Abstract
Heretofore unpublished enthalpy data which were used in the derivation of smooth enthalpy and heat-capacity data for NBS SRM 720 (α-Al2O3, heal eapacily and enthalpy standard) are presented along with some details of the high-temperature experiments. Recent NBS low-temperature measurements on SRM 720 are smoothed by a least-squares spline technique and a revised table of certified values for enthalpy and heal capacity of α-Al2O3 from 10 K to near the melting point (2250 K) is presented.
Keywords: Aluminum oxide, corundum, drop calorimetry, enthalpy, heat capacity, high temperature, Standard Reference Material, synthetic sapphire
1. Introduction
Standard Reference Material 720 (α-Al2O3) has been offered by the NBS Office of Standard Reference Materials since 1970 [1]1 as a heat-capacity and enthalpy standard certified in the temperature range 273.15 K to 2250 K. The relative enthalpy data, whose smoothed representation appears in [1], were obtained in two different types of drop calorimeter: a Bunsen ice calorimeter was used from 273.15 K to 1173.15 K. [2] and an adiabatic receiving calorimeter from 1173.15 K to 2250 K. The smoothed relative enthalpy values of [1] rely as well for the absolute ice-point enthalpy (H273.15 K — HO K) upon a much earlier low-temperature heat-capacity study [3] on the “Calorimetry Conference Sample” of pure α-Al2O3.
A detailed presentation of the original ice-calorimeter enthalpy data along with a description of the smoothing procedure used to obtain the enthalpy and heat-capacity values appearing in [1] has been given in [2]. Unfortunately, the original receiving-calorimeter enthalpy data were never published due to the death of one of the principal experimenters and subsequent personnel changes.
The present work presents this enthalpy data and describes certain aspects of the experiments in the receiving calorimeter. In addition, it documents a re-smoothing of the NBS low-temperature heat-capacity data [4] on SRM 720 obtained since 1970. New smooth heat-capacity data for SRM 720 in the temperature interval 10 K to 2250 K are presented. The present smooth enthalpy data above 273.15 K differ by less than 0.01% from the corresponding valnes given in [1].
2. High-Temperature Enthalpy Data, 1173 K to 2250 K
Enthalpy data above 1173.15 K were obtained by S. Ishihara in a high-temperature adiabatic receiving calorimeter. Some physical and operational details of this apparatus have been given in [5] and [6]. These experiments were carried out with the calorimeter and furnace containing purified argon gas at about 0.1 atm. pressure. The single-crystal segments of α-Al2O3 were contained in a molybdenum capsule (mass: 9.26727 g) with a close-fitting, though not hermetically-sealed, lid. The capsule was suspended from a doubled and twisted loop of 8-mil tungsten wire by a small tantalum hook (hook mass: 0.56097 g). Thirteen evenly-spaced temperatures from 1173 K to 2250 K were chosen, and one day’s experiments consisted of four enthalpy measurements at a single one of these temperatures. The temperature for any particular day was chosen randomly with the aid of a table of random numbers. The first and last enthalpy measurements of a day were made on the same system (either the empty capsule or the same capsule filled with α-Al2O3). Experience has shown that this method of scheduling one day’s experiments makes it possible to take into account small changes in the pyrometer characteristics or sample capsule mass changes due either to interaction with the sample or with the carbon atmosphere created by the induction furnace susceptor.
Results of the high-temperature measurements are given in table 1. The initial sample capsule temperatures are given in column 1. These were measured with an automatic optical pyrometer which was focused on the bottom of the sample capsule through a small aperture in the furnace susceptor. A separate output signal from the pyrometer was used to control the furnace temperature. Column 3 gives the measured heat to the calorimeter at 298.15 K. The aetual terminal temperature of the ealorimeter and capsule in the equilibrating period after an experiment was usually less than 320 K. In order to reference all heat data to 298.15 K, it was necessary to add to each measured heat a correction equal to the enthalpy of the capsule (plus sample and carbon contamination, if present) at the terminal temperature of the calorimeter relative to 298. ? 5 K. These corrections ranged from one to two percent of the measured heat. The enthalpy data necessary to calculate the corrections for carbon, tantalum, aluminum oxide, and molybdenum, were taken from references [7], [8], [9] and [2], respectively. The heat content of the aluminum oxide constituted about 55 percent of the total measured heat at all temperatures. The differing values for specimen mass (column 4) indicate that in some experiments different amounts of α-Al2O3 were used, though the difference can correspond to at most one or two small pieces of specimen. In the correction of specimen mass data for atmospheric buoyancy, a density of 3.97 g cm−3 for α-Al2O3 was used. The molar enthalpy values in column 5 were obtained from net heat values (F-C differences from column 3) by division by the applieable specimen mass (column 4).
Table 1.
Heat-Content Data for α-Al2O3(s), SRM 720.
| Furnace Temperature T68 | Date (1969) | Heat to Calorimeter at 298.15 K | Speeimen Mass | HT – H298.15 | Deviation from eq. (3) of [2] | |
|---|---|---|---|---|---|---|
| K | J | molb | J mol−1 | J mol−1 | % | |
| F 5386.39a | 0.0295095 | 99871.6 | + 24.06 | +0.024 | ||
| 1173.18 | 24 Mar. | C 2439.23 | ||||
| C 2439.35 | ||||||
| F 5384.10 | 0.0295094 | 99794.3 | − 53.24 | −0.053 | ||
| F 5681.19 | 0.0303910 | 103184.8 | − 0.57 | −0.001 | ||
| 1199.25 | 7 Apr. | C 2545.30 | ||||
| C 2545.25 | ||||||
| F 5680.87 | 0.0303914 | 103174.6 | − 10.77 | −0.010 | ||
| C 2855.86 | ||||||
| 1299.16 | 4 Apr. | F 6474.38 | 0.0311633 | 116114.8 | + 41.71 | +0.036 |
| F 6474.87 | 0.0311633 | 116156.8 | + 83.71 | +0.072 | ||
| C 2855.04 | ||||||
| C 3060.22 | ||||||
| 1401.65 | 10 Mar. | F 6474.38 | 0.0317601 | 129563.7 | +123.18 | +0.095 |
| F 7175.51 | 0.0317601 | 129553.7 | +113.38 | +0.088 | ||
| C 3060.87 | ||||||
| C 3467.73 | ||||||
| 1501.15 | 19 Mar. | F 7914.37 | 0.0311937 | 142549.3 | + 3.60 | +0.003 |
| F 7913.75 | 0.0311937 | 142612.1 | + 66.40 | +0.047 | ||
| C 3565.15 | ||||||
| F 8441.51 | 0.0303913 | 156264.1 | − 66.65 | −0.043 | ||
| 1604.90 | 6 Mar. | C 3692.44 | ||||
| C 3691.51 | ||||||
| F 8353.12 | 0.0298344 | 156254.7 | − 76.05 | −0.049 | ||
| C 4002.05 | ||||||
| 1702.22 | 4 Mar. | F 9003.15 | 0.0295487 | 169249.4 | −110.34 | −0.065 |
| F 9003.65 | 0.0295487 | 169251.4 | −108.34 | −0.064 | ||
| C 4002.49 | ||||||
| C 4461.57 | ||||||
| 1799.86 | 27 Mar. | F10147.78 | 0.0311923 | 182307.8 | −210.67 | −0.115 |
| F10147.78 | 0.0311923 | 182307.8 | −207.47 | −0.114 | ||
| F 4461.18 | ||||||
| F10946.89 | 0.0311712 | 196456.3 | + 12.42 | +0.006 | ||
| 1902.65 | 17 Mar. | C 4823.11 | ||||
| C 4823.88 | ||||||
| F10947.56 | 0.0311671 | 196479.3 | + 35.42 | +0.018 | ||
| F11264.44 | 0.0295326 | 210507.7 | +242.33 | +0.115 | ||
| 2004.13 | 12 Mar. | C 5047.60 | ||||
| C 5047.53 | ||||||
| F11264.44 | 0.0295218 | 210509.9 | +244.53 | +0.116 | ||
| C 5125.11 | ||||||
| 2101.61 | 21 Apr. | Fl 1697.26 | 0.0293640 | 223816.6 | +214.15 | +0.096 |
| F11692.47 | 0.0293223 | 223971.5 | +369.05 | +0.165 | ||
| C 5125.11 | ||||||
| C | ||||||
| 2203.28 | 16 Apr. | Fc | 237748.5 | +173.77 | +0.073 | |
| F | 237625.0 | + 50.27 | +0.021 | |||
| C | ||||||
| F13886.37 | 0.0315029 | 244676.5 | −322.90 | −0.132 | ||
| 2257.11 | 14 Mar. | C 6178.35 | ||||
| C 6177.04 | ||||||
| F13855.68 | 0.0313787 | 244708.7 | −290.70 | −0.119 | ||
“C” prefixes data for empty capsule.
“F” prefixes data for capsule and sample.
Molecular Weight = 101.9613.
Original heat and mass data are available but it was not possible to trace the corrections applied to yield the molar values (col. 5), which were used in evaluation of the smoothing function (eq. (3) of [2]).
The present, high-temperature enthalpy results (table 1) and those from [2] in the range 273.15 K to 1173.15 K were represented by a single smoothing funetion derived by the method of least squares (eq. (3) of [2]). The last two columns of table 1 give the absolute and percent deviation of the present high-temperature data from this equation.
3. Low-Temperature Heat Capacity Data, 8.6 K to 273.15 K
Chang [4] has measured in an automated adiabatic calorimeter [10] the low-temperature heat capacity of α-Al2O3 chosen from the same NBS SRM 720 lot as was the material for the high-temperature measurements presented above. A piecewise representation of this low-temperature heat-capacity data, smooth in derivatives to order two, has been obtained using a least-squares spline-fitting technique. The value of this function, as well as its first and second derivatives, match precisely at 273.15 K corresponding values derived from the function [2] representing the enthalpy above 273.15 K.
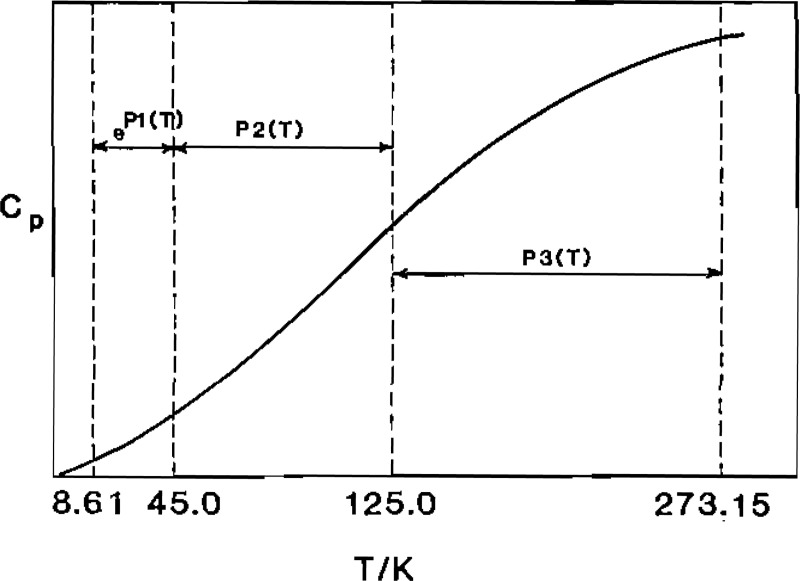
The piecewise representation is illustrated in figure 1., where P1, P2, and P3 are polynomials of the form
| (1) |
T0 is a reference temperature, different for each temperature interval.
| (1a) |
| (1b) |
| (1c) |
Figure 1.
Temperature ranges for spline fit of low-temperature heat-capacity data for Al2O3.
The low-temperature heat-capacity data [4] are represented by these smoothing functions with computed percent standard deviations (Sc) for each of the three fitting intervals as follows:
According to Chang [4], “It is estimated that the accuracy of the smoothed (low-temperature) values are better than 0.1% at temperatures between 100 and 350 K … Below 100 K, the inaccuracy is estimated to become progressively larger, reaching perhaps 1% around 50 K and 10% around 10 K.”
4. Enthalpy of SRM 720 (α-Al2O3), 10 K to 2250 K
Smooth heat-capacity values were computed from eqs (1), above. Below 8.6 K, a T3 dependence of heat capacity was assumed. Using these values, the heat-capacity functions were integrated to obtain the absolute enthalpy of α-Al2O3 in the temperature range 10 K to 273.15 K. Above 273.15 K, enthalpy and heat-capacity values were derived from the following equation (reproduced for convenience from [2]):
| (2) |
Table 2 presents these smooth heat-capacity and enthalpy data for α-Al2O3.
Table 2.
Enthalpy and heat capacity of standard reference material 720.
| Temp | HT − HOK | Cp | Temp. | HT − HOK | Cp |
|---|---|---|---|---|---|
| K | J mol−1b | J mol−1 K−1 | K | J mol−1 | J mol−1 K−1 |
| 440 | 22953 | 100.69 | |||
| 10 | 0.023 | 0.0091 | 450 | 23965 | 101.71 |
| 15 | 0.115 | 0.0307 | 460 | 24987 | 102.68 |
| 20 | 0.364 | 0.0732 | 470 | 26018 | 103.60 |
| 25 | 0.898 | 0.146 | 480 | 27059 | 104.48 |
| 30 | 1.905 | 0.265 | 490 | 28108 | 105.33 |
| 35 | 3.646 | 0.443 | 500 | 29165 | 106.13 |
| 40 | 6.460 | 0.697 | 510 | 30230 | 106.90 |
| 45 | 10.77 | 1.046 | 520 | 31303 | 107.64 |
| 50 | 17.11 | 1.506 | 530 | 32383 | 108.35 |
| 60 | 38.18 | 2.793 | 540 | 33470 | 109.02 |
| 70 | 74.68 | 4.592 | 550 | 34563 | 109.67 |
| 80 | 131.7 | 6.901 | 560 | 35663 | 110.29 |
| 90 | 214.2 | 9.678 | 570 | 36769 | 110.89 |
| 100 | 326.6 | 12.855 | 580 | 37881 | 111.46 |
| 110 | 472.4 | 16.347 | 590 | 38998 | 112.02 |
| 120 | 654.3 | 20.07 | 600 | 40121 | 112.55 |
| 130 | 874.3 | 23.95 | 610 | 41249 | 113.06 |
| 140 | 1133.7 | 27.93 | 620 | 42382 | 113.55 |
| 150 | 1433.1 | 31.95 | 630 | 43520 | 114.02 |
| 160 | 1772.7 | 35.95 | 640 | 44663 | 114.48 |
| 170 | 2152.0 | 39.90 | 650 | 45810 | 114.92 |
| 180 | 2570.3 | 43.75 | 660 | 46961 | 115.35 |
| 190 | 3026.7 | 47.50 | 670 | 48117 | 115.76 |
| 200 | 3519.9 | 51.12 | 680 | 49276 | 116.16 |
| 210 | 4048.7 | 54.61 | 690 | 50440 | 116.55 |
| 220 | 4611.6 | 57.95 | 700 | 51607 | 116.92 |
| 230 | 5207.1 | 61.14 | 720 | 53953 | 117.64 |
| 240 | 5833.9 | 64.18 | 740 | 56313 | 118.32 |
| 250 | 6490.3 | 67.00 | 760 | 58685 | 118.96 |
| 260 | 7175.0 | 69.82 | 780 | 61071 | 119.56 |
| 270 | 7886.3 | 72.42 | 800 | 63468 | 120.14 |
| 273.15 | 8115.6 | 73.21 | 820 | 65876 | 120.69 |
| 280 | 8622.8 | 74.87 | 840 | 68295 | 121.21 |
| 290 | 9383.2 | 77.20 | 860 | 70724 | 121.71 |
| 298.15 | 10020 | 79.01 | 880 | 73163 | 122.20 |
| 300 | 10166 | 79.41 | 900 | 75612 | 122.66 |
| 310 | 10971 | 81.51 | 920 | 78070 | 123.11 |
| 320 | 11796 | 83.49 | 940 | 80536 | 123.55 |
| 330 | 12641 | 85.37 | 960 | 83011 | 123.97 |
| 340 | 13503 | 87.16 | 980 | 85495 | 124.37 |
| 350 | 14383 | 88.84 | 1000 | 87986 | 124.77 |
| 360 | 15280 | 90.45 | 1020 | 90486 | 125.16 |
| 370 | 16192 | 91.97 | 1040 | 92992 | 125.53 |
| 380 | 17119 | 93.41 | 1060 | 95507 | 125.90 |
| 390 | 18060 | 94.7a | 1080 | 98028 | 126.26 |
| 400 | 19014 | 96.08 | 1100 | 100560 | 126.61 |
| 410 | 19982 | 97.32 | 1120 | 103090 | 126.95 |
| 420 | 20961 | 98.50 | 1140 | 105640 | 127.29 |
| 430 | 21951 | 99.62 | 1160 | 108180 | 127.61 |
| 1180 | 110740 | 127.94 | 1700 | 179080 | 134.31 |
| 1200 | 113300 | 128.25 | 1750 | 185810 | 134.73 |
| 1250 | 119730 | 129.01 | 1800 | 192550 | 135.13 |
| 1300 | 126200 | 129.74 | 1850 | 199320 | 135.50 |
| 1350 | 132710 | 130.43 | 1900 | 206100 | 135.89 |
| 1400 | 139240 | 131.08 | 1950 | 212900 | 136.18 |
| 1450 | 145810 | 131.70 | 2000 | 219720 | 136.50 |
| 1500 | 152410 | 132.29 | 2050 | 226550 | 136.80 |
| 1550 | 159040 | 132.84 | 2100 | 233400 | 137.10 |
| 1600 | 165700 | 133.36 | 2150 | 240260 | 137.41 |
| 1650 | 172380 | 133.85 | 2200 | 247140 | 137.73 |
| 2250 | 254030 | 138.06 | |||
Temperatures expressed on IPTS-68 seale.
Molecular Weight = 101.9613.
Footnotes
Figures in brackets indicate literature references at the end of this paper.
Contributor Information
D. A. Ditmars, National Bureau of Standards, Washington, DC 20234.
S. Ishihara, National Bureau of Standards, Washington, DC 20234.
S. S. Chang, National Bureau of Standards, Washington, DC 20234.
G. Bernstein, National Bureau of Standards, Washington, DC 20234.
E. D. West, Calorimetrics, Inc., P.O. Box 4146, Boulder, CO 80302
5. References
- [1].NBS Certificate, Standard Reference Material 720, Synthetic Sapphire (Al2O3); August 26, 1970. [Google Scholar]
- [2].Dilmars D. A.; Douglas T. B., J. Res. Nat, Bur. Stand. (U.S.), 75A (Phys. and Chem.), No. 5, 401 (Sep-Oct 1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Furukawa G. T.; Douglas T. B.; McCoskey R. E.; Ginnings D. C., J. Res. Nat. Bur. Stand. (U.S.), 57, No. 2, 67 (1956) RP2694, [Google Scholar]
- [4].Chang S. S., Proceedings of the Seventh Symposium on Thermophysieal Properties, ASME; NBS, Gaithersburg, MD, May 10–12, 1977, 83 (1977). [Google Scholar]
- [5].West E. D.; Ishihara S., A ealorimetric determination of the enthalpy of graphite from 1200 to 2600 K in Advances in Thermophysical Properties at Extreme Temperatures and Pressures, p. 146, ASME, New York, (1965). See also NBS Special Publication No. 300. vol. 6, p, 220 (1970). [Google Scholar]
- [6].Ditmars D. A.; Cezairliyan A.; Ishihara S.; Douglas T. B.; NBS Special Publication 260–55 (1977).
- [7].Kelley K. K., “Contributions to the data on theoretical metallurgy, XIII. High-temperature heat-eontent, heat-capacity, and entropy data for the elements and inorganie compounds,” U.S. Bureau of Mines Bulletin; 584, (1960). [Google Scholar]
- [8].Sterrelt K. F.; Wallace W. E., J. Am. Chem. Soc. 80, 3176 (1958). [Google Scholar]
- [9].Reilly M. L.; Furukawa G. T., Critical analysis of the heat-eapacity data of the literature and evaluation of thermodynamie properties of Cr, Mo, and W from 0 to 300 K, NSRDS-NBS (to be published). [Google Scholar]
- [10].Chang S. S., Proceedings of the Seventh Symposium on Thermophysical Properties, ASME; NBS, Gaithersburg, MD., May 10–12, 1977, 75 (1977). [Google Scholar]