Abstract
Understanding the effect of molecular pathways involved in the age-dependent deterioration of stem cell function is critical for developing new therapies. The overexpression of Klotho (KL), an antiaging protein, causes treated animal models to enjoy extended life spans. Now, the question stands: Does KL deficiency accelerate stem cell aging and telomere shortening? If so, what are the specific mechanisms by which it does this, and is cycloastragenol (CAG) treatment enough to restore telomerase activity in aged stem cells? We found that KL deficiency diminished telomerase activity by altering the expression of TERF1 and TERT, causing impaired differentiation potential, pluripotency, cellular senescence, and apoptosis in stem cells. Telomerase activity decreased with KL-siRNA knockdown. This suggests that both KL and telomeres regulate the stem cell aging process through telomerase subunits TERF1, POT1, and TERT using the TGFβ, Insulin, and Wnt signaling. These pathways can rejuvenate stem cell populations in a CD90-dependent mechanism. Stem cell dysfunctions were largely provoked by KL deficiency and telomere shortening, owing to altered expression of TERF1, TGFβ1, CD90, POT1, TERT, and basic fibroblast growth factor (bFGF). The CAG treatment partially rescued telomerase deterioration, suggesting that KL plays a critical role in life-extension by regulating telomere length and telomerase activity.
Keywords: Adipose stem cells, Telomerase enzyme, Klotho, Antiaging genes, Telomere, CD90, Cycloastragenol, Pluripotency
Stem cells are promising therapeutic cells that stand against the erosive effect of diseases and aging (1,2). However, even stem cell function declines with aging (1,3). It is currently known that aging is accompanied by a decrease in telomere length and, as a result, loss of telomerase activity (4–6). Understanding the molecular pathways involved in this age-dependent deterioration of stem cell functions and telomerase activity is critical for developing new therapies. However, it is not well understood how stem cell aging and telomerase activity may be molecularly related to important regulatory proteins.
One such protein that has been implicated in stem cell aging and telomerase activity is Klotho (KL). KL is an antiaging protein predominantly produced in the kidney, with shedding of the secreted Klotho (sKL) into the systemic circulation (7,8). KL expression level decreases with age, and is associated with increased risk of age-related diseases (6,9,10). Prior studies have shown that KL regulates life-extension, and derives much of its antiaging capacities from regulation of the telomerase activity in stem cells (11,12). For example, one study demonstrated that mice enjoyed extended life spans when KL was overexpressed (12,13). Conversely, KL deficiency has been shown to induce premature aging (12). Another study demonstrated that KL regulates telomerase activity (14). However, the specific molecular mechanisms behind KL-regulated life-extension on telomerase are not well characterized, particularly within stem cell aging. No studies have yet directly addressed whether or not stimulation via increasing telomere length and telomerase activity has specific beneficial effects in aged stem cells that have undergone KL deficiency.
The aim of this study was to investigate how impaired telomerase activity, induced by KL deficiency, affects the stem cell aging process. To address this aim, we used siRNA knockdown to induce KL deficiency in adipose-derived stem cell populations (ADSC). We found that KL deficiency severely impaired telomere length and telomerase activity in stem cells, altered expression of ADSC and telomerase-associated proteins, and impacted major signaling pathways such as Wnt and insulin. We also investigated the ability of cycloastragenol (CAG), a relatively new aglycone with antiaging properties, to rescue the effects of KL deficiency.
Materials and Methods
Generation of KspKL-/- Mice
We used Cre-Lox recombination technology to generate kidney KL null mice as described previously (15). Briefly, kidney-specific cre and KL floxed mice were bred to generate kidney-specific conditional Klotho null (KspKL-/-) mice. Mice without Cre expression (klothoflox/flox) were used as controls. Male KspKL-/- and their inbred wild-type controls were housed in a room with an ambient temperature of 23 ± 2°C and a 12-hour dark/light cycle at the University of Oklahoma Health Sciences Center (OUHSC) animal care facility. Animal experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee at the OUHSC.
ADSC Isolation
Subcutaneous adipose tissue was extracted from 12-month-old mice, following euthanasia. The animals were then divided into two groups, KL-/-, and wild type (n = 6). Adipose tissue was then immediately digested into individual cells using 0.1% (w/v) collagenase type 1A (Sigma-Aldrich, USA) in 30mL phosphate-buffered saline (PBS) for 1.5hours. The tissue samples were incubated at 37°C for 60 minutes in PBS containing 2 mg/mL of collagenase and shacked (60 cycles/min) for 60 minutes. After centrifugation, the floated lipid layer was discarded and the stromal vascular fraction was collected, washed, and resuspended in Dulbecco’s modified Eagle medium medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin and seeded into tissue culture flask (all from Sigma-Aldrich). After 24 hours, the nonadherent cells were discarded by changing the medium. Cells were incubated at 37°C in a humidified atmosphere consisting of 95% air and 5% CO2 until confluence was reached. Adherent cells when reached about 70% confluency, they were harvested and expanded further until passages 3.
FACS Analysis
Passage 3 (P3), ADSC were trypsinized (1% trypsin-EDTA, Sigma-Aldrich) and then centrifuged at 1,000×g for 5minutes. To analyze cell-surface markers, 1 × 105 ADSC were washed in cold PBS supplemented with 0.5% BSA, and labeled with phycoerythrin (PE) or fluorescein isothiocyannate (FITC)-conjugated antibodies. According to the minimal criteria as recommended by international society for cellular therapy, expression of CD11b, CD34, CD44, CD45, CD73, CD90, CD105, CD106, and CD166 (All from BD Biosciences, USA), was analyzed. To measure KL level in mice tissues, we used mouse Klotho beta (Clone Phe53-Leu995, R&D Systems, USA). Fibroblast growth factor 23 (FGF23) antibody (Clone OABF01602, Aviva Systems Biology, USA) was used to measure the expression of FGF23 protein. For pluripotency markers of Sox2, Oct4, SSEA1, and Nanog (R&D Systems), the single cell’s suspension in PBS having 1% fetal bovine serum, was stained with primary purified rat anti-mouse. The cells were then incubated at 4°C for 30minutes. To determine the level of nonspecific binding, fluorochrome-conjugated isotype control antibodies (R&D Systems) were used. Flow cytometry was performed using Stratedigm Analyzer (Stratedigm, CA).
MTT Assay and Growth Kinetics
Metabolic activity of ADSC was analyzed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) as described elsewhere (16). Briefly, ADSC were seeded in 96-well plates (ThermoFisher, USA) at concentration of 5 × 103 cells/well in stem cells culture medium and incubated under standard conditions. At each time point, MTT reagent (Sigma-Aldrich) was added to each well at 0.5 mg/mL and incubated for subsequent 2 hours until colored formazan compounds were visible. The supernatants were aspirated and DMSO was added to dissolve dark precipitates. Optical density was measured using an automatic reader for microtiter plates (BioTech Instruments Inc., USA). For evaluation of growth kinetics and population doublings of ADSC, cells were seeded in tissue culture flasks in culture medium with 10% fetal bovine serum under standard conditions. On each day, the viable cells were counted.
Immunofluorescence for Determination of Pluripotency Markers in ADSC
An immunofluorescence analyses were performed to know expression of pluripotency markers, including Nanog, Oct4, Sox2, and stage-specific embryonic antigen (SSEA)-1. ADSC were seeded at concentration of 5 × 104 cells/coverslip, were allowed to adhere and when reached 80% confluency, then medium was removed and washed with PBS. Afterwards, cultured cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X (Sigma, USA) in PBS for 10 minutes. After washing with PBS, cells were treated with 1% bovine serum albumin (BSA) in PBS to block nonspecific staining, and then labeled with primary antibody (anti-Nanog, anti-Oct4, anti-SOX2, anti-SSEA-1; Cell Signaling Technology, USA) for 1 hour at room temperature. Samples were incubated in 1% BSA in PBS served as negative control. Afterwards, cells were washed in PBS, and incubated with an appropriate secondary antibody (anti-mouse-TRITC, anti-rabbit-FITC [Cell signaling technology] and 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Santa Cruz Biotechnology, USA) for 1 hour in dark. The coverslips were washed in PBS and mounted on glass plates. The samples were analyzed and photographed by an epifluorescence microscope.
Calcium Determination
To determine the membrane bound calcium, ADSC were seeded into six-well plate and finally spectroscopically recorded as described elsewhere (17). Briefly, after decanting the medium the cell-layer was washed twice with PBS (pH 7.4). To dissolve the mineral 4 mL 0.1 M HCl were added. The calcium concentration was measured spectroscopically at 600 nm by using a calorimetric assay (Sigma-Aldrich) and a standard solution containing calcium (10 mg/dL Calcium, Sigma-Aldrich) for calibration.
Western Blot
Western blotting was performed as described elsewhere (18). Briefly, cells were lysed in RIPA buffer (50 mM Tris, pH 7.5, 0.3 M NaCl, 0.5% Triton X-100, 0.1% sodium azide) containing protease inhibitors (Sigma). Then, centrifuged at 18,500 ×g for 15 minutes. The pellet was discarded and the supernatant was kept for further analysis. Protein concentration was measured using a bicinchoninic acid (BCA) assay kit (Thermo scientific, USA). Loading buffer (Biorad, USA) and reducing agents were added and then an equivalent microgram of proteins was loaded for each group. Primary antibodies were used against secreted KL (R&D, USA), SIRT1, TGFβ1, SMAD2/3, BMP2, CDK4, FGF (Santa Cruz Biotechnology), IGF, FGF2, SMAD4, SOX2, CD90 (Cell Signaling, USA), and β-actin (dilution: 1:10,000; Abcam, USA) as loading control. Separation was performed under nonreducing conditions. After transfer to nitro cellulose membranes (Bio-Rad, USA), antibody incubations and development were performed using Chemiluminescence kit and ChemiDoc (Bio-Rad) were used for membrane exposure.
Senescence-Associated β-Galactosidase Assay
ADSC were cultured in six-well plates for 3 days, then fixed with 4% paraformaldehyde and stained with 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal) overnight. The staining solution consisted of: PBS, citric acid/phosphate buffer (final concentrations: 20 mM citric acid, 40 mM sodium phosphate, pH 5.5), 5 mM potassium-ferricyanide, 5 mM potassium-ferrocyanide, 150 mM NaCl, 2 mM MgCl2, and 1 mg/mL X-Gal.
Reactive Oxygen Species (ROS) Detection
ROS measurement was done as described previously (19). Briefly, after harvesting and washing, cells were incubated for 30 minutes at 37°C in the dark with oxidative stress detection reagent and superoxide detection reagent (Abcam). After washing with PBS three times, PBS was added and fluorescence from the cells was read using flow cytometric analyzer. ROS expression levels were calculated in %, and unstained cells were applied as unlabeled control. ROS inducer (Pyocyanin 1 µM) was used to generate a positive control.
Telomere Length and Activity Analysis in ADSC Derived from Klotho Knockout and Wild-Type Animals
To analyze the telomere length, in ADSC derived from Klotho knockout and wild-type animals, DNA was isolated, quantified, and examined by Southern blotting. To determine telomerase activity, the sample protein concentrations were adjusted to 0.1 µg/µL with lysis buffer, and 0.1 µg of the protein extract was poured into a tube containing elongation buffer, which was modified with 30 mM Tris-HCl (pH 8.3) and 0.25 mg BSA. The reaction mixture was brought to a final volume of 20 µL and incubated for 30 minutes at 30°C. To amplify the elongated G-rich strand of telomere repeats, 10 µL of the prior mixture was used for PCR using FastStart Taq DNA polymerase (Roche, USA), according to the manufacturer’s instructions. The mixture was subjected to 30 cycles of 95°C for 30 seconds, 50°C for 30 seconds, and 72°C for 1 minute using a TS primer (5′ AATCCGTCGAGCAGAGTT 3′) plus a CX (5′ CCCTAACCCTAACCCTAACCCTAA 3′) reverse primer. The extracts from ADSC were heat-inactivated at 85°C for 15 minutes and used as negative controls. RNase-treated samples were also used as telomerase-negative controls. The TeloTAGGG Telomerase PCR ELISA kit (Roche) was used to determine telomerase activity accordingly to manufacturer’s instructions. The average absorbance value (A) for each sample was calculated and to calculate the difference in absorbance (ΔA), we subtracted the mean of the absorbance of the negative controls from the mean sample absorbance. The samples were identified as telomerase positive if the difference in absorbance (ΔA = Asample − A neg. control) was higher than 0.2 units.
siRNA Knockdown of KL Expression in ADSC
To know the role of siRNA inhibition of KL in aging stem cells, ADSC transfected with siRNA of KL for 48 hours (siRNA was generated against KL from Qiagen). ADSC with scrambled siRNA was chosen as controls. The sequence of the annealed double-strand siRNA that we used was: 5′-ATGGGCATAGGTGATCGTAAA-3′-siRNA.
ADSC were seeded at a density of 2 × 105 cells/well in six-well plates and cultured for 2 days. After 2 days, ADSC were transfected with siRNA using siRNA transfection reagents (Life Technologies, USA) according to the manufacturer’s recommendation. Briefly, 20 nM siRNA was mixed with 200 μL transfection medium and then was added slowly to the cultured cells. Control ADSC received scrambled siRNA. After transfection (48 hours), ADSC were harvested for subsequent assays. Gene and protein silencing were monitored at PCR and western blot level.
Telomerase-Related Protein Array in ADSC
To investigate telomerase-related proteins in the ADSC, telomerase-associated protein array (R&D Systems) was performed according to the manufacturer’s recommendations with little in house modification. Briefly, the normal ADSC, and KL-/-ADSC with siRNA, were homogenized in PBS using protease inhibitors cocktail (Sigma-Aldrich) and subsequently centrifuged at at 4°C (10,000 × g for 8 minutes), and 300 µg of total protein concentrations were quantified using BCA assay kit (Sigma-Aldrich). The already calculated concentration of protein in 200 µL was added to a membrane spotted with antibodies against telomerase-related proteins. After being incubated overnight at 4°C, the membranes were treated with streptavidin-horseradish peroxidase and were visualized using an enhanced chemiluminescence detection system (Bio-Rad) on an image analyzer using ChemiDoc (Bio-Rad). After acquisition, the protein spots were quantified using commercial software (R&D Systems), and data were exported as an excel sheet for further processing.
Quantitative PCR
Total RNAs were isolated using RNeasy (Qiagen), following manufacturer recommendation. A total of 2 μg RNA was used for first-strand cDNA synthesis using superscript first-strand synthesis kit (Invitrogen, USA). Taqman gene expression assays, assay Mm00440940_m1 for Pparg, Mm00445878_m1 for Fabp4, Mm00501584_m1 for Runx2, Mm01340178_m1 for Bmp2, Mm00448840_m1 for Sox9, Mm01309565_m1 for Col2a1, Mm00493681_m1 for Cd90, Mm00502002_m1 for Kl, Mm01244861_m1 for Ucp1, Mm99999915_g1 for Gapdh control were purchased from Applied Biosystems (USA). Real-time PCR was done as reported (20). The sequences of other primers were retrieved from Primerbank (pga.mgh.harvard.edu/primerbank/) and synthesized by Life technologies. Taqman fast universal master mix (2×), was used for 40 cycles of PCR in a 20 µL reaction volume using the CFX96 thermal cycler (Bio-Rad). Quadruplicates of the reaction were performed and Ct values were detected using CFX manager software (Bio-Rad) using the following program: 1 cycle at 95°C for 3 minutes and 45 cycles at 95°C for 15 seconds and 60°C for 60 seconds. The Ct values were exported into Microsoft excel program for further analysis.
Statistical Analysis
Data are represented as mean ± SEM unless otherwise specified. Differences in quantitative data between experimental groups were examined by one‐way analysis of variance (ANOVA) followed by the Bonferroni post‐test using Prism software (GraphPad, La Jolla, CA). For two group analysis, Student’s t test was used. For all analysis, statistically significant values: *p = .05; **p = .01; ***p = .001, were considered significant.
Results
Isolation, Proliferation, and Characterization of ADSC
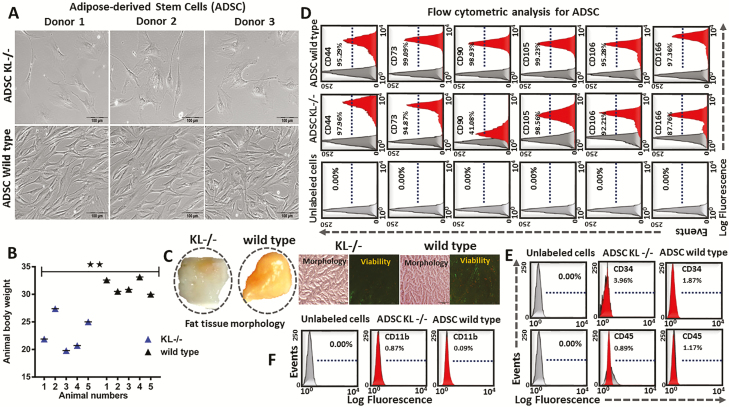
To characterize the ADSC, Adipose tissues were sectioned, pooled, and then processed to isolate ADSC from both Klotho knockout (KL-/-) and wild-type mice (n = 6). Morphological appearance showed flattened features for KL-/--derived ADSC, and more fibroblastic-like features for wild-type-derived ADSC (Figure 1A). The total animal body weight was decreased in the KL-/- group compared to the wild-type mice group (Figure 1B). Fat morphology was found to be white in appearance for KL-/- group, while wild-type mice showed more brownish fat content (Figure 1C). Isolated ADSC showed positive expression of typical mesenchymal markers including CD44, CD73, CD90, CD105, CD106, and CD166, and negative expression of hematopoietic cells markers including CD11B, CD34, and CD45 (Figure 1D–F).
Figure 1.
Adipose-derived stem cells (ADSC) were isolated, cultured, and verified by flow for their CD markers. (A) Morphology of cultured ADSC, at passage 3 (P3). (B) Animal body weight of KL-/- and wild-type mice. (C) Representative images showing gross morphology of fat tissue, and microscopic view of fat sections along with viability staining (Fluorescein diacetate [FDA] and Propidium iodide [PI]), in KL-/- and wild-type group. (D–F) Flow cytometric analysis of ADSC makers (single cell suspensions of P3 cells were stained with conjugated fluorescent dyes: CD11b-PE, CD34-PE, CD44-PE, CD45-PE, CD73-PE, CD90-PE, CD105-PE, CD106-FITC, and CD166-PE). Positively or negatively stained cells are expressed as a % in the middle of the frame. Mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001, scale bar: 100 μm.
Association of KL and Telomeres in Stem Cells Aging
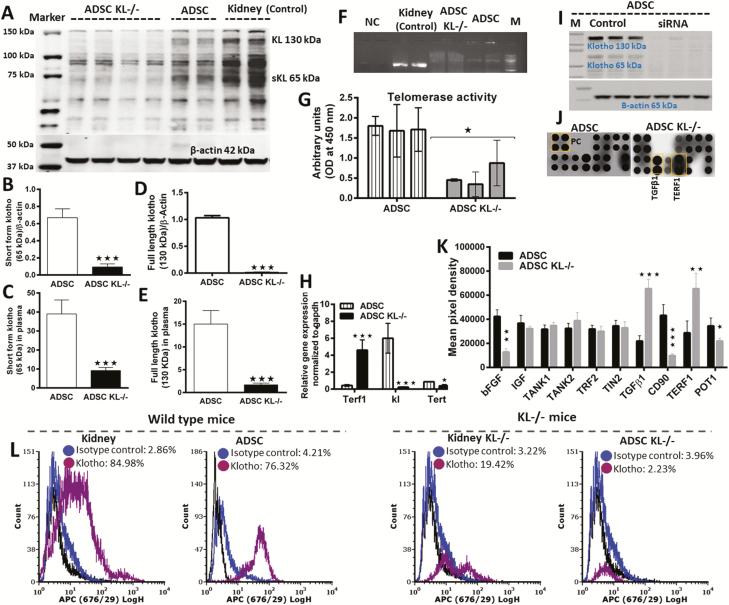
To determine the relationship between KL deficiency and decreased telomerase activity, we measured KL level both in ADSC and in the plasma of KL-/- mice (Figure 2). We found a significantly lower level of full-length (130 kDa) and secreted KL (65 kDa) in knockdown mice plasma and in isolated KL-/- ADSC (Figure 2A–E), suggesting that KL-/- ADSC diminished the amount of KL in circulation and in ADSC. Next, we set out to address the mechanisms linking KL and telomerase (Tert and terf1) expression in stem cell aging. To achieve this, we used specific siRNA to inhibit KL expression in the cultured ADSC and then measured telomerase activity. PCR (Figure 2F), western blot (Figure 2I), and flow cytometry (Figure 2L) results confirmed the complete knockdown of KL in the kidney as well as in cultured ADSC. Next, we performed ELISA to measure telomerase activity and found a significantly reduced level of telomerase (Figure 2G), confirming an association between KL and telomerase activity in KL-/- ADSC. To link this association with molecular mechanisms of aging, we conducted protein arrays of normal stem cells and then compared the results with the protein profile of KL-siRNA knockdown ADSC. Through a protein blot and its quantification, we found a significantly higher expression of telomeric repeat binding factor 1 (TERF1) and a significantly lower expression of protection of telomeres (POT1) protein (Figure 2J and K). Lower KL activity in KL-/-ADSC (Figure 2F and I) was associated with significantly shorter telomeres and lower telomerase activity (Figure 2G and H). Concomitant with this, the higher expression of Terf1 gene in KL-/-ADSC had the lower expression of telomerase activity as shown by real-time PCR of the telomerase reverse transcriptase (Tert) gene (Figure 2H). Interestingly, both KL deficiency and impaired telomerase activity (Figure 2) positively correlated with shortened telomeres, an effect that could be linked to reduced stem cell proliferation and aging. In summary, our results indicate that KL and telomerase association via Tert and Terf1 expression regulate the aging process in ADSC. We also observed higher expression of TGFβ1 and lower expression of CD90 and basic fibroblast growth factor (bFGF) growth factors in KL-/-ADSC (Figure 2J and K), which may also link KL and telomerase activity to stem cells aging. Short telomeres due to KL deficiency also led to aging in stem cells.
Figure 2.
Comparative klotho expression and protein profile of adipose-derived stem cells (ADSC) with or without klotho knockdown with siRNA. (A) Western blot showing full-length klotho (130 kDa) and short form klotho protein (65 kDa). (B,C) Comparative expression of short form klotho in ADSC and in plasma of KL-/- and wild-type mice. (D,E) Comparative expression of full-length klotho in ADSC and in plasma of KL-/- and wild-type mice. (F) PCR confirmation for klotho siRNA knockdown. (G) Telomerase activity in ADSC, and in klotho knockdown ADSC with siRNA. (H) Gene level confirmation of klotho siRNA knockdown and its effects on Terf1 and Tert expression. (I) Western blot confirming klotho siRNA knockdown. (J,K) Protein profile of telomerase activity in ADSC and siRNA knockdown ADSC for klotho. (L) Kidney and ADSC showing protein expression of KL measured by flow cytometry in wild-type mice and KL-/- mice. Mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001.
KL Impact on Proliferation, Growth Kinetics, and Metabolic Activity in ADSC
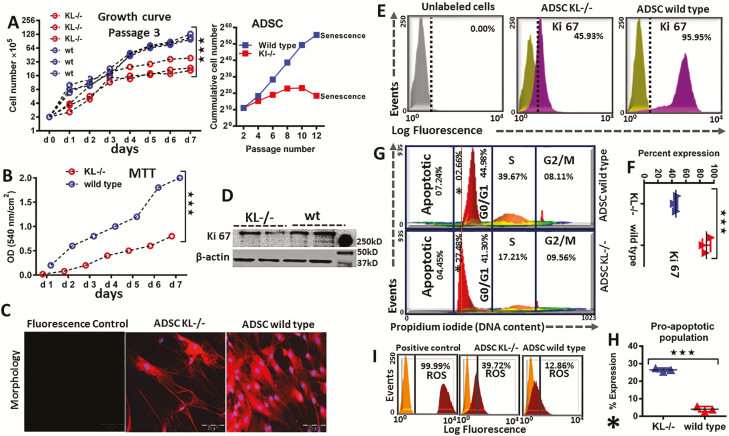
To elucidate KL effect on stem cell proliferation and growth, the number of cultured ADSC was measured by direct cell counting (Figure 3A). The proliferation rate of cultured ADSC was higher in a time-dependent manner when compared to the KL-/-ADSC group (Figure 3A). In accordance with this finding, the cumulative cell number of ADSC was also significantly higher (Figure 3A) in comparison to KL-/-ADSC group, as confirmed by higher proliferation and greater metabolic activity (Figure 3B). KL-/-ADSC showed the lowest metabolic activity, reduced proliferation, earlier senescence, and altered flattened morphology (Figure 3B and C). Wild-type ADSC displayed healthier and more fibroblastic-like features with active cells divisions than cultured ADSC (Figure 3B and C). KL-/-ADSC demonstrated low expression of Ki-67 (a known cell proliferation marker protein), as confirmed by western blot (Figure 3D), flow cytometric analysis (Figure 3E), and % comparative expression of Ki-67 between the KL-/-ADSC and the wild-type group (Figure 3F). To track markers of later stages of proliferation, a cell cycle analysis was performed using propodium iodide, which showed a major shift in histogram of the KL-/-ADSC group towards an apoptotic valley (Figure 3G). Making a new sub gate of proapoptotic cells, the KL-/-ADSC group showed a higher % of proapoptotic cells compared to the wild-type group (Figure 3H). To determine the causative factor of increased apoptosis in the KL-/-ADSC group, a ROS analysis was carried out and an elevated level of ROS generation was observed in KL-/-ADSC group (Figure 3I).
Figure 3.
Proliferation and metabolic activity of adipose-derived stem cells (ADSC) in KL-/- and wild-type animals. (A) Proliferation and growth curve of ADSC: Cells were counted daily in P3, and their cumulative cells number was recorded until senescence in P12, in KL-/- and wild-type group. (B) 5 × 103 cells/well were seeded and metabolic activity was measured by MTT assay. (C) Representative images showing morphology of P3 ADSC in KL-/- and wild-type mice. (D) Western blot of Ki-67 antigen (a nuclear protein and cellular proliferation marker). (E) Flow cytometric analysis of Ki 67, (F) and % comparative representation of Ki-67 in KL-/- and wild-type ADSC. (G) Cell cycle analysis was performed to determine healthy versus unhealthy ADSC, (H), which showing higher proapoptotic population in % for KL-/- group compared to wild type. (I) Flow cytometric analysis was carried out to monitor the reactive oxygen species (ROS), in KL-/- versus wild-type ADSC and ROS inducer (Pyocyanin 1 µM) was used to generate positive control. mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001, scale bar: 200 μm.
Role of KL in Pluripotency and Cellular Senescence
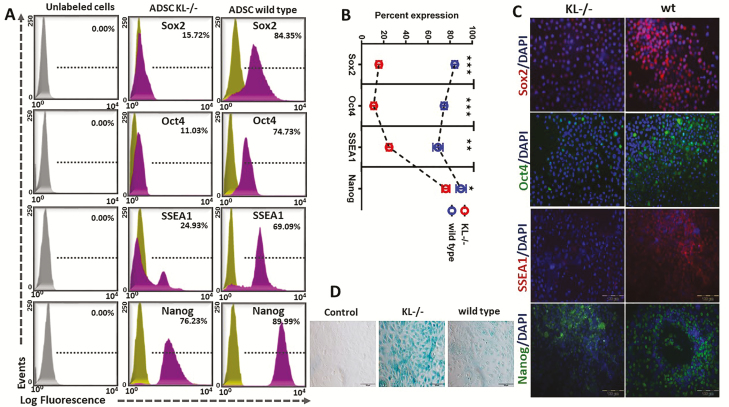
It has been well established that the role of telomere attrition is to limit the replicative capacity of cells in a culture (21). But how does telomerase activity become impaired by KL deficiency and does KL impact the process of pluripotency and cellular senescence? To answer this question, we analyzed pluripotent-markers like transcription factors such as Sox2, Oct4, SSEA1, and Nanog that are highly expressed in embryonic stem cells and in adult stem cells (22). Downregulation of these transcription factors is reported as the loss of pluripotency, impairment of self-renewal, and the stimulation of the differentiation process (22). Expression of these factors is vital to maintain the pluripotent state of stem cells. We studied the expression of these transcription factors using P0 cultured ADSC and observed a significantly higher expression of Sox2, Oct4, SSEA1, and Nanog pluripotent-markers in wild-type ADSC than in KL-/-ADSC (Figure 4A–C). Fluorescence analysis using antigen-specific probes identified higher expression of pluripotency-associated markers (Sox2, Oct4, SSEA1, and Nanog) in wild-type ADSC (Figure 4C). KL-/-ADSC showed a limited expression of pluripotency markers, which could potentially be a cause of cellular senescence and aging. Cellular senescence studies were also conducted and a greater rate of cellular senescence was noted in the KL-/-ADSC group when compared to the wild-type group (Figure 4D).
Figure 4.
Pluripotent-markers expression of adipose-derived stem cells (ADSC) in KL-/- and wild-type animals. (A) ADSC were analyzed for the expression of pluripotent-markers of Sox2, Oct4, stage-specific embryonic antigen (SSEA1), and Nanog. Representative histograms revealed lower in % expression of pluripotent-markers in KL-/-ADSC compared to wild type. (B) Histogram peaks showed lesser in % expression, of Sox2, Oct4, SSEA1 and Nanog in KL-/--derived ADSC. (C) Immunofluorescence labeled anti-mouse primary antibodies were used to determine the protein expression of Sox2, Oct4, SSEA1, and Nanog markers to know the level/intensity of expression in KL-/- group and wild-type ADSC. Nuclei were counter stained with DAPI (blue). (D) Senescence β-Galactosidase staining was used to differentiate between senescent and healthy cells in KL-/- and wild-type-derived ADSC. Mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001, scale bar: 100 μm.
Further Mechanistic Approach to Study the Role of KL Knockdown in ADSC
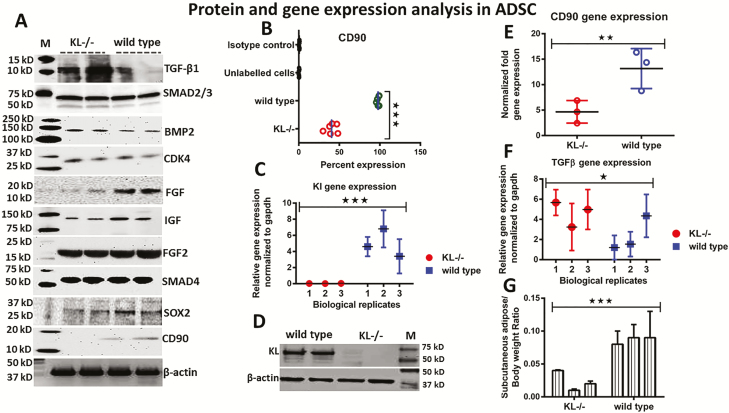
To further investigate the role of KL in stem cell regulation and aging, important targets were selected using bioinformatics and gene mining tools (23,24). Western blot analysis showed a higher expression of TGFβ1, Smad2/3, Smad4, Bmp2, and FGF2 proteins in KL-/-ADSC compared to wild-type ADSC (Figure 5A), while protein expression of FGF, IGF, Sox2, and CD90 was significantly downregulated in KL-/-ADSC in comparison to wild-type ADSC (Figure 5A). Additionally, we measured the % expression of CD90 by flow analysis, and found about a 50% reduction in its expression in KL-/-ADSC versus wild-type ADSC (Figure 5B). RT-PCR and western blot analysis were performed to ensure the status of KL expression. These results demonstrated significant downregulation of KL mRNA expression (Figure 5C), and the protein expression of KL was almost absent in KL-/-ADSC in comparison to wild-type ADSC (Figure 5D). To examine whether KL and telomeres accounted for the association of targets TERF1, TERT, TGFβ1, bFGF, and CD90 with stem cell aging, we further analyzed CD90 in our models. On a molecular level, CD90 gene expression was downregulated (Figure 5E), while TGFβ1 gene expression was upregulated in KL-/-ADSC (Figure 5F) compared to wild-type ADSC. We also observed lower fat deposits in KL-/-ADSC compared to wild-type ADSC (Figure 5G).
Figure 5.
Western blot and RT-PCR analysis. (A) Western blot was performed to analyze the expression of TGF-β1, Smad2/3, BMP2, CDK4, FGF, IGF, FGF2, Smad4, Sox2, CD90, and actin, in KL-/-ADSC and wild-type adipose-derived stem cells (ADSC). (B) Flow cytometric analysis showing lower % expression of CD90 in KL-/-ADSC. (C) Expression of KL mRNA in KL-/-ADSC compared to wild type. (D) Western blot confirmed the absence of KL protein in KL-/-ADSC while wild-type ADSC showed higher expression. (E) RT-PCR showing the expression of mRNA of CD90 (F) and TGF-β1 in KL-/- and wild-type-derived ADSC. (G) Subcutaneous adipose and animal body weight ratio in KL-/- and wild-type ADSC. Mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001.
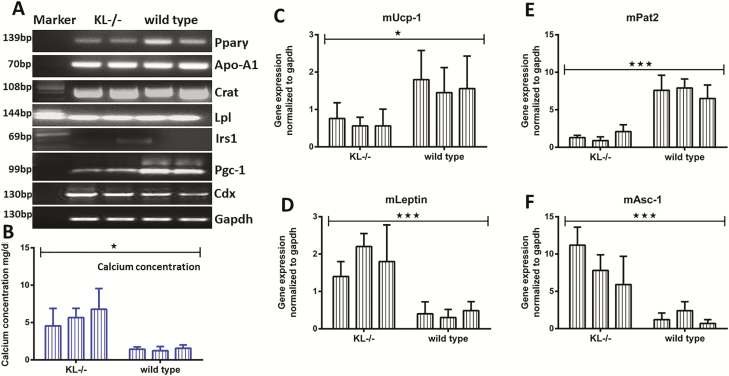
Thus, the investigative window was expanded to identify the additional targets of stem cell aging. As a result, some major targets were recorded as having an elevated gene expression in KL-/-ADSC. These targets were Apo-A1, Crat, Lpl, and Cdx and FGF23 (Figure 6A, Supplementary Figure 6). In contrast, the targets Pparg, Iris1, and Pgc-1 demonstrated a downregulated gene expression pattern in KL-/-ADSC and most likely had an association with KL (Figure 6A). FGF23 expression changed inversely to KL expression (Supplementary Figure 6). In Figures 1D,5B, and 5E, we recorded a remarkable reduction in CD90 expression on both a proteomic and genetic level for KL-/-ADSC. The expression of CD90 is associated with calcium concentration and mineralization (17). Consistent with this, we observed higher calcium level in KL-/-ADSC (Figure 6B). Due to the distinctive difference in fat morphology between WT and KL-/-ADSC as shown in Figure 1C, we investigated the expression of various brown and white fat-specific genes, which is also known to play an important role in functional classification of fat and energy metabolism. We noted a downregulated expression of the brown fat-specific genes of Ucp1 (Figure 6C), Pat2 (Figure 6E), and significantly upregulated expression of white fat-specific genes of Leptin (Figure 6D), and Asc-1 (Figure 6F) in KL-/-ADSC compared to wild-type ADSC. These genes play a crucial role in lipid homeostasis and energy production for vital processes (25) and may even regulate the mechanism of aging in stem cells.
Figure 6.
Expression of gene targets in KL-/-ADSC and wild-type adipose-derived stem cells (ADSC). (A) As shown on agarose gel, gene expression of Pparg, Irs1, and Pgc-1 was downregulated, while upregulated for Apo-A1, Crat, Lpl, and Cdx genes in KL-/-ADSC. (B) Membrane bound calcium concentration of ADSC was determined in KL-/- and wild-type group. (C) RT-PCR was performed for (C) mUcp-1, (D) mLeptin, (E) mPat2, (F) and mAsc-1, genes. Mean ± SEM, statistically significant values are: *p = .05; **p = .01; ***p = .001.
Stem Cells Treated with Telomerase Activator CAG for Telomere Restoration
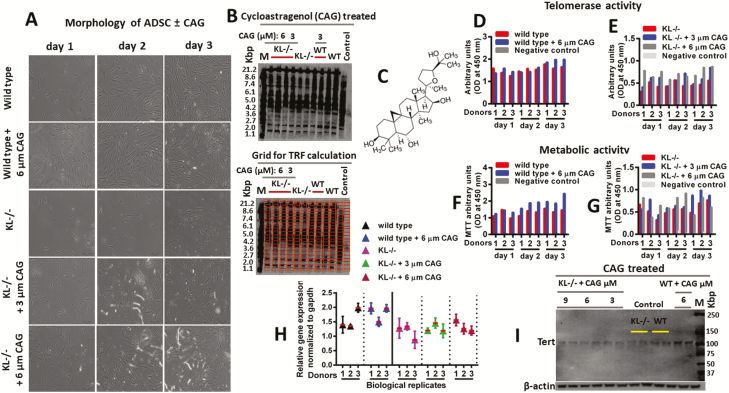
To examine the restorative properties of CAG, KL-/-ADSC were treated with the telomerase activator CAG to restore the telomere length and telomerase activity. We found that CAG treatment successfully restored normal morphology of ADSC (Figure 7A), enhanced telomere length (Figure 7B), and boosted telomerase activity (Figure 7D and E). Treated KL-/-ADSC with CAG displayed higher cellular proliferation and metabolic activity as shown by MTT assay (Figure 7F and G). In addition, CAG treatment improved telomerase gene expression, and increased catalytic activity of TERT (Figure 7H, 7I, and Figure 8). Restoration of telomere length and telomerase activity in stem cells by using 6-µm CAG treatment was sufficient to renew cellular proliferation abilities and partially rescued the regenerative power of KL-/-ADSC (Figure 8). The CAG treatment improved aging-related deformities in KL-deficient ADSC and partially preserved telomere length and telomerase activity in stem cells.
Figure 7.
Telomere length and telomerase activity of adipose-derived stem cells (ADSC). (A) Representative microscopic images, showing morphology of wild-type ADSC, KL-/-ADSC, and KL-/-ADSC treated with 3 or 6 µM cycloastragenol (CAG). (B) Southern blot assay showing the telomere length size for untreated and treated samples with CAG. The blot-grid image was used for Telomere Restriction Fragment (TRF) calculation (C) Chemical structure of the CAG compound used for treatment. (D,E) Telomerase activity, and (F,G) Metabolic activity of the untreated and CAG-treated samples. (H) PCR and (I) western blot of telomerase activity in the untreated and CAG-treated samples.
Figure 8.
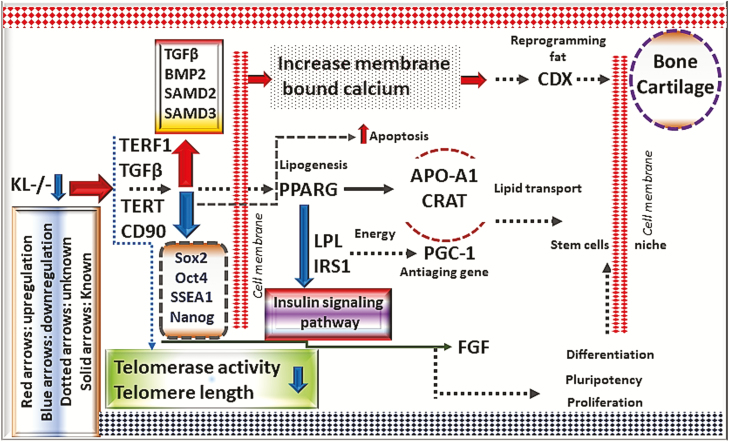
Flow sheet diagram showing that how Klotho (KL) impacts telomerase activity and telomeres length using different signaling pathways of TGFβ, insulin, and Wnt in adipose-derived stem cells proliferation, differentiation, reprogramming, pluripotency, stem cells niche, self-renewal, and stem cells aging.
Discussion
Our findings suggest that KL is associated with telomerase activity and telomere length and can directly affect the aging process of stem cells. Stem cell dysfunction is provoked by KL deficiency and telomere shortening and could also be one of the mechanisms responsible for human aging. While reporting the current evidence linking impaired-KL expression with telomere shortening and stem cells aging, we noticed deteriorating telomerase activity in KL-/-ADSC in the presence of decreased KL expression, “an antiaging protein,” suggesting that KL plays a role in life-extension by regulating telomere length and telomerase activity. Telomere-deficient ADSC were then treated with CAG which stimulated partial improvement in telomerase activity along with growth and proliferation recovery. These findings could have broad therapeutic implications for aging-related disease like cardiovascular, diabetes, and other degenerative health issues. Since aging is a primary risk factor and emerging health threat to age-related morbidities and is an economic burden on the entire health care system, research related to this topic is of utmost urgency (7,26). Here, we reported that KL does not accomplish the life-extension job on its own; instead, KL also needs some degree of telomerase activity to prolong the lifespan of stem cells. Furthermore, KL has a strong communicative cross link with telomeres for the regulation and proliferation of stem cells and represents a new therapeutic avenue in aging-related diseases. It is still debated whether soluble or full-length KL serves as an antiaging hormone, since a receptor for soluble KL has not been identified to date (27). Additionally, a recent paper suggests that soluble KL only acts with fibroblast growth factor 23 (FGF23) and serves as a circulating FGF23 coreceptor, but has no FGF23-independent functions (27,28). The aging suppressor protein KL binds to the fibroblast growth factor receptor and maintains serum phosphate levels within normal range by increasing renal phosphate excretion (29). In our study, KL-/- ADSC showed higher levels of FGF23 expression, suggesting that both KL and FGF23 work in conjugation with the process of stem cells aging.
In the process of aging, KL could possibly establish a genetic or epigenetic connection with telomeres and coordinate stem cell reprogramming to a more youthful state (30). To validate our experimental hypothesis, we used KL-/-ADSC and examined their cellular processes of proliferation, growth kinetics, metabolic activity, cellular senescence, pluripotency, and reactive oxygen species. Using this information, we then identified the major targets (TGFβ, insulin, Wnt, and telomeres signaling pathways), since it is believed that they affect the aging activity of stem cells in accordance with KL deficiency (1,31). KL-/-ADSC starts senescence after passage 8, while wild-type ADSC showed higher growth even after passage 12. We observed a longer life span and a higher metabolic activity in wild-type ADSC, whereas KL expression was normal. Due to these results, augmenting KL or its effects are promising tools of enhancing the growth of stem cells and their pluripotency.
We also found higher gene expression of fat-specific markers, like Fabp4 and Ppar-γ, in wild-type-derived ADSC compared to KL-/-ADSC. Ppar-γ is a transcription factor involved in adipogenesis. It has recently been demonstrated to have a direct effect on KL expression and, hence, have a role in regulating the process of aging (16). Aging is an unavoidable but an extendable process (31). During stem cell aging, both exogenous and endogenous factors contribute to this course of biological aging and KL is one of those factors (31). In this study, we found an alteration in expression of main targets of insulin signaling pathways, like IGF-1, FGF, and others. In recent studies, this has been concluded to indicate that KL probably uses its antiaging traffic on CD90, TGFβ1, insulin, Wnt, and telomeres signaling pathways (11,32). Recently, it has become evident that telomeres and telomerase are main components of the processes that control stem cell aging mechanisms (5,33,34). Our study identified KL as a novel master regulator of telomere length, which could be an important consideration in delaying aging-related morbidities.
It has been an established fact that stem cell depletion is associated with senescence in aged tissues (30,35). Unlike apoptotic cells, senescent cells remain alive, and upregulate cell cycle arrest genes and additional inhibitors (35), as shown in Figures 3–5. As a result, senescent cells secrete a panel of bioactive proteolytic enzymes, inflammatory cytokines, growth factors, and ROS species (35), which may further speed up the aging process by pushing the stem cell population toward a valley of death, as shown in Figures 3–5.
Some external stimuli are also required to regulate stem cell proliferation and aging (36). Our study demonstrates that such external signals could be coming from KL-interlinked telomerase activity using TGFβ1, bFGF, and CD90 signaling. We observed a significant reduction in CD90 expression of KL-/-ADSC, which led to an increase in calcium concentration and mineralization as documented elsewhere (17). Stem cell aging is affected not only by its origin and growth condition, but also by many different cellular pathways that disrupt stem cell functions (37). We demonstrate that KL has a strong communicative link with telomerase activity in the process of stem cells aging, and represents a new therapeutic avenue in age-related diseases.
To maintain stem cell populations, it is essential to sustain a pool of stem cells through multiple rounds of tissue regeneration (36,38). In accordance with this, we observed significantly higher expression of TGFβ1 protein and Terf1 mRNA in KL-/-ADSC, which is necessary to maintain the telomerase activity for sustained stem cells proliferation. TGFβ1, along with other regenerative factors, takes part in the regulation and proliferation of stem cells (32). In addition, TGFβ1 also secrets inflammatory mediators that promote the health of stem cells to hinder aging (11,32,35). Accumulation of these active factors toxifies the stem cell’s niche and causes a further buildup of senescent cells in aging tissues. This can be another driver of chronic inflammation that enhances the apoptotic and degradative signals for unprotected proteolytic activity for neighboring cells, and in this way promotes senescence and inflammation (11,35).
Published evidence indicates that stem cell aging is affected by other pathways as well, that also use a shared cross-talk in the determination of stem cell function (39). For example, the mTOR pathway has been shown to play a major role in stem cell aging via ROS and autophagy (39). Stem cells appear to be particularly sensitive to elevated ROS levels. As we observed a higher level of ROS in KL-deficient ADSC, we note that such an elevated level of ROS species could contribute to accumulation of senescent cells and other toxic metabolites, endangering the life of cells and leading to limited stem cell function.
Stem cell aging, caused by accumulated toxic metabolites, cellular senescence, ROS, and metabolic stress, contributes to a decline in stem cells functions and depletion of stem cell pool (34,35,39). However, using KL overexpression alone or in combination with telomeres can cause aging phenotypes to be reversed, restoring the regenerative functions of stem cells in some cases (8,40,41). Such regenerative factors hold promise for the treatment of many diseases, including cardiovascular, renal and neural degeneration (26,42,43). Whether these factors certainly restore stem cell functions to a youthful state or instead induce a state of “pseudo-youth” in which the reprogrammed cells retain an epigenetic memory of their origin remains a debated question.
Aging is also associated with cardiovascular disease, neuropathy, cancer, and many other diseases (9,10,44). Uncovering the underlying molecular mechanism of this process on a cellular level would help modern medicine advance towards new therapeutic options. Stem cells and KL are not necessarily the “fountain of life,” but they are powerful tools that may speed up the journey of unraveling new strategies that open new avenues for futuristic therapeutic options.
Nonetheless, KL and telomere overexpression are both crucial for life-extension, generating much excitement about the development of novel “rejuvenating” interventions that could extend the number of healthy years in our lives (1,45). Even small gains in this area of research are a step toward dramatically lessening the burden of the aging population on the health care system and economy.
Knowledge about the aging of stem cells has exploded in the past decade and numerous studies demonstrate links between KL and aging (12,34). Nevertheless, the exact mechanisms of how KL accomplishes this task remain elusive. Current findings suggest that telomere-induced premature stem cells aging might be influenced by KL deficiency. Telomere shortening has been a reported cause of stem cell aging and KL impairment which advances this aging journey (31). We investigated the hypothesis that KL impacts aging by moderating telomere length and telomerase activity. This manuscript provides evidence that KL deficiency is highly associated with lower proliferation, reduced pluripotency, higher oxidative stress, lower telomerase activity, and shorter telomere length, all known determinants of stem cells aging. In addition, we treated KL-deficient ADSC with CAG, which is known to stimulate telomerase activity and cell repair (46). In response, this CAG treatment (6 µm) successfully attenuated pluripotency, cell loss, apoptosis, cellular senescence, promoted cell proliferation, increased cell survival growth rate, enhanced telomerase expression, and partially rescued telomere length in stem cells. These valuable findings hold promising implications for understanding how, at the cellular level, both KL and telomerase activity may promote an earlier onset of age-related diseases.
Funding
This work was supported by the National Institute of Health (NIH), USA. The supported NIH grants number are R01 HL118558, DK093403, HL122166, HL116863, and AG049780.
Conflict of Interest
None reported.
Authors’ contributions
M.U.: Conceptualization, data mining, data analysis, and writing of research manuscript. M.U. and Z.S. were responsible for the investigation, conceptualization, manuscript analysis, editing suggestions, and final approval. Both authors read and approved the final manuscript.
Ethics approval and consent to participate: All the experimental procedures were performed at the University of Oklahoma Health Sciences Center (OUHSC) animal care facility. Animal experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee at the OUHSC.
Authors’ information: Department of Physiology, BMSB, College of Medicine University of Oklahoma Health Sciences Center (OUHSC), and Biomedical Research Center, BRC, 940 Stanton L. Young Blvd. Oklahoma City, OK 73126-0901, USA
Supplementary Material
Acknowledgments
The authors are grateful for the assistance of National Institute of Health (NIH) for funding and professors working at the department of physiology especially Dr. Shirley Wang and Dr. Yi Lin University of Oklahoma Health Science Center, and Sriya Jonnakuti for professional editing of the manuscript. This work could not be done without them. The authors also want to thank experts working at Biomedical Research Center, University of Oklahoma.
References
- 1. Nurkovic J, Volarevic V, Lako M, Armstrong L, Arsenijevic N, Stojkovic M. Aging of stem and progenitor cells: mechanisms, impact on therapeutic potential, and rejuvenation. Rejuvenation Res. 2016;19:3–12. doi: 10.1089/rej.2015.1676 [DOI] [PubMed] [Google Scholar]
- 2. Rando TA, Wyss-Coray T. Stem cells as vehicles for youthful regeneration of aged tissues. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S39–S42. doi: 10.1093/gerona/glu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2017;72:1513–1522. doi: 10.1093/gerona/glx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vidacek NŠ, Nanic L, Ravlic S, et al. Telomeres, nutrition, and longevity: can we really navigate our aging?J Gerontol A Biol Sci Med Sci. 2017;73:39–47. doi: 10.1093/gerona/glx082 [DOI] [PubMed] [Google Scholar]
- 5. Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180 [DOI] [PubMed] [Google Scholar]
- 6. Vetter VM, Meyer A, Karbasiyan M, Steinhagen-Thiessen E, Hopfenmuller W, Demuth I. Epigenetic clock and relative telomere length represent largely different aspects of aging in the Berlin Aging Study II (BASE-II). J Gerontol A Biol Sci Med Sci. 2018; doi:10.1093/gerona/gly184 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuo Z, Lei H, Wang X, Wang Y, Sonntag W, Sun Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr). 2011;33:261–274. doi: 10.1007/s11357-010-9176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng Y, Wang PH, Zhang M, Du JR. Aging-related renal injury and inflammation are associated with downregulation of Klotho and induction of RIG-I/NF-κB signaling pathway in senescence-accelerated mice. Aging Clin Exp Res. 2016;28:69–76. doi: 10.1007/s40520-015-0371-y [DOI] [PubMed] [Google Scholar]
- 11. Bian A, Neyra JA, Zhan M, Hu MC. Klotho, stem cells, and aging. Clin Interv Aging. 2015;10:1233–1243. doi: 10.2147/CIA.S84978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113–122. doi:10.3904/kjim.2011.26.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salech F, Varela-Nallar L, Arredondo SB, et al. Local Klotho enhances neuronal progenitor proliferation in the adult hippocampus. J Gerontol A Biol Sci Med Sci. 2017. doi:10.1093/gerona/glx248 [DOI] [PubMed] [Google Scholar]
- 14. Vera E, Bernardes de Jesus B, Foronda M, Flores JM, Blasco MA. Telomerase reverse transcriptase synergizes with calorie restriction to increase health span and extend mouse longevity. PLoS One. 2013;8:e53760. doi: 10.1371/journal.pone.0053760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, et al. Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis. 2006;44:225–232. doi: 10.1002/dvg.20207 [DOI] [PubMed] [Google Scholar]
- 16. Fan J, Sun Z. The antiaging gene klotho regulates proliferation and differentiation of adipose-derived stem cells. Stem Cells. 2016;34:1615–1625. doi: 10.1002/stem.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiesmann A, Bühring HJ, Mentrup C, Wiesmann HP. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006;2:8. doi: 10.1186/1746-160X-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Y, Sun Z. Antiaging gene Klotho attenuates pancreatic β-cell apoptosis in type 1 diabetes. Diabetes. 2015;64:4298–4311. doi: 10.2337/db15-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40(Database issue):D1144–D1149. doi: 10.1093/nar/gkr1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludlow AT, Spangenburg EE, Chin ER, Cheng WH, Roth SM. Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J Gerontol A Biol Sci Med Sci. 2014;69:821–830. doi: 10.1093/gerona/glt211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trivanović D, Jauković A, Popović B, et al. Mesenchymal stem cells of different origin: comparative evaluation of proliferative capacity, telomere length and pluripotency marker expression. Life Sci. 2015;141:61–73. doi: 10.1016/j.lfs.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 23. Fernández JM, Hoffmann R, Valencia A. iHOP web services. Nucleic Acids Res. 2007;35(suppl_2):W21–W26. doi: 10.1093/nar/gkm298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maier H, Döhr S, Grote K, et al. LitMiner and WikiGene: identifying problem-related key players of gene regulation using publication abstracts. Nucleic Acids Res. 2005;33(Web Server issue):W779–W782. doi: 10.1093/nar/gki417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ullah M, Sittinger M, Ringe J. Extracellular matrix of adipogenically differentiated mesenchymal stem cells reveals a network of collagen filaments, mostly interwoven by hexagonal structural units. Matrix Biol. 2013;32:452–465. doi: 10.1016/j.matbio.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 26. Martín-Núñez E, Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Implications of Klotho in vascular health and disease. World J Cardiol. 2014;6:1262–1269. doi: 10.4330/wjc.v6.i12.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen G, Liu Y, Goetz R, et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466. doi: 10.1038/nature25451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inci A, Sari F, Coban M, et al. Soluble Klotho and fibroblast growth factor 23 levels in diabetic nephropathy with different stages of albuminuria. J Investig Med. 2016; 64:1128–1133. doi:10.1136/jim-2016-000142 [DOI] [PubMed] [Google Scholar]
- 29. Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract. 2014;128:1–10. doi: 10.1159/000365787 [DOI] [PubMed] [Google Scholar]
- 30. Li T, Shi Y, Wang P, et al. Smg6/Est1 licenses embryonic stem cell differentiation via nonsense-mediated mRNA decay. EMBO J. 2015;34:1630–1647. doi: 10.15252/embj.201489947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ullah M, Sun Z. Stem cells and anti-aging genes: double-edged sword-do the same job of life extension. Stem Cell Res Ther. 2018;9:3. doi: 10.1186/s13287-017-0746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010;223:658–666. doi: 10.1002/jcp.22064 [DOI] [PubMed] [Google Scholar]
- 33. Vidacek NŠ, Nanic L, Ravlic S, et al. Telomeres, nutrition, and longevity: can we really navigate our aging?J Gerontol A Biol Sci Med Sci. 2017;73:39–47. doi: 10.1093/gerona/glx082 [DOI] [PubMed] [Google Scholar]
- 34. Sun MUaZ. Antiaging Gene “Klotho” deficiency accelerates stem cells aging by impairing telomere. The FASEB Journal. 2017;31. [Google Scholar]
- 35. Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- 36. Artandi SE, Blau HM, de Haan G, et al. Stem cells and aging: what’s next?Cell Stem Cell. 2015;16:578–581. [DOI] [PubMed] [Google Scholar]
- 37. Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meena JK, Cerutti A, Beichler C, et al. Telomerase abrogates aneuploidy-induced telomere replication stress, senescence and cell depletion. EMBO J. 2015;34:1371–1384. doi: 10.15252/embj.201490070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santos-Dias A, MacKenzie B, Oliveira-Junior MC, Moyses RM, Consolim-Colombo FM, Vieira RP. Longevity protein klotho is induced by a single bout of exercise. Br J Sports Med. 2017;51:549–550. doi: 10.1136/bjsports-2016-096139 [DOI] [PubMed] [Google Scholar]
- 41. Gołembiewska E, Stępniewska J, Kabat-Koperska J, Kędzierska K, Domański M, Ciechanowski K. The Role of Klotho protein in chronic kidney disease: studies in animals and humans. Curr Protein Pept Sci. 2016;17:821–826. doi:10.2174/1389203717666160526123646 [DOI] [PubMed] [Google Scholar]
- 42. Yu L, Meng W, Ding J, Cheng M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem Biophys Res Commun. 2016;473:455–461. doi: 10.1016/j.bbrc.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Geest KS, Lorencetti PG, Abdulahad WH, et al. Aging-dependent decline of IL-10 producing B cells coincides with production of antinuclear antibodies but not rheumatoid factors. Exp Gerontol. 2016;75:24–29. doi: 10.1016/j.exger.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 45. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi:10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ip FC, Ng YP, An HJ, et al. Cycloastragenol is a potent telomerase activator in neuronal cells: implications for depression management. Neurosignals. 2014;22:52–63. doi: 10.1159/000365290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.