Abstract
As a semi-aquatic plant, rice requires water for proper growth, development, and orientation of physiological processes. Stress is induced at the cellular and molecular level when rice is exposed to drought or periods of low water availability. Plants have existing defense mechanisms in planta that respond to stress. In this review we examine the role played by miRNAs in the regulation and control of drought stress in rice through a summary of molecular studies conducted on miRNAs with emphasis on their contribution to drought regulatory networks in comparison to other plant systems. The interaction between miRNAs, target genes, transcription factors and their respective roles in drought-induced stresses is elaborated. The cross talk involved in controlling drought stress responses through the up and down regulation of targets encoding regulatory and functional proteins is highlighted. The information contained herein can further be explored to identify targets for crop improvement in the future.
Keywords: miRNA, drought, rice, hormone, osmoregulation, antioxidant defense, senescence, growth and development
1. Introduction
miRNAs function in controlling expression levels of target genes regulating physiological and developmental processes in plants, animals, and microbes [1,2]. They are able to regulate multiple targets and thence act as master regulators of processes such as growth, photosynthesis, respiration, and response to biotic and environmental stresses. As a master switch, miRNA may be exploited to control or improve agronomic traits such as resistance and tolerance towards stresses [3,4,5]. The miRNA biogenesis begins with the transcription of miRNA genes through RNA polymerase II that generates primary miRNA transcripts via DICER like 1 (DCL1). DCL1 then further processes precursor miRNAs to miRNA duplexes in the nuclei. The miRNA is then transferred to the cytoplasm where through ARGONAUTE (AGO), it regulates the cleavage of mRNA [6,7]. As part of the regulatory network, miRNAs work closely with target genes and transcription factors. The interaction between these components are important for the regulation of plant developmental processes and is absolutely essential in moderating gene expression in plants [8]. The interaction of these miRNAs, their target genes, and the transcription factors therein differ between organisms and exhibit differential expression spatially and temporally. As part of the regulatory network, miRNAs work closely with target genes and various transcription factors such as APETALA 2 (AP2), GRF (Growth-Regulating Factor), NAC (NAM, ATAF1, and CUC2), NF-Y (Nuclear transcription factor Y), MYB, TCP (TEOSINTE BRANCHED/CYCLOIDEA/PCF), SPL (SQUAMOSA-promoter binding transcription factor) and WRKY [9]. The overlap between miRNAs and their targets in multiple processes show that there is cross talk between various physiological and developmental processes in plants [10,11]. From the studies conducted in various plant systems, one can conclude that miRNAs are regulated by multiple factors and are dynamic [12,13].
Rice is a staple diet of most Asian countries. Various countries grow rice for local consumption, export, and income. With the growing population, the demand made on the supply of rice is high. Therefore, breeding programs are directed towards increasing yield in response to both biotic and abiotic stresses [14,15]. One such stress that affects yield is drought. It is therefore important to understand how drought affects rice and its yield. Drought-prone as well as poorly irrigated regions require low water utilization efficiency in their crops. The lack of water can affect development and physiological processes within the plant and result in detrimental outcomes. It is therefore important to identify factors that regulate against drought and to find key components, such as miRNAs that may be used in the development of drought resistant plants. Plants have evolved several mechanisms that enable them to withstand stresses. These mechanisms include root elongation, osmotic stress regulation, regulation of photosynthesis and respiration, senescence, antioxidative stress modulation, and various hormonal fine-tuning of physiological and developmental processes within the plant. Studies have shown that miRNAs are important modulators of drought tolerance in plants where it influences the cleavage of several drought responsive genes and thence inhibits their translation. The regulatory information derived from these components control the networking between miRNA and their target genes [16]. However, to date, the information on miRNAs, target genes and the cross talk between pathways is not well understood in plants, especially in rice. Therefore, further systematic elucidation is required to understand the regulatory networks and useful strategies for crop improvement [13,17,18,19].
In this review we look into the role of miRNA in regulating drought stress in rice. The summary of information obtained from a systematic review is useful in understanding the role of miRNAs in drought response and to identify key modulators that may be targeted for rice crop improvement in the near future. The miRNAs identified in rice will also be compared with reports from other plant systems to observe the similarities and differences in the mechanisms of regulation in drought. By understanding the contribution of each miRNA in influencing their target genes and processes, we are better equipped to understand the complexities involved in managing drought. Some of these miRNAs while mitigating stress are also responsible for yield elevation. The following sections will elucidate their involvement in hormonal, developmental, and stress management during drought in rice and compare them with other plant systems. Key miRNAs for further functional analysis will also be identified.
2. Hormonal Regulation of Drought
2.1. Abscisic Acid (ABA) in Drought Stress Signaling
Abscisic acid (ABA) plays a dominant role in drought regulation by addressing water deficit and mediating stress response through the activation of appropriate genes and stomatal movement [20,21]. In plants, abiotic stresses are both regulated by ABA dependent and non-dependent pathways [22], which are controlled by their respective regulatory elements [23]. However, it was not until the isolation of ABA hypersensitive mutant, that the role of miRNA in regulating ABA responses was recognized. Through mutant analysis, several genes that are involved in miRNA biogenesis pathways such as DCL1 (Dicer-like 1), HASTY, HEN1 (Small RNA 2′-O-methyltransferase), HYL1 (double-stranded RNA-binding protein 1), and SE (Serrate RNA effector molecule) were identified. Mutants of dcl1 and hen1 were shown to be sensitive to ABA during germination while the se and hasty mutants were sensitive to high osmosis and salt stresses [24]. Collectively we note that the members of the miRNA biogenesis may control drought responses in plants through various cellular and molecular processes that are controlled via a series of feedback regulations [25,26].
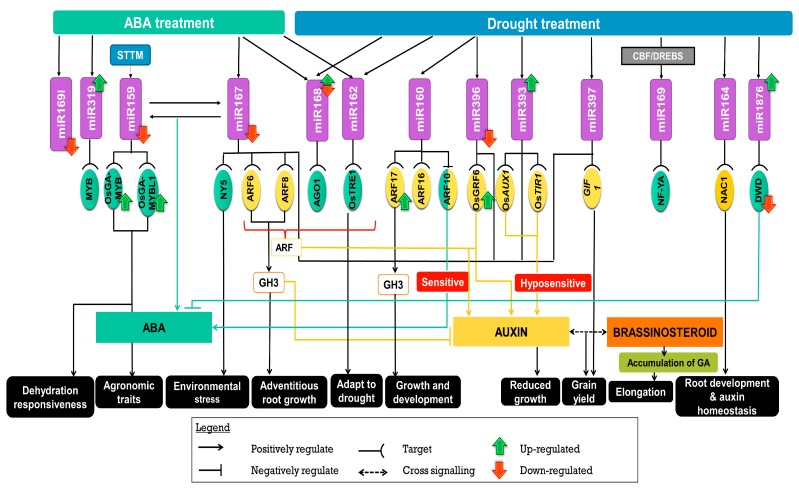
Transcription factors are key regulators of various processes in plants. MYB is implicated in several studies as a transcription factor that regulates dehydration responsiveness in plants [27]. ABA, through miRNAs, regulates genes that may aid in drought tolerance. Zhao et al. (2017) demonstrated that Short Tandem Target Mimic (STTM) suppresses miR159 expression, and subsequently increased the expression of two target genes, OsGAMYB and OsGAMYBL1 resulting in plants with short stature and smaller organs [28]. Further in a study conducted by Sunkar and Zhu (2007), miR159 was reported to be regulated by ABA in rice [29]. On the other hand, following inoculation with a root endophytic fungus Piriformospora indica, MYB targeting of miR159 was significantly induced in rice under drought [30]. In Arabidopsis, ABA and drought treatment resulted in elevated levels of miR159 which in turn cleaves MYB33 and MYB101 transcripts [31]. Further when overexpressed, miR159 suppresses MYB33 leading to ABA hyposensitivity. DWD (DDB1 binding WD40) is another protein that has been implicated in ABA regulated drought response. While MYB positively regulates ABA signaling, DWD regulates ABA negatively [32]. However, the up regulation of mi1876 reduces DWD activity thus reducing DWD’s negative effect on the regulation of ABA in rice roots. Under drought conditions, miR1876 was differentially expressed in rice [33]. However, miR1876 was not reported in Arabidopsis thaliana or other plant systems. Tian et al. (2015) through next generation sequencing (NGS) of Osaba1, an ABA deficient mutant, showed the influence of miR162b, an ABA responsive miRNA and its target gene OsTRE1 (trehalase 1) in its adaptive role under drought stress and ABA treatment. The up regulation of OsTRE1 caused the down regulation of miR162b which reduces trehalose accumulation leading to higher sensitivity to drought in rice [34] (Figure 1). Likewise, miR162 was down regulated in cotton but up regulated in plant systems such as Arabidopsis, barley (Hordeum vulgare), and maize (Zea mays) when subjected to drought [35].
Figure 1.
The regulation of microRNA under drought stress by phytohormones in rice. The legend above provides elaboration on the functions depicted by each arrow. The above miRNAs were identified post treatment with ABA and drought. Their up or down regulation resulted in a positive or negative effect on target transcription factors (auxin response factor (ARF), NAC, MYB, Nuclear factor Y (NFY), and DDB1 binding WD40 (DWD)). These interactions resulted in either an increase or decrease in the expression of drought stress related genes such as GH3. Hormones such as auxin, Abscisic acid (ABA), gibberellin (GA), and brassinosteroid (BR) play an important role in drought response. The processes affected by the interaction between the miRNA-Transcription Factor-gene complex is elaborated within the text.
Further, miR167 that targets auxin response factor (ARF) was shown to be down regulated by ABA in rice [36] and up regulated in Arabidopsis, wheat, and maize under water stress [37,38]. In maize, miR167d was down regulated under drought treatment, increasing the accumulation of Phospholipase D (PLD) which inhibits stress response [39]. PLD activation is vital for ABA signaling which regulates stomatal movement and responses in other plant systems including rice [40]. However, it is unknown whether PLD is targeted by miR167 in these plant systems. The ABRE motif containing miR168 is present in multiple species such as poplar, tobacco, Arabidopsis, maize, as well as in rice. Its AGO1 target is up regulated in Arabidopsis, in response to abiotic stimuli such as cold, high salinity, and drought [34] and down regulated in rice during drought [41,42]. NFY (Nuclear factor Y) directed ABA signaling was also implicated in drought stress tolerance in several of the following instances. For example, NFY5 is targeted by miR169a to withstand environmental stresses like drought [43,44]. NFY5A transcripts are highly expressed in stomatal guard cells and vascular tissues where they have control over the guard cell aperture. This transcript exerts control over several drought-responsive genes e.g., superoxide dismutase (SOD), glutathione transferase (GT) and peroxidases (POD) [44]. miR169a was down regulated in response to drought and ABA treatment resulting in induced levels of NFY5A transcript in Arabidopsis and tomato [45,46]. Further, miR169g was reported to be up regulated in roots and shoots of rice plants and is down regulated in Arabidopsis and Medicago under drought stress [47]. The expression of OsNF-YA7, a rice NFY transcription was induced in drought treatment but remained unchanged when treated with ABA proposing an ABA independent drought tolerance [48]. Previously, the miR169/NF-YA complex has been implicated in the regulation of plant stress responses [43]. Macovei et al. (2012) further noted that miR169 is regulated by CBF/DREBs (dehydration-responsive element) suggesting a role for miR169 in drought stress of rice [49,50]. The presence of DREs within the promoter of MIR169g enhances its role in drought [50]. Wheat miR169 on the other hand targets CCAAT-box transcription factor [51]. Interestingly, NF-YA is reported to bind to CCAAT-box transcription factor suggesting that the regulation of CCAAT-box transcription factor under drought is NF-YA independent in wheat. While miR169g was strongly up regulated, miR393 was transiently induced in rice [50]. When both miR167 and miR169 were down regulated, miR319 was up regulated in response to ABA in rice under normal conditions [36] (Figure 1). While no target was discovered for miR319 in the study conducted by Liu et al. (2009) [36], another study reported that TEOSINTE BRANCHED/CYCLOIDEA/PCF (TCP) could be a possible target [52]. miR319 that targets MYB family transcription factors are differentially expressed under drought stress in rice. gma-miR319c was up regulated during tillering and inflorescence-forming stages five days after water withholding (DAW) [53] but down regulated at six DAW [54]. Meanwhile, the overexpression of osa-miR319 that targets TCP TF in creeping bentgrass (Agrostis stolonifera) results in thicker and expanded leaves coated with increased leaf wax for drought tolerance [55]. We note that some miRNAs can interact or regulate other miRNAs to perform certain functions. For instance, MYB positively regulates ABA signaling through interaction with miR159 and miR167 in rice seedlings [9,56].
2.2. Auxin in Drought Stress Signaling
Water stress induced by drought results in inhibited growth and development allowing for diversion of energy and nutrients to enable plants to adapt to stress [57]. Auxin’s regulation and control of growth and development of drought stress in plants is modulated through several miRNA families. There are several transcription factor families such as auxin response factors (ARFs), NAC-domain and AGO1 [58] that regulate auxin-induced changes in tissues and organs. In tomato, miR160 was down regulated under drought condition whereas its target, ARF10 was significantly induced, implicating auxin’s role in stress responses [59]. The repression of ARF10 by miR160 in tomato has resulted in narrow leaf blades and low density of stomata. miR160 targets ARF10 and ARF16 while miR167 regulates ARF6 and ARF8 in rice [60,61]. Under drought stress, both miR160 and miR167 were down regulated in rice roots. In other crops such as wheat and cowpea, miR160 is found to be up regulated. As miR160 negatively regulates both ARF10 and ARF16, it is plausible that the down regulation of miR160 is crucial to increase root length by auxin under drought [62]. By negatively regulating ARF10, miR160 regulates ABA induced seed germination in Arabidopsis. Further, the miR167a-j regulates ARF6 and ARF8 which results in adventitious root formation in response to drought [63]. ARF6 and ARF8 negatively regulates auxin by controlling GH3 gene expression [64,65]. Mallory et al. (2005) presented that miR160 results in increased levels of ARF17 causing the accumulation of GH3 genes resulting in dramatic changes to growth and development in Arabidopsis [66,67]. NAC1 on the other hand is cleaved by miR164 which down regulates auxin signals related to lateral root development in tea plants under drought stress [68,69]. miR164 targeted NAC genes and was found to negatively regulate drought tolerance in rice [70]. Liu et al. (2008) in their study showed that miR160 and miR167 are involved in ABA responses in plants [71]. ABA down regulates miR167 in rice implicating a role in ARF induction and accumulation. Together with ABA, the auxin responsive ARFs jointly regulate drought stresses in rice, where the miRNAs in roots regulate auxin, nutrition, and stresses through feedback regulation between miR167 and ARFs [56]. miR160 on the other hand negatively regulates ARF10 resulting in ABA sensitivity which implies cross talk between both hormones [72]. The miR167-ARF8-GH3 pathway is highly conserved in rice [73] (Figure 1).
In Arabidopsis, miR393 is required for homeostasis of TIR1/AFB2 Auxin Receptor (TAAR) gene expression, which is important for various processes including drought tolerance [53]. In rice, it targets OsTIR1 (Transport inhibitor response 1) and OsAUX1 (auxin transporter 1) to restrict growth. Further, different miRNAs connected to auxin signaling have been shown to affect developmental activities such as tillering and branching. miR156 regulates yield (OsSPL14) while miR393a, miR396b, miR397, and miR167 promotes panicle branching and better grain yield [16,74,75]. The up regulation of miR397 in rice negatively regulates OsLAC (laccase) which is associated to brassinosteroids and grain yield increase [75]. miR397 was also induced under drought conditions in rice [28] and Arabidopsis where it targets the laccase genes [76]. Majority of the miRNAs above target GRF-interacting factor 1 (GIF1), which are responsible for cell proliferation and growth [35,77,78]. The suppression of miR396 up regulates auxin responsive genes, where OsGRF6 positively regulates auxin signaling in rice [16,79]. Contrary to the above, miR396 is up regulated in drought-stressed Arabidopsis [71] and tobacco [59]. Cross talk and co-expression network of miR396 and miR397 provides evidence that there is a network between brassinosteroid (BR) and auxin in regulating growth and yield in rice [80]. Besides BR, there is also the accumulation of gibberellin (GA) that is able to stimulate elongation through the interaction of miRNAs and target genes such as SCR (Scarecrow), DELLA, and GRF. BR cross talks with auxin and or GA to control yield in rice [81] (Figure 1).
3. The Role of miRNA in Growth and Development during Drought
Hormones largely regulate growth and development [82]. However, key players such as miR396 repress GRF activity [83,84] when overexpressed to enhance drought tolerance as well as leaf development in Medicago truncatula and Arabidopsis [36,47]. In rice, miR396 was down regulated under drought conditions and up regulated following inoculation with P. indica, resulting in down regulation of GRF. This lowered cell proliferation and growth which subsequently reduced transpiration for drought tolerance [30].
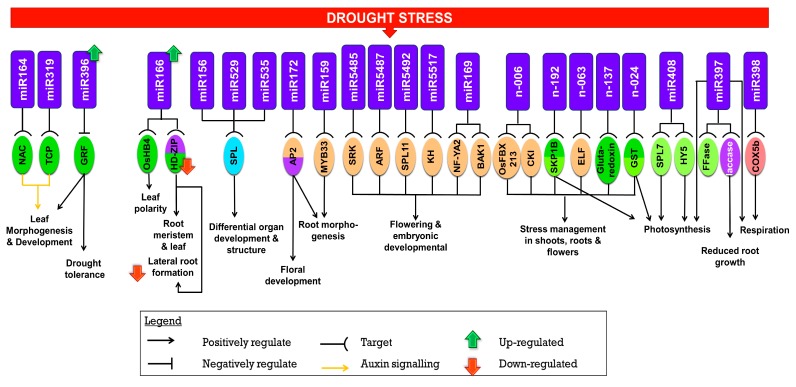
miR156 targets SPL that remains constant through embryogenic [85], trichome [86], flower, and fruit developmental stages [35]. Transcriptome studies in wild type and miR156 overexpressing rice lines showed a temporal change in expression of young to old leaves. Gene expression changes in these overexpressing lines resulted in rapid maturation and tillering of plants. miR156 was significantly induced in Arabidopsis and rice, where it negatively regulates SPL9 to increase abiotic stress tolerance such as salinity and drought [87]. In another study, the overexpression of miR156 up regulated OsSPL14 in rice which is associated with an ideal plant architecture where a point mutation of this gene reduces the number of tillers, increases yield, and contributes to resistance against lodging [74]. Together with miR529 and miR535, miR156 regulates SPLs that orchestrates differential organ development and structure. Overall the miR156-SPL, miR159-MYB33, and miR172-AP2 are involved in the regulation of leaf morphogenesis, floral organ development, root morphogenesis, and drought stress response, respectively [88,89,90]. Under drought, miR159a/b is down regulated resulting in an increase in MYB expression and reduction in growth. In addition, MYB has been shown to play a role in programmed cell death [79,91]. ARFs (10, 16, and 17) were regulated by miR160 [92] where when up regulated, miR160 reduced ARF (10 and 16) expression and produced shorter roots. However increased ARF16 activity resulted in reduced lateral root growth which is advantageous in energy conservation during drought [33,93]. ARF17 on the other hand inhibits adventitious root formation [94] (Figure 2).
Figure 2.
The regulation of microRNA under drought stress involving growth, development, photosynthesis and respiration. The legend above provides elaboration on the functions depicted by each arrow. The above miRNAs were identified under drought stress and their regulation resulted in a positive or negative effect on target transcription factors (ARF, NAC, GRF, HD-ZIP, SPL, MYB, NFY) or genes. These interactions resulted in either an increase or decrease in the expression of genes related to growth and development, photosynthesis, and respiration for better adaptation under drought. The interactions between the miRNAs-Transcription Factors-gene complex has been elaborated on within the text. The putative microRNAs (n-006, n-192, n-063, n-137, and n-024) are novel microRNAs from rice.
Enlarged root and drought tolerance was observed in rice plants over-expressing OsNAC10 which results in higher yields [95]. The architectural changes to the roots are driven by miR166. Further, Zhang et al. (2018) provided molecular and genetic evidence that miR166 targets OsHB4 that regulates leaf morphology and vascular development [96]. Leaf rolling in drought exposed plants is a result of vascular constriction by miR166 on the xylem. In Acacia, miR166 was reported to regulate HD (Homeo Domain)-ZIP transcript genes for xylem development [97]. In addition, miR166 has been implicated in the development of leaf polarity by targeting HD-ZIP genes where their expression is differential in abaxial and adaxial regions of leaves [98]. The miR166 family has a greater tendency to regulate lateral root development under drought stress rather than under drought signaling. Further, miR166 via the posttranscriptional regulation of HD-ZIP results in cell development of roots, meristem, and leaves in M. truncatula [99]. Liu et al. (2007) reported that HD-ZIP III positively regulates lateral root formation [100]. However, when miR166 levels are elevated, HD-ZIP III is down regulated resulting in a reduction of lateral root formation under drought [99]. The knockdown of miRNA166 in rice led to morphological changes associated with drought tolerance such as leaf rolling and constriction of xylem [96] (Figure 2). This miRNA was either down or up regulated in roots and shoots in response to drought in different plant systems [10,101]. Together with miR167 and miR390, this protein acts on ARFs and controls root formation [33]. Additionally, during mineral deficiency in rice, miR167, miR394, and miR399 regulate physiological changes to the adventitious root growth in drought stress [102] (Figure 2).
Drought also affects the reproductive tissues in plants. Cheah et al. (2017) reported that four inflorescences specific miRNA, miR5485, miR5487, miR5492, and miR5517 which targets SRK (S-domain receptor kinase), ARF, SPL11, and KH (K homology) domain respectively and two non-inflorescences specific miRNA, miR169d, and miR169f.2 that targets BAK1 (Brassinosteroid Insensitive 1-Associated Kinase I) and NFYA2. These targets mostly include flowering and embryogenic developmental genes under drought condition in rice [26]. miR5485, miR5487, miR5492, and miR5517 were not reported in other plant systems (miRbase). CBF/DREBs regulates the expression of miR169g, which in turn is induced in roots and shoots [103]. APETALA2 (AP2) genes that are involved in floral organ identity were also targeted by miR172 in response to both biotic and abiotic stresses [98]. Further, drought responsive novel miRNAs (n-006, n-192, n-002, n-063, n-137, n-024) have been reported in rice to target several important genes such as CK1 (CYCLIN-DEPENDENT KINASE INHIBITOR), ELF (EARLY FLOWERING PROTEIN), GLUTAREDOXIN 2, GST (GLUTATHIONE S-TRANSFERASE), GPI-ANCHORED PROTEIN, OsFBX213 (F-box domain containing protein 213), and SKP1-LIKE PROTEIN 1B. These genes have roles in stress management in shoots, roots, and flowers. The expression profile analyses of these genes observed in vegetative and reproductive tissue may suggests possible functions for these genes in response to drought [104] (Figure 2).
4. The Role of miRNAs on Photosynthesis and Respiration during Drought
Drought suppresses photosynthetic activity and electron transport while enhancing respiration [105,106]. As a consequence of the above, drought reduces carbon dioxide fixation and starch storage in plants [107]. Therefore, maintaining a good rate of C-H synthesis is important to protect against drought in plants. As photosynthesis occurs in the leaf, leaf architecture is crucial in contributing to modulating photosynthesis and development. Leaf growth induces a tri-directional growth in plants directing the flow from source to distal, abaxial, and lateral tissues. In rice, the flag leaf is the location of photosynthates which is utilized for plant growth and development [108]. High levels of novel n-024 and n-063 were found in the flag leaf that targets GLUTATHIONE S-TRANSFERASE and EARLY FLOWERING PROTEIN respectively. Both these miRNAs and their targets are potential candidates for crop improvement [75]. In Arabidopsis, miR408 was also highly expressed in leaf tissues and is regulated by SPL7 and HY5 which is implicated in copper and light signaling. Their roles in copper and light is tied to copper apportioning to chloroplasts and higher levels of plantacyanin which is linked to light reaction of photosynthesis implicating a role for miR408 as a photosynthetic regulator in Arabidopsis [109]. In rice, miR408 cleaves OsUCL8 (plastocyanin-like protein) to positively regulate photosynthesis and grain yield [10]. The level of miR408 transcript is high in drought tolerant cultivars and low in drought sensitive lines [104].
The NFY transcription family are associated in the regulation of photosynthesis under drought stress where they are stipulated to work in concert with miR167 and miR169 [110] (Figure 2). In rice, miR397 is down regulated under drought [54,111] whereas miR397b is up regulated by ABA and drought in Arabidopsis. In tolerant soybean however, miR397a/b is down regulated during water stress conditions [112]. This transcript targets β-fructofuranosidase (FFase) and modulates metabolic activity of starch and sucrose in plants [113]. The fluctuation in the levels of miR397 expression (up or down regulation) regulates photosynthesis and respiration. In addition, miR397 targets laccase, which explains why reduced root growth is observed in dehydrated knockout mutants [114,115,116]. Yet another modulator, miR398, regulates respiration in rice by targeting cytochrome C oxidase subunit V (COX5b), which acts as an electron transporter in respiration [41,114]. However, further investigation is required to establish the functionality of miR398 in abiotic stress (Figure 2).
5. The Role of miRNAs in Stress Modulation
5.1. miRNAs and Senescence
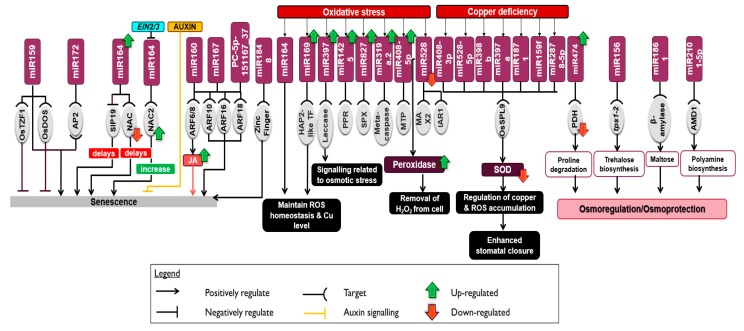
Senescence effects are most clearly seen in the leaf and is a means by which plants moderate stress. We found that there are ABA regulated miRNA like miR172a, miR172c, and miR172d in leaves, which target AP2 transcription factors to regulate the process of senescence in rice. In senescence resistant cultivars these miRNAs are expressed at high levels. Two zinc finger transcription factors; OsTZF1 (CCCH-tandem zinc finger protein 1), and OsDOS (delay of the onset of senescence protein) that are targeted by miR159 negatively regulate senescence and therefore provide tolerance against abiotic stresses like drought in rice [116,117]. In addition to the Zn-finger factors, there are several ARFs (ARF8, 10, 16, 18) that regulate miR160a, miR167, and PC-5p-151167_37 in the regulation of senescence. ARF6 and ARF8 regulate the synthesis of jasmonate, which leads to increased expression of SAGs (Senescence Associated Genes) and elevated levels of JA (jasmonic acid) biosynthesis. This could be a possible mechanism used by miR167 to mediate senescence-resistance via JA signaling. Together with miR167 and miR172, miR159 mediates senescence resistance in rice [118]. Further, miR164 was highly expressed in rice leaves where it negatively regulates SIP19 (salicylic acid-induced protein 19), which delays senescence [119]. High levels of miR164a, miR164b, miR164d, and miR164e expression decreased NAC1 and NAC21/22 expression thus resulting in delayed leaf senescence in Arabidopsis [18]. NAC genes are also targeted by miR164 in rice to negatively regulate drought tolerance [70]. It is believed that miR164 uses this mechanism to regulate senescence in rice. Another regulator of senescence in rice is EIN2. Mutants of this gene showed delayed senescence in rice. It is believed that EIN2 and EIN3 inhibits miR164 and results in the induction of NAC2, which leads to elevated levels of senescence. Auxin on the other hand negatively regulates senescence in leaves [120]. While the process of senescence is poorly understood, key miRNAs involved in orchestrating the delay or hastening of senescence in rice are miR159, miR160, miR164, miR167, miR172, and miR1848. These miRNAs regulate leaf senescence through a well-orchestrated phytohormone signaling pathways [119] (Figure 3).
Figure 3.
The regulation of microRNA under drought stress involving senescence, antioxidant defense and osmoregulation. The legend above provides elaboration on the functions of each arrow. The above miRNAs were identified under drought stress and their regulation resulted in a positive or negative effect on target transcription factors (ARF, NAC, ZF, HAP-2, SPL) or genes. These interactions resulted in either an increase or decrease in the expression of genes related to senescence, oxidative stress, and osmoprotection for better adaptation under drought.
5.2. miRNAs in Antioxidant Defense
Reactive oxygen species (ROS) are produced in response to drought stress in various cellular compartments such as chloroplast and peroxisomes [121]. Elevated levels of ROS are potentially toxic to cells if not kept under check. Therefore, significant tolerance may be afforded by ROS detoxification in rice. Plants have evolved a defense mechanism that consists of low-molecular-weight antioxidants and antioxidative enzymes that facilitate ROS scavenging. Enzymes like superoxide dismutase (SOD), peroxidase (POD), catalase, glutathione reductase, and ascorbate peroxidase (APX) defend against these toxic radicals [122,123]. Li et al. (2011) in their study of oxidative stress in rice identified seven miRNAs responsible for the regulation of H2O2 stress (miR169, miR319a.2, miR397, miR408-5p, miR528, miR1425, miR827) that target HAP2-like transcription factor, metacaspase, laccase, MTP (Monosaccharide transport protein), MAX2 (F-box/LRR-repeat MAX2), and IAA-alanine resistance protein 1 (IAR1) like, PPR (Pentatricopeptide repeat protein) and SPX (SYG1/Pho81/XPR1 proteins) domain respectively [124]. All these miRNAs except miR528, miR319a.2, and miR408-5p, were up regulated in response to oxidative stress. miR319a.2 targets metacaspase, which is involved in programmed cell death in plants. Down regulation of miR528 resulted in increased SOD activity in rice. The same has been noted in other cereal crops like maize and wheat [39,125]. This therefore implies that miR528 is involved in detoxification during drought stress. In Arabidopsis however, miR398 was repressed and resulted in the up regulation of SOD [126] (Figure 3).
In a study between resistant Nagina and sensitive Pusa Basmati, miR164 and miR169 formed part of a regulatory node to maintain ROS homeostasis and rice copper levels through interplay of Cu-transporters, enzymes, and transcription factors [127]. Through copper mediated deficiency, certain cultivar specific miRNAs (miR159f, miR397a, miR398b, miR408-3p, miR528-5p, miR1871, and miR2878-5p) down regulated genes that are involved in the regulation of copper (e.g., SODs), ultimately leading to enhanced resistance towards ROS and stomatal closure in the resistant cultivars. This was achieved via the OsSPL9 transcription factor [17]. In drought a number of peroxidases are repressed in rice roots (LOC_Os05g04470.1, LOC_Os07g48030.1, LOC_Os07g48060.1, LOC_Os04g59260.1). In drought tolerant wheat, peroxidase levels were elevated in root cell walls. However, peroxidases act in contradiction. While peroxidases are essential for maintaining tolerance against drought stress, high levels of OH− in cells may result in loss of structural integrity implying that a correct balance in peroxide levels is essential at managing stress [128,129] (Figure 3).
5.3. miRNA in Osmotic Stress during Drought
Plants also handle drought stress through the accumulation of osmoprotectants that are able to provide cell stability and turgor pressure [111,130]. Though these osmoregulators may vary from plant to plant, one osmolyte that plays a multifunctional role in defense is proline. Proline synthesis and degradation is responsible for the regulation of drought stress. Up regulation of miR474 has been referenced to control proline degradation in rice [54] by decreasing the expression of PDH (Pyruvate dehydrogenase), which results in improved drought tolerance. Trehalose, a non-reducing disaccharide sugar, is also associated to abiotic stress tolerance [131] through its ability to replace water molecules and rehydrate membranes and biomolecules [132,133]. miR156 was reported to be elevated in mutant Arabidopsis of trehalose-6-phosphate synthase (tps1-2) [134], a key enzyme for trehalose biosynthesis [135] (Figure 3).
Under drought stress, plants are conditioned to undergo lower photosynthetic activity. To support respiration in such conditions, starch degradation by β-amylase is required which increases the level of maltose that plays a role as an osmoprotectant [136]. Based on degradome data, miR1861 was found to target a transcript-encoding β-amylase (Os10g32810) in rice [137]. Polyamines, which are osmoprotectants, were implicated in drought stress tolerance [138]. Target mRNAs of miR2101-5p encoding an S-adenosylmethionine decarboxylase proenzyme (AMD1) is an important enzyme for polyamine synthesis in plants [139].
In an in silico study conducted by Umate and Tuteja (2010), 12 miRNAs that targeted helicase genes in rice were identified [140]. Out of these 12, miR164, miR408, and miR414 were down regulated and known to affect DEAD-box helicases (DBH) that play a role in up regulating the response to drought and salinity stress in plants [141]. miR156, miR159, and miR396 are highly regulated in Arabidopsis however the same miRNAs are severely down regulated in rice [33]. Further, a DUF1645 domain containing OsSGL (Stress tolerance and Grain Length) protein in Arabidopsis and an overexpressing rice lines of the same exhibited tolerance to osmotic stresses through accumulation of proline. In addition, when RNA-Seq analysis was conducted on the overexpressing rice lines, an abundance of stress related genes were found at enhanced levels where scavenging enzymes for ROS were among the most dominant [142]. A differential expression profiling study on drought leaves at vegetative stage conducted by Cheah et al. (2015) revealed that miR397a/b targets osmotic stress-activated protein kinase and were found to be up regulated in IR64 while down regulated in Vandana and Aday Sel [114] (Figure 3).
6. Differences and Similarities in Drought Response between Rice and Other Plant Systems
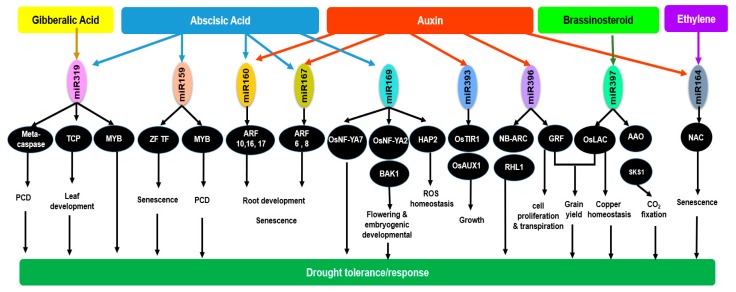
Based on the exhaustive information provided in this review, key conserved miRNAs that play an important role in drought stress in rice and other plant systems (miR160, miR164, miR167, miR169, miR319, miR393, miR396, and miR397) and their regulation thereof of various biological processes is summarized in Figure 4 [39,47,76,104,143,144]. As depicted in Figure 4, all these processes, which include programmed cell death, senescence, ROS homeostasis, growth and development, photosynthesis, and yield are interrelated and are required for the survival and well-being of plants. In the following paragraphs we elaborate on the similarities and differences observed between rice and other plant systems in responding to drought based on these key miRNAs.
Figure 4.
The regulation of drought tolerance and response in rice. The regulation of drought tolerance and response by key miRNAs via auxin, ABA, GA, BR, and ethylene. Processes like programmed cell death, senescence, ROS homeostasis, growth and development, photosynthesis, and yield are regulated by miRNAs through its respective targets for better drought tolerance. The colored arrows show the regulation of miRNA by different phytohormones (yellow: GA, blue: ABA, orange: auxin, green: BR, purple: ethylene). The black arrows indicate the regulation of target genes/transcription factors by miRNA and the regulation of drought responses by the target genes/transcription factors. The black ovals represent target genes/transcription factors).
6.1. Hormones Are Central to Drought Regulation
As anticipated, in both rice and other plant system the various physiological processes affected by drought are tightly regulated by an interplay between miRNA and hormones. In plants, hormones play an integral role in drought stress regulation and response. Accordingly, Figure 4 shows that rice, and in general plants, mitigate drought response through cross talk and interplay between ABA, IAA, ETH, and BR. While all these hormones interact to alleviate the stress induced by drought, ABA is the most crucial hormone for drought stress regulation followed by auxin and ethylene. In Section 2.1 and Section 2.2 it is stated that the increase and decrease in the levels of hormones are tightly linked to their miRNAs targets (Figure 4) [36]. However, there are miRNAs that are regulated in an ABA independent manner and certain miRNAs with no reported hormone targets (Figure 4). These miRNAs require further study to determine if hormones or signal molecules play a role in their regulation. We also provided an in-depth overview on the miRNAs-Transcription Factors-genes involved in the hormonal regulation of root and shoot growth, photosynthesis, respiration, and stress modulation (Section 2, Section 3, Section 4 and Section 5; Figure 1, Figure 2, Figure 3 and Figure 4). Hormone associated regulation of miRNAs, target genes, and transcription factors modulate plant vegetative and reproductive development. In drought resistant plants, a proper regulation of these hormone-driven processes by miRNAs results in its survivability and productivity. The opposite is observed in drought susceptible plants. The changes to the structure of the plants to adapt to drought stress will be elaborated in the following section. However, one interesting finding from analyzing the involvement of miRNAs response to hormones in plants is that similar miRNAs may either be up or down regulated in different plant systems due to variation in developmental stages, growth conditions, or stresses applied. These miRNAs seem to work in pairs where there are sets of miRNAs that are up regulated while others are down regulated implying a possible feedback regulation in most processes. For example, when miR167 and miR169 were down regulated, miR319 was up regulated in response to ABA in rice under normal conditions. Nonetheless, though the role of hormone in regulation of drought stress is well-defined, functional analyses of these target miRNAs are required to provide a clear picture on how these biological processes are fine-tuned by these conserved and novel miRNAs.
6.2. The Semi-Aquatic Rice Versus Terrestrial Plants
Further through our review at the miRNA level, we are able to observe some similarities and differences between rice and terrestrial plants in their response towards drought stress. Being a semi-aquatic plant, rice requires constant water supply making it more sensitive to drought as compared to other terrestrial crops which are better adapted to thrive under water deficit conditions. Root architecture plays an important role in drought tolerance and may differ according to plant systems. As a monocotyledonous plant, rice has fibrous root systems which is different from that of the dicotyledonous Arabidopsis with tap roots. Arabidopsis is more likely to be resistant to drought than rice, as taproot enables the plant to reach for deep underground water. miR160 and miR167 are two miRNAs that play an important role in root architecture control via auxin. In severe drought conditions, plants are generally designed to save all energy avoiding processes that are not relevant to its survival. One such process in rice is the adventitious root formation under water deficit. The down regulation of miR160 in rice under drought condition contributes to reduced lateral root growth and inhibits adventitious root formation in both rice and Arabidopsis. However, miR160 was differentially expressed in various studies conducted on wheat, implying that the root growth regulation via auxin may be modulated by the level of stress imposed. Meanwhile, the inhibition of adventitious root formation induced by the down regulation of miR167 under drought in rice is in contrast with Arabidopsis and wheat implying that under stress conditions root growth regulation by miR167 is different in rice as compared to Arabidopsis and wheat. Hence inhibition of adventitious root and reduced lateral root is vital for efficient expenditure of energy, water use, and generating tolerance towards a stressful environment [5] (Figure 2; Figure 4; Section 3).
Plants experience oxidative stress and senescence followed by the accumulation of osmoprotectants under drought stress. The regulation of these processes seem to be conserved across all plant systems. miR169 was up regulated under oxidative [124] and drought stress [90] to slow down respiration and cell differentiation. Accumulation of ROS is one of the earliest events observed upon drought stress. Hence, miR169 helps in scavenging the excessive ROS to alleviate the negative effects of drought stress. In addition, copper plays an important role in maintaining ROS homeostasis. In copper deficient conditions, miR408, miR528, miR398, miR397, miR1871, miR159, and miR2878 were up regulated which increased the ROS accumulation resulting in stomatal closure. Through stomatal closure, respiration is reduced and thus results in increased photosynthesis. This results in an increase towards yield through the activation of miRNAs such as miR408 and miR397. Apart from that, miRNA that targets senescence related proteins and transcription factors are up regulated to delay senescence to reduce yield loss with decrased drought tolerance. For example, miR164 was up regulated for delayed senescence and targets NAC that negatively regulates drought tolerance. miRNA that are involved in the synthesis of osmoprotectants, such as proline and trehalose, were also induced, indicating that the plants are programmed to protect against cellular and molecular harm. Jointly, the regulation of miRNA helps to mitigate stresses induced by drought. Overall, this implies that the above stress responses which are important for plants survival and homeostasis, are conserved across all plant systems [145,146].
Under severe drought conditions, stomata may remain closed completely depending on the plant species. The stomata may be intermittently opened and closed in tolerant plant species for processes such as photosynthesis and carbon fixation [147]. Stomatal movement is usually regulated by ABA to restrict water loss through transpiration via the interaction between miR169 and MYB. The down regulation of miR169 in Arabidopsis under drought stress enhances stomatal closure. However, miR169 was up regulated in rice and differentially expressed in wheat to confer drought tolerance. This indicates that rice may be less tolerant to drought as compared to Arabidopsis which has better stomatal aperture control which prevents excessive water loss through respiration and transpiration [147]. Other than changes in stomata, there are other observable changes such as leaf rolling in drought stressed plants. Leaf rolling is also one of the earliest signs observed in plant under drought stress which serves as an adaptive response to prevent water loss. The knockdown of miR166 in rice is associated with leaf development and leaf rolling under drought stress. miR166 was also down regulated in drought resistant wheat [143]. However, the suppression of miR166 in Arabidopsis resulted in other developmental defects and late flowering with good tolerance against drought [148]. Besides, similar miRNAs in different plant systems may regulate different organs under drought. For example, down regulation of miR393 represses GRF activity, lowers cell proliferation, and reduces transpiration in rice. In Arabidopsis, however, the up regulated miR393 cleaves auxin related genes leading to the inhibition of lateral root growth via ABA, while resulting in differential expression in wheat without any clear function [38] (Figure 4). This shows the versatility of some of the miRNA players in drought response and regulation.
Further, drought stress can affect grain yield as reproductive processes and grain filling are disrupted due to lack of resources which directs the plant to undergo early senescence where the duration of seed-filling is reduced and the translocation of assimilates from source to sink is enhanced [144]. The induction of miR397 under normal conditions contributes to higher grain yield and panicle branching through their positive involvement in photosynthesis (Figure 2; Figure 4; Section 4). However, under drought, miR397 is down regulated in rice and up regulated in Arabidopsis and wheat. This implies that rice is more sensitive to water deficit and tends to retard growth as part of its programmed response to conserve energy and survive. Flag leaf is a vital part of the plant that provides photosynthates for panicles and carbohydrate for grain filling [107]. In addition to the conserved miR397, two novel miRNAs namely n-024 and n-063 were significantly induced in flag leaf during drought in rice [103]. These novel miRNAs were also implicated in rice yield and not reported in other plant systems (Figure 2).
7. Conclusions and Future Prospects
This review summarizes findings regarding miRNAs and their versatile function in targeting genes and transcription factors to repress the effect of drought in rice. The information is compared with reported findings from other plant systems. It appears that these miRNAs are part of a regulatory network that control metabolic pathways in response to drought stress, highlighting their functional role in drought tolerance or drought avoidance. In particular, these modulators control vital processes in plants including growth, development, photosynthesis, respiration, and other drought-induced stresses [149].
In the recent years, a vast number of drought-responsive miRNAs have been discovered using NGS [150] and their functional analyses provide a better understanding of mechanisms by which plants counter this stress. However, since a multitude of drought-responsive miRNAs have been observed in rice and other plant species, further research on their functions, signaling pathways, and gene networks is required for a better understanding of the role played by miRNAs in drought. In addition, further exploration of regulatory pathways upstream of miRNA and downstream of the target genes will increase our understanding of drought stress adaptation in rice and other plant systems.
Globally, from the various studies conducted at the miRNA level in different plant systems, miRNAs appear to be involved in the regulation and expression of multiple target genes. There is also evidence of cross talk between processes and pathways associated with drought stress response in rice. Here we are able to conclude that miRNAs provide a link between environment and plant development based on miRNA-mediated response to drought stress. These modulators have great potential for development of resistant varieties, with improved agronomic traits through next generation technologies and genetic engineering.
Through a systematic review of literature on the role of miRNAs in drought stress modulation in rice and other plant systems, we have identified a few miRNAs that have potential for manipulation and use in crop improvement. As observed in drought sensitive plants, stress retards growth and development and this has a direct consequence on yield [21,31,33,62]. Therefore, it is crucial to identify key players of growth and development that can be used in breeding for drought tolerant plants. The growth and developmental process is tightly linked to the photosynthetic ability of the plant where, when affected, it interferes with its source and sink ability. This results in changes in growth such as reduced lateral root formation as well as enlarged root, affected flowering stages, low seed setting ability, and overall lower yield. miR408, miR156, miR167, miR393, miR396, miR1432, miR444, miR172, and miR397 have been reported to influence growth and yield in rice and other plant systems. Additionally, miRNAs such as miR156, miR159, miR160, miR164, miR167, miR169, miR319, miR396, and miR397 have been reported to reduce negative effects of drought through regulation of hormones, development, physiology, and stress in rice (Figure 1, Figure 2, Figure 3 and Figure 4). As these miRNAs play a role in regulating multiple processes, further dissection of these miRNAs is crucial for the development of drought resistant rice [151,152].
Funding
This research was funded by a grant awarded by Universiti Kebangsaan Malaysia (DCP-2017-004/1) to Kalaivani Nadarajah.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Baldrich P., Campo S., Wu M.T., Liu T.T., Hsing Y.I.C., Segundo B.S. MicroRNA-mediated regulation of gene expression in the response of rice plants to fungal elicitors. RNA Biol. 2015;12:847–863. doi: 10.1080/15476286.2015.1050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebert L.F., MacRae I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2018;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djami-Tchatchou A.T., Sanan-Mishra N., Ntushelo K., Dubery I.A. Functional roles of microRNAs in agronomically important plants—potential as targets for crop improvement and protection. Front. Plant Sci. 2017;8:378. doi: 10.3389/fpls.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A., Pan X., Najeeb U., Tan D.K.Y., Fahad S., Zahoor R., Luo H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018;51:47. doi: 10.1186/s40659-018-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalanotto C., Cogoni C., Zardo G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal D., Sharma N., Sharma V., Sopory S., Sanan-Mishra N. Role of microRNAs in rice plant under salt stress. Ann. Biol. 2016;168:2–18. doi: 10.1111/aab.12241. [DOI] [Google Scholar]
- 8.Willmann M.R., Poethig R.S. Conservation and evolution of miRNA regulatory programs in plant development. Curr. Opin. Plant Biol. 2007;10:503–511. doi: 10.1016/j.pbi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samad A.F., Sajad M., Nazaruddin N., Fauzi I.A., Murad A., Zainal Z., Ismail I. MicroRNA and transcription factor: Key players in plant regulatory network. Front. Plant Sci. 2017;8:565. doi: 10.3389/fpls.2017.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Zhang J., Yan J., Gou F., Mao Y., Tang G., Botella J.R., Zhu J.K. Short tandem target mimic rice lines uncover functions of miRNAs in regulating important agronomic traits. Proc. Natl. Acad. Sci. USA. 2017;114:5277–5282. doi: 10.1073/pnas.1703752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagliarani C., Vitali M., Ferrero M., Vitulo N., Incarbone M., Lovisolo C., Valle G., Schubert A. The accumulation of miRNAs differentially modulated by drought stress is affected by grafting in grapevine. Plant Physiol. 2017;173:2180–2195. doi: 10.1104/pp.16.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Yi F., Xie S., Liu Y., Qi X., Yu J. Genome-wide characterization of microRNA in foxtail millet (Setaria italica) BMC Plant Biol. 2013;13:212. doi: 10.1186/1471-2229-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A., Sandhu N., Dixit S., Yadav S., Swamy B., Shamsudin N.A.A. Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice. 2018;11:35. doi: 10.1186/s12284-018-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahim H., Bhuiyan M., Lim L., Sabu K., Saad A., Azhar M., Wickneswari R. Identification of quantitative trait loci for blast resistance in BC. Genet. Mol. Res. 2012;11:3277–3289. doi: 10.4238/2012.September.12.11. [DOI] [PubMed] [Google Scholar]
- 16.Hu J., Zeng T., Xia Q., Qian Q., Yang C., Ding Y., Chen L., Wang W. Unravelling miRNA regulation in yield of rice (Oryza sativa) based on differential network model. Sci. Rep. 2018;8:8498. doi: 10.1038/s41598-018-26438-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balyan S., Kumar M., Mutum R.D., Raghuvanshi U., Agarwal P., Mathur S., Raghuvanshi S. Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22. Sci. Rep. 2017;7:15446. doi: 10.1038/s41598-017-15450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Cai Z., Gan S. Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ. 2004;27:521–549. doi: 10.1111/j.1365-3040.2003.01158.x. [DOI] [Google Scholar]
- 19.Yue E., Liu Z., Li C., Li Y., Liu Q., Xu J.H. Overexpression of miR529a confers enhanced resistance to oxidative stress in rice (Oryza sativa L.) Plant Cell Rep. 2017;36:1171–1182. doi: 10.1007/s00299-017-2146-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B., Pan X., Cannon C.H., Cobb G.P., Anderson T.A. Conservation and divergence of plant microRNA genes. Plant J. 2006;46:243–259. doi: 10.1111/j.1365-313X.2006.02697.x. [DOI] [PubMed] [Google Scholar]
- 21.Daszkowska-Golec A., Szarejko I. Abiotic Stress-Plant Responses and Applications in Agriculture. IntechOpen; London, UK: 2013. The molecular basis of ABA-mediated plant response to drought. [Google Scholar]
- 22.Khan S.A., Li M.Z., Wang S.M., Yin H.J. Revisiting the role of plant transcription factors in the battle against abiotic stress. Int. J. Mol. Sci. 2018;19:1634. doi: 10.3390/ijms19061634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A., Pramanik K., Sharma R., Gantait S., Banerjee J. In-silico study of biotic and abiotic stress-related transcription factor binding sites in the promoter regions of rice germin-like protein genes. PLoS ONE. 2019;14:e0211887. doi: 10.1371/journal.pone.0211887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J.F., Yuan L.J., Shao Y., Du W., Yan D.W., Lu Y.T. The disturbance of small RNA pathways enhanced abscisic acid response and multiple stress responses in Arabidopsis. Plant Cell Environ. 2008;31:562–574. doi: 10.1111/j.1365-3040.2008.01786.x. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y., Shao C., Wang H., Chen M. The regulatory activities of plant microRNAs: A more dynamic perspective. Plant Physiol. 2011;157:1583–1595. doi: 10.1104/pp.111.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheah B.H., Jadhao S., Vasudevan M., Wickneswari R., Nadarajah K. Identification of functionally important microRNAs from rice inflorescence at heading stage of a qDTY4. 1-QTL bearing Near Isogenic Line under drought conditions. PLoS ONE. 2017;12:e0186382. doi: 10.1371/journal.pone.0186382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Kereamy A., Bi Y.M., Ranathunge K., Beatty P.H., Good A.G., Rothstein S.J. The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS ONE. 2012;7:e52030. doi: 10.1371/journal.pone.0052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y., Wen H., Teotia S., Du Y., Zhang J., Li J., Sun H., Tang G., Peng T., Zhao Q. Suppression of microRNA159 impacts multiple agronomic traits in rice (Oryza sativa L.) BMC Plant Biol. 2017;17:215. doi: 10.1186/s12870-017-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunkar R., Zhu J.K. Micro RNAs and short-interfering RNAs in plants. J. Integr. Plant Biol. 2007;49:817–826. doi: 10.1111/j.1744-7909.2007.00499.x. [DOI] [Google Scholar]
- 30.Mohsenifard E., Ghabooli M., Mehri N., Bakhshi B. Regulation of miR159 and miR396 mediated by Piriformospora indica confer drought tolerance in rice. J. Plant Mol. Breed. 2017;5:10–18. [Google Scholar]
- 31.Reyes J.L., Chua N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007;49:592–606. doi: 10.1111/j.1365-313X.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.H., Terzaghi W., Deng X.W. DWA3, an Arabidopsis DWD protein, acts as a negative regulator in ABA signal transduction. Plant Sci. 2011;180:352–357. doi: 10.1016/j.plantsci.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Bakhshi B., Fard E.M., Nikpay N., Ebrahimi M.A., Bihamta M.R., Mardi M., Salekdeh G.H. MicroRNA signatures of drought signaling in rice root. PLoS ONE. 2016;11:e0156814. doi: 10.1371/journal.pone.0156814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian C., Zuo Z., Qiu J.-L. Identification and characterization of ABA-responsive microRNAs in rice. J. Genet. Genom. 2015;42:393–402. doi: 10.1016/j.jgg.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Xie F., Zhang B. micro RNA evolution and expression analysis in polyploidized cotton genome. Plant Biotechnol. J. 2015;13:421–434. doi: 10.1111/pbi.12295. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q., Zhang Y.C., Wang C.Y., Luo Y.C., Huang Q.J., Chen S.Y., Zhou H., Qu L.H., Chen Y.Q. Expression analysis of phytohormone-regulated microRNAs in rice, implying their regulation roles in plant hormone signaling. FEBS Lett. 2009;583:723–728. doi: 10.1016/j.febslet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Pandey B., Gupta O.P., Pandey D.M., Sharma I., Sharma P. Identification of new stress-induced microRNA and their targets in wheat using computational approach. Plant Signal. Behav. 2013;8:e23932. doi: 10.4161/psb.23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H., Able A.J., Able J.A. SMARTER de-stressed cereal breeding. Trends Plant Sci. 2016;21:909–925. doi: 10.1016/j.tplants.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Wei L., Zhang D., Xiang F., Zhang Z. Differentially expressed miRNAs potentially involved in the regulation of defense mechanism to drought stress in maize seedlings. Int. J. Plant Sci. 2009;170:979–989. doi: 10.1086/605122. [DOI] [Google Scholar]
- 40.Zhang Y., Zhu H., Zhang Q., Li M., Yan M., Wang R., Wang L., Welti R., Zhang W., Wang X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Y., Tao Y., Zhu C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013;64:3077–3086. doi: 10.1093/jxb/ert164. [DOI] [PubMed] [Google Scholar]
- 42.Wen M., Xie M., He L., Wang Y., Shi S., Tang T. Expression variations of miRNAs and mRNAs in rice (Oryza sativa) Genome Biol. Evol. 2016;8:3529–3544. doi: 10.1093/gbe/evw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumimoto R.W., Adam L., Hymus G.J., Repetti P.P., Reuber T.L., Marion C.M., Hempel F.D., Ratcliffe O.J. The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta. 2008;228:709–723. doi: 10.1007/s00425-008-0773-6. [DOI] [PubMed] [Google Scholar]
- 44.Li W.X., Oono Y., Zhu J., He X.J., Wu J.M., Iida K., Lu X.Y., Cui X., Jin H., Zhu J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones-Rhoades M.W., Bartel D.P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Zou Z., Gong P., Zhang J., Ziaf K., Li H., Xiao F., Ye Z. Over-expression of microRNA169 confers enhanced drought tolerance to tomato. Biotechnol. Lett. 2011;33:403–409. doi: 10.1007/s10529-010-0436-0. [DOI] [PubMed] [Google Scholar]
- 47.Wang T., Chen L., Zhao M., Tian Q., Zhang W.H. Identification of drought-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. BMC Genom. 2011;12:367. doi: 10.1186/1471-2164-12-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee D.K., Kim H.I., Jang G., Chung P.J., Jeong J.S., Kim Y.S., Bang S.W., Jung H., Do Choi Y., Kim J.K. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 2015;241:199–210. doi: 10.1016/j.plantsci.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Macovei A., Gill S.S., Tuteja N. microRNAs as promising tools for improving stress tolerance in rice. Plant Signal. Behav. 2012;7:1296–1301. doi: 10.4161/psb.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao B., Liang R., Ge L., Li W., Xiao H., Lin H., Ruan K., Jin Y. Identification of drought-induced microRNAs in rice. Biochem. Biophys. Res. Commun. 2007;354:585–590. doi: 10.1016/j.bbrc.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Gasparis S., Yanushevska Y., Nadolska-Orczyk A. Bioinformatic identification and expression analysis of new microRNAs from wheat (Triticum aestivum L.) Acta Physiol. Plant. 2017;39:236. doi: 10.1007/s11738-017-2530-6. [DOI] [Google Scholar]
- 52.Schommer C., Palatnik J.F., Aggarwal P., Chételat A., Cubas P., Farmer E.E., Nath U., Weigel D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008;6:e230. doi: 10.1371/journal.pbio.0060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windels D., Bielewicz D., Ebneter M., Jarmolowski A., Szweykowska-Kulinska Z., Vazquez F. miR393 is required for production of proper auxin signalling outputs. PLoS ONE. 2014;9:e95972. doi: 10.1371/journal.pone.0095972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L., Liu Y., Liu Z., Kong D., Duan M., Luo L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010;61:4157–4168. doi: 10.1093/jxb/erq237. [DOI] [PubMed] [Google Scholar]
- 55.Zhou M., Luo H. Role of microRNA319 in creeping bentgrass salinity and drought stress response. Plant Signal. Behav. 2014;9:1375–1391. doi: 10.4161/psb.28700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D., Song Y., Chen Z., Yu D. Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol. Plant. 2009;136:223–236. doi: 10.1111/j.1399-3054.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 57.Chaves M., Oliveira M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004;55:2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- 58.Sorin C., Bussell J.D., Camus I., Ljung K., Kowalczyk M., Geiss G., McKhann H., Garcion C., Vaucheret H., Sandberg G. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005;17:1343–1359. doi: 10.1105/tpc.105.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouzroud S., Gouiaa S., Hu N., Bernadac A., Mila I., Bendaou N., Smouni A., Bouzayen M., Zouine M. Auxin Response Factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum) PLoS ONE. 2018;13:e0193517. doi: 10.1371/journal.pone.0193517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J., Si W., Deng Q., Li P., Yang S. Rapid evolution of avirulence genes in rice blast fungus Magnaporthe oryzae. BMC Genet. 2014;15:45. doi: 10.1186/1471-2156-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S.B., Xie Z.Z., Hu C.G., Zhang J.Z. A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 2016;7:47. doi: 10.3389/fpls.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakhshi B., Salekdeh G.H., Bihamta M.R., Tohidfar M. Characterization of Three Key MicroRNAs in Rice Root Architecture under Drought Stress using In silico Analysis and Quantitative Real-time PCR. Biosci. Biotechnol. Res. Asia. 2014;11:555–565. doi: 10.13005/bbra/1306. [DOI] [Google Scholar]
- 63.Gleeson M., Constantin M., Carroll B.J., Mitter N. MicroRNAs as regulators of adventitious root development. J. Plant Biochem. Biotechnol. 2014;23:339–347. doi: 10.1007/s13562-014-0269-3. [DOI] [Google Scholar]
- 64.Reed J.W., Wu M.F., Reeves P.H., Hodgens C., Yadav V., Hayes S., Pierik R. Three Auxin Response Factors Promote Hypocotyl Elongation. Plant Physiol. 2018;178:864–875. doi: 10.1104/pp.18.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C., Feng B., Chen T., Zhang X., Tao L., Fu G. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017;83:313–323. doi: 10.1007/s10725-017-0296-x. [DOI] [Google Scholar]
- 66.Tian G.W., Mohanty A., Chary S.N., Li S., Paap B., Drakakaki G., Kopec C.D., Li J., Ehrhardt D., Jackson D., et al. High-Throughput Fluorescent Tagging of Full-Length Arabidopsis Gene Products in Planta. Plant Physiol. 2004;135:25–38. doi: 10.1104/pp.104.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallory A.C., Bartel D.P., Bartel B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell. 2005;17:1360–1375. doi: 10.1105/tpc.105.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Y., Zhao S., Zhu C., Chang X., Yue C., Wang Z., Lin Y., Lai Z. Identification of drought-responsive miRNAs and physiological characterization of tea plant (Camellia sinensis L.) under drought stress. BMC Plant Biol. 2017;17:211. doi: 10.1186/s12870-017-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eckardt N.A. MicroRNAs Regulate Auxin Homeostasis and Plant Development. Plant Cell. 2005;17:1335–1338. doi: 10.1105/tpc.105.033159. [DOI] [Google Scholar]
- 70.Fang Y., Xie K., Xiong L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J. Exp. Bot. 2014;65:2119–2135. doi: 10.1093/jxb/eru072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H.H., Tian X., Li Y.J., Wu C.A., Zheng C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14:836–843. doi: 10.1261/rna.895308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu P.P., Montgomery T.A., Fahlgren N., Kasschau K.D., Nonogaki H., Carrington J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007;52:133–146. doi: 10.1111/j.1365-313X.2007.03218.x. [DOI] [PubMed] [Google Scholar]
- 73.Yang J.H., Han S.J., Yoon E.K., Lee W.S. Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells. Nucleic Acids Res. 2006;34:1892–1899. doi: 10.1093/nar/gkl118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao Y., Wang Y., Xue D., Wang J., Yan M., Liu G., Dong G., Zeng D., Lu Z., Zhu X., et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y.C., Yu Y., Wang C.Y., Li Z.Y., Liu Q., Xu J., Liao J.Y., Wang X.J., Qu L.H., Chen F. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat. Biotechnol. 2013;31:848. doi: 10.1038/nbt.2646. [DOI] [PubMed] [Google Scholar]
- 76.Sunkar R., Zhu J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee B.H., Ko J.H., Lee S., Lee Y., Pak J.H., Kim J.H. The Arabidopsis GRF-INTERACTING FACTOR gene family performs an overlapping function in determining organ size as well as multiple developmental properties. Plant Physiol. 2009;151:655–668. doi: 10.1104/pp.109.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Xu M.H., Yuan C.Y., Wu F.L., Chen S.B., Xue K. MicroRNA-139-5p inhibits cell proliferation and invasion by targeting insulin-like growth factor 1 receptor in human non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015;8:3864. [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H., Guo S., Xu Y., Li C., Zhang Z., Zhang D., Xu S., Zhang C., Chong K. OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol. 2014;165:160–174. doi: 10.1104/pp.114.235564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang C., Bai M.Y., Chong K. Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 2014;33:683–696. doi: 10.1007/s00299-014-1578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niu Y., Zhao T., Xu X., Li J. Genome-wide identification and characterization of GRAS transcription factors in tomato (Solanum lycopersicum) PeerJ. 2017;5:3955. doi: 10.7717/peerj.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mallory A.C., Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006;38:S31–S36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 83.Debernardi J.M., Rodriguez R.E., Mecchia M.A., Palatnik J.F. Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions. PLoS Genet. 2012;8:e1002419. doi: 10.1371/journal.pgen.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez R.E., Ercoli M.F., Debernardi J.M., Breakfield N.W., Mecchia M.A., Sabatini M., Cools T., De Veylder L., Benfey P.N., Palatnik J.F. MicroRNA miR396 Regulates the Switch between Stem Cells and Transit-Amplifying Cells in Arabidopsis Roots. Plant Cell. 2015;27:3354–3366. doi: 10.1105/tpc.15.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nodine M.D., Bartel D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010;24:2678–2692. doi: 10.1101/gad.1986710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu N., Cai W.J., Wang S., Shan C.M., Wang L.J., Chen X.Y. Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana. Plant Cell. 2010;22:2322–2335. doi: 10.1105/tpc.109.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui L.G., Shan J.X., Shi M., Gao J.P., Lin H.X. The miR156-SPL 9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014;80:1108–1117. doi: 10.1111/tpj.12712. [DOI] [PubMed] [Google Scholar]
- 88.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 89.Gocal G.F., Sheldon C.C., Gubler F., Moritz T., Bagnall D.J., MacMillan C.P., Li S.F., Parish R.W., Dennis E.S., Weigel D., et al. GAMYB-like genes, flowering, and gibberellin signaling in Arabidopsis. Plant Physiol. 2001;127:1682–1693. doi: 10.1104/pp.010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu W.W., Meng J., Cui J., Luan Y.S. Characterization and Function of MicroRNA(∗)s in Plants. Front. Plant Sci. 2017;8:2200. doi: 10.3389/fpls.2017.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alonso-Peral M.M., Li J., Li Y., Allen R.S., Schnippenkoetter W., Ohms S., White R.G., Millar A.A. The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol. 2010;154:757–771. doi: 10.1104/pp.110.160630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiong L., Wang R.G., Mao G., Koczan J.M. Identification of Drought Tolerance Determinants by Genetic Analysis of Root Response to Drought Stress and Abscisic Acid. Plant Physiol. 2006;142:1065–1074. doi: 10.1104/pp.106.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J.W., Wang L.J., Mao Y.B., Cai W.J., Xue H.W., Chen X.Y. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell. 2005;17:2204–2216. doi: 10.1105/tpc.105.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gutierrez L., Bussell J.D., Păcurar D.I., Schwambach J., Păcurar M., Bellini C. Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of AUXIN RESPONSE FACTOR Transcripts and MicroRNA Abundance. Plant Cell. 2009;21:3119–3132. doi: 10.1105/tpc.108.064758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeong J.S., Kim Y.S., Baek K.H., Jung H., Ha S.H., Do Choi Y., Kim M., Reuzeau C., Kim J.K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010;153:185–197. doi: 10.1104/pp.110.154773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J., Zhang H., Srivastava A.K., Pan Y., Bai J., Fang J., Shi H., Zhu J.K. Knockdown of Rice MicroRNA166 Confers Drought Resistance by Causing Leaf Rolling and Altering Stem Xylem Development. Plant Physiol. 2018;176:2082–2094. doi: 10.1104/pp.17.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ong S.S., Wickneswari R. Characterization of microRNAs expressed during secondary wall biosynthesis in Acacia mangium. PLoS ONE. 2012;7:e49662. doi: 10.1371/journal.pone.0049662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rubio-Somoza I., Weigel D. MicroRNA networks and developmental plasticity in plants. Trends Plant Sci. 2011;16:258–264. doi: 10.1016/j.tplants.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Boualem A., Laporte P., Jovanovic M., Laffont C., Plet J., Combier J.P., Niebel A., Crespi M., Frugier F. MicroRNA166 controls root and nodule development in Medicago truncatula. Plant J. 2008;54:876–887. doi: 10.1111/j.1365-313X.2008.03448.x. [DOI] [PubMed] [Google Scholar]
- 100.Hawker N.P., Bowman J.L. Roles for Class III HD-Zip and KANADI Genes in Arabidopsis Root Development. Plant Physiol. 2004;135:2261–2270. doi: 10.1104/pp.104.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khraiwesh B., Zhu J.K., Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta. 2012;1819:137–148. doi: 10.1016/j.bbagrm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grewal R.K., Saraf S., Deb A., Kundu S. Differentially expressed microRNAs link cellular physiology to phenotypic changes in rice under stress conditions. Plant Cell Physiol. 2018;59:2143–2154. doi: 10.1093/pcp/pcy136. [DOI] [PubMed] [Google Scholar]
- 103.Ferdous J., Hussain S.S., Shi B.J. Role of microRNAs in plant drought tolerance. Plant Biotechnol. J. 2015;13:293–305. doi: 10.1111/pbi.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mutum R.D., Kumar S., Balyan S., Kansal S., Mathur S., Raghuvanshi S. Identification of novel miRNAs from drought tolerant rice variety Nagina 22. Sci. Rep. 2016;6:30786. doi: 10.1038/srep30786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basu S., Ramegowda V., Kumar A., Pereira A. Plant adaptation to drought stress. F1000Research. 2016;5 doi: 10.12688/f1000research.7678.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galle A., Florez-Sarasa I., Thameur A., de Paepe R., Flexas J., Ribas-Carbo M. Effects of drought stress and subsequent rewatering on photosynthetic and respiratory pathways in Nicotiana sylvestris wild type and the mitochondrial complex I-deficient CMSII mutant. J. Exp. Bot. 2010;61:765–775. doi: 10.1093/jxb/erp344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S. Sustainable Agriculture. Springer; Berlin/Heidelberg, Germany: 2009. Plant drought stress: Effects, mechanisms and management; pp. 153–188. [Google Scholar]
- 108.Berwal M.K., Goyal P., Chugh L., Kumar R. Impact of Flag Leaf Removal on Grain Development and Nutrients Deposition in Pearl Millet Developing Grains. Vegetos. 2017:31. doi: 10.4172/2229-4473.1000370. [DOI] [Google Scholar]
- 109.Zhang H., Zhao X., Li J., Cai H., Deng X.W., Li L. MicroRNA408 is critical for the HY5-SPL7 gene network that mediates the coordinated response to light and copper. Plant Cell. 2014;26:4933–4953. doi: 10.1105/tpc.114.127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Zhao S.L., Li J.L., Hu X.H., Wang H., Cao X.L., Xu Y.J., Zhao Z.X., Xiao Z.Y., Yang N. Osa-miR169 negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Front. Plant Sci. 2017;8:2. doi: 10.3389/fpls.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shriram V., Kumar V., Devarumath R.M., Khare T.S., Wani S.H. MicroRNAs As Potential Targets for Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2016;7:817. doi: 10.3389/fpls.2016.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh I., Smita S., Mishra D.C., Kumar S., Singh B.K., Rai A. Abiotic Stress Responsive miRNA-Target Network and Related Markers (SNP, SSR) in Brassica juncea. Front. Plant Sci. 2017;8:1943. doi: 10.3389/fpls.2017.01943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheah B.H., Nadarajah K., Divate M.D., Wickneswari R. Identification of four functionally important microRNA families with contrasting differential expression profiles between drought-tolerant and susceptible rice leaf at vegetative stage. BMC Genom. 2015;16:692. doi: 10.1186/s12864-015-1851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan L., Zhao H., Yu Q., Bai L., Dong L. miR397/Laccase gene mediated network improves tolerance to fenoxaprop-p-ethyl in Beckmannia syzigachne and Oryza sativa. Front. Plant Sci. 2017;8:879. doi: 10.3389/fpls.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kong Z., Li M., Yang W., Xu W., Xue Y. A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol. 2006;141:1376–1388. doi: 10.1104/pp.106.082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pomeranz M.C., Hah C., Lin P.C., Kang S.G., Finer J.J., Blackshear P.J., Jang J.C. The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol. 2010;152:151–165. doi: 10.1104/pp.109.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim P.O., Woo H.R., Nam H.G. Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci. 2003;8:272–278. doi: 10.1016/S1360-1385(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 119.Xu X., Bai H., Liu C., Chen E., Chen Q., Zhuang J., Shen B. Genome-wide analysis of microRNAs and their target genes related to leaf senescence of rice. PLoS ONE. 2014;9:e114313. doi: 10.1371/journal.pone.0114313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma N., Ma C., Liu Y., Shahid M.O., Wang C., Gao J. Petal senescence: A hormone view. J. Exp. Bot. 2018;69:719–732. doi: 10.1093/jxb/ery009. [DOI] [PubMed] [Google Scholar]
- 121.Cruz de Carvalho M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Loggini B., Scartazza A., Brugnoli E., Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1100. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 124.Li T., Li H., Zhang Y.X., Liu J.Y. Identification and analysis of seven H2O2-responsive miRNAs and 32 new miRNAs in the seedlings of rice (Oryza sativa L. ssp. indica) Nucleic Acids Res. 2011;39:2821–2833. doi: 10.1093/nar/gkq1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kantar M., Lucas S.J., Budak H. miRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta. 2011;233:471–484. doi: 10.1007/s00425-010-1309-4. [DOI] [PubMed] [Google Scholar]
- 126.Sunkar R., Kapoor A., Zhu J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–2065. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andrés-Colás N., Carrió-Seguí A., Abdel-Ghany S.E., Pilon M., Peñarrubia L. Expression of the Intracellular COPT3-Mediated Cu Transport Is Temporally Regulated by the TCP16 Transcription Factor. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Csiszár J., Gallé Á., Horváth E., Dancsó P., Gombos M., Váry Z., Erdei L., Györgyey J., Tari I. Different peroxidase activities and expression of abiotic stress-related peroxidases in apical root segments of wheat genotypes with different drought stress tolerance under osmotic stress. Plant Physiol. Biochem. 2012;52:119–129. doi: 10.1016/j.plaphy.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014;5:771. doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slama I., Abdelly C., Bouchereau A., Flowers T., Savouré A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015;115:433–447. doi: 10.1093/aob/mcu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roelofs D., Aarts M., Schat H., Van Straalen N. Functional ecological genomics to demonstrate general and specific responses to abiotic stress. Funct. Ecol. 2008;22:8–18. doi: 10.1111/j.1365-2435.2007.01312.x. [DOI] [Google Scholar]
- 132.Crowe J.H., Crowe L.M. Preservation of mammalian cells-learning nature’s tricks. Nat. Biotechnol. 2000;18:145–146. doi: 10.1038/72580. [DOI] [PubMed] [Google Scholar]
- 133.Richards A.B., Krakowka S., Dexter L.B., Schmid H., Wolterbeek A.P., Waalkens-Berendsen D.H., Shigoyuki A., Kurimoto M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002;40:871–898. doi: 10.1016/S0278-6915(02)00011-X. [DOI] [PubMed] [Google Scholar]
- 134.Wahl V., Ponnu J., Schlereth A., Arrivault S., Langenecker T., Franke A., Feil R., Lunn J.E., Stitt M., Schmid M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science. 2013;339:704–707. doi: 10.1126/science.1230406. [DOI] [PubMed] [Google Scholar]
- 135.Eastmond P.J., Van Dijken A.J., Spielman M., Kerr A., Tissier A.F., Dickinson H.G., Jones J.D., Smeekens S.C., Graham I.A. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002;29:225–235. doi: 10.1046/j.1365-313x.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- 136.Kaplan F., Guy C.L. β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004;135:1674–1684. doi: 10.1104/pp.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peng T., Sun H., Du Y., Zhang J., Li J., Liu Y., Zhao Y., Zhao Q. Characterization and expression patterns of microRNAs involved in rice grain filling. PLoS ONE. 2013;8:e54148. doi: 10.1371/journal.pone.0054148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sengupta A., Chakraborty M., Saha J., Gupta B., Gupta K. Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. Springer; Berlin/Heidelberg, Germany: 2016. Polyamines: Osmoprotectants in plant abiotic stress adaptation; pp. 97–127. [Google Scholar]
- 139.Minocha R., Majumdar R., Minocha S.C. Polyamines and abiotic stress in plants: A complex relationship1. Front. Plant Sci. 2014;5 doi: 10.3389/fpls.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Umate P., Tuteja R. Architectures of the unique domains associated with the DEAD-box helicase motif. Cell Cycle. 2010;9:4228–4235. doi: 10.4161/cc.9.20.13635. [DOI] [PubMed] [Google Scholar]
- 141.Macovei A., Tuteja N. Different expression of miRNAs targeting helicases in rice in response to low and high dose rate γ-ray treatments. Plant Signal. Behav. 2013;8:e25128. doi: 10.4161/psb.25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cui Y., Wang M., Zhou H., Li M., Huang L., Yin X., Zhao G., Lin F., Xia X., Xu G. OsSGL, a Novel DUF1645 Domain-Containing Protein, Confers Enhanced Drought Tolerance in Transgenic Rice and Arabidopsis. Front. Plant Sci. 2016;7:2001. doi: 10.3389/fpls.2016.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akdogan G., Tufekci E.D., Uranbey S., Unver T. miRNA-based drought regulation in wheat. Funct. Integr. Genom. 2016;16:221–233. doi: 10.1007/s10142-015-0452-1. [DOI] [PubMed] [Google Scholar]
- 144.Barrera-Figueroa B.E., Gao L., Diop N.N., Wu Z., Ehlers J.D., Roberts P.A., Close T.J., Zhu J.K., Liu R. Identification and comparative analysis of drought-associated microRNAs in two cowpea genotypes. BMC Plant Biol. 2011;11:127. doi: 10.1186/1471-2229-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jajic I., Sarna T., Strzalka K. Senescence, stress, and reactive oxygen species. Plants. 2015;4:393–411. doi: 10.3390/plants4030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sade N., del Mar Rubio-Wilhelmi M., Umnajkitikorn K., Blumwald E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2017;69:845–853. doi: 10.1093/jxb/erx235. [DOI] [PubMed] [Google Scholar]
- 147.Pirasteh-Anosheh H., Saed-Moucheshi A., Pakniyat H., Pessarakli M. Water Stress and Crop Plants. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2016. Stomatal responses to drought stress; pp. 24–40. [Google Scholar]
- 148.Jung J.H., Park C.M. MIR166/165 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta. 2007;225:1327–1338. doi: 10.1007/s00425-006-0439-1. [DOI] [PubMed] [Google Scholar]
- 149.Raza A., Razzaq A., Mehmood S.S., Zou X., Zhang X., Lv Y., Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants. 2019;8:34. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shuai P., Liang D., Zhang Z., Yin W., Xia X. Identification of drought-responsive and novel Populus trichocarpa microRNAs by high-throughput sequencing and their targets using degradome analysis. BMC Genom. 2013;14:233. doi: 10.1186/1471-2164-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar A., Dixit S., Ram T., Yadaw R., Mishra K., Mandal N. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014;65:6265–6278. doi: 10.1093/jxb/eru363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lemoine R., La Camera S., Atanassova R., Dédaldéchamp F., Allario T., Pourtau N., Bonnemain J.L., Laloi M., Coutos-Thévenot P., Maurousset L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013;4:272. doi: 10.3389/fpls.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]