Abstract
Background
Effective integration of concurrent sensory information is crucial for successful locomotion. This study aimed to determine the association of multisensory integration with mobility outcomes in aging.
Methods
A total of 289 healthy older adults (mean age 76.67 ± 6.37 years; 53% female participants) participated in a visual–somatosensory simple reaction time task. Magnitude of multisensory effects was assessed using probability models, and then categorized into four multisensory integration classifications (superior, good, poor, or deficient). Associations of multisensory integration with falls and balance (unipedal stance) were tested at cross-section and longitudinally using Cox proportional hazards models.
Results
At baseline, the prevalence of falls in the previous year was 24%, and 52% reported an incident fall over a mean follow-up period of 24 ± 17 months. Mean unipedal stance time was 15 ± 11 seconds. Magnitude of multisensory integration was a strong predictor of balance performance at cross-section (β = 0.11; p < .05). Of the cohort, 31% had superior, 26% had good, 28% had poor, and 15% had deficient multisensory effects. Older adults with superior multisensory integration abilities were significantly less likely to report a fall in the past year (17%), compared to the rest of the cohort (28%; χ2 = 4.01; p = .04). Magnitude of multisensory integration was an incremental predictor of incident falls (adjusted hazard ratio = 0.24; p = .01), over and above balance and other known fall risk factors.
Conclusions
Our study highlights the clinical relevance of multisensory integration in aging; worse visual–somatosensory integration is associated with worse balance and increased risk of incident falls.
Keywords: Multisensory integration, Brain aging, Sensory, Balance, Falls
Human brains are designed to simultaneously process multiple sensory inputs, so as to engender the most appropriate response to environmental cues. Concurrent sensory stimulation provides redundant information that gives rise to faster detection responses (1). Efficient interactions between somatosensory, visual, and auditory inputs are crucial for functional independence and successful completion of activities of daily living. Yet, the nature of multisensory integration effects and their contribution to clinical outcomes is not fully understood.
Multisensory integration effects are commonly assessed using psychophysical reaction time (RT) data to measure a phenomenon referred to as a redundant signal effect (2). The redundant signal effect is a response time benefit where RTs to multisensory stimuli are typically faster than RTs to constituent unisensory stimuli. These behavioral multisensory integration effects are well documented in young adults, but not as well in older adults (3–6). In a study contrasting integration processes in old and young adults across auditory–visual, auditory–somatosensory, and visual–somatosensory combinations, we revealed that older adults exhibited the greatest RT facilitation when presented with visual–somatosensory (VS) stimulation. We hypothesized that RT facilitation would vary given known age-related alterations in sensory processing (7) and discovered that level of RT facilitation was linked to balance (8), falls (8), and participation in physical activities (9).
The method of using RT facilitation as a proxy for integrative effects has been challenged by Couth and colleagues (10) who posit that basing integration effects solely on RT differences is likely insufficient. Instead, the authors recommend examination of cumulative distribution functions (CDFs) of multisensory and unisensory RTs that account for individual differences in multisensory processing. Given our previously established association of VS RT facilitation effects with balance, and the fact that balance requires efficient interaction of musculoskeletal and sensory systems (11) known to be compromised in aging (12,13), this study aimed to develop a robust phenotype of VS integration and establish its relationship with balance. Poor balance is a major predictor of falls, which is the leading cause of injury and death in older Americans (12). Despite the identification of links between multisensory and balance processes (8,14–17), the clinical relevance has not been established. Hence, our second objective was to determine whether VS integration could predict incident falls (18,19).
Methods
Participants
A total of 378 participants enrolled in the Central Control of Mobility in Aging study in New York completed a multisensory simple RT experiment between June 2011 and January 2018. Central Control of Mobility in Aging eligibility criteria required that participants be 65 years of age and older, reside in lower Westchester county, and speak English. Exclusion criteria included inability to independently ambulate, dementia, significant bilateral vision and/or hearing loss, active neurological or psychiatric disorders that would interfere with evaluations, recent or anticipated medical procedures that would affect mobility, and/or receiving hemodialysis treatment (see also 20,21). The presence of dementia was excluded using reliable cut scores from the AD8 Dementia Screening Interview (cutoff score greater than or equal to 2; 22,23); and the Memory Impairment Screen MIS (cutoff score less than 5; 24); and confirmed at consensus clinical case conferences.
Additional exclusion criteria included the presence of severe unilateral impairments in vision (eg, glaucoma, macular degeneration, detached retina, and monocular blindness; n = 23) or unilateral deafness (n = 4). All Central Control of Mobility in Aging participants were required to have bilateral visual acuity that was better or equal to 20/100 as measured by the Snellen eye chart. Individuals that were unable to hear a 2,000 Hz tone at 25 dB in both ears were not included. Presence or absence of neuropathy was diagnosed by study clinicians (8,9), and participants with severe neuropathy (unable to feel somatosensory stimulation) were not included. Additional exclusion criteria included inadequate multisensory performance (n = 47; see later) and missing mobility assessments (n = 15).
After exclusions, the eligible sample consisted of 289 older adults (mean age 76.67 ± 6.37 years; 53% female participants). All participants provided written informed consent to the experimental procedures, which were approved by the institutional review board (Protocol # 2016-6936) of the Albert Einstein College of Medicine.
Stimuli, Task, and Responses
Participants completed a simple RT paradigm using three sensory conditions that were presented bilaterally: two unisensory (visual and somatosensory) and one multisensory (simultaneous VS). Participants were instructed to respond to all stimuli by pressing a stationary foot pedal as quickly as possible (Figure 1). The three stimulus conditions were presented randomly with equal frequency and consisted of three blocks of 45 trials (135 trials in total). Anticipatory effects were prevented by using an interstimulus interval that varied randomly from 1 to 3 seconds. Performance accuracy was defined as the number of accurate stimulus detections divided by 45 trials per condition. Each block was separated by a 20 second break to reduce fatigue and facilitate concentration.
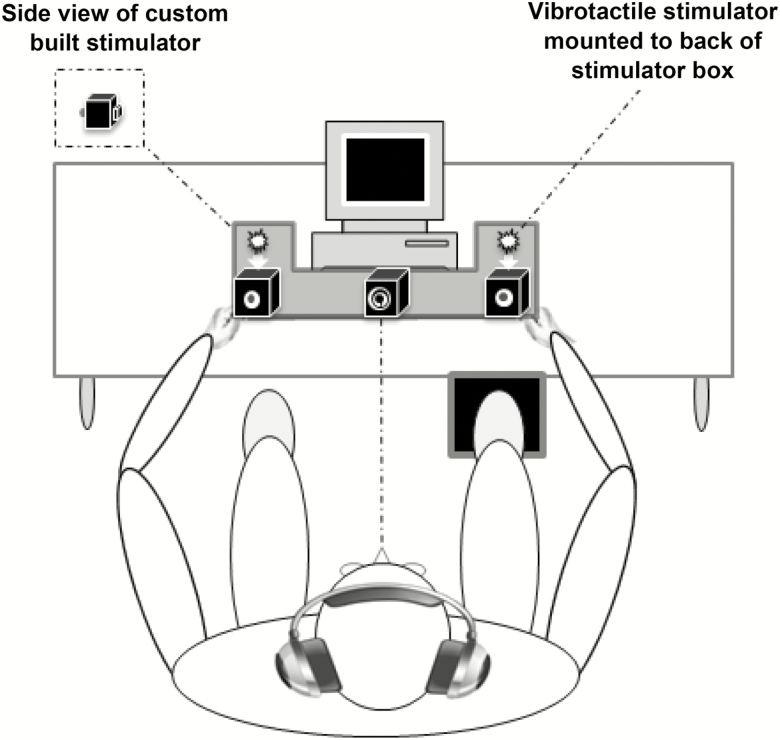
Figure 1.
Experimental Apparatus. Participants were required to make speeded responses to bilateral visual, somatosensory, and visual–somatosensory stimuli by pressing a foot pedal located under their right foot (see also 9).
As described previously (9), visual and somatosensory stimuli were delivered through a custom-built stimulus generator (Zenometrics LLC; Peekskill, NY) that consisted of two control boxes, each housing a 15.88 cm diameter blue light-emitting diodes and a 30.48 mm × 20.32 mm × 12.70 mm plastic housing containing a vibrator motor with 0.8 G vibration amplitude. The devices were connected to a network control center, which allowed direct control for each device through the testing computer’s parallel port. The devices were cycled on and off at precise predetermined intervals in any combination. A transistor–transistor logic (5 V, duration 100 ms) pulse was used to trigger the visual and somatosensory stimuli through E-Prime 2.0 software.
Control boxes were mounted to an experimental apparatus, which participants rested their hands on comfortably, with index fingers placed over the vibratory motors on the back of the box and their thumb on the front of the box, under the light-emitting diode (Figure 1). A third dummy control box was placed in the center of the actual control boxes, at an equidistant length (28 cm) and contained a bull’s eye sticker with a central circle of 0.4 cm diameter that served as the fixation point. To ensure that the somatosensory stimuli were inaudible, each participant was provided with headphones over which continuous white noise was played.
Participants with unreliable data [accuracy less than 70% on any one condition (n = 45) and extremely long RTs > 1,000 ms (n = 2)] were excluded (8,9). To facilitate comparisons to other multisensory studies, the overall RT facilitation effect (ie, RT difference between the multisensory VS condition and the fastest unisensory condition) is presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of Study Sample Overall and by Classification*
| Variable | Overall cohort (n = 289) | Superior integrators (n = 90) | Good integrators (n = 76) | Poor integrators (n = 79) | Deficient integrators (n = 44) |
|---|---|---|---|---|---|
| % Of study cohort | 100 | 31 | 26 | 28 | 15 |
| % Female | 53 | 46 | 62 | 56 | 48 |
| % Caucasian | 78 | 83 | 67 | 76 | 86 |
| % Moderate visual impairment | 27 | 22 | 26 | 34 | 27 |
| % With neuropathy | 5 | 4 | 5 | 5 | 5 |
| % With prevalent falls | 24 | 17 | 29 | 25 | 30 |
| % With incident falls | 52 | 42 | 46 | 54 | 80 |
| Time to fall (d) | 726.85 (515.28) 60–1,994 | 769.27 (561.18) 88–1,944 | 759.63 (529.74) 120–1,714 | 719.91 (493.70) 60–1,691 | 595.91 (414.28) 60–1,431 |
| Age (y) | 76.67 (6.37) 65–93 | 76.39 (6.08) 65–92 | 75.78 (5.88) 66–91 | 77.03 (6.92) 65–93 | 78.14 (6.60) 67–92 |
| Education (y) | 14.96 (2.89) 5–21 | 15.13 (2.61) 8–21 | 14.82 (3.00) 5–21 | 15.00 (2.98) 8–21 | 14.77 (3.15) 8–21 |
| GHS (0–10) | 1.18 (0.95) 0–4 | 0.90 (0.86) 0–3 | 1.18 (0.93) 0–4 | 1.41 (0.91) 0–3 | 1.34 (1.10) 0–4 |
| RBANS total score (65–135) | 94.48 (11.81) 65–132 | 95.83 (11.70) 76–130 | 96.08 (11.69) 65–118 | 92.38 (11.12) 65–132 | 92.77 (12.98) 70–126 |
| Overall RT (ms) | 398.65 (101.90) 243–945 | 398.72 (101.80) 258–822 | 381.50 (76.67) 243–764 | 403.40 (108.93) 248–781 | 419.60 (123.77) 278–945 |
| Somatosensory RT (ms) | 439.18 (112.12) 252–895 | 441.19 (119.80) 273–880 | 421.72 (86.72) 271–743 | 442.42 (114.13) 252–848 | 459.42 (129.45) 289–895 |
| Visual RT (ms) | 399.13 (109.15) 233–1050 | 399.81 (98.79) 262–805 | 382.73 (98.27) 233–1050 | 405.10 (121.67) 250–901 | 415.37 (122.83) 275–915 |
| VS RT (ms) | 358.39 (100.55) 213–1019 | 355.67 (94.95) 239–781 | 341.77 (66.85) 226–617 | 362.64 (111.55) 213–793 | 385.02 (132.02) 245–1019 |
| RT facilitation (ms) | 32.18 (34.93) –135–132 | 38.45 (24.05) –11–115 | 33.23 (25.17) –45–84 | 27.96 (45.19) –135–132 | 25.15 (44.67) –123–112 |
| VS integration† | 0.04 (0.14) –0.32–0.49 | 0.16 (0.09) 0.04–0.41 | 0.08 (0.09) –0.05–0.49 | –0.04 (0.11) –0.31–0.26 | –0.11 (0.08) –0.32– –0.01 |
| Unipedal stance time (s) | 15.14 (11.16) 0–30 | 16.43 (10.72) 0–30 | 16.83 (11.04) 0–30 | 13.49 (11.66) 0–30 | 12.57 (10.79) 0–30 |
Notes: GHS = global health score; RBANS = Repeatable Battery for Assessment of Neuropsychological Status; RT = reaction time; VS = visual–somatosensory.
*Values are presented as mean ± SD for continuous variables and % for dichotomous variable, followed by the range.
†Area under the curve of the cumulative distribution function difference wave over the 0–10 percentile.
Quantification of Multisensory Integration Using the Race Model Inequality
When two sources of sensory information are presented concurrently, they offer synergistic information that give rise to faster responses (redundant signals effect) (1). Race models, commonly implemented to examine multisensory effects, are robust probability (P) models that compare the CDF of combined unisensory visual (V) and unisensory somatosensory (S) RTs with an upper limit of one [min [P(RTV ≤ t) + P(RTS ≤ t), 1] to the CDF of multisensory VS RTs [P(RTVS≤ t)] (2,25,26). For any latency t, the race model inequality (RMI) holds when the CDF of the actual multisensory condition [P(RTVS ≤ t)] is less than or equal to the predicted CDF [min (P(RTV ≤ t) + P(RTS ≤ t), 1)]. Note that CDFs take all RTs into account. Acceptance of the earlier described RMI suggests that unisensory signals are processed in parallel, such that the fastest unisensory signal produces the actual response (ie, the “winner” of the race). However, when the actual CDF is greater than the predicted CDF, the RMI is rejected, and the RT facilitation is the result of multisensory interactions that allow signals from redundant information to integrate or combine nonlinearly.
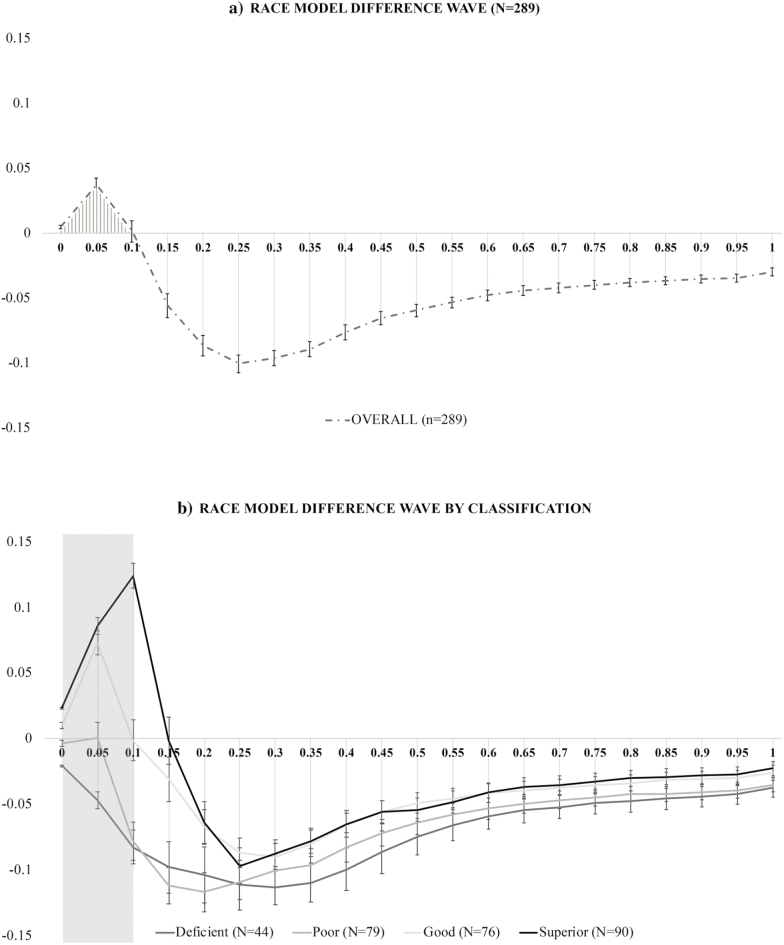
To establish a reliable and measurable phenotype of VS integration, the RMI was first tested using Gondan’s permutation test over the fastest quartile (0%–25%) of responses. A robust violation was observed (tmax = 12.83, tcrit = 1.88, p < .001) (27,28). Slow and omitted responses (< 5% for each condition) were set to infinity rather than excluded. For each participant, RTs were sorted by condition in ascending order and the RT range was calculated across the three stimulus conditions and quantized into 20 bins from the fastest RT (or zero percentile) to the slowest RT (100th percentile) in 5% increments (0%, 5%, …, 95%, 100%). The group-averaged difference between actual and predicted CDFs is presented in Figure 2a where positive values are indicative of a violated RMI in favor of multisensory integration.
Figure 2.
Test of the Race Model. The cumulative distribution function difference waves over the trajectory of averaged responses for: (a) the entire study cohort and (b) each of the four multisensory integration classifications.
In addition, we used a data driven approach to determine which portion of the difference wave best characterized the VS integration effect of the entire cohort. The results indicated that only the fastest 10% of all RTs (first three percentile bins) revealed a race model violation. Thus, the CDF difference values for these three violated percentile bins (0%, 5%, and 10%) were examined on an individual basis and used to (a) determine multisensory integration classification given known differential VS integration abilities in aging (8,9) and (b) calculate the area under the curve that served as our independent variable of “magnitude of VS integration.” Multisensory integration classification was assigned based on the number of violated percentile bins (values greater than zero for 0, 1, 2, or 3 bins) during the 0–10th percentile. Classification definition was assigned as follows: if all percentile bins violated the RMI the individual was considered a “superior” integrator; if two bins violated the RMI, the individual was considered a “good” integrator; if one bin violated the RMI, the individual was considered a “poor” integrator; and if zero bins violated the RMI, the person was considered a “deficient” integrator. Figure 2b depicts race model difference waves by integration classification and illustrates a clear degradation of violation by classification. Note that the magnitude of VS integration as measured by area under the curve during the fastest 10% of responses represents a continuous measure; however, for ease of data interpretation, participants were divided into four groups.
Static Balance and Falls
We selected balance and falls to assess the clinical relevance of multisensory effects in older adults. Static balance was assessed using the unipedal stance time test (29,30). Unipedal stance time is a widely used test of balance: lower scores are associated with neuropathy (29) and predicts falls (30) in the elderly participants. This test was administered twice, and participants’ stance time on one leg for a maximum of 30 seconds served as the outcome measure.
Fall information was collected every 2–3 months over the telephone and during yearly in-house visits. Falls were operationalized as unintentionally coming down to the floor or to a lower level not due to a major intrinsic or extrinsic event (31). Trained research assistants interviewed participants by telephone using a structured questionnaire to reduce variability between testers. If the participant endorsed a fall, further information regarding the number of falls and whether an injury was sustained was collected. High reliability in fall ascertainment using this method has been reported in our previous work (32). All participants completed at least one fall interview following their baseline assessment.
Clinical Evaluation
Global cognitive status was assessed using the Repeatable Battery for Assessment of Neuropsychological Status (33). Global health scores (range 0–10) were obtained from dichotomous rating (presence or absence) of physician diagnosed diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive pulmonary disease, angina, and myocardial infarction (3,7–9).
Statistical Analysis
Data were inspected descriptively and graphically, and the normality of model assumptions was formally tested. Descriptive statistics (M ± SD) were calculated for continuous variables. The distribution of maximum unipedal stance time was skewed; therefore, a natural log transformation was applied to achieve normality, and all statistical analyses used the transformed value.
Linear regression analyses were performed with maximum unipedal stance time as the dependent variable and magnitude of VS integration as the independent variable in an unadjusted model. Additional covariates were entered in a stepwise manner. In Step 2, overall RT (average of all RTs regardless of stimulus condition) was added as an index of processing speed. In Step 3, age, gender, and ethnicity were added. In Step 4, additional independent variables included the presence of moderate visual loss, presence of mild neuropathy, and global health score.
A chi-square analysis was conducted to determine differences in fall prevalence between superior versus inferior (good, poor, and deficient) multisensory integrators. Cox proportional hazard model was used to compute hazard ratios with 95% confidence intervals (CI) to predict incident falls based on magnitude of VS integration. Time to fall was recorded as number of days from baseline study date to the interview date when the fall was endorsed. If the participant did not report a fall, the follow-up time was defined as the number of days from the baseline in-house visit to the last date of contact. Cox models were run unadjusted and then adjusted for age, gender, ethnicity, global health score, presence of moderate visual impairment equal or worse than 20/70 (n = 79) (34), presence of mild neuropathy (n = 15), overall RT, unipedal stance time, and history of falls during the 1-year time interval before assessment. Proportional hazards assumptions of all models were tested graphically and analytically and were adequately met. All data analyses were run using IBM’s Statistical Package for the Social Sciences (SPSS), version 21.
Results
Demographics are presented in Table 1 for the entire cohort and by classification. Group-averaged RTs to the VS condition were faster than group-averaged RTs to the unisensory–somatosensory (β = 80.76, 95% CI = 74.56 to 87.03, p < .001) and unisensory–visual conditions (β = 40.74, 95% CI= 34.23 to 47.26, p < .001).
Our results demonstrated significant RMI violation over the fastest quartile of RTs using an established permutation test (28), in favor of robust VS integration effects. Using a data-driven approach, difference values between actual and predicted CDFs were individually calculated for the group-averaged violated bins (0%, 5%, and 10%). Values for these percentile bins were used to calculate the magnitude of VS integration and determine integration classification. On the basis of our operational definition integration, our sample consisted of 90 superior, 76 good, 79 poor, and 44 deficient integrators. On average, compared to superior and good integrators, poor and deficient integrators were older and manifested more medical comorbidities at baseline. They also demonstrated longer RTs and less RT facilitation. Difference waves (actual minus predicted CDFs) for each multisensory classification are presented in Figure 2b where the shaded rectangular overlay represents the group-averaged portion of the violated RMI (0–10 percentile) identified in Figure 2a.
Maximal unipedal stance time was highest for superior and good integrators (16.43 and 16.83 seconds) and lowest for poor and deficient integrators (13.49 and 12.57 seconds). Results from the linear regression analyses (Table 2) reveal that VS integration is associated with maximum unipedal stance time (β = 0.15, p ≤ .013). Magnitude of VS integration remained associated with maximum unipedal stance time even after controlling for additional covariates in models 2 through 4 (β = 0.11, p < .05). Only 17% of the 90 superior integrators reported a prevalent fall, whereas, collectively 28% of the 199 good, poor, and deficient integrators reported a prevalent fall in the previous (χ2 = 4.06; p < .05).
Table 2.
Summary of Linear Regression Model for Predicting Mean Unipedal Stance Time*
| Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | 95% Confidence interval for B | |||
|---|---|---|---|---|---|---|---|---|
| B | Std. error | β | Lower bound | Upper bound | ||||
| 1 | VS integration | 2.47 | .99 | 0.15 | 2.50 | 0.013 | 0.52 | 4.41 |
| 2 | VS integration | 2.15 | .98 | 0.13 | 2.19 | 0.029 | 0.22 | 4.07 |
| Overall RT | 0.00 | .00 | –0.18 | –3.09 | 0.002 | –0.01 | 0.00 | |
| 3 | VS integration | 1.96 | .96 | 0.12 | 2.03 | 0.043 | 0.06 | 3.86 |
| Overall RT | 0.00 | .00 | –0.17 | –2.94 | 0.004 | –0.01 | 0.00 | |
| Age | –0.07 | .02 | –0.20 | –3.53 | 0.000 | –0.12 | –0.03 | |
| Gender | –0.14 | .28 | –0.03 | –0.52 | 0.605 | –0.69 | 0.40 | |
| Ethnic | 0.36 | .32 | 0.06 | 1.10 | 0.274 | –0.28 | 0.99 | |
| 4 | VS integration | 1.93 | .97 | 0.11 | 1.99 | 0.048 | 0.02 | 3.84 |
| Overall RT | 0.00 | .00 | –0.16 | –2.69 | 0.008 | –0.01 | 0.00 | |
| Age | –0.07 | .02 | –0.20 | –3.40 | 0.001 | –0.11 | –0.03 | |
| Gender | –0.16 | .28 | –0.03 | –0.58 | 0.564 | –0.71 | 0.39 | |
| Ethnic | 0.36 | .33 | 0.07 | 1.11 | 0.266 | –0.28 | 1.00 | |
| Visual impairment | –0.08 | .30 | –0.02 | –0.25 | 0.802 | –0.67 | 0.52 | |
| Neuropathy | –1.02 | .62 | –0.09 | –1.64 | 0.102 | –2.24 | 0.20 | |
| GHS | –0.09 | .14 | –0.04 | –0.61 | 0.540 | –0.37 | 0.19 | |
Notes: GHS = global health score; RT = reaction time; VS = visual–somatosensory.
*Natural log of unipedal stance time (s). Bold values are statistically significant.
Over a mean study follow-up period of 24 ± 17 months, 151 participants (52%) reported an incident fall. Of the fallers, the mean time to fall was 537 days (range 60–1,994 days). Compared to the 138 older adults who did not report an incident fall (time to censor = 935 days [range 88–1,714]), the 151 incident fallers at baseline were older (77.89 vs 75.35 years; p = .001), had worse unipedal stance times (13.54 vs 16.89 seconds; p = .010), and reported more prevalent falls (35% vs 12%; χ2 = 20.38; p= .001). Non-fallers demonstrated greater magnitude of VS integration compared to fallers (0.02 vs 0.06; p = .008). In fully-adjusted Cox models, VS integration predicted incident falls (hazard ratio = 0.24; p = .014; see also Table 3); individuals with greater VS integration were 76% less likely to experience an incident fall than those with lower magnitude of VS integration. The interaction term of VS integration * maximal unipedal stance time was not predictive of falls (hazard ratio = 0.79; p = .46).
Table 3.
Visual–Somatosensory (VS) Integration and Incident Fall Risk: Cox Model Results
| Unadjusted model | Fully adjusted model | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | p-Value | aHR (95% CI) | p-Value |
| VS integration | .22 (0.07–0.68) | .009 | 0.24 (0.08 to 0.75) | .014 |
Note: CI = confidence interval; HR = hazard ratio; VS = visual–somatosensory. Bold values are statistically significant.
Discussion
The main objectives of this study were to (a) identify a robust phenotype of VS integration processes in a large cohort of healthy, non-demented older adults using probability models and (b) determine whether VS integration is associated with important clinical aging outcomes. This study strategically used multisensory stimulation comparable to commercially available electronic devices such as cellular phones and a simple RT task free of higher-order cognitive and perceptual processes in an attempt to tap into early, basic multisensory processing.
Our findings reveal robust, but differential VS integration effects. On the basis of our operational definition, 31% of this study sample was superior VS integrators, whereas 26%, 28%, and 15% were considered good, poor, and deficient VS integrators, respectively. Our results demonstrate an association between magnitude of VS integration (quantified by the amount area under the curve in the CDF difference wave) and balance. Older adults with superior and good integration abilities demonstrated better balance performance than those with poor and deficient integration abilities. To the best of our knowledge, this is the first study to demonstrate that older adults with worse ability to integrate VS information (ie, less magnitude of VS integration) report more incident falls—a significant translational advance in the field of multisensory and aging research.
In a previous study, we examined differential VS integration effects and its relationship with balance and prevalent falls using RT facilitation as a proxy for multisensory integration in 70 older adults. Our results revealed that magnitude of VS integration in this smaller sample was associated with balance and prevalent falls in the past 1 year. However, the directionality of this association was seemingly paradoxical; larger RT facilitation was associated with worse balance and increased falls (8). Unlike this study, the experimental apparatus used in our earlier study relied on less realistic visual (asterisks presented on a computer monitor) and somatosensory (electrical pulse) stimulations. In a similar study examining 147 older adults exposed to the same multisensory apparatus as this study, older adults with larger RT facilitation reported less participation in physical activities than those with less RT facilitation (9). Thus, differential VS integration abilities have been previously identified by our group, but operationalized based solely on RTs. We have since implemented several methodological modifications. One major advance was the proposal of a new, more robust, operational definition of VS integration based on magnitude of race model violation given claims that these tests take individual differences of all RTs into account (10). Furthermore, in an effort to avoid skewing the CDF, data-trimming procedures were strategically avoided (27).
Multisensory integration is an integral aspect of functioning and mobility (18,19,35). Research on animals and young adults reveals that efficient sensory integration depends on intact feedback and feedforward neuronal loops between cortical (primary sensory regions, multisensory areas [eg, superior temporal sulcus, motor regions], and subcortical regions [thalamus]) (36). The thalamus plays an important role in the integration of sensory information, through cortico-cortical and cortical–subcortical transmissions (37) Cortico-cortical and cortico-thalamic loops required for intact multisensory integration and balance performance are notoriously compromised with aging. Although multisensory integration and balance showed significant associations at baseline, the magnitude of VS integration had incremental predictive validity for falls over balance and other known fall risk factors; indicating that deficient multisensory processing may contribute to falls through other mechanisms or pathways.
Encouragingly, researchers have demonstrated the effect of combined multisensory and fall-prevention training in enhancing balance (postural control), functional ability, and confidence in daily activities, in relatively small samples of fall-prone older adults using the sensory organization test (38). Hu and Woollacott provide support that concurrent VS training on a platform sensory test can improve postural control in a small cohort of relatively healthy older adults (39). The authors posited that the basis of improving postural stability is likely linked to a central integrative mechanism where visual, somatosensory, and vestibular inputs converge. This area could be the superior temporal sulcus given its known association with multisensory processing (35); however, further research is necessary to confirm this speculation and set the stage for developing interventions.
This study is not without its limitations. Given known alterations in unisensory processing with increasing age, no healthy young control group was included. Unipedal stance test though widely used clinically does not capture all aspects of balance. Further, other types of sensation may also contribute to balance. Future investigations should consider using more sophisticated measures of both static and dynamic balance, as well as other multisensory combinations. We sought to establish the clinical relevance of multisensory integration, but it may still be too early to implement multisensory testing in routine clinical practice though the testing protocol is short and does not require expensive equipment.
Conclusion
In summary, we identified differential VS integration abilities using a robust proxy of multisensory integration. We reveal the clinical relevance of multisensory integration in aging in the context of balance and fall prediction. We described a protective effect of multisensory integration; whereby greater ability to successfully integrate visual and somatosensory information was associated with lesser likelihood of falling. This study highlights inefficient VS integration as a potential novel mechanism for falls in older adults, independent of other known fall risk factors.
Funding
This work was supported by the National Institute on Aging at the National Institute of Health (K01AG049813 to J.R.M.), (R01AG044007 to J.V.), and (R01AG036921 to Dr. Roee Holtzer). Additional funding was supported by the Resnick Gerontology Center of the Albert Einstein College of Medicine.
Conflict of Interest
There are no conflicts of interest to report in relation to the current article.
Acknowledgements
Special thanks to our participants, research assistants, and Mr. David Graves for their outstanding assistance with this project.
References
- 1. Kinchla R. Detecting target elements in multielement arrays: A confusability model. Perception & psychophysics. 1974;15:149–158. doi: 10.3758/BF03205843 [DOI] [Google Scholar]
- 2. Miller J. Divided attention: Evidence for coactivation with redundant signals. Cogn Psychol. 1982;14(2):247–279. [DOI] [PubMed] [Google Scholar]
- 3. Mahoney JR, Li PC, Oh-Park M, Verghese J, Holtzer R. Multisensory integration across the senses in young and old adults. Brain research. 2011;1426:43–53. doi: 10.1016/j.brainres.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hugenschmidt CE, Mozolic JL, Laurienti PJ. Suppression of multisensory integration by modality-specific attention in aging. Neuroreport. 2009;20(4):349–353. doi: 10.1097/WNR.0b013e328323ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laurienti PJ, Burdette JH, Maldjian JA, Wallace MT. Enhanced multisensory integration in older adults. Neurobiology of aging. 2006;27(8):1155–1163. doi: 10.1016/j.neurobiolaging.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 6. Peiffer AM, Mozolic JL, Hugenschmidt CE, Laurienti PJ. Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport. 2007;18(10):1077–1081. doi: 10.1097/WNR.0b013e3281e72ae7. [DOI] [PubMed] [Google Scholar]
- 7. Dumas K, Holtzer R, Mahoney JR. Visual-Somatosensory Integration in Older Adults: Links to Sensory Functioning. Multisensory Research. 2016;29(4–5):397–420. doi: 10.1163/22134808-00002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahoney JR, Holtzer R, Verghese J. Visual-somatosensory integration and balance: evidence for psychophysical integrative differences in aging. Multisens Res. 2014;27(1):17–42. doi: 10.1163/22134808-00002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahoney JR, Dumas K, Holtzer R. Visual-Somatosensory Integration is linked to Physical Activity Level in Older Adults. Multisensory Research. 2015;28(1–2):11–29. doi: 10.1163/22134808-00002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couth S, Gowen E, Poliakoff E. Using race model violation to explore multisensory responses in older adults: Enhanced multisensory integration or slower unisensory processing?Multisensory Research. 2017;31(3–4):151–174. [DOI] [PubMed] [Google Scholar]
- 11. Shumway-Cook A, Woollacott M.. Motor Control. Fourth Edition New York: Lippincott, Williams, & Wilkins; 2012. [Google Scholar]
- 12. Dillon CF, Gu Q, Hoffman H, Ko CW.. Vision, hearing, balance, and sensory impairment in Americans aged 70 years and over: United States, 1999–2006. NCHS data brief, no 31. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 13. Lord S, Sherrington C, Menz H, Close JC.. Falls in Older People: Risk factors and strategies for prevention. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 14. Setti A, Burke KE, Kenny RA, Newell FN. Is inefficient multisensory processing associated with falls in older people?Exp Brain Res. 2011;209(3):375–384. doi: 10.1007/s00221-011-2560-z. [DOI] [PubMed] [Google Scholar]
- 15. Stapleton J, Setti A, Doheny EP, Kenny RA, Newell FN. A standing posture is associated with increased susceptibility to the sound-induced flash illusion in fall-prone older adults. Exp Brain Res. 2013;232(2):423–434. doi: 10.1007/s00221-013-3750-7. [DOI] [PubMed] [Google Scholar]
- 16. Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging?Psychology and aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- 17. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychology and aging. 1994;9(3):339–355. doi: 10.1037/0882-7974.9.3.339 [DOI] [PubMed] [Google Scholar]
- 18. Wallace MT. The Impact of Multisensory Alterations in Human Developmental Disabilities and Disease: The Tip of the Iceberg? In: Stein BE, ed. The New Handbook of Multisensory Processing. Cambridge, MA: The MIT Press; 2012:645–656. [Google Scholar]
- 19. Meyer GF, Noppeney U. Multisensory integration: from fundamental principles to translational research. Exp Brain Res. 2011;213(2–3):163–166. doi: 10.1007/s00221-011-2803-z. [DOI] [PubMed] [Google Scholar]
- 20. Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age. 2013;36(1):373–381. doi: 10.1007/s11357-013-9570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holtzer R, Mahoney J, Verghese J. Intraindividual Variability in Executive Functions but Not Speed of Processing or Conflict Resolution Predicts Performance Differences in Gait Speed in Older Adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;69(8):980–986. doi: 10.1093/gerona/glt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 23. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 24. Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52(2):231–238. doi: 10.1212/WNL.52.2.231 [DOI] [PubMed] [Google Scholar]
- 25. Maris G, Maris E. Testing the race model inequality: A nonparametric approach. Journal of Mathematical Psychology. 2003;47(5–6): 507–514. doi: 10.1016/S0022-2496(03)00062-2. [DOI] [Google Scholar]
- 26. Colonius H, Diederich A. The race model inequality: interpreting a geometric measure of the amount of violation. Psychol Rev. 2006;113(1):148–154. doi: 10.1037/0033-295X.113.1.148. [DOI] [PubMed] [Google Scholar]
- 27. Gondan M, Minakata K. A tutorial on testing the race model inequality. Attention, perception & psychophysics. 2016;78(3):723–735. doi: 10.3758/s13414-015-1018-y. [DOI] [PubMed] [Google Scholar]
- 28. Gondan M. A permutation test for the race model inequality. Behavior research methods. 2010;42(1):23–28. doi: 10.3758/brm.42.1.23. [DOI] [PubMed] [Google Scholar]
- 29. Hurvitz EA, Richardson JK, Werner RA. Unipedal stance testing in the assessment of peripheral neuropathy. Archives of physical medicine and rehabilitation. 2001;82(2):198–204. doi: 10.1053/apmr.2001.17830. [DOI] [PubMed] [Google Scholar]
- 30. Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Archives of physical medicine and rehabilitation. 2000;81(5):587–591. doi: 10.1016/S0003-9993(00)90039-X [DOI] [PubMed] [Google Scholar]
- 31. Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. The New England journal of medicine. 1994;331(13):821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 32. Verghese J HR, Lipton RB, Wang C. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J Gerontol A Biol Sci Med Sci. 2009;64(8):896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duff K, Humphreys Clark JD, O'Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: sensitivity, specificity, and positive and negative predictive powers. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. BMC medicine. 2006;4:7. doi: 10.1186/1741-7015-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calvert GA, Spence C, Stein BE eds. The Handbook of Multisensory Processes. Cambridge, Massachusetts: The MIT Press; 2004. [Google Scholar]
- 36. Schroeder CE, Foxe JJ. Multisensory convergence in early cortical processing. In: Calvert GA, Spence C, Stein BE eds. The Handbook of Multisensory Processes. Massachusetts: MIT Press; 2004:295–309. [Google Scholar]
- 37. Sherman SM. Thalamic relays and cortical functioning. Progress in brain research. 2005;149:107–126. doi: 10.1016/s0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 38. Allison LK, Kiemel T, Jeka JJ. Sensory-Challenge Balance Exercises Improve Multisensory Reweighting in Fall-Prone Older Adults. Journal of neurologic physical therapy : JNPT. 2018;42(2):84–93. doi: 10.1097/npt.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 39. Hu MH, Woollacott MH. Multisensory training of standing balance in older adults: I. Postural stability and one-leg stance balance. Journal of gerontology. 1994;49(2):M52–61. doi: 10.1093/geronj/49.2.M52 [DOI] [PubMed] [Google Scholar]