Abstract
Rett syndrome (RTT) is a neurodevelopmental disorder, affecting 1 in 10,000 girls. Intellectual disability, loss of speech and hand skills with stereotypies, seizures and ataxia are recurrent features. Stringent diagnostic criteria distinguish classical Rett, caused by a MECP2 pathogenic variant in 95% of cases, from atypical girls, 40–73% carrying MECP2 variants, and rarely CDKL5 and FOXG1 alterations. A large fraction of atypical and RTT-like patients remain without genetic cause. Next Generation Sequencing (NGS) targeted to multigene panels/Whole Exome Sequencing (WES) in 137 girls suspected for RTT led to the identification of a de novo variant in STXBP1 gene in four atypical RTT and two RTT-like girls. De novo pathogenic variants—one in GABRB2 and, for first time, one in GABRG2—were disclosed in classic and atypical RTT patients. Interestingly, the GABRG2 variant occurred at low rate percentage in blood and buccal swabs, reinforcing the relevance of mosaicism in neurological disorders. We confirm the role of STXBP1 in atypical RTT/RTT-like patients if early psychomotor delay and epilepsy before 2 years of age are observed, indicating its inclusion in the RTT diagnostic panel. Lastly, we report pathogenic variants in Gamma-aminobutyric acid-A (GABAa) receptors as a cause of atypical/classic RTT phenotype, in accordance with the deregulation of GABAergic pathway observed in MECP2 defective in vitro and in vivo models.
Keywords: atypical RTT, STXBP1, GABAa receptors genes, NGS
1. Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder, with an incidence of approximately 1 in 10,000 live births, most frequently affecting girls during early childhood [1]. RTT patients develop normally during the first 6–18 months of life, but over time they manifest a motor and psychomotor regression and gradually develop a severe condition associated with motor, cognitive and behavioral impairment. Up to 95% of classical RTT cases are accounted for by mutations in the Methyl CpG-binding protein 2 gene (MECP2) [2], mapping at Xq28 and encoding a multifunctional protein whose expression impacts many fundamental biological processes [3]. The MeCP2 protein acts as epigenetic “reader” [4] which by binding with methylated DNA, interacting with corepressors and recruiting histone deacetylases to methylated genes, leads to their silencing [5], but it can also dampen transcriptional noise genome-wide, altering global chromatine structure [6]. MeCP2 is also a transcriptional activator through its interaction with the c-AMP responsive element-binding protein 1 (CREB1), has a role in alternative splicing in a DNA methylation-dependent manner and in microRNA processing and may influence global translation through modulation of the AKT/mTOR pathway [7,8,9]. In RTT patients, the differential expression of multiple genes related to intracellular signaling, modulation of cytoskeleton plasticity and cell metabolism [9] support the involvement of MeCP2 in neural development and synaptic function. Interestingly, perturbations in the genes acting in GABAergic circuits [10] could result in neuron hyperexcitability, which in turn is potentially responsible for epilepsy reported in 60–80% of RTT patients [11].
Similar to the classical form, atypical or variant RTT present many RTT clinical features, but do not necessarily meet all the distinctive signs of the disorder; however highly stringent criteria allow proper definition of the variant forms [12]. A fraction ranging from 5% to 8% of classic RTT [2,13] and 42% to 73% of variant RTT patients [13,14] are negative for MECP2 mutation. Among them, individuals with early seizure onset variant RTT [15,16], who manifest epilepsy before regression, have mutations in the cyclin-dependent kinase-like 5 gene (CDKL5), and patients with congenital RTT, who show gross early abnormal development, have molecular defects in the forkhead box G1 gene FOXG1 [17]. However, a subset of patients who receive the clinical diagnosis of RTT remain negative for mutation in all the aforementioned genes. Next generation sequencing (NGS) and particularly Whole Exome Sequencing (WES) have emerged as a powerful tool to identify new genes involved in rare genetic diseases [18] and to give a diagnosis to patients without a known genetic cause. Thanks to these technological advances, in the last few years, several uncommon causative genes for classic or variant Rett syndrome or similar phenotypes (RTT-like) have been discovered [11,14,19,20,21,22,23,24]. Among the novel genes, several have been previously associated with developmental delay, often in comorbidity with epilepsy. An emerging concept is that some genes causing epileptic encephalopathy may be responsible for more complex neurodevelopmental disorders (DEE, Developmental and Epileptic Encephalopathies), where both ID and epilepsy contribute to the clinical phenotype [25,26,27].
Based on these premises, we expected that a proportion of patients with RTT phenotype and epilepsy might be mutated in one of the genes involved in DEE.
In order to identify new genes responsible for the RTT phenotype, we processed by WES 26 girls with a RTT/RTT-like phenotype negative to the conventional genetic test for RTT and by NGS Custom multigene Panel a cohort of 78 patients with pediatric age onset seizures, that included 11 patients with RTT/RTT-like phenotype.
The combined approaches allowed the identification of a genetic cause different from customarily studied genes in RTT syndrome in six patients. Following this result, processing 100 patients with RTT/RTT-like syndrome or syndromes in differential diagnosis by using an NGS custom panel enriched in the new genes allowed the detection of two additional cases. Lastly, all the molecularly defined patients were clinically re-evaluated to highlight the occurrence of distinctive phenotypic traits that might specifically guide the molecular investigation of the genes identified in this study.
2. Results
Overall, the NGS experiments identified eight patients carrying a pathogenic variant in genes alternative to the customarily tested genes in RTT syndrome. Table 1 summarizes the patients’ clinical features, the pathogenic variant and the NGS approach. The clinical features have been grouped according to Neul classification [12] under separated entries distinguishing main and supportive criteria. Epilepsy characteristics and neurological disturbances not specifically related to RTT are reported in Table S1. All patients showed epilepsy during their lifetime. Six patients (No. 1–6) exhibited a pathogenic variant in the STXBP1 gene (OMIM 602926), one patient (No. 7) showed a pathogenic variant in GABRG2 (OMIM 137164), while another one (No. 8) had a pathogenic variant in GABRB2 (OMIM 600232).
Table 1.
Patient’s clinical features grouped according to Neul classification, the pathogenic variants and the Next generation sequencing (NGS) approach.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Sex, Current Age (years) | F (18 y) | F (11 y) | F (19 y) | F (29 y) | F (7y) | F (9 y) | F (38 y) | F (42 y) | |
| Molecular Approach | NGS-pediatric epilepsy | WES-RTT | WES-RTT | NGS -pediatric epilepsy | NGS-diagnostic | NGS-diagnostic | NGS -pediatric epilepsy | WES-RTT | |
| Mutation/Inheritance Pattern | STXBP1 NC_000009.11: g.130423471 C>T, NM_003165.3: c.416C>T: p.(Pro139Leu), de novo | STXBP1 NC_000009.11: g.130435529 C>T, NM_003165.3: c.1099C>T: p.(Arg367Ter), de novo | STXBP1 NC_000009.11: g.130416077 T>C, (NM_003165.3): c.169+2T>C, r.([169_170 ins [gc;169+3_169+1168]; 169_170ins [gc; 169+3_169+1334]]),p.(Ile57Serfs7*) de novo | STXBP1 NC_000009.11: g.130428548 T>C, NM_003165.3: c.767T>C, p.(Leu256Pro), de novo | STXBP1 NC_000009.11: g.130444840 G>A, (NM_003165.3) c.1702+1G>A, r. [1585_1702del117] p.(Glu530_Gly 568del) de novo | STXBP1 NC_000009.11: g.130438188 C>T, NM_003165.3: c.1216 C>T, p.(Arg406Cys), de novo | GABRG2 NC_000005.9: g.161576128_161576129 delinsGG, NM_000816.3: c.937_938 delinsGG, p.(Leu313Gly), de novo mosaic | GABRB2 NC_000005.9: g.160758063 C>T, NM_021911.2: c.904G>A p.(Val302Met), de novo | |
| Regression (age indicated) Followed by Recovery or Stabilization | No cdv | No cdv | No cdv | No cdv | No | Yes (6 months) | Yes (12 months) | Yes (9 months) | |
| Main Criteria | Partial or Complete Loss of Acquired Purposeful Hand Skills | No: not lost, but NevAcq (grabs food and takes it to her mouth) | No: not lost, but NevAcq (grasping and manipulation disturbed by involuntary movements) | No: not lost, but NevAcq (grasping disturbed by tremors and stereotypies) | No | No: very limited hand skills | No: not lost, but Nev completely Acq | Yes: very limited hand skills | Yes: (since 2 years leaves behind and drops things) |
| Partial or Complete loss of Acquired Spoken Language | No: not lost, but NevAcq | No: not lost, vocalisms and only ten words | No: not lost, but NevAcq | No: not lost, only a few words | No: not lost, but Nev Acq, only vocalisms | No: not lost, but NevAcq(vocalism) | No: not lost, but NevAcq | Yes (only “Mum” and “Dad”, then lost) | |
| Gait Abnormalities: Impaired or Absence of Ability | Yes (ataxic-dyspraxic, unstable and only for short distances: since 4 years) | Yes (ataxic with axillary support: since 4 years) | Yes absent (only standing with axillary support) | Yes ataxic (walking with enlarged base and out of rotation of feet: since 3 years) | Yes (walking with enlarged base/ not apraxic: since 3 years) | Yes (Nev Acq) | Yes (ataxic: since 6 years) | Yes (apraxic, slow but autonomous, since 16 months, climbs the stairs) | |
| Stereotypic Hand Movements (type) | Yes frequent (brings her hands to mouth and bites fingers) | Yes (not typical for RTT, beats her head: since 3 years) | Yes (hand washing) | Yes (hand rocking) | Yes (hand washing, clapping, tapping right hand on table/books, tapping the forehead with the right upper limb, upper limb flickering) | Yes (upper limbs tremors, upper limb flickering, and dyskinesias) | Yes (tapping her right hand on her teeth: since 18 months), | Yes (upper limbs flickering) | |
| Exclusion Criteria | Brain Injury: Peri or Postnatal Trauma, Neurometabolic Disease or Severe Infection | No | No | No | No | No | No | No | No |
| Grossly Abnormal Psychomotor Development in First 6 Months of Life: Exam at the Birth | hypotonia | normal | normal | hypotonia, hyperexcitability, inconsolable crying | normal | normal | normal | normal | |
| Supportive Criteria | Breathing Disturbances | No | Yes | No | No | No | No | Yes (mild cyanosis and apneas) | Yes (hyperventilation) |
| Bruxism when Awake | No | No | Yes | No | Yes | Yes | Yes | Yes (significant) | |
| Impaired Sleep Pattern | Yes (sleeplessness and nocturnal agitation) | Yes (seizures) | Yes (nocturnal bruxism) | No | Yes (several and prolonged nocturnal awakenings) | No | Yes | No | |
| Abnormal Muscle Tone | Yes (proximal hypotonia) | Yes | Yes | No | No | Yes (axial hypotonia, hypertonus of the limbs) | Yes mild hypertonus (hypotonia in the first years of life) | No | |
| Peripheral Vasomotor Disturbances | No | No | Yes | No | No | No | cold and bluish hands and feet without trophic changes | No | |
| Scoliosis/Kyphosis | Yes (lumbar hyperlordosis) | Yes (mild) | Yes | No | No | No | Yes (mild kyphosis) | No (only scoliotic attitude) | |
| Growth Retardation | No | No | No | hypostaturism and obesity | No | Yes | Yes mild | No | |
| Small Cold Hands/Feet | Yes | No | Yes | No (but short and stubby fingers) | No | Yes (small, not cold) | Yes | Yes (cold feet) | |
| Inappropriate Laughing /Screaming Spells | Yes | No | Yes (screams) | No | Yes | nd | Yes frequent | Yes rare | |
| Diminished Response to Pain | Yes | No | No | nd | Yes | nd | nd | No | |
| Intense Eye Communication | No | Yes | No | No | Yes | No | Yes | Yes | |
| Microcephaly: if Yes Indicate if Acquired | Yes acquired | No | No | No | No | Yes acquired | No | Yes acquired | |
| Clinical Diagnosis at Referral | RTT atypical | RTT atypical Hanefeld | RTT atypical congenital | RTT-like-EOEE (West>Lennox-Gastaut) | RTT atypical | RTT-like (myoclonic epileptic encephalopathy) | RTT atypical | RTT classic | |
WES-RTT= Whole Exome Sequencing on Rett Syndrome; RTT = Rett Syndrome; NGS = Next generation Sequencing; WES = Whole Exome sequencing; cdv = Congenital Developmental Delay; nd = not done; Nev Acq = Never Acquired.
2.1. STXBP1 Variants
Two out of the six variants in STXBP1 (patients No. 3 and 4) had never been described in the literature and the two splicing variants of patients No. 3 and 5 had never been studied at transcript level.
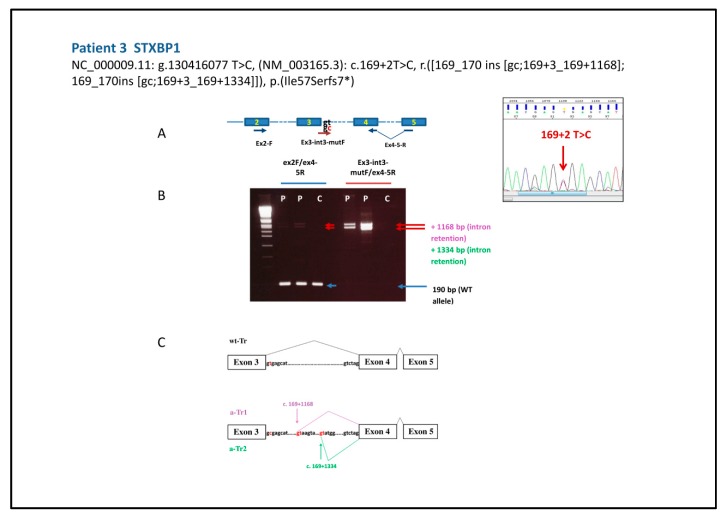
Therefore, in order to corroborate the pathogenicity of these latter variants, we characterized the transcripts obtained by peripheral blood. To evaluate the effect of the mutation NC_000009.11: g.130416077 T>C, (NM_003165.3): c.169+2 T>C of patient 3 (Figure 1) we set up two distinct PCRs on STXBP1 cDNA: one with a reverse primer including the splice junction and a part of the two flanking exons 4 and 5 and the forward in exon 2, and another with a forward primer complementary to the intronic sequence, to check for possible splicing retention phenomena, as described for a different mutation hitting the same splice site [28]. The second primer set allowed us to highlight that the variant gives rise to two aberrant transcripts, a-Tr1 and a-Tr2, retaining part of IVS3 (r.([169_170ins[gc;169+3_169+1168];169_170ins[gc;169+3_169+1334]])). For both transcripts the predicted outcome is a protein prematurely truncated 57 aminoacids after the end of exon 3 (p. (Ile57Serfs7*)) and completely missing the functional domains of the Syntaxin–binding Protein 1.
Figure 1.
(A) Schematic representation of STXBP1 gene primer pairs used for characterization of patient 3 variant and on the right the electropherogram with the heterozygous variant. (B) The 2% agarose gel shows the wt amplicon of 190 bp in the first PCR (ex2F/ex4–5R) (blue arrow) and a weak signal of two aberrant fragments only in the proband (P). Two long amplicons obtained by a primer pair selective for the aberrant transcript (Ex3-int3-mutF/ex4–5R) are well visible in the proband’s lanes (P) (red arrows) and not in the control DNA lane (C). (C) Schematic of the mis-splicing caused by the c.169 + 2T> C mutation inferred by sequencing of the two amplicons that correspond to two aberrant intron-retaining transcripts resulting from the use of two alternative donor sites (in red) 1168 and 1134 nt downstream from the end of exon 3.
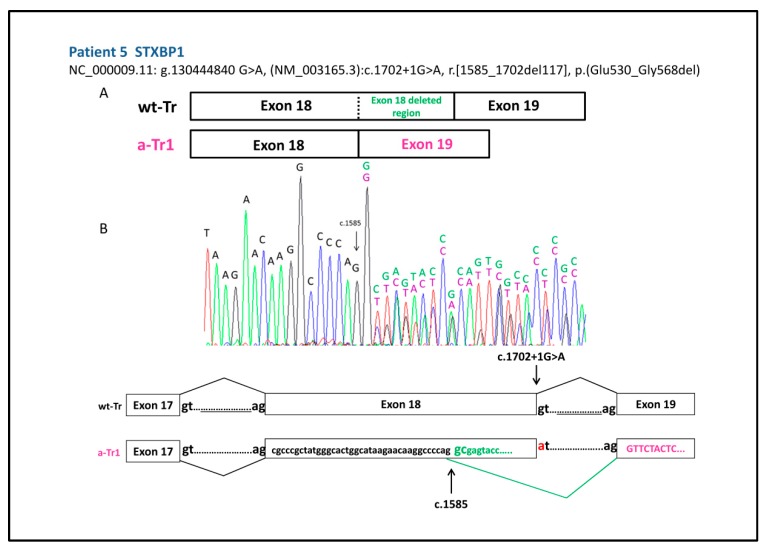
In patient 5, the splice variant NC_000009.11: g.130444840 G>A, (NM003165.3) c.1702+ 1G>A in STXBP1 gene generates an aberrant transcript due to the choice of a new donor site within exon 18 stronger than the aberrant one, with deletion of the last 117 bp of this exon (r.[1585_1702del117]) predicting a protein, with an in frame deletion of 38 amino acids (p.(Glu530_Gly568del)), devoid of a large portion of the functional D2 domain (Figure 2).
Figure 2.
(A) Schematic representation of the wild type (wt-Tr) and the aberrant transcript (a-Tr1) generated by variant NC_000009.11: g.130444840 G>A, (NM003165.3), c.1702 + 1G>A of patient 5, resulting in the deletion of the last 117 bp of exon 18. (B) cDNA electropherogram showing the wt transcript (black and green letters) and the aberrant transcript (black and purple letters) produced through the choice of a new donor site within exon 18 at position c.1585 (arrowed).
The de novo variant yet unreported NC_000009.11: g.130428548 T>C, NM_003165.3, c. 767 T>C p.(Leu256Pro) of patient 4 is classified as likely pathogenic in ClinVar and predicted as pathogenic using Align GVGD, SIFT, Mutation Taster and Polyphen by Alamut software (Figure S1).
The remaining variants already reported in the literature are specified in Table 1.
2.2. Clinical Features of Girls with STXBP1 Variants
Table 1 and Table S1 detail the phenotypic traits of the patients with STXBP1 mutation. In summary, the clinical re-examination took into account the RTT criteria revisited by Neul [12], the epileptic profile and the phenotypic traits usually observed in STXBP1-mutated patients, such as movement disorders. Although the patients had been referred to the molecular study as atypical RTT (early seizures onset RTT or congenital) or RTT-like (see Table 1 “Clinical Diagnosis” at referral) and all presented hand stereotypies (which in 2 patients are typical for RTT such as “hand washing”), absence or walking/gait abnormalities and absence of purposeful hands skills and language, only patient 6 had a true regression, distinguishing them from RTT. The occurrence of supportive criteria for RTT varies from patient to patient, apart from hypotonia which recurs in four out of six patients. Movements disorders, typical of STXBP1-mutated patients are frequent (tremors and dyskinesia in 5/6 patients). Epilepsy was referred in all of the six girls but: a) the age of seizures onset was very variable, occurring later than expected for STXBP1-EE (on average at six weeks) [25] in three out of six patients; b) the type of crisis was heterogeneous and only in one patient (No. 4) the onset was typical for West syndrome; c) the pharmacological response varied from drug resistance to responsiveness. Only patient 4 showed the typical hypsarythmic pattern associated with West syndrome.
2.3. GABAa Receptors Genes Variants
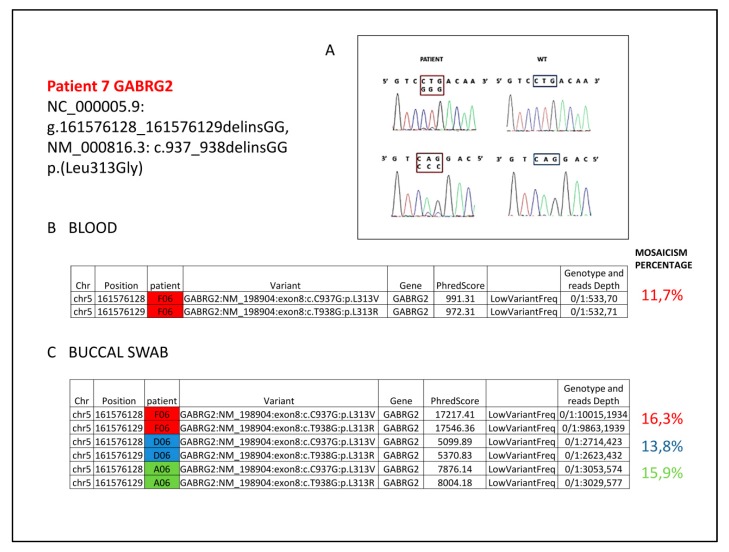
The patient No. 7 carried a pathogenic variant in GABRG2 never described in literature. The variant is a double nucleotide substitution in the first and second nucleotides of the CTG codon (Leu) in position 313 of the GABRG2 gene (Figure 3). The analysis by True Seq Custom Amplicon (see File S1) on genomic DNA from peripheral blood showed that the two substitutions are in cis and create the novel GGG codon (Gly) (according to HGVD nomenclature guidelines the deletion/insertion format is preferred, therefore variant is NC_000005.9: g.161576128_161576128 delinsGG, NM_000816.3: c.937_938delinsGG, p.(Leu313Gly)). NGS approach showed a read count for the variant equal to about 12% of the total (Figure 3B), barely detectable even with Sanger Sequencing (Figure 3A). The analysis on buccal swab DNA by Nextera approach using three different pairs of primers (see File S2), showed a percentage similar to that observed in blood (Figure 3C), excluding the occurrence of a partial allelic drop-out and supporting a real mosaicism condition. This variant is predicted as pathogenetic by the Alamut software. Parents’ analysis on genomic DNA from both blood and buccal swab carried out by Sanger and Nextera sequencing proved the de novo origin of the mutation.
Figure 3.
(A) Patient electropherograms show the mosaic double nucleotide substitution in the first and second position of the CTG codon (framed) with the mutated GGG codon (framed) replacing the aminoacid Leu at position 313 of the GABRG2 gene with Gly. (B) Annovar table revealing the low-rate (12%) mosaicism for the double base substitution in DNA from peripheral blood. (C) Nextera-XT-Library-prep protocol performed with three different pairs of primers (red-blue-green) shows on DNA from buccal swab a mosaic mutation percentage comparable to that obtained on blood.
The de novo variant in GABRB2, NC_000005.9: g.160758063 T>C, NM_021911.2: c.904G>A p.(Val302Met) of patient 8, although unreported in literature, is present in ClinVar as probably pathogenetic and is localized in the same region (the extracytoplasmic loop between the TM2 and TM3 chains) of the mutational hot spots p.Lys303Arg and p.Lys303Asn [26,29] and close to the p.Ala304Val mutation [26,30].The pathogenicity is indicated by the in silico predictions using Alamut software (Figure S2).
2.4. Clinical Features of Girls with Variants in GABAa Receptors Genes
According to the Neul criteria [12], the two patients with pathogenic variants in GABAa Receptors genes can be classified as RTT, since they presented a true regression, patient 7 as atypical RTT (3 primary criteria and> 5 supportive criteria) and patient 8 as classical RTT (4 primary criteria). In two patients epilepsy is not triggered by fever and in patient No. 8 the age of onset of seizures was not very early.
3. Discussion
The widespread use of NGS targeted to multi-gene panels or to the whole exome has shown that a notable number of patients referred with a suspected syndromic diagnosis turned out to be carriers of a pathogenic variant in genes not classically associated with the initial clinical diagnosis. The molecular definition of syndromes affecting the neurodevelopment associated with epilepsy remains a challenge, often hampered by the genetic heterogeneity [31]. In any case, a clinical diagnosis based on stringent diagnostic criteria commonly recognized by the scientific community should remain an initial milestone. Furthermore, the clinical diagnosis of Rett syndrome (RTT OMIM 3127520) is advantaged by the definition of stringent clinical criteria, which upon revision [12] allow classification and distinguishing classical RTT from both early onset epilepsy and congenital atypical RTT, the latter two mainly ascribed to disease causing variants in the CDKL5 and FOXG1 genes. On the other hand, when molecular tests exclude the involvement of the three canonical genes, the most frequent choice is a wider investigation usually starting with NGS targeted to multi gene panels including those associated with epilepsy and neurological disorders and often ending with the more expensive and time-consuming WES. According to recent literature [11,14], more than 69 genes from these studies turned out to be candidate genes for RTT/RTT-like phenotypes.
The availability of a large cohort of girls referred to our lab with suspected RTT and found negative for the canonical RTT genes, prompted us to perform NGS targeted to gene panels and/or the whole exome, a common strategy which succeeded in identifying so far in our cohort pathogenic variants in the STXBP1 gene and in two different subunits of GABAa receptor in six and two girls, respectively.
In keeping with the role of these genes, all eight patients experienced early-onset seizures (from the neonatal period to the second year of age). In order to pinpoint the existence of distinctive phenotypic traits helpful in addressing the molecular investigation of these three genes and designing a proper therapy, the clinical history and features of all eight girls were reviewed by their child neurologists and clinical geneticists and compared to the RTT diagnosis criteria.
STXBP1 encodes the presynaptic protein Munc18-1, a protein binding and stabilizing the complex of SNARE (Soluble NSF Attachment Protein REceptor) proteins, actively involved in the fusion between synaptic vesicles and the presynaptic membrane, thus favoring neuronal exocytosis [32]. Functional in vitro studies and experiments on Stxbp1 mouse models [33] demonstrated that instability of defective Munc18-1 protein and the consequent haploinsufficiency may explain the mechanism underlying the STXBP1 encephalopathy (EIEE4, OMIM 612164). GABAergic more than glutamatergic neurons seem impaired, probably resulting in an imbalanced excitability in the neocortex, responsible for an abnormal epileptic activity [34,35]. The phenotype of patients with a STXBP1 pathogenic variant ranges [25] from the generic early onset epilepsy encephalopathy, to Ohathara syndrome [36,37], EME [38], West syndrome [39,40], Dravet syndrome [41], but also includes intellectual disability (ID) in the absence of epilepsy [42,43,44], classic MECP2-negative RTT and atypical RTT [45]. As the degree of ID does not appear to correlate with the severity of the seizures and/or the age of onset of epilepsy, STXBP1-EE is not thought to be a simple Early Onset Epileptic Encephalopaty-EOEE, but a more complex neurodevelopmental disorder (DEE, Developmental and Epileptic Encephalopaty), where both ID (often occurring before the onset of epilepsy) and epilepsy play a synergic role in the phenotype evolution [25,26,27]. Considering that only four STXBP1 positive girls, two with a classical RTT [46,47], one with atypical RTT [48] and one male with RTT-like phenotype [21] had been reported, the patients described herein increase the overall number of RTT/RTT-like patients caused by pathogenic variants in STXBP1. Variants in patients No. 3 and 4 have never been reported in literature, while concerning the remaining mutations [25,49,50], one (p.(Pro139Leu)) is a mutational hotspot [51,52,53].
A review of clinical history of our patients and a comparison with the described cases highlighted the fact that all our STXBP1 girls share hand stereotypies and abnormal, impaired ataxic or absent gait and do not have purposeful use of hands and language, which has never, or in a very limited way, been acquired and has not been lost. Furthermore, only patient 6 presented a regression. Since the presence of regression is a fundamental criterion for the diagnosis of RTT syndrome, according to the Neul criteria, our cases should not be classified as RTT, but RTT-like. However, MECP2-positive patients presenting abnormal signs or deviant developmental profiles before 6 months who subsequently acquired supportive criteria have been reported [54,55] (defined as atypical congenital RTT) and an eventual regression before 6 months of age is difficult to identify also because ”typically the family and the primary clinician is not concerned about development until after 6 months of age” [12,56], suggesting that at the least our STXBP1 patients 1, 2, 3 and 5, who show two main criteria and >5 supportive criteria might be included in the subset of atypical/congenital RTT variants. Moving to the epileptic phenotype, the West syndrome history experienced by three of the four reported RTT patients with STXBP1 variants [21,46,47,48], was not observed in our cases, with the exception of patient 4.
Furthermore, our STXBP1 patients did not resemble the RTT Hanefeld variant due to mutations in CDKL5 gene and characterized by a typical course of epilepsy [57], nor did they seem to share a common epilepsy history and the age of seizure onset appeared to be quite different. Drug resistance, common in RTT Hanefeld variant patients [58] was present only in one out of our six cases. Moderate to severe ID is evident in all six patients, bruxism during wakefulness is present in three out of six girls, a characteristic shared with RTT and STXBP1-DEE patients [59]. The small number of reported cases with STXBP1 mutations and RTT/RTT-like diagnosis does not allow the definition of a correlation between mutation type and severity of the phenotype or epileptic profile. On the other hand, studies on a wide cohort of STXBP1-positive patients did not reveal any genotype–phenotype correlation [25]. In conclusion, the identification of six patients with a pathogenic defect in this gene from a RTT/RTT-like cohort of 137 patients (4.4%) suggests the performing of STXBP1 analysis when a congenital phenotype, characterized by the main criteria for RTT syndrome and associated with epilepsy is observed. Seizures may appear after developmental delay and are not necessarily drug resistant or associated with West phenotype.
We also identified a patient with a mutation in GABRG2 which encodes the gamma subunit of the heteropentameric GABA type A (GABAa) receptor in the form α2β2ɤ. It represents the most abundant inhibitory receptor subtype in the CNS [60], and the primary mediator of fast inhibitory synaptic transmission. The gamma subunit oligomerizes in the endoplasmic reticulum (ER) with the other subunits and is required for postsynaptic GABAa receptor clustering [61,62]. To date, only about twenty mutations associated with different epileptic phenotypes have been described, from mild (FS, Febrile Seizures or CAE, Childhood Absence Epilepsy) to moderate (GEFS +, Generalized Epilepsy with Febrile Seizures +), to severe [61] Dravet syndrome (DS) [63], or early onset encephalopathy phenotype [64,65], but never underlying the RTT phenotype. Clinical heterogeneity may depend primarily on how the mutation interferes with the assembly of the mutant with the other subunits, causing mechanisms of haploinsufficiency or dominant negative suppression [61]. A genotype-phenotype correlation study had previously associated missense mutations with milder clinical phenotypes and truncating variants with more severe phenotypes [61], but recent reports [63,64] identified several missense mutations in epileptic encephalopathy and DS as well. The mosaic p.(Leu313Gly) variant of patient 7 (see Figure 3), never described in literature, changes a highly conserved residue in the TM2 also located in the pore-lining region likewise the p.(Pro302Leu) mutation, which is associated with the severe phenotype of DS and that determine a dramatic whole-cell current reduction, resulting in hyperexcitability [63]. Moreover, a decrease in maximal response to GABA was observed for mutations located in TM2 [64]. The key location of the mutation together with the in silico predictions support its pathogenicity with regard to the epileptic phenotype, highlighting a new genotype-phenotype correlation according to which missense mutations associated with severe phenotype are located in the pore forming region of the channel, while the missense mutations affecting the N-ter or the extracytoplasmatic loop region underlie a mild phenotype. However, the condition of the mosaic mutation (described for this gene in Stosser 2018 [66] in an epileptic male scarcely defined in the phenotype, with a mutation percentage similar to that of our patient, but with localization in the N-terminal region) could be compatible with a milder phenotype, at least for the epileptic features. Although the condition of disability appears severe in our patient, she is now seizure-free. More generally, the evidence of mosaic status, at the limits of Sanger sequencing sensitivity, suggests the opportunity to retest negative patients by the more sensitive NGS approach, mainly for genes involved in epilepsy where mosaic mutations seem to be frequent [66,67,68].
Lastly, patient 8 carries the de novo unreported mutation in the GABRB2 gene, p.Val302Met. GABRB2 encodes the beta subunit of the GABAa ionotropic heteropentameric receptor, which also includes the ɤ subunit encoded by GABRG2, with which it shares the structure, common to all the GABAa receptors subunits [69]. To date, only 13 mutations have been described in this gene, clustered mainly in the 3 TM1–TM3 transmembrane domains and in the linker regions between them, responsible for IECEE2 (Infantile or Early Childhood Epilepsy Encephalopaty type 2, OMIM 617829), a neurodevelopmental disorder characterized by the onset of epilepsy in infancy or childhood, developmental delay and variable ID [26,30,70,71]. To our knowledge, only one other patient, carrying the p.Ala304Val mutation which is very close to the residue mutated in our patient, has a phenotype classified as atypical RTT [30].
According to Neul’s criteria, patients 7 and 8 can be classified RTT, since they exhibited regression, unlike those mutated in STXBP1. In the reported GABRG2 patients, the onset of seizures, beyond the severity of the clinical picture, is often triggered by fever, data not registered in patients 7 and 8. With regards to GABRB2, epilepsy is present in almost all the patients so far described, but the age of onset in patient 8 is later (2 years) (Table S1) than that (within the first year of life) reported [26]. Although replication of these findings in more patients is needed for a correct genotype-phenotype correlation and to establish differences and similarities with RTT patients mutated in classic associated genes, our data suggests testing this gene in negative RTT cases that meet Neul criteria for classic and atypical forms with epilepsy presenting not necessarily at very early onset.
The identification of mutated patients in GABRB2 and GABRG2 is in agreement with the observation of deregulation of the GABAergic pathway in murine Mecp2-deficient models [72,73] and iPSCs-derived neurons of MECP2 mutated patients [10]. The evidence that haploinsufficiency of STXBP1 also affects more inhibitory GABAergic than glutamatergic neurons [35] supports the hypothesis that the alteration of the GABAergic pathway is of primary importance in the RTT phenotype and the genes acting in this pathway may represent a target for mutational screening in RTT/RTT-like patients, as well as a key gene network in the future treatment of the disorder.
4. Materials and Methods
4.1. Patients
Twenty-six girls (aged from 5 to 38 years), accurately selected by a number of different Italian neuropsychiatric departments, classified as follows: 14 as Classical Rett, five as Atypical Early Onset, five as congenital Rett and two females as Rett-like, according to Neul classification, were tested in 2013 by a WES experiment.
Seventy-eight patients (72 singleton plus 3 pairs of siblings, of which 49 females and 29 males, aged from 1 to 49 years) characterized by a) neonatal, infantile or childhood onset seizures, and/or or b) drug resistant epilepsy and/or c) progressive worsening of the clinical course associated with epilepsy and/or d) autistic traits and/or e) intellectual disability, were tested by NGS Custom Panel for pediatric epilepsy; 11 of them were classified by clinicians as RTT/RTT-like.
One hundred patients referred to our lab with clinical suspicion of RTT/RTT-like, were screened by NGS Custom panel in use in IAI laboratory since 2016 for diagnostics of RTT and syndromes in differential diagnosis.
All positive patients were referred to our laboratory by a number of Italian neuropsychiatric departments.
A written consent was signed for all patients by their families. The study was approved by IRCCS Istituto Auxologico Italiano Ethical Committee on 03/04/2012 (protocol number 2012_04_03_05-Project MOH 08C208 and AIRETT) and on 12/03/2013 (protocol number 2013_03_12_14-Project MOH 08C305).
4.2. Methods
Genomic DNA was extracted from peripheral blood leukocytes using Freedom Evo TECAN extractor.
4.2.1. WES
The WES experiment was performed on Illumina Hi Scan SQ platform and technology (Illumina, San Diego, CA, USA); the True Seq Exome Enrichment Kit enables the enrichment of the coding portion as well as the adjacent intronic and 5’-3’-untranslated regions.
Sequencing Data Analysis
The text files of exome sequences were aligned to the Human assembly GRCh37/h19, using the Burrows-Wheeler alignment tool, BWA [74] to generate Binary Alignment Map (BAM files). Variant calling was performed using the Genome Analysis Tool Kit (GATK) [75] and Genotyping identification was performed using GATK’s Unified Genotyper [76]. Variants were subsequently annotated for their existence in dbSNP, also harboring all properties described in dbSNP for known variants (e.g., GMAF). The possible impact of variations was evaluated using SnpEff [77]. Additional bioinformatics tools were applied (SIFT score, Polyphen prediction, GERP score) using the NSFP database [78], to assess their potential noxious effect.
The coverage at each nucleotide was extracted from each subject’s exome BAM file. The average exome sequence coverage per individual was 40 X ranging from 20 X to 67 X at the target exome sequence.
Selection of Potentially Causative Variants
We utilized a custom-built interpretation scheme to identify possible causative variations, based on several parameters including minor allele frequency, conservation, mutation type, predicted pathogenicity, presence (in public databases or in literature) in genes already known to be associated with phenotypes sharing common aspects with RTT or in genes involved in pathway relevant to the development and features of the central nervous system. The prioritization was carried out using specific bioinformatic tools as ToppGene (toppgene.cchmc.org) [79] and DAVIDgene (david.abcc.ncifcrf.gov). Before proceeding to Single Nucleotide Variation (SNV) validation by Sanger Sequencing, prioritized genes were further investigated individually by literature updates (www.ncbi.nlm.nih.gov/pubmed) and through a range of bioinformatic websites providing further information about locus-specific databases, protein structure, function, expression and interacting networks (www.genome.jp/kegg/, ncbi.nlm.nih.gov/gene, www.genecards.org/).
4.2.2. NGS Custom Panel for Pediatric Epilepsy
A Custom Illumina Panel covering coding regions and their intron–exon boundaries (flanking 20 nucleotides) of 33 genes (see Supplementary File S3) causing neonatal, infantile or childhood onset seizures and/or autism was created in 2014 for sequencing using the True Seq Custom Amplicon protocol according to the reference guide and analyzed in-house on an Illumina MiSeq instrument (Illumina, San Diego, CA, USA).
4.2.3. NGS Custom Panel for Diagnostic Analysis
Genomic sequencing of whole coding region and intron-exon junctions of 35 genes involved in RTT and syndromes in differential diagnosis (see Supplementary File S3) was performed by Illumina Nextera Rapid Capture Enrichment protocol, following the manufacturer’s instructions.
For both approaches of NGS Custom Panel, the genomic regions with a coverage of less than 20 X were analyzed by Sanger Sequencing or Nextera-XT-Library-prep protocol (Illumina) using MiSeq Instrument for sequencing.
4.2.4. Variants Validation
The variants identified by all three experiments were validated by Sanger Sequencing using a Big-Dye® Terminator v3.1 Cycle Sequencing Kit and analyzed in an Applied Biosystems Abi Prism 3500 Sequencer. The primers used for Sanger sequencing are shown in Table S2. Sanger sequencing was also performed on DNA from patients’ parents. We analyzed microsatellite parental inheritance in order to confirm the correct family relationship and avoid any possible sampling error. Microsatellite analysis was performed using the commercial kit ChromoQuant® QF-PCR (CyberGene AB, Solna, Sweden) which evaluates polymorphic loci on chromosomes X, Y, 13, 18 and 21, according to the manufacturer’s protocol and analyzed on an ABI Prism 3500 sequencer (Applied Biosystems, Foster City, CA, USA).
For STXBP1, splicing variants total RNA was extracted from peripheral blood leukocytes using Tempus Spin RNA Isolation Kit (Applied Biosystems-Termofisher, Waltham, NA, USA) and reverse transcribed to cDNA by SuperScript VILO Kit (Invitrogen-Termofisher, Waltham, NA, USA). cDNA was amplified by Go Taq Hot Start Polymerase (Promega, Madison, WI, USA) and sequenced using the Big DyeTerminator v.3.1 Cycle Sequencing Kit (Applied Biosystems) with primers: STXBP1ex2F 5’AAGAAGAAGGGGGAATGGAA3’, ex4-5R 5’GAGAGTGGACGGACTTCTCG3’ and Ex3-int3-mutF 5’AGGCATAACGAgcgagca, STXBP1 ex15-16F 5’ACCGATTCCACGCTGCGTCG3’, STXBP1ex19R 5’CCATTGTTGGAGCCTGATCC3’ and run on ABI PRISM 3500 sequencer (Applied Biosystems).
In the case of patient No. 7, Sanger Sequencing and Nextera-XT_library-prep protocol was also conducted on DNA extracted from buccal swab collected with Oragene-DNA OG575, using prepIT L2P kit (DNA genotek, Ottawa, Canada). Three different pairs of primers were used to validate the variant on saliva and blood in the patient and her parents: GABRG2EX8TrueSEQredF 5’AGTCTCACGAGTGACTCAGTTACCCAA3’, 5’GABRG2EX8TrueSEQredR GTTATGGCCTGGCTAAACTCATACATG3’, GABRG2EX8blueF 5’tccctgtattctccatggca3’, GABRG2EX8blueR 5’TTGTCCTTGCTTGGTTTCCG3’, GABRG2EX8greenF 5’ttcccattgctgaaactgcc3’, GABRG2EX8greenR 5’CCTTGCTTGGTTTCCG3’.
Acknowledgments
We would like to thank our patients, their families and AIRETT.
Abbreviations
| RTT | Rett Syndrome |
| MECP2 | Methyl CpG-binding protein 2 |
| CREB1 | cAMP Responsive Element Binding Protein 1 |
| mTOR | Mammalian Target of Rapamycin |
| GABA | Gamma-aminobutyric acid |
| CDKL5 | Cyclin-dependent kinase-like 5 |
| FOXG1 | Forkhead box G1 gene |
| NGS | Next generation sequencing |
| WES | Whole Exome Sequencing |
| DEE | Developmental and Epileptic Encephalopaty |
| ID | Intellectual Disability |
| EE | Epileptic Encephalopaty |
| EOEE | Early Onset Epileptic Encephalopaty |
| EIEE | Early Infantil Epileptic Encephalopaty |
| STXBP1 | Syntaxin-binding protein 1 |
| GABRB2 | Gamma-aminobutyric acid type A receptor beta2 |
| GABRG2 | Gamma-aminobutyric acid type A receptor gamma2 |
| EME | Early Myoclonic Encephalopathy |
| SNARE | Soluble NSF Attachment Protein REceptor |
| Munc18-1 | Mammalian uncoordinated-18-1 |
| CNS | Central Nervous System |
| FS | Febrile Seizures |
| CAE | Childhood Absence Epilepsy |
| GEFS + | Generalized Epilepsy with Febrile Seizures plus |
| DS | Dravet syndrome |
| TM | Transmembrane Domain |
| ER | Endoplasmic Reticulum |
| IECEE2 | Infantile or Early Childhood Epilepsy Encephalopaty type 2 |
| NTS | Nucleus of the solitary tract |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/15/3621/s1.
Author Contributions
S.R. conceived the project of WES experiment, F.C. conceived the project of NGS Custom Panel for Pediatric Epilepsy, M.M. (Maura Masciadri), S.R. and F.C. conceived the NGS Custom Panel for Diagnostic Analysis. M.M. (Margherita Marchi) and I.C. prioritized, selected and validated the causative variants of WES experiment. F.C. selected and validated the causative variants of NGS Custom Panel for Pediatric Epilepsy. V.G. performed experiments by Nextera-XT-Library-prep protocol of NGS Custom Panel for Pediatric Epilepsy. M.M. (Maura Masciadri) selected and validated the causative variants of NGS Custom Panel for Diagnostic Analysis. D.G. handled bioinformatics data analysis. M.T.B., A.V., A.P., M.N.S., L.S., B.S., S.M., E.V., G.P., M.P., I.M. selected and clinically evaluated the patients. F.C. prepared the figures and Tables collecting clinical data and wrote the manuscript with the help of S.R. Lastly A.V., A.P. and L.L. corrected and edited the manuscript.
Funding
The study has been supported by MOH Grant to Istituto Auxologico Italiano (08C208 and 08C305) and by RETT Italian Association (AIRETT) National Grant 2012.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Laurvick C.L., de Klerk N., Bower C., Christodoulou J., Ravine D., Ellaway C., Williamson S., Leonard H. Rett syndrome in Australia: A review of the epidemiology. J. Pediatrics. 2006;148:347–352. doi: 10.1016/j.jpeds.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Glaze D.G., Percy A.K., Motil K.J., Lane J.B., Isaacs J.S., Schultz R.J., Barrish J.O., Neul J.L., O’Brien W.E., Smith E.O. A study of the treatment of Rett syndrome with folate and betaine. J. Child. Neurol. 2009;24:551–556. doi: 10.1177/0883073808327827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrhart F., Coort S.L., Cirillo E., Smeets E., Evelo C.T., Curfs L.M. Rett syndrome - biological pathways leading from MECP2 to disorder phenotypes. Orphanet J. Rare Dis. 2016;11:158. doi: 10.1186/s13023-016-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weissman J., Naidu S., Bjornsson H.T. Abnormalities of the DNA methylation mark and its machinery: An emerging cause of neurologic dysfunction. Semin. Neurol. 2014;34:249–257. doi: 10.1055/s-0034-1386763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nan X., Ng H.H., Johnson C.A., Laherty C.D., Turner B.M., Eisenman R.N., Bird A. Transcriptional repression by the methyl-CpG-binding protein MECP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 6.Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maunakea A.K., Chepelev I., Cui K., Zhao K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013;23:1256–1269. doi: 10.1038/cr.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng T.L., Wang Z., Liao Q., Zhu Y., Zhou W.H., Xu W., Qiu Z. MECP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev. Cell. 2014;28:547–560. doi: 10.1016/j.devcel.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Bedogni F., Rossi R.L., Galli F., Cobolli Gigli C., Gandaglia A., Kilstrup-Nielsen C., Landsberger N. Rett syndrome and the urge of novel approaches to study MeCP2 functions and mechanisms of action. Neurosci. Biobehav. Rev. 2014;46:187–201. doi: 10.1016/j.neubiorev.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Landucci E., Brindisi M., Bianciardi L., Catania L.M., Daga S., Croci S., Frullanti E., Fallerini C., Butini S., Brogi S., et al. iPSC -derived neurons profiling reveals GABAergic circuit disruption and acetylated alpha-tubulin defect which improves after iHDAC6 treatment in Rett syndrome. Exp. Cell Res. 2018;368:225–235. doi: 10.1016/j.yexcr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Operto F.F., Mazza R., Pastorino G.M.G., Verrotti A., Coppola G. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019;9:e01250. doi: 10.1002/brb3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neul J.L., Kaufmann W.E., Glaze D.G., Christodoulou J., Clarke A.J., Bahi-Buisson N., Leonard H., Bailey M.E., Schanen N.C., Zappella M., et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percy A.K. Rett syndrome: Recent research progress. J. Child. Neurol. 2008;23:543–549. doi: 10.1177/0883073807309786. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhart F., Sangani N.B., Curfs L.M.G. Current developments in the genetics of Rett and Rett-like syndrome. Curr. Opin. Psychiatry. 2018;31:103–108. doi: 10.1097/YCO.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 15.Mari F., Azimonti S., Bertani I., Bolognese F., Colombo E., Caselli R., Scala E., Longo I., Grosso S., Pescucci C., et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum. Mol. Genet. 2005;14:1935–1946. doi: 10.1093/hmg/ddi198. [DOI] [PubMed] [Google Scholar]
- 16.Scala E., Ariani F., Mari F., Caselli R., Pescucci C., Longo I., Meloni I., Giachino D., Bruttini M., Hayek G., et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J. Med. Genet. 2005;42:103–107. doi: 10.1136/jmg.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariani F., Hayek G., Rondinella D., Artuso R., Mencarelli M.A., Spanhol-Rosseto A., Pollazzon M., Buoni S., Spiga O., Ricciardi S., et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X., Petrovski S., Xie P., Ruzzo E.K., Lu Y.F., McSweeney K.M., Ben-Zeev B., Nissenkorn A., Anikster Y., Oz-Levi D., et al. Whole-exome sequencing in undiagnosed genetic diseases: Interpreting 119 trios. Genet. Med. 2015;17:774–781. doi: 10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucariello M., Vidal E., Vidal S., Saez M., Roa L., Huertas D., Pineda M., Dalfo E., Dopazo J., Jurado P., et al. Whole exome sequencing of Rett syndrome-like patients reveals the mutational diversity of the clinical phenotype. Hum. Genet. 2016;135:1343–1354. doi: 10.1007/s00439-016-1721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Percy A.K., Lane J., Annese F., Warren H., Skinner S.A., Neul J.L. When Rett syndrome is due to genes other than MECP2. Transl. Sci. Rare Dis. 2018;3:49–53. doi: 10.3233/TRD-180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes F., Barbosa M., Ameur A., Soares G., de Sa J., Dias A.I., Oliveira G., Cabral P., Temudo T., Calado E., et al. Identification of novel genetic causes of Rett syndrome-like phenotypes. J. Med. Genet. 2016;53:190–199. doi: 10.1136/jmedgenet-2015-103568. [DOI] [PubMed] [Google Scholar]
- 22.Kulikovskaja L., Sarajlija A., Savic-Pavicevic D., Dobricic V., Klein C., Westenberger A. WDR45 mutations may cause a MECP2 mutation-negative Rett syndrome phenotype. Neurol. Genet. 2018;4:e227. doi: 10.1212/NXG.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriksen M.W., Ravn K., Paus B., von Tetzchner S., Skjeldal O.H. De novo mutations in SCN1A are associated with classic Rett syndrome: A case report. Bmc Med. Genet. 2018;19:184. doi: 10.1186/s12881-018-0700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allou L., Julia S., Amsallem D., El Chehadeh S., Lambert L., Thevenon J., Duffourd Y., Saunier A., Bouquet P., Pere S., et al. Rett-like phenotypes: Expanding the genetic heterogeneity to the KCNA2 gene and first familial case of CDKL5-related disease. Clin. Genet. 2017;91:431–440. doi: 10.1111/cge.12784. [DOI] [PubMed] [Google Scholar]
- 25.Stamberger H., Nikanorova M., Willemsen M.H., Accorsi P., Angriman M., Baier H., Benkel-Herrenbrueck I., Benoit V., Budetta M., Caliebe A., et al. STXBP1 encephalopathy: A neurodevelopmental disorder including epilepsy. Neurology. 2016;86:954–962. doi: 10.1212/WNL.0000000000002457. [DOI] [PubMed] [Google Scholar]
- 26.Hamdan F.F., Myers C.T., Cossette P., Lemay P., Spiegelman D., Laporte A.D., Nassif C., Diallo O., Monlong J., Cadieux-Dion M., et al. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am. J. Hum. Genet. 2017;101:664–685. doi: 10.1016/j.ajhg.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshe S.L., et al. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamdan F.F., Piton A., Gauthier J., Lortie A., Dubeau F., Dobrzeniecka S., Spiegelman D., Noreau A., Pellerin S., Cote M., et al. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Ann. Neurol. 2009;65:748–753. doi: 10.1002/ana.21625. [DOI] [PubMed] [Google Scholar]
- 29.Baldridge D., Heeley J., Vineyard M., Manwaring L., Toler T.L., Fassi E., Fiala E., Brown S., Goss C.W., Willing M., et al. The exome clinic and the role of medical genetics expertise in the interpretation of exome sequencing results. Genet. Med. 2017;19:1040–1048. doi: 10.1038/gim.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajan S.A., Jhangiani S.N., Muzny D.M., Gibbs R.A., Lupski J.R., Glaze D.G., Kaufmann W.E., Skinner S.A., Annese F., Friez M.J., et al. Enrichment of mutations in chromatin regulators in people with Rett syndrome lacking mutations in MECP2. Genet. Med. 2017;19:13–19. doi: 10.1038/gim.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McTague A., Howell K.B., Cross J.H., Kurian M.A., Scheffer I.E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15:304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- 32.Dawidowski D., Cafiso D.S. Munc18-1 and the Syntaxin-1 n terminus regulate open-closed states in a t-SNARE complex. Structure. 2016;24:392–400. doi: 10.1016/j.str.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacevic J., Maroteaux G., Schut D., Loos M., Dubey M., Pitsch J., Remmelink E., Koopmans B., Crowley J., Cornelisse L.N., et al. Protein instability, haploinsufficiency, and cortical hyper-excitability underlie STXBP1 encephalopathy. Brain. 2018;141:1350–1374. doi: 10.1093/brain/awy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang J.Q. Defects at the crossroads of GABAergic signaling in generalized genetic epilepsies. Epilepsy Res. 2017;137:9–18. doi: 10.1016/j.eplepsyres.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toonen R.F., Wierda K., Sons M.S., de Wit H., Cornelisse L.N., Brussaard A., Plomp J.J., Verhage M. Munc18-1 expression levels control synapse recovery by regulating readily releasable pool size. Proc. Natl. Acad. Sci. USA. 2006;103:18332–18337. doi: 10.1073/pnas.0608507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitsu H., Kato M., Shimono M., Senju A., Tanabe S., Kimura T., Nishiyama K., Yoneda Y., Kondo Y., Tsurusaki Y., et al. Association of genomic deletions in the STXBP1 gene with Ohtahara syndrome. Clin. Genet. 2012;81:399–402. doi: 10.1111/j.1399-0004.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 37.Mastrangelo M., Peron A., Spaccini L., Novara F., Scelsa B., Introvini P., Raviglione F., Faiola S., Zuffardi O. Neonatal suppression-burst without epileptic seizures: Expanding the electroclinical phenotype of STXBP1-related, early-onset encephalopathy. Epileptic Disord. 2013;15:55–61. doi: 10.1684/epd.2013.0558. [DOI] [PubMed] [Google Scholar]
- 38.Aravindhan A., Shah K., Pak J., Veerapandiyan A. Early-onset epileptic encephalopathy with myoclonic seizures related to 9q33.3-q34.11 deletion involving STXBP1 and SPTAN1 genes. Epileptic Disord. 2018;20:214–218. doi: 10.1684/epd.2018.0969. [DOI] [PubMed] [Google Scholar]
- 39.Deprez L., Weckhuysen S., Holmgren P., Suls A., Van Dyck T., Goossens D., Del-Favero J., Jansen A., Verhaert K., Lagae L., et al. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–1165. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- 40.Otsuka M., Oguni H., Liang J.S., Ikeda H., Imai K., Hirasawa K., Imai K., Tachikawa E., Shimojima K., Osawa M., et al. STXBP1 mutations cause not only Ohtahara syndrome but also West syndrome--Result of Japanese cohort study. Epilepsia. 2010;51:2449–2452. doi: 10.1111/j.1528-1167.2010.02767.x. [DOI] [PubMed] [Google Scholar]
- 41.Carvill G.L., Weckhuysen S., McMahon J.M., Hartmann C., Moller R.S., Hjalgrim H., Cook J., Geraghty E., O’Roak B.J., Petrou S., et al. GABRA1 and STXBP1: Novel genetic causes of Dravet syndrome. Neurology. 2014;82:1245–1253. doi: 10.1212/WNL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamdan F.F., Gauthier J., Dobrzeniecka S., Lortie A., Mottron L., Vanasse M., D’Anjou G., Lacaille J.C., Rouleau G.A., Michaud J.L. Intellectual disability without epilepsy associated with STXBP1 disruption. Eur. J. Hum. Genet. 2011;19:607–609. doi: 10.1038/ejhg.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell I.M., Yatsenko S.A., Hixson P., Reimschisel T., Thomas M., Wilson W., Dayal U., Wheless J.W., Crunk A., Curry C., et al. Novel 9q34.11 gene deletions encompassing combinations of four mendelian disease genes: STXBP1, SPTAN1, ENG, and TOR1a. Genet. Med. 2012;14:868–876. doi: 10.1038/gim.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N., et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: An exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 45.Khaikin Y., Mercimek-Mahmutoglu S. STXBP1 encephalopathy with epilepsy. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. Genereviews. GeneReviews® [Internet]; Washington, DC, USA: 2016. [Google Scholar]
- 46.Yuge K., Iwama K., Yonee C., Matsufuji M., Sano N., Saikusa T., Yae Y., Yamashita Y., Mizuguchi T., Matsumoto N., et al. A novel STXBP1 mutation causes typical Rett syndrome in a Japanese girl. Brain Dev. 2018;40:493–497. doi: 10.1016/j.braindev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Romaniello R., Saettini F., Panzeri E., Arrigoni F., Bassi M.T., Borgatti R. A de-novo STXBP1 gene mutation in a patient showing the Rett syndrome phenotype. Neuroreport. 2015;26:254–257. doi: 10.1097/WNR.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 48.Olson H.E., Tambunan D., LaCoursiere C., Goldenberg M., Pinsky R., Martin E., Ho E., Khwaja O., Kaufmann W.E., Poduri A. Mutations in epilepsy and intellectual disability genes in patients with features of Rett syndrome. Am. J. Med. Genet. 2015;167:2017–2025. doi: 10.1002/ajmg.a.37132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen N.M., Conroy J., Shahwan A., Lynch B., Correa R.G., Pena S.D., McCreary D., Magalhaes T.R., Ennis S., Lynch S.A., et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia. 2016;57:e12–e17. doi: 10.1111/epi.13250. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald T.W., Gerety S.S., Jones W.D., van Kogelenberg M., King D.A., McRae J., Morley K.I., Parthiban V., Al-Turki S., Ambridge K., et al. Deciphering Developmental Disorders, S. Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barcia G., Chemaly N., Gobin S., Milh M., Van Bogaert P., Barnerias C., Kaminska A., Dulac O., Desguerre I., Cormier V., et al. Early epileptic encephalopathies associated with STXBP1 mutations: Could we better delineate the phenotype? Eur. J. Med. Genet. 2014;57:15–20. doi: 10.1016/j.ejmg.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Keogh M.J., Daud D., Pyle A., Duff J., Griffin H., He L., Alston C.L., Steele H., Taggart S., Basu A.P., et al. A novel de novo STXBP1 mutation is associated with mitochondrial complex I deficiency and late-onset juvenile-onset parkinsonism. Neurogenetics. 2015;16:65–67. doi: 10.1007/s10048-014-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson H.E., Kelly M., LaCoursiere C.M., Pinsky R., Tambunan D., Shain C., Ramgopal S., Takeoka M., Libenson M.H., Julich K., et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann. Neurol. 2017;81:419–429. doi: 10.1002/ana.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi Y., Ohashi T., Akasaka N., Tohyama J. Congenital variant of Rett syndrome due to an intragenic large deletion in MECP2. Brain Dev. 2012;34:601–604. doi: 10.1016/j.braindev.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Rajaei S., Erlandson A., Kyllerman M., Albage M., Lundstrom I., Karrstedt E.L., Hagberg B. Early infantile onset “congenital” Rett syndrome variants: Swedish experience through four decades and mutation analysis. J. Child. Neurol. 2011;26:65–71. doi: 10.1177/0883073810374125. [DOI] [PubMed] [Google Scholar]
- 56.Einspieler C., Marschik P.B. Regression in Rett syndrome: Developmental pathways to its onset. Neurosci. Biobehav. Rev. 2019;98:320–332. doi: 10.1016/j.neubiorev.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 57.Bahi-Buisson N., Kaminska A., Boddaert N., Rio M., Afenjar A., Gerard M., Giuliano F., Motte J., Heron D., Morel M.A., et al. The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia. 2008;49:1027–1037. doi: 10.1111/j.1528-1167.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 58.Pintaudi M., Calevo M.G., Vignoli A., Parodi E., Aiello F., Baglietto M.G., Hayek Y., Buoni S., Renieri A., Russo S., et al. Epilepsy in Rett syndrome: Clinical and genetic features. Epilepsy Behav. 2010;19:296–300. doi: 10.1016/j.yebeh.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 59.Rezazadeh A., Uddin M., Snead O.C., 3rd, Lira V., Silberberg A., Weiss S., Donner E.J., Zak M., Bradbury L., Scherer S.W., et al. STXBP1 encephalopathy is associated with awake bruxism. Epilepsy Behav. 2019;92:121–124. doi: 10.1016/j.yebeh.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Sarto-Jackson I., Sieghart W. Assembly of GABA(a) receptors (review) Mol. Membr. Biol. 2008;25:302–310. doi: 10.1080/09687680801914516. [DOI] [PubMed] [Google Scholar]
- 61.Kang J.Q., MacDonald R.L. Molecular pathogenic basis for GABRG2 mutations associated with a spectrum of epilepsy syndromes, from generalized absence epilepsy to Dravet syndrome. Jama Neurol. 2016;73:1009–1016. doi: 10.1001/jamaneurol.2016.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alldred M.J., Mulder-Rosi J., Lingenfelter S.E., Chen G., Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAa receptors and gephyrin. J. Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernandez C.C., Kong W., Hu N., Zhang Y., Shen W., Jackson L., Liu X., Jiang Y., Macdonald R.L. Altered channel conductance states and gating of GABAa receptors by a pore mutation linked to Dravet syndrome. eNeuro. 2017;4 doi: 10.1523/ENEURO.0251-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen D., Hernandez C.C., Shen W., Hu N., Poduri A., Shiedley B., Rotenberg A., Datta A.N., Leiz S., Patzer S., et al. De novo GABRG2 mutations associated with epileptic encephalopathies. Brain. 2017;140:49–67. doi: 10.1093/brain/aww272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou F., McWalter K., Schmidt L., Decker A., Picker J.D., Lincoln S., Sweetser D.A., Briere L.C., Harini C., Members of the Undiagnosed Diseases N., et al. Expanding the phenotypic spectrum of GABRG2 variants: A recurrent GABRG2 missense variant associated with a severe phenotype. J. Neurogenet. 2017;31:30–36. doi: 10.1080/01677063.2017.1315417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stosser M.B., Lindy A.S., Butler E., Retterer K., Piccirillo-Stosser C.M., Richard G., McKnight D.A. High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genet. Med. 2018;20:403–410. doi: 10.1038/gim.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers C.T., Hollingsworth G., Muir A.M., Schneider A.L., Thuesmunn Z., Knupp A., King C., Lacroix A., Mehaffey M.G., Berkovic S.F., et al. Parental mosaicism in "de novo" epileptic encephalopathies. New Engl. J. Med. 2018;378:1646–1648. doi: 10.1056/NEJMc1714579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muir A.M., King C., Schneider A.L., Buttar A.S., Scheffer I.E., Sadleir L.G., Mefford H.C. Double somatic mosaicism in a child with Dravet syndrome. Neurol. Genet. 2019;5:e333. doi: 10.1212/NXG.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacob T.C., Moss S.J., Jurd R. Gaba(a) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srivastava S., Cohen J., Pevsner J., Aradhya S., McKnight D., Butler E., Johnston M., Fatemi A. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am. J. Med. Genet. 2014;164:2914–2921. doi: 10.1002/ajmg.a.36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishii A., Kang J.Q., Schornak C.C., Hernandez C.C., Shen W., Watkins J.C., Macdonald R.L., Hirose S. A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J. Med. Genet. 2017;54:202–211. doi: 10.1136/jmedgenet-2016-104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao H.T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H.C., Heintz N., et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen C.Y., Di Lucente J., Lin Y.C., Lien C.C., Rogawski M.A., Maezawa I., Jin L.W. Defective GABAergic neurotransmission in the nucleus tractus solitarius in MECP2-null mice, a model of Rett syndrome. Neurobiol. Dis. 2018;109:25–32. doi: 10.1016/j.nbd.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., Philippakis A.A., del Angel G., Rivas M.A., Hanna M., et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cingolani P., Platts A., Wang le L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila Melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu X., Jian X., Boerwinkle E. Dbnsfp v2.0: A database of human non-synonymous SNVs and their functional predictions and annotations. Hum. Mutat. 2013;34:E2393–E2402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J., Bardes E.E., Aronow B.J., Jegga A.G. ToppGene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.