Abstract
This cohort study analyzes the medical records of 47 721 patients with various cancer types with POLE/POLD1 mutations to evaluate whether the mutations were associated with immunotherapy outcomes.
Immune-checkpoint inhibitor (ICI) therapy, including antibodies targeting programmed cell death protein 1(PD-1), programmed death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte–associated protein 4 (CTLA4), has demonstrated impressive clinical efficacy in controlling advanced cancers. Recent studies have identified several positive predictive markers for ICI, including high levels of microsatellite instability (MSI-high), PD-L1 overexpression, and elevated tumor mutation burden (TMB).1 The genes that encode DNA polymerase epsilon (POLE) and delta 1 (POLD1) are essential for proofreading and fidelity in DNA replication.2 Their germline or somatic mutations can lead to DNA-repair deficiencies and carcinogenesis via a DNA hypermutated molecular phenotype.3,4 An association between POLE or POLD1 mutations and clinical benefit to ICI has been observed in several case reports.5,6 However, to our knowledge, a comprehensive analysis of POLE or POLD1 mutation frequency and their predictive value for ICI treatment outcome has not yet been reported. In this study, we conducted a combined analysis using a large data set and found that POLE/POLD1 mutations are promising potential predictive biomarkers for positive ICI outcomes.
Methods
All the patients and mutation data were selected from the cBioPortal database (https://www.cbioportal.org). All nonsynonymous mutations including missense, frame-shift, nonsense, nonstop, splice site, and translation start site changes of POLE/POLD1 were considered. To compare the tumor mutation burden (TMB) between different groups, a subset generated from MSK-IMPACT was selected to ensure the TMB could be comparable. The TMB was calculated with the total number of mutations divided by the number of bases in the target panel. For survival analysis, Kaplan-Meier survival curves were generated and compared using the log-rank test. All data were analyzed from December 25, 2018, to January 21, 2019. This study was deemed exempt from institutional board approval and patient informed consent was waived because all patient data were deidentified.
Results
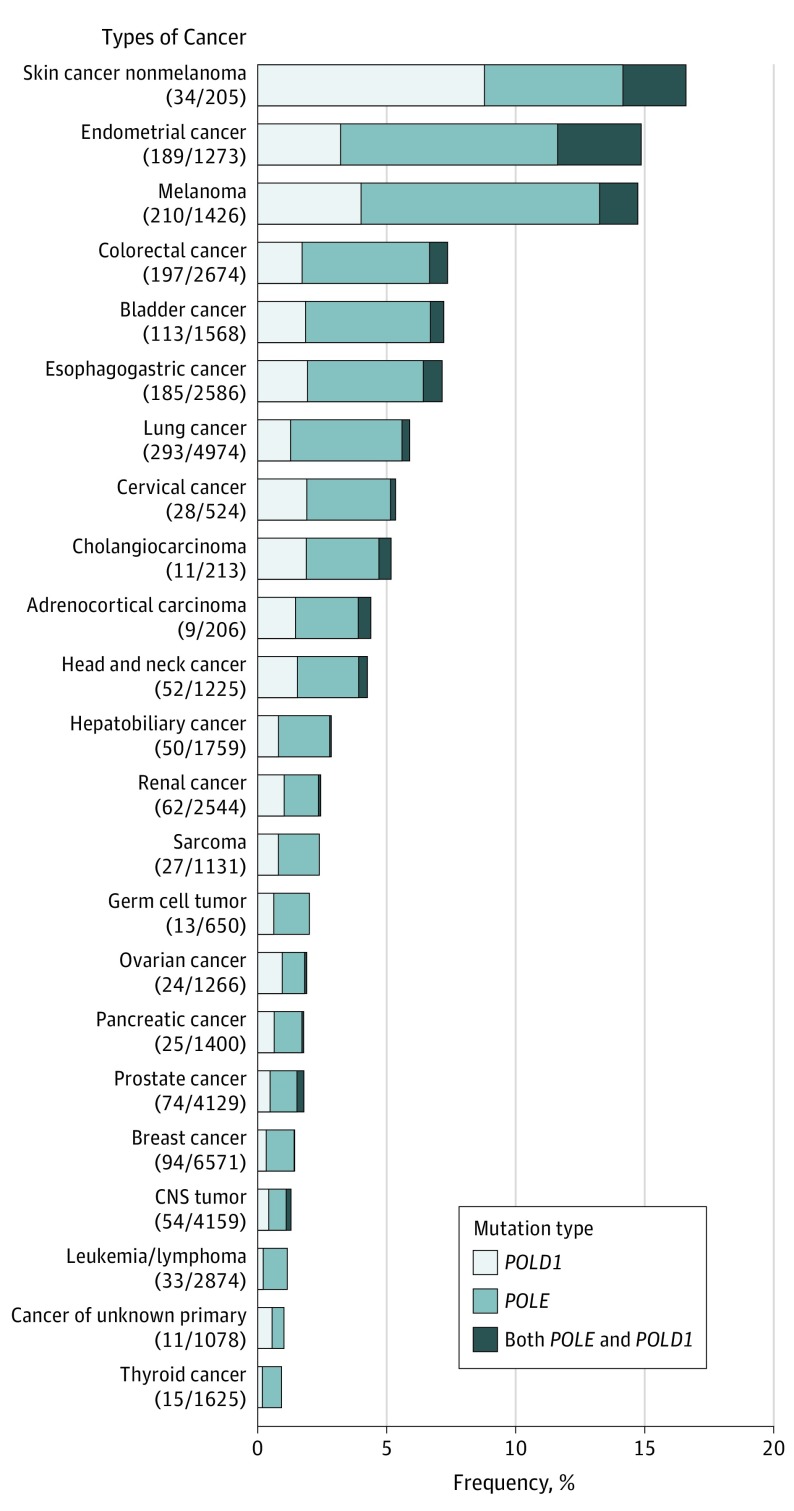
The prevalence of POLE/POLD1 mutations in 47 721 patients with different cancer types is summarized in Figure 1, with patients with nonmelanoma skin cancer having the highest levels of POLE/POLD1 mutations (16.59%). Across all 47 721 patients, the mutational frequencies of POLE and POLD1 were 2.79% and 1.37%, respectively. The TMB of patients with these mutations was substantially higher than in those without the mutations in most of the cancer types.
Figure 1. Prevalence of POLE/POLD1 Mutations in 47 721 Patients With Different Cancer Types.
CNS indicates central nervous system.
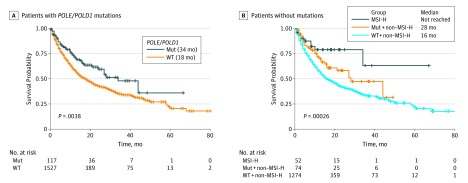
We further investigated the association between POLE/POLD1 mutations and overall survival (OS) in the ICI treatment cohort.1 As shown in Figure 2, patients with either POLE or POLD1 mutations showed a significantly longer OS of 34 months vs 18 months in the wild-type population (log-rank test, χ2 = 8.4; P = .004). Seventy-four out of 100 patients with POLE/POLD1 mutations were microsatellite stable (MSS) or had low levels of microsatellite instability (MSI-L). When cancer types and MSI status were adjusted for a multivariable Cox regression analysis, POLE/POLD1 mutations were an independent risk factor for identifying patients who benefited from ICI treatment (P = .047; hazard ratio, 1.41; 95% CI, 1.00-1.98). Analysis of POLE/POLD1 mutations could identify patients who can benefit from ICI treatment besides those with MSI-H (Figure 2). No significant differences in OS were observed between patients with MSI-H and those patients with POLE/POLD1 mutations who were non-MSI-H. Notably, the patients with POLE exonuclease domain mutation or with other mutations showed no difference in levels TMB or OS.
Figure 2. Overall Survival of Patients With POLE/POLD1 Mutations vs Those Without or With MSI-H.
MSI-H indicates patients who have high levels of microsatellite instability; Mut indicates patients with POLE/POLD1 mutations; WT indicates patients without mutations.
Discussion
From a cohort of 47 721 patients with different types of cancer, a high frequency of POLE/POLD1 mutations were observed not only in endometrial cancer and colorectal cancer, but also skin cancer, esophagogastric cancer, bladder cancer, lung cancer and others. We also observed that POLE or POLD1 mutations were a negative prognostic marker and might be used to predict a survival benefit from ICI therapy across diverse cancer types. Nonsynonymous mutations in POLE/POLD1 not found in the exonuclease domain had similar associations with the OS of patients receiving ICI treatment, suggesting that mutations in all exons of these 2 genes should be integrated into predictive biomarker panels for ICI therapy. Based on these data and rationale, we have initiated a phase 2 clinical trial for patients with solid cancer and POLE/POLD1 mutations who are non-MSI-H to test the treatment outcomes of toripalimab, a PD1 antibody.
References
- 1.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi: 10.1038/s41588-018-0312-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayner E, van Gool IC, Palles C, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nat Rev Cancer. 2016;16(2):71-81. doi: 10.1038/nrc.2015.12 [DOI] [PubMed] [Google Scholar]
- 3.Palles C, Cazier JB, Howarth KM, et al. ; CORGI Consortium; WGS500 Consortium . Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45(2):136-144. doi: 10.1038/ng.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li HD, Cuevas I, Zhang M, et al. Polymerase-mediated ultramutagenesis in mice produces diverse cancers with high mutational load. J Clin Invest. 2018;128(9):4179-4191. doi: 10.1172/JCI122095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126(6):2334-2340. doi: 10.1172/JCI84940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]