Abstract
Background
Elderly patients are underrepresented in clinical trials but comprise the majority of heart failure patients. Data on age-specific use of heart failure therapy are limited. The European Society of Cardiology heart failure guidelines provide no age-specific treatment recommendations. We investigated practice-based heart failure management in a large registry at heart failure outpatient clinics.
Design and methods
We studied 8351 heart failure with reduced ejection fraction patients at 34 Dutch outpatient clinics between 2013 and 2016. The mean age was 72.3 ± 11.8 years and we divided age into three categories: less than 60 years (13.9%); 60–74 years (36.0%); and 75 years and over (50.2%).
Results
Elderly heart failure with reduced ejection fraction patients (≥75 years) received significantly fewer beta-blockers (77.8% vs. 84.2%), renin–angiotensin system inhibitors (75.2% vs. 89.7%), mineralocorticoid receptor antagonists (50.6% vs. 59.6%) and ivabradine (2.9% vs. 9.3%), but significantly more diuretics (88.1% vs. 72.6%) compared to patients aged less than 60 years (Pfor all trends < 0.01). Moreover, the prescribed target dosages were significantly lower in elderly patients. Also, implantable cardioverter defibrillator (18.9% vs. 44.1%) and cardiac resynchronisation therapy device (14.6% vs. 16.7%) implantation rates were significantly lower in elderly patients. A similar trend in drug prescription was observed in patients with heart failure with mid-range ejection fraction as in heart failure with reduced ejection fraction.
Conclusion
With increasing age, heart failure with reduced ejection fraction patients less often received guideline-recommended medication prescriptions and also in a lower dosage. In addition, a lower percentage of implantable cardioverter defibrillator and cardiac resynchronisation therapy device implantation in elderly patients was observed.
Keywords: Heart failure, HFrEF, guideline adherence, age, treatment
Introduction
Chronic heart failure (HF) is a major healthcare problem, associated with a poor prognosis, high morbidity and mortality.1 Optimising medical and device therapy according to the guidelines improves prognosis.2 Therefore, adherence to the guidelines, such as the rate of drug prescription and dosage, are often used as benchmarks of quality of care. Approximately 1–2% of the global adult population is diagnosed with HF.3 Due to an aging population and better survival of underlying heart diseases, these numbers are expected to rise even further.4 Elderly patients are a major part of the HF population, with approximately 80% older than 65 years, and 40–50% even aged 75 years or older.2,5
In elderly patients, HF is the leading cause of hospitalisation and is associated with high morbidity and mortality, resulting in an enormous burden on hospital resources.6 Due to the high prevalence of comorbidities in elderly patients, optimising HF management remains even more challenging.7 Until now, randomised clinical trials investigating HF therapy did not include large number of elderly patients,8 with the exception of the SENIORS trial.9 In fact, patients enrolled in these trials were on average 10 years younger than in daily clinical practice,3 and elderly patients were clearly underrepresented.10 A few registries have shown a lower prescription rate in the elderly but lack size.8,11 Despite the ongoing discussion on optimal therapy in elderly HF patients, there is no European Society of Cardiology (ESC) recommended age-specific guidelines for HF treatment,2 and data in groups of patients with advanced age are scarce.
Therefore, we investigated age-related differences in HF therapy in a large-scale cross-sectional registry in 34 Dutch HF clinics, reflecting actual practice-based HF care at outpatient clinics including large numbers of elderly patients.
Methods
The design and methods of the CHECK–HF (Chronisch Hartfalen ESC – richtlijn Cardiologische praktijk Kwaliteitsproject HartFalen) registry have been published in detail elsewhere.12,13 Briefly, the CHECK–HF registry consists of 10,910 patients with chronic HF from a total of 34 participating Dutch centres, participating in the inclusion for this cross-sectional observational cohort. Between 2013 and 2016, all centres included patients diagnosed with HF according to 2012 ESC guidelines on HF,2 based on symptoms and echo parameters, who were seen at the outpatient HF clinic (96%) or general cardiology outpatient clinic (4%) if no specific HF clinic was present.
Baseline patient characteristics, aetiology of HF, comorbidities, basic echocardiographic and electrocardiographic parameters, laboratory markers, pacemaker, implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy (CRT) treatment, as well as prescription rates of medication (drug name, dosage and frequency and total daily dose), were recorded. Furthermore, contraindication and intolerance rates were collected.
Ivabradine was only considered indicated on top of optimal treatment with beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs) (or angiotensin II receptor blockers (ARBs)) and mineralocorticoid receptor antagonists (MRAs) (or ARBs), and if patients were in sinus rhythm, left ventricular ejection fraction (LVEF) of 35% or less, heart rate of 70 beats/minute or greater and were still symptomatic (New York Heart Assocation (NYHA) ≥II), or already received ivabradine. Target doses of guideline-recommended HF therapy are presented in Supplementary Table 1.
Based on echocardiographic results, patients were classified based on LVEF or visual assessment of the function of the left ventricle function as heart failure with reduced ejection fraction (HFrEF, LVEF <50% (n = 8360 (76.6%))), and according to 2016 ESC HF guidelines as heart failure with mid-range ejection fraction (HFmrEF) (LVEF 40–49% (n = 1574 (14.4%))) in those with available measurement of ejection fraction. In addition, HFpEF was classified as LVEF of 50% or greater in 2267 (20.8%) patients. In 274 (2.5%) patients, recording of the left ventricular function in the database was insufficient to classify patients into HF type, in nine patients (0.1%) age was missing in the database, and they were excluded from this analysis. In the current analyses, we focus on age-related treatment differences in guideline recommended HF therapies, including device therapy and lifestyle interventions, in HFrEF and HFmEF patients only.
Statistical analysis
Continuous data are expressed as mean value ± SD or median and interquartile range, depending on the distribution of the data, and compared by the one-way analysis of variance (ANOVA) or Mann–Whitney U-test. Categorical data are expressed as counts and percentages, and compared by the Pearson chi-square test. In order to investigate whether the observed age-related differences were independent of potential clinical predictors, univariable and multivariable logistic regression were used. Results of these regression analyses are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). A two-sided P value of 0.05 was considered statistically significant.
In model 1, we adjusted for gender only. In model 2, we further adjusted for NYHA and LVEF. In model 3, we further included all comorbidities which were significantly related to the outcome variable at statistical level P value less than 0.05 using stepwise entry method in binary logistic regression. In the specific device therapy-related analysis, QRS duration was an additional variable in univariable analysis we included by entry method in the models. Age was entered per 10 years into the models.
In a total of 8.9% of all predicting values data were missing. These missing data were imputed using multiple imputation. If the missing variables showed a monotone pattern of missing values, the monotone method was used, otherwise, an iterative Markov chain Monte Carlo method was used with a number of 10 iterations. A total of five imputations was performed, and the pooled data were analysed. The imputed data were only used for the multivariable analysis. For all reported data of the multivariable analysis, we compared crude and imputed P values as well as the ORs and CIs in order to analyse whether imputation changed the results, and if no significant changes occurred we only presented the imputed values in the main analyses. All analyses were performed with SPSS statistical package version 24.0 (SPSS Inc., Chicago, IL, USA).
Results
HFrEF patients (n = 8351) were on average 72.3 ± 11.8 years old, with 13.9% less than 60 years of age, 36.0% between 60 and 74 years, and 50.2% 75 years or older; 63.9% were men. Most patients were in NYHA class II and approximately half of the patients had an ischaemic cause of their HF (Table 1).
Table 1.
Patient characteristics in HFrEF patients.
| HFrEF (n = 8351)‡ |
||||
|---|---|---|---|---|
| Age <60 years (n = 1206) | Age 60–74 years (n = 3105) | Age ≥75 years (n = 4040) | P value | |
| Age (years) | 51.3 ± 7.1 | 68.0 ± 4.2 | 81.8 ± 4.7 | <0.01 |
| Male gender | 763 (63.6) | 2163 (70.0) | 2388 (59.3) | <0.01 |
| BMI, kg/m2 | 28.7 ± 6.1 | 27.9 ± 5.4 | 26.2 ± 4.4 | <0.01 |
| NYHA | ||||
| I | 322 (26.9) | 569 (18.5) | 421 (10.6) | <0.01 |
| II | 667 (55.7) | 1845 (60.0) | 2176 (54.6) | |
| III | 192 (16.0) | 618 (20.1) | 1295 (32.5) | |
| IV | 16 (1.3) | 42 (1.4) | 91 (2.3) | |
| LVEF, % | 30.4 ± 10.4 | 31.6 ± 10.0 | 34.2 ± 10.8 | <0.01 |
| Cause of HF | ||||
| Ischaemic cause of HF | 435 (37.1) | 1630 (54.0) | 2113 (54.3) | <0.01 |
| Non-ischaemic cause of HF | 738 (62.9) | 1390 (46.0) | 1779 (45.7) | |
| Systolic BP, mmHg | 123.1 ± 20.0 | 126.2 ± 20.6 | 126.0 ± 20.9 | <0.01 |
| Diastolic BP, mmHg | 74.3 ± 11.5 | 72.5 ± 11.2 | 69.3 ± 11.1 | <0.01 |
| Heart rate, bpm | 72.8 ± 13.8 | 71.8 ± 14.2 | 71.9 ± 13.6 | 0.09 |
| Atrial fibrillation | 87 (7.3) | 678 (22.1) | 1341 (33.6) | <0.01 |
| LBBB | 156 (12.9) | 490 (15.8) | 767 (19.0) | <0.01 |
| QRS ≥130 ms | 289 (27.8) | 957 (37.2) | 1525 (46.0) | <0.01 |
| eGFR, ml/min | 79.3 ± 22.8 | 64.8 ± 23.6 | 50.8 ± 21.6 | <0.01 |
| eGFR | ||||
| <30 ml/min | 23 (3.0) | 154 (7.1) | 490 (16.5) | <0.01 |
| 30–59 ml/min | 116 (15.2) | 774 (35.8) | 1552 (52.4) | |
| ≥60 ml/min | 622 (81.7) | 1,231 (57.0) | 921 (31.1) | |
| Comorbidities | ||||
| Hypertension | 306 (29.1) | 1097 (39.4) | 1573 (43.2) | <0.01 |
| Diabetes mellitus | 252 (23.9) | 848 (30.4) | 1072 (29.4) | <0.01 |
| COPD | 118 (11.2) | 546 (19.6) | 717 (19.7) | <0.01 |
| OSAS | 95 (9.0) | 246 (8.8) | 154 (4.2) | <0.01 |
| Thyroid disease | 57 (5.4) | 209 (7.5) | 290 (8.0) | 0.02 |
| Renal insufficiency† | 191 (20.3) | 1214 (47.1) | 2543 (72.9) | <0.01 |
HFrEF: heart failure with reduced ejection fraction; BMI: body mass index; NYHA: New York Heart Association classification; LVEF: left ventricular ejection fraction; HF: heart failure; BP: blood pressure; LBBB: left bundle branch block; eGFR: estimated glomerular filtration rate; COPD: chronic obstructive pulmonary disease; OSAS: obstructive sleep apnoea syndrome.
†Defined as eGFR <60 mL/min or a history of renal failure.
‡In nine patients data on age were missing.
Elderly HFrEF patients had significantly more renal insufficiency, more often atrial fibrillation, thyroid disease, chronic obstructive pulmonary disease, diabetes mellitus and hypertension and less often obstructive sleep apnoea syndrome when compared to younger patients (P < 0.01, for all) (Table 1).
Pharmacological therapy in HFrEF
Elderly patients less often received beta-blockers, renin–angiotensin system (RAS) inhibitors, MRAs and ivabradine, but significantly more diuretics than younger patients (Table 2). These differences gradually increased with age.
Table 2.
Percentage of HF therapy use in HFrEF and HFmrEF patients.
| Pharmacotherapy |
Device therapy |
|||||||
|---|---|---|---|---|---|---|---|---|
| Beta-blockers | RAS inhibitors | MRAs | Ivabradine* | Diuretics | ICD | CRT | Pacemaker | |
| ESC Guideline 2012 | ||||||||
| HFrEF | ||||||||
| <60 Years | 978 (84.2) | 1042 (89.7) | 692 (59.6) | 112 (9.3) | 843 (72.6) | 417 (44.1) | 158 (16.7) | 13 (1.4) |
| 60–74 Years | 2492 (81.5) | 2627 (85.9) | 1639 (53.6) | 153 (4.9) | 2440 (79.8) | 1018 (41.2) | 500 (20.2) | 102 (4.1) |
| ≥75 Years | 3103 (77.8) | 2999 (75.2) | 2017 (50.6) | 119 (2.9) | 3,513 (88.1) | 612 (18.9) | 473 (14.6) | 446 (13.8) |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| ESC Guideline 2016 | ||||||||
| HFrEF | ||||||||
| <60 Years | 834 (84.5) | 888 (90.0) | 630 (63.8) | 101 (9.9) | 746 (75.7) | 385 (45.9) | 145 (17.3) | 9 (1.1) |
| 60–74 Years | 2073 (81.5) | 2209 (86.9) | 1397 (55.0) | 133 (5.2) | 2048 (80.6) | 950 (44.7) | 463 (21.8) | 82 (3.9) |
| ≥75 Years | 2473 (78.6) | 2393 (76.1) | 1629 (51.8) | 101 (3.2) | 2783 (88.5) | 565 (21.8) | 428 (16.5) | 335 (12.9) |
| P value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| HFmrEF | ||||||||
| <60 Years | 144 (82.3) | 154 (88.0) | 62 (35.4) | 11 (5.9) | 97 (55.4) | 32 (29.9) | 13 (12.1) | 4 (3.7) |
| 60–74 Years | 419 (81.0) | 418 (80.9) | 242 (46.8) | 20 (3.8) | 392 (76.0) | 68 (19.8) | 37 (10.8) | 20 (5.8) |
| ≥75 Years | 630 (74.7) | 606 (71.9) | 388 (46.0) | 18 (2.1) | 730 (86.6) | 47 (7.2) | 45 (6.9) | 111 (17.0) |
| P value | <0.01 | <0.01 | 0.02 | 0.02 | <0.01 | <0.01 | 0.05 | <0.01 |
CRT: cardiac resynchronisation therapy; ESC: European Society of Cardiology; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart failure with mid-range ejection fraction; ICD: implantable cardioverter defibrillator; MRAs: mineralocorticoid receptor antagonists; RAS: renin–angiotensin syndrome.
If ivabradine is indicated (n = 500), patients with HFrEF according to the 2012 ESC Guideline received 78.3%, 75.0% and 77.8% (<60, 60–74 and ≥75 years, respectively, P = 0.73) ivabradine.
Patients received all three of the HF medications (beta-blockers, RAS inhibitors and MRAs), if indicated, in 47.8%, 38.7% and 29.6% of the patients in the three age groups (<60 years, 60–74 years and ≥75 years, respectively), two out of three were prescribed in 39.9%, 45.4% and 47.6%, one out of three was prescribed in 10.2%, 14.0% and 19.5%, and none of these medications were prescribed in 2.1%, 1.9% and 3.3%, respectively (P < 0.01). Supplementary Figure 1 shows the use of RAS inhibitors divided into ACEIs and ARBs.
The total reported contraindication or intolerance rates were 3.2% (beta-blockers), 4.6%, (RAS inhibitors), 4.7% (MRAs) and 1.7% (ivabradine) (Table 3). The reported contraindication or intolerance rates in elderly patients were significantly higher for beta-blockers, RAS inhibitors and MRAs (P < 0.01). However, in a substantial number of patients the reason for not receiving RAS inhibitors or MRAs was not specified in the patients’ charts.
Table 3.
Reasons for not prescribing HF medication in HFrEF patients.
| Contraindicated or intolerance | No reason specified | |
|---|---|---|
| Beta-blockers | ||
| Total population | 262 (3.2) | 971 (11.8) |
| <60 Years | 21 (1.8) | 109 (9.4) |
| 60–74 Years | 90 (2.9) | 300 (9.8) |
| ≥75 Years | 150 (3.8) | 562 (14.1) |
| RAS inhibitors | ||
| Total population | 380 (4.6) | 1161 (14.1) |
| <60 Years | 21 (1.8) | 99 (8.5) |
| 60–74 Years | 105 (3.4) | 327 (10.7) |
| ≥75 Years | 254 (6.4) | 735 (18.4) |
| MRAs | ||
| Total population | 387 (4.7) | 3479 (42.3) |
| <60 Years | 25 (2.2) | 445 (38.3) |
| 60–74 Years | 115 (3.8) | 1305 (42.7) |
| ≥75 Years | 247 (6.2) | 1724 (43.2) |
| Ivabradine* | ||
| Total population | 143 (1.7) | 7691 (93.6) |
| <60 Years | 12 (1.0) | 1038 (89.3) |
| 60–74 Years | 52 (1.7) | 2854 (93.3) |
| ≥75 Years | 79 (2.0) | 3790 (95.0) |
HF: heart failure; HFrEF: heart failure with reduced ejection fraction.
If indicated (n = 500) 22.6%, 23.5% and 22.2% (<60, 60–74 and ≥75 years, respectively) of patients did not receive ivabradine with no specified reason.
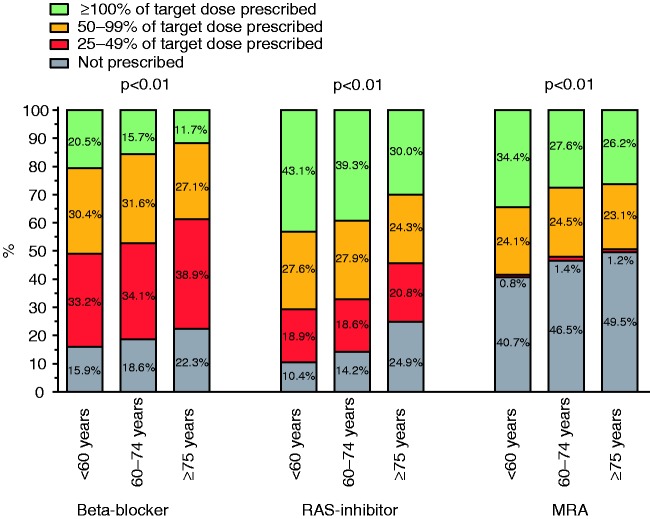
Elderly patients less often received the recommended target dose of beta-blockers, RAS inhibitors and MRAs than the younger patient groups (P < 0.01, for all) (Figure 1). Fifty per cent or greater of the target dose of all three of the HF medication groups (beta-blockers, RAS inhibitors and MRAs) was achieved in 25.4%, 17.7% and 11.0% of the patients (<60 years, 60–74 years and ≥75 years, respectively); 50% or greater of the target dose of two out of three medications in 38.6%, 40.6% and 35.7%, respectively; 50% or greater of the target dose of none out of three medication in 27.1%, 32.2% and 38.3%, respectively. Younger patients more often received 50% or greater of the target dose of all three guideline-recommended medications than elderly patients, P < 0.01.
Figure 1.
Percentages of target dose prescribed in heart failure with reduced ejection fraction.
After multivariable adjustment, the probability of receiving a beta-blocker, RAS inhibitor, MRA and ivabradine decreases for each 10-year increase in age by 10% (MRAs), 12% (beta-blockers), 29% (RAS inhibitors) and 21% (ivabradine), whereas the probability of receiving diuretics increases by 32% (Table 4). Multiple imputation did not change these findings. The age differences in HF therapy, adjusted for the differences in comorbidities, are presented in Table 4.
Table 4.
Multivariable analysis: likelihood of receiving guideline recommended therapy per 10 years of age in patients with HFrEF.
| Univariable |
Multivariable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||
| OR | P value | OR | P value | OR | P value | OR | P value | |
| Guideline recommended pharmacotherapy | ||||||||
| Beta-blockers | 0.87 (0.83–0.92) | <0.01 | 0.87 (0.83–0.91) | <0.01 | 0.88 (0.83–0.92) | <0.01 | 0.83 (0.79–0.88) | <0.01 |
| RAS inhibitors | 0.67 (0.64–0.71) | <0.01 | 0.67 (0.64–0.71) | <0.01 | 0.71 (0.67–0.75) | <0.01 | 0.75 (0.71–0.80) | <0.01 |
| MRAs | 0.93 (0.89–0.96) | <0.01 | 0.93 (0.91–0.94) | <0.01 | 0.90 (0.86–0.93) | <0.01 | 0.86 (0.83–0.90) | <0.01 |
| Ivabradine* | 0.72 (0.67–0.78) | <0.01 | 0.72 (0.67–0.77) | <0.01 | 0.69 (0.64–0.75) | <0.01 | 0.69 (0.62–0.75) | <0.01 |
| Diuretics | 1.42 (1.35–1.48) | <0.01 | 1.41 (1.38–1.45) | <0.01 | 1.32 (1.26–1.39) | <0.01 | 1.15 (1.09–1.21) | <0.01 |
| Guideline recommended device therapy | ||||||||
| ICD | 0.63 (0.60–0.66) | <0.01 | 0.63 (0.60–0.66) | <0.01 | 0.61 (0.57–0.65) | <0.01 | 0.62 (0.57–0.67) | <0.01 |
| CRT | 0.88 (0.83–0.92) | <0.01 | 0.88 (0.86–0.90) | <0.01 | 0.83 (0.78–0.88) | <0.01 | 0.75 (0.71–0.80) | <0.01 |
| Pacemaker | 2.29 (2.07–2.53) | <0.01 | 2.29 (2.17–2.41) | <0.01 | 2.17 (1.94–2.41) | <0.01 | 2.25 (2.00–2.53) | <0.01 |
COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; eGFR: estimated glomerular filtration rate; HFrEF: heart failure with reduced ejection fraction; ICD: implantable cardioverter defibrillator; MRAs: mineralocorticoid receptor antagonists; NYHA: New York Heart Association; OR: odds ratio; OSAS: obstructive sleep apnoea syndrome; RAS: renin–angiotensin syndrome.
Model 1 included age and gender.
Model 2 included age, gender, NYHA classification, left ventricular ejection fraction (and QRS for device therapy).
Model 3 included age, gender, NYHA classification, left ventricular ejection fraction (QRS duration for device therapy), hypertension, diabetes mellitus, COPD, OSAS, thyroid disease, renal insufficiency (defined as eGFR <60 mL/min or a history of renal insufficiency) and atrial fibrillation.
For ivabradine atrial fibrillation was not included in the model; if ivabradine was indicated (n = 500) the ORs were 1.00 (0.85–1.18), 1.00 (0.92–1.09), 0.97 (0.82–1.15) and 0.97 (0.80–1.17) for univariable, model 1–3, respectively, P > 0.70.
The percentage of fluid and sodium restriction recommendations are presented in Supplementary Figure 2.
Device implantation in HFrEF
Elderly patients received significantly more pacemakers, but fewer ICD and CRT devices, compared to younger patients (Table 2). After adjustment for multiple clinical parameters, the chance of receiving an ICD and CRT device decreases by 39% and 17%, respectively, for every 10-year increase in age (Table 4). After multiple imputation, the described differences did not change.
General therapy in subgroups of HFmrEF
HFmrEF patients were on average 73.7 ± 11.7 years old, and 58.4% were men. The differences in baseline characteristics between HFrEF and HFmrEF patients are shown in Supplementary Table 2. Beta-blockers (82.3% vs. 74.7%, P < 0.01), RAS inhibitors (88.0% vs. 71.9%, P < 0.01) and ivabradine (5.9% vs. 2.1%, P = 0.02) were less often prescribed in patients aged 75 years and older compared to patients less than 60 years, while MRAs (35.4% vs. 46.0%, P = 0.02) and diuretics (55.4% vs. 86.6%, P < 0.01) were more often prescribed (Table 2). The inferences of the HFmrEF group are comparable to the findings in HFrEF.
Discussion
This large practice-based clinical registry of 8351 HF patients including a relatively large group of elderly patients demonstrates that aged HFrEF patients less often receive guideline-recommended therapy. Furthermore, the prescribed dosages as a percentage of the target dose, especially to elderly patients, are lower than recommended.
Pharmacological therapy
Previous recent large registries demonstrated an age-related decline of ESC HF guidelines recommended HF therapy, especially in patients older than 75 years.11,14–17 However, these registries are older and were not using the ESC HF guidelines of 2012. Our results also demonstrate an age-related decline, but in contrast to these earlier registries, the decline in our study started already in patients older than 60 years of age and seems to be continuous, indicating that the decline is not restricted to the very old.
It has been suggested that the higher rate of comorbidities or the different aetiology of HF might be an explanation for the age-associated decline in drug prescription.16 Although we demonstrated significant differences in comorbidities between age groups, these differences were not large enough to explain the observed differences in prescription rates as shown in our multivariable analysis. In chronic HF patients, chronic obstructive pulmonary disease frequently coexists and symptoms overlap, and while getting more prevalent with increasing age, adequate treatment of underlying diseases gets even more challenging.18
Frailty in elderly patients is highly prevalent and is associated with a worse prognosis19 and might explain in some part the lower prescription rate in elderly patients; however, this could not be tested in our registry as no information on frailty was available.
Although elderly patients constitute a large part of the general HF population, patients aged 75 years of age and older are underrepresented in large randomised clinical trials.2,5,11 Thereby the positive effect of the HF medication in the elderly HF population is not yet properly investigated. This might be another explanation for the decline in prescription rates in elderly patients. However, the decline appears to be not limited to the very old, but to be a continuum, starting at a younger age than was previously assumed, indicating that the decline cannot be fully explained by lack of evidence in the elderly alone.
In contrast to the HF medication, diuretics, fluid and sodium restrictions are more often used in elderly patients. However, after adjustment in the multivariable analysis for comorbidities, the influence of age is largely reduced, in contrast to the other recommendations. This might indicate that the use of diuretics, fluid and sodium restrictions can partially be explained by worse renal function in elderly patients.
Despite the fact that elderly patients less often received guideline-recommended pharmacological therapy, we still observed an overall high prescription rate in all age groups, compared to the CHAMP–HF registry.20 Importantly, when HF medication is prescribed, the actual dosages are significantly lower in elderly than in younger patients, which could potentially lead to a worse outcome. As has been shown, good adherence to the guidelines, with prescription of at least 50% of the recommended dosage, is associated with better clinical outcomes.21
Despite relative good guideline adherence, there still seems to be room for further improvement, especially in the prescribed dosages, and in the elderly population. As previously demonstrated, the uptitration of HF medication is possible, even in elderly patients.22 However, evidence on the effect of HF therapy in patients aged 75 years and older is very limited,22,23 and appropriate prospective trials are urgently needed to address the important question as to whether treatment should differ depending on age.
Device therapy
Elderly patients less often received a ICD or CRT device, and more frequently received a pacemaker. These results are in line with recent publications, showing a decline of the CRT device and ICD implantation rate in older patients11,14,16 and an increase of the pacemaker implantation rate.11
The age differences in implantation rates might be explained by more perceived or actual comorbidities or contraindications, including non-HF-related comorbidities such as cognitive and mobility impairments.16 It has been shown that elderly HF patients have a higher non-cardiac mortality rate compared with younger HF patients.24 This might negatively influence the benefits and cost-effectiveness of implanted devices in the elderly. However, after multivariable analysis, the age-related differences remained. Also, device implantation, such as ICDs, has been shown to be effective and even warranted in elderly patients if life expectancy is longer than one year.24 Still, a recent study in patients with non-ischaemic cardiomyopathy found a strong relationship between reduced mortality by ICD and age, with only younger patients having any benefit in post-hoc analysis.25 Furthermore, assumption of a higher risk of complications due to the implantation procedure in elderly patients might explain the lower implantation rates. However, as recently reported there are no differences in the number of complications in elderly patients compared with younger patients.26 Finally, the perception that quality of life is seen as more important for elderly patients than a prolonged survival period might result in the lower implantation rates of a ICD. However, the preference of patients to prefer longevity over optimal quality of life was found to be surprisingly high and not individually predictable even at a high age.27
The use of a CRT device not only reduces morbidity and mortality, but also symptoms and improves quality of life, also in elderly patients.28 In addition, it can lead to a rise in blood pressure and protect against bradycardia.29 These gains may lead to a better adherence to recommended HF medication, such as beta-blockers.29 Thus, there is no evidence that a CRT device may be less important in HFrEF patients at an older age. As elderly patients are more often in need of a pacemaker, as shown in our results, and a CRT device holds positive treatment effects for elderly patients, it might be beneficial to treat these patients with biventricular CRT pacing instead of right ventricular pacing using a pacemaker.
Limitations and strengths
Our study has some limitations. CHECK–HF has a cross-sectional design with no follow-up data on patient outcomes. In addition, for some important variables data were missing, which might influence the results. However, imputation of missing data did not influence the results. The strengths of the CHECK–HF registry include the large scale, a reflection of the true practice of outpatient HF management in The Netherlands representative of western European countries. A further strength is the availability of a large number of elderly patients with detailed information on medication prescription and dosage.
Conclusion
In this large Dutch registry of a real-world outpatient HF population, HFrEF patients in a higher age group less often received guideline-recommended HF drugs, at lower dosages and less often ICD and CRT device therapy. The differences cannot be fully explained by clinical variables, comorbidities or higher reported contraindications or intolerance. Our study indicates the need to focus especially on elderly HF patients, in order to optimise their medical therapy, and further uptitrate their dosages or reflect on policy and accept lower age-adjusted target doses in elderly patients as they do not tolerate higher dosages.
Author contribution
HPBLR, GCML, AWH and JJB contributed to the conception or design of the work. PRG, MWFVG, IA, LO, AHMM, JFV and JJB contributed to the acquisition, analysis, or interpretation of data for the work. JFV and JJB drafted the manuscript. HPBLR, GCML, AWH, PRG, MWFVG, IA, LO and AHMM critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Supplemental Material
Supplemental Material for Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction by Jesse F Veenis, Hans-Peter Brunner-La Rocca, Gerard CM Linssen, Peter R Geerlings, Marco WF Van Gent, Ismail Aksoy, Liane Oosterom, Arno HM Moons, Arno W Hoes and Jasper J Brugts in European Journal of Preventive Cardiology
Acknowledgements
The authors greatly acknowledge the participation of HF nurses and cardiologists of all participating sites for including patients and entering patient data.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Servier, The Netherlands, funded the inclusion of data and software programme. The steering committee (HPBLR, GCML, AWH, JJB) received no funding for this project. This analysis was initiated by the authors and was designed, conducted, interpreted and reported independently of the sponsor. The current study had no other funding source or any with a participating role in outcome assessment, or writing of the manuscript. All authors had joint responsibility for the decision to submit for publication. All authors have no other conflict of interest to report.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Chioncel O, Lainscak M, Seferovic PM, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M, Ulrichsen SP, Pedersen L, et al. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail 2016; 18: 490–499. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno M, Kajimoto K, Sato N, et al. Clinical profile, management, and mortality in very-elderly patients hospitalized with acute decompensated heart failure: an analysis from the ATTEND registry. Eur J Intern Med 2016; 27: 80–85. [DOI] [PubMed] [Google Scholar]
- 6.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Clin Geriatr Med 2007; 23: 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Vidan MT, Blaya-Novakova V, Sanchez E, et al. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016; 18: 869–875. [DOI] [PubMed] [Google Scholar]
- 8.Abete P, Testa G, Della-Morte D, et al. Treatment for chronic heart failure in the elderly: current practice and problems. Heart Fail Rev 2013; 18: 529–551. [DOI] [PubMed] [Google Scholar]
- 9.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26: 215–225. [DOI] [PubMed] [Google Scholar]
- 10.Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007; 297: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 11.Ozieranski K, Balsam P, Tyminska A, et al. Heart failure in elderly patients: differences in clinical characteristics and predictors of 1-year outcome in the Polish ESC–HF Long-Term Registry. Pol Arch Med Wewn 2016; 126: 502–513. [DOI] [PubMed] [Google Scholar]
- 12.Brugts JJ, Linssen GCM, Hoes AW, et al. CHECK–HF investigators. Real-world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the Chronic Heart failure ESC guideline-based Cardiology practice Quality project (CHECK–HF) registry. Neth Heart J 2018; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunner-La Rocca HP, Linssen GC, Smeele FJ, et al. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK–HF Registry. JACC Heart Fail 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 14.Forman DE, Cannon CP, Hernandez AF, et al. Influence of age on the management of heart failure: findings from Get With the Guidelines-Heart Failure (GWTG–HF). Am Heart J 2009; 157: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 15.Hulsmann M, Berger R, Mortl D, et al. Influence of age and in-patient care on prescription rate and long-term outcome in chronic heart failure: a data-based substudy of the EuroHeart Failure Survey. Eur J Heart Fail 2005; 7: 657–661. [DOI] [PubMed] [Google Scholar]
- 16.Lund LH, Benson L, Stahlberg M, et al. Age, prognostic impact of QRS prolongation and left bundle branch block, and utilization of cardiac resynchronization therapy: findings from 14,713 patients in the Swedish Heart Failure Registry. Eur J Heart Fail 2014; 16: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 17.Pulignano G, Del Sindaco D, Tavazzi L, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN–CHF Registry). Am Heart J 2002; 143: 45–55. [DOI] [PubMed] [Google Scholar]
- 18.Griffo R, Spanevello A, Temporelli PL, et al. Frequent coexistence of chronic heart failure and chronic obstructive pulmonary disease in respiratory and cardiac outpatients: evidence from SUSPIRIUM, a multicentre Italian survey. Eur J Prev Cardiol 2017; 24: 567–576. [DOI] [PubMed] [Google Scholar]
- 19.Vigorito C, Abreu A, Ambrosetti M, et al. Frailty and cardiac rehabilitation: a call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol 2017; 24: 577–590. [DOI] [PubMed] [Google Scholar]
- 20.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP–HF Registry. J Am Coll Cardiol 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 21.Komajda M, Cowie MR, Tavazzi L, et al. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017; 19: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 22.Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME–CHF) randomized trial. JAMA 2009; 301: 383–392. [DOI] [PubMed] [Google Scholar]
- 23.Brunner-La Rocca HP, Eurlings L, Richards AM, et al. Which heart failure patients profit from natriuretic peptide guided therapy? A meta-analysis from individual patient data of randomized trials. Eur J Heart Fail 2015; 17: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 24.Ermis C, Zhu AX, Vanheel L, et al. Comparison of ventricular arrhythmia burden, therapeutic interventions, and survival, in patients <75 and patients > or =75 years of age treated with implantable cardioverter defibrillators. Europace 2007; 9: 270–274. [DOI] [PubMed] [Google Scholar]
- 25.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 26.Killu AM, Wu JH, Friedman PA, et al. Outcomes of cardiac resynchronization therapy in the elderly. Pacing Clin Electrophysiol 2013; 36: 664–672. [DOI] [PubMed] [Google Scholar]
- 27.Brunner-La Rocca HP, Rickenbacher P, Muzzarelli S, et al. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J 2012; 33: 752–759. [DOI] [PubMed] [Google Scholar]
- 28.Hoth KF, Nash J, Poppas A, et al. Effects of cardiac resynchronization therapy on health-related quality of life in older adults with heart failure. Clin Interv Aging 2008; 3: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranda JM, Jr, Woo GW, Conti JB, et al. Use of cardiac resynchronization therapy to optimize beta-blocker therapy in patients with heart failure and prolonged QRS duration. Am J Cardiol 2005; 95: 889–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction by Jesse F Veenis, Hans-Peter Brunner-La Rocca, Gerard CM Linssen, Peter R Geerlings, Marco WF Van Gent, Ismail Aksoy, Liane Oosterom, Arno HM Moons, Arno W Hoes and Jasper J Brugts in European Journal of Preventive Cardiology