Abstract
A concise and efficient approach to synthesizing coumarin-fused pyrazolo[3,4-b]pyridine via silica sulfuric acid (SSA) catalyzed three-component domino reaction under microwave irradiation has been demonstrated. Participation of various alcohols in construction of coumarin derivatives has been described for the first time. Short reaction time, high yields, one-pot procedure, usage of eco-friendly catalyst, and solvent are the key features of this method.
Keywords: coumarin; pyrazolo[3,4-b]pyridine; synthesis; silica sulfuric acid
1. Introduction
As one of the most important heterocyclic compounds, coumarin was widely found in nature products [1,2], and several synthetic coumarins [3] with a variety of pharmacophoric groups at C-3, C-4, and C-7 positions have been intensively screened for various biological activities like AChE inhibitors [4,5,6], anticancer [7,8,9], anticoagulant [10,11], anti-HIV [12,13,14], antitubercular [15,16], anti-inflammatory [17,18], antioxidant [19], antibacterial [20], antihypertensive [21], anticonvulsant [22], antifungal [23], and antihyperglycemic [24]. A recent literature survey suggests quite a few coumarin derivatives have been patented for their biological properties (Figure 1). Besides the high biological activity, coumarin is also considered to be a functional material [25,26] such as receptors [27,28,29], signaling units in sensors and biosensors, as well as in advanced photophysical systems [30,31].
Figure 1.
General structures of coumarin molecules possessing biological activity.
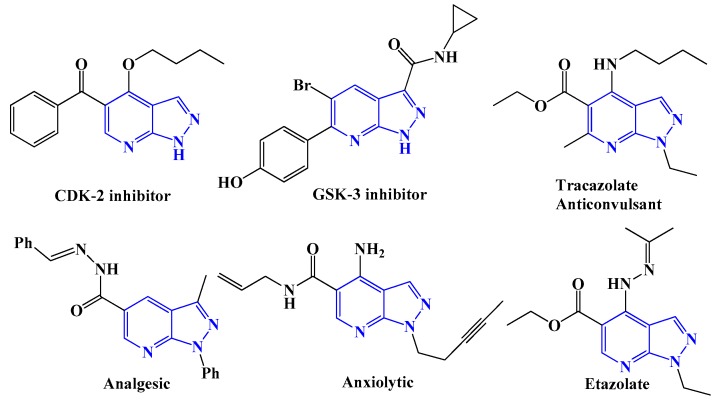
Among various nitrogen-containing heterocyclic compounds, pyrazolo[3,4-b]pyridine is recognized as important drug molecular skeleton in recent years due to a wide varieties of biological activities (Figure 2), such as antimicrobial [32,33], anti-inflammatory [34,35], anti-proliferative [36,37], and many other [38,39] important effects.
Figure 2.
Biologically active compounds having pyrazolo[3,4-b]pyridine unit.
Therefore, development and introduction of a convenient, efficient method for the synthesis of coumarin-fused pyrazolo[3,4-b]pyridine is highly desirable for their immense pharmacological potential. As a part of our research on the synthesis of novel functionalized heterocyclic derivatives [40,41,42,43,44,45,46], in the current paper, we report a novel three-component domino reaction for the synthesis of functionalized coumarin-fused pyrazolo[3,4-b]pyridine derivatives using silica sulfuric acid as the catalyst. It worth mentioning that participation of alcohols in construction of coumarin derivatives is described for the first time.
2. Results and Discussion
In the early literature reports of our group [44], the coumarino[4,3-d]pyrazolo[3,4-b]pyridine derivative (3a) was synthesized by the reaction of 3-acylcoumarin (1a) with 5-aminopyrazole (2a) catalyzed by silica sulfuric acid (SSA) in EtOH at 90 °C for 20 min under microwave irradiation (Scheme 1).
Scheme 1.
Synthesis of coumarino[4,3-d]pyrazolo[3,4-b]pyridine derivative 3a.
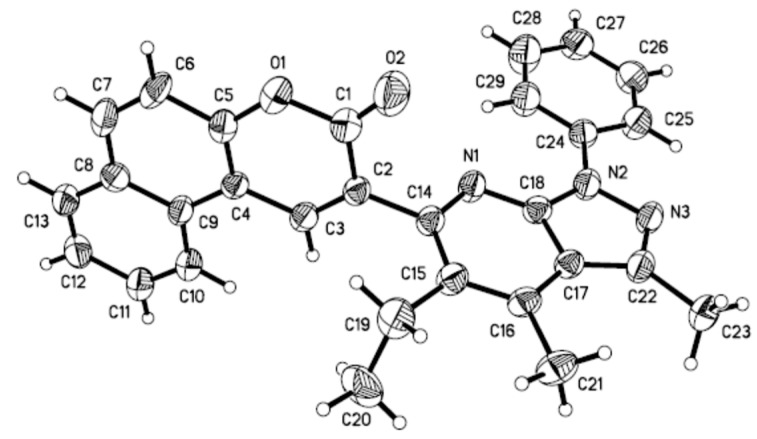
According to our previously reported synthetic procedure, we speculate that the coumarin derivative 6a could be obtained from the 2-butyryl-3H-benzo[f]chromen-3-one (4a) and 3-methyl-1-phenyl-1H-pyrazol-5-amine (2a) used as the starting materials. However, product 6a was not available as expect (Scheme 2-1). Considering the steric hindrance effect of the reaction, when ethanol and ethylene glycol (EG) as mixed solvent (volume ratio of EG/EtOH = 1:1) was added to the reaction, and further increasing the temperature (120 °C), a new product 7a formed unexpectedly (Scheme 2-2), which was identified by 1H-NMR, 13C-NMR, HRMS analysis. Moreover, we also obtained the single crystal of 7a suitable for X-ray analysis (Figure 3) [47]. To our surprise, the solvent ethanol also participated in this reaction and a novel coumarin derivative was constructed.
Scheme 2.
New multicomponent domino reactions.
Figure 3.
Crystal structure of 7a.
In order to achieve the optimal conditions of three-component reaction, a series of catalysts, solvents, and temperature were screened, as shown in Table 1. Some other acid catalysts such as p-TsOH, HClO3S, H2SO4, SiO2-H2SO4 (Table 1. entries 1, 3–5) and base catalysts such as K2CO3, NaOH, Cs2CO3 (Table 1, entries 6–8) were tested. However, none of them gave better results, lead to the identification of SSA as the most effective catalyst (Table 1. entry 2). To further increase the yield of desired product 7a, different solvents were evaluated. The results revealed that EtOH and EG as mixed solvents greatly improved the transformation, in control to EtOH, PEG, glycerol, and DMF as a single solvent (Table 1, entries 2, 9–12). When the volume ratio of EG/EtOH = 3:1, the yield of 7a could further increase to 68% (Table 1, entry 15). Much to our delight, we observed that increasing of the temperature to 140 °C resulted in affording 7a in 84% yield (Table 1, entry 20).
Table 1.
Optimizing the reaction conditions for the synthesis of 7a under microwave a.
| Entry | Catalyst | Solvent (v/v) | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | p-TsOH (20 mol%) | EG/EtOH=1:1 | 120 | trace |
| 2 | SSA (0.25 g) | EG/EtOH=1:1 | 120 | 58 |
| 3 | HClO3S (5 mol%) | EG/EtOH=1:1 | 120 | 36 |
| 4 | SiO2-H2SO4 (0.25 g) | EG/EtOH=1:1 | 120 | - |
| 5 | H2SO4 (20 mol%) | EG/EtOH=1:1 | 120 | - |
| 6 | K2CO3 (20 mol%) | EG/EtOH=1:1 | 120 | - |
| 7 | NaOH (20 mol%) | EG/EtOH=1:1 | 120 | - |
| 8 | Cs2CO3 (20 mol%) | EG/EtOH=1:1 | 120 | - |
| 9 | SSA (0.25 g) | EtOH | 110 | 20 |
| 10 | SSA (0.25 g) | PEG/EtOH = 1:1 | 120 | 45 |
| 11 | SSA (0.25 g) | Glycerol/EtOH = 1:1 | 120 | 32 |
| 12 | SSA (0.25 g) | DMF/EtOH = 1:1 | 120 | 24 |
| 13 | SSA (0.25 g) | EG/EtOH=1:2 | 120 | 21 |
| 14 | SSA (0.25 g) | EG/EtOH = 2:1 | 120 | 55 |
| 15 | SSA (0.25 g) | EG/EtOH = 3:1 | 120 | 68 |
| 16 | SSA (0.25 g) | EG/EtOH = 4:1 | 120 | 57 |
| 17 | SSA (0.25 g) | EG/EtOH = 3:1 | 100 | trace |
| 18 | SSA (0.25 g) | EG/EtOH = 3:1 | 110 | trace |
| 19 | SSA (0.25 g) | EG/EtOH = 3:1 | 130 | 78 |
| 20 | SSA (0.25 g) | EG/EtOH = 3:1 | 140 | 84 |
| 21 | SSA (0.25 g) | EG/EtOH = 3:1 | 150 | 76 |
a Reaction conditions: 4a (0.5 mmol), 2a (0.5 mmol), 5a (1.0 mL), 45 min; b GC yield of 7a determined using tridecane as internal standard.
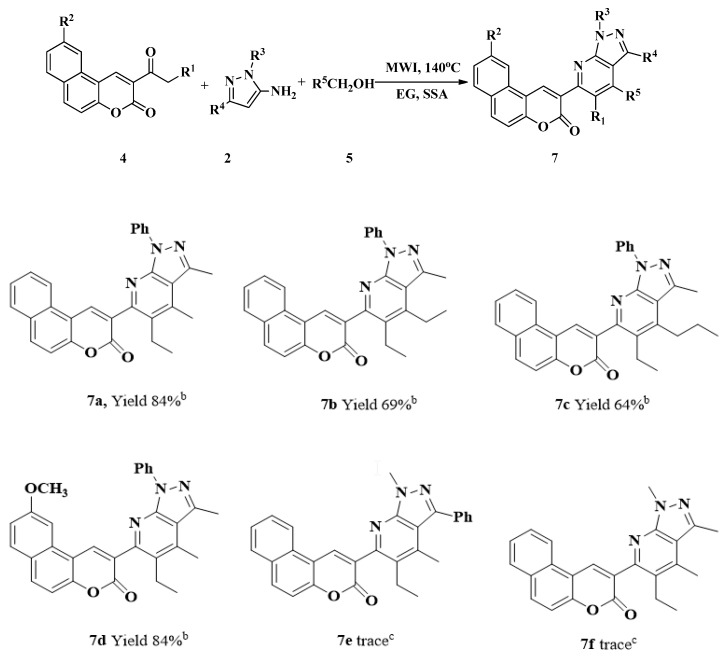
With optimal conditions in hand, the corresponding novel coumarin-fused pyrazolo[3,4-b]pyridine derivatives 7 were synthesized (Scheme 3).
Scheme 3.
Synthesis of coumarin-fused pyrazolo[3,4-b]pyridine derivatives 7 a. a Reaction conditions: arylbenzo[f]chromen-3-one 4 (0.5 mmol), enaminone 2 (0.5 mmol), alkyl alcohol 5 (1.0 mL), EG (3 mL) and SSA (0.25 g), 140 °C,45 min; b Isolated yield; c 2 h.
As illustrated in Scheme 3, the substrate scope of the transformation was examined using arylbenzo[f]chromen-3-one 4, enaminone 2, and alkyl alcohol 5 as staring materials. Notably, electronic effects had an important impact on this reaction. When the substituent R3 was electron-donating group, such as Me, the desired products could not be obtained at all (7e, 7f).
To further expand the scope of substrates, aryl alcohols (8) instead of alkyl alcohols (5) were also tested. It was found that aryl alcohols were well tolerated under the optimal reaction conditions, the corresponding products were afforded in moderate to good yields. When substituent R3 was electron-withdrawing groups (Ph), the yields were good and no more than 1 h cost (Table 2, entries 1–19). However, the substituents R3 was electron-donating groups (CH3) (Table 2, entries 20–22), the yields were lower and the reaction time was longer. Unfortunately, When R3 and R4 was electron rich group, such as Me, the reaction could not proceed successfully (Table 2, entry 23).
Table 2.
Synthesis of coumarin-fused pyrazolo[3,4-b]pyridine derivatives 9 a.

| Entry | Product | R1 | R2 | R3 | R4 | Ar | Time (h) | Isolated yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9a | CH2CH3 | H | Ph | CH3 | C6H5 | 1 | 69 |
| 2 | 9b | CH2CH3 | H | Ph | CH3 | 4-CH3C6H4 | 1 | 74 |
| 3 | 9c | CH2CH3 | H | Ph | CH3 | 4-OCH3C6H4 | 0.75 | 77 |
| 4 | 9d | CH2CH3 | H | Ph | CH3 | 3-OCH3C6H4 | 0.75 | 76 |
| 5 | 9e | CH2CH3 | H | Ph | CH3 | 4-BrC6H4 | 0.75 | 71 |
| 6 | 9f | CH2CH3 | H | Ph | CH3 | Pyridine-4-yl | 1 | 70 |
| 7 | 9g | CH2CH3 | H | Ph | CH3 | Furan-2-yl | 1 | 76 |
| 8 | 9h | CH2CH3 | OCH3 | Ph | CH3 | C6H5 | 1 | 62 |
| 9 | 9i | CH2CH3 | OCH3 | Ph | CH3 | 4-CH3C6H4 | 1 | 70 |
| 10 | 9j | CH2CH3 | OCH3 | Ph | CH3 | 4-OCH3C6H4 | 1 | 72 |
| 11 | 9k | CH3 | H | Ph | CH3 | C6H5 | 1 | 68 |
| 12 | 9l | CH3 | H | Ph | CH3 | 4-CH3C6H4 | 1.25 | 70 |
| 13 | 9m | CH3 | H | Ph | CH3 | 4-OCH3C6H4 | 1.25 | 74 |
| 14 | 9n | CH3 | H | Ph | CH3 | 3-OCH3C6H4 | 1.25 | 72 |
| 15 | 9o | CH3 | OCH3 | Ph | CH3 | 4-CH3C6H4 | 1.25 | 67 |
| 16 | 9p | CH3 | OCH3 | Ph | CH3 | 4-OCH3C6H4 | 1.25 | 70 |
| 17 | 9q | CH3 | OCH3 | Ph | CH3 | C6H5 | 1.25 | 60 |
| 18 | 9r | H | H | Ph | CH3 | C6H5 | 1.5 | 56 |
| 19 | 9s | H | H | Ph | CH3 | 4-OCH3C6H4 | 1.5 | 58 |
| 20 | 9t | CH2CH3 | H | CH3 | Ph | C6H5 | 2 | 55 |
| 21 | 9u | CH3 | OCH3 | CH3 | Ph | C6H5 | 2 | 50 |
| 22 | 9v | CH3 | OCH3 | CH3 | Ph | 4-OCH3C6H4 | 2 | 45 |
| 23 | 9w | CH2CH3 | H | CH3 | CH3 | 4-OCH3C6H4 | 2.5 | trace |
a Reaction conditions: arylbenzo[f]chromen-3-one 4 (0.5 mmol), enaminone 2 (0.5 mmol), aryl alcohols 8 (1.0 mL), EG (3 mL) and SSA (0.25 g), 140 °C.
To gain insight into the mechanism of this one-spot three-component reaction process, some additional experiments were performed. When benzaldehyde (10) was added to the reaction instead of phenylmethanol (8a) under standard conditions, 73% yield of desired product (9a) could be obtained, and reaction time reduced from 1 h to 15 min (Scheme 4A), and when butyraldehyde (11) was added to the reaction 50% yield of desired product (7c) could be obtained (Scheme 4B). The reaction did not proceed successfully without SSA catalyzed. Just phenylmethanol (8a) was heated to 140 °C, directly with the catalyst of SSA, benzaldehyde (10) and benzoic acid (12) could be detected by GC-MS (Scheme 4C). We speculated that the benzaldehyde was most likely the key intermediate in this protocol.
Scheme 4.
Preliminary mechanistic studies. (A) Synthesis of coumarin-fused pyrazolo[3,4-b]pyridine derivatives 9a. (B) Synthesis of coumarin-fused pyrazolo[3,4-b]pyridine derivatives 7c. (C) Reaction of phenylmethanol with the catalyst of SSA.
Herein, we propose the following mechanism for the reaction (Scheme 5). SSA catalyzed alkyl alcohol 5 to afford the corresponding aldehyde, then the intermediate A is formed by means of a Knoevenagel condensation of aldehyde and arylbenzo[f]chromen-3-one (4). The intermediate A is activated by SSA, which subsequently undergoes Michael addition with enaminone (2) via attack of the nucleophilic C-4 of the intermediate A to give intermediate B, which transformed to more-stable intermediate C. Then, intermediate C tautomerizes to intermediate D, which undergoes intramolecular nucleophilic addition to form intermediate E. In the last step, loss of H2O affords the desired product.
Scheme 5.
Proposed mechanism for this reaction.
3. Conclusions
In conclusion, we have developed a protocol for the facile synthesis of various potentially biologically active coumarin-fused pyrazolo[3,4-b]pyridine derivatives, based on a novel three-component domino reaction under microwave irradiation. Using this method, coumarin derivatives could be rapidly constructed in moderate-to-good yields with short reaction time. Further study to deeply understand the reaction mechanism is currently underway in our lab.
4. Experimental Section
4.1. General
All reagents were purchased from commercial suppliers (Aladdin, Shanghai, China) and used without further purification. Microwave irradiation was carried out with Initiator 2.5 Microwave Synthesizers from Biotage, Uppsala, Sweden. The reaction temperatures were measured by infrared detector during microwave heating. Melting points are uncorrected. IR spectra were recorded on a Tensor 27 spectrometer (Bruker Corp., Karlsruhe, Germany) in KBr with absorptions in cm−1. 1H-NMR (400 MHz) and 13C-NMR (75 MHz or 100 MHz) spectra were recorded on a Varian Inova-400 MHz or Varian Inova-300 MHz (Varian, CA, America) in CDCl3, DMSO-d6 or CF3COOD as solution. J values are in hertz. Chemical shifts are expressed in parts per million downfield from interal standard TMS. High-resolution mass spectra (HRMS) for all the compounds were determined on Bruker MicrOTOF-QII mass spectrometer (Bruker Corp., Karlsruhe, Germany) with ESI resource. X-ray diffraction analysis was recorded on a Smart-1000 diffractometer (PANalytical B.V., Holland).
4.2. General Procedure for the Synthesis of Products 4 Are Represented as Follows
Typically, 2-hydroxy-1-naphthaldehyde (5 mmol), ethyl 3-oxopentanoate or ethyl 3-oxohexanoate or ethyl acetoacetate (5 mmol) and piperidine (0.5 mmol) were introduced in a 20 mL vial with ethanol (10 mL) as solution. Subsequently, the reaction vial was closed and then prestirred for 10 s. The mixture was irradiated at 90 °C for 10 min. After the completion, the reaction mixture was then cooled to room temperature and concentrated in vacuo to remove the solvent. The residue was then washed with water, filtered, dried, and the precipitate was purified by recrystallization from 95% EtOH to give the products of 4. The analytical data for represent compounds are shown below. 1H-NMR and 13C-NMR spectra of compounds 4 in Supplementary Materials.
4.2.1. 2-Butyryl-3H-benzo[f]chromen-3-one (4a)
Yellow solid; yield 89%; m.p.: 127–129 °C; IR (KBr): ν 1734, 1626, 1557, 1513, 1383, 1109, 864 cm−1; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 9.21 (s, 1H, ArH), 8.91 (s, 1H, ArH), 8.06 (d, J = 8.8 Hz, 1H, ArH), 7.83 (d, J = 8.8 Hz, 1H, ArH), 7.57–7.56 (m, 1H, ArH), 7.20 (d, J = 9.2 Hz, 1H, ArH), 7.12 (dd, J1 = 8.8 Hz, J2 = 2.0 Hz, 1H, ArH), 3.00 (t, J = 7.2 Hz, 2H, CH2), 1.63–1.58 (m, 2H, CH2), 0.93 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 197.5, 159.1, 158.9, 156.4, 142.8, 136.6, 132.1, 131.5, 124.6, 121.9, 118.6, 113.0, 111.4, 104.8, 43.9, 17.3, 14.1;
4.2.2. 2-Butyryl-9-methoxy-3H-benzo[f]chromen-3-one (4b)
Yellow solid, yield 88%; m.p.: 125–128 °C; IR (KBr) ν: 1730, 1667, 1601, 1556, 1513, 1386, 1365, 1196, 948, 836 cm−1; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 9.01 (s, 1H, ArH), 7.86 (d, J = 8.8 Hz, 1H, ArH), 7.68 (d, J = 8.8 Hz, 1H, ArH), 7.40 (s, 1H, ArH), 7.14 (t, J = 8.4 Hz, 2H, ArH), 3.94 (s, 3H, CH3O), 3.12 (t, J = 8.4 Hz, 2H, CH2); 1.76–1.70 (m, 2H, CH2), 1.00 (t, J = 8.4 Hz, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 197.6, 159.9, 158.7, 156.0, 142.5, 135.3, 131.2, 130.2, 124.7, 121.1, 117.9, 113.1, 111.4, 100.7, 55.5, 44.0, 16.9, 13.3.
4.2.3. 2-Propionyl-3H-benzo[f]chromen-3-one (4c)
Yellow solid, yield 87%; m.p.: 134–136 °C; IR (KBr): ν 1732, 1662, 1601, 1556, 1524, 1387, 1365, 1196, 945, 823 cm−1; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 9.15 (s, 1H, ArH), 8.74 (s, 1H, ArH), 7.90 (d, J = 8.8 Hz, 1H, ArH), 7.66 (d, J = 8.8 Hz, 1H, ArH), 7.41–7.40 (m, 1H, ArH), 7.04 (d, J = 8.8 Hz, 1H, ArH), 6.95 (dd, J1 = 8.8 Hz, J2 = 2.0 Hz, 1H, ArH), 3.14–3.08 (m, 2H, CH2), 1.08 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 198.4, 159.9, 159.4, 156.7, 143.3, 135.9, 131.9, 130.8, 125.3, 121.8, 118.7, 113.7, 112.1, 102.0, 35.2, 10.7.
4.2.4. 9-Methoxy-2-propionyl-3H-benzo[f]chromen-3-one (4d)
Yellow solid, yield 87%; m.p.: 125–128 °C; IR (KBr): ν 1730, 1667, 1601, 1556, 1513, 1386, 1365, 1196, 948, 836 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 9.19 (s, 1H, ArH), 8.14 (d, J = 9.2 Hz, 1H, ArH), 7.90 (d, J = 9.2 Hz, 1H, ArH), 7.79 (s, 1H, ArH), 7.32 (t, J = 8.8 Hz, 1H, ArH), 7.21 (dd, J1 = 8.8 Hz, J2 = 2.0 Hz, 1H, ArH), 3.98 (s, 3H, CH3O), 3.10–3.05 (m, 2H, CH2), 1.09 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 198.7, 160.4, 158.8, 156.1, 143.0, 136.2, 131.9, 131.2, 125.4, 122.8, 118.6, 113.9, 112.1, 102.4, 56.2, 35.4, 8.4.
4.2.5. 2-Acetyl-3H-benzo[f]chromen-3-one (4e)
Yellow solid, yield 88%; m.p.: 189–190 °C [48]; IR (KBr): ν 2959, 1696, 1622, 1562, 1384, 1227, 1206, 857 cm−1.
4.3. General Procedure for the Synthesis of Products 7 and 9 Are Represented as Follows
Typically, benzo[f]chromen-3-one 4 (0.5 mmol), enaminone 2 (0.5 mmol), alkyl alcohol 5 (1.0 mL) or aryl alcohols 8 (1.0 mL) and SSA (0.25 g) were introduced in a 5 mL vial with ethylene glycol (3 mL) as solution. Subsequently, the reaction vial was closed and then prestirred for 10 s. The mixture was irradiated at 140 °C. The reaction was monitored by TLC. After the completion, the reaction mixture was then cooled to room temperature and diluted with cold water (30 mL), and extracted with CH2Cl2 (3 × 30 mL). The extracts were washed with water (3 × 50 mL) and dried over anhydrous Na2SO4. After evaporation of the solvent under reduced pressure, the precipitate was collected and purified by recrystallization from 95% EtOH or by flash column chromatography (petroleum ether:ethyl acetate = 8:1) to give the products 7 or 9. The analytical data for represent compounds are shown below. 1H-NMR and 13C-NMR spectra of compounds 7 and 9 in Supplementary Materials.
4.3.1. 2-(5-Ethyl-3,4-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (7a)
White solid, m.p.: 258–260 °C; IR (KBr, cm−1) ν: 2960, 1722, 1629, 1572, 1507, 1415, 1387, 1315, 1290, 1248, 1211, 1096, 989, 906, 815, 787, 713, 691, 605; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.07 (s, 1H, ArH), 8.58 (d, J = 8.0 Hz, 1H, ArH), 8.23 (t, J = 8.0 Hz, 3H, ArH), 8.08 (d, J = 8.0 Hz, 1H, ArH), 7.69–7.61 (m, 3H, ArH), 7.44 (t, J = 8.0 Hz, 2H, ArH), 7.20 (t, J = 7.2 Hz, 1H, ArH), 2.78–2.66 (m, 8H, 2 × CH3 + CH2), 1.05 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 156.0, 148.8, 146.2, 145.4, 140.5, 139.1, 135.6, 134.1, 132.6, 132.0, 131.2, 130.8, 130.2, 129.4, 128.5, 126.5, 122.3, 121.3, 116.7, 116.2, 22.7, 17.0, 14.3, 13.4; HRMS: m/z cacld. for C29H24N3O2 [M + H]+ 446.1869, Found 446.1853.
4.3.2. 2-(4,5-Diethyl-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (7b)
White solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2974, 1719, 1688, 1656, 1628, 1596, 1628, 1571, 1546, 1506, 1413, 1357, 1204, 1071, 909, 817, 752, 694, 676, 589; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.16 (s, 1H, ArH), 8.66 (d, J = 8.4 Hz, 1H, ArH), 8.29 (d, J = 9.2 Hz, 1H, ArH), 8.21 (d, J = 7.6 Hz, 2H, ArH), 8.11 (d, J = 8.0 Hz, 1H, ArH), 7.73–7.63 (m, 3H, ArH), 7.47 (t, J = 8.0 Hz, 2H, ArH), 7.23 (t, J = 7.6 Hz, 1H, ArH), 3.51–3.48 (m, 2H, CH2), 3.17–3.14 (m, 2H, CH2), 2.79 (s, 3H, CH3), 1.35 (t, J = 7.2 Hz, 3H, CH3), 1.09 (t, J = 7.6 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.2, 154.3, 153.8, 149.4, 148.1, 142.4, 139.6, 134.1, 130.5, 130.3, 129.6, 129.0, 128.3, 126.8, 125.7, 123.2, 120.5, 117.2, 115.9, 113.5, 100.0, 22.2, 21.6, 16.6, 16.2, 15.5; HRMS: m/z cacld. for C30H25N3O2 (M)+ 459.1947, Found 459.1946.
4.3.3. 2-(5-Ethyl-3-methyl-1-phenyl-4-propyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (7c)
White solid, m.p.: 242–245 °C; IR (KBr, cm−1) ν: 2974, 2880, 2703, 2545, 1789, 1722, 1665, 1573, 1503, 1439, 1414, 1389, 1359, 1320, 1288, 1248, 1217, 1155, 1091, 915, 858, 813, 792, 745, 695, 641, 610; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.14 (s, 1H, ArH), 8.63 (d, J = 8.8 Hz, 1H, ArH), 8.26 (d, J = 8.8 Hz, 1H, ArH), 8.22 (d, J = 8.0 Hz, 2H, ArH), 8.09 (d, J = 8.0 Hz, 1H, ArH), 7.70–7.63 (m, 3H, ArH), 7.46 (t, J = 7.6 Hz, 2H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 3.07–3.06 (m, 2H, CH2), 2.76–2.73 (m, 5H, CH3 + CH2), 1.72–1.69 (m, 2H, CH2), 1.13 (s, 3H, CH3), 1.07 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 165.7, 155.9, 148.1, 146.2, 145.9, 140.8, 139.1, 135.0, 134.1, 132.6, 131.9, 131.2, 130.7, 130.2, 129.3, 128.5, 126.5, 121.6, 121.3, 116.3, 33.4, 26.2, 22.2, 14.5, 13.7; HRMS: m/z cacld. for C31H28N3O2 [M + H]+ 474.2182, Found 474.2210.
4.3.4. 2-(5-Ethyl-3,4-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (7d)
White solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2975, 2026, 1795, 1728, 1628, 1574, 1509, 1230, 1091, 989, 917, 840, 794, 751, 686, 610; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.21 (s, 1H, ArH), 8.24–8.17 (m, 3H, ArH), 8.01–7.96 (m, 2H, ArH), 7.50–7.45 (m, 3H, ArH), 7.27–7.21 (m, 2H, ArH), 3.90 (s, 3H, OCH3), 2.80–2.79 (m, 8H, CH2 + 2 × CH3), 1.07 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.3, 160.1, 154.4, 154.0, 149.0, 143.1, 142.4, 140.0, 139.7, 133.8, 131.4, 131.0, 129.5, 127.5, 125.6, 125.5, 120.3, 118.7, 116.9, 114.3, 102.6, 56.3, 22.4, 16.1, 15.4, 15.0; HRMS: m/z cacld. for C30H26N3O3 [M + H]+ 476.1974, Found 476.1980.
4.3.5. 2-(5-Ethyl-3-methyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9a)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 3032, 2978, 2888, 2763, 1725, 1049, 958, 815, 756, 699, 679, 588; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.16 (s, 1H, ArH), 9.15–9.14 (m, 2H, ArH), 8.88–8.87 (m, 1H, ArH), 8.65–8.64 (m, 1H, ArH), 8.59–8.55 (m, 4H, ArH), 8.51–8.47 (m, 6H, ArH), 8.38–8.37 (m, 2H, ArH), 3.77–3.76 (m, 2H, CH2), 3.05 (s, 3H, CH3), 1.91 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 163.7, 156.1, 149.4, 146.8, 146.4, 140.8, 139.1, 135.8, 134.3, 132.8, 132.6, 132.0, 131.6, 131.2, 130.8, 130.2, 129.9, 129.5, 128.5, 127.8, 126.5, 121.9, 121.4, 116.8, 116.3, 113.6, 22.9, 14.3, 12.5; HRMS: m/z cacld. for C34H26N3O2 [M + H]+ 508.2025, Found 508.2025.
4.3.6. 2-(5-Ethyl-3-methyl-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9b)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2968, 1972, 1779, 1572, 1505, 1413, 1360, 1207, 1088, 961, 898, 806, 758, 728, 690, 642; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.13 (s, 1H, ArH), 9.15–9.09 (m, 2H, ArH), 8.87–8.84 (m, 1H, ArH), 8.63–8.60 (m, 1H, ArH), 8.54–8.40 (m, 9H, ArH), 8.25–8.24 (m, 2H, ArH), 3.76–3.74 (m, 2H, CH2), 3.37 (s, 3H, CH3), 3.06 (s, 3H, CH3), 1.88–1.87 (m, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.8, 155.1, 148.6, 145.6, 145.4, 142.0, 139.8, 138.2, 135.0, 133.3, 131.7, 131.1, 130.3, 129.9, 129.6, 129.3, 128.7, 128.5, 127.6, 126.9, 125.6, 121.0, 120.4, 115.9, 115.4, 112.7, 21.9, 19.5, 13.4, 11.7; HRMS: m/z cacld. for C35H28N3O2 [M + H]+ 522.2182, Found 522.2180.
4.3.7. 2-(5-Ethyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9c)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2967, 1711, 1597, 1571, 1505, 1412, 1286, 1249, 1211, 1048, 982, 897, 849, 806, 758, 690, 641, 587; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.29 (s, 1H, ArH), 8.28–8.23 (m, 2H, ArH), 7.99 (d, J = 8.4 Hz, 1H, ArH), 7.76 (t, J = 7.6 Hz, 1H, ArH), 7.69–7.56 (m, 7H, ArH), 7.50 (d, J = 8.4 Hz, 2H, ArH), 7.35 (d, J = 8.4 Hz, 2H, ArH), 4.06 (s, 3H, OCH3), 2.93–2.88 (m, 2H, CH2), 2.23 (s, 3H, CH3), 1.02 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.9, 160.9, 160.5, 155.2, 148.4, 145.8, 145.7, 140.0, 138.3, 135.3, 133.5, 131.8, 131.2, 130.4, 130.0, 129.4, 129.2, 128.7, 127.7, 125.8, 125.3, 121.3, 120.6, 116.0, 115.5, 114.9, 112.8, 55.1, 22.1, 13.4, 12.0; HRMS: m/z cacld. for C35H28N3O3 [M + H]+ 538.2131, Found 538.2111.
4.3.8. 2-(5-Ethyl-4-(3-methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9d)
Yellow solid, m.p.: >300 °C;.IR (KBr, cm−1) ν: 2965, 2023, 1785, 1712, 1573, 1504, 1382, 1357, 1285, 1158, 1136, 1046, 782, 759, 712, 689, 588; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.23 (s, 1H, ArH), 8.66 (d, J = 8.4 Hz, 1H, ArH), 8.29–8.23 (m, 3H, ArH), 8.10 (d, J = 8.0 Hz, 1H, ArH), 7.73–7.62 (m, 3H, ArH), 7.54–7.47 (m, 3H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.14–7.12 (m, 1H, ArH), 7.03–7.01 (m, 2H, ArH), 3.84 (s, 3H, OCH3), 2.58–2.56 (m, 2H, CH2), 1.96 (s, 3H, CH3), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.2, 159.6, 154.2, 153.9, 148.7, 145.3, 142.9, 140.1, 139.6, 137.3, 134.3, 130.5, 130.4, 130.1, 129.6, 129.5, 129.4, 129.0, 127.8, 126.8, 125.9, 123.1, 121.4, 120.6, 117.2, 115.8, 114.6, 113.5, 55.8, 22.5, 16.0, 14.2; HRMS: m/z cacld. for C35H27N3O3 (M)+ 537.2052, Found 537.2053.
4.3.9. 2-(4-(4-Bromophenyl)-5-ethyl-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9e)
Yellow solid, m.p.: >300 °C;.IR (KBr, cm−1) ν: 2968, 2032, 1775, 1721, 1574, 1385, 1357, 1285, 1166, 1047, 782, 759, 712, 681, 588; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.19 (s, 1H, ArH), 8.57–8.53 (m, 2H, ArH), 8.43 (d, J = 9.2 Hz, 1H, ArH), 8.06 (d, J = 8.0 Hz, 1H, ArH), 7.86 (t, J = 7.6 Hz, 1H, ArH), 7.78–7.23 (m, 10H, ArH), 2.79 (s, 2H, CH2), 2.54(s, 3H, CH3), 1.35 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 165.7, 159.3, 157.7, 152.3, 151.4, 144.7, 141.6, 138.9, 134.8, 132.3, 131.0, 129.7, 126.7, 125.2, 121.4, 118.1, 113.1, 111.4, 111.3, 109.6, 107.5, 21.9, 17.0, 14.6; HRMS: m/z cacld. for C34H24BrN3O2 (M)+ 585.1052, Found 585.1057.
4.3.10. 2-(5-Ethyl-3-methyl-1-phenyl-4-(pyridin-4-yl)-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9f):
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2965, 1972, 1783, 1573, 1505, 1413, 1362, 1089, 961, 898, 805, 758, 693, 642; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.09 (s, 1H, ArH), 9.11–9.05 (m, 2H, ArH), 8.80 (d, J = 8.0 Hz, 1H, ArH), 8.60–8.35 (m, 10H, ArH), 8.21–8.19 (m, 2H, ArH), 3.72–3.70 (m, 2H, CH2), 3.01 (s, 3H, CH3), 1.83 (t, J = 6.8 Hz, 3H, CH3);13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.8, 155.0, 148.5, 145.5, 145.4, 142.0, 139.7, 138.1, 135.0, 133.3, 131.6, 131.0, 130.2, 129.8, 129.5, 129.2, 128.7, 128.5, 127.5, 126.8, 125.5, 121.0, 120.4, 115.8, 115.3, 112.6, 21.8, 13.3, 11.6; HRMS: m/z cacld. for C33H25N4O2 [M + H]+ 509.1978, Found 509.1963.
4.3.11. 2-(5-Ethyl-4-(furan-2-yl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9g):
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2966, 1720, 1629, 1566, 1412, 1383, 1264, 1084, 959, 852, 797, 766, 724, 691, 640, 617; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.24 (s, 1H, ArH), 8.21 (d, J = 9.2 Hz, 1H, ArH), 7.95 (d, J = 8.8 Hz, 1H, ArH), 7.65–7.63 (m, 2H, ArH), 7.62–7.55 (m, 6H, ArH), 7.46 (d, J = 9.2 Hz, 1H, ArH), 7.39 (m, 3H, ArH), 2.93–2.87 (m, 2H, CH2), 2.21 (s, 3H, CH3), 1.03 (t, J = 7.6 Hz, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 163.0, 160.2, 156.0, 148.7, 145.7, 142.1, 140.0, 137.9, 135.2, 133.5, 131.9, 131.5, 130.7, 130.5, 129.8, 129.0, 127.1, 126.9, 125.8, 121.2, 118.0, 115.4, 113.7, 112.2, 22.1, 13.6, 11.8; HRMS: m/z cacld. for C32H24N3O3 [M + H]+ 498.1818, Found 498.1831.
4.3.12. 2-(5-Ethyl-3-methyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9h)
White solid, m.p.: 248–250 °C; IR (KBr, cm−1) ν: 2968, 1724, 1631, 1573, 1507, 1434, 1414, 1384, 1354, 1281, 1241, 1135, 1105, 980, 960, 905, 827, 789, 758, 705, 692, 636; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.32 (s, 1H, ArH), 8.24 (d, J = 8.0 Hz, 2H, ArH), 8.17 (d, J = 9.2 Hz, 1H, ArH), 8.00–7.97 (m, 2H, ArH), 7.61–7.57 (m, 3H, ArH), 7.51–7.46 (m, 5H, ArH), 7.27–7.24 (m, 2H, ArH), 3.91 (s, 3H, OCH3), 2.54–2.53 (m, 2H, CH2), 1.89 (s, 3H, CH3), 0.86 (t, J = 7.6 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.3, 160.2, 154.5, 154.4, 148.7, 145.5, 142.8, 140.6, 139.5, 135.9, 134.0, 131.5, 131.0, 130.5, 129.6, 129.1, 129.0, 128.9, 127.0, 125.9, 125.7, 120.6, 118.7, 115.8, 114.3, 112.8, 102.7, 56.3, 22.5, 15.8, 14.2; HRMS: m/z cacld. for C35H28N3O3 [M + H]+ 538.2131, Found 538.2122.
4.3.13. 2-(5-Ethyl-3-methyl-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9i)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2966, 1720, 1628, 1570, 1417, 1383, 1264, 1084, 959, 904, 832, 796, 761, 725, 691, 678, 640, 602; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.24 (s, 1H, ArH), 8.20 (d, J = 9.2 Hz, 1H, ArH), 7.95 (d, J = 8.8 Hz, 1H, ArH), 7.65–7.54 (m, 8H, ArH), 7.46 (d, J = 9.2 Hz, 1H, ArH), 7.39 (d, J = 7.6 Hz, 3H, ArH), 4.04 (s, 3H, OCH3), 2.92–2.87 (m, 2H, CH2), 2.52 (s, 3H, CH3), 2.20 (s, 3H, CH3), 1.02 (t, J = 7.6 Hz, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.9, 160.1, 155.9, 148.6, 145.6, 145.5, 142.0, 139.8, 137.8, 135.1, 133.4, 131.8, 131.4, 130.6, 130.4, 129.6, 128.9, 127.0, 126.8, 125.7, 121.1, 117.9, 115.3, 113.6, 112.1, 55.3, 22.0, 19.6, 13.5, 11.8; HRMS: m/z cacld. for C36H30N3O3 [M + H]+ 552.2287, Found 552.2246.
4.3.14. 2-(5-Ethyl-4-(4-methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9j)
White solid, m.p.: 256–258 °C; IR (KBr, cm−1) ν: 2965, 2145, 1735, 1717, 1629, 1572, 1463, 1381, 1286, 1227, 1077, 960, 887, 884, 805, 691, 604, 567; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.33 (s, 1H, ArH), 8.23 (d, J = 8.0 Hz, 2H, ArH), 8.19 (d, J = 8.8 Hz, 1H, ArH), 8.01–7.99 (m, 2H, ArH), 7.52–7.48 (m, 3H, ArH), 7.39–7.38 (m, 2H, ArH), 7.28–7.25 (m, 2H, ArH), 7.16 (d, J = 8.8 Hz, 2H, ArH), 3.92 (s, 3H, OCH3), 2.87 (s, 3H, OCH3), 2.56–2.55 (m, 2H, CH2), 1.95 (s, 3H, CH3), 0.87 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.3, 160.2, 159.7, 154.6, 154.4, 148.8, 145.5, 142.9, 140.6, 139.6, 134.0, 131.5, 131.0, 130.9, 130.4, 129.7, 127.8, 127.1, 125.9, 125.7, 120.6, 118.8, 116.2, 114.3, 112.8, 102.7, 56.4, 55.7, 22.5, 15.8, 14.5; HRMS: m/z cacld. for C36H30N3O4 [M + H]+ 568.2236, Found 568.2248.
4.3.15. 2-(3,5-Dimethyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9k)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2934, 2173, 1710, 1598, 1572, 1438, 1278, 965, 909, 820, 791, 692, 651, 633, 585; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.33 (s, 1H, ArH), 8.29–8.24 (m, 2H, ArH), 8.00–7.97 (m, 1H, ArH), 7.72–7.61 (m, 11H, ArH), 7.48–7.47 (m, 2H, ArH), 2.42 (s, 3H, CH3), 2.22 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 155.3, 148.3, 146.2, 145.8, 139.9, 138.5, 133.5, 132.4, 131.8, 131.1, 130.8, 130.4, 130.0, 129.4, 129.3, 129.0, 128.7, 127.7, 126.9, 125.7, 120.6, 120.4, 115.9, 115.5, 112.9, 14.5, 12.0; HRMS: m/z cacld. for C33H24N3O2 [M + H]+ 494.1869, Found 494.1887.
4.3.16. 2-(3,5-Dimethyl-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9l)
Yellow solid, m.p.: 286–290 °C; IR (KBr, cm−1) ν: 3078, 2187, 1719, 1626, 1606, 1575, 1507, 1447, 1380, 1212, 1093, 963, 813, 790, 741, 685; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.35 (s, 1H, ArH), 8.31 (d, J = 9.2 Hz, 1H, ArH), 8.27 (d, J = 7.6 Hz, 1H, ArH), 8.02 (d, J = 8.4 Hz, 1H, ArH), 7.79 (t, J = 7.2 Hz, 1H, ArH), 7.72–7.57 (m, 9H, ArH), 7.39 (d, J = 7.6 Hz, 2H, ArH), 2.55 (s, 3H, CH3), 2.46 (s, 3H, CH3), 2.29 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 154.3, 147.5, 145.2, 144.5, 141.3, 138.9, 137.5, 132.5, 130.8, 130.2, 129.4, 129.0, 128.8, 128.4, 128.3, 128.1, 127.7, 126.7, 126.0, 124.7, 119.6, 119.5, 114.9, 114.4, 18.6, 14.0, 10.9; HRMS: m/z cacld. for C34H26N3O2 [M + H]+ 508.2025, Found 508.2020.
4.3.17. 2-(4-(4-Methoxyphenyl)-3,5-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo [f]chromen-3-one (9m)
White solid, m.p.: 258–260 °C; IR (KBr, cm−1) ν: 2904, 2342, 1735, 1631, 1574, 1427, 1367, 1240, 1200, 1158, 1103, 1061, 849, 818, 759, 712, 668, 589; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.20 (s, 1H, ArH), 8.66 (d, J = 8.4 Hz, 1H, ArH), 8.28 (t, J = 7.6 Hz,3H, ArH), 8.11 (d, J = 8.0 Hz, 1H, ArH), 7.75–7.63 (m, 3H, ArH), 7.50 (t, J = 8.0 Hz, 2H, ArH), 7.37 (d, J = 8.4 Hz, 2H, ArH), 7.26 (t, J = 7.2 Hz, 1H, ArH), 7.16 (d, J = 8.4 Hz, 2H, ArH), 3.87 (s, 3H, OCH3), 2.13 (s, 3H, CH3), 2.02 (s, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 159.8, 159.6, 154.2, 154.0, 149.0, 145.3, 142.7, 140.3, 139.6, 134.3, 130.6, 130.5, 129.6 129.5, 128.1, 126.7, 125.8, 124.9, 123.1, 120.6, 117.1, 115.9, 114.4, 113.6, 55.7, 16.3, 14.7; HRMS: m/z cacld. for C34H26N3O3 [M + H]+ 524.1974, Found 524.1978.
4.3.18. 2-(4-(3-Methoxyphenyl)-3,5-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9n)
White solid, m.p.: 260–263 °C; IR (KBr, cm−1) ν: 2970, 2372, 1718, 1573, 1505, 1410, 1362, 1279, 1239, 1142, 1054, 1019, 988, 970, 877, 815, 786, 744, 714, 670, 586; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.19 (s, 1H, ArH), 8.66 (d, J = 8.4 Hz, 1H, ArH), 8.29–8.25 (m, 3H, ArH), 8.10 (d, J = 8.0 Hz, 1H, ArH), 7.75–7.63 (m, 3H, ArH), 7.52–7.48 (m, 3H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.13–7.11 (m, 1H, ArH), 7.00–6.98 (m, 2H, ArH), 3.84 (s, 3H, OCH3), 2.13 (s, 3H, CH3), 2.00 (s, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 159.7, 159.6, 154.3, 154.0, 148.9, 145.2, 142.7, 140.4, 139.6, 137.6, 134.3, 130.5, 130.3, 129.6, 129.5, 129.4, 129.0, 128.1, 126.8, 125.9, 124.5, 123.1, 121.3, 120.6, 117.1, 115.5, 114.7, 114.6, 113.6, 55.8, 16.2, 14.4; HRMS: m/z cacld. for C34H26N3O3 [M + H]+ 524.1974, Found 524.1978.
4.3.19. 2-(3,5-Dimethyl-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9o)
Yellow solid, m.p.: 288–290 °C; IR (KBr, cm−1) ν: 2929, 1718, 1631, 1600, 1346, 1239, 1204, 1173, 1149, 1125, 1019, 852, 827, 795, 749, 690, 643, 606; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.28 (s, 1H, ArH), 8.22 (d, J = 8.8 Hz, 1H, ArH), 7.96 (d, J = 8.8 Hz, 1H, ArH), 7.65–7.62 (m, 6H, ArH), 7.56–7.54 (m, 2H, ArH), 7.46 (d, J = 8.8 Hz, 1H, ArH), 7.41–7.35 (m, 3H, ArH), 4.04 (s, 3H, OCH3), 2.52 (s, 3H, CH3), 2.44 (s, 3H, CH3), 2.26 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 159.0, 158.9, 155.0, 147.4, 145.2, 144.4, 141.2, 138.8, 137.0, 132.4, 130.7, 130.4, 129.6, 129.4, 128.8, 128.2, 128.1, 126.0, 125.8, 124.6, 119.4, 116.6, 115.2, 114.1, 112.5, 54.2, 18.5, 14.0, 10.9; HRMS: m/z cacld. for C35H28N3O3 [M + H]+ 538.2131, Found 538.2130.
4.3.20. 9-Methoxy-2-(4-(4-methoxyphenyl)-3,5-dimethyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9p)
Yellow solid, m.p.: 287–289 °C; IR (KBr, cm−1) ν: 1716, 1630, 1611, 1571, 1513, 1464, 1385, 1246, 1107, 1033, 960, 832, 795, 754, 691; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 9.29 (s, 1H, ArH), 8.23 (d, J = 8.8 Hz, 1H, ArH), 7.56 (d, J = 8.8 Hz, 1H, ArH), 7.65–7.63 (m, 6H, ArH), 7.48–7.42 (m, 3H, ArH), 7.39–7.35 (m, 3H, ArH), 4.07–4.05 (m, 6H, 2 × OCH3), 2.46 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 159.6, 159.0, 155.0, 147.2, 145.1, 144.6, 138.9, 137.0, 132.4, 130.7, 130.4, 129.6, 129.3, 128.3, 128.1, 125.8, 124.6, 116.6, 114.0, 112.5, 54.3, 54.0, 14.0, 11.1; HRMS: m/z cacld. for C35H28N3O4 [M + H]+ 554.2080, Found 554.2093.
4.3.21. 2-(3,5-Dimethyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9q)
Yellow solid, m.p.: 252–254 °C; IR (KBr, cm−1) ν: 2961, 1725, 1629, 1582, 1557, 1435, 1397, 1335, 1290, 1250, 1219, 1196, 999, 906, 819, 797, 753, 695, 625; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 9.29 (s, 1H, ArH), 8.28–8.26 (m, 2H, ArH), 8.17 (d, J = 9.2 Hz, 1H, ArH), 8.01–7.98 (m, 2H, ArH), 7.62–7.57 (m, 3H, ArH), 7.52–7.43 (m, 5H, ArH), 7.28–7.25 (m, 2H, ArH), 3.92 (s, 3H, OCH3), 2.11 (s, 3H, CH3), 1.95 (s, 3H, CH3); 13C-NMR (75 MHz, DMSO-d6) δ (ppm): 160.2, 159.7, 154.6, 154.5, 148.9, 145.3, 142.6, 140.8, 139.6, 136.2, 134.0, 131.5, 131.1, 129.7, 129.2, 129.1, 127.4, 125.9, 125.7, 124.5, 120.7, 118.7, 115.5, 114.3, 112.9, 102.8, 56.3, 16.2, 14.5; HRMS: m/z cacld. for C34H26N3O3 [M + H]+ 524.1974, Found 524.1988.
4.3.22. 2-(3-Methyl-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9r)
Yellow solid, m.p.: 268–270 °C; IR (KBr, cm−1) ν: 2935, 2355, 1729, 1667, 1553, 1092, 891, 818, 746, 694, 657, 631, 585; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.22 (s, 1H, ArH), 8.61–8.57 (m, 2H, ArH), 8.46 (d, J = 9.2 Hz, 1H, ArH), 8.09 (d, J = 8.0 Hz, 1H, ArH), 7.90 (t, J = 7.6 Hz, 1H, ArH), 7.82–7.76 (m, 11H, ArH), 7.70 (d, J = 8.8 Hz, 1H, ArH), 2.58 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 163.5, 159.1, 155.0, 147.5, 145.0, 144.8, 140.3, 138.7, 132.9, 132.4, 131.1, 130.4, 130.3, 130.0, 129.8, 128.8, 128.3, 127.9, 127.4, 127.3, 122.6, 120.1, 114.3, 114.1, 113.5, 11.9; HRMS: m/z cacld. for C32H22N3O2 [M + H]+ 480.1712, Found 480.1726.
4.3.23. 2-(4-(4-Methoxyphenyl)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9s)
Yellow solid, m.p.: >300 °C; IR (KBr, cm−1) ν: 2988, 2355, 1987, 1730, 1512, 1089, 1066, 959, 810, 809, 788, 765, 689, 654, 633, 599; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 11.03 (s, 1H, ArH), 9.43 (d, J = 8.4 Hz, 1H, ArH), 9.34–9.30 (m, 2H, ArH), 8.94 (d, J = 8.0 Hz, 1H, ArH), 8.75 (t, J = 7.6 Hz, 1H, ArH), 7.66–7.63 (m, 8H, ArH), 8.54 (t, J = 9.2 Hz, 1H, ArH), 8.23 (d, J = 8.8 Hz, 2H, ArH), 4.95 (s, 3H, OCH3), 3.50 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 164.3, 159.2, 155.9, 148.2, 145.7, 145.3, 141.1, 139.8, 133.6, 131.3, 130.9, 130.6, 129.7, 128.8, 128.2, 126.6, 123.5, 120.9, 117.1, 115.1, 114.6, 55.1, 13.1; HRMS: m/z cacld. for C33H24N3O3 [M + H]+ 510.1818, Found 510.1835.
4.3.24. 2-(5-Ethyl-1-methyl-3,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9t)
Yellow solid, m.p.: 285–288 °C; IR (KBr, cm−1) ν: 2396, 1732, 1574, 1353, 1099, 1515, 1088, 1076, 959, 810, 803, 704; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.26 (s, 1H, ArH), 9.25 (d, J = 8.8 Hz, 2H, ArH), 8.95 (d, J = 8.0 Hz, 1H, ArH), 8.73 (t, J = 7.6 Hz, 1H, ArH), 8.63 (t, J = 7.6 Hz, 1H, ArH), 8.46 (d, J = 7.6 Hz, 1H, ArH), 8.26 (t, J = 7.6 Hz, 1H, ArH), 8.21–8.14 (m, 3H, ArH), 8.09–8.02 (m, 4H, ArH), 7.94 (d, J = 7.6 Hz, 2H, ArH), 5.31 (s, 3H, CH3), 3.87–3.85 (m, 2H, CH2), 1.84 (t, J = 7.2 Hz, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.9, 155.3, 150.3, 146.0, 145.4, 140.3, 138.2, 134.3, 131.2, 131.1, 130.2, 129.9, 129.5, 129.3, 128.5, 128.3, 127.9, 127.8, 127.6, 127.1, 120.5, 119.3, 116.3, 115.5, 34.9, 21.8, 13.1; HRMS: m/z cacld. for C34H26N3O2 [M + H]+ 508.2025, Found 508.2027.
4.3.25. 2-(1,5-Dimethyl-3,4-diphenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-9-methoxy-3H-benzo[f]chromen-3-one (9u)
Yellow solid, m.p.: 260–262 °C; IR (KBr, cm−1) ν: 2697, 2551, 1783, 1708, 1628, 1567, 1511, 1469, 1441, 1387, 1330, 1218, 1149, 1017, 976, 898, 845, 796, 756, 725, 702, 601; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.10 (s, 1H, ArH), 9.03 (d, J = 9.2 Hz, 1H, ArH), 8.76 (d, J = 8.8 Hz, 1H, ArH), 8.66–8.65 (m, 1H, ArH), 8.28 (d, J = 8.8 Hz, 1H, ArH), 8.20 (d, J = 9.2 Hz, 1H, ArH), 8.12 (d, J = 7.6 Hz, 1H, ArH), 8.05–7.99 (m, 3H, ArH), 7.91–7.87 (m, 4H, ArH), 7.81 (d, J = 7.6 Hz, 2H, ArH), 5.18 (s, 3H, OCH3), 4.85 (s, 3H, CH3), 3.21 (s, 3H, CH3). 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.3, 160.0, 156.0, 150.1, 146.1, 145.8, 140.3, 138.0, 131.5, 131.4, 130.6, 130.3, 129.5, 128.5, 128.3, 128.2, 128.0, 127.9, 127.2, 126.7, 117.5, 113.5, 112.2, 112.1, 103.0, 55.2, 34.8, 15.1. HRMS: m/z cacld. for C34H26N3O3 [M + H]+ 554.2080, Found 554.2093.
4.3.26. 9-Methoxy-2-(4-(4-methoxyphenyl)-1,5-dimethyl-3-phenyl-1H-pyrazolo[3,4-b]pyridin-6-yl)-3H-benzo[f]chromen-3-one (9v)
Yellow solid, m.p.: 240–244 °C; IR (KBr, cm−1) ν: 2932, 1720, 1624, 1608, 1564, 1512, 1463, 1383, 1353, 1289, 1249, 1208, 1173, 1025, 970, 902, 836, 801, 698, 664, 607; 1H-NMR (400 MHz, CF3COOD) δ (ppm): 10.15 (s, 1H, ArH), 9.09 (d, J = 9.2 Hz, 1H, ArH), 8.82 (d, J = 9.2 Hz, 1H, ArH), 8.72–8.71 (m, 1H, ArH), 8.34 (d, J = 9.2 Hz, 1H, ArH), 8.26 (d, J = 9.2 Hz, 1H, ArH), 8.17–8.13 (m, 1H, ArH), 8.02–7.95 (m, 4H, ArH), 7.89–7.87 (m, 2H, ArH), 7.69–7.66 (m, 2H, ArH), 5.23 (s, 3H, OCH3), 4.90 (s, 3H, OCH3), 4.72 (s, 3H, CH3), 3.31 (s, 3H, CH3); 13C-NMR (75 MHz, CF3COOD) δ (ppm): 162.4, 160.3, 156.1, 150.0, 146.0, 145.9, 140.3, 138.1, 131.5, 130.4, 129.4, 128.7, 128.1, 128.0, 127.6, 126.8, 125.1, 117.5, 114.2, 113.5, 55.2, 55.1, 34.9, 15.1; HRMS: m/z cacld. for C35H28N3O4 [M + H]+ 524.1974, Found 524.1972.
Supplementary Materials
The following are available online, Crystal date of compound 7a [47], 1H NMR and 13C NMR Spectra of all compounds and GC-MS spectra of Scheme 4B.
Author Contributions
W.L. conceived the synthetic route, supervised the project and wrote the paper; J.Z. and J.W. designed the experiments; C.Z. and X.H. performed all synthetic work in the laboratory.
Funding
This research was supported financially by the Natural Science Foundation of China (no. 21502074), Qing Lan Project of Jiangsu Province, Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX18_0985 and SJCX19_0766) and CAMS lnitiative for Innovative Medicine (2016-I2M-3-014).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4 and 7 are available from the authors.
References and Note
- 1.Revankar H.M., Bukhari S.N.A., Kumar G.B., Qin H.L. Coumarins scaffolds as COX inhibitors. Bioorg. Chem. 2017;71:146–159. doi: 10.1016/j.bioorg.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Dandriyal J., Singla R., Kumar M., Jaitak V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016;119:141–168. doi: 10.1016/j.ejmech.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 3.Ibrar A., Shehzadi S.A., Saeed F., Khan I. Developing hybrid molecule therapeutics for diverse enzyme inhibitory action: Active role of coumarin-based structural leads in drug discovery. Bioorg. Med. Chem. 2018;26:3731–3762. doi: 10.1016/j.bmc.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Singla S., Piplani P. Coumarin derivatives as potential inhibitors of acetylcholinesterase: Synthesis, molecular docking and biological studies. Bioorg. Med. Chem. 2016;24:4587–4599. doi: 10.1016/j.bmc.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 5.Hamulakova S., Janovec L., Soukup O., Jun D., Kuca K. Synthesis, in vitro acetylcholinesterase inhibitory activity and molecular docking of new acridine-coumarin hybrids. Int. J. Biol. Macromol. 2017;104:333–338. doi: 10.1016/j.ijbiomac.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Lan J.S., Ding Y., Liu Y., Kang P., Hou J.W., Zhang X.Y., Xie S.S., Zhang T. Design, synthesis and biological evaluation of novel coumarin-N-benzyl pyridinium hybrids as multi-target agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;139:48–59. doi: 10.1016/j.ejmech.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 7.Emami S., Dadashpour S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015;102:611–630. doi: 10.1016/j.ejmech.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Kaur M., Kohli S., Sandhu S., Bansal Y., Bansal G. Coumarin: A promising scaffold for anticancer agents. Anti Cancer Agents Med. Chem. 2015;15:1032–1048. doi: 10.2174/1871520615666150101125503. [DOI] [PubMed] [Google Scholar]
- 9.Klenkar J., Molnar M. Natural and synthetic coumarins as potential anticancer agents. J. Chem. Pharm. Res. 2015;7:1223–1238. [Google Scholar]
- 10.Poole S.K., Poole C.F. Thin-layer chromatographic method for the determination of the principal polar aromatic flavour compounds of the cinnamons of commerce. Analyst. 1994;119:113–120. doi: 10.1039/an9941900113. [DOI] [Google Scholar]
- 11.Riveiro M.E., De Kimpe N., Moglioni A., Vazquez R., Monczor F., Shayo C., Davio C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010;17:1325–1338. doi: 10.2174/092986710790936284. [DOI] [PubMed] [Google Scholar]
- 12.Patil A.D., Freyer A.J., Eggleston D.S., Haltiwanger R.C., Bean M.F., Taylor P.B., Caranfa M.J., Breen A.L., Bartus H.R. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the malaysian tree, calophyllum inophyllum linn. J. Med. Chem. 1993;36:4131–4138. doi: 10.1021/jm00078a001. [DOI] [PubMed] [Google Scholar]
- 13.Spino C., Dodier M., Sotheeswaran S. Anti-HIV coumarins from calophyllum seed oil. Bioorg. Med. Chem. Lett. 1998;8:3475–3478. doi: 10.1016/S0960-894X(98)00628-3. [DOI] [PubMed] [Google Scholar]
- 14.Kostova I., Mojzis J. Biologically active coumarins as inhibitors of HIV-1. Future HIV Ther. 2007;1:315–329. doi: 10.2217/17469600.1.3.315. [DOI] [Google Scholar]
- 15.Shin E., Choi K.M., Yoo H.S., Lee C.K., Hwang B.Y., Lee M.K. Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol. Pharm. Bull. 2010;33:1610–1614. doi: 10.1248/bpb.33.1610. [DOI] [PubMed] [Google Scholar]
- 16.Keri R.S., Sasidhar B.S., Nagaraja B.M., Santos M.A. Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. Eur. J. Med. Chem. 2015;100:257–269. doi: 10.1016/j.ejmech.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Piller N.B. A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema. Br. J. Exp. Pathol. 1975;56:554–560. [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal Y., Sethi P., Bansal G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013;22:3049–3060. doi: 10.1007/s00044-012-0321-6. [DOI] [Google Scholar]
- 19.Whang W.K., Park H.S., Ham I., Oh M., Namkoong H., Kim H.K., Hwang D.W., Hur S.Y., Kim T.E., Park Y.G. Natural compounds, fraxin and chemicals structurally related to fraxin protect cells from oxidative stress. Exp. Mol. Med. 2005;37:436–446. doi: 10.1038/emm.2005.54. [DOI] [PubMed] [Google Scholar]
- 20.Rosselli S., Maggio A.M., Faraone N., Spadaro V., Morris-Natschke S.L., Bastow K.F., Lee K.H., Bruno M. The cytotoxic properties of natural coumarins isolated from roots of ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 2009;4:1701–1706. doi: 10.1177/1934578X0900401219. [DOI] [PubMed] [Google Scholar]
- 21.Crichton E.G., Waterman P.G. Dihydromammea C/OB: A new coumarin from the seed of mammea Africana. Phytochemistry. 1978;17:1783–1786. doi: 10.1016/S0031-9422(00)88695-1. [DOI] [Google Scholar]
- 22.Baek N.I., Ahn E.M., Kim H.Y., Park Y.D. Furanocoumarins from the root of Angelica dahurica. Arch. Pharm. Res. 2000;23:467–470. doi: 10.1007/BF02976574. [DOI] [PubMed] [Google Scholar]
- 23.Teng M.C., Lin H., Ko F.N., Wu T.S., Huang T.F. The relaxant action of osthole isolated from Angelica pubescens in guinea-pig trachea. Naunyn Schmiedeberg’s. Arch. Pharmacol. 1994;349:202–208. doi: 10.1007/BF00169838. [DOI] [PubMed] [Google Scholar]
- 24.Fort D., Rao K., Jolad S., Luo J., Carlson T., King S. Antihyperglycemic activity of teramnus labialis (fabaceae) Phytomedicine. 2000;6:465–467. doi: 10.1016/S0944-7113(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y.H., Xu J.R., Wang Q., Li M. Coupling coumarin to gold nanoparticles by DNA chains for sensitive detection of DNase I. Anal. Biochem. 2018;555:50–54. doi: 10.1016/j.ab.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Chesterman J.P., Hughes T.C., Amsden B.G. Reversibly photo-crosslinkable aliphatic polycarbonates functionalized with coumarin. Eur. Polym. J. 2018;105:186–193. doi: 10.1016/j.eurpolymj.2018.05.038. [DOI] [Google Scholar]
- 27.Sarkar N., Datta A., Das S., Bhattacharyya K. Solvation dynamics of coumarin 480 in micelles. J. Phys. Chem. 1996;100:15483–15486. doi: 10.1021/jp960630g. [DOI] [Google Scholar]
- 28.Arzhantsev S., Ito N., Heitz M., Maroncelli M. Solvation dynamics of coumarin 153 in several classes of ionic liquids: Cation dependence of the ultrafast component. Chem. Phys. Lett. 2003;381:278–286. doi: 10.1016/j.cplett.2003.09.131. [DOI] [Google Scholar]
- 29.Lang B., Angulo G., Vauthey E. Ultrafast solvation dynamics of coumarin 153 in imidazolium-based ionic liquids. J. Phys. Chem. A. 2006;110:7028–7034. doi: 10.1021/jp057482r. [DOI] [PubMed] [Google Scholar]
- 30.Birau M.M., Wang Z.Y. A dual-mode molecular switch based on a chiral binaphthol-coumarin compound. Tetrahedron Lett. 2000;41:4025–4028. doi: 10.1016/S0040-4039(00)00576-1. [DOI] [Google Scholar]
- 31.Deng G.W., Xu H.J., Kuang L., He C.C., Li B.K., Yang M., Zhang X.L., Li Z.H., Liu J.L. Novel nonlinear optical chromophores based on coumarin: Synthesis and properties studies. Opt. Mater. 2019;88:218–222. doi: 10.1016/j.optmat.2018.11.035. [DOI] [Google Scholar]
- 32.Goda F.E., Abdel-Azizb A.A.M., Attef O.A. Synthesis, antimicrobial activity and conformational analysis of novel substituted pyridines: BF3-promoted reaction of hydrazine with 2-alkoxy pyridines. Bioorg. Med. Chem. 2004;12:1845–1852. doi: 10.1016/j.bmc.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Foks H., Pancechowska-Ksepko D., Kędzia A., Zwolska Z., Janowiec M., Augustinowicz-Kopeć E. Synthesis and antibacterial activity of 1H-pyrazolo[3,4-b] pyrazine and-pyridine derivatives. Farmaco. 2005;60:513–517. doi: 10.1016/j.farmac.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Bharate S.B., Mahajan T.R., Gole Y.R., Nambiar M., Matan T.T., Kulkarni-Almeida A., Balachandran S., Junjappa H., Balakrishnan A., Vishwakarma R.A. Synthesis and evaluation of pyrazolo[3,4-b] pyridines and its structural analogues as TNF-α and IL-6 inhibitors. Bioorg. Med. Chem. 2008;16:7167–7176. doi: 10.1016/j.bmc.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 35.De Mello H., Echevarria A., Bernardino A.M., CantoCavalheiro M., Leon L.L. Antileishmanial pyrazolopyridine derivatives: Synthesis and structure-activty relationship analysis. J. Med. Chem. 2004;47:5427–5432. doi: 10.1021/jm0401006. [DOI] [PubMed] [Google Scholar]
- 36.Misra R.N., Rawlins D.B., Xiao H., Shan W., Bursuker I., Kellar K.A., Mulheron J.G., Sack J.S., Tokarski J.S., Kimball S.D., et al. 1H-Pyrazolo[3,4-b] pyridine inhibitors of cyclin-dependent kinases: Highly potent 2,6-difluorophenacyl analogues. Bioorg. Med. Chem. Lett. 2003;13:2405–2408. doi: 10.1016/S0960-894X(03)00381-0. [DOI] [PubMed] [Google Scholar]
- 37.Lin R., Connolly P.J., Lu Y., Chiu G., Li S., Yu Y., Huang S., Li X., Emanuel S.L., Middleton S.A., et al. Synthesis and evaluation of pyrazolo[3,4-b] pyridine CDK1 inhibitors as anti-tumor agents. Bioorg. Med. Chem. Lett. 2007;17:4297–4302. doi: 10.1016/j.bmcl.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 38.Parker W.B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 2009;109:2880–2893. doi: 10.1021/cr900028p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miliutina M., Janke J., Hassan S., Zaib S., Iqbal J., Lecka J., Sévigny J., Villinger A., Friedrich A., Lochbrunner S., et al. A domino reaction of 3-chlorochromones with aminoheterocycles. Synthesis of pyrazolopyridines and benzofuropyridines and their optical and ecto-5′-nucleotidase inhibitory effects. Org. Biomol. Chem. 2018;16:717–732. doi: 10.1039/C7OB02729J. [DOI] [PubMed] [Google Scholar]
- 40.Lin W., Hu X.X., Song S., Cai Q., Wang Y., Shi D.Q. Microwave-assisted synthesis of novel hetero[5]helicene-like molecules and coumarin derivatives. Org. Biomol. Chem. 2017;15:7909–7916. doi: 10.1039/C7OB01742A. [DOI] [PubMed] [Google Scholar]
- 41.Wang H.Y., Liu X.C., Feng X., Huang Z.B., Shi D.Q. GAP chemistry for pyrrolyl coumarin derivatives: A highly efficient one-pot synthesis under catalyst-free conditions. Green Chem. 2013;15:3307–3311. doi: 10.1039/c3gc41799a. [DOI] [Google Scholar]
- 42.Wang H.Y., Shi D.Q. Efficient synthesis of functionalized dihydro-1H-indol-4(5H)-ones via one-pot three-component reaction under catalyst-free conditions. ACS Comb. Sci. 2013;15:261–266. doi: 10.1021/co4000198. [DOI] [PubMed] [Google Scholar]
- 43.Liu X.C., Lin W., Wang H.Y., Huang Z.B., Shi D.Q. Improved and efficient synthesis of chromeno[4,3-d]pyrazolo[3,4-b]pyridine-6(3H)-ones and their fluorescence properties. J. Heterocycl. Chem. 2014;51:1036–1044. doi: 10.1002/jhet.2104. [DOI] [Google Scholar]
- 44.Wang J.X., Lin W., Liu H.T., Hu M.H., Feng X., Ren J.F., Huang Z.B., Shi D.Q. An efficient synthesis of coumarino[4,3-d]pyrazolo[3,4-b]-pyridine derivatives catalyzed by silica sulfuric acid under microwave irradiation. Chin. J. Org. Chem. 2015;35:927–933. doi: 10.6023/cjoc201409020. [DOI] [Google Scholar]
- 45.Lin W., Cai Q., Zheng C.Z., Zheng Y.X., Shi D.Q. Synthesis of functionalized coumarino[4,3-d]pyrazolo[3,4-b]pyridine derivatives and their selective recognition for Zn2+ Chin. J. Org. Chem. 2017;37:2392–2398. doi: 10.6023/cjoc201702032. [DOI] [Google Scholar]
- 46.Lin W., Zheng Y.X., Wang Y.Z., Shi D.Q. An efficient synthesis of functionalized chromeno[4,3-d]pyrazolo[3,4-b]pyridine derivatives. Heterocycles. 2016;92:2235–2243. [Google Scholar]
- 47.Crystallographic Data for 7a Have been Deposited at the Cambridge Crystallographic Data Centre (CCDC 1881623) [(accessed on 30 July 2019)]; Available online: www.ccdc.cam.ac.uk/conts/retrieving.html.
- 48.Bogdal D. Coumarins: Fast synthesis by knoevenagel condensation under microwave irradiation. J. Chem. Res. Synop. 1998;12:468–469. doi: 10.1039/a801724g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.