Abstract
Background:
Although several studies have evaluated the change of cognitive performance after severe carotid artery stenosis, the results still remain elusive. The objective of this study was to assess changes in cognitive function, depressive symptoms and Health Related Quality of Life (HRQoL) after carotid stenosis revascularisation and Best Medical Treatment (BMT).
Methods:
Study involved 213 patients with ≥70% carotid stenosis who underwent assessment of cognitive function using Montreal Cognitive Assessment scale (MoCA), depressive symptoms - using Patient Health Questionnaire-9 (PHQ-9) and HRQoL - using Medical Outcome Survey Short Form version 2 (SF-36v2). The assessment was performed before and at 6 and 12 months follow-up periods in patients who had Carotid Endarterectomy (CEA), Carotid Artery Stenting (CAS) or received BMT only.
Results:
Improvement in the total MoCA scores was observed after 6 and 12 months (p<0.001, Kendall's W=0.28) in the CEA group. In the CAS group - after 12 months (p=0.01, Kendall's W=0.261) whereas in the BMT group - no significant changes (p=0.295, Kendall's W=0.081) were observed. Reduction of depressive symptoms was not found in any of the study groups. Comparing mean SF-36v2 scores in the CEA group, there was no significant difference in any of 10 subscales. Likewise in the CAS group - no significant difference in 9 of 10 subscales (p=0.028, η2=0.343) was observed. Three subscales worsened in the BMT group during the 1-year follow-up period.
Conclusion:
Patients with severe carotid stenosis who underwent revascularisation enhanced their cognitive performance without exerting significant change of depressive symptoms. Preoperative HRQoL may be maintained for at least one year in the CEA group.
Keywords: Cognition, depression, quality of life, carotid stenosis, endarterectomy, stenting, medical treatment
1. INTRODUCTION
Carotid artery stenosis is a well-known causal risk factor for ischaemic stroke. Approximately 10-15% of all strokes follow thromboembolism from previously asymptomatic >50% internal carotid artery stenosis [1]. In addition to functional disability, stroke patients frequently go on to develop cognitive impairment and depression. The prevalence of post-stroke cognitive impairment ranges from 20% to 80% [2], whereas the prevalence of post-stroke depression has been reported as 31% at any time point within 5 years following stroke [3]. Therefore, not only functional disabilities but also post-stroke cognitive impairment and depression significantly affect Health Related Quality of Life (HRQoL).
However, there is less knowledge and evidence regarding neuropsychological symptoms in patients with severe asymptomatic carotid stenosis. Several pathogenetic mechanisms for the development of cognitive impairment such as microembolism, hypoperfusion and reduced cerebrovascular reserve [4, 5] have been proposed, but the definite effect of revascularisation on cognitive function in patients with severe carotid stenosis is still unknown because the results of studies on the topic remain controversial [6]. However, the questions of whether carotid stenosis causes cognitive impairment and whether carotid interventions improve cognitive function have been discussed lately in clinical practice. For the first time, this topic has been mentioned in the guidelines of the European Society for Vascular Surgery [7] as well. While the results from controlled randomised CREST-2 trial are awaited in years to come [8], the current study demonstrates not only the change in cognitive function, depressive symptoms and HRQoL after revascularisation but also the change in the Best Medical Treatment (BMT) group in patients with severe carotid stenosis.
Advances in prevention and healthcare have increased life expectancy and produced a shift in the burden of diseases worldwide. Therefore, besides cognitive impairment, vascular depression as a subtype of late-life depression is of great interest because of its clinical significance and complex basis, which may affect outcomes in the depressed elderly and increase the risk of cognitive impairment and poor quality of life [9]. Therefore, the goal of contemporary management is not only to reduce stroke risk and to extend life expectancy but also to ensure a sufficiently high long-term HRQoL [10].
The aim of this study was to assess long-term changes in cognitive function, depressive symptoms and HRQoL after carotid stenosis revascularisation and BMT. We hypothesised that patients with severe carotid stenosis would have improvement of cognitive function, depressive symptoms and HRQoL after revascularisation whereas the improvement would not be seen in the BMT group.
2. MATERIALS AND METHODS
2.1. Participants and Study Design
Between March 2015 and October 2017, 213 patients with severe carotid stenosis (≥ 70% luminal narrowing) were recruited from the Stroke Unit, Outpatient Stroke Clinic and Cardiology Department at Pauls Stradins Clinical University Hospital for this prospective observational study. The inclusion criteria for all consenting patients were as follows: age 18 years or older, severe extracranial carotid stenosis ≥70%, and an indication for carotid artery revascularisation. Carotid stenosis in all patients was estimated with computed tomography angiography and defined according to the North American Symptomatic Carotid Artery Endarterectomy Trial (NASCET) criteria [11]. Carotid stenosis was considered symptomatic if a minor stroke (NIHSS <3, modified Rankin Scale (mRS) <2 [12]), transient ischaemic attack (TIA) or amaurosis fugax had occurred within 6 months prior to inclusion [13]. Asymptomatic carotid stenoses were defined as having no previous clinical ipsilateral minor stroke, TIA or amaurosis fugax within the last 6 months [14]. The exclusion criteria were major stroke (NIHSS ≥4, mRS 3-5), carotid stenosis <70%, progressive cerebral pathology (tumour, multiple sclerosis, trauma or a history of cerebral surgery, Alzheimer's disease, Parkinson's disease), patients with known antidepressant therapy and refusal to attend long term follow-up.
Management of severe carotid stenosis was conducted independently of the study by the treating physician and patient based on patient preference and characteristics according to American Heart Association guidelines [15]. Patients who refused to receive Carotid Endarterectomy (CEA) or Carotid Artery Stenting (CAS) were enrolled in the BMT group. Therefore, 3 cohorts were formed from the study sample: 1) patients who underwent CEA, 2) patients who underwent CAS, and 3) patients who received BMT only.
All patients were assessed 1-3 days before revascularisation by a trained neurologist-baseline visit 1 (V1). For patients who refused to receive revascularisation of carotid stenosis, evaluation was performed 1 day before discharge from the hospital. All patients underwent clinical and cognitive assessment, evaluation of depressive symptoms and HRQoL. All patients in this study received recommendations to use pharmacological treatment after discharge from hospital including antiplatelet agents, statins or other hypolipidaemic medications, antihypertensive treatment to attain blood pressure <140/90mmHg, strict control of hyperglycaemia if diabetic, counselling for smoking cessation, weight control, and regular physical exercise according to guidelines [16].
Patients were asked to come to the follow-up visits at 6 (visit 2-V2) and 12 (visit 3-V3) months ± 14 days after revascularisation of carotid stenosis or after inclusion time point for those who received BMT only. Six-months and one-year follow-up periods were chosen to gain insight into evolution of cognitive, depressive symptoms, HRQoL and long-term outcome of patients after carotid stenosis treatment. In addition to assessment of clinical, neurological, cognitive, depressive symptoms, evaluation of extracranial carotid arteries was also performed by duplex ultrasound to assess the rate of restenosis at 6-months and 1-year follow-up time periods by a single experienced neurosonographer.
2.2. Clinical Assessment
At the baseline visit (V1), basic demographic characteristics (age, sex, education), anthropometric and lifestyle characteristics (weight, height, smoking), data on comorbidities (history of stroke or TIA, Coronary Artery Disease (CAD), Arterial Hypertension (AH), chronic heart failure, Atrial Fibrillation (AF), Diabetes Mellitus (DM), Peripheral Artery Disease (PAD) and others), use of medications and neurological examination results were recorded on a standardised form during an interview. Participants were classified as smokers if they were current smokers or had quit smoking within 5 years before enrolment. History of TIA or minor stroke was collected from previous medical records. Regular use of medications was also recorded on a standardised form. After the examination of neurological status, patients were required to undergo a standardised cognitive assessment and to complete questionnaires, which assessed depressive symptoms and HRQoL in the presence of a single neurologist who was blinded to patient data.
At V2 and V3, aside from vascular risk factors, new comorbidities, neurological status and medication compliance, cognitive function, depressive symptoms and HRQoL were also reassessed.
Besides the change in cognitive function, depression and HRQoL, the primary (ischaemic or haemorrhagic stroke, Myocardial Infarction (MI) or death during the periprocedural period or within 30 days after the baseline visit) and the secondary (stroke, acute coronary syndrome or death due to different causes within a year, except for the first 30 days) outcomes also were evaluated.
2.3. Cognitive Assessment
The cognitive assessment was performed using the Latvian or Russian version of Montreal Cognitive Assessment Scale (MoCA), according to the patient's native language and instructions given by the authors [17]. The MoCA test was chosen because it has been approved as a valid 10-minute cognitive screening tool for vascular cognitive impairment [18-21]. The MoCA test is divided into 7 subscores that assess 7 cognitive domains: visuospatial/executive, naming, attention, language, abstraction, memory, orientation and an additional point that is given to each patient who has an educational experience of 12 years or less. The MoCA scores range from 0-30, and a final total score of 26 and above is considered normal [18].
2.4. Assessment of Depressive Symptoms
Assessment of depressive symptoms was performed using the Patient Health Questionnaire-9 (PHQ-9), which has been compared with other questionnaires that screen depressive symptoms. PHQ-9 is proposed to be an acceptable tool [22-24]. Moreover, the PHQ-9 has been used in vascular depression [25] and post-stroke depression [26, 27] studies. The PHQ-9 is a self-reporting 9-item questionnaire, and its scores range from 0 to 27 because each of the 9 questions can be scored from 0 (“not at all”) to 3 (“nearly every day”). A PHQ-9 score of 10 or greater is recommended as a screening cut point, because it has sensitivity for major depression of 88% a specificity of 88% [28]. Therefore, patients were categorised into two groups according to PHQ-9 score. PHQ-9 scores lower than 10 denoted no relevant depressive symptoms. Scores of 10 or higher indicated relevant depressive symptoms. In this study validated Latvian and Russian versions of the PHQ-9 depression scale [29] were used.
2.5. Assessment of Health Related Quality of Life
HRQoL was measured using the Medical Outcome Survey Short Form 36 version 2 (SF-36v2) in Latvian and Russian languages [30]. The SF-36v2 includes 36 items that are grouped into eight subscales: Physical Functioning (PF); role limitations due to physical problems or Role-Physical (RP); Bodily Pain (BP); General Health (GH); Vitality (VT); Social Functioning (SF); role limitations due to emotional problems or Role-Emotional (RE); and Mental Health (MH). In addition, the SF-36v2 provides summary scales for overall physical and mental health: Physical Component Summary (PCS) and Mental Component Summary (MCS) scores. For each item, scores are coded, summed and transformed into a scale from 0 (worst possible health state measured by the questionnaire) to 100 (best possible health state). A difference of 5 to 10 points is considered a clinically important change for an individual subject (a smaller difference may be important for group comparisons) [31].
2.6. Treatment type of Carotid Stenosis
All CEA procedures were performed by experienced board-certified vascular surgeons. Decision on which technique of plaque removal (regular endarterectomy or eversion endarterectomy) and administration of shunting during operation was taken independently of the study by the treating surgeon. All CEA procedures were performed under general anaesthesia.
All patients who underwent CAS were given acetylsalicylic acid (100 mg/day) and clopidogrel (75 mg/day) at least 5 days prior the intervention or a loading dose (300 mg) 4 to 5 hours prior to the procedure. CAS was performed under local anaesthesia. Distal protection devices and self-expanding stents were used in all CAS procedures. After CAS all patients were recommended a lifelong application of 100 mg acetylsalicylic acid and 75 mg clopidogrel for a minimum of 3 months after CAS, as well as vascular risk factor modification according to guidelines [16].
In the BMT group as well as in the CEA and CAS groups, in addition to antiplatelet agents, statins or other hypolipidaemic medications, antihypertensive treatment, strict control of hyperglycaemia if diabetic, counselling for smoking cessation, weight control, and regular physical exercises were recommended [16].
2.7. Statistical Analysis
Descriptive statistics were used to analyse the demographics and clinical characteristics of the population. Continuous variables were described as the median and Interquartile Range (IQR) or as the means (Standard Deviation (SD)). As majority of the variables were not normally distributed and there was imbalance between groups, non-parametric statistics were mainly used to evaluate variables. We used the Pearson's Chi-squared test to compare baseline categoric variables between the groups. For detection of differences among three treatment groups (CEA, CAS, BMT), the Kruskal-Wallis test was applied. Changes in continuous variables at V1, V2 and V3 in each treatment group were calculated using the Friedman's test followed by the least significant difference post hoc test using the Wilcoxon signed rank test for paired continuous data and the McNemar test for paired categorical data. To understand whether differences were statistically meaningful, we calculated Cramer's V for the Pearson's Chi-squared test, partial eta squared (η2) for Kruskal-Wallis and ANOVA (analysis of variance) tests; Kendall's coefficient of concordance (Kendall's W) for Friedman's test, coefficient r for Wilcoxon signed rank test. Raw scores of cognitive, depression and HRQoL tests were used for the analysis. In the study of HRQoL, the results were expressed as the means ± SD according to suggestions by Shan et al. [32]. Therefore, one-way ANOVA was used for comparison of SF-36v2 mean scores between treatment groups and repeated measures ANOVA for comparing changes at follow-up visits in each group. A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics (version 23 for Windows, IBM Corp., Somers, NY, USA).
3. RESULTS
3.1. Characteristics of Patients
Initially 213 patients were recruited in the 3 following groups: patients who underwent CEA (n=159), patients who underwent CAS (n=29) and patients who received BMT only (n=25).
There was no statistically significant difference by gender - in each treatment group men were more common than women (p=0.226, Cramer's V=0.118). There were 95 (60%) men in the CEA group, 20 (69%) in the CAS group and 19 (76%) in the BMT group. The median age in all three groups was similar: in the CEA group-71 (IQR: 63-75); in the CAS group-71 (IQR: 63.4-78) and in the BMT group -74 (IQR: 67-78) years (p=0.171, effect size η2=0.01). Comparing age differences between men and women in each group, we observed a statistically significant difference only in the CEA group where women were older than men (p<0.001, η2=0.1) (Fig. 1).
Fig. (1).
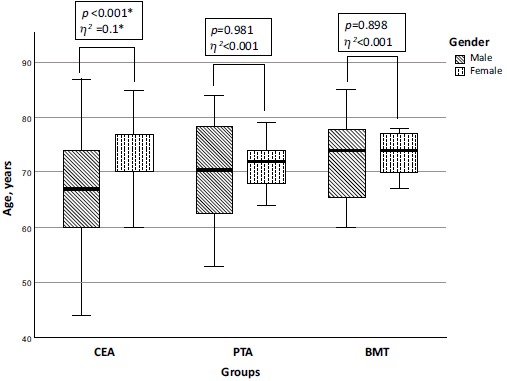
Age differences in the carotid endarterectomy, carotid stenting and best medical treatment groups at baseline. Comparison of median age in males and females at baseline. Significant differences between male and female median age were in the CEA group where females were older than males. No significant age differences were observed in the CAS and BMT groups at baseline. Bar display median, boxes represent IQR, and whiskers display total range.
The difference between treatment groups regarding clinical and neurological characteristics, cardiovascular risk factors was not statistically significant, except for CAD and chronic heart failure, AF and DM. Atrial fibrillation was more common in the BMT group (p=0.001, Cramer's V=0.255), but DM - in the CAS group (p<0.001, Cramer's V=0.281). Although CAD and chronic heart failure were more common in the CAS group (p=0.048 and p=0.034), the statistical effect size of the differences was small. The demographic, lifestyle and clinical characteristics of the patients in each group are presented in Table 1.
Table 1. Characteristics of patients with severe carotid stenosis in each treatment group.
| - |
CEA
n=159 |
CAS
n=29 |
BMT
n=25 |
p-value | Effect Size |
|---|---|---|---|---|---|
| Neurological Characteristics | |||||
| Asymptomatic, n (%) | 118 (74.2%) | 20 (69%) | 13 (52%) | 0.072 | Cramer's V 0.165 |
| Symptomatic | - | - | - | ||
| Stroke, (NIHSS<3) | 22 (13.8%) | 6 (20.7%) | 10 (40%) | ||
| TIA | 16 (10.1%) | 2 (6.9%) | 1 (4%) | ||
| Amaurosis fugax | 3 (1.9%) | 1 (3.4%) | 1 (4%) | ||
| Stenosis Side, n (%) | |||||
| Right | 66 (41.5%) | 12 (41.4%) | 8 (32.0%) | 0.845 | Cramer's V 0.057 |
| Left | 52 (32.7%) | 8 (27.6%) | 10 (40.0%) | ||
| Bilateral | 41 (25.8%) | 9 (31.0%) | 7 (28%) | ||
| Cardiovascular Risk Factors | |||||
| Coronary artery disease | 68 (42.8%) | 19 (65.5%) | 9 (36.0%) | 0.048* | Cramer's V 0.169 |
| Chronic heart failure | - | - | - | 0.034* | Cramer's V 0.197 |
| Class II | 30 (18.9%) | 9 (31%) | 10 (40%) | ||
| Class III | 10 (6.3%) | 4 (13.8%) | 2 (8%) | ||
| Class IV | 0 | 1 (3.4%) | 0 | ||
| Hypertension | |||||
| Stage 2 | 102 (64.2%) | 17 (58.6%) | 16 (64%) | 0.709 | Cramer's V 0.094 |
| Stage 3 | 25 (15.7%) | 5 (17.2%) | 5 (20%) | ||
| Atrial fibrillation | 15 (9.4%) | 6 (20.7%) | 9 (36%) | 0.001* | Cramer's V 0.255† |
| Peripheral artery disease | 49 (30.8%) | 11 (39.3%) | 5 (20%) | 0.314 | Cramer's V 0.105 |
| Diabetes mellitus | 21 (13.2%) | 13 (44.8%) | 4 (16%) | <0.001* | Cramer's V 0.281† |
| Smoking | |||||
| Non - smoker | 54 (34%) | 9 (44%) | 11 (44%) | 0.764 | Cramer's V 0.066 |
| Current smoker | 72 (45.3%) | 12 (41.4%) | 10 (40%) | ||
| Former smoker | 33 (20.7%) | 8 (27.6%) | 4 (16%) | ||
| BMI (mean, SD) | 27.12 (4.26) | 27.67 (4.27) | 27.29 (3.59) | 0.805 | η2=0.002 |
| <18.4 | 1 (0.6%) | 0 | 0 | 0.835 | Cramer's V 0.081 |
| 18.5-24.9 | 48 (30.2%) | 8 (27.6%) | 4 (16%) | ||
| 25.0-29.9 | 74 (46.5%) | 13 (44.8%) | 14 (56%) | ||
| ≥30 | 36 (22.6%) | 8 (27.6%) | 7 (28%) | ||
Abbreviations: CEA - carotid endarterectomy group; CAS - carotid artery stenting group; BMT - best medical treatment group; NIHSS - National Institute of Health Stroke Scale; TIA- transient ischaemic attack; BMI - body mass index; SD - standard deviation.
* p<0,05; † effect size Cramer's V=0,3 (medium).
During the one-year follow up period, primary and secondary outcomes were observed in 14 patients. The causes of primary outcome in the CEA group were perioperative disabling stroke (n=2), large perioperative MI (n=1), and perioperative intracerebral haemorrhage (ICH) (n=1); in the CAS group, periprocedural infection with sepsis and death (n=1); but in the BMT group, there were no primary outcome events during the first 30 days after the initiation of the study. The causes for secondary outcome in the CEA group were death (n=3) and contralateral disabling stroke (n=2); in the CAS group death (n=2) was due to traumatic ICH and acute CAD but in the BMT group one patient had ipsilateral disabling stroke and one patient had acute CAD. The data of all these patients were analysed until the time point when the patient was unable to continue the study. However, there were some patients who did not want to continue to participate in the study during the follow-up period due to social background. In telephone interviews (at V2 or V3), no vascular event or death was reported for subjects who interrupted the study. Therefore, in the CEA group at the beginning, there were 159 patients; after 6 months - only 132 patients continued to participate in the study, but after 12 months, there were 128 patients. In the CAS group at the beginning, there were 29 patients; after 6 months, there were only 27 patients, but after 12 months, there were 25 patients. In the BMT group at the beginning, there were 25 patients; after 6 months - 24, but after 12 months, only 22 patients continued the study.
3.2. Characteristics of Cognitive Function
During cognitive assessment at the beginning of the study, there was no significant difference of median total MoCA scores as well as of median MoCA subtest scores between all treatment groups (p=0.728, η2=0.003). The median total MoCA score in the CEA group was 25 (IQR: 22-27), in the CAS group - 24 (IQR: 21-26) and in the BMT group - 25 (IQR: 22-26).
In the CEA group patients performed significantly better on the total MoCA scores after 6 and 12 months (p<0.001, Kendall's W=0.28). There was a statistically significant difference in median total MoCA scores between V1 and V2, as well as between V1 and V3. Although the difference in median total MoCA scores between V2 and V3 was statistically significant (p=0.001), the statistical effect size was small (r=0.2) (Fig. 2).
Fig. (2).
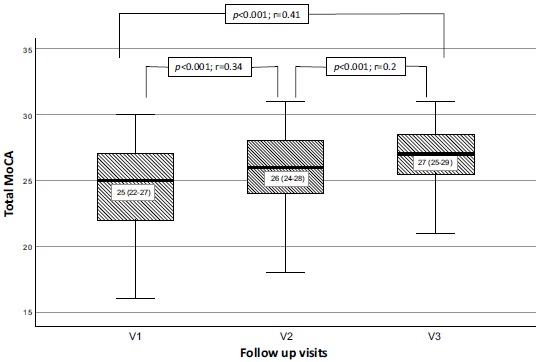
Median total MoCA scores at baseline, 6 and 12 months in carotid endarterectomy group. Significant increase of median total MoCA score as a measure of cognitive function at 6 and 12 months after successful CEA in patients with severe carotid stenosis. The difference of median MoCA scores between V2 and V3 is statistically significant, but the statistical effect size is small (r=0.2).
Comparing median MoCA subtest scores during the follow-up period, there was statistically significant improvement in attention (p=0.035, Kendall's W=0.033), language (p<0.001, Kendall's W=0.075), abstraction (p<0.001, Kendall's W=0.076) and delayed recall (p<0.001, Kendall's W=0.217) subtest scores; however, the statistical effect size was small. There was no decline in any of the MoCA subtest scores (Table 2).
Table 2. Median MoCA subtest scores at baseline, after 6 and 12 months in the carotid endarterectomy group.
| - | V1 | V2 | V3 | p-value | Effect Size |
|---|---|---|---|---|---|
| Total MoCA | 25 (22-27) | 26 (24-28) | 27 (25-29) | <0.001* | 0.28† |
| VSE | 4 (3-5) | 4 (3-5) | 4 (4-5) | 0.254 | 0.013 |
| Naming | 3 (3-3) | 3 (3-3) | 3 (3-3) | 0.135 | 0.019 |
| Attention | 5 (6-6) | 5 (6-6) | 5 (6-6) | 0.035* | 0.033 |
| Language | 2 (1-2) | 2 (1-2) | 2 (1-3) | <0.001* | 0.075 |
| Abstraction | 2 (1-2) | 2 (1-2) | 2 (2-2) | <0.001* | 0.076 |
| Delayed recall | 3 (1-4) | 4 (2-5) | 4 (3-5) | <0.001* | 0.217 |
| Orientation | 6 (6-6) | 6 (6-6) | 6 (6-6) | 0.103 | 0.023 |
Abbreviations: CEA - carotid endarterectomy; V1 - baseline visit before endarterectomy, V2 - visit 2 (6 months after endarterectomy), V3- visit 3 (12 months after endarterectomy); VSE - visuospatial/executive functions.
Median values (IQR: Q1-Q3); * p<0,05; † Kendall's W effect size ≥ 0,3 (medium).
Patients in the CAS group also improved in total MoCA scores during 1-year follow-up (p=0.01, Kendall's W=0.261). The difference between median total MoCA scores was statistically significant only between V1 and V3 (p=0.034, r=0.44). The median total MoCA scores before CAS, 6 and 12 months after CAS are presented in Fig. (3).
Fig. (3).
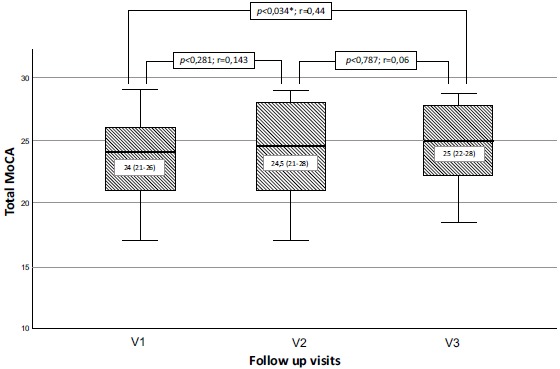
Median total MoCA scores at baseline, 6 and 12 months in carotid artery stenting group. Significant increase of median total MoCA score as a measure of cognitive function at 12 months after successful CAS in patients with severe carotid stenosis. No significant change of median MoCA score was observed between baseline and 6 months and between 6 and 12 months. Bar display median, boxes represent IQR, and whiskers display total range.
Comparing median MoCA subtest scores during the follow-up period, there was statistically significant improvement only in visuospatial/executive (p=0.01) subtest scores, but the statistical effect size was small (Kendall's W=0.24) (Table 3).
Table 3. Median MoCA subtest scores at baseline, after 6 and 12 months in the carotid artery stenting group.
| - | V1 | V2 | V3 | p-value | Effect size |
|---|---|---|---|---|---|
| Total MoCA | 24 (21-26) | 24.5 (21-28) | 25 (22-28) | 0.01* | 0.261† |
| VSE | 3 (2-5) | 4 (3-5) | 4 (3-5) | 0.01* | 0.24 |
| Naming | 3 (3-3) | 3 (3-3) | 3 (3-3) | 0.368 | 0.053 |
| Attention | 5 (4-6) | 6 (5-6) | 5 (4-6) | 0.572 | 0.029 |
| Language | 2 (1-2) | 1 (1-2) | 1 (1-2) | 0.917 | 0.005 |
| Abstraction | 2 (1-2) | 1 (1-2) | 2 (1-2) | 0.289 | 0.065 |
| Delayed recall | 3 (0.75-4) | 4 (1.75-4) | 3 (1.75-4) | 0.144 | 0.108 |
| Orientation | 6 (6-6) | 6 (6-6) | 6 (6-6) | 0.368 | 0.053 |
Abbreviations: CAS - carotid artery stenting; V1 - baseline visit before carotid stenting, V2 - visit 2 (6 months after carotid stenting), V3- visit 3 (12 months after carotid stenting); VSE - visuospatial/executive functions; Median values (IQR:Q1- Q3); * p<0,05; † Kendall's W effect size ≥ 0,3 (medium).
Bar display median, boxes represent IQR, and whiskers display total range.
Patients in the BMT group did not show statistically significant changes in total MoCA scores in a 1-year period (p=0.295, Kendall's W=0.081): at the beginning of the study, the total MoCA score was 25 (IQR: 22-26), after 6 months - 26 (IQR: 23-27) and after 12 months - 26 (IQR: 23-28). Comparing median MoCA subtest scores during the follow-up period, we found statistically significant improvement specifically in memory (p=0.027) subtest scores, but the sta-tistical effect size was small (Kendall's W=0.242) (Table 4).
Table 4. Median MoCA subtest scores at baseline, after 6 and 12 months in the best medical treatment group.
| - | V1 | V2 | V3 | p-value | Effect Size |
|---|---|---|---|---|---|
| Total MoCA | 25 (22-26) | 26 (23-27) | 26 (23-28) | 0.295 | 0.081 |
| VSE | 4 (3-4) | 4 (3-5) | 4 (2-5) | 0.973 | 0.002 |
| Naming | 3 (3-3) | 3 (3-3) | 3 (3-3) | 1.0 | <0.001 |
| Attention | 6 (6-6) | 6 (5-6) | 6 (5-6) | 0.507 | 0.045 |
| Language | 2 (1-2) | 1 (1-2) | 2 (1-3) | 0.531 | 0.042 |
| Abstraction | 2 (1-2) | 2 (2-2) | 2 (2-2) | 0.229 | 0.098 |
| Delayed recall | 2 (2-3) | 3 (2-4) | 4 (3-5) | 0.027* | 0.242 |
| Orientation | 6 (6-6) | 6 (6-6) | 6 (6-6) | 0.999 | >0.099 |
Abbreviations: BMT - best medical treatment group; V1 - baseline visit, recruitment in the study, V2 - visit 2 (6 months after recruitment), V3- visit 3 (12 months after recruitment); VSE - visuospatial/executive functions; Median values (IQR:Q1- Q3); * p<0.05.
3.3. Characteristics of Depressive Symptoms
At the beginning of the study, the median PHQ-9 scores were similar in all groups: in the CEA group: 5 (IQR: 2-9), in the CAS group: 6 (IQR: 2-10) and in the BMT group: 6 (IQR: 3-10); p=0.3, η2=0.0142.
In the CEA group, the median PHQ-9 scores did not change during the follow-up period (p=0.543, Kendall's W=0.008). Likewise, there was no statistically significant difference between the frequencies of depressive symptoms (PHQ-9 screening cut point ≥ 10) before and 6 or 12 months after CEA (p=0.485, Kendall's W=0.007). In our analysis, from those patients who had depressive symptoms before CEA, more than half of them did not feel depressed after 6 months (n=18; 58.1%) as well as after 12 months (n=16 (59.2%)). However, the differences between patients who remained depressed and those whose symptoms did get better at 6 (p=0.17) and 12 months (p=0.557) were not statistically significant. The changes in the frequency of depressive symptoms after 6 and 12 months in the CEA group are presented in Fig. (4).
Fig. (4).
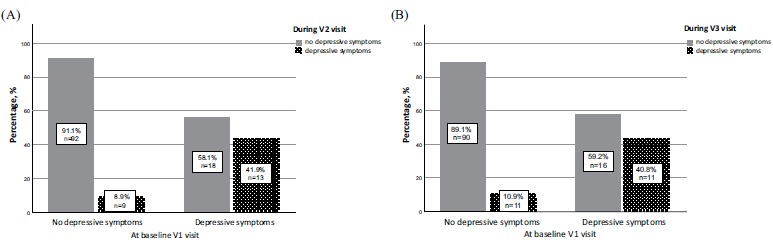
Changes in the frequency of depressive symptoms after 6 and 12 months in the carotid endarterectomy group. (A) Graph of percentage and number of patients with and without depressive symptoms at baseline and 6 months after CEA. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 6 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 6 months. (B) Graph of percentage and number of patients with and without depressive symptoms at baseline and 12 months after CEA. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 12 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 12 months.
In the CAS group, median PHQ-9 scores did not change (p=0.17, Kendall's W=0.093). There was no statistically significant difference between the frequencies of depressive symptoms before and 6 or 12 months after CAS (p=0.165, Kendall's W=0.095). In the analysis of depressive symptoms after 6 months, only one from 8 patients who had depressive symptoms before CAS did get better. Likewise, 12 months after CAS, depressive symptoms were not observed in 3 from 8 patients. The differences between patients who remained depressed and those whose symptoms did get better at 6 (p=0.375) and 12 months (p=0.97) were not statistically significant. The changes in the frequency of depressive symptoms after 6 and 12 months in the CAS group are presented in Fig. (5).
Fig. (5).
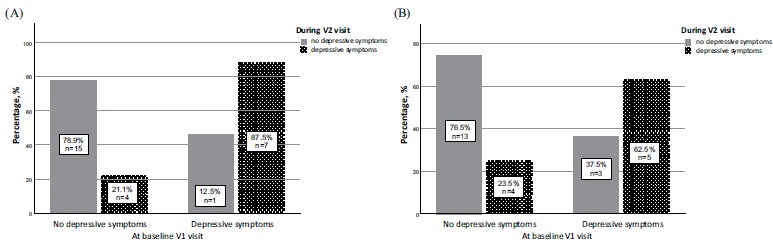
Changes in the frequency of depressive symptoms after 6 and 12 months in the carotid artery stenting group. (A) Graph of percentage and number of patients with and without depressive symptoms at baseline and 6 months after CAS. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 6 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 6 months. (B) Graph of percentage and number of patients with and without depressive symptoms at baseline and 12 months after CAS. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 12 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 12 months.
In the BMT group median PHQ-9 scores did not change during the follow-up period compared with baseline (p=0.64, Kendall's W=0.03). In addition, in the analysis of the frequencies of depressive symptoms, there was no statistically significant difference between baseline and 6 or 12 months after initiation of BMT (p=0.819, Kendall's W=0.013). In the analysis, from those who had depressive symptoms at baseline, only two out of 6 patients were free from depressive symptoms after 6 months and 2 out of 7 after 12 months. The differences between patients who remained depressed and those whose symptoms did get better at 6 (p=0.687) and 12 months (p=0.243) were not statistically significant. The changes in the frequency of depressive symptoms after 6 and 12 months in the BMT group are presented in Fig. (6).
Fig. (6).
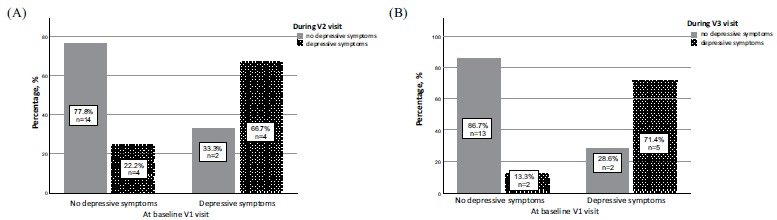
Changes in the frequency of depressive symptoms after 6 and 12 months in the best medical treatment group. (A) Graph of percentage and number of patients with and without depressive symptoms at baseline and 6 months after BMT. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 6 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 6 months. (B) Graph of percentage and number of patients with and without depressive symptoms at baseline and 12 months after BMT. The graph depicts number of patients who did not have depressive symptoms at baseline but felt depressed after 12 months. Likewise, number of patients who had depressive symptoms at baseline but did get better or remained depressed after 12 months.
3.4. Characteristics of Health Related Quality of Life
At baseline, SF-36v2 subscale scores were similar in all three study groups. The lowest mean scores in all groups were for GH, PCS and MCS, and the highest were for SF in the CEA and BMT groups. Although mean SF-36v2 scores for PF and BP were significantly lower in the CAS group than in CEA and BMT groups (p<0.05), the statistical effect size was small (Table 5).
Table 5. Mean SF-36v2 scores in patients with severe carotid stenosis at baseline.
| - |
CEA
(n=159) |
CAS
(n=29) |
BMT
(n=25) |
p- value |
Effect Size
η2 |
|---|---|---|---|---|---|
| Physical Functioning (PF) | 66.6 (22.4) | 53.4 (23) | 57 (30) | 0.036* | 0.031 |
| Role-Physical (RP) | 55.4 (26.7) | 47.7 (25.7) | 57.1 (27.4) | 0.158 | 0.017 |
| Bodily Pain (BP) | 60 (27) | 48.4 (27.2) | 66.7 (26.4) | 0.009* | 0.044 |
| General Health (GH) | 47.6 (17) | 44.7 (19.5) | 47.5 (25.6) | 0.297 | 0.015 |
| Vitality (VT) | 56.7 (18.6) | 49.7 (20.7) | 60.4 (20.1) | 0.173 | 0.017 |
| Social Functioning (SF) | 71.7 (25.3) | 65.1 (27.2) | 75.8 (19.2) | 0.671 | 0.004 |
| Role-Emotional (RE) | 65 (27.7) | 57.9 (27.3) | 71.7 (23.1) | 0.231 | 0.014 |
| Mental Health (MH) | 63.5 (17.5) | 62.6 (21.7) | 71.3 (15) | 0.238 | 0.014 |
| Physical Component Summary (PCS) | 44 (8.5) | 39.7 (8.3) | 42.1 (8.9) | 0.09 | 0.023 |
| Mental Component Summary (MCS) | 45.5 (10.1) | 44.5 (10.9) | 50.1 (7.8) | 0.285 | 0.012 |
Results: mean (±SD); *p<0.05; CEA - carotid endarterectomy group; CAS - carotid artery stenting group; BMT - best medical treatment group; SD - standard deviation.
Comparing mean SF-36v2 scores in CEA group during the follow-up period, there was no statistically significant difference in any of 10 subscales. The lowest mean SF-36v2 scores for GH, PCS and MCS and the highest mean SF-36v2 scores for SF remained unchanged after 6 and 12 months. Likewise, comparing mean SF-36v2 scores in CAS group, there was no statistically significant difference in 9 of 10 subscales during the follow-up period, except for BP (p=0.028, η2=0.343). Before revascularisation, the mean BP score was 48.4 (27.2), after 6 months - 54.3 (30) and after 12 months - 45.2 (17.8). The lowest mean SF-36v2 scores for RP, GH, VT, PCS and MCS remained unchanged after 6 and 12 months. For PF and SF there were decrements in the mean SF-36v2 scores after 12 months, but the changes were not statistically significant. Nevertheless, in the BMT group there were statistically significant differences in RP mean scores during the follow-up period (p=0.039, η2=0.392)
where at the beginning of the study mean RP score was 57.1 (27.4), but after 6 months - 34.6 (20.4) and after 12 months 37.5 (24.4). Likewise, mean SF-36v2 scores for bodily pain were significantly different during follow-up periods (p=0.051, η2=0.368): at the beginning of the study, the mean BP score was 66.7 (26.4), but after 6 months it was 57.3 (25.1) and after 12 months - 64.5 (24.3). There were statistically significant changes in MCS mean scores during the follow-up period as well: at the baseline, the mean MCS score was 50.1 (7.8), but after 6 months, it was 47.9 (7.3) and after 12 months - 44.5 (7.8). The lowest mean scores for GH, PCS, MCS and the highest scores for SF in the BMT group remained unchanged during the follow-up periods. The results of the mean SF-36v2 scores during the follow-up periods are summarised in Fig. (7).
Fig. (7).
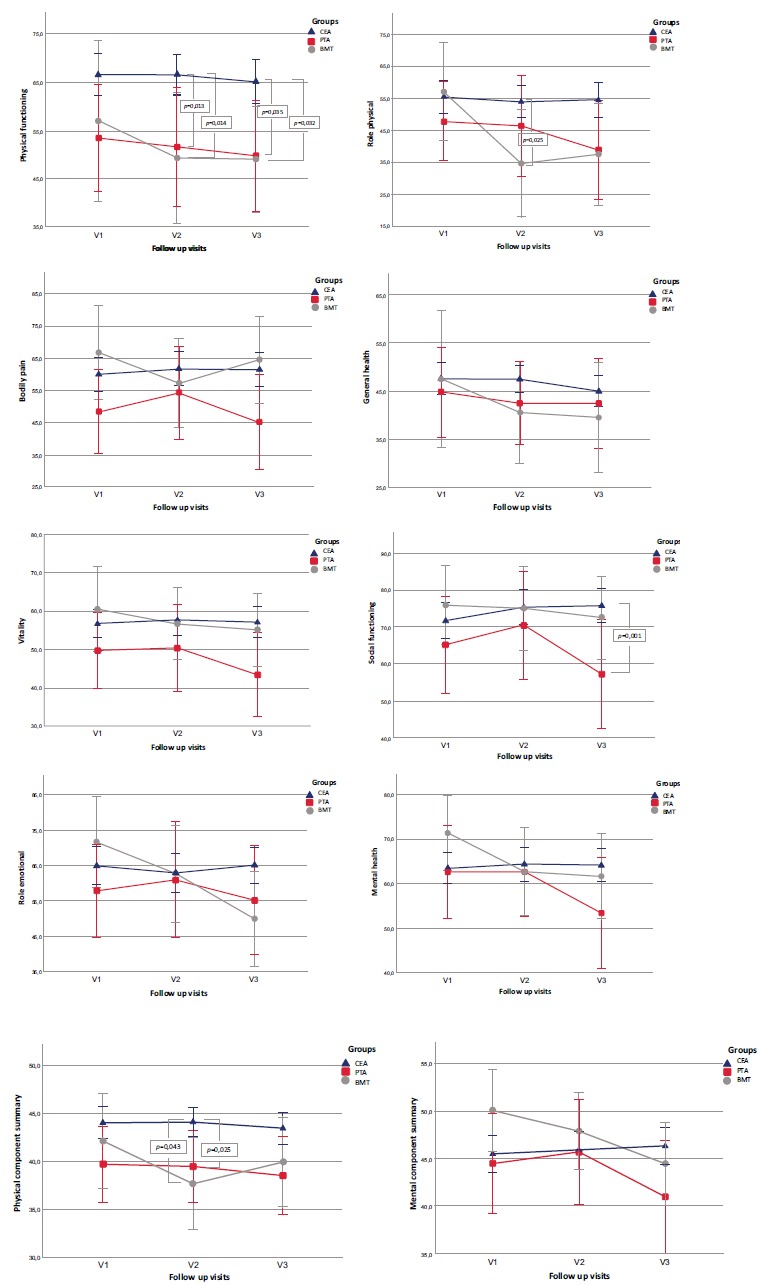
Results of mean SF-36v2 scores during the follow-up period in all study groups. Trend in SF-36v2 scores from baseline to 1 year. Higher scores indicate better quality of life. Significant differences (p<0.05) in scores were not observed in the CEA group during 1 year follow up period. In the CAS group SF-36v2 scores did not change during the follow up period except for bodily pain (p=0.02) where worst scores were after 12 months. In the BMT significant differences in scores were noted in 3 of 10 subscales (role physical, bodily pain and mental component summary). Compared with CAS and BMT group, CEA group patients had better scores at 6 months for 3 of the 10 SF-36v2 subscales (physical functioning, role physical and physical component summary). Plotted values at each timepoint represent means and standard deviation derived from the analysis of covariance. CEA = blue, CAS = red, BMT = grey. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper).
Compared with CAS and BMT group, CEA group patients had better scores at 6 months for 3 of the 10 SF-36v2 subscales: for PF (p=0.001, η2=0.076), RP (p=0.012, η2=0.05) and for PCS (p=0.005, η2=0.061). By 12 months of follow-up, although 7 of the 10 subscales (PF, RP, VT, SF, RE, MH and MCS) had better scores in the CEA group (p<0.05), the effect size of statistical significance was medium only for PF (p=0.004, η2=0.065) and SF (p=0.002, η2=0.072) domains.
4. DISCUSSION
Although older studies do not show changes in cognitive function after CEA or CAS [33], the current study indicates significant improvement of cognitive function after revascularisation of severe carotid stenosis, except in the BMT group, where the observed improvement was not statistically significant. The significant improvement in revascularisation groups could be explained by “mechanical” improvement of blood flow and subsequent restoration of cerebral perfusion after CEA or CAS in addition to BMT. As cognitive decline was not observed in the BMT group, it may indicate that there could be benefits not only from “mechanical” revascularisation of carotid stenosis by removal of atherosclerotic plaque, but also from BMT regarding cognitive function because BMT alters the pathogenetic mechanisms of cerebral small vessel disease. Reducing atherosclerotic load in cerebral small vessels, which is a cause for white matter burden [34], it could positively affect cognitive performance. Therefore, combined medical and surgical or endovascular interventions may halt or reverse cognitive impairment.
On the other hand, the probability of a practice or learning effect of the MoCA test may also be one of the causes or explanations for the improvement of cognitive performance. To minimise the practice effect, we chose follow-up time 6 and 12 months. Although Plessers et al. have shown a practice effect of repeated neurocognitive testing [35], these results remain equivocal. In their research cognitive impairment was observed at baseline in the study group as well as in the control group. The control group consisted of patients with PAD who shared the same cardiovascular risk factors as the patients with severe carotid stenosis. The results are inconclusive because it is unknown whether the improvement of cognitive function in the study and control groups could be explained only by the practice effect or by fact that there was a comprehensive management of cardiovascular risk factors in both study groups, which reduces the risk of cognitive decline itself [36] or by both practice and management of cardiovascular risk factors.
In the analysis of MoCA subtest scores during the follow-up period, slight discrepancy between treatment groups was detected. In the CEA group MoCA subtest analysis showed significant improvement in multiple domains as attention, language, abstraction and delayed recall (p<0.04); in the CAS group, improvement was observed only in a visuospatial/executive subtest (p=0.01); in the BMT group - in a delayed-recall subtest (p=0.027). However, the effect size was small for all statistically significant improvements. The disparity of improvement in various domains could be explained by partial overlap of some subtests for assessment of main cognitive domains in the MoCA scale. For example, the two-item abstraction and phonemic fluency tasks are part of executive function assessment as well [18]. Therefore, we may state that in addition to attention, language and memory, executive function may also be partly improved in the CEA group. Likewise, the findings of MoCA subtests in the CEA group could be congruent with the improvement of MoCA subtests after CAS and in the BMT group. On the other hand, the statistical effect size was small in each group, thus these findings could change if the study populations were larger. In addition, MoCA as a screening tool is not as sensitive as comprehensive sophisticated standardised neuropsychological cognitive assessment tests, which could reveal subtler changes in cognitive domains compared with a screening tool.
In the literature, there are only few studies that have evaluated and compared long-term changes in cognitive function after carotid stenosis revascularisation (CEA, CAS) and BMT. Although methodological differences make meaningful comparison of results across studies challenging, main conclusions of these findings are similar. In the studies where cognitive function was assessed, improvement of total MoCA score in a year after revascularisation (CEA, CAS), but not in the BMT group was observed. Furthermore, the CEA group showed improvement in executive and memory MoCA subtest scores, which is partially congruent with our findings [37]. The improvement of these domains is consistent with results that show association between reduced perfusion of anterior circulation and worse executive and memory function [38]. Furthermore, the revascularisation and improvement of blood flow in the middle cerebral artery is associated with greater improvement in attention and executive function [39]. In the studies where changes in cognitive function in a year after CEA or CAS versus the control group was assessed with the MoCA test, the results indicated that cognitive function might improve or at least do not decline in symptomatic and asymptomatic elderly patients with severe carotid stenosis who had revascularisation [40, 41]. Comparing studies where long-term effects of different carotid stenosis treatment methods (CEA, CAS or BMT) on cognitive function were assessed, their results also show that revascularisation of carotid stenosis improves long-term cognitive performance, independent of treatment type [42-46]. Except, older studies have suggested that there is no difference between cognitive function before and after carotid stenosis revascularisation (CEA, CAS) [47, 48]. How-
ever, comparing the treatment effect of carotid stenosis in older studies, the results of these studies must be interpreted with caution because in recent decades not only pharmacological management of cerebrovascular disease but also the technical equipment and skills of revascularisation have been improved [42].
In 1997, Alexopoulus et al. [49] suggested the “vascular depression” hypothesis, which is supported by the comorbidity of depression, vascular disease and vascular risk factors and by the association of ischaemic lesions to distinctive behavioural symptoms. A recent update of the vascular depression hypothesis confirmed that vascular depression can be regarded as a distinct subtype of late life depression characterised by specific clinical presentation and association with vascular risk factors and a variety of cerebrovascular lesions, as shown by structural magnetic resonance imaging (MRI). The mechanisms how vascular disease may influence the development and course of depression are mechanistic disconnection, inflammation and hypoperfusion [9]. Therefore, one of the aims of our study was to assess the relationship between severe carotid stenosis and depressive symptoms. Most patients with severe carotid stenosis also have several cardiovascular risk factors (AH, dyslipidaemia, vascular comorbidities, DM), which itself may cause white matter damage. If the small vessels are already impaired, autoregulation of cerebral blood flow is affected as well. Therefore, the presence of severe carotid stenosis and impaired cerebrovascular reserve reduces the cerebral perfusion pressure even more [50]. Development of depression may be decreased by revascularisation of carotid stenosis which increases the cerebral perfusion pressure and reduces ischaemic lesion due to hypoperfusion. In contrast to Mlekusch et al. study where a significant reduction of depressive symptoms was found in patients who underwent CAS [51], results of our study did not show statistically significant differences in the frequencies of depressive symptoms at 6 or 12 months not only in the BMT group but also in the CEA and CAS groups. Therefore, we argue about the direct causal relationship between severe carotid stenosis and depression and beneficial effects on the course of depressive symptoms after revascularisation. Nevertheless, other studies have also evaluated change in depressive symptoms using variable scales such as Beck Depression and Anxiety Scale [45], Hamilton Depression Rating Scale [43-47]. Geriatric Depression Scale [52], Hospital Anxiety and Depression Scale [42], where no statistically significant differences in mood or depressive symptoms over follow up periods or between CEA and CAS groups were observed. Therefore, if we suppose that vascular depression may share similar pathogenetic mechanisms with cognitive impairment, lack of reversibility of depressive symptoms could suggest that depression may be a marker for more severe brain structural damage or dysfunction where these changes are no more reversible [53]. These irreversible changes could also explain why patients with vascular depression have poor response to depression treatment [9]. However, this hypothesis is unclear and needs further investigations.
In this study, we found that patients undergoing CEA had similar mean values of all SF-36v2 domains at 6 and 12 months compared to pre-procedure levels. In CAS group mean SF-36v2 scores also did not change during the follow-up period except for BP where the worst scores were after 12 months. However, in the BMT group measures of RP, BP and MCS worsened after 6 and 12 months compared to SF-36v2 scores at the beginning of the study. So far, literature review and meta-analysis have shown that CEA and CAS maintain preoperative HRQoL for at least 1 year which is partially congruent with our findings [32, 54]. In our study, most of the patients in the CEA and CAS groups were asymptomatic, whereas symptomatic patients had TIA or minor stroke that was not disabling. Therefore, it is reasonable not expect superior HRQoL compared to baseline, particularly for previously asymptomatic patients [32]. However, in the BMT group, HRQoL worsened. Martin et al. reported that patients with TIA or minor stroke in the CEA cohort rated significantly improved changes in GH perception [55]. Therefore, the interpretation of our findings could highlight some anxieties over future ischaemic events or doubts of treatment choice in patients who refused revascularisation of carotid stenosis. As majority of patients had symptomatic carotid stenosis in the BMT group, probable reason for decreased values of RP could be gradual worsening of neurological deficit due to chronic hypoperfusion of the brain where brain plasticity is more restricted; the same could also be referred to patients with severe asymptomatic carotid stenosis. BMT alone cannot reduce the degree of stenosis and improve the perfusion of the entire hemisphere, build collateral conduits for blood flow or limit the effects of encephalomalacia and neuronal loss caused by chronic ischaemia [56].
Comparing HRQoL between treatment groups during the follow-up period, we found that patients undergoing CEA had better HRQoL during the 6 and 12 months after carotid revascularisation relative to patients undergoing CAS or receiving BMT only. Six months after CEA, these benefits were most pronounced for measures of overall PF, RP and PCS. Whereas at 12 months - for PF and SF. Several studies have compared HRQoL after CEA versus CAS in patients with severe carotid stenosis. Most of these studies report that there are no differences between CEA and CAS at 1 year with similar HRQoL for CEA and CAS in all domains of SF-36v2 [57-59]. The comment for this discrepancy with our findings could be that in the CAS group we had patients with more cardiovascular risk factors as in the CEA group. It is known that HRQoL is poorer in patients with cardiovascular risk factors compared to other chronic illnesses, where CAD imposes one of the greatest decrements across a broad range of domains of functioning and perceived HRQoL [60, 61]. In addition, in the CAS group during the follow-up, BP worsened, which may have affected scores of the overall PCS.
Several limitations of this study should be acknowledged. First, this was an observational cohort study where patients were treated according to preference and consequently selection has occurred by choosing a treatment modality. The aim of our study was to assess changes in cognitive function, depressive symptoms and HRQoL after carotid revascularisation and BMT not to compare carotid stenosis treatment modalities and their effect on outcomes. Therefore randomized controlled trials are needed to confirm our findings as well as to evaluate effect of revascularisation on cognitive function, depressive symptoms and HRQoL. Second, our study had non-uniform sample sizes across study groups and was not completely balanced with regard to comorbidities, having disproportionate percentage of cardiovascular comorbidities in the CAS group and ischaemic events in the BMT group. However, to reduce the probability of incorrect results of statistical significance due to different sample sizes, we calculated effect sizes to quantify the magnitude of the difference between study groups. Third, the lack of brain imaging may be a cause of incorrect classification of symptomatic or asymptomatic carotid stenosis because mild symptoms may be unnoticed by the patient. Besides there is a question whether new ischaemic lesions on early diffusion weighted imaging after revascularisation may affect cognitive function because some studies have observed partial reversibility of these lesions [62]. Therefore MRI imaging in randomized controlled trials after revascularisation could give insight whether and how MRI changes or differences affect the results of cognitive function.
However, this study to our knowledge has larger BMT control group than any previous trials. In addition, we evaluated not only long-term changes in cognition, but also long-term changes in depressive symptoms and HRQoL in patients with severe (≥ 70%) carotid stenosis after revascularisation and in the BMT group. Therefore the results and suggestion of our study should be confirmed in further studies.
CONCLUSION
Patients with severe carotid stenosis who underwent revascularisation enhanced their cognitive performance without exerting significant change of depressive symptoms. Preoperative HRQoL may be maintained for at least one year in the CEA group whereas the presence or absence of HRQoL change after carotid stenting remains equivocal.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Tatjana Muravska for her substantial contribution in data collection.
LIST OF ABBREVIATIONS
- AF
Atrial Fibrillation
- AH
Arterial Hypertension
- ANOVA
Analysis of Variance
- BMT
Best Medical Treatment
- BP
Bodily Pain
- CAD
Coronary Artery Disease
- CAS
Carotid Artery Stenting
- CEA
Carotid Artery Endarterectomy
- CREST-2 trial
Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial 2
- DM
Diabetes Mellitus
- GH
General Health
- HRQoL
Health Related Quality of Life
- ICH
Intracerebral Hemorrhage
- IQR
Interquartile Range
- MCS
Mental Component Summary
- MH
Mental Health
- MI
Myocardial Infarction
- MoCA
Montreal Cognitive Assessment Scale
- MRI
Magnetic Resonance Imaging
- mRS
Modified Rankin Scale
- NASCET
North American Symptomatic Carotid Artery Endarterectomy Trial
- NYHA
New York Heart Association
- NIHSS
National Institute of Health Stroke Scale
- PAD
Peripheral Artery Disease
- PCS
Physical Component Summary
- PF
Physical Functioning
- PHQ-9
Patient Health Questionnaire-9
- SD
Standard Deviation
- r
Statistical Coefficient (Statistical Effect Size for Wilcoxon Signed Rank Test)
- RE
Role Limitations Due to Emotional Problems or Role Emotional
- RP
Role Limitations Due to Physical Problems or Role-physical
- SF
Social Functioning
- SF-36v2
Medical Outcome Survey Short Form 36 Version 2
- TIA
Transient Ischaemic Attack
- V1
Baseline Visit 1
- V2
Visit 2 (after 6 months)
- V3
Visit 3 (after 12 months)
- VT
Vitality
- η2
Partial Eta Squared, Statistical Coefficient (Statistical Effect Size for Kruskal-Wallis and ANOVA Tests).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical permission was granted by the Riga Stradins University Ethics Committee, Riga, Lativa.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are base of this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants before revascularisation or initiation of best medical treatment.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
FUNDING
None.
AUTHORS' CONTRIBUTION
EP, EM, DK, AM participated in the conception and design of the study. EP coordinated the study. EP and IK participated in data collection. ER performed the analyses. EP wrote the first draft of the manuscript. All authors participated in the writing and revision of the successive drafts of the manuscript. All authors have read and approved the final manuscript.
REFERENCES
- 1.Naylor A.R. Why is the management of asymptomatic carotid disease so controversial? Surgeon. 2015;13(1):34–43. doi: 10.1016/j.surge.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Sun J-H., Tan L., Yu J-T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014;2(8):80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackett M.L., Pickles K., Part I. Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke. 2014;9(8):1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 4.Wang T., Mei B., Zhang J. Atherosclerotic carotid stenosis and cognitive function. Clin. Neurol. Neurosurg. 2016;146:64–70. doi: 10.1016/j.clineuro.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Lal B.K., Dux M.C., Sikdar S., et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J. Vasc. Surg. 2017;66(4):1083–1092. doi: 10.1016/j.jvs.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Paraskevas K.I., Lazaridis C., Andrews C.M., et al. Comparison of cognitive function after carotid artery stenting versus carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2014;47(3):221–231. doi: 10.1016/j.ejvs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Naylor A.R., Ricco J.B., de Borst G.J., et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018;55(1):3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Mott M., Koroshetz W., Wright C.B. CREST-2: Identifying the best method of stroke prevention for carotid artery stenosis: National Institute of Neurological Disorders and Stroke Organizational Update. Stroke. 2017;48(5):e130–e131. doi: 10.1161/STROKEAHA.117.016051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizenstein H.J., Baskys A., Boldrini M., et al. Vascular depression consensus report – a critical update. BMC Med. 2016;14(1):161. doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smedt D., Clays E., De Bacquer D. Measuring health-related quality of life in cardiac patients. Eur. Heart J. 2016;2(3):149–150. doi: 10.1093/ehjqcco/qcw015. [DOI] [PubMed] [Google Scholar]
- 11.North American Symptomatic Carotid Endarterectomy Trial Collaborators1, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M. Beneficial effect of carotid endarter-ectomy in simptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 12.Fischer U., Baumgartner A., Arnold M., et al. What is a minor stroke? Stroke. 2010;41(4):661–666. doi: 10.1161/STROKEAHA.109.572883. [DOI] [PubMed] [Google Scholar]
- 13.Warlow C. MRC European carotid surgery trial: Interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337(8752):1235–1243. [PubMed] [Google Scholar]
- 14.Halliday A.W., Thomas D., Mansfiel A. The Asymptomatic Carotid Surgery Trial (ACST) rationale and design. Eur. J. Vasc. Surg. 1994;8(6):703–710. doi: 10.1016/s0950-821x(05)80650-4. [DOI] [PubMed] [Google Scholar]
- 15.Brott T.G., Halperin J.L., Abbara S., et al. 2011 ASA/ACCF/AHA/ AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: A report of the American College of Cardiology Foundation/American Heart Association Task F. Circulation. 2011;124(4):e54–e130. doi: 10.1161/CIR.0b013e31820d8c98. [DOI] [PubMed] [Google Scholar]
- 16.Kernan W.N., Ovbiagele B., Black H.R., et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine Ziad. MoCA Instruction Latvian. Available from: http: //www.mocatest.org/wp-content/uploads/2015/tests-instructions/MoCA-Instruction-Latvian.pdf.
- 18.Nasreddine Z., Phillips N., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Pendlebury S.T., Mariz J., Bull L., et al. MoCA, ACE-R, and MMSE versus the national institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke. 2012;43(2):464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bocti C., Legault V., Leblanc N., et al. Vascular cognitive impairment: most useful subtests of the Montreal Cognitive Assessment in minor stroke and transient ischemic attack. Dement. Geriatr. Cogn. Disord. 2013;36(3-4):154–162. doi: 10.1159/000351674. [DOI] [PubMed] [Google Scholar]
- 21.Koski L. Validity and applications of the montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc. Dis. 2013;36(1):6–18. doi: 10.1159/000352051. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. Gen. Hosp. Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Kung S., Alarcon R.D., Williams M.D., et al. Comparing the Beck Depression Inventory-II (BDI-II) and Patient Health Questionnaire (PHQ-9) depression measures in an integrated mood disorders practice. J. Affect. Disord. 2013;145(3):341–343. doi: 10.1016/j.jad.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Beutel M.E., Wiltink J., Kirschner Y., et al. History of depression but not current depression is associated with signs of atherosclerosis: data from the Gutenberg Health Study. Psychol. Med. 2014;44(05):919–925. doi: 10.1017/S0033291713001542. [DOI] [PubMed] [Google Scholar]
- 26.Williams L.S., Brizendine E.J., Plue L., et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36(3):635–638. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 27.De Man-Van Ginkel J.M., Hafsteinsdottir T., et al. An efficient way to detect poststroke depression by subsequent administration of a 9-item and a 2-item patient health questionnaire. Stroke. 2012;43(3):854–856. doi: 10.1161/STROKEAHA.111.640276. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K., Spitzer R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002;32(9):509–515. [Google Scholar]
- 29.Vrublevska J., Trapencieris M., Rancans E. Adaptation and validation of the Patient Health Questionnaire-9 to evaluate major depression in a primary care sample in Latvia. Nord. J. Psychiatry. 2018;72(2):112–118. doi: 10.1080/08039488.2017.1397191. [DOI] [PubMed] [Google Scholar]
- 30.Optum. SF-36 Health Survey Latvian and Russian version-Optum.com. 2015 [cited 2015 Mar 3]. Available from: https: //campaign.optum.com/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html.
- 31.Ware J.E. Features of the Short Form Surveys. An excerpt from the user’s manual for the SF-36v2 health survey. 2nd ed. 2007. Deciding Which Short Form Survey to Use. [Google Scholar]
- 32.Shan L., Shan J., Saxena A., Robinson D. Quality of life and functional status after carotid revascularisation: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2015;49(6):634–645. doi: 10.1016/j.ejvs.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 33.De Rango P., Caso V., Leys D., et al. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: A systematic review. Stroke. 2008;39(11):3116–3127. doi: 10.1161/STROKEAHA.108.518357. [DOI] [PubMed] [Google Scholar]
- 34.Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 35.Plessers M., Van Herzeele I., Hemelsoet D., et al. Prospective comparison of cognitive effects of carotid endarterectomy versus carotid stenting with flow reversal or distal filters. J. Clin. Exp. Neuropsychol. 2015;37(8):834–841. doi: 10.1080/13803395.2015.1060952. [DOI] [PubMed] [Google Scholar]
- 36.Baumgart M., Snyder H.M., Carrillo M.C., et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe J., Ogata T., Higashi T., Inoue T. Cognitive Change 1 Year after CEA or CAS compared with medication. J. Stroke Cerebrovasc. Dis. 2017;26(6):1297–1305. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Alosco M.L., Gunstad J., Jerskey B.A., et al. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav. 2013;3(6):626–636. doi: 10.1002/brb3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghogawala Z., Amin-Hanjani S., Curran J., et al. The effect of carotid endarterectomy on cerebral blood flow and cognitive function. J. Stroke Cerebrovasc. Dis. 2013;22(7):1029–1037. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Baracchini C., Mazzalai F., Gruppo M., et al. Carotid endarterectomy protects elderly patients from cognitive decline: A prospective study. Surgery. 2012;151(1):99–106. doi: 10.1016/j.surg.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Yan Y., Yuan Y., Liang L., et al. Influence of carotid artery stenting on cognition of elderly patients with severe stenosis of the internal carotid artery. Med. Sci. Monit. 2014;20:1461–1468. doi: 10.12659/MSM.890847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wapp M., Everts R., Burren Y., et al. Cognitive improvement in patients with carotid stenosis is independent of treatment type. Swiss Med. Wkly. 2015;145:1–7. doi: 10.4414/smw.2015.14226. [DOI] [PubMed] [Google Scholar]
- 43.Carta M.G., Lecca M.E., Saba L., et al. Patients with carotid atherosclerosis who underwent or did not undergo carotid endarterectomy: outcome on mood, cognition and quality of life. BMC Psychiatry. 2015;15:277. doi: 10.1186/s12888-015-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dempsey R.J., Jackson D.C., Wilbrand S.M., et al. The preservation of cognition 1 yr after carotid endarterectomy in patients with prior cognitive decline. Neurosurgery. 2018;82(3):322–328. doi: 10.1093/neuros/nyx173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J.J., Schwartz S., Wen J., et al. Comparison of neurocognitive outcomes after carotid endarterectomy and carotid artery stenting. Am. Surg. 2015;81(10):1010–1014. [PubMed] [Google Scholar]
- 46.Kougias P., Collins R., Pastorek N., et al. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J. Vasc. Surg. 2015;62(2):355–362. doi: 10.1016/j.jvs.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 47.Aleksic M., Huff W., Hoppmann B., et al. Cognitive function remains unchanged after endarterectomy of unilateral internal carotid artery stenosis under local anaesthesia. Eur. J. Vasc. Endovasc. Surg. 2006;31(6):616–621. doi: 10.1016/j.ejvs.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Altinbas A., Van Zandvoort M.J.E., Van Den Berg E., et al. Cognition after carotid endarterectomy or stenting: A randomized comparison. Neurology. 2011;77(11):1084–1090. doi: 10.1212/WNL.0b013e31822e55b9. [DOI] [PubMed] [Google Scholar]
- 49.Alexopoulos G.S., Meyers B.S., Young R.C., et al. “Vascular depression” hypothesis. Arch. Gen. Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 50.Gupta A., Chazen J.L., Hartman M., et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke. 2012;43(11):2884–2891. doi: 10.1161/STROKEAHA.112.663716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mlekusch W., Mlekusch I., Minar E., et al. Is there improvement of “vascular depression” after carotid artery stent placement? Radiology. 2006;240(2):508–514. doi: 10.1148/radiol.2402051043. [DOI] [PubMed] [Google Scholar]
- 52.Feliziani F.T., Polidori M.C., De Rango P., et al. Cognitive performance in elderly patients undergoing carotid endarterectomy or carotid artery stenting: A twelve-month follow-up study. Cerebrovasc. Dis. 2010;30(3):244–251. doi: 10.1159/000319066. [DOI] [PubMed] [Google Scholar]
- 53.Hare D.L., Toukhsati S.R., Johansson P., Jaarsma T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 54.Chabowski M., Grzebien A., Ziomek A., et al. Quality of life after carotid endarterectomy: A review of the literature. Acta Neurol. Belg. 2017;117(4):829–835. doi: 10.1007/s13760-017-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin P., Fotopoulou M., Baker G., Humphrey P. Health-related quality of life after transient ischemic attack and minor stroke: is medical or surgical treatment influential? J. Stroke Cerebrovasc. Dis. 1998;7(1):70–75. doi: 10.1016/s1052-3057(98)80024-4. [DOI] [PubMed] [Google Scholar]
- 56.Bauer A.M., Bain M.D., Rasmussen P.A. Chronic cerebral ischemia: where “evidence-based medicine” fails patients. World Neurosurg. 2015;84(3):714–718. doi: 10.1016/j.wneu.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 57.CaRESS Steering Committee. Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-Year results. J. Vasc. Surg. 2005;42(2):213–219. doi: 10.1016/j.jvs.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Stolker J.M., Mahoney E.M., Safley D.M., et al. Health-related quality of life following carotid stenting versus endarterectomy: Results from the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at HIgh Risk for Endarterectomy) trial. JACC Cardiovasc. Interv. 2010;3(5):515–523. doi: 10.1016/j.jcin.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Cohen D.J., Stolker J.M., Wang K., et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: Results from CREST (Carotid Revascularization Endarterectomy versus Stenting Trial). J. Am. Coll. Cardiol. 2011;58(15):1557–1565. doi: 10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swenson J.R. Quality of life in patients with coronary artery disease and the impact of depression. Curr. Psychiatry Rep. 2004;6(6):438–445. doi: 10.1007/s11920-004-0008-x. [DOI] [PubMed] [Google Scholar]
- 61.Martinelli L.M.B., Mizutani B.M., Mutti A., et al. Quality of life and its association with cardiovascular risk factors in a community health care program population. Clinics (São Paulo) 2008;63(6):783–788. doi: 10.1590/S1807-59322008000600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grunwald I.Q., Papanagiotou P., Reith W., et al. Influence of carotid artery stenting on cognitive function. Neuroradiology. 2010;52:61–66. doi: 10.1007/s00234-009-0618-4. [DOI] [PubMed] [Google Scholar]


