Abstract
Breast Milk (BM) is the best source of nutrition for newborns, especially if premature. In fact, its beneficial impact on short- and the long-term neonatal outcome has was deeply described.
Unfortunately, BM could not be always so safe, especially due to the possible presence of maternal viruses that can be shed and transferred to the breastfed neonate. Among these, Cytomegalovirus (CMV) can potentially lead to a serious and acute illness, mostly in case of low gestational age.
Some studies also report the association of CMV-acquired infection to an increased risk of structural and functional brain modifications and neurological impairment.
Due to these reasons, a strategy to remove CMV from BM with a minimal or absent impact on its beneficial components would be desirable.
Up to now, pasteurization, freezing, ultraviolet- C or microwave irradiation are the available techniques; they show different levels of efficacy and variable effects on BM composition, even if many studies are still needed to fully clarify these implications.
In this review, we provide an update of the current evidence about these topics. We focus on the factors promoting CMV shedding through BM; moreover, the possible occurrence of a severe disease in preterm neonates is also described. Finally, we investigate the potential effects showed on BM properties by the strategies that prevent or reduce viral transmission, therefore influencing newborns’ health, and the new techniques which could show a relevant role in the next future, such as metabolomics.
Keywords: Breast milk, cytomegalovirus, preterm newborns, pasteurization, freezing, irradiation
1. INTRODUCTION
The beneficial impact of Breast Milk (BM) has been deeply investigated and demonstrated by many authors, especially on extremely low birth weight (ELBW) newborns in neonatal intensive care unit (NICU) [1-4].
Due to these reasons, BM represents the ideal nutrition source and exclusive breastfeeding is recommended by the American Academy of Pediatrics (AAP) until at least 6 months of post-natal life [5, 6].
BM exerts several short- and long-term positive effects. In fact, it has shown anti-inflammatory and anti-infective functions, it promotes immune system formation and can also support the development of immature organs [7].
BM also confers protection against prematurity-related retinopathy (ROP) and necrotizing enterocolitis (NEC) [8]. Finally, BM has been related to beneficial effects in the successive phases of life. It can give protection against the development of obesity, diabetes and cardiovascular disease and improve newborn’s neurological development [7]; regarding this, some recent data also demonstrated the association of BM with a higher intelligence quotient (IQ) and educational outcome at thirty years of age [9].
However, it is known that BM could not be always so safe, especially taking into account the possible presence of maternal viruses that can be transmitted to the newborn. For example, maternal Cytomegalovirus (CMV) can be shed in BM and transferred to the breastfed neonate. This acquired infection can lead to a serious and acute illness, especially in very premature infants. Therefore, a strategy to improve the removal of CMV from maternal milk without reducing or altering its beneficial components should be desirable [10].
In this perspective, many efforts have been already made and continue to be performed, through several experimental studies and clinical trials, in order to improve our knowledge on this topic [1, 6, 11, 12].
Due to the high prevalence of BM-acquired infection, inactivating virus concentration in BM could represent a beneficial option for preterms [6, 13, 14].
In this paper, we describe all the factors promoting CMV shedding through BM; moreover, we investigate the possible occurrence of a severe disease in preterm neonates and finally we focus on the available strategies to prevent viral transmission.
2. CMV INFECTION: EPIDEMIOLOGY
CMV is a ubiquitous infection among humans [8, 15]. It consists of a beta-herpesvirus which can persist lifelong in CD34+ precursors, representing a chronic infection [15-17].
Several genes of its genome can interact and take benefits from the human immune system and inflammatory patterns [15, 17].
An increased rate of CMV infection seems to depend on many factors; among these, a low socioeconomic status, social habits or customs, and origin in a developing country or in a region showing a high viral prevalence seem to have a potential role. Person to person transmission can occur through breastfeeding, care of neonates or young children, sexual contacts. In conclusion, the contact with body fluids is needed for CVM acquisition [15].
The prevalence of CMV-seropositive women in reproductive age seems to be lower in the United States and Western Europe (about 40-60%), compared with Asia and Africa (until 90%) [15, 16, 18].
In literature, BM acquired infection ranges from 5.7% to 60%, depending on the study and on the technique used for CMV inactivation [6, 19-21].
CMV is also associated with the congenital infection presenting the highest prevalence in developed countries, occurring in approximately 0.6-0.7% of the newborns [15, 22-24].
Moreover, CMV seems the principal cause of perinatal acquired infection in the world [6]; this risk is higher for ELBWs belonging to countries or ethnicity where a higher prevalence of CMV-IgG positive women is reported [1, 25], since the majority of such mothers undergo reactivation during lactation and shed the virus in BM without any appearance of infection signs [1, 13, 26-28].
3. CMV INFECTION: BRIEF HISTORY OF BREAST MILK TRANSMISSION
In 1970s, the observation of some neonates negative for CMV at birth and acquiring it during the first month of life suggested the possibility of a postnatal transmission. At first, this was attributed only to maternal genital secretion shedding [10]. Since 1972s, Hayes et al. [29] and Reynolds et al. [30] searched CMV in BM samples belonging to CMV-seropositive mothers, both finding its presence in about 27% of these.
Successive studies found a prevalence of CMV in BM ranging from 13 to 50% of the analyzed samples, according to the kind of study and to the method used for viral detection [10, 13, 26, 31-34].
CMV-seronegative mothers did not shed virus in BM. On the contrary, among CMV-seropositive mothers, the percentage of viral shedding attested between 32 and 96% [10, 13, 26, 32, 33, 35].
BM is not the only source of CMV transmission. In fact, in 1979, it was described the first case of transmission through blood transfusion [16, 36]; this was subsequently and successfully avoided through the use of CMV-IgG- negative blood [16, 37].
CMV transmission to full term babies was initially described as a form of natural immunization, associated with minimal or absent signs of disease [16, 32].
Unfortunately, BM acquired infection can cause a severe disease in preterm babies [16].
4. CMV INFECTION: MOTHER TO CHILD TRANSMISSION ROUTES
The transmission of CMV from mother to child is a key passage in maintaining the high human prevalence showed by such virus. It can occur through three routes: the first is transplacental route; the second is peri-natal route, during the delivery. Thirdly, transmission via BM is also possible [8, 15].
Therefore, the last two ways are not related to the acute illness or to the damage involving central nervous system (CNS) which are described after the occurrence of congenital infection [15].
The modalities of transmission are the following three, presented below and reported in Fig. (1).
Fig. (1).
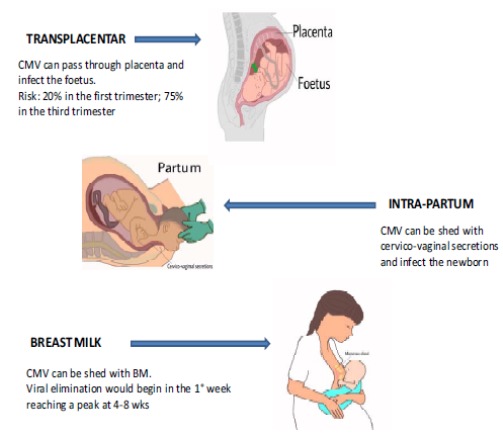
CMV-mother to child transmission routes.
4.1. Transplacental Transmission
Transmission through this route occurs in about 35% of the pregnancies in which the mother undergoes primary infection. For the mother, this usually results in an asymptomatic infection and is detected only through CMV-antibody screening [15, 38]. The risk of transmission to the foetus increases according to the gestational age (GA). In fact, it results in approximately 20% during the first trimester of gestation and 75% if maternal infection occurs in the last trimester [15, 39-41]. Especially when acquired during the first trimester, it can lead to a severe CNS damage, developmental disability, cognitive impairment or cerebral palsy, sensorineural hearing loss or vision impairment [15, 23, 42, 43].
About 50% among the newborns who acquire congenital infection and show signs of intrauterine infection at birth will probably develop disabilities. Instead, among the asymptomatic neonates at birth, about 5-15% will undergo the same disabilities [15, 22].
A recurrent infection, due to reactivation of maternal latent CMV infection, is also possible during pregnancy [10, 15, 44]. Therefore, the transmission to the child can occur both if the mother has been infected previously or during the pregnancy; the first modality is related to maternal reactivation, even if she was immune at the time of conception. Congenital CMV infection is an important cause of neonatal morbidity and mortality [10, 15].
4.2. Intrapartum Transmission
As previously reported, an intrapartum CMV transmission is also possible, due to viral elimination through genital secretions in CMV-seropositive mothers. According to Pass et al. [15], these babies eliminate CMV virus at 3-6 weeks of life [15]. In general, the newborns acquiring the virus through this route would not develop a serious illness, except if they are very preterm or Low Birth Weight (LBW) [13, 15, 45].
4.3. Breast Milk transmission
The first source of post-natal CMV transmission is through BM. This route mostly regards ELBWs [1, 8, 13, 23, 46-50].
Such modality of transmission represents the source of infection for the majority of the infants infected during the first year of life [15, 32]. This has a great impact on CMV seroprevalence in the population, due to the fact that infected infants can also shed the virus in their fluids and secretions and transmit it to caregivers or other babies [15].
Maternal immunity or latent infection does not prevent viral shedding, due to the possibility of viral reactivation [8, 13, 15, 16, 23, 51-53].
The moment in which viral shedding begins, its variations and the end moment can be highly variable among the subjects. All the mechanisms of this reactivation are not currently known [16].
However, it is well known that CMV DNA undergoes reactivation and therefore is detectable in BM of about 95% of CMV-seropositive mothers, according to polymerase chain reaction (PCR) detection [6, 13, 15, 16, 26, 28, 52, 54, 55].
CMV reactivation is promoted in case of a low immunological status, stress, interleukin Il-6 stimulus, DNA-damage but also in healthy mothers during lactation [16, 32]. In this last case, viral shedding in BM is observable without systemic findings of viral load, as an example in blood or maternal genital secretions [16, 27, 56, 57].
Macrophages of BM are the cells responsible for CMV reactivation, even if the presence of infected cells seems not indispensable for viral transmission [16, 27, 58].
In conclusion, although the possibility to acquire CMV via BM is well known [6, 49, 59-61], not all the mechanism of viral reactivation in BM, the role of milk cells and free-virus in transmission have been fully clarified and described [13].
5. CMV INFECTION: KINETICS OF VIRAL EXCRETION
The kinetics of CMV viral shedding in BM has been deeply investigated and defined as a unimodal and self-limited process [6].
According to many authors, viral shedding would begin during the first week of lactation, reaching a peak at 4-8 weeks. Successively, from 9-12 postnatal weeks, it would reduce significantly [1, 6, 28, 49, 59] and it would end at 3 months of lactation approximately [16]. According to other studies, viral shedding would be detectable since the first day until nine months after delivery [10, 23, 26, 34, 62, 63].
According to Hamprecht et al. [16], CMV can be detected in colostrum since the third day of lactation.
The elimination of a high viral load, an early viral excretion and a long period of breastfeeding may represent risk factors associated with an increased CMV transmission to the child [6, 8].
Moreover, it was also detected an inverse association between CMV IgG avidity and viral shedding rate [16, 64].
Some reported data suggest that colostrum could not be infectious. In this perspective, pasteurization starting from the second week after birth until the eight postnatal could be an efficacious approach to reduce ELBW CMV infection via BM [1].
On the contrary, in the trial performed by Romero-Gomez et al. [6], 45.9% of 90 tested samples of BM was positive for CMV until 3 days post-delivery, with a viral load in colostrum comparable with mature milk 4 weeks postpartum; these findings result in agreement with the results of Chiavarini et al. [20] (54,4% of 57 colostrum samples positive for CMV DNA), suggesting that colostrum could not be so safe regarding CMV transmission [6, 20, 23].
Some BM components seem able to exert various effects on viral transmission. i.e., IgA and antiviral lipids present in BM seem to be protective against CMV transmission; moreover, in some experiments, Vitamin A, monolaurin and lactoferrin inhibited CMV growth in BM in vitro. On the contrary, prostaglandin promoted CMV growth in vitro [10, 62].
Co-infection HIV + CMV determines an increased risk of viral shedding with cervicovaginal secretion and therefore a higher rate of intrapartum mother to child CMV-transmission [15, 65-67].
Moreover, viral CMV-shedding via BM is increased and potentially associated with EBV transmission too [16, 68].
In addition, CMV transmission from HIV-positive mothers seems to increase the risk of HIV transmission too, and it also accelerates its progression [15, 69].
A higher maternal CMV level in BM and a low number of CD4+ lymphocytes are risk factors associated with an increased rate of CMV transmission [16, 70].
6. CMV INFECTION: CAUSES OF EXTREMELY LOW BIRTH WEIGHT NEWBORN’S SUSCEPTIBILITY
Postnatal infection is usually asymptomatic in full term babies [6, 8, 10, 13, 15, 23, 26, 32, 33, 71, 72].
This is due to the protection conferred by maternal IgG antibodies, passively acquired after 28 weeks of gestation. Very preterm babies do not have this protection, and therefore result susceptible to develop a severe infection both acquiring the virus through BM, both via cervical secretions during delivery [1, 10, 23].
Many authors report the occurrence of a severe disease in preterms which acquired CMV post-natally, especially in the most immature newborns [6, 10, 13, 23, 26, 73-76].
A symptomatic disease can also occur in case of increased susceptibility due to a deficient immune function of the mother, fetus or newborn [15].
Preterms born at GA lower than 30-32 weeks and/or showing a BW lower than 1000 gr present a higher risk of CMV acquisition via BM [3, 8, 13, 77-80]; in fact, a lower GA and a reduced birth weight are considered risk factors for CMV infection acquisition [1, 6, 15, 61].
7. CMV INFECTION: CLINICAL FEATURES
In healthy adults or child, the initial infection is generally asymptomatic. However, viremia and viral shedding through secretions can persist for months, or even years in some cases (viral elimination in urine, saliva, genital fluids). Moreover, latent CMV DNA persists in the host without signs of replication. The intermittent occurrence of viral shedding is possible long-life, especially during periods of impaired immune response. In fact, it reduces the ability in CVM replication-control [15].
Preterms, newborns prenatally infected, patients affected by congenital, acquired or drug-related immunodeficiencies, or even other immune diseases, are at risk of disseminating infections with a serious clinical manifestation [15].
Acquired infection in full-term newborns rarely causes a symptomatic disease [6, 14].
According to the trial of Hamprecht et al. [13], 52% of 33 infected infants presented neutropenia, thrombocytopenia or hepatopathy and in 12% of the cases showed sepsis-like symptoms [6, 13].
Very premature babies can show signs of a severe disease because of their immature immune system and because the transfer of maternal IgG begins at 28 weeks of GA. Moreover, in this category of newborns, acquired antibody’s level decreases rapidly [10]. This gives more susceptibility to develop a serious illness after CMV acquisition [1, 3, 10, 13, 47, 73, 81-83].
Postnatally BM acquired CMV infection, in preterms, can be a symptomatic infection varying from a self-limiting disease to a serious and even mortal illness, especially in newborns showing less than 32 weeks of GA. Moreover, the infected infants show an increased risk of Bronchodysplasia (BDP) [1, 16, 84-86].
The clinical presentation of acquired CMV-symptoms in preterm newborns is currently indicated with the expression sepsis-like symptoms; these are showed by about 13,7-15% of the preterms (this percentage reached 80% among the newborns with a GA lower than 26 weeks) and include, as reported through several trials, respiratory impairment, pneumonia, apnea, bradycardia, hepatosplenomegaly, hepatitis, gray pallor, distending bowels, haematological changes as neutropenia and thrombocytopenia and/or absolute lymphocytosis, cholestasis, impaired liver function and increase in liver enzymes, a mild increase in C-reactive Proteins (CRP) [6, 8, 10, 13, 15, 16, 21, 26, 33, 45, 76], and even death [10].
These symptoms are mostly self-limited, without showing the impact on neonatal outcome. It has not been reported an increase in intracranial hemorrage, periventricular leukomalacia, retinopathy of prematurity (ROP) and NEC rate [16].
However, in few cases and small case series, a more serious illness has been described and even death in VLBW, pneumonia requiring ventilation, hepatitis, NEC, gastrointestinal involvement, bloody diarrhea, bowel occlusion and volvulus. In some cases, antiviral treatment has been needed [3, 16, 45, 87-90].
In the multicenter study of Balcells et al. [23], the newborns with acquired CMV infection resulted smaller and showed a lower GA.
The percentage of clinical manifestation’s occurrence after a post-natal infection varies from 0% to 75% depending on the GA of the infected newborn [8, 13, 21, 45, 59, 91].
Moreover, infected preterms may be at risk of long-term sequelae, but few studies are available and this correlation has been not clarified yet [8].
In conclusion, ELBWs infants show the highest risk of developing a severe post-natal CMV infection and probably also long-term sequelae [1, 26, 46, 81].
8. CMV INFECTION: DIAGNOSIS
In the congenital infection, diagnosis is allowed by the detection of CMV in urine samples within the first three weeks of life. This can be made both through the detection of viruria (by culturing) (viruria) or through PCR (DNAuria) [23, 92]. Moreover, congenital infection can be defined as CMV IgM positivity within the first week of life [8], although this method results less sensitive, due to the frequent occurrence of false negative [23].
In the acquired infection, the screening performed with real-time PCR (rtPCR), which amplifies DNA from urine, shows more sensitivity and specificity if compared with cell culturing [23, 93].
The acquired infection is defined as a positive CMV-DNA PCR on urine sample (or CMV viruria) [8], after a previous test performed in the first 14 days of life and which gave a negative result [1, 6, 23].
The lowest limit of detection attests at 360 copies/ml [1, 94]. RtPCR on BM, according to Romero Gomez et al. [6], could help to find samples from mothers showing a high viral shedding; therefore, this could help to decide if BM should undergo any treatment (i.e. freezing), especially under 28 weeks of GA.
9. CMV IN BREAST MILK: METHODS FOR INACTIVATION
Several strategies have been developed and deeply investigated to inactivate CMV in BM, prior to use it to fed neonates [6].
Information about the available BM treatment techniques to reduce CMV transmission are not fully clarified. More studies would be needed to determine the best strategy to eliminate the risk of contagious, leaving BM’s properties unmodified [1].
The actual knowledge on this topic is reported in the following paragraphs and synthesized in Table 1.
Table 1. Description of the available techniques, its efficacy in CMV inactivation and action on breast milk composition.
| Tecnique | Definition | Efficacy on CMV Inactivation | Effect on BM Components |
|---|---|---|---|
| Long-term Pasteurization | Heating: • 62,5 °C for 30 min [6, 10, 95, 96] • 63 °C for 30 min [16] |
It induces the complete CMV eradication. Maximally efficacious in preventing CMV transmission [1, 6, 10, 16, 28, 90, 95-97, 106] |
It modifies the composition reducing nutritional, immunological and endocrinological properties [1, 6, 23, 28, 98, 99, 107], damaging Ig and lymphocytes [10], lactoferrin, lysozyme and secretory IgA, bioactive factors, hormons [96] |
| Short-term Pasteurization | Heating: • 70 °C for 5 min [10] • 72 °C for 10 sec [1,100] • 62 °C for 5 sec [16] |
It seems promising in inactivating CMV and preventing its transmission [10, 16, 28, 90, 95] |
It preserves nutritional and immunological components, enzymes, hormones, growth factors and CMV-specific Ab [10, 16, 28, 90, 95] |
| Freezing | Freeze thawing: • - 20°C for a time ranging from 18 h to 10 d [3, 10, 16, 23, 28, 101-103] |
It can reduce viral concentration, without completely eliminating it [1, 3, 10, 16, 20, 23, 28, 101-103, 108]. In vitro studies (several days) showed the complete CMV elimination, but there are still discordant results in this field [1, 6, 28, 61, 97] | It does not modify composition and nutritional and immunological properties [1, 3, 6, 10, 16, 23, 28, 98, 99, 101-103, 108] |
| UV-Irradiation | • Uv at 250 nm for several time intervals (10-50 sec) at the distance of 1, 2, 3, 4 cm from the light [96] |
It seems promising in eradicating CMV [16, 96, 104] However, some CMV related proteins are not fully eradicated and that a little viral gene transcription can still occur [96] |
It seems to preserve beneficial components, such as lactoferrin, lysozyme and secretory IgA [96] |
| Microwave-Irradiation | • High power (750 W) • Low power (500 W) for 30 sec [104] |
It seems promising in eradicating CMV [16, 96, 104] • High power irradiation induced the complete inactivation of CMV. • Low power irradiation showed a failure rate of 13% [104] |
Few studies investigate its effects on immunological, bioactive, cellular and nutritive components. It would induce a little decrease in the total content of Ig, without modifying fatty acids’ composition [104, 105] |
CMV = Cytomegalovirus; BM = Breast milk; min = minutes; sec = seconds; d = days; Ig = immunoglobulins; Ab = antibodies; Uv = ultraviolet; nm = nanometers; cm = centimeters; W = watts.
9.1. Pasteurization
According to several authors, both short-term and long-term pasteurization result efficacious in preventing CMV transmission. However, only the first method allows the conservation of nutritional properties and immunological components of BM, such as enzymes, hormones, growth factors and CMV-specific antibodies [16, 28, 90, 95].
9.1.1. Long-term Pasteurization
Romero-Gomez et al. [6], Bryant et al. [10] and Lyoid and colleagues [95, 96] define long-term pasteurization as the heating of BM for 30 min at 62,5 °C, while Hamprecht et al. [16] refer to the heat treatment at 63 °C for 30 minutes.
All the authors agree with the observation that this method can produce a microbiologically safe product, completely inactivating CMV, degrading CMV-RNA [6, 28, 97] and reducing bacterial loads by 105 colony forming units per mL [96]. Unfortunately, it alters BM composition reducing its nutritional, immunological and endocrinological properties [6, 23, 98, 99]. It has also been shown to damage immunoglobulins and lymphocytes [10].
9.1.2. Short-term Pasteurization
There is not a univocal definition of short-term pasteurization. Some authors consider it the heat treatment of BM at 70 °C for 5 minutes [10]. Others refer to the treatment at 72 °C for 10 seconds [1, 100].
Finally, it can also be defined as the treatment at 62 °C for 5 seconds [16].
However, it seems to be promising due to its efficacy in inactivating CMV in BM without modifying its nutritional and immunological properties. More studies are needed to fully clarify these observations [10].
9.2. Freezing
Many authors affirm that freeze-thawing at – 20 °C for a time varying from 18 hours to 10 days can reduce viral concentration, without completely eliminating it. Differently, from pasteurization, it does not modify BM constituents and beneficial nutritional and immunological properties [3, 10, 16, 23, 28, 101-103].
However, some in vitro studies performed freezing BM at -20°C for several days, sometimes demonstrated even the complete elimination of CMV, but there are still discordant results in this field [1, 6, 28, 61, 97]. Moreover, these studies confirmed that this technique does not induce alterations in BM composition [6, 98, 99].
The recent multicenter study performed by Balcells et al. [23] evidenced as milk freezing reduces the CMV infection rate; this technique revealed a useful strategy to prevent BM transmission with a reduction rate of about 71% if started within the first week of lactation. However, it does not completely eliminate BM infectivity and in their study, some patients acquired CMV even if fed with frozen BM; in these cases, freezing was started after the first week of lactation [20, 23]. They conclude underlying the needs of more studies to completely understand these implications.
9.3. Novel Techniques
Ultraviolet- C irradiation and Microwave irradiation are two recently evaluated-techniques, which seem to be promising in eradicating CMV from BM [16, 96, 104].
Ultraviolet- C (UV-C) irradiation at 250 nm seems to preserve components such as lactoferrin, lysozyme and secretory IgA, instead of long-term pasteurization. However, after this treatment, it seems that some CMV related proteins are not fully eradicated and that a little viral gene transcription could still occur. The meaning and the implications of this finding have still to be clarified [96].
Moreover, in the study of Lyoid et al. [96], UV-C irradiation was evaluated for several time intervals at the distance of 1, 2, 3 and 4 cm from UV light; the reported data suggest the necessity to define, through more studies, the appropriate dose and conditions for an optimal UV-C irradiation.
Microwave irradiation has been recently evaluated in a small trial by Ben-Shoshan and colleagues [104]; they compared low power- (500 W) and high power-irradiation techniques (750 W), performed for 30 seconds. In their results, high power-irradiation caused the complete inactivation of CMV, instead of the low power-method, which showed a failure rate of 13%.
These authors affirm that the high power-irradiation may represent a valid alternative to the other available methods, even in there are few studies investigating the effects produced by this technique on BM immunological, bioactive, cellular and nutritive components [104]. According to some authors, it would induce a little decrease in the total content of immunoglobulin, without modifying fatty acids’ composition. However, up to now, this topic has been poorly studied [104, 105].
In conclusion, many trials are needed to clarify the effects of these novel techniques on BM properties [16, 96, 104].
9.4. Trial Comparing the Described Techniques
Two techniques were compared in the retrospective study conducted by Soo Yoo et al. [1] on 385 ELBWs. The authors evaluated the efficacy of BM pasteurization (63 °C for 30 min) versus freeze-thawing in the prevention of CMV transmission.
The results obtained confirmed that the first technique allows the complete CMV eradication [1, 97, 106, 28]. Unfortunately, it severely impairs BM good properties and benefits [1, 28, 107].
On the contrary, the second one preserves nutritional and immunological features of BM but does not completely eradicate CMV and therefore viral transmission [1, 61, 108].
In the same study, the risk of CMV transmission via frozen BM depended on the total quantity of frozen milk administered [1, 79]. Eight percent of the infants fed with frozen milk for more than 3 days acquired CMV infection. However, the global CMV transmission was reduced to almost 6-22% [52, 59, 82, 109].
BM irradiation was compared with freezing in a small trial performed by Ben-Shoshan et al. [104]. They described a lower CMV elimination rate using the 1-day freezing technique (20%) and 3-day freezing (7%), instead of high-power irradiation.
10. ACQUIRED CMV INFECTION: OUTCOME AND FOLLOW-UP
Although acquired infection was thought, as first, not associated with long-term sequelae [10, 110, 111], some studies have reported the increased risk of a neurological impairment [10, 37, 112].
Up to now, the outcome of the early post-natal acquired CMV infection on neurodevelopment and hearing function is still not fully defined, although it has been studied by several authors [23, 113].
In some studies, long-term effects have not been reported; on the contrary, in other case series, a higher risk of sensorineural deafness or long-term neurologic functions impairment has been described [23].
It seems that a severe post-natal acquired infection causing symptoms and clinical manifestations could more likely lead to an impairment in the long-term prognosis, especially taking into account its occurrence in the critical and susceptible category of premature [23, 64].
Below we report some interesting studies on this topic, which result useful to improve our current knowledge on the outcome of this infection.
There are few studies investigating long-term outcome associated with CMV acquired the infection in VLBWs and ELBWs, giving controversial results [1, 8].
In the retrospective study of Soo Yoo et al. [1], a follow up at 2 years of corrected age has been evaluated. In their sample, a high proportion of the symptomatic preterms developed severe consequences, such as a moderate or severe BDP.
In the same study, the growth parameters and neurodevelopmental status were not different if compared with a control group. Moreover, no hearing loss was reported [1, 21].
In the study of Jim et al. [8], involving VLBW newborns which acquired CMV infection via BM, the outcome at 12 and 24 months of corrected age has been evaluated. In this group, anthropometry, clinical outcome including the incidence of BDP, intraventricular hemorrage (IVH), periventricular leucomalacia (PVL), ROP, cerebral palsy (CP) and even length of hospitalisation, psychomotor or mental development did not result impaired if compared with a control group of premature. Moreover, death for CMV-directly related problems has not been reported and none of the patients required antiviral treatment during the infection. A permanent sensorineural hearing loss was not detected in the evaluated babies.
Other follow-up studies evidenced a comparable outcome among post-natal CMV infected and noninfected preterm infants [16, 73, 110, 114, 115].
According to these studies, CMV acquired infection does not seem to modify clinical outcome and in particular neurocognitive development, sensorineural hearing function or children growth [8].
However, contrasting results have been reported by other authors. In fact, neurological sequelae have been described after three years of age in some infected preterms [112, 116]. More recently, other authors reported an impaired cognitive function and also neurological sequelae in some adolescents, ex-preterms, who early acquired a symptomatic CMV infection [117]. Therefore, current data cannot exclude the possibility of a negative impact on long-term outcome [16].
It must also be considered the presence of several confounding factors that might influence the results of these long-term to follow up. For example the occurrence of comorbidities, different strategies of treatment, parental cares, a low socio-economic status and even the outcome related to prematurity itself [8].
10.1. Magnetic Resonance Image Studies
Some findings of not completely clear meaning have also been reported analyzing cerebral Magnetic Resonance Image (MRI) of some ex-preterm infants, infected by CMV in the early life. In some of these patients, a different diffusion pattern involving the occipital regions has been described during functional studies, if compared with healthy controls. However, their neurodevelopmental outcome after one year of life was comparable [16, 118].
Moreover, structural and functional studies detected some differences. The volume of several areas resulted in less developed in infected subjects, especially grey matter. This has been confirmed also in long-term evaluations performed during adolescence. A difference has also been reported in the activation of the left hippocampus and right anterior cingulated cortex during performances regarding the language [16, 119].
In conclusion, even if a clear pattern of brain damage has not been detected and more studies are needed to correlate an early acquired CMV infection to long-term structural and functional brain alterations, these cannot be excluded up to now [16].
11. FUTURE PERSPECTIVES
11.1. Prevention
The prevention of post-natal acquired infection should regard both an eventual prevention of maternal infection and the reduction of maternal transmission [15].
Because the transmission due to maternal reinfection/recurrent infections varies from 0,1-1% to higher rates in countries with a higher CMV prevalence, an efficacious strategy to prevent mother to child transmission could be therefore the prevention of maternal primary infection [15].
To reduce the infection occurrence, some measures would be introduced or improved, such as washing hands, reducing the contact with babies’ fluids, cleaning surfaces after the contact with body fluids, the use of condoms to reduce sexually transmitted infections [15, 120].
Regarding the prevention of transmission, another proposed strategy was the use of donated BM from seronegative mothers; unfortunately, this is usually constituted by mature milk, whose composition is different from the BM produced by preterm’s mothers and could result inadequate for these neonates [10].
11.2. Metabolomics
Interesting data were recently reported with metabolomic studies, both investigating amniotic fluid (AF) [121, 122] and neonatal or maternal urine; in fact, metabolome seems a very promising instrument in several obstetric diseases, since it has been shown the ability to describe the complex interaction between mother and fetus, also taking into account the central role played by the placenta [123].
According to the study of Fattuoni et al., this evaluation allowed to discriminate AF belonging to CMV infected pregnant women who had transmitted the virus to the foetus, comparing it from the samples collected from infected women who resulted ‘non-transmitters’ of the same virus. The analyzed metabolites, in these samples, have also shown different profiles if compared with those of healthy pregnant women. Finally, different concentrations of these mediators in AF were also detected between symptomatic and asymptomatic infected newborns, potentially allowing the early diagnosis of infected newborns. In this perspective, precocious and specific biomarkers could be early detected and a targeted therapeutic approach may be performed, potentially improving the outcome of congenital or acquired infection and therefore avoiding the CMV-related sequelae [121-123].
11.3. Vaccination
Vaccines are under development but are not still available. A trial regarded a vaccination with a CMV viral Glycoprotein B which showed an efficacy of 50%. However, the protective effect reduced during the time [15].
12. CURRENT MANAGEMENT
Currently, a standardized algorithm of decision to perform the treatment of CMV-IgG seropositive mothers of premature neonates does not exist, and each NICU follows its recommendations, according to the national decision-making address, which should take into account the beneficial effects of breastfeeding extremely preterm newborns but even the risks connected with the possible viral transmission.
According to the AAP, a decision of BM treatment before administration should be taken especially for such premature babies born less than 32 weeks of GA from CMV-IgG positive mothers; these guidelines recognize long- and short-term pasteurization as safe techniques, due to their efficacy in viral inactivation, while freezing results a technique reducing but not eliminating viral load, according to the evidence reported in such review [124].
According to the Spanish group of pediatric infectivology, pasteurization of BM must be performed only on donor milk in human milk banks, since it reduces its beneficial properties. Moreover, in these guidelines, BM from CMV-IgG seropositive mothers should be frozen in premature newborns showing LBW, after the first week of lactation and with the highest efforts to not interfere with lactation. The authors conclude that freezing does not eliminate viral transmission but may reduce cases of severe infection and should also be considered in case of BM acquired infection in order to reduce viral shedding and improve infected newborn outcome [125].
In Sweden, according to Omarsdottir and co-workers, in many units, newborns showing less than 32 weeks of GA are initially fed with fresh milk and, after the first days of life, if the mother results seropositive for CMV-IgG, frozen BM is administered, while pasteurization is avoided. However, even these authors affirm that a consensus should be needed, especially to protect extremely preterm neonates [126].
Moreover, in case of maternal CMV-IgG seropositivity, The Committee for Nutrition of the Austrian Society of Paediatrics and Adolescent Medicine recommends BM pasteurization up to GA of 35 weeks, the French Society for the Safety of Nutrition recommend fresh BM only after 32 weeks of GA (above 1500 g), the guidelines of the Swedish National Board of Health and Welfare recommend freezing in neonates born before than 32 GA [21].
Finally, Picaud and working group of the French Neonatal Society on fresh human milk use in preterm infants, recently reviewed the available literature and guidelines from many countries and concluded, in accordance with the last recommendations, that newborns showing less than 28 weeks of GA or weighting less than 1000 gr should be fed with pasteurized BM up to 31+6 weeks of GA, both in case of positive or unknown maternal detection of CMV-IgG. In their opinion, this practice can be started after three days of lactation, due to a relative safety of colostrum [127].
In some cases, newborns cannot be fed with their mothers’ milk, due to a lacking lactation in preterm delivering mothers or to such conditions contraindicating breastfeeding with the milk of the newborns’ own mothers. In this perspective, the efforts made by human milk banks may result very useful. In fact, these structures collect and process samples from volunteer donors, accurately selected and screened, to provide BM also to such a vulnerable category of newborns. To avoid viral transmission through this nutrition route, potentially regarding also CMV, donor human milk must undergo long-term pasteurization; moreover, bacterial cultures performed before than the pasteurization must reveal no growth of pathogenic organisms (such as S aureus, group B Streptococcus, lactose-fermenting coliforms), no more than 100 000 colony-forming units/mL of normal skin bacteria and no viable bacteria after pasteurization and alternative treatments should not be recommended.
Currently, US donor milk banks refer to the Human Milk Banking Association of North America and BM is processed according to the US Food and Drug Administration and the Centers for Disease Control and Prevention guidelines [124].
CONCLUSION
BM is the best available source of nutrition for newborns, especially for the vulnerable category of prematures [8].
Post-natal acquired CMV infection is frequent among preterms and can be very compromising for the newborn short- and maybe long-term outcome. Therefore, it would be very important to perform efficacious methods to avoid CMV transmission via BM [8].
The studies and results mentioned in this review, however, show some limits. It should be taken into account that not all the methods to detect CMV show the same efficacy, also depending, i.e., on the available PCR kits. Moreover, differences can be also related to the analyzed samples [23, 128].
The two strategies that must be improved to avoid CMV transmission are, currently, a reduction of maternal rate of infection during pregnancy and an optimization of the strategies to inactivate the virus in BM (such as pasteurization, freezing and irradiation) [15, 42].
It is well known that pasteurization modifies BM components; therefore, also its impact on newborn outcome should be evaluated [1]. Moreover, beneficial effects of BM freezing and irradiation should be deeply understood and also its effects on BM properties and even on newborn’s outcome [23].
According to the most recent evidence, fresh BM could be administered only in newborns showing more than 32 weeks of GA or weighting more than 1000 gr. In case of more preterm neonates, BM should undergo pasteurization after the first 3 days of lactation in case of maternal positivity for CMV-IgG. However, in our opinion and taking into account the results presented above, neither colostrum can be considered so safe and should not be administered without treatment.
In the next future, the clarification of the real impact of CMV acquired infection on preterm’s outcome, especially regarding neurological sequelae, would be desirable [15].
For example, innovative techniques such as metabolomics could improve the approach to mother to child-transmitted infection.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AF
Amniotic Fluid
- BM
Breast Milk
- BDP
Bronchodysplasia
- CNS
Central Nervous System
- CP
Cerebral Palsy
- CMV
Cytomegalovirus
- DNA
Deoxyribonucleic acid
- ELBW
Extremely Low Birth Weight
- GC-MS
Gas Chromatography-Mass Spectrometry
- GA
Gestational Age
- HIV
Human Immunodeficiency Virus
- IQ
Intelligence Quotient
- IVH
Intraventricular Hemorrage
- LBW
Low Birth Weight
- NEC
Necrotizing Enterocolitis
- NICU
Neonatal Intensive Care Unit
- PVL
Periventricular Leucomalacia
- PCR
Polymerase Chain Reaction
- ROP
Prematurity-related Retinopathy
- rtPCR
Real Time C-reactive Proteins
- RNA
Ribonucleic acid
- UV-C
Ultraviolet- C
- WKS
Weeks
AUTHORS’ CONTRIBUTIONS
Flaminia Bardanzellu: preparing the manuscript, review of the literature. Vassilios Fanos: a review of the literature, final approval. Alessandra Reali: review of the literature, final approval.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Yoo H.S., Sung S.I., Jung Y.J., Lee M.S., Han Y.M., Ahn S.Y., Chang Y.S., Park W.S. Prevention of cytomegalovirus transmission via breast milk in extremely low birth weight infants. Yonsei Med. J. 2005;56:998–1006. doi: 10.3349/ymj.2015.56.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence R.A. The evidence for growth standards and iron in moderation and exclusive breastfeeding. Breastfeed. Med. 2006;1:205–206. doi: 10.1089/bfm.2006.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Hamprecht K., Maschmann J., Jahn G., Poets C.F., Goelz R. Cytomegalovirus transmission to preterm infants during lactation. J. Clin. Virol. 2008;41:198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Vohr B.R., Poindexter B.B., Dusick A.M., et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120:953–959. doi: 10.1542/peds.2006-3227. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Breastfeeding and the use of human milk. Pediatrics. 2012;129:827–841. [Google Scholar]
- 6.Romero-Gomez M.P., Cabrera M., Montes-Bueno M.T., et al. Evaluation of cytomegalovirus infection in low-birth weight children by breast milk using a real-time polymerase chain reaction assay. J. Med. Virol. 2015;87:845–850. doi: 10.1002/jmv.24101. [DOI] [PubMed] [Google Scholar]
- 7.Bardanzellu F., Fanos V., Reali A. “Omics” in human colostrum and mature milk: Looking to old data with new eyes. Nutrients. 2017;9:83. doi: 10.3390/nu9080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jim W.T., Chiu N.C., Ho C.S., et al. Outcome of preterm infants with postnatal Cytomegalovirus infection via breast milk. Medicine (Baltimore) 2015;94:e1835. doi: 10.1097/MD.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victora C.G., Horta B.L., Loret de Mola C., et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: A prospective birth cohort study from Brazil. Lancet Glob. Health. 2015;3:e199–e205. doi: 10.1016/S2214-109X(15)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant P., Morley C., Garland S., Curtis N. Cytomegalovirus transmission from breast milk in premature babies: Does it matter? Arch. Dis. Child. Fetal Neonatal Ed. 2002;87:F75–F77. doi: 10.1136/fn.87.2.F75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner L.M., Auerbach K.G. Breast milk and breastfeeding jaundice. Adv. Pediatr. 1987;34:249–274. [PubMed] [Google Scholar]
- 12.Dougherty D., Luther M. Birth to breast-a feeding care map for the NICU: helping the extremely low birth weight infant navigate the course. Neonatal Netw. 2008;27:371–377. doi: 10.1891/0730-0832.27.6.371. [DOI] [PubMed] [Google Scholar]
- 13.Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 14.Omarsdottir S., Casper C., Zweygberg Wirgart B., Grillner L., Vanpee M. Transmission of cytomegalovirus to extremely preterm infants through breast milk. Acta Paediatr. 2007;96:492–494. doi: 10.1111/j.1651-2227.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 15.Pass R.F., Anderson B. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. J. Pediatric Infect. Dis. Soc. 2014;3:2–6. doi: 10.1093/jpids/piu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamprecht K., Goelz R. Postnatal cytomegalovirus infection through human milk in preterm infants transmission, clinical presentation, and prevention. Clin. Perinatol. 2017;44:121–130. doi: 10.1016/j.clp.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Schleiss M.R. Congenital cytomegalovirus infection: Molecular mechanisms mediating viral pathogenesis. Infect. Disord. Drug Targets. 2011;11:449–465. doi: 10.2174/187152611797636721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manicklal S., Emery V.C., Lazzarotto T., Boppana S.B., Gupta R.K. The ‘Silent’ Global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013;26:86. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H.C., Enright A., Benitz W.E., Madan A. Postnatal cytomegalovirus infection from frozen breast milk in preterm, low birth weight infants. Pediatr. Infect. Dis. J. 2007;26:276. doi: 10.1097/01.inf.0000254412.66944.3e. [DOI] [PubMed] [Google Scholar]
- 20.Chiavarini M., Bragetti P., Sensini A., et al. Breastfeeding and transmission of cytomegalovirus to preterm infants. Case report and kinetic of CMV-DNA in breast milk. Ital. J. Pediatr. 2011;37:6. doi: 10.1186/1824-7288-37-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurath S., Halwachs-Baumann G., Müller W., Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin. Microbiol. Infect. 2010;16:1172–1178. doi: 10.1111/j.1469-0691.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 22.Dollard S., Grosse S.D., Ross D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 23.Balcells C., Botet F., Gayete S., et al. Vertically transmitted cytomegalovirus infection in newborn preterm infants. J. Perinat. Med. 2016;44:485–490. doi: 10.1515/jpm-2015-0325. [DOI] [PubMed] [Google Scholar]
- 24.Ross S.A., Boppana S.B. Congenital cytomegalovirus infection: Outcome and diagnosis. Semin. Pediatr. Infect. Dis. 2005;16:44–49. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Bae C.W. Maternal diseases and breastfeeding. Hanyang Med. Rev. 2010;3:60–67. [Google Scholar]
- 26.Vochem M., Hamprecht K., Jahn G., Speer C.P. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr. Infect. Dis. J. 1998;17:53–58. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hamprecht K., Witzel S., Maschmann J., et al. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: Comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J. Clin. Virol. 2003;28:303–316. doi: 10.1016/s1386-6532(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 28.Hamprecht K., Maschmann J., Muller D., et al. Cytomegalovirus (CMV) inactivation in breast milk: Reassessment of pasteurization and freeze-thawing. Pediatr. Res. 2004;56:529–535. doi: 10.1203/01.PDR.0000139483.35087.BE. [DOI] [PubMed] [Google Scholar]
- 29.Hayes K., Danks D.M., Gibas H., Jack I. Cytomegalovirus in human milk. N. Engl. J. Med. 1972;287:177–178. doi: 10.1056/NEJM197207272870407. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds D.W., Stagno S., Hosty T.S., Tiller M., Alford C.A. Maternal cytomegalovirus excretion and perinatal infection. N. Engl. J. Med. 1973;289:1–5. doi: 10.1056/NEJM197307052890101. [DOI] [PubMed] [Google Scholar]
- 31.Hamprecht K., Vochem M., Baumeister A., Boniek M., Speer C.P., Jahn G. Detection of cytomegaloviral DNA in human milk cells and cell free milk whey by nested PCR. J. Virol. Methods. 1998;70:167–176. doi: 10.1016/s0166-0934(97)00179-1. [DOI] [PubMed] [Google Scholar]
- 32.Stagno S., Reynolds D.W., Pass R.F., Alford C.A. Breast milk and the risk of cytomegalovirus infection. N. Engl. J. Med. 1980;302:1073–1076. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 33.Dworsky M., Yow M., Stagno S., Pass R.F., Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72:295–299. [PubMed] [Google Scholar]
- 34.Boeckh M., Boivin G. Quantitation of cytomegalovirus: Methodologic aspects and clinical applications. Clin. Microbiol. Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlfors K., Ivarsson S.A. Cytomegalovirus in breast milk of Swedish milk donors. Scand. J. Infect. Dis. 1985;17:11–13. doi: 10.3109/00365548509070413. [DOI] [PubMed] [Google Scholar]
- 36.Benson J.W., Bodden S.J., Tobin J.O. Cytomegalovirus and blood transfusion in neonates. Arch. Dis. Child. 1979;54:538–541. doi: 10.1136/adc.54.7.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeager A.S., Grumet F.C., Hafleigh E.B., Arvin A.M., Bradley J.S., Prober C.G. Prevention of transfusion acquired cytomegalovirus infections in newborn infants. J. Pediatr. 1981;98:281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 38.Stagno S., Pass R.F., Dworsky M.E., Alford C.A. Maternal cytomegalovirus infection and perinatal transmission. Clin. Obstet. Gynecol. 1982;25:563–576. doi: 10.1097/00003081-198209000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths P.D., Baboonian C. A prospective study of primary cytomegalovirus infection during pregnancy: Final report. Br. J. Obstet. Gynaecol. 1984;91:307–315. doi: 10.1111/j.1471-0528.1984.tb05915.x. [DOI] [PubMed] [Google Scholar]
- 40.Leurez-Ville M., Sellier Y., Salomon L.J., Stirnemann J.J., Jacquernard F., Wille Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: A retrospective cohort. Clin. Infect. Dis. 2013;56:1428–1435. doi: 10.1093/cid/cit059. [DOI] [PubMed] [Google Scholar]
- 41.Gindes L., Teperberg-Oikawa M., Pardo J., Rahav G. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830–835. doi: 10.1111/j.1471-0528.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 42.Pass R.F., Zhang C., Evans A., et al. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liesnard C., Donner C., Brancart F., Gosselin F., Delforge M.L., Rodesch F. Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstet. Gynecol. 2000;95:881–888. doi: 10.1016/s0029-7844(99)00657-2. [DOI] [PubMed] [Google Scholar]
- 44.Preece P.M., Pearl K.N., Peckham C.S. Congenital cytomegalovirus infection. Arch. Dis. Child. 1984;59:1120–1126. doi: 10.1136/adc.59.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzieri T.M., Dollard S.C., Josephson C.D., Schmid T.S., Bialek S.R. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131:1937–1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nijman J., de Vries L.S., Koopman-Esseboom C., Uiterwaal C.S., van Loon A.M., Verboon-Maciolek M.A. Postnatally acquired cytomegalovirus infection in preterm infants: A prospective study on risk factors and cranial ultrasound findings. Arch. Dis. Child. Fetal Neonatal Ed. 2012;97:F259–F263. doi: 10.1136/archdischild-2011-300405. [DOI] [PubMed] [Google Scholar]
- 47.Maschmann J., Hamprecht K., Dietz K., Jahn G., Speer C.P. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin. Infect. Dis. 2001;33:1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 48.Nijman J., van Loon A.M., de Vries L.S., et al. Urine viral load and correlation with disease severity in infants with congenital or postnatal cytomegalovirus infection. J. Clin. Virol. 2012;54:121–124. doi: 10.1016/j.jcv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi S., Kimura H., Oshiro M., et al. Transmission of cytomegalovirus via breast milk in extremely premature infants. J. Perinatol. 2011;31:440–445. doi: 10.1038/jp.2010.150. [DOI] [PubMed] [Google Scholar]
- 50.Okulu E., Akin I.M., Atasay B., Ciftçi E., Arsan S., Türmen T. Severe postnatal cytomegalovirus infection with multisystem involvement in an extremely low birth weight infant. J. Perinatol. 2012;32:72–74. doi: 10.1038/jp.2011.58. [DOI] [PubMed] [Google Scholar]
- 51.Josephson C.D., Caliendo A.M., Easley K.A., et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: A prospective cohort study. JAMA Pediatr. 2014;168:1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jim W.T., Shu C.H., Chiu N.C., et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr. Infect. Dis. J. 2004;23:845–851. doi: 10.1097/01.inf.0000137571.35541.55. [DOI] [PubMed] [Google Scholar]
- 53.Kenneson A., Cannon M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 54.Hotsubo T., Nagata N., Shimada M., Yoshida K., Fujinaga K., Chiba S. Detection of human cytomegalovirus DNA in breast milk by means of polymerase chain reaction. Microbiol. Immunol. 1994;38:809–811. doi: 10.1111/j.1348-0421.1994.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 55.Meier J., Lienicke U., Tschirch E., Kruger D.H., Wauer R.R., Prösch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J. Clin. Microbiol. 2005;43:1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Numazaki K., Chiba S., Asanuma H. Transmission of cytomegalovirus. Lancet. 2001;357:1799–1800. doi: 10.1016/S0140-6736(00)04913-8. [DOI] [PubMed] [Google Scholar]
- 57.Mosca F., Pugni L., Barbi M., Binda S. Transmission of cytomegalovirus. Lancet. 2001;357:1800. doi: 10.1016/S0140-6736(00)04914-X. [DOI] [PubMed] [Google Scholar]
- 58.Hamprecht K., Maschmann J., Vochem M., Speer C.P., Jahn G. Transmission of cytomegalovirus to preterm infants by breastfeeding. In: Prösch S., Cinatl J., Scholz M., editors. New aspects of CMV-related immunopathology, 24. Basel, Switzerland: Karger P; 2003. pp. 43–52. [Google Scholar]
- 59.Yasuda A., Kimura H., Hayakawa M., et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003;111:1333–1336. doi: 10.1542/peds.111.6.1333. [DOI] [PubMed] [Google Scholar]
- 60.Schanler R.J. Suitability of human milk for the low-birth weight infant. Clin. Perinatol. 1995;22:207–222. [PubMed] [Google Scholar]
- 61.Maschmann J., Hamprecht K., Weissbrich B., Dietz K., Jahn G., Speer C.P. Freeze-thawing of breast milk does not prevent cytomegalovirus transmission to a preterm infant. Arch. Dis. Child. Fetal Neonatal Ed. 2006;91:F288–F290. doi: 10.1136/adc.2004.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke N.M., May J.T. Effect of antimicrobial factors in human milk on rhinoviruses and milk-borne cytomegalovirus in vitro. J. Med. Microbiol. 2000;49:719–723. doi: 10.1099/0022-1317-49-8-719. [DOI] [PubMed] [Google Scholar]
- 63.Asanuma H., Numazaki K., Nagata N., Hotsubo T., Horino K., Chiba S. Role of milk whey in the transmission of human cytomegalovirus infection by breast milk. Microbiol. Immunol. 1996;40:201–204. doi: 10.1111/j.1348-0421.1996.tb03335.x. [DOI] [PubMed] [Google Scholar]
- 64.Ehlinger E.P., Webster E.M., Kang H.H., et al. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J. Infect. Dis. 2011;204:1672–1682. doi: 10.1093/infdis/jir632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke L.M., Durr A., Feldman J., Sierra M.F., Daidone B.J., Landesman S.H. Factors associated with cytomegalovirus infection among human immunodeficiency virus type 1-seronegative and -seropositive women from an urban minority community. J. Infect. Dis. 1996;173:77–82. doi: 10.1093/infdis/173.1.77. [DOI] [PubMed] [Google Scholar]
- 66.Roxby A.C., Atkinson C., Asbjornsdottir K., et al. Maternal valacyclovir and infant cytomegalovirus acquisition: A randomized controlled trial among HIV-infected women. PLoS One. 2014;9:87855. doi: 10.1371/journal.pone.0087855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sagar M. Origin of the transmitted virus in HIV infection: infected cells versus cell free virus. J. Infect. Dis. 2014;210:S667–S673. doi: 10.1093/infdis/jiu369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viljoen J., Tuaillon E., Nagot N., et al. Cytomegalovirus, and possibly Epstein–Barr virus, shedding in breast milk is associated with HIV-1 transmission by breastfeeding. AIDS. 2015;29:145–153. doi: 10.1097/QAD.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 69.Kovacs A., Schluchter M., Easley K., et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. N. Engl. J. Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slyker J., Farquhar C., Atkinson C., et al. Compartmentalized cytomegalovirus replication and transmission in the setting of maternal HIV-1 infection. Clin. Infect. Dis. 2014;58:564–572. doi: 10.1093/cid/cit727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peckham C.S., Johnson C., Ades A., Pearl K., Chin K.S. Early acquisition of cytomegalovirus infection. Arch. Dis. Child. 1987;62:780–785. doi: 10.1136/adc.62.8.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minamishima I., Ueda K., Minematsu T., et al. Role of breast milk in acquisition of cytomegalovirus infection. Microbiol. Immunol. 1994;38:549–552. doi: 10.1111/j.1348-0421.1994.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 73.Capretti M.G., Lanari M., Lazzarotto T., et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: A prospective study. J. Pediatr. 2009;154:842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 74.Richter D., Hampl W., Pohlandt F. Vertical transmission of cytomegalovirus, most probably by breast milk, to an infant with Wiskott-Aldrich syndrome with fatal outcome. Eur. J. Pediatr. 1997;156:854–855. doi: 10.1007/s004310050729. [DOI] [PubMed] [Google Scholar]
- 75.Stagno S., Brasfield D.M., Brown M.B., et al. Infant pneumonitis associated with cytomegalovirus, Chlamydia, Pneumocystis, and Ureaplasma: a prospective study. Pediatrics. 1981;68:322–329. [PubMed] [Google Scholar]
- 76.Ballard R.A., Drew W.L., Hufnagle K.G., Riedel P.A. Acquired cytomegalovirus infection in preterm infants. Am. J. Dis. Child. 1979;133:482–485. doi: 10.1001/archpedi.1979.02130050026005. [DOI] [PubMed] [Google Scholar]
- 77.Hamprecht K., Goeiz R., Maschmann J. Breast milk and cytomegalovirus infection in preterm infants. Early Hum. Dev. 2005;81:989–996. doi: 10.1016/j.earlhumdev.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Pietrasanta C., Ghirardi B., Manca M.F., et al. Herpesviruses and breast milk. Pediatr. Med. Chir. 2014;36:5. doi: 10.4081/pmc.2014.5. [DOI] [PubMed] [Google Scholar]
- 79.Jim W.T., Shu C.H., Chiu N.C., et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr. Infect. Dis. J. 2009;28:891–894. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 80.Schleiss M.R. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev. Med. Virol. 2006;16:73–82. doi: 10.1002/rmv.484. [DOI] [PubMed] [Google Scholar]
- 81.Numazaki K. Human cytomegalovirus infection of breast milk. FEMS Immunol. Med. Microbiol. 1997;18:91–98. doi: 10.1111/j.1574-695X.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 82.Doctor S., Friedman S., Dunn M.S., et al. Cytomegalovirus transmission to extremely low-birthweight infants through breast milk. Acta Paediatr. 2005;94:53–58. doi: 10.1111/j.1651-2227.2005.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 83.Cheong J.L., Cowan F.M., Modi N. Gastrointestinal manifestations of postnatal cytomegalovirus infection in infants admitted to a neonatal intensive care unit over a five year period. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89:F367–F369. doi: 10.1136/adc.2003.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawyer M.H., Edwards D.K., Spector S.A. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am. J. Dis. Child. 1987;141:303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- 85.Kelly M.S., Benjamin D.K., Puopolo K.M., et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169:153785. doi: 10.1001/jamapediatrics.2015.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopes A.A., Belhabri S., Karaoui L. Clinical findings and autopsy of a preterm infant with breast milk-acquired cytomegalovirus infection. AJP Rep. 2016;6:198–202. doi: 10.1055/s-0036-1593627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakabayashi H., Mizuno K., Kohda C., et al. Low HCMV DNA copies can establish infection and result in significant symptoms in extremely preterm infants: A prospective study. Am. J. Perinatol. 2012;29:377–382. doi: 10.1055/s-0031-1300971. [DOI] [PubMed] [Google Scholar]
- 88.Mehler K., Oberthuer A., Lang-Roth R., et al. High rate of symptomatic cytomegalovirus infection in extremely low gestational age preterm infants of 22-24 weeks’ gestation after transmission via breast milk. Neonatology. 2014;105:27–32. doi: 10.1159/000355306. [DOI] [PubMed] [Google Scholar]
- 89.Hamele M., Flanagan R., Loomis C.A., Stevens T., Fairchok M.P. Severe morbidity and mortality with breast milk associated cytomegalovirus infection. Pediatr. Infect. Dis. J. 2010;29:84–86. doi: 10.1097/INF.0b013e3181b6dbb5. [DOI] [PubMed] [Google Scholar]
- 90.Goelz R., Hihn E., Hamprecht K., et al. Effects of different CMV-heat-inactivation methods on growth factors in human breast milk. Pediatr. Res. 2009;65:458–461. doi: 10.1203/PDR.0b013e3181991f18. [DOI] [PubMed] [Google Scholar]
- 91.Miron D., Brosilow S., Felszer K., et al. Incidence and clinical manifestations of breast milk-acquired cytomegalovirus infection in low birth weight infants. J. Perinatol. 2005;25:299–303. doi: 10.1038/sj.jp.7211255. [DOI] [PubMed] [Google Scholar]
- 92.Baquero-Artigao F. Grupo de estudio de la infección congénita por citomegalovirus de la Sociedad Española de Infectología Pediátrica. Consensus document from the Spanish Society of Paediatric Infectious Diseases (SEIP) on the diagnosis and treatment of congenital cytomegalovirus infection. An Pediatr (Barc) 2009;71:535–47. doi: 10.1016/j.anpedi.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 93.Reina J., Weber I., Riera E., Busquets M., Morales C. Usefulness of a real-time quantitative Polymerase-Chain Reaction (PCR) assay for the diagnosis of congenital and postnatal cytomegalovirus infection. An. Pediatr. (Barc.) 2014;80:299–303. doi: 10.1016/j.anpedi.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 94.Klein J.O., Wilson C.B., Nizet V., Maldonado Y.A. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia: Elsevier; 2011. [Google Scholar]
- 95.Peila C., Moro G.E., Bertino E., et al. The effect of Holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients. 2016;8:477. doi: 10.3390/nu8080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lloyd M.L., Hod N., Jayaraman J., et al. Inactivation of cytomegalovirus in breast milk using ultraviolet-C irradiation: opportunities for a new treatment option in breast milk banking. PLoS One. 2016;11:0161116. doi: 10.1371/journal.pone.0161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dworsky M., Stagno S., Pass R.F., Cassady G., Alford C. Persistence of cytomegalovirus in human milk after storage. J. Pediatr. 1982;101:440–443. doi: 10.1016/s0022-3476(82)80081-4. [DOI] [PubMed] [Google Scholar]
- 98.Ford J.E., Law B.A., Marshall V.M., Reiter B. Influence of the heat treatment of human milk on some of its protective constituents. J. Pediatr. 1977;90:29–35. doi: 10.1016/s0022-3476(77)80759-2. [DOI] [PubMed] [Google Scholar]
- 99.Evans T.J., Ryley H.C., Neale L.M., Dodge J.A., Lewarne V.M. Effect of storage and heat on antimicrobial proteins in human milk. Arch. Dis. Child. 1978;53:239–241. doi: 10.1136/adc.53.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldblum R.M., Dill C.W., Albrecht T.B., Alford E.S., Garza C., Goldman A.S. Rapid high-temperature treatment of human milk. J. Pediatr. 1984;104:380–385. doi: 10.1016/s0022-3476(84)81099-9. [DOI] [PubMed] [Google Scholar]
- 101.Curtis N., Chau L., Garland S., Tabrizi S., Alexander R., Morley C.J. Cytomegalovirus remains viable in naturally infected breast milk despite being frozen for 10 days. Arch. Dis. Child. Fetal Neonatal Ed. 2005;90:F529–F530. doi: 10.1136/adc.2004.067769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hosseini M., Esmaili H.A., Abdoli O.S., et al. Evaluation of the freeze-thawing method in reducing viral load of Cytomegalovirus in breast milk of mothers of preterm infants. Breastfeed. Med. 2016;11:557–560. doi: 10.1089/bfm.2016.0107. [DOI] [PubMed] [Google Scholar]
- 103.Stronati M., Lombardi G., Di Comite A., Fanos V. Breastfeeding and cytomegalovirus infections. J. Chemother. 2007;19:49–51. doi: 10.1080/1120009x.2007.11782446. [DOI] [PubMed] [Google Scholar]
- 104.Ben-Shoshan M., Mandel D., Lubetzky R., Dollberg S., Mimouni F.B. Eradication of cytomegalovirus from human milk by microwave irradiation: A pilot study. Breastfeed. Med. 2016;11:186–187. doi: 10.1089/bfm.2016.0016. [DOI] [PubMed] [Google Scholar]
- 105.Tacken K.J., Vogelsang A., van Lingen R.A., Slootstra J., Dikkeschei B.D., van Zoeren-Grobben D. Loss of triglycerides and carotenoids in human milk after processing. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F447–F445. doi: 10.1136/adc.2008.153577. [DOI] [PubMed] [Google Scholar]
- 106.Friis H., Andersen H.K. Rate of inactivation of cytomegalovirus in raw banked milk during storage at -20 degrees C and pasteurisation. Br. Med. J. (Clin. Res. Ed.) 1982;285:1604–1605. doi: 10.1136/bmj.285.6355.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ewaschuk J.B., Unger S., O’Connor D.L., et al. Effect of pasteurization on selected immune components of donated human breast milk. J. Perinatol. 2011;31:593–598. doi: 10.1038/jp.2010.209. [DOI] [PubMed] [Google Scholar]
- 108.Forsgren M. Cytomegalovirus in breast milk: Reassessment of pasteurization and freeze-thawing. Pediatr. Res. 2004;56:526–528. doi: 10.1203/01.PDR.0000143155.84802.A3. [DOI] [PubMed] [Google Scholar]
- 109.Mussi-Pinhata M.M., Yamamoto A.Y., do Carmo Rego M.A., Pinto P.C., da Motta M.S., Calixto C. Perinatal or early-postnatal cytomegalovirus infection in preterm infants under 34 weeks gestation born to CMV-seropositive mothers within a high-seroprevalence population. J. Pediatr. 2004;145:685–688. doi: 10.1016/j.jpeds.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 110.Kumar M., Nankervis G.A., Jacobs I.B. Congenitally and postnatally acquired cytomegalovirus infections: long-term follow-up. J. Pediatr. 1984;104:674–679. doi: 10.1016/s0022-3476(84)80942-7. [DOI] [PubMed] [Google Scholar]
- 111.Granstrom M., Leinikki P., Santavuori P., Pettay O. Perinatal cytomegalovirus infection in man. Arch. Dis. Child. 1977;52:354–359. doi: 10.1136/adc.52.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paryani S.G., Yeager A.S., Hosford-Dunn H., et al. Sequelae of acquired Cytomegalovirus infection in premature and sick term infants. J. Pediatr. 1985;107:451–456. doi: 10.1016/s0022-3476(85)80533-3. [DOI] [PubMed] [Google Scholar]
- 113.Bevot A., Hamprecht K., Krageloh-Mann I., Brosch S., Goelz R., Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101:e167–e172. doi: 10.1111/j.1651-2227.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 114.Vollmer B., Seibold-Weiger K., Schmitz-Salue C., et al. Postnatally acquired cytomegalovirus infection via breast milk: Effects on hearing and development in preterm infants. Pediatr. Infect. Dis. J. 2004;23:322–327. doi: 10.1097/00006454-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 115.Luck S., Sharland M. Postnatal cytomegalovirus: Innocent bystander or hidden problem? Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F58–F64. doi: 10.1136/adc.2007.131623. [DOI] [PubMed] [Google Scholar]
- 116.Goeltz R., Meisner C., Bevot A., Hamprecht K., Kraegeloh-Mann I., Poets C.F. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal. 2013;98:430–433. doi: 10.1136/archdischild-2012-303384. [DOI] [PubMed] [Google Scholar]
- 117.Brecht K.F., Goelz R., Bevot A., Krageloh-Mann I., Wilke M., Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long term neuropsychological sequelae. J. Pediatr. 2015;166:834–839. doi: 10.1016/j.jpeds.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 118.Nijman J., Gunkel J., de Vries L.S., et al. Reduced occipital fractional anisotropy on cerebral diffusion tensor imaging in preterm infants with postnatally acquired cytomegalovirus infection. Neonatology. 2013;104:143–150. doi: 10.1159/000351017. [DOI] [PubMed] [Google Scholar]
- 119.Dorn M., Lidzba K., Bevot A., Goelz R., Hauser T.K., Wilke M. Long-term neurobiological consequences of early postnatal hCMV infection in former preterms: A functional MRI study. Hum. Brain Mapp. 2014;35:2594–2606. doi: 10.1002/hbm.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Centers for Disease Control and Prevention. Preventing Congenital CMV Infection Available at: http://www.cdc. gov/cmv/prevention. html 2011.
- 121.Barberini L., Noto A., Saba L., et al. Multivariate data validation for investigating primary HCMV infection in pregnancy. Data Brief. 2016;9:220–230. doi: 10.1016/j.dib.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fattuoni C., Palmas F., Noto A., et al. Primary HCMV infection in pregnancy from classic data towards metabolomics: An exploratory analysis. Clin. Chim. Acta. 2016;460:23–32. doi: 10.1016/j.cca.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 123.Fanos V., Atzori L., Makarenko K., Melis G.B., Ferrazzi E. Metabolomics application in maternal-fetal medicine. BioMed Res. Int. 2013:720514. doi: 10.1155/2013/720514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kimberlin D.W., Brady M.T., Jackson M.A. Red Book: Report of the committee on infectious diseases. 31st edition. AAP committee on infectious diseases; 2018. pp. 117–20. [Google Scholar]
- 125.Alarcón Allen A., Baquero-Artigao F. Grupo de estudio de la infección por citomegalovirus de la Sociedad Espanola de Infectología Pediátrica. Revisión y recomendaciones sobre la prevención, diagnóstico y tratamiento de la infección posnatal por citomegalovirus. An. Pediatr. (Barc.) 2011;74:52.e1–52.e13. doi: 10.1016/j.anpedi.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 126.Omarsdottir S., Casper C., Navér L., et al. Cytomegalovirus Infection and Neonatal Outcome in Extremely Preterm Infants After Freezing of Maternal Milk. Pediatr. Infect. Dis. J. 2015;34:482–489. doi: 10.1097/INF.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 127.Picaud J.C., Buffin R., Gremmo‐Feger G., Rigo J., Putet G., Casper C. Working group of the French Neonatal Society on fresh human milk use in preterm infants. Review concludes that specific recommendations are needed to harmonise the provision of fresh mother’s milk to their preterm infants. Acta Paediatr. 2018;107:1145–1155. doi: 10.1111/apa.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Binnicker M.J., Espy M.E. Comparison of six real-time PCR assays for qualitative detection of cytomegalovirus in clinical specimens. J. Clin. Microbiol. 2013;51:3749–3752. doi: 10.1128/JCM.02005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


