fujiwara T., Fukushi J., Yamamoto S., Matsumoto Y., Setsu N., Oda Y., Yamada H., Okada S., Watari K., Ono M., et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am. J. Pathol. 2011;179:1157–1170. doi: 10.1016/j.ajpath.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handl M., Hermanova M., Hotarkova S., Jarkovsky J., Mudry P., Shatokhina T., Vesela M., Sterba J., Zambo I. Clinicopathological correlation of tumor-associated macrophages in Ewing sarcoma. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc. 2018;162:54–60. doi: 10.5507/bp.2017.049. [DOI] [PubMed] [Google Scholar]
- 41.Han Q., Shi H., Liu F. CD163(+) M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016;34:101–106. doi: 10.1016/j.intimp.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Chang C.-H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J.W., et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis D.M., Pruitt H., Jain N., Ciccaglione M., McCaffery J.M., Xia Z., Weber K., Eisinger-Mathason T.S.K., Gerecht S. A Feedback Loop between Hypoxia and Matrix Stress Relaxation Increases Oxygen-Axis Migration and Metastasis in Sarcoma. Cancer Res. 2019;79:1981–1995. doi: 10.1158/0008-5472.CAN-18-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh H., Kadomatsu T., Tanoue H., Yugami M., Miyata K., Endo M., Morinaga J., Kobayashi E., Miyamoto T., Kurahashi R., et al. TET2-dependent IL-6 induction mediated by the tumor microenvironment promotes tumor metastasis in osteosarcoma. Oncogene. 2018;37:2903–2920. doi: 10.1038/s41388-018-0160-0. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins A.G., Basrur V., da Veiga Leprevost F., Pedersen E., Sperring C., Nesvizhskii A.I., Lawlor E.R. The Ewing Sarcoma Secretome and Its Response to Activation of Wnt/beta-catenin Signaling. Mol. Cell. Proteom. 2018;17:901–912. doi: 10.1074/mcp.RA118.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volchenboum S.L., Andrade J., Huang L., Barkauskas D.A., Krailo M., Womer R.B., Ranft A., Potratz J., Dirksen U., Triche T.J., et al. Gene expression profiling of Ewing sarcoma tumours reveals the prognostic importance of tumour–stromal interactions: A report from the Children’s Oncology Group. J. Pathol. Clin. Res. 2015;1:83–94. doi: 10.1002/cjp2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sand L.G.L., Berghuis D., Szuhai K., Hogendoorn P.C.W. Expression of CCL21 in Ewing sarcoma shows an inverse correlation with metastases and is a candidate target for immunotherapy. Cancer Immunol. Immunother. 2016;65:995–1002. doi: 10.1007/s00262-016-1862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piperno-Neumann S., Le Deley M.-C., Rédini F., Pacquement H., Marec-Bérard P., Petit P., Brisse H., Lervat C., Gentet J.-C., Entz-Werlé N., et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–1080. doi: 10.1016/S1470-2045(16)30096-1. [DOI] [PubMed] [Google Scholar]
- 49.Paydas S., Bagir E.K., Deveci M.A., Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med. Oncol. 2016;33:93. doi: 10.1007/s12032-016-0807-z. [DOI] [PubMed] [Google Scholar]
- 50.Lussier D.M., O’Neill L., Nieves L.M., McAfee M.S., Holechek S.A., Collins A.W., Dickman P., Jacobsen J., Hingorani P., Blattman J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015;38:96–106. doi: 10.1097/CJI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen J.K., Cote G.M., Choy E., Yang P., Harmon D., Schwab J., Nielsen G.P., Chebib I., Ferrone S., Wang X., et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol. Res. 2014;2:690–698. doi: 10.1158/2326-6066.CIR-13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundara Y.T., Kostine M., Cleven A.H.G., Bovée J.V.M.G., Schilham M.W., Cleton-Jansen A.-M. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: A rationale for T-cell-based immunotherapy. Cancer Immunol. Immunother. 2017;66:119–128. doi: 10.1007/s00262-016-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duchartre Y., Kim Y.-M., Kahn M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polakis P. Wnt Signaling in Cancer. Cold Spring Harb Perspect. Biol. 2012;4:a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai D., Wells K., Arcaroli J., Vanderbilt C., Aisner D.L., Messersmith W.A., Lieu C.H. Targeting the WNT Signaling Pathway in Cancer Therapeutics. Oncologist. 2015;20:1189–1198. doi: 10.1634/theoncologist.2015-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Major M.B., Camp N.D., Berndt J.D., Yi X., Goldenberg S.J., Hubbert C., Biechele T.L., Gingras A.-C., Zheng N., Maccoss M.J., et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 57.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Kim S.-E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M.V., MacDonald B.T., Zhang X., Garcia Abreu J., Peng L., et al. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu C., Li Y., Semenov M., Han C., Baeg G.-H., Tan Y., Zhang Z., Lin X., He X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 60.Robertson H., Hayes J.D., Sutherland C. A partnership with the proteasome; the destructive nature of GSK3. Biochem. Pharmacol. 2018;147:77–92. doi: 10.1016/j.bcp.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamos J.L., Weis W.I. The β-catenin destruction complex. Cold Spring Harb Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chodaparambil J.V., Pate K.T., Hepler M.R.D., Tsai B.P., Muthurajan U.M., Luger K., Waterman M.L., Weis W.I. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 2014;33:719–731. doi: 10.1002/embj.201387188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li V.S.W., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J.R., Maurice M.M., Mahmoudi T., et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Lien W.-H., Fuchs E. Wnt some lose some: Transcriptional governance of stem cells by Wnt/-catenin signaling. Genes Dev. 2014;28:1517–1532. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh N., Hossain U., Mandal A., Sil P.C. The Wnt signaling pathway: A potential therapeutic target against cancer. Ann. N. Y. Acad. Sci. 2019;1443:54–74. doi: 10.1111/nyas.14027. [DOI] [PubMed] [Google Scholar]
- 68.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishibashi M. Screening for natural products that affect Wnt signaling activity. J. Nat. Med. 2019:1–9. doi: 10.1007/s11418-019-01320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le P.N., McDermott J.D., Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabatabai R., Linhares Y., Bolos D., Mita M., Mita A. Targeting the Wnt Pathway in Cancer: A Review of Novel Therapeutics. Target Oncol. 2017;12:623–641. doi: 10.1007/s11523-017-0507-4. [DOI] [PubMed] [Google Scholar]
- 72.Tran F.H., Zheng J.J. Modulating the wnt signaling pathway with small molecules: Modulating the Wnt Signaling Pathway. Protein Sci. 2017;26:650–661. doi: 10.1002/pro.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X., Hao J. Development of anticancer agents targeting the Wnt/β-catenin signaling. Am. J. Cancer Res. 2015;5:2344–2360. [PMC free article] [PubMed] [Google Scholar]
- 74.Harb J., Lin P.-J., Hao J. Recent Development of Wnt Signaling Pathway Inhibitors for Cancer Therapeutics. Curr. Oncol. Rep. 2019;21:12. doi: 10.1007/s11912-019-0763-9. [DOI] [PubMed] [Google Scholar]
- 75.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen C., Zhao M., Tian A., Zhang X., Yao Z., Ma X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget. 2015;6:17570–17583. doi: 10.18632/oncotarget.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iwaya K., Ogawa H., Kuroda M., Izumi M., Ishida T., Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin. Exp. Metastasis. 2003;20:525–529. doi: 10.1023/A:1025821229013. [DOI] [PubMed] [Google Scholar]
- 79.Liu W., Zhao Z., Wang Y., Li W., Su Q., Jia Q., Zhang J., Zhang X., Shen J., Yin J. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. 2018;9:343. doi: 10.1038/s41419-018-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Y., Guan G.-F., Chen J., Hu B., Sun C., Ma Q., Wen Y.-H., Qiu X.-C., Zhou Y. Aberrant CXCR4 and β-catenin expression in osteosarcoma correlates with patient survival. Oncol. Lett. 2015;10:2123–2129. doi: 10.3892/ol.2015.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai Y., Mohseny A.B., Karperien M., Hogendoorn P.C.W., Zhou G., Cleton-Jansen A.-M. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J. Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- 82.Cleton-Jansen A.-M., Anninga J.K., Briaire-de Bruijn I.H., Romeo S., Oosting J., Egeler R.M., Gelderblom H., Taminiau A.H.M., Hogendoorn P.C.W. Profiling of high-grade central osteosarcoma and its putative progenitor cells identifies tumourigenic pathways. Br. J. Cancer. 2009;101:1909–1918. doi: 10.1038/sj.bjc.6605405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du X., Yang J., Yang D., Tian W., Zhu Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer. 2014;14:450. doi: 10.1186/1471-2407-14-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimozaki S., Yamamoto N., Domoto T., Nishida H., Hayashi K., Kimura H., Takeuchi A., Miwa S., Igarashi K., Kato T., et al. Efficacy of glycogen synthase kinase-3β targeting against osteosarcoma via activation of β-catenin. Oncotarget. 2016;7:77038–77051. doi: 10.18632/oncotarget.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jie X.-X., Zhang X.-Y., Xu C.-J. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications. Oncotarget. 2017;8:81558–81571. doi: 10.18632/oncotarget.18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei P., Ding D., Xie J., Wang L., Liao Q., Hu Y. Expression profile of Twist, vascular endothelial growth factor and CD34 in patients with different phases of osteosarcoma. Oncol. Lett. 2015;10:417–421. doi: 10.3892/ol.2015.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharili A.-S., Allen S., Smith K., Hargreaves J., Price J., McGonnell I. Expression of Snail2 in long bone osteosarcomas correlates with tumour malignancy. Tumour Biol. 2011;32:515–526. doi: 10.1007/s13277-010-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen A., Zhang Y., Yang H., Xu R., Huang G. Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J. Surg. Oncol. 2012;105:830–834. doi: 10.1002/jso.23012. [DOI] [PubMed] [Google Scholar]
- 89.Yang G., Yuan J., Li K. EMT transcription factors: Implication in osteosarcoma. Med. Oncol. 2013;30:697. doi: 10.1007/s12032-013-0697-2. [DOI] [PubMed] [Google Scholar]
- 90.Verrecchia F., Rédini F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay Between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018;8:133. doi: 10.3389/fonc.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuxe J., Vincent T., de Garcia Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 92.Tian H., Zhou T., Chen H., Li C., Jiang Z., Lao L., Kahn S.A., Duarte M.E.L., Zhao J., Daubs M.D., et al. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J. Orthop. Res. 2019;37:1638–1648. doi: 10.1002/jor.24244. [DOI] [PubMed] [Google Scholar]
- 93.Wang S., Zhang D., Han S., Gao P., Liu C., Li J., Pan X. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway. Sci. Rep. 2017;7:6215. doi: 10.1038/s41598-017-06353-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Fan S., Gao X., Chen P., Li X. Carboxypeptidase E-ΔN promotes migration, invasiveness, and epithelial-mesenchymal transition of human osteosarcoma cells via the Wnt-β-catenin pathway. Biochem. Cell Biol. 2018:1–8. doi: 10.1139/bcb-2018-0236. [DOI] [PubMed] [Google Scholar]
- 95.Zeng Q., Li Z., Zhao X., Guo L., Yu C., Qin J., Zhang S., Zhang Y., Yang X. Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial-mesenchymal transition. Oncol. Rep. 2019;41:543–551. doi: 10.3892/or.2018.6835. [DOI] [PubMed] [Google Scholar]
- 96.Cai Z., Cao Y., Luo Y., Hu H., Ling H. Signalling mechanism(s) of epithelial-mesenchymal transition and cancer stem cells in tumour therapeutic resistance. Clin. Chim. Acta. 2018;483:156–163. doi: 10.1016/j.cca.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 97.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M., Yang S.X., Ivy S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adhikari A.S., Agarwal N., Wood B.M., Porretta C., Ruiz B., Pochampally R.R., Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siclari V.A., Qin L. Targeting the osteosarcoma cancer stem cell. J. Orthop. Surg. Res. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martins-Neves S.R., Corver W.E., Paiva-Oliveira D.I., van den Akker B.E.W.M., Briaire-de-Bruijn I.H., Bovée J.V.M.G., Gomes C.M.F., Cleton-Jansen A.-M. Osteosarcoma Stem Cells Have Active Wnt/β-catenin and Overexpress SOX2 and KLF4. J. Cell. Physiol. 2016;231:876–886. doi: 10.1002/jcp.25179. [DOI] [PubMed] [Google Scholar]
- 101.Cai W., Xu Y., Yin J., Zuo W., Su Z. miR-552-5p facilitates osteosarcoma cell proliferation and metastasis by targeting WIF1. Exp. Ther. Med. 2019;17:3781–3788. doi: 10.3892/etm.2019.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y., Wang Y., Yang H., Zhao L., Song R., Tan H., Wang L. MicroRNA-873 targets HOXA9 to inhibit the aggressive phenotype of osteosarcoma by deactivating the Wnt/β-catenin pathway. Int. J. Oncol. 2019;54:1809–1820. doi: 10.3892/ijo.2019.4735. [DOI] [PubMed] [Google Scholar]
- 103.Liu Y., Bao Z., Tian W., Huang G. miR-885-5p suppresses osteosarcoma proliferation, migration and invasion through regulation of β-catenin. Oncol. Lett. 2019;17:1996–2004. doi: 10.3892/ol.2018.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren J., Yang M., Xu F., Chen J. microRNA-758 inhibits the malignant phenotype of osteosarcoma cells by directly targeting HMGA1 and deactivating the Wnt/β-catenin pathway. Am. J. Cancer Res. 2019;9:36–52. [PMC free article] [PubMed] [Google Scholar]
- 105.Xia P., Gu R., Zhang W., Shao L., Li F., Wu C., Sun Y. MicroRNA-377 exerts a potent suppressive role in osteosarcoma through the involvement of the histone acetyltransferase 1-mediated Wnt axis. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28843. [DOI] [PubMed] [Google Scholar]
- 106.Li C., Wang F., Wei B., Wang L., Kong D. LncRNA AWPPH promotes osteosarcoma progression via activation of Wnt/β-catenin pathway through modulating miR-93-3p/FZD7 axis. Biochem. Biophys. Res. Commun. 2019;514:1017–1022. doi: 10.1016/j.bbrc.2019.04.203. [DOI] [PubMed] [Google Scholar]
- 107.Lin C.H., Ji T., Chen C.-F., Hoang B.H. Wnt signaling in osteosarcoma. Adv. Exp. Med. Biol. 2014;804:33–45. doi: 10.1007/978-3-319-04843-7_2. [DOI] [PubMed] [Google Scholar]
- 108.Pridgeon M.G., Grohar P.J., Steensma M.R., Williams B.O. Wnt Signaling in Ewing Sarcoma, Osteosarcoma, and Malignant Peripheral Nerve Sheath Tumors. Curr. Osteoporos. Rep. 2017;15:239–246. doi: 10.1007/s11914-017-0377-9. [DOI] [PubMed] [Google Scholar]
- 109.Pedersen E.A., Menon R., Bailey K.M., Thomas D.G., Van Noord R.A., Tran J., Wang H., Qu P.P., Hoering A., Fearon E.R., et al. Activation of Wnt/beta-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Cancer Res. 2016;76:5040–5053. doi: 10.1158/0008-5472.CAN-15-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scannell C.A., Pedersen E.A., Mosher J.T., Krook M.A., Nicholls L.A., Wilky B.A., Loeb D.M., Lawlor E.R. LGR5 is Expressed by Ewing Sarcoma and Potentiates Wnt/β-Catenin Signaling. Front. Oncol. 2013;3:81. doi: 10.3389/fonc.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uren A., Wolf V., Sun Y.-F., Azari A., Rubin J.S., Toretsky J.A. Wnt/Frizzled signaling in Ewing sarcoma. Pediatr. Blood Cancer. 2004;43:243–249. doi: 10.1002/pbc.20124. [DOI] [PubMed] [Google Scholar]
- 112.Endo Y., Beauchamp E., Woods D., Taylor W.G., Toretsky J.A., Uren A., Rubin J.S. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol. Cell. Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Navarro D., Agra N., Pestaña A., Alonso J., González-Sancho J.M. The EWS/FLI1 oncogenic protein inhibits expression of the Wnt inhibitor DICKKOPF-1 gene and antagonizes beta-catenin/TCF-mediated transcription. Carcinogenesis. 2010;31:394–401. doi: 10.1093/carcin/bgp317. [DOI] [PubMed] [Google Scholar]
- 114.Hayashi M., Baker A., Goldstein S.D., Albert C.M., Jackson K.W., McCarty G., Kahlert U.D., Loeb D.M. Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma. Oncotarget. 2017;8:78265–78276. doi: 10.18632/oncotarget.19432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown H.K., Schiavone K., Gouin F., Heymann M.-F., Heymann D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018;102:174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pederson L., Ruan M., Westendorf J.J., Khosla S., Oursler M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA. 2008;105:20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Westendorf J.J., Kahler R.A., Schroeder T.M. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 118.Zhong Z., Zylstra-Diegel C.R., Schumacher C.A., Baker J.J., Carpenter A.C., Rao S., Yao W., Guan M., Helms J.A., Lane N.E., et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc. Natl. Acad. Sci. USA. 2012;109:E2197–E2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weivoda M.M., Ruan M., Hachfeld C.M., Pederson L., Howe A., Davey R.A., Zajac J.D., Kobayashi Y., Williams B.O., Westendorf J.J., et al. Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways. J. Bone Miner. Res. 2016;31:65–75. doi: 10.1002/jbmr.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sadanandam A., Futakuchi M., Lyssiotis C.A., Gibb W.J., Singh R.K. A cross-species analysis of a mouse model of breast cancer-specific osteolysis and human bone metastases using gene expression profiling. BMC Cancer. 2011;11:304. doi: 10.1186/1471-2407-11-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bu G., Lu W., Liu C.-C., Selander K., Yoneda T., Hall C., Keller E.T., Li Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int. J. Cancer. 2008;123:1034–1042. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bjørnland K., Flatmark K., Pettersen S., Aaasen A.O., Fodstad O., Maelandsmo G.M. Matrix metalloproteinases participate in osteosarcoma invasion. J. Surg. Res. 2005;127:151–156. doi: 10.1016/j.jss.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 123.Kunz P., Sähr H., Lehner B., Fischer C., Seebach E., Fellenberg J. Elevated ratio of MMP2/MMP9 activity is associated with poor response to chemotherapy in osteosarcoma. BMC Cancer. 2016;16:223. doi: 10.1186/s12885-016-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang M., Zhang X. Association of MMP-2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2015;8:14965–14970. [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou J., Liu T., Wang W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine. 2018;97:e13051. doi: 10.1097/MD.0000000000013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mateo E.C., Motta F.J.N., de Paula Queiroz R.G., Scrideli C.A., Tone L.G. Protein expression of matrix metalloproteinase (MMP-1, -2, -3, -9 and -14) in Ewing family tumors and medulloblastomas of pediatric patients. J. Pediatr. Genet. 2012;1:181–187. doi: 10.3233/PGE-2012-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ye C., Yu X., Liu X., Zhan P., Nie T., Guo R., Liu H., Dai M., Zhang B. Beclin-1 knockdown decreases proliferation, invasion and migration of Ewing sarcoma SK-ES-1 cells via inhibition of MMP-9. Oncol. Lett. 2018;15:3221–3225. doi: 10.3892/ol.2017.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo Y., Zi X., Koontz Z., Kim A., Xie J., Gorlick R., Holcombe R.F., Hoang B.H. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J. Orthop. Res. 2007;25:964–971. doi: 10.1002/jor.20356. [DOI] [PubMed] [Google Scholar]
- 129.Liu B., Li G., Wang X., Liu Y. A furin inhibitor downregulates osteosarcoma cell migration by downregulating the expression levels of MT1-MMP via the Wnt signaling pathway. Oncol. Lett. 2014;7:1033–1038. doi: 10.3892/ol.2014.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang F., Chen A., Chen J., Yu T., Guo F. Influence of β-catenin small interfering RNA on human osteosarcoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011;31:353–358. doi: 10.1007/s11596-011-0380-9. [DOI] [PubMed] [Google Scholar]
- 131.Zhang F., Chen A., Chen J., Yu T., Guo F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac. J. Cancer Prev. 2011;12:239–245. [PubMed] [Google Scholar]
- 132.Lowy C.M., Oskarsson T. Tenascin C in metastasis: A view from the invasive front. Cell Adh. Migr. 2015;9:112–124. doi: 10.1080/19336918.2015.1008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Olsen J.J., Pohl S.Ö.-G., Deshmukh A., Visweswaran M., Ward N.C., Arfuso F., Agostino M., Dharmarajan A. The Role of Wnt Signalling in Angiogenesis. Clin. Biochem. Rev. 2017;38:131–142. [PMC free article] [PubMed] [Google Scholar]
- 134.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 135.Mavrogenis A.F., Vottis C.T., Megaloikonomos P.D., Agrogiannis G.D., Theocharis S. Neovascularization in Ewing’s sarcoma. Neoplasma. 2018;65:317–325. doi: 10.4149/neo_2018_170410N264. [DOI] [PubMed] [Google Scholar]
- 136.Chen D., Zhang Y.-J., Zhu K., Wang W.-C. A systematic review of vascular endothelial growth factor expression as a biomarker of prognosis in patients with osteosarcoma. Tumour Biol. 2013;34:1895–1899. doi: 10.1007/s13277-013-0733-z. [DOI] [PubMed] [Google Scholar]
- 137.Yang J., Yang D., Sun Y., Sun B., Wang G., Trent J.C., Araujo D.M., Chen K., Zhang W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer. 2011;117:4925–4938. doi: 10.1002/cncr.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fuchs B., Inwards C.Y., Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin. Cancer Res. 2004;10:1344–1353. doi: 10.1158/1078-0432.CCR-03-0038. [DOI] [PubMed] [Google Scholar]
- 139.Van der Schaft D.W.J., Hillen F., Pauwels P., Kirschmann D.A., Castermans K., Egbrink M.G.A.O., Tran M.G.B., Sciot R., Hauben E., Hogendoorn P.C.W., et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65:11520–11528. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 140.Reddy K., Zhou Z., Schadler K., Jia S.-F., Kleinerman E.S. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol. Cancer Res. 2008;6:929–936. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reddy K., Cao Y., Zhou Z., Yu L., Jia S.-F., Kleinerman E.S. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing’s sarcoma vasculature. Angiogenesis. 2008;11:257–267. doi: 10.1007/s10456-008-9109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Easwaran V., Lee S.H., Inge L., Guo L., Goldbeck C., Garrett E., Wiesmann M., Garcia P.D., Fuller J.H., Chan V., et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- 143.Zhang X., Gaspard J.P., Chung D.C. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 144.Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 145.Dufourcq P., Leroux L., Ezan J., Descamps B., Lamazière J.-M.D., Costet P., Basoni C., Moreau C., Deutsch U., Couffinhal T., et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: Role in neovessel formation. Am. J. Pathol. 2008;172:37–49. doi: 10.2353/ajpath.2008.070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duplàa C., Jaspard B., Moreau C., D’Amore P.A. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence for a role in endothelial cell growth control. Circ. Res. 1999;84:1433–1445. doi: 10.1161/01.RES.84.12.1433. [DOI] [PubMed] [Google Scholar]
- 147.Muley A., Majumder S., Kolluru G.K., Parkinson S., Viola H., Hool L., Arfuso F., Ganss R., Dharmarajan A., Chatterjee S. Secreted frizzled-related protein 4: An angiogenesis inhibitor. Am. J. Pathol. 2010;176:1505–1516. doi: 10.2353/ajpath.2010.090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao S., Kurenbekova L., Gao Y., Roos A., Creighton C.J., Rao P., Hicks J., Man T.-K., Lau C., Brown A.M.C., et al. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene. 2015;34:5069. doi: 10.1038/onc.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liao D., Johnson R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 150.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D., Semenza G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krock B.L., Skuli N., Simon M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zeng W., Wan R., Zheng Y., Singh S.R., Wei Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011;313:129–136. doi: 10.1016/j.canlet.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X.-D., Wu Q., Yang S.-H. Effects of siRNA-mediated HIF-1α gene silencing on angiogenesis in osteosarcoma. Pak. J. Med. Sci. 2017;33:341–346. doi: 10.12669/pjms.332.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aryee D.N.T., Niedan S., Kauer M., Schwentner R., Bennani-Baiti I.M., Ban J., Muehlbacher K., Kreppel M., Walker R.L., Meltzer P., et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Cancer Res. 2010;70:4015–4023. doi: 10.1158/0008-5472.CAN-09-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang D., Cui G., Sun C., Lei L., Lei L., Williamson R.A., Wang Y., Zhang J., Chen P., Wang A., et al. Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-β axis. Biochem. Biophys. Res. Commun. 2019;512:360–366. doi: 10.1016/j.bbrc.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 156.Zhang B., Li Y.-L., Zhao J.-L., Zhen O., Yu C., Yang B.-H., Yu X.-R. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma. Biomed. Pharmacother. 2018;105:1–9. doi: 10.1016/j.biopha.2018.03.165. [DOI] [PubMed] [Google Scholar]
- 157.Li Y., Zhang W., Li S., Tu C. Prognosis value of Hypoxia-inducible factor-1α expression in patients with bone and soft tissue sarcoma: A meta-analysis. Springerplus. 2016;5:1370. doi: 10.1186/s40064-016-3064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang S., Ren T., Huang Y., Bao X., Sun K., Shen D., Guo W. BMPR2 and HIF1-α overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chin. J. Cancer Res. 2017;29:447–454. doi: 10.21147/j.issn.1000-9604.2017.05.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren H.-Y., Zhang Y.-H., Li H.-Y., Xie T., Sun L.-L., Zhu T., Wang S.-D., Ye Z.-M. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: A meta-analysis. Onco Targets Ther. 2016;9:1477–1487. doi: 10.2147/OTT.S95490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Demir R., Dimmler A., Naschberger E., Demir I., Papadopoulos T., Melling N., Sturzl M., Hohenberger W. Malignant progression of invasive tumour cells seen in hypoxia present an accumulation of beta-catenin in the nucleus at the tumour front. Exp. Mol. Pathol. 2009;87:109–116. doi: 10.1016/j.yexmp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 161.Kaidi A., Williams A.C., Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 162.Scholten D.J., Timmer C.M., Peacock J.D., Pelle D.W., Williams B.O., Steensma M.R. Down regulation of Wnt signaling mitigates hypoxia-induced chemoresistance in human osteosarcoma cells. PLoS ONE. 2014;9:e111431. doi: 10.1371/journal.pone.0111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Casey D.L., Lin T.-Y., Cheung N.-K.V. Exploiting Signaling Pathways and Immune Targets Beyond the Standard of Care for Ewing Sarcoma. Front. Oncol. 2019;9:537. doi: 10.3389/fonc.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qian B.-Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang Y., Ye Y.-C., Chen Y., Zhao J.-L., Gao C.-C., Han H., Liu W.-C., Qin H.-Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu T., Fang X.-C., Ding Z., Sun Z.-G., Sun L.-M., Wang Y.-L. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5:682–687. doi: 10.1016/j.fob.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Assal A., Kaner J., Pendurti G., Zang X. Emerging targets in cancer immunotherapy: Beyond CTLA-4 and PD-1. Immunotherapy. 2015;7:1169–1186. doi: 10.2217/imt.15.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koirala P., Roth M.E., Gill J., Chinai J.M., Ewart M.R., Piperdi S., Geller D.S., Hoang B.H., Fatakhova Y.V., Ghorpade M., et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci. Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koirala P., Roth M.E., Gill J., Piperdi S., Chinai J.M., Geller D.S., Hoang B.H., Park A., Fremed M.A., Zang X., et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016;6:30093. doi: 10.1038/srep30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spurny C., Kailayangiri S., Jamitzky S., Altvater B., Wardelmann E., Dirksen U., Hardes J., Hartmann W., Rossig C. Programmed cell death ligand 1 (PD-L1) expression is not a predominant feature in Ewing sarcomas. Pediatr. Blood Cancer. 2018;65:e26719. doi: 10.1002/pbc.26719. [DOI] [PubMed] [Google Scholar]
- 171.McCaughan G.J.B., Fulham M.J., Mahar A., Soper J., Hong A.M., Stalley P.D., Tattersall M.H.N., Bhadri V.A. Programmed cell death-1 blockade in recurrent disseminated Ewing sarcoma. J. Hematol. Oncol. 2016;9:48. doi: 10.1186/s13045-016-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang L., Zhang Q., Chen W., Shan B., Ding Y., Zhang G., Cao N., Liu L., Zhang Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE. 2013;8:e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yin S.J., Wang W.J., Zhang J.Y. Expression of B7-H3 in cancer tissue during osteosarcoma progression in nude mice. Genet. Mol. Res. 2015;14:14253–14261. doi: 10.4238/2015.November.13.9. [DOI] [PubMed] [Google Scholar]
- 174.Goldsberry W.N., Londoño A., Randall T.D., Norian L.A., Arend R.C. A Review of the Role of Wnt in Cancer Immunomodulation. Cancers. 2019;11:771. doi: 10.3390/cancers11060771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luke J.J., Bao R., Sweis R.F., Spranger S., Gajewski T.F. WNT/β-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019;25:3074–3083. doi: 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 177.De Ruiz Galarreta M., Bresnahan E., Molina-Sanchez P., Lindblad K.E., Maier B., Sia D., Puigvehi M., Miguela V., Casanova-Acebes M., Dhainaut M., et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019 doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dhupkar P., Gordon N., Stewart J., Kleinerman E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7:2654–2664. doi: 10.1002/cam4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Galluzzi L., Spranger S., Fuchs E., López-Soto A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]