Abstract
Metalloproteins and metalloenzymes play important roles in biological systems by using the limited metal ions, complexes, and clusters that are associated with the protein matrix. The design of artificial metalloproteins and metalloenzymes not only reveals the structure and function relationship of natural proteins, but also enables the synthesis of artificial proteins and enzymes with improved properties and functions. Acknowledging the progress in rational design from single to multiple active sites, this review focuses on recent achievements in the design of artificial metalloproteins and metalloenzymes with metal clusters, including zinc clusters, cadmium clusters, iron–sulfur clusters, and copper–sulfur clusters, as well as noble metal clusters and others. These metal clusters were designed in both native and de novo protein scaffolds for structural roles, electron transfer, or catalysis. Some synthetic metal clusters as functional models of native enzymes are also discussed. These achievements provide valuable insights for deep understanding of the natural proteins and enzymes, and practical clues for the further design of artificial enzymes with functions comparable or even beyond those of natural counterparts.
Keywords: metalloproteins, metalloenzymes, protein design, metalclusters, synthetic models
1. Introduction
Metalloproteins and metalloenzymes play important roles in biological systems, including electron transfer, O2 binding and delivery, and catalysis, etc. [1,2,3,4,5,6,7,8,9,10,11]. Despite the functional diversity, the cofactor or prosthetic group of native metalloproteins/metalloenzymes are made of about 14 metal ions, and several types of metal complexes (such as heme) or metal clusters (such as iron–sulfur clusters) [4,5,10], which may limit the functionalities of native metalloenzymes. To overcome these limitations, it is desirable to rationally design artificial metalloproteins and metalloenzymes, which have received great attention for several decades, and achieved significant progresses, such as for artificial oxidases and reductases, etc. [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. These progresses not only reveal the key structural elements responsible for the activity of native enzymes, but also endows the ability to create artificial enzymes with improved properties and functions.
In the design of artificial metalloproteins and metalloenzymes, many strategies have been developed, such as redesign of the active metal site by fine-tuning the cofactor–protein interactions through site/loop-directed mutagenesis [30,31,32], the design of new metal-binding sites [33,34,35,36,37,38], the incorporation of unnatural amino acids and non-native cofactors into native or de novo protein scaffolds [20,25,39,40,41], and the directed evolution of metalloenzymes [23,42], as well as the use of post-translational modifications (PTMs) [43,44,45,46,47,48,49,50]. Other materials such as nanoparticles and hydrogels have also been used for the design of enzyme mimics [51,52,53]. Moreover, these designs have been achieved for metalloproteins and metalloenzymes with single to multiple active sites by construction of the mononuclear site via metal substitution or incorporation, the design of homodinuclear or heterodinuclear sites and the reconstitution of metal complexes, as well as the design of dual and multiple active sites in single, dimeric proteins, and protein oligomers and polymers [15]. The progress in this field has also been well reviewed very recently [21,22,23,24,25,26,27,28,29].
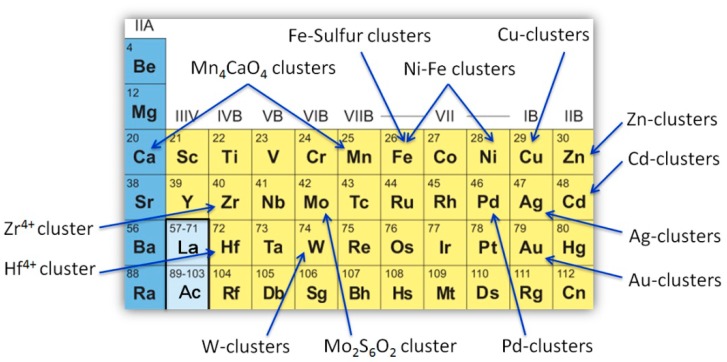
Comparatively, there has been less progress in the design of artificial metalloproteins and metalloenzymes with metal clusters, especially for those formed by multiple metal ions. In 2016, Fehl and Davis critically reviewed the progress in the design of heterogeneous catalysis by using proteins or polypeptides as templates for synthetic metalloclusters, including iron–sulfur clusters, di-iron clusters, and nickel–iron clusters [54]. In 2018, Ueno et al. highlighted the research on the functionalization of protein crystals with metal ions, complexes, and nanoparticles [55]. In this review, we summarize the very recent progress in the rational design of artificial metalloproteins and metalloenzymes by focusing on the design of multi-metal clusters, as well as some synthetic metal clusters as functional models of native enzymes. The mapping of the diverse metal clusters that have been designed to date in the periodic table is shown in Figure 1. These achievements provide not only valuable insights into the structure–function relationship of native metalloproteins and metalloenzymes, but also practical clues for creating more advanced artificial metalloenzymes, which will ultimately stimulate the growth and expansion of this field.
Figure 1.
Artificial metalloproteins and metalloenzymes with diverse metal clusters shown in the periodic table.
2. Artificial Metalloproteins with Metal Clusters for Structural Roles
2.1. Zinc Clusters
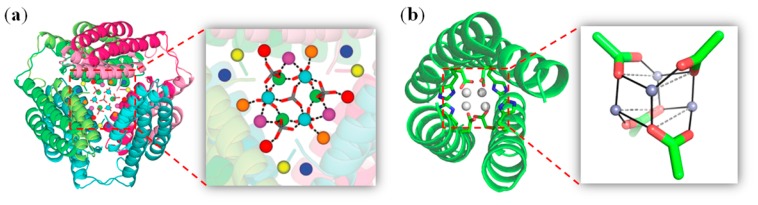
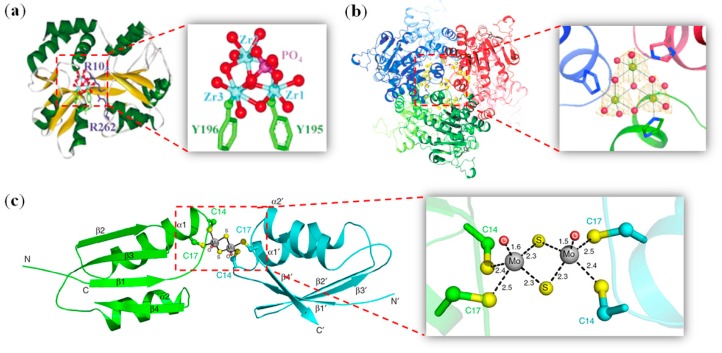
Zinc ions play crucial roles in both protein scaffolds and protein–protein interfaces, acting as catalytic sites or supporting quaternary protein structures [56]. Tezcan et al. showed that by design of azinc-binding site on the protein surface, such as on c-type cytochrome b562 (Cytcb562) with a covalently attached heme, the protein assembles in ordered structures as dictated by the binding of Zn2+ ions [57]. Both structural zinc sites (3-His-1-Asp) and catalytic zinc sites (2-His-1-Glu-1-H2O) can be designed in Cytcb562 assembly, which confer a stable and active artificial hydrolase both in vitro and in vivo [58]. Note that Cytcb562 is a four-helix bundle protein, and can form a dimer by domain-swapping, wherethe N-terminal two helices of one protomer may interact with the C-terminal two helices of the other protomer. Moreover, Hirota et al. observed that three domain-swapped Cytcb562 dimers can further form a unique nanocage, witha novel Zn–SO4 cluster (15 Zn2+ and 7 SO42− ions) inside the cavity, as shown in the X-ray crystal structure (Figure 2a) [59]. In addition to the coordination between Zn2+ and SO42− ions, the Zn2+ ions in the Zn–SO4 cluster were also coordinated by the amino acid side chains of the dimers, which stabilized the cage structure. Additional stabilization effects were contributed from a hinge loop thatconnected two four-helix bundle units.
Figure 2.
(a) Cage structure of three domain-swapped Cytcb562 dimers (PDB code 5AWI), and a close-up view of the Zn2+ and SO42− ions in the internal cavity. Reprinted with permission from Ref. [59], Copyright 2016 The Royal Society of Chemistry; (b) Crystal structure of 4DH1 (PDB code 5WLL), and a close-up view of the designed tetranuclear zinc cluster. Reprinted with permission from Ref. [63], Copyright 2018 American Chemical Society.
As an alternative to native proteins, de novodesigned proteins such as helical bundles provide ideal scaffolds for the design of artificial metalloproteins by the incorporation of metal ions, metal complexes, or metal clusters [36,60]. For example, Pecoraro et al. designed a Zn2+-binding site (3-His-1-H2O) inthree-helical bundles, which confers an impressive hydrolase activity toward the hydration of CO2 with an efficiency comparable to that of native carbonic anhydrases [61]. DeGrado et al. designed a dinuclear zinc site in four-helical bundles, with two Zn2+ ions bridged by two Glu residues and coordinated by additional His and Glu residues. With a suitable substrate-binding pocket, this de novo protein was able to stabilize the radical semiquinone form of catechols for weeks, which is otherwise unstable in aqueous solution [62]. Recently, Lombardi et al. designed a tetranuclear zinc cluster within four-helical bundles (named 4D/EH1/2), which consist of four Zn2+ ions and four carboxyl oxygens from Asp/Glu (Figure 2b) [63]. Additional ligands were provided by His residues, which were further stabilized by second-shell and third-shell interactions, forming a fully connected H-bond network. By optimizing the amino acid sequence, the peptide can be designed to form a tetramer in aqueous solution in the absence of metal ions, which subsequently binds Zn2+ ions and forms a tetranuclear cluster, suggesting an impact of the designed geometry to the metal cluster [64].
2.2. Cadmium Clusters
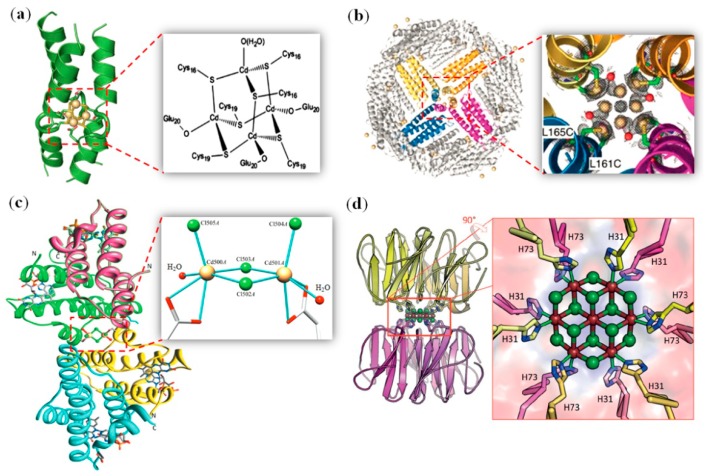
Similar to a tetranuclear copper cluster (Cu4S4) that can be designed within a four-helical bundle [65,66], a tetranuclear cadmium cluster can also be designed, such as in the interior of a three-helical bundle, by using a metal-binding motif of CXXCE [67]. As shown by X-ray crystallography (Figure 3a), the tetra-Cd2+ clusteris a tetrahedral adamantane-like cluster, with four Cd2+ ions bridged by six Cys residues and coordinated by three Glu residues, as well as an additional water molecule, i.e., [Cd4(μ2-S·Cys)6(O2C·Glu)3(H2O)], resulting in a very stable de novo designed metalloprotein.
Figure 3.
(a) X-ray crystal structure of a tetranuclear Cd–thiolate cluster (PDB code 4G1A), and the coordination structure. Reprinted with permission from Ref. [67], Copyright 2013 Elsevier; (b) X-ray crystal structure of apo-L161C/L165C-Fr with Cd ions bound (PDB code 6JEE), and a close-up view of the fourfold axis channel. Reprinted with permission from Ref. [70], Copyright 2019 The Royal Society of Chemistry; (c) X-ray crystal structure of tetrameric hsALR (PDB code 3R7C), and a close-up view of the Cd2Cl4O6 cluster. Reprinted with permission from Ref. [71]. Copyright 2012, International Union of Crystallography; (d) X-ray crystal structure of nvPizza2-S61H58 (PDB code 5CHB), and a close-up view of the Cd7Cl12 cluster. Reprinted with permission from Ref. [72], Copyright 2015 Wiley-VCH.
Ferritin (Fr) is a cage-like protein for iron storage, which is formed by the assembly of 24 subunits [68]. As a result, the structure is highly symmetrical, with twofold, threefold, and fourfold symmetry axes. The threefold axis channel is hydrophilic, and can be used for metal penetration. Differently, the fourfold axis channel has a hydrophobic microenvironment. Inspired by the use of de novofour-helical bundles for the design of metal-binding sites [69], Ueno et al. introduced two Cys residues (L161C/L165C or L168C/L69C mutation) at the fourfold axis channel of apo-Fr, which leads to the formation of four or eight binding sites for Cd3+ ions, as revealed by the X-ray crystal structures [70]. For example, the X-ray structure of apo-L161C/L165C-Fr showed that a Cd8-cluster is located at the fourfold axis channel (Figure 3b), which is coordinated by Cys161 and/or Cys165 with a distance of 2.4 to 2.5 Å, as well as water molecules (2.4 Å). The Cd–Cd distance was found to be 3.4 Å, which agrees with thoseof the Cd–S cluster in natural metallothionein (3.4 to 4.4 Å), suggesting the possible formation of a metal–metal bond, although the occupancies of both Cd ions were less than one (0.6 and 0.2, respectively). This study suggests that not only the threefold axis channel, but also thefourfold axis channelof Fr can be used for design of metal clusters, with the coordination structure controlled by the assembly interfaces of protein cages.
In addition to the Cd–sulfur cluster, cadmium can form clusters with inorganic ions such as chloride ions. Rose et al. found that when crystallized, the protein augmenter of liver regeneration containing a 14-residue hexahistidine purification tag (termed hsALR) in the presence of a high concentration of CdCl2 (50 mM), the protein formed a tetramer composed of two homodimers, which was bridged by a novel Cd2Cl4O6 cluster with coordination by two Asp residues, as well as two water molecules (Figure 3c) [71]. Moreover, the formation of a cadmium chloride cluster can be rationally designed by usage of the protein interface. As designed by Voet et al., an artificial β-propeller protein named Pizza forms a trimer with threefold symmetry. By the further design of metal-binding sites using His as ligands, the new version of nvPizza2-S61H58 was found to accumulate cadmium and chloride ions by dimerization of the trimer, forming a novel Cd7Cl12 cluster at the threefold symmetric axis (Figure 3d) [72]. The cadmium ions were coordinated by the His residues from each subunit, as well as chloride ions, resulting in a lattice that is nearly identical to that of crystalline cadmium chloride. This study suggests that symmetric proteins may be used for the biomineralization of nanocrystals with useful properties.
2.3. Noble Metal Clusters
In general, noble metals refer to eightelements, including ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), osmium (Os), iridium (Ir), platinum (Pt), and gold (Au). They tend to form metal clusters with a sub-nanometer size (<2 nm), which have a diverse array of applications in biology, energy, and the environment [73]. Moreover, the formation of noble metal clusters with desirable sizes can be controlled by rationally designed metal-binding proteins. For example, Morozov and Ogawa [74] designed helical bundles to form trimers, tetramers, and hexamers, which can bind six, eight, and 12 Ag+ ions, respectively. Upon chemical reduction, a series of Ag0 nanoclusters with predictable sizes can be formed, which display strong visible fluorescence with a number-dependent emission energy. Recently, Cortajarena et al. developed a simple strategy to design proteins for the sustainable synthesisof metal nanoclusters by the introduction of a dihistidine coordination site [75]. Using this approach, they obtained metal nanoclusters such as Cu cluster (15 ± 1 atoms), Ag cluster (nine ± 1 atoms), and Au cluster (five ± 1 atoms) within the protein scaffold of a 34-amino acid helix–turn–helix motif, which showed photostable luminescence both in vitro and in vivo, thereby allowing biomedical applications such as cell imaging and labeling.
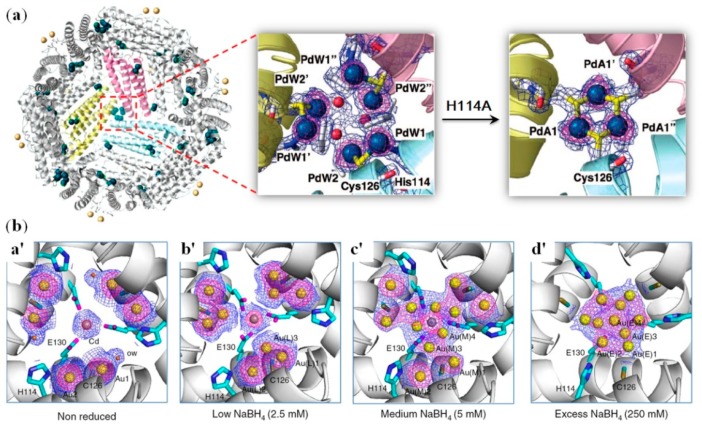
As shown in the previous section, Fr is a highly symmetrical cage-like protein. In its threefold axis channel, the presence of both His and Cys residues allows the coordination of noble metal ions, such as Pd2+ and Au3+ ions. These metal ions can also be designed to form metal clusters. Ueno et al. showed that metal complexes of PdII(allyl) (allyl = η3-C3H5) can be accumulated in the threefold axis channel of apo-Fr, forming a thiol-bridged dinuclear structure, which was stabilized by the coordination of His114 and a water molecule (Figure 4a, middle) [76]. When His114 was replaced with a non-coordinating Ala residue, a unique thiolato trinuclear Pd-cluster was formed, with a six-membered ring structure (Figure 4a, right). This observation suggests that the threefold axis channel of apo-Fr can be modified to acquire metal ions and form novel metal clusters.
Figure 4.
(a) Pd(allyl) complexes binding to the threefold channel of apo-Fr, which form a trinuclear Pd-cluster as a result of H114A mutation. Reprinted with permission from Ref. [76], Copyright 2008 American Chemical Society. (b) X-ray crystal structures Au-loaded apo-Frs in the absence and presence of different concentrations of NaBH4, showing the process of formation of an Au-cluster at the threefold channel of apo-Fr [77].
Moreover, as shown in Figure 3b, Ueno et al. observed the formation of a gold sub-nanocluster at the threefold axis channel of apo-Fr [77]. By the introduction of two surface Cys residues, the apo-E45C/R52C-Fr protein with Au3+ ions loaded were first cross-linked together to form stable crystals, which showed the binding of multiple Au3+ ions at the threefold axis channel with His114, Cys126, and H2O as the ligands, as well as a Cd2+ ion, due to the use of (NH4)2SO4/CdSO4 as the precipitant for crystallization (Figure 4ba′). Upon the reduction of these Au3+ ions by using different concentrations of NaBH4, the X-ray structural analysis showed the gradual movement of the Au3+ ions and the ultimate formation of a sub-nano gold cluster (totally 10 Au3+ ions), which accompanied significant conformational changes of the His114 residue (Figure 4(bb′–bd′)). Therefore, these observations deepened our understanding of metal cluster formation as well as its interactions with the protein microenvironments.
2.4. Other Metal Clusters
In addition to those metal clusters mentioned in previous sections, other non-nativemetal clusters can also be formed in the protein scaffolds with a structural role, which were either rationally designed or found in experiments. For example, Sadler et al. showed that an ferric–ion-binding protein from Neisseria gonorrhoeae (nFbp) may form trinuclear or pentanuclear oxo-clusters of Hf4+ and Zr4+ using a di-tyrosyl cluster nucleation motif (Tyr195-Tyr196) [78,79]. An X-ray crystal structure of Zr3–nFbp was shown in Figure 5a, which revealed an oxo-Zr3 phosphate cluster that was coordinated directly by both Tyr195 and Tyr196 to two of the three ZrIV ions, whereas the O atoms of the cluster were H-bonded to surrounding amino acids [79]. Müller et al.observed several types of polynucleartungsten oxide clusters (W2, W3, W6, W7, and W7+x) in the binding cavity of a Mo/W-storage protein, and a close-up view of the oxo-W3-cluster was shown in Figure 5b [80].
Figure 5.
(a) X-ray structure of Zr3–nFbp showing the coordination geometry. Reprinted with permission from Ref. [79]. Copyright 2004 Wiley–VCH; (b) Structure of the Mo/WSto protein of A. vinelandii, and a close-up view of the oxo-W3-cluster. Reprinted with permission from Ref. [80], Copyright 2007 Wiley-VCH; (c) X-ray structure of a dimer of the metal-binding domain ofcellular copper efflux protein ATP7B (WLN4), and a close-up view of a cluster Mo2S6O2 at the interface [81].
Recently, Liu et al. found that ammoniumtetrathiomolybdate ([(NH4)2MoS4], TM) can induce the dimerization of the metal-binding domain ofcellular copper efflux protein ATP7B (WLN4), due to the formation of a unique sulfur-bridged Mo2S6O2 at the interface, as shown by the X-ray crystal structure in Figure 5c [81].The detailed structure showed that the Mo atom formstwo Mo=O double bonds, as indicated by the distances between Mo and O (1.5 and 1.6 Å), which is in a pyramidalgeometry, with coordination from six S atoms (four Cys and two inorganic S atoms) at a distance of 2.3 to 2.5 Å.
3. Artificial Metalloproteins/Metalloenzymes with Metal Clusters for ElectronTransfer
Nature has evolved diverse electron-transfer centers for metalloproteins, which includes single metal ions such as iron and copper, metal complexes such as b-type and c-type hemes, and metal clusters such as different iron–sulfur clusters ([2Fe-2S], [3Fe-4S] or [4Fe-4S]), with a broad range of reduction potentialsfrom −700mV to +800 mV [7]. Due to their important rolesof electron transfer in biology, as well as serving as catalysts, iron–sulfur clusters are of considerable interest in the design of artificial metalloproteins and metalloenzymes, which aimed at gaining insights into the complex nature systems, and possible applications in industry and energy [82,83]. With a deep understanding of structural principles for natural iron–sulfur proteins, the design of artificial iron–sulfur proteins hasobtained significant progress [83].
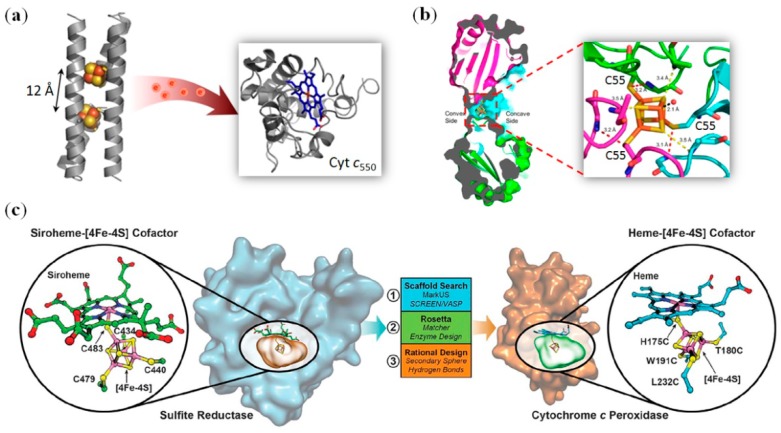
In a pioneer work, with the help of computer-aided design, Goldren et al. introduced four Cys residues into the hydrophobic core of thioredoxin, followed by the incorporation of a [4Fe-4S] cluster into the protein scaffold [84]. Similarly, by the introduction of eight Cys residues in the hydrophobic core of a dimeric three-helix bundle (domain-swapped dimer, DSD), Ghirlanda et al. that two cubane-type [4Fe-4S] clusters was readily bound by in situ reconstitution, which increases the thermal stability relative to that of apo-protein. Meanwhile, the two clusters were separated by 29–34 Å, and were not efficientin electron transfer [85]. In a second generation of protein design, these authors shortened the distance between two clusters to 12 Å (Figure 6a), which is a distance comparable to native intercluster distance (≤15 Å), such as in ferredoxin [86]. In addition to structural mimicking, the designed protein, named DSD-Fdm, showed a reduction potential (–479 mV, [4Fe-4S]2+/1+) within the lower range of natural ferredoxins. As a result, the reduced protein was able to transfer electrons to ferric Cytc550 (Figure 6a), with a stoichiometry of Cytc550/DSD-Fdm = 2/1, which closely mimics the function of natural ferredoxins. Note that the protein reduction potential could be fine-tuned by ligand replacement. For example, the replacement of one of the Cys ligands with Ser or Leu increased thereduction potentialto −4 mV and 12 mV, respectively [87]. More recently, Falkowskia et al. designed artificial symmetric ferredoxins that bind two [4Fe-4S] clusters and exhibit reduction potentials ranging from −405 mV to −515 mV, which are capable of shuttling electrons in vivo, such as through designed cellular pathways in E. coli [88].
Figure 6.
(a) Rational design of two [4Fe-4S] clusters in domain-swapped dimer(DSD)-Fdm that transfer electrons to ferric Cyt c. Reprinted with permission from [86]. Copyright 2014 American Chemical Society; (b)X-ray structure of BMC-T1-S55C and a close-up view of the [4Fe-4S] cluster (PDB code 5DII). Reprinted with permission from [89]. Copyright 2014 American Chemical Society; (c) Rational design of a [4Fe-4S] cluster in cytochrome c peroxidase (CcP) that closely mimics the active site of native sulfite reductase (SiR). Reprinted with permission from [98]. Copyright 2018 AAAS.
Similar to other metal clusters mentioned in previous sections, the iron–sulfur cluster can also be designed within the protein interface if it forms a suitable binding pocket. For example, Kerfeld et al. redesigned the interface of a trimeric bacterial microcompartment (BMC) shell protein by introducing a single Cys at position 55, namely BMC-T1-S55C [89]. The designed protein was found to spontaneously incorporate a [4Fe-4S] cluster at the threefold symmetry axisof the trimer when the protein was expressed in vivo. As shown by X-ray structure (Figure 6b), the cluster was coordinated by three Cys55 residues, as well as an additional water molecule, which provides the first structural information for a designed [4Fe-4S] cluster within a protein scaffold. Moreover, the cluster exhibited a reduction potential of −370 mV (pH 7.5); this value is close to that of the minimal ferredoxin maquette (−350 mV, pH 8) [90], whereas it is more positive than that of dimeric ferredoxin maquette (−479 mV, pH 7.5) [86]. This comparison suggests that greaterexposure of the [4Fe-4S] cluster to the solution leads to more positive potential, which in turn suggests that the reduction potential of the [4Fe-4S] cluster can be fine-tuned by modifying the environment hydrophobicity.
Metalloenzymes with multi-cofactors are efficient in catalyzing multi-electron reactions, such as heme-copper oxidases (HCO, CuB-heme dinuclear center) [91,92,93], nitric oxide reductases (NOR, FeB-heme dinuclear center) [34,94,95,96], and sulfite reductase (SiR, heme-[4Fe-4S] center) [48,97,98]. It is full of challenges to reproduce both the structure and function of these native enzymes by the design of artificial enzymes with a heteronuclear cofactor. Lu et al. have made significant achievements in this field. In addition to the design of both the structural and functional model of HCO and NOR in the scaffold of Mb, namely CuBMb [92] and FeBMb 34], respectively, they recently designed an artificial SiR in the scaffold of CcO (Figure 6c) [98]. With the help of the Rosetta matcher, four residues were selected and mutated to Cys (H75C, T180C, W191C, and L232C) to coordinate to the iron of [4Fe-4S], where Cys175 acts as an axial ligand the heme iron. As shown by the modeling structure in Figure 6c, the designed enzyme closely mimics the active site of native SiR. To further optimize the microenvironment of the heme active site to improve substrate binding and catalysis, triple mutations (W51K, H52R, and P145K) were performed, which enhances the reductase activity by ~5.3-fold. Moreover, an H-bond was further introduced to the [4Fe-4S] cluster by D235N or D235C mutation, resulting in a 17-fold and 63-fold increase over that of the first generation, which indicates that both the microenvironment and H-bond interactions are key factors affecting the catalytic efficiency. Note that the highest rate of sulfite reduction achieved was 21.8 min−1, which is ~18% that of a native SiR (121 min−1). This study demonstrates that the rational design of a [4Fe-4S] cluster in combination with secondary interactions may confer upon artificial enzymesan ability to performmulti-electron and multi-proton reduction.
4. Artificial Metalloenzymes with Metal Clusters for Catalysis
4.1. Iron–Sulfur Clusters
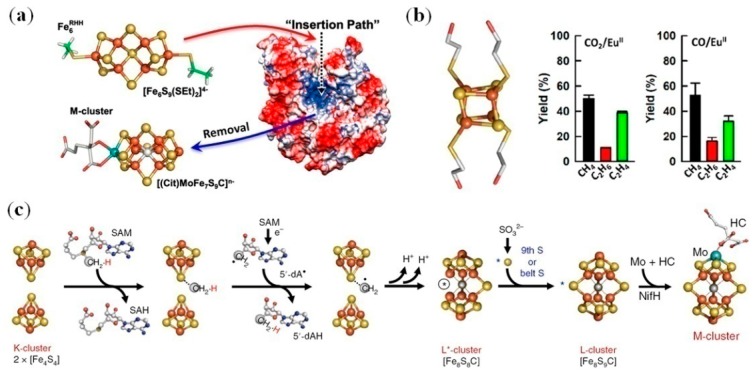
As mentioned in the previous section, iron–sulfur clusters play rolesof both electron transfer and catalysis in biology. For example, nitrogenase catalyzes the reduction of nitrogento ammonia, as well as other substrates (C2H2, CN−, etc.), at its cofactor center of [(Cit)MoFe7S9C]n− (designated M-cluster), where electrons were transferred from a [4Fe–4S] cluster via an [8Fe–7S] cluster (designated P-cluster) [99]. The M-cluster consists of [Mo–3Fe–3S] and [4Fe–3S] subclusters, as bridged by three S atoms and one carbide (C4−) atom (Figure 7a) [100]. To mimic this unique geometry and the anionic nature, Holm et al. synthesized a hexanulcear, dithiolateiron–sulfur cluster, [Fe6S9(SEt)2]4−, which was designated Fe6RHH (Figure 7) [101]. To reveal whether the M-cluster mimic could be combined with a protein scaffold to afford a functional enzyme, Hu et al. removed the native M-cluster from the catalytic component of nitrogenase, followed by incorporation of the Fe6RHH cluster into the apo-protein (Figure 7a) [102]. The reconstituted artificial enzyme was able to catalyze the reduction of C2H2 to C2H4 in the presence of the reductase component of nitrogenase and ATP, with an activity comparable to that of the native enzyme. Moreover, in the absence of ATP and presence of a strong reductant, europium(II) diethylenetriaminepentaacetic acid (EuII-DTPA), the artificial enzyme can catalyze the reduction of CN− to C1-C3 hydrocarbons, with CH4 being the major product. This study demonstrates that the functional artificial enzyme can be obtained by the combination of a synthetic cluster with an appropriate protein scaffold.
Figure 7.
(a) An artificial metalloenzyme constructed by replacement of the M-cluster with a synthesized [Fe6S9(SEt)2]4− cluster in nitrogenase scaffold. Reprinted with permission from [102]. Copyright 2015 Wiley-VCH; (b) The structure of [4Fe-4S(SCH2CH2OH)4]2− cluster (left), and its catalysis of CO2/CO reduction in the presence of europium(II) diethylenetriaminepentaacetic acid (EuII-DTPA) (right) [104]. Copyright 2018 Nature press; (c) The proposed mechanism of l-cluster assembly in nitrogenase, followed by the maturation of M-cluster. SAM: S-adenosyl-l-methionine; NifH: the reductase component of Mo-nitrogenase;HC:homocitrate. Reprinted with permission from [103]. Copyright 2018 Macmillan Publishers.
Recently, thesemi-synthetic approach was extended to reveal the assembly mechanism of the M-cluster. For determination of the ninth sulfur source of the l-cluster [Fe8S9C], Hu et al. synthesized a water-stable and soluble [4Fe-4S] cluster with four –SCH2CH2OH ligands (designated [4Fe-4S]syn, Figure 7b, left) that were exchangeable by Cys residues from protein, and incorporated it into the apo-protein of nitrogenase [103]. The results showed that SO32−, instead of S2− or SO42−, gives rise to the ninth sulfur, with the formation of a new [Fe8S8C] cluster intermediate, namely the L*-cluster. Then, the M-cluster was maturated by the insertion of Mo and homocitrate (HC) (Figure 7c). Moreover, the cluster of [4Fe-4S]syn was found to be able to catalyze the reduction of C1 substrates such as CO2 and CO in the presence of EuII-DTPA, producing hydrocarbons (CH4, C2H4, and C2H6) in an aqueous buffer (Figure 7b, right) [104,105]. These observations indicate an inherent catalytic feature for the [4Fe-4S] cluster, which may thus be used for the design of other artificial metalloenzymes.
4.2. Copper–Sulfur Clusters
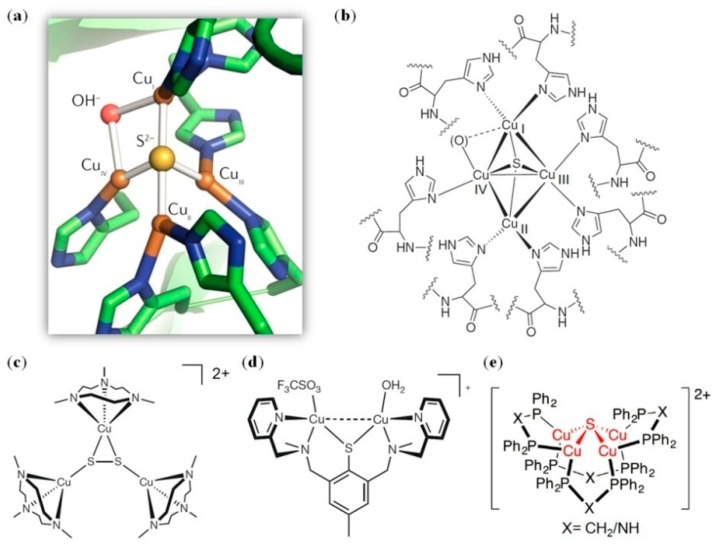
Copper is an essential element in biological systems. When associated with a protein matrix by coordination with His, Cys, and/or Met, it plays diverse functions including O2 binding, electron transfer, and catalysis [106,107]. Copper centers have various types, such as the CuA and CuB centers found in cytochrome c oxidase (CcO) that contain two and one copper atom, performing electron-transfer and catalysis functions, respectively [106,107]. It was revealed that the axial Met ligand and the surrounding amino acids influence the reduction potential of CuA via coordination, H-bond, and hydrophobic interactions [107,108,109], and in the active site of the CuB center, a novel Tyr–His cross-link was found to fine-tune the protein reactivity [110,111]. In addition, the CuZ center was discovered in N2O reductase (N2OR), which containsfour copper atoms forming a distorted tetrahedral geometry, with an inorganic sulfur ion (S2-) as a bridging ligand (Cu4S cluster, Figure 8a, PDB code 1QNI [112]). The copper ions (CuI-IV) were coordinated by seven His residues, and the CuI–CuIV edge was bridged by one unknown O ligand (Figure 8a,b), which was proposed to be the active site where the substrate binds and reduces to N2 and H2O (N2O + 2e− + 2H+ → N2 + H2O) [113,114].
Figure 8.
(a) The X-ray structure of the Cuz center in N2OR from P. nautica (PDB code 1QNI [112]); (b) The coordination geometry of the Cuz center in native N2OR; (c–e) The structures of the synthetic functional models of the native Cuz center [117,118,119].
It is full of challenges to design an artificial enzyme with a Cuz center, and no report is availablein the literature yet. Meanwhile, some progresses have been made in the design of synthetic model complexes mimicking the structure and function of the Cuz cluster in native N2OR [115,116]. For example, Cramer et al. reported the first functional model of Cuz with a [Cu3(μ3-S2)] core (Figure 8c), which exhibited spectroscopic features similar to those of N2OR, and can reduce N2O to N2 under mild conditions such as at room temperature [117]. Instead of using a bridging S2− ligand, Esmieu et al. synthesized a dissymmetric mixed-valentdicopper(II,I) complex using thiolates, which contains a [Cu2S] core with labile triflate and water molecules at the copper centers (Figure 8d) [118]. This simple dicopper complex was shown to be a functional model of the Cuz cluster, by the reduction of N2O to N2at room temperature. Recently, Mankad et al. reported a tetranuclear copper cluster with a [Cu4(μ4-S)] core (Figure 8e), in its one-hole (S=1/2) redox state [119]. The one-hole clusterwas shown to reduce N2O and produce N2, which is the first model closely mimicking the one-electron reduced form of native Cuz (termed Cuz*, CuI4S). Therefore, although these model complexes work in organic solvents such as CH2Cl2 and acetone, they are very useful for gaining the structure and function relationship of native N2OR. Moreover, the incorporation of these model complexes in suitable protein scaffolds might be able to produce artificial metalloenzymes working in physiological conditions.
4.3. Other Metal Clusters
The oxygen-evolving center (OEC) in photosystem II has received much attention in the last decade, which contains a Mn4CaO5 cluster for water oxidation (Figure 9a). Many effects have been directed to synthetic mimics of the OEC. For example, Agapie et al. have rationally synthesized a [Mn3CaO4]6+ cubane that structurally models the Mn3Ca subsite of OEC [120]. Zhang et al. have synthesized a Mn4CaO4 cluster closely mimicking the native OEC, not only in the metal–oxygen core, but also in the coordinating amino acids with ligands of acetate and pyridine (Figure 9b) [121]. Note that the two water ligands on the Ca2+ ion could be mimicked not only by acetate, but also by polar solvents such as acetonitrile and DMF, as structurally characterized recently by Zhang et al. [122]. Moreover, the artificial Mn4CaO4 cluster underwent four redox transitions similar to that of native OEC, which thus provides an ideal model for studying the structure and function relationship of native OEC, as well as an artificial enzyme model for applications in water oxidation.
Figure 9.
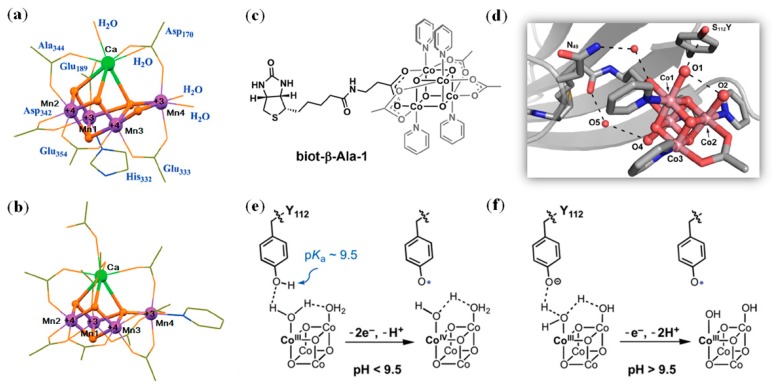
(a) The X-ray crystal structure of native oxygen-evolving center (OEC) showing the core Mn4CaO5 cluster and the ligands; (b) The X-ray crystal structure of a synthesized Mn4CaO4 cluster; Reprinted with permission from [121], Copyright 2015 AAAS; (c) The chemical structure of biotinylated Co4O4 cluster, biot-β-Ala-1; (d) The X-ray crystal structure of 2xm-S112Y-Sav with biot-β-Ala-1 bound (PDB code 6AUE); (e–f) Proposed mechanisms for the multi-e−/multi-H+ reactivity of the designed enzyme at pH <9.5 or pH >9.5.Reprinted with permission from [123]. Copyright 2018 American Chemical Society.
Since the above-synthesized cluster was not incorporated into a protein scaffold, the secondary coordination sphere interactions could hardly be fine-tuned. Meanwhile, in a native photosystem II system, Tyr residue facilitates protein-coupled electron transfer (PCET) between the OEC center and light-harvesting chlorophylls (P680). It is challengeable to design an artificial metalloenzyme with tunable PCET properties. Recently, Tilley et al. synthesized a biotinylated Co4O4 cluster (Figure 9c) and incorporated it into a Sav protein by employing the biotin-streptavidin technology [123]. By introduction of a proximal Tyr (S112Y mutation), the X-ray crystal structure revealed that Tyr112 forms an H-bond interaction with the water molecule that acts as an axial ligand of the Co ion (Figure 9d). As a result, a chemistry of multi-e−/multi-H+ was observed in electronchemical studies, with a transition of the mechanism at pH 9.5 corresponding to the pKa value (~9.5) of Tyr (Figure 9e,f). Note that in the absence of the proximal Tyr (Ser112 or Phe112), only 1e−/1H+ chemistry was observed, which suggests that the secondary sphere interactions, as provided by the redox active Tyr residue, are critical for fine-tuning the multi-e−/multi-H+ reactivity for the artificial enzyme [123]. Note that the introduction ofa redox active residue such as Tyr and Trp in the heme distal pocketor on the protein surface was also shown to fine-tune the reactivity of artificial peroxidases designed in the protein scaffold of myoglobin [46,124,125].
5. Conclusions and Perspectives
In summary, artificial metalloproteins and metalloenzymes with diverse metalclusters have been rationally designed in recent years, in which the clustersare formed either in the protein pocket, between the interface of protein dimers, trimers, and oligomers, or within the scaffold of de novo designed proteins. As shown in Figure 1, the metal ions used for the design of metalclusters are not only those in natural biological systems, such as the first-row transition metals (Mn, Co, Fe, Cu, and Zn, Figure 2 and Figure 6, Figure 7, Figure 8 and Figure 9), but also non-native metal ions, such as those of the second-row transition metals (Zr, Mo, Pd, Ag, and Cd, Figure 3, Figure 4a and Figure 5a,c) and the third-row transition metals (Hf, W, and Au, Figure 4b and Figure 5b). Although only several amino acids (Cys/Met, His, and Asp/Glu) act as the ligand for the metal clusters, the bridging atoms/ligands are diverse, which are usually inorganic anions such as S2−, SO42−, Cl−, O2−, and OH−, and amino acids (Cys/Asp/Glu) (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9), as well as synthetic ligands such as –SCH2CH2OH (Figure 7b). In most cases, these metal clusters were found to play a structural role, which stabilize the protein 3D structures, dimers, trimers, or oligomers. Moreover, the metal clusters, especially iron–sulfur clusters, have been successfully designed to play roles of both electron-transfer and catalysis (Figure 6 and Figure 7). Within this progress, some functional model complexes have been rationally designed and synthesized, which closely mimic the active metal clusters in more complex native metalloenzymes, such as the Cuz center in N2OR (Figure 8b–e) and Mn4CaO5 cluster in photosystem II (Figure 9a,b), respectively.
Currently, the design of artificial metalloproteins and metalloenzymes with metal clusters is still at the stage of providing insights into the structure and functional relationship for native metalloenzymes, and some synthetic models are still waiting for incorporation into suitable protein scaffolds to work in physiological conditions. Comparatively, artificial metalloenzymes with a single active site have been designed to exhibit catalytic parameters similar to those of native enzymes [50,98,126,127], or even with a much higher catalytic efficiency [128], which have potential applications in the future. Moreover, some artificial metalloenzymes may catalyze reactions beyond the functionalities of natural enzymes, such as the catalytic formation of a C–Si bond by an engineered Cyt c [129]. By the construction of multi-metal clusters in protein scaffolds, the catalysis of multi-electron/multi-proton reactions has been achieved [98,123], which otherwise can hardly be achieved by using a single metal center, suggesting a more promising application in biotransformations. As suggested by Martinez et al., oxidoreductases are on their way from laboratory to industry [130]. We are confident that in the near future, more advanced artificial metalloenzymes with metal clusters will be rationally designed andexplored for practical applications in different fields, such as in biological medicine, biofuel generation, and environmental protection.
Acknowledgments
I thank all the co-workers for their studies described in this review.
Funding
The work on the heme protein design from my group was supported by the National Natural Science Foundation of China (31370812, 21101091) and the double first-class construct program of the University of South China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Riordan J.F., Vallee B.L. The functional roles of metals in metalloenzymes. Adv. Exp. Med. Biol. 1974;48:33–57. doi: 10.1007/978-1-4684-0943-7_2. [DOI] [PubMed] [Google Scholar]
- 2.Karlin K.D. Metalloenzymes, structural motifs, and inorganic models. Science. 1993;261:701–708. doi: 10.1126/science.7688141. [DOI] [PubMed] [Google Scholar]
- 3.Holm R.H., Kennepohl P., Solomon E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 4.Waldron K.J., Rutherford J.C., Ford D., Robinson N.J. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 5.Valdez C.E., Smith Q.A., Nechay M.R., Alexandrova A.N. Mysteries of metals in metalloenzymes. Acc. Chem. Res. 2014;47:3110–3117. doi: 10.1021/ar500227u. [DOI] [PubMed] [Google Scholar]
- 6.Poulos T.L. Heme enzyme structure and function. Chem. Rev. 2014;114:3919–3962. doi: 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Chakraborty S., Hosseinzadeh P., Yu Y., Tian S., Petrik I., Bhagi A., Lu Y. Metalloproteins containing cytochrome, iron-sulfur, or copper redox centers. Chem. Rev. 2014;114:4366–4469. doi: 10.1021/cr400479b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y.-W. Understanding the choice of copper by heme-copper oxidase using biosynthetic models in myoglobin. Inorg. Chem. Front. 2017;4:918–920. doi: 10.1039/C6QI00603E. [DOI] [Google Scholar]
- 9.Huang X., Groves J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018;118:2491–2553. doi: 10.1021/acs.chemrev.7b00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eom H., Song W.J. Emergence of metal selectivity and promiscuity in metalloenzymes. J. Biol. Inorg. Chem. 2019;24:517–531. doi: 10.1007/s00775-019-01667-0. [DOI] [PubMed] [Google Scholar]
- 11.Pernil R., Schleiff E. Metalloproteins in the Biology of Heterocysts. Life. 2019;9:E32. doi: 10.3390/life9020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y., Yeung N., Sieracki N., Marshall N.M. Design of functional metalloproteins. Nature. 2009;460:855–862. doi: 10.1038/nature08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y.-W., Sawyer E.B., Wang J. Rational heme protein design: All roads lead to Rome. Chem. Asian. J. 2013;8:2534–2544. doi: 10.1002/asia.201300291. [DOI] [PubMed] [Google Scholar]
- 14.Nastri F., Chino M., Maglio O., Bhagi-Damodaran A., Lu Y., Lombardi A. Design and engineering of artificial oxygen-activating metalloenzymes. Chem. Soc. Rev. 2016;45:5020–5054. doi: 10.1039/C5CS00923E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y.-W. Rational design of metalloenzymes: From single to multiple active sites. Coord. Chem. Rev. 2017;336:1–27. doi: 10.1016/j.ccr.2017.01.001. [DOI] [Google Scholar]
- 16.Schwizer F., Okamoto Y., Heinisch T., Gu Y., Pellizzoni M.M., Lebrun V., Reuter R., Kohler V., Lewis J.C., Ward T.R. Artificial Metalloenzymes: Reaction Scope and Optimization Strategies. Chem. Rev. 2018;118:142–231. doi: 10.1021/acs.chemrev.7b00014. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Griffith W.P., Davis I., Shin I., Wang J., Li F., Wang Y., Wherritt D.J., Liu A. Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase. Nat. Chem. Biol. 2018;14:853–860. doi: 10.1038/s41589-018-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirota S., Lin Y.-W. Design of artificial metalloproteins/metalloenzymes by tuning noncovalent interactions. J. Biol. Inorg. Chem. 2018;23:7–25. doi: 10.1007/s00775-017-1506-8. [DOI] [PubMed] [Google Scholar]
- 19.Hu C., Chan S.I., Sawyer E.B., Yu Y., Wang J. Metalloprotein design using genetic code expansion. Chem. Soc. Rev. 2014;43:6498–6510. doi: 10.1039/C4CS00018H. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y., Hu C., Xia L., Wang J. Artificial Metalloenzyme Design with Unnatural Amino Acids and Non-Native Cofactors. ACS Catal. 2018;8:1851–1863. doi: 10.1021/acscatal.7b03754. [DOI] [Google Scholar]
- 21.Oohora K., Onoda A., Hayashi T. Hemoproteins Reconstituted with Artificial Metal Complexes as Biohybrid Catalysts. Acc. Chem. Res. 2019;52:945–954. doi: 10.1021/acs.accounts.8b00676. [DOI] [PubMed] [Google Scholar]
- 22.Shoji O., Aiba Y., Watanabe Y. Hoodwinking Cytochrome P450BM3 into Hydroxylating Non-Native Substrates by Exploiting Its Substrate Misrecognition. Acc. Chem. Res. 2019;52:925–934. doi: 10.1021/acs.accounts.8b00651. [DOI] [PubMed] [Google Scholar]
- 23.Reetz M.T. Directed Evolution of Artificial Metalloenzymes: A Universal Means to Tune the Selectivity of Transition Metal Catalysts? Acc. Chem. Res. 2019;52:336–344. doi: 10.1021/acs.accounts.8b00582. [DOI] [PubMed] [Google Scholar]
- 24.Natoli S.N., Hartwig J.F. Noble−Metal Substitution in Hemoproteins: An Emerging Strategy for Abiological Catalysis. Acc. Chem. Res. 2019;52:326–335. doi: 10.1021/acs.accounts.8b00586. [DOI] [PubMed] [Google Scholar]
- 25.Mirts E.N., Bhagi-Damodaran A., Lu Y. Understanding and Modulating Metalloenzymes with Unnatural Amino Acids, Non-Native Metal Ions, and Non-Native Metallocofactors. Acc. Chem. Res. 2019;52:935–944. doi: 10.1021/acs.accounts.9b00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardi A., Pirro F., Maglio O., Chino M., DeGrado W.F. De Novo Design of Four-Helix Bundle Metalloproteins: One Scaffold, Diverse Reactivities. Acc. Chem. Res. 2019;52:1148–1159. doi: 10.1021/acs.accounts.8b00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Wang C., Cong Z. Strategies for Substrate-Regulated P450 Catalysis: From Substrate Engineering to Co-catalysis. Chemistry. 2019;25:6853–6863. doi: 10.1002/chem.201806383. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y.-W. Rational design of heme enzymes for biodegradation of pollutants toward a green future. Biotechnol. Appl. Biochem. 2019 doi: 10.1002/bab.1788. [DOI] [PubMed] [Google Scholar]
- 29.Nastri F., D’Alonzo D., Leone L., Zambrano G., Pavone V., Lombardi A. Engineering Metalloprotein Functions in Designed and Native Scaffolds. Trends Biochem. Sci. 2019 doi: 10.1016/j.tibs.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Sligar S.G., Egeberg K.D., Sage J.T., Morikis D., Champion P.M. Alteration of heme axial ligands by site-directed mutagenesis: A cytochrome becomes a catalytic demethylase. J. Am. Chem. Soc. 1987;109:7896–7897. doi: 10.1021/ja00259a056. [DOI] [Google Scholar]
- 31.Hay M., Richards J.H., Lu Y. Construction and characterization of an azurin analog for the purple copper site in cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 1996;93:461–464. doi: 10.1073/pnas.93.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying T., Zhong F., Wang Z.H., Li W., Tan X., Huang Z.X. A route to novel functional metalloproteins via hybrids of cytochrome P450 and cytochrome c. Chem. Bio. Chem. 2011;12:707–710. doi: 10.1002/cbic.201000631. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y., Berry S.M., Pfister T.D. Engineering novel metalloproteins: Design of metal-binding sites into native protein scaffolds. Chem. Rev. 2001;101:3047–3080. doi: 10.1021/cr0000574. [DOI] [PubMed] [Google Scholar]
- 34.Yeung N., Lin Y.-W., Gao Y.G., Zhao X., Russell B.S., Lei L., Miner K.D., Robinson H., Lu Y. Rational design of a structural and functional nitric oxide reductase. Nature. 2009;462:1079–1082. doi: 10.1038/nature08620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pordea A. Metal-binding promiscuity in artificial metalloenzyme design. Curr. Opin. Chem. Biol. 2015;25:124–132. doi: 10.1016/j.cbpa.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Peacock A.F. Recent advances in designed coiled coils and helical bundles with inorganic prosthetic groups-from structural to functional applications. Curr. Opin. Chem. Biol. 2016;31:160–165. doi: 10.1016/j.cbpa.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Shu X.-G., Su J.-H., Du K.-J., You Y., Gao S.-Q., Wen G.-B., Tan X., Lin Y.-W. Rational Design of Dual Active Sites in a Single Protein Scaffold: A Case Study of Heme Protein in Myoglobin. Chem. Open. 2016;5:192–196. doi: 10.1002/open.201500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rittle J., Field M.J., Green M.T., Tezcan F.A. An efficient, step-economical strategy for the design of functional metalloproteins. Nat. Chem. 2019;11:434–441. doi: 10.1038/s41557-019-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y. Design and engineering of metalloproteins containing unnatural amino acids or non-native metal-containing cofactors. Curr. Opin. Chem. Biol. 2005;9:118–126. doi: 10.1016/j.cbpa.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Lewis J.C. Metallopeptide catalysts and artificial metalloenzymes containing unnatural amino acids. Curr. Opin. Chem. Biol. 2015;25:27–35. doi: 10.1016/j.cbpa.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sreenilayam G., Moore E.J., Steck V., Fasan R. Stereoselective Olefin Cyclopropanation under Aerobic Conditions with an Artificial Enzyme Incorporating an Iron-Chlorin e6 Cofactor. ACS Catal. 2017;7:7629–7633. doi: 10.1021/acscatal.7b02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. Engl. 2018;57:4143–4148. doi: 10.1002/anie.201708408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Y.-W. The broad diversity of heme-protein cross-links: An overview. Biochim. Biophys. Acta. 2015;1854:844–859. doi: 10.1016/j.bbapap.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Yan D.-J., Li W., Xiang Y., Wen G.-B., Lin Y.-W., Tan X. A novel tyrosine-heme C-O covalent linkage in F43Y myoglobin: A new post-translational modification of heme proteins. Chem. Bio. Chem. 2015;16:47–50. doi: 10.1002/cbic.201402504. [DOI] [PubMed] [Google Scholar]
- 45.Yan D.-J., Yuan H., Li W., Xiang Y., He B., Nie C.-M., Wen G.B., Lin Y.-W., Tan X. How a novel tyrosine-heme cross-link fine-tunes the structure and functions of heme proteins: A direct comparative study of L29H/F43Y myoglobin. Dalton Trans. 2015;44:18815–18822. doi: 10.1039/C5DT03040D. [DOI] [PubMed] [Google Scholar]
- 46.Li L.-L., Yuan H., Liao F., He B., Gao S.-Q., Wen G.-B., Tan X., Lin Y.-W. Rational Design of Artificial Dye-decolorizing Peroxidases using Myoglobin by Engineering Tyr/Trp in the Heme Center. Dalton Trans. 2017;46:11230–11238. doi: 10.1039/C7DT02302B. [DOI] [PubMed] [Google Scholar]
- 47.Cheng H.-M., Yuan H., Wang X.-J., Xu J.-K., Gao S.-Q., Wen G.-B., Tan X., Lin Y.-W. Formation of Cys-heme cross-link in K42C myoglobin under reductive conditions with molecular oxygen. J. Inorg. Biochem. 2018;182:141–149. doi: 10.1016/j.jinorgbio.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y.-W. Structure and function of heme proteins regulated by diverse post-translational modifications. Arch. Biochem. Biophys. 2018;641:1–30. doi: 10.1016/j.abb.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Yin L.-L., Yuan H., Du K.-J., He B., Gao S.-Q., Wen G.-B., Tan X., Lin Y.-W. Regulation of both the structure and function by a de novo designed disulfide bond: A case study of heme proteins in myoglobin. Chem. Commun. 2018;54:4356–4359. doi: 10.1039/C8CC01646A. [DOI] [PubMed] [Google Scholar]
- 50.Liu C., Yuan H., Liao F., Wei C.-W., Du K.-J., Gao S.-Q., Tan X., Lin Y.-W. Unique Tyr-heme double cross-links in F43Y/T67R myoglobin: An artificial enzyme with a peroxidase activity comparable to that of native peroxidases. Chem. Commun. 2019;55:6610–6613. doi: 10.1039/C9CC02714A. [DOI] [PubMed] [Google Scholar]
- 51.Chen M., Wang Z., Shu J., Jiang X., Wang W., Shi Z.-H., Lin Y.-W. Mimicking a Natural Enzyme System: Cytochrome c Oxidase-Like Activity of Cu2O Nanoparticles by Receiving Electrons from Cytochrome c. Inorg. Chem. 2017;56:9400–9403. doi: 10.1021/acs.inorgchem.7b01393. [DOI] [PubMed] [Google Scholar]
- 52.Wang X., Wei C., Su J.-H., He B., Wen G.-B., Lin Y.-W., Zhang Y. A Chiral Ligand Assembly That Confers One-Electron O2 Reduction Activity for a Cu2+-Selective Metallohydrogel. Angew. Chem. Int. Ed. 2018;57:3504–3508. doi: 10.1002/anie.201801290. [DOI] [PubMed] [Google Scholar]
- 53.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., Qin L., Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes(II) Chem. Soc. Rev. 2019;18:1004–1076. doi: 10.1039/C8CS00457A. [DOI] [PubMed] [Google Scholar]
- 54.Fehl C., Davis B.G. Proteins as templates for complex synthetic metalloclusters: Towards biologically programmed heterogeneous catalysis. Proc. Math. Phys. Eng. Sci. 2016;472:20160078. doi: 10.1098/rspa.2016.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abe S., Maity B., Ueno T. Functionalization of protein crystals with metal ions, complexes and nanoparticles. Curr. Opin. Chem. Biol. 2018;43:68–76. doi: 10.1016/j.cbpa.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Song W.J., Sontz P.A., Ambroggio X.I., Tezcan F.A. Metals in protein-protein interfaces. Annu. Rev. Biophys. 2014;43:409–431. doi: 10.1146/annurev-biophys-051013-023038. [DOI] [PubMed] [Google Scholar]
- 57.Radford R.J., Brodin J.D., Salgado E.N., Tezcan F.A. Expanding the utility of proteins as platforms for coordination chemistry. Coord. Chem. Rev. 2011;255:790–803. doi: 10.1016/j.ccr.2010.10.010. [DOI] [Google Scholar]
- 58.Song W.J., Tezcan F.A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science. 2014;346:1525–1528. doi: 10.1126/science.1259680. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto T., Kuribayashi M., Nagao S., Shomura Y., Higuchi Y., Hirota S. Domain-swapped cytochrome cb562 dimer and its nanocage encapsulating a Zn-SO4 cluster in the internal cavity. Chem. Sci. 2015;6:7336–7342. doi: 10.1039/C5SC02428E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tebo A.G., Pecoraro V.L. Artificial metalloenzymes derived from three-helix bundles. Curr. Opin. Chem. Biol. 2015;25:65–70. doi: 10.1016/j.cbpa.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cangelosi V.M., Deb A., Penner-Hahn J.E., Pecoraro V.L. A de novo designed metalloenzyme for the hydration of CO2. Angew. Chem. Int. Ed. Engl. 2014;53:7900–7903. doi: 10.1002/anie.201404925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulas G., Lemmin T., Wu Y., Gassner G.T., DeGrado W.F. Designed metalloprotein stabilizes a semiquinone radical. Nat. Chem. 2016;8:354–359. doi: 10.1038/nchem.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang S.-Q., Chino M., Liu L., Tang Y., Hu X., DeGrado W.F., Lombardi A. De Novo Design of Tetranuclear Transition Metal Clusters Stabilized by Hydrogen-Bonded Networks in Helical Bundles. J. Am. Chem. Soc. 2018;140:1294–1304. doi: 10.1021/jacs.7b08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chino M., Zhang S.Q., Pirro F., Leone L., Maglio O., Lombardi A., DeGrado W.F. Spectroscopic and metal binding properties of a de novo metalloprotein binding a tetrazinc cluster. Biopolymers. 2018;109:e23339. doi: 10.1002/bip.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kharenko O.A., Kennedy D.C., Demeler B., Maroney M.J., Ogawa M.Y. Cu(I) Luminescence from the Tetranuclear Cu4S4 Cofactor of a Synthetic 4-Helix Bundle. J. Am. Chem. Soc. 2005;127:7678–7679. doi: 10.1021/ja042757m. [DOI] [PubMed] [Google Scholar]
- 66.Xie F., Sutherland D.E.K., Stillman M.J., Ogawa M.Y. Cu(I) binding properties of a designed metalloprotein. J. Inorg. Biochem. 2010;104:261–267. doi: 10.1016/j.jinorgbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Zaytsev D.V., Morozov V.A., Fan J., Zhu X., Mukherjee M., Ni S., Kennedy M.A., Ogawa M.Y. Metal-binding properties and structural characterization of a self-assembled coiled coil: Formation of a polynuclear Cd-thiolate cluster. J. Inorg. Biochem. 2013;119:1–9. doi: 10.1016/j.jinorgbio.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Theil E.C. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr. Opin. Chem. Biol. 2011;15:304–311. doi: 10.1016/j.cbpa.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joh N.H., Wang T., Bhate M.P., Acharya R., Wu Y., Grabe M., Hong M., Grigoryan G., DeGrado W.F. De novo design of a transmembrane Zn(2)(+)-transporting four-helix bundle. Science. 2014;346:1520–1524. doi: 10.1126/science.1261172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abe S., Ito N., Maity B., Lu C., Lu D., Ueno T. Coordination design of cadmium ions at the 4-fold axis channel of the apo-ferritin cage. Dalton Trans. 2019;48:9759–9764. doi: 10.1039/C9DT00609E. [DOI] [PubMed] [Google Scholar]
- 71.Florence Q., Wu C.K., Habel J., Swindell J.T., 2nd, Wang B.C., Rose J.P. The structure of augmenter of liver regeneration crystallized in the presence of 50 mM CdCl2 reveals a novel Cd2Cl4O6 cluster that aids in crystal packing. Acta Crystallogr. D Biol. Crystallogr. 2012;68 Pt 9:1128–1133. doi: 10.1107/S0907444912022561. [DOI] [PubMed] [Google Scholar]
- 72.Voet A.R., Noguchi H., Addy C., Zhang K.Y., Tame J.R. Biomineralization of a Cadmium Chloride Nanocrystal by a Designed Symmetrical Protein. Angew. Chem. Int. Ed. Engl. 2015;54:9857–9860. doi: 10.1002/anie.201503575. [DOI] [PubMed] [Google Scholar]
- 73.Mathew A., Pradeep T. Noble Metal Clusters: Applications in Energy, Environment, and Biology. Part. Part. Syst. Charact. 2014;31:1017–1053. doi: 10.1002/ppsc.201400033. [DOI] [Google Scholar]
- 74.Morozov V.A., Ogawa M.Y. Controlled formation of emissive silver nanoclusters using rationally designed metal-binding proteins. Inorg. Chem. 2013;52:9166–9168. doi: 10.1021/ic400760v. [DOI] [PubMed] [Google Scholar]
- 75.Aires A., Llarena I., Moller M., Castro-Smirnov J., Cabanillas-Gonzalez J., Cortajarena A.L. A Simple Approach to Design Proteins for the Sustainable Synthesis of Metal Nanoclusters. Angew. Chem. Int. Ed. 2019;58:6214–6219. doi: 10.1002/anie.201813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe S., Niemeyer J., Abe M., Takezawa Y., Ueno T., Hikage T., Erker G., Watanabe Y. Control of the coordination structure of organometallic palladium complexes in an apo-ferritin cage. J. Am. Chem. Soc. 2008;130:10512–10514. doi: 10.1021/ja802463a. [DOI] [PubMed] [Google Scholar]
- 77.Maity B., Abe S., Ueno T. Observation of gold sub-nanocluster nucleation within a crystalline protein cage. Nat.Commun. 2017;8:14820. doi: 10.1038/ncomms14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexeev D., Zhu H., Guo M., Zhong W., Hunter D.J.B., Yang W., Campopiano D.J., Sadler P.J. A novel protein–mineral interface. Nat. Struct. Mol. Biol. 2003;10:297–302. doi: 10.1038/nsb903. [DOI] [PubMed] [Google Scholar]
- 79.Zhong W., Alexeev D., Harvey I., Guo M., Hunter D.J., Zhu H., Campopiano D.J., Sadler P.J. Assembly of an oxo-zirconium(IV) cluster in a protein cleft. Angew. Chem. Int. Ed. Engl. 2004;43:5914–5918. doi: 10.1002/anie.200460806. [DOI] [PubMed] [Google Scholar]
- 80.Schemberg J., Schneider K., Demmer U., Warkentin E., Muller A., Ermler U. Towards biological supramolecular chemistry: A variety of pocket-templated, individual metal oxide cluster nucleations in the cavity of a mo/w-storage protein. Angew. Chem. Int. Ed. Engl. 2007;46:2408–2413. doi: 10.1002/anie.200604858. [DOI] [PubMed] [Google Scholar]
- 81.Fang T., Chen W., Sheng Y., Yuan S., Tang Q., Li G., Huang G., Su J., Zhang X., Zang J., et al. Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein. Nat.Commun. 2019;10:186. doi: 10.1038/s41467-018-08102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fontecave M. Iron-sulfur clusters: Ever-expanding roles. Nat. Chem. Biol. 2006;2:171–174. doi: 10.1038/nchembio0406-171. [DOI] [PubMed] [Google Scholar]
- 83.Nanda V., Senn S., Pike D.H., Rodriguez-Granillo A., Hansen W.A., Khare S.D., Noy D. Structural principles for computational and de novo design of 4Fe-4S metalloproteins. Biochim. Biophys. Acta. 2016;1857:531–538. doi: 10.1016/j.bbabio.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coldren C.D., Hellinga H.W., Caradonna J.P. The rational design and construction of a cuboidal iron-sulfur protein. Proc. Natl. Acad. Sci. USA. 1997;94:6635–6640. doi: 10.1073/pnas.94.13.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roy A., Sarrou I., Vaughn M.D., Astashkin A.V., Ghirlanda G. De novo design of an artificial bis [4Fe-4S] binding protein. Biochemistry. 2013;52:7586–7594. doi: 10.1021/bi401199s. [DOI] [PubMed] [Google Scholar]
- 86.Roy A., Sommer D.J., Schmitz R.A., Brown C.L., Gust D., Astashkin A., Ghirlanda G. A de novo designed 2[4Fe-4S] ferredoxin mimic mediates electron transfer. J. Am. Chem. Soc. 2014;136:17343–17349. doi: 10.1021/ja510621e. [DOI] [PubMed] [Google Scholar]
- 87.Sommer D.J., Roy A., Astashkin A., Ghirlanda G. Modulation of cluster incorporation specificity in a de novo iron-sulfur cluster binding peptide. Biopolymers. 2015;104:412–418. doi: 10.1002/bip.22635. [DOI] [PubMed] [Google Scholar]
- 88.Mutter A.C., Tyryshkin A.M., Campbell I.J., Poudel S., Bennett G.N., Silberg J.J., Nanda V., Falkowski P.G. De novo design of symmetric ferredoxins that shuttle electrons in vivo. Proc. Natl. Acad. Sci. USA. 2019;116:14557–14562. doi: 10.1073/pnas.1905643116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aussignargues C., Pandelia M.E., Sutter M., Plegaria J.S., Zarzycki J., Turmo A., Huang J., Ducat D.C., Hegg E.L., Gibney B.R., et al. Structure and Function of a Bacterial Microcompartment Shell Protein Engineered to Bind a [4Fe-4S] Cluster. J. Am. Chem. Soc. 2016;138:5262–5270. doi: 10.1021/jacs.5b11734. [DOI] [PubMed] [Google Scholar]
- 90.Gibney B.R., Mulholland S.E., Rabanal F., Dutton P.L. Ferredoxin and ferredoxin-heme maquettes. Proc. Natl. Acad. Sci. USA. 1996;93:15041–15046. doi: 10.1073/pnas.93.26.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferguson-Miller S., Babcock G.T. Heme/Copper Terminal Oxidases. Chem. Rev. 1996;96:2889–2908. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 92.Sigman J.A., Kwok B.C., Lu Y. From Myoglobin to Heme-Copper Oxidase: Design and Engineering of a CuB Center into Sperm Whale Myoglobin. J. Am. Chem. Soc. 2000;122:8192–8196. doi: 10.1021/ja0015343. [DOI] [Google Scholar]
- 93.Bhagi-Damodaran A., Michael M.A., Zhu Q., Reed J., Sandoval B.A., Mirts E.N., Chakraborty S., Moënne-Loccoz P., Zhang Y., Lu Y. Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases. Nat. Chem. 2017;9:257–263. doi: 10.1038/nchem.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y.-W., Yeung N., Gao Y.-G., Miner K.D., Lei L., Robinson H., Lu Y. Introducing a 2-His-1-Glu nonheme iron center into myoglobin confers nitric oxide reductase activity. J. Am. Chem. Soc. 2010;132:9970–9972. doi: 10.1021/ja103516n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Y.-W., Yeung N., Gao Y.-G., Miner K.D., Tian S., Robinson H., Lu Y. Roles of glutamates and metal ions in a rationally designed nitric oxide reductase based on myoglobin. Proc. Natl. Acad. Sci. USA. 2010;107:8581–8586. doi: 10.1073/pnas.1000526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hino T., Matsumoto Y., Nagano S., Sugimoto H., Fukumori Y., Murata T., Iwata S., Shiro Y. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science. 2010;330:1666–1670. doi: 10.1126/science.1195591. [DOI] [PubMed] [Google Scholar]
- 97.Crane B.R., Siegel L.M., Getzoff E.D. Sulfite reductase structure at 1.6 A: Evolution and catalysis for reduction of inorganic anions. Science. 1995;270:59–67. doi: 10.1126/science.270.5233.59. [DOI] [PubMed] [Google Scholar]
- 98.Mirts E.N., Petrik I.D., Hosseinzadeh P., Nilges M.J., Lu Y. A designed heme-[4Fe-4S] metalloenzyme catalyzes sulfite reduction like the native enzyme. Science. 2018;361:1098–1101. doi: 10.1126/science.aat8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sickerman N.S., Hu Y., Ribbe M.W. Nitrogenases. Methods Mol. Biol. 2019;1876:3–24. doi: 10.1007/978-1-4939-8864-8_1. [DOI] [PubMed] [Google Scholar]
- 100.Lee S.C., Lo W., Holm R.H. Developments in the biomimetic chemistry of cubane-type and higher nuclearity iron-sulfur clusters. Chem. Rev. 2014;114:3579–3600. doi: 10.1021/cr4004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paech C., Reynolds J.G., Singer T.P., Holm R.H. Structural identification of iron-sulfur clusters of the respiratory chain-linked NADH dehydrogenase. J. Biol. Chem. 1981;256:3167–3170. [PubMed] [Google Scholar]
- 102.Tanifuji K., Lee C.C., Ohki Y., Tatsumi K., Hu Y., Ribbe M.W. Combining a Nitrogenase Scaffold and a Synthetic Compound into an Artificial Enzyme. Angew. Chem. Int. Ed. Engl. 2015;54:14022–14025. doi: 10.1002/anie.201507646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tanifuji K., Lee C.C., Sickerman N.S., Tatsumi K., Ohki Y., Hu Y., Ribbe M.W. Tracing the ‘ninth sulfur’ of the nitrogenase cofactor via a semi-synthetic approach. Nat. Chem. 2018;10:568–572. doi: 10.1038/s41557-018-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stiebritz M.T., Hiller C.J., Sickerman N.S., Lee C.C., Tanifuji K., Ohki Y., Hu Y. Ambient conversion of CO2 to hydrocarbons by biogenic and synthetic [Fe4S4] clusters. Nat. Catal. 2018;1:444–451. doi: 10.1038/s41929-018-0079-4. [DOI] [Google Scholar]
- 105.Lee C.C., Stiebritz M.T., Hu Y. Reactivity of [Fe4S4] Clusters toward C1 Substrates: Mechanism, Implications, and Potential Applications. Acc. Chem. Res. 2019;52:1168–1176. doi: 10.1021/acs.accounts.9b00063. [DOI] [PubMed] [Google Scholar]
- 106.Rubino J.T., Franz K.J. Coordination chemistry of copper proteins: How nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012;107:129–143. doi: 10.1016/j.jinorgbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 107.Wilson T.D., Yu Y., Lu Y. Understanding copper-thiolate containing electron transfer centers by incorporation of unnatural amino acids and the CuA center into the type 1 copper protein azurin. Coord. Chem. Rev. 2013;257:260–276. doi: 10.1016/j.ccr.2012.06.015. [DOI] [Google Scholar]
- 108.Marshall N.M., Garner D.K., Wilson T.D., Gao Y.G., Robinson H., Nilges M.J., Lu Y. Rationally tuning the reduction potential of a single cupredoxin beyond the natural range. Nature. 2009;462:113–116. doi: 10.1038/nature08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosseinzadeh P., Marshall N.M., Chacon K.N., Yu Y., Nilges M.J., New S.Y., Tashkov S.A., Blackburn N.J., Lu Y. Design of a single protein that spans the entire 2-V range of physiological redox potentials. Proc. Natl. Acad. Sci. USA. 2016;113:262–267. doi: 10.1073/pnas.1515897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miner K.D., Mukherjee A., Gao Y.G., Null E.L., Petrik I.D., Zhao X., Yeung N., Robinson H., Lu Y. A designed functional metalloenzyme that reduces O2 to H2O with over one thousand turnovers. Angew. Chem. Int. Ed. Engl. 2012;51:5589–5592. doi: 10.1002/anie.201201981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu X., Yu Y., Hu C., Zhang W., Lu Y., Wang J. Significant increase of oxidase activity through the genetic incorporation of a tyrosine-histidine cross-link in a myoglobin model of heme-copper oxidase. Angew. Chem. Int. Ed. Engl. 2012;51:4312–4316. doi: 10.1002/anie.201108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown K., Tegoni M., Prudencio M., Pereira A.S., Besson S., Moura J.J., Moura I., Cambillau C. A novel type of catalytic copper cluster in nitrous oxide reductase. Nat. Struct. Biol. 2000;7:191–195. doi: 10.1107/S0108767300022571. [DOI] [PubMed] [Google Scholar]
- 113.Dell’Acqua S., Pauleta S.R., Moura I., Moura J.J. The tetranuclear copper active site of nitrous oxide reductase: The CuZ center. J. Biol. Inorg. Chem. 2011;16:183–194. doi: 10.1007/s00775-011-0753-3. [DOI] [PubMed] [Google Scholar]
- 114.Johnston E.M., Dell’Acqua S., Ramos S., Pauleta S.R., Moura I., Solomon E.I. Determination of the active form of the tetranuclear copper sulfur cluster in nitrous oxide reductase. J. Am. Chem. Soc. 2014;136:614–617. doi: 10.1021/ja411500p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Timmons A.J., Symes M.D. Converting between the oxides of nitrogen using metal-ligand coordination complexes. Chem. Soc. Rev. 2015;44:6708–6722. doi: 10.1039/C5CS00269A. [DOI] [PubMed] [Google Scholar]
- 116.Lehnert N., Dong H.T., Harland J.B., Hunt A.P., White C.J. Reversing nitrogen fixation. Nat. Rev. Chem. 2018;2:278–289. doi: 10.1038/s41570-018-0041-7. [DOI] [Google Scholar]
- 117.Bar-Nahum I., Gupta A.K., Huber S.M., Ertem M.Z., Cramer C.J., Tolman W.B. Reduction of nitrous oxide to dinitrogen by a mixed valent tricopper-disulfido cluster. J. Am. Chem. Soc. 2009;131:2812–2814. doi: 10.1021/ja808917k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Esmieu C., Orio M., Torelli S., Le Pape L., Pécaut J., Lebrun C., Ménage S. N2O reduction at a dissymmetric Cu2S-containing mixed-valent center. Chem. Sci. 2014;5:4774–4784. doi: 10.1039/C4SC01487A. [DOI] [Google Scholar]
- 119.Johnson B.J., Antholine W.E., Lindeman S.V., Graham M.J., Mankad N.P. A One-Hole Cu4S Cluster with N2O Reductase Activity: A Structural and Functional Model for CuZ. J. Am. Chem. Soc. 2016;138:13107–13110. doi: 10.1021/jacs.6b05480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanady J.S., Tsui E.Y., Day M.W., Agapie T. A synthetic model of the Mn(3)Ca subsite of the oxygen-evolving complex in photosystem II. Science. 2011;333:733–736. doi: 10.1126/science.1206036. [DOI] [PubMed] [Google Scholar]
- 121.Zhang C., Chen C., Dong H., Shen J.R., Dau H., Zhao J. Inorganic chemistry. A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science. 2015;348:690–693. doi: 10.1126/science.aaa6550. [DOI] [PubMed] [Google Scholar]
- 122.Chen C., Chen Y., Yao R., Li Y., Zhang C. Artificial Mn4Ca Clusters with Exchangeable Solvent Molecules Mimicking the Oxygen-Evolving Center in Photosynthesis. Angew. Chem. Int. Ed. Engl. 2019;58:3939–3942. doi: 10.1002/anie.201814440. [DOI] [PubMed] [Google Scholar]
- 123.Olshansky L., Huerta-Lavorie R., Nguyen A.I., Vallapurackal J., Furst A., Tilley T.D., Borovik A.S. Artificial Metalloproteins Containing Co4O4 Cubane Active Sites. J. Am. Chem. Soc. 2018;140:2739–2742. doi: 10.1021/jacs.7b13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liao F., Yuan H., Du K.J., You Y., Gao S.Q., Wen G.-B., Lin Y.-W., Tan X. Distinct roles of a tyrosine-associated hydrogen-bond network in fine-tuning the structure and function of heme proteins: Two cases designed for myoglobin. Mol. Biosyst. 2016;12:3139–3145. doi: 10.1039/C6MB00537C. [DOI] [PubMed] [Google Scholar]
- 125.Liu C., Xu J., Gao S.-Q., He B., Wei C.-W., Wang X.-J., Wang Z., Lin Y.-W. Green and efficient biosynthesis of indigo from indole by engineered myoglobins. RSC Adv. 2018;8:33325–33330. doi: 10.1039/C8RA07825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu Y., Cui C., Liu X., Petrik I.D., Wang J., Lu Y. A Designed Metalloenzyme Achieving the Catalytic Rate of a Native Enzyme. J. Am. Chem. Soc. 2015;137:11570–11573. doi: 10.1021/jacs.5b07119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dydio P., Key H.M., Nazarenko A., Rha J.Y.E., Seyedkazemi V., Clark D.S., Hartwig J.F. An artificial metalloenzyme with the kinetics of native enzymes. Science. 2016;354:102–106. doi: 10.1126/science.aah4427. [DOI] [PubMed] [Google Scholar]
- 128.Yin L.-L., Yuan H., Liu C., He B., Gao S.-Q., Wen G.-B., Tan X., Lin Y.-W. A Rationally Designed Myoglobin Exhibits a Catalytic Dehalogenation Efficiency More than 1000-Fold That of a Native Dehaloperoxidase. ACS Catal. 2018;8:9619–9624. doi: 10.1021/acscatal.8b02979. [DOI] [Google Scholar]
- 129.Kan S.B., Lewis R.D., Chen K., Arnold F.H. Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life. Science. 2016;354:1048–1051. doi: 10.1126/science.aah6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martinez A.T., Ruiz-Duenas F.J., Camarero S., Serrano A., Linde D., Lund H., Vind J., Tovborg M., Herold-Majumdar O.M., Hofrichter M., et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017;35:815–831. doi: 10.1016/j.biotechadv.2017.06.003. [DOI] [PubMed] [Google Scholar]