Abstract
Background:
Data on the effectiveness of definitive oral (PO) antibiotics for BSIs in preparation for discharge from hospital are lacking, particularly for Gram-positive bacterial BSIs (GP-BSI). The objective of this study was to determine rates of treatment failure based on bioavailability of PO antimicrobial agents used for GP-BSI.
Methods:
This was a single-center, retrospective cohort study of adult inpatients admitted to an academic medical center over a three-year period. Patients with a non-staphylococcal GP-BSI who received intravenous antibiotics and were then switched to PO antibiotics for at least a third of their treatment course were included. The cohort was stratified into high (⩾90%) and low (<90%) bioavailability groups. The primary endpoint was the proportion of patients experiencing clinical failure in each group. Secondary endpoints included clinical failure stratified by antibiotic group, bactericidal versus bacteriostatic PO agents, and organism.
Results:
A total of 103 patients met criteria for inclusion, which failed to reach the a priori power calculation. Of the patients included, 26 received high bioavailability agents and 77 received low bioavailability agents. Infections originated largely from a pulmonary source (30%) and were caused primarily by streptococcal species (75%). Treatment failure rates were 19.2% in the high bioavailability group and 23.4% in the low bioavailability group (p = 0.66). Clinical failure stratified by subgroups also did not yield statistically significant differences.
Conclusions:
Clinical failure rates were similar among patients definitively treated with high or low bioavailability agents for GP-BSI, though the study was underpowered to detect such a difference.
Keywords: bacteremia, bioavailability, Gram-positive, oral antibiotics
Introduction
Bloodstream infections (BSIs) are associated with significant morbidity and mortality, and are the 11th leading cause of death in the United States (US).1 In an epidemiological study of nosocomial BSIs in the US, 65% of episodes were caused by Gram-positive bacterial organisms.2 Treatment of Gram-positive bacterial BSIs typically involves a course of intravenous (IV) therapy, requiring administration via IV lines, prolonged hospitalization, increased treatment costs, and increased risk of line-related infections.3,4 There is a paucity of evidence-based data on the effectiveness of definitive antimicrobial regimens, specifically oral (PO) options, for Gram-positive bacterial BSIs in preparation for discharge from hospital. The 2007 Infectious Diseases Society for America (IDSA) guideline for community acquired pneumonia (CAP) suggests that a switch to PO therapy is reasonable, even in patients with bacteremia, once clinical stability is achieved5; however, this recommendation is based on a small study in which only 9% of patients had concomitant bacteremia, with no information about which PO antibiotics were used.6 A similar, randomized study by Oosterheert and colleagues assessing the effectiveness of early PO antibiotic use in severe CAP showed the majority of patients received amoxicillin/clavulanic acid. However, the specific agents used in the 9% of patients with concomitant bacteremia was not specifically mentioned.7
The practice of transitioning from IV to PO antimicrobial agents with high bioavailability for the completion of treatment of BSI is largely based on expert opinion. Providers often analyze patient-specific factors in clinical context to determine agent selection and appropriate timing of PO antibiotics, due to the lack of conclusive data demonstrating efficacy of one PO agent over another. In an evidence-based review by Hale and colleagues, the authors point to susceptibility data, source control, stable hemodynamics, and the use of highly bioavailable agents as important considerations prior to switching to PO therapy in Gram-positive bacterial BSI.8 More data for the transition from IV to PO antibiotics in Gram-negative bacterial BSIs have been published than in Gram-positive bacterial BSI, but with conflicting results. A study by Kutob and colleagues examined treatment failure rates based on bioavailability of PO antimicrobial agents prescribed for definitive therapy of Gram-negative bacterial BSI, and found that the risk of treatment failure increased as bioavailability of the PO regimen declined.9 In contrast, a study published by Mercuro and colleagues found no difference in clinical success between PO stepdown therapy with beta-lactams or fluoroquinolones for Gram-negative bacterial BSI.10
The purpose of this study was to examine the rate of clinical treatment failure based on bioavailability of PO agents and to determine risk factors for failure. This study was designed to help guide selection of the most optimal antimicrobial agents in solely Gram-positive bacterial BSIs, due to the gap in literature on this topic. We hypothesized that patients treated with agents with lower bioavailability would be more likely to experience clinical failure than those treated with higher bioavailability agents.
Methods
Study design and setting
This was a single-center, retrospective cohort study of adult inpatients admitted to an academic medical center in Charlotte, NC, between 1 September 2014 and 31 August 2017. Patients with a Gram-positive bacterial BSI caused by a prespecified list of organisms who received IV antibiotics and were then switched to PO antibiotics for discharge were included. Patients were identified through a Theradoc® information systems (Premier, Inc., Charlotte, NC, USA) generated report. The electronic medical record was used to gather and collect all necessary data, and approval for the study was obtained through the institutional review board. The data were entered and managed in a secure Research Electronic Data Capture (REDCapTM) database.11
Patient selection
Adult patients (>18 years old) were included in the study if they had a positive blood culture for Streptococcus spp., Enterococcus spp., Peptostreptococcus, or Clostridium spp.; if they received appropriate antibiotic therapy; and if at least one-third of their total course of antibiotics received were PO, including both inpatient and upon discharge. Appropriate antibiotic therapy was defined as antibiotics adequately dosed for the patient’s creatinine clearance (CrCl) with in vitro activity against the isolate based on the Clinical and Laboratory Standards Institute criteria. CrCl and dose were assessed at the initiation of IV and PO antibiotics, and again at hospital discharge. Patients were excluded if they had bacteremia caused by Staphylococcus aureus, coagulase-negative Staphylococcus, or had a prior episode of bacteremia due to the same organism within the past 90 days. Patients were also excluded if the organism identified was determined by a treating clinician to be a contaminant; if the patient had a polymicrobial infection with an organism not listed previously; if the patient was never admitted for IV treatment; if the patient had a catheter-related BSI, concomitant meningitis, osteomyelitis, or endocarditis; or if they expired during hospitalization.
Study objectives
The primary outcome of this study was clinical failure in patients receiving high versus low bioavailability agents. High bioavailability agents were defined as those with >90% bioavailability and included clindamycin, doxycycline, fluoroquinolones, linezolid, metronidazole, and trimethoprim/sulfamethoxazole. Low bioavailability agents were <90% bioavailable, including the aminopenicillins, penicillins, and cephalosporins. Clinical failure was defined as all-cause mortality within 90 days of diagnosis of BSI, 90-day hospital readmission from date of previous discharge due to infectious process, switch back to IV therapy due to lack of improvement on PO therapy, or recurrent BSI due to the original organism within 90 days of switch to PO therapy. To determine all-cause mortality, both medical records and death certificate databases were searched. Secondary endpoints assessed included clinical failure stratified by antibiotic group, organism, and bactericidal versus bacteriostatic agents. Bacteriostatic agents were defined as clindamycin, doxycycline, and linezolid. Duration of hospitalization, clinical failure in patients who had documented microbiological clearance versus those who did not, and source of infection were also assessed.
Statistical analysis
Descriptive statistics including means and standard deviations, or counts and percentages, were calculated for all variables. The primary analysis was a chi-squared test comparing the proportion of patients experiencing clinical failure in those receiving high versus low bioavailability agents. To assess potential risk factors of treatment failure, univariate logistic regression models were used to calculate odds ratios and 95% confidence intervals. To compare baseline characteristics and other secondary outcomes between study groups, Student’s t test was used for normally distributed data, the Wilcoxon rank sum test was used for ordinal data or continuous data that were not normally distributed, and the chi-square test or Fisher’s exact test was used for categorical data. A two-tailed p value of less than 0.05 was considered statistically significant. SAS Enterprise Guide®, version 6.1, was used for all analyses (SAS Institute Inc., Cary, NC, USA).
Sample size was based on a chi-square test comparing the proportion of patients experiencing clinical failure in those receiving high versus low bioavailability agents. To detect a proportional difference of 10%, and assuming a 4% rate of clinical failure for patients treated with agents known to have higher bioavailability, 128 patients were required per treatment group for a power of 80% and alpha of 0.05. These failure rates were based on earlier studies in Gram-negative bacteremia as well as clinical expertise.9
Results
Over the 3-year study period, 597 adult patients with non-Staphylococcal Gram-positive bacterial BSIs were identified, with 103 patients being eligible for inclusion (Figure 1). The median patient age was 59, with 51% being female, and 47% and 43% being White and African American, respectively (Table 1). Out of 103 included patients, 26 received PO treatment with highly bioavailable agents, whereas 77 patients received low bioavailability agents. In this study, 19% of the patients were actively immunocompromised, defined as an absolute neutrophil count <500/mm3, chemotherapy within 30 days of BSI, human immunodeficiency virus and CD4 count <200/mm3, transplant recipients, receiving immunosuppressive medications, or receipt of prednisone >20 mg or equivalent for at least 7 days. Baseline characteristics between groups were well balanced, apart from 46% of the high bioavailability group having a history of cancer compared with 20% of the low bioavailability group (p = 0.008). The predominant source of infection was pulmonary (30%) with the predominant organism being a streptococcal species (75%) (Table 2). Patients were treated for a median of 15 days with 9 days administered orally and 7 days administered in the hospital.
Figure 1.
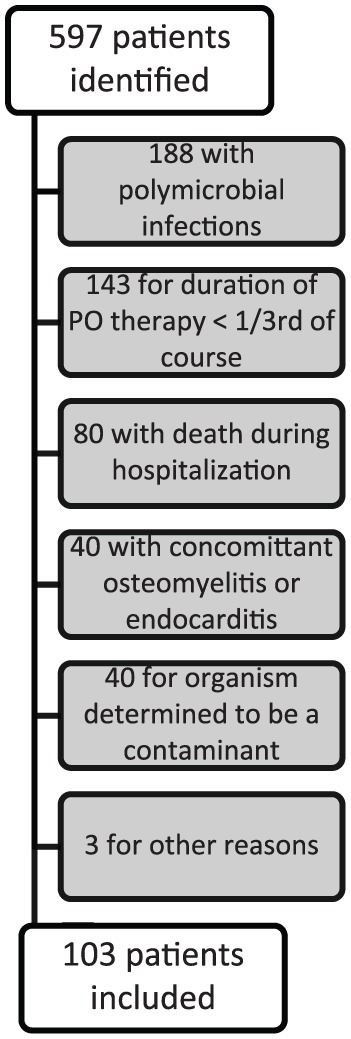
Summary of patient exclusion.
Table 1.
Baseline demographics.
| Clinical characteristics of patients with Gram-positive bloodstream infection by bioavailability group | ||||
|---|---|---|---|---|
| Variable | Bioavailability |
p-value | ||
| Total (n = 103) | High (n = 26) | Low (n = 77) | ||
| Age (years) [median (IQR)] | 59 (43–72) | 62 (46–70) | 57 (41–73) | 0.516 |
| Weight (kg) [median (IQR)] | 75 (68–90) | 78 (68–86) | 75 (66–90) | 0.846 |
| Female sex [n (%)] | 52 (50.5) | 12 (46.2) | 40 (51.9) | 0.609 |
| Race [n (%)] | – | – | – | 0.308 |
| White | 48 (46.6) | 15 (57.7) | 33 (42.9) | |
| African American | 44 (42.7) | 9 (34.6) | 35 (45.5) | |
| Asian | 3 (2.9) | 2 (7.7) | 1 (1.3) | |
| American Indian | 2 (1.9) | – | 2 (2.6) | |
| Other | 4 (3.9) | – | 4 (5.2) | |
| Unknown | 2 (1.9) | – | 2 (2.6) | |
| Immunocompromised [n (%)] | 20 (19.4) | 4 (15.4) | 16 (20.8) | 0.548 |
| Pitt bacteremia score [median (IQR)] | 1 (0–2) | 1 (0–2) | 1 (1–2) | 0.059 |
| Charlson Comorbidity index [median (IQR)] | 4 (2–7) | 4.5 (3–7) | 4 (2–6) | 0.258 |
| Diabetes mellitus [n (%)] | 24 (23.3) | 5 (19.2) | 19 (24.7) | 0.570 |
| Moderate-severe renal disease [n (%)] | 19 (18.4) | 4 (15.4) | 15 (19.5) | 0.775 |
| Moderate-severe liver disease [n (%)] | 9 (8.7) | 3 (11.5) | 6 (7.8) | 0.689 |
| Cancer [n (%)] | 27 (26.2) | 12 (46.2) | 15 (19.5) | 0.008 |
| Source of infection [n (%)] | 0.132 | |||
| Urinary | 10 (9.7) | 2 (7.7) | 8 (10.4) | |
| Pulmonary | 31 (30.1) | 8 (30.8) | 23 (29.9) | |
| Intra-abdominal | 9 (8.7) | 4 (15.4) | 5 (6.5) | |
| Skin/soft tissue | 20 (19.4) | 1 (3.8) | 19 (24.7) | |
| Other | 9 (8.7) | 3 (11.5) | 6 (7.8) | |
| Unknown | 24 (23.3) | 8 (30.8) | 16 (20.8) | |
| Appropriate antibiotic duration (days) [median (IQR)] | – | – | – | – |
| IV | 5 (4–7) | 4 (3–6) | 5 (4–7) | 0.117 |
| PO | 9 (7–12) | 9.5 (7–14) | 9 (8–11) | 1.000 |
| Total | 15 (13–17) | 14 (11–16) | 15 (14–17) | 0.183 |
| Confirmed negative blood cultures [n (%)] | 77 (74.8) | 22 (84.6) | 55 (71.4) | 0.181 |
| Days to blood culture clearance [median (IQR)] | 2.6 (1.8–2.9) | 2.4 (1.5–2.8) | 2.6 (1.8–3.0) | 0.495 |
| ID consult involvement [n (%)] | 53 (51.5) | 14 (53.8) | 39 (50.6) | 0.778 |
| Length of stay (days) [median (IQR)] | 7 (5–10) | 8 (5–10) | 6 (5–9) | 0.534 |
ID, infectious diseases; IQR, interquartile range.
Table 2.
Microbiology.
| Microbiology and treatment of Gram-positive bloodstream infection | |||||
|---|---|---|---|---|---|
| Bacteria | Total |
Bioavailability |
Pharmacodynamic property |
||
| (n = 103) | High (n = 26) n (%) |
Low (n = 77) n (%) |
Bactericidal (n = 96) n (%) |
Bacteriostatic (n = 7) n (%) |
|
| Streptococcus species | 77 (75) | 17 (65) | 60 (78) | 72 (75) | 5 (71) |
| Group A Streptococcus | 7 (6.8) | 1 (3.8) | 6 (7.8) | 6 (6.3) | 1 (14.3) |
| Group B Streptococcus | 16 (15.5) | 3 (11.5) | 13 (16.9) | 15 (15.6) | 1 (14.3) |
| Group C Streptococcus | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| Group G Streptococcus | 3 (2.9) | – | 3 (3.9) | 3 (3.1) | – |
| Viridans group Streptococcus | 17 (16.5) | 2 (7.7) | 15 (19.5) | 15 (15.6) | 2 (28.6) |
| S. pneumoniae | 27 (26.2) | 9 (34.6) | 18 (23.4) | 27 (28.1) | – |
| S. bovis | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| S. anginosus | 2 (1.9) | 2 (7.7) | – | 1 (1) | 1 (14.3) |
| S. constellatus | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| S. intermedius | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| S. mitis | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| Enterococcus species | 10 (10) | 1 (4) | 9 (12) | 9 (9) | 1 (14) |
| E. faecalis | 9 (8.7) | – | 9 (11.7) | 9 (9.4) | – |
| E. faecium | 1 (1) | 1 (3.8) | – | – | 1 (14.3) |
| Peptostreptococcus species | 8 (8) | 2 (8) | 6 (8) | 8 (8) | – |
| Clostridium species | 8 (8) | 6 (23) | 2 (3) | 7 (7) | 1 (14) |
| C. perfringens | 1 (1) | 1 (3.8) | – | 1 (1) | – |
| C. sordelli | 1 (1) | – | 1 (1.3) | 1 (1) | – |
| C. septicum | 2 (1.9) | 2 (7.7) | – | 1 (1) | 1 (14.3) |
| C. ramosum | 1 (1) | 1 (1) | – | 1 (1) | – |
| Individual species not specified | 3 (2.9) | 2 (7.7) | 1 (1.3) | 3 (3.1) | – |
Overall, 23 out of the 103 patients experienced clinical failure (Table 3). In the high bioavailability group, 4 of the 26 patients were readmitted due to an infectious process, and 1 patient in the group expired for a total of five failures. In the low bioavailability group, 15 of the 77 were readmitted due to infection, with 1 of the 15 expiring, 5 requiring a switch back to IV therapy due to lack of improvement, and 2 having recurrent bacteremia due to the original organism. An additional three patients in the low bioavailability group died within 90 days of diagnosis for a total of 18 failures. Upon stratifying failure rates based on bioavailability, 19.2% of the high bioavailability group failed, while 23.4% of the low bioavailability group failed (p = 0.66). When assessing for risk factors for failure between bacteriostatic versus bactericidal agents, high versus low bioavailability agents, medication class, and organism, there were no statistically significant associations identified (Table 4).
Table 3.
Primary outcomes.
| Outcomes | Bioavailability |
p-value | ||
|---|---|---|---|---|
| Total (n = 103) | High (n = 26) n (%) |
Low (n = 77) n (%) |
||
| Clinical Failure | 23 (22.3) | 5 (19.2) | 18 (23.4) | 0.661 |
| 90-day readmission | 47 (45.6) | 13 (50) | 34 (44.2) | 0.605 |
| Infection related | 19 (18.4) | 4 (15.4) | 15 (19.5) | 0.775 |
| 90-day all-cause mortality | 5 (4.9) | 1 (3.8) | 4 (5.2) | 1.000 |
| Switch from PO back to IV therapy | 5 (4.9) | – | 5 (6.5) | 0.327 |
| Recurrent bloodstream infection within 90 days of switch to PO therapy | 2 (1.9) | – | 2 (2.6) | 1.000 |
Table 4.
Univariate analysis for risk factors of treatment failure.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Medication class | – | – |
| Penicillin VK | 1.429 (0.259–7.897) | 0.683 |
| Aminopenicillins | 0.643 (0.25–1.654) | 0.360 |
| Cephalosporins | 2.063 (0.715–5.947) | 0.180 |
| Fluoroquinolones | 0.285 (0.035–2.335) | 0.242 |
| SMX/TMP | N/A* | N/A* |
| Clindamycin | 0.864 (0.092–8.129) | 0.898 |
| Doxycycline | N/A* | N/A* |
| Linezolid | N/A* | N/A* |
| Metronidazole | 1.429 (0.259–7.897) | 0.683 |
| Organism | – | – |
| Streptococcus species | 1.281 (0.423–3.884) | 0.661 |
| Enterococcus species | 0.857 (0.169–4.348) | 0.852 |
| Peptostreptococcus species | 1.175 (0.221–6.253) | 0.850 |
| Clostridium species | 0.474 (0.055–4.065) | 0.496 |
| Source | – | – |
| Urinary | 1.565 (0.371–6.605) | 0.542 |
| Pulmonary | 0.577 (0.193–1.726) | 0.325 |
| Intra-abdominal | 0.993 (0.192–5.144) | 0.994 |
| Skin/Soft Tissue | 1.204 (0.385–3.759) | 0.750 |
| Other | N/A* | 0.968 |
| Unknown | 1.75 (0.616–4.969) | 0.293 |
| Length of hospital stay ⩾5 versus <5 days | 6.852 (0.865–54.249) | 0.068 |
| Bacteriostatic vs bactericidal | 1.429 (0.259–7.897) | 0.683 |
| Bioavailability high vs low | 0.78 (0.257–2.366) | 0.661 |
| Blood culture clearance yes versus no | 0.712 (0.255–1.987) | 0.517 |
Unable to be calculated due to small sample size
SMX/TMP, sulfamethoxazole/trimethoprim.
Discussion
In this study, no difference in rates of clinical failure between the high (19.2%) and low (23.4%) bioavailability groups were observed. Additionally, there were no statistically significant differences in secondary endpoints. Failure rates in this study were higher than those seen in prior studies for the treatment of Gram-negative bacteremia. Kutob examined 362 cases of Gram-negative bacteremia treated with PO antibiotics and reported failure rates of 2%, 12%, and 14% for high, moderate, and low bioavailability agents respectively (p = 0.02).9 Similar to Kutob, Mercuro studied PO treatment of Gram-negative bacteremia with beta-lactams compared with fluoroquinolones in 224 patients. This resulted in failure rates of 13% for each group (p = 0.96).10 Apart from focusing on Gram-negative as opposed to Gram-positive organisms, these studies also had dissimilar stratifications of bioavailability, different definitions of clinical failure, and unique patient populations. In our study, 19% of patients were immunocompromised, with 9% having moderate-to-severe liver disease. In the Kutob study, these were the only two statistically significant risk factors for failure seen outside of bioavailability, and, in their study, only 9% were immunocompromised, with 3% having liver cirrhosis.9 This could indicate that our patient population had a higher burden of illness and may have been more infection prone. In the study by Mercuro, 5% of patients had cirrhosis, and the number of immunocompromised patients was not stated.10 Mercuro found diabetes with complications as their largest risk factor for treatment failure, which entailed 23% of their population, a similar proportion to our study.10
Infections in our study were largely from a pulmonary source and caused by a streptococcal species, whereas prior studies had high rates of urinary sources of infection due to Escherichia coli.9,10 It is important to note differences in treatment of these organisms, especially when considering use of low bioavailability agents. In general, streptococcal species have much lower minimum inhibitory concentrations for beta-lactam antibiotics when compared with the Enterobacteriaceae, making pharmacodynamic targets more easily achievable, even with use of agents with lower bioavailability.12 With the majority of patients in this study having a streptococcal pneumonia as their source of BSI, these results could be taken into consideration when recommending treatment options in conjunction with clinical experience, microbiological data, and previous literature. Ramirez found that, in patients with Streptococcus pneumoniae bacteremia from a pulmonary source, there was no difference in clinical outcomes between patients who received PO switch therapy versus those who completed a course of IV only therapy after showing clinical improvement.6 Although the specific antibiotics used were not mentioned, together these studies provide rationale for treating highly susceptible organisms such as Streptococcus pneumoniae with targeted antimicrobial agents such as beta-lactams.
In this study, median length of therapy was approximately 2 weeks in both groups, similar to other studies in Gram-negative bacterial infections, and which recent literature suggests may not be needed in lower inoculum infections with absence of a deep seeded source.9–10,13,14 However, to our knowledge, there are no published studies in Gram-positive bacterial infections to support this extrapolation. Due to the prolonged duration in both groups, it is unknown how a shorter duration of therapy would affect failure rates between groups, or if the similar failure rates were due to the 2-week course. With most patients receiving low bioavailability agents, it is clear that use of these agents was common practice at our institution for treatment of Gram-positive bacterial BSI after initial IV antibiotics.
To our knowledge, this is the first study of IV to PO switch therapy for the completion of treatment of Gram-positive bacteremia. Similar studies are available in Gram-negative bacteremia; however, there are conflicting results regarding whether high bioavailability agents are more successful in treating BSI.9,10,15 This study adds to the literature in an area of uncertainty, but must be interpreted with its limitations.
Due to the retrospective nature of this study, the medical record was relied upon and the data extracted were dependent upon documentation accuracy. There was no randomization of the treatment groups and the groups were unevenly split, with 25% receiving high bioavailability agents and 75% receiving low bioavailability agents. We were also unable to confirm adherence to the treatment regimen upon discharge due to the study design and had to rely on discharge medication reconciliations with the assumption of adherence. Although death certificate databases were searched and medical records postdischarge were examined, some clinical failures may have gone undetected if they were admitted to a hospital outside of the center’s healthcare system. For the five patients who were confirmed to have expired during the study period, cause of death (e.g. whether or not infection-related) was unable to be determined. Due to the strict exclusion criteria and the unexpectedly small sample size, power was not met, making the validity of these results uncertain. To power our study to detect the 4% difference in clinical failure that was seen, over 300 patients in each group would have been required. As our data set was run through a Theradoc® generated report in conjunction with our rapid diagnostic blood culture testing, our data set was able to go back only as far as September of 2014 when these processes were implemented.
Despite these limitations, this study is the first of its kind in Gram-positive bacterial BSI. The patients in this trial did receive a long duration of PO therapy, with a median of 9 out of 15 (60%) days of therapy being PO, and patients were followed for 90 days postdiagnosis. As the primary objective of this trial was to determine the effectiveness of PO antibiotics, this is a major strength of the data. All patients received appropriately dosed and active antibiotics for the duration of their course. Despite no statistically significant differences, relevant risk factors were chosen to determine their effects on treatment outcomes, including bioavailability and pharmacodynamic properties. This study is hypothesis-generating for future, large-scale studies.
Conclusion
There were no differences in outcomes between the use of high or low bioavailability agents for the treatment of Gram-positive bacterial BSI. There were also no risk factors observed that increased the risk of failure based on the type of agent used or the organism and source that were being treated. Due to its limitations, conclusions cannot be drawn solely from this study; however, it is hypothesis-generating in an area where there is a paucity of data. These results set the stage for future, large-scale studies to validate the results of this study.
Acknowledgments
The authors would like to thank Jing Zhao, MD, PhD from the Center for Outcomes Research and Evaluation (CORE) for help with statistical analysis.
Appendix
Appendix A.
Oral bioavailability of various antimicrobial agents per package insert.
| Agent | % Bioavailable |
|---|---|
| Penicillin VK | 25–60 |
| Aminopenicillins | |
| Ampicillin | 50 |
| Amoxicillin | 70–77 |
| Amoxicillin/clavulanate | 70–77 |
| Cephalosporins | |
| First-generation | |
| Cephalexin | 80 |
| Third-generation | |
| Cefpodoxime | 41–64 |
| Cefdinir | 16–25 |
| Cefixime | 40–52 |
| TMP/SMX | 90 |
| Clindamycin | 90 |
| Doxycycline | 100 |
| Linezolid | 100 |
| Metronidazole | 100 |
| Fluoroquinolones* | |
| Levofloxacin | 100 |
| Moxifloxacin | 90 |
Ciprofloxacin not included given lack of reliable activity against Gram-positive organisms.
Appendix B.
Appropriate renal dose adjustments.
| Package insert, American Journal of Kidney Diseases, and institution specific recommendations | ||
|---|---|---|
| Drug | Normal dose | Renal adjustment (CrCl in mL/min) |
| Penicillin VK | 250–500 mg Q6–8H | None |
| Ampicillin | 500 mg Q6H | 10–50: 500 mg Q6–12H <10: 500 mg Q12–24H |
| Amoxicillin | 500 mg Q8H 875 mg Q12H |
10–30: 500 mg Q12H <10: 500 mg Q24H Should not use 875 mg tablet if CrCl <30 |
| Amoxicillin/clavulanate | 500 mg Q8 875 mg Q12H 2000 mg ER Q12H |
10–30: 500 mg Q12H <10: 500 mg Q24H ER tab should not be used if CrCl <30 |
| Cephalexin | 500 mg Q6H | 15–29: 500 mg Q8–12H 1–14: 500 mg Q24H |
| Cefadroxil | 1–2 g daily divided | 25–50: 500 mg Q12H 10–25: 500 mg Q24H <10: 500 mg Q36H |
| Cefpodoxime | 200–400 mg Q12H | <30: 200–400 mg Q24H HD: 3x week after HD OR 200 mg once, 100 mg 12 h later, then 100 mg q24h |
| Cefixime | 200 mg Q12H 400 mg Q24H |
<20: 200 mg Q24H HD: use not recommended |
| TMP/SMX | 10–15 mg/kg Q6–8H | 15–30: 50% reduction <15: use not recommended |
| Clindamycin | 300–450 Q6H | None |
| Doxycycline | 100 mg Q12H | None |
| Linezolid | 600 mg Q12H | None |
| Metronidazole | 500 mg Q6–8H | None |
| Levofloxacin | 500–750 mg Q24H | 750 mg
20–49: 750 mg Q48H 10–19: 750 × 1, 500 mg Q48H 500 mg 20–49: 500 × 1, 250 mg Q24H 10–19: 500×1, 250 mg Q48H |
| Moxifloxacin | 400 mg Q24H | None |
Appendix C.
Oral antibiotics prescribed.
| Agent | n | Most common dosing regimen | n (%) |
|---|---|---|---|
| High bioavailability | – | ||
| Fluoroquinolones | – | ||
| Moxifloxacin | 1 | 400 mg Q24H | 1 (100) |
| Levofloxacin | 11 | 750 mg Q24H | 7 (64) |
| Linezolid | 1 | 600 mg Q12H | 1 (100) |
| Doxycycline | 1 | 100 mg Q12H | 1 (100) |
| Metronidazole | 7 | 500 mg Q8H | 7 (100) |
| Clindamycin | 5 | 450 mg Q6H | 2 (40) |
| TMP/SMX | 1 | 160/800 mg Q12H | 1 (100) |
| Low bioavailability | – | ||
| Penicillins | – | ||
| Penicillin VK | 7 | 500 mg Q6H | 6 (87) |
| Ampicillin | 2 | 500 mg Q6H | 2 (100) |
| Amoxicillin | 24 | 500 mg Q8H | 16 (67) |
| Amoxicillin/Clav | 23 | 875 mg Q12H | 19 (83) |
| Cephalosporins | – | ||
| Cephalexin | 12 | 500 mg Q6H | 9 (75) |
| Cefpodoxime | 6 | 200 mg Q12H | 5 (83) |
| Cefdinir | 2 | 300 mg Q12H | 2 (100) |
| Cefixime | 1 | 200 mg Q12H | 1 (100) |
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Nicholas J. Quinn  https://orcid.org/0000-0002-9703-5845
https://orcid.org/0000-0002-9703-5845
Contributor Information
Nicholas J. Quinn, Pharmacy Department, Atrium Health, Charlotte, NC, USA
Jamielynn C. Sebaaly, Pharmacy Department, Atrium Health, Charlotte, NC, USA Wingate University School of Pharmacy, Wingate, NC, USA
Bianka A. Patel, Pharmacy Department, Atrium Health, Charlotte, NC, USA
David A. Weinrib, Division of Infectious Diseases, Atrium Health, Charlotte, NC, USA
William E. Anderson, Center for Outcomes Research and Evaluation, Atrium Health, Charlotte, NC, USA
Danya G. Roshdy, Carolinas Medical Center, 1000 Blythe Blvd. Charlotte, NC 28203, USA.
References
- 1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19: 501–509. [DOI] [PubMed] [Google Scholar]
- 2. Wisplinghoff H, Bischoff T, Tallent SMet al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39: 309–317. [DOI] [PubMed] [Google Scholar]
- 3. Mermel LA. Short-term peripheral venous catheter–related bloodstream infections: a systematic review. Clin Infect Dis 2017; 65: 1757–1762. [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Cao S, Marsh Net al. Infection risks associated with peripheral vascular catheters. J Infect Prev 2016; 17: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandell LA, Wunderink RG, Anzueto Aet al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl. 2): S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161: 848–850. [DOI] [PubMed] [Google Scholar]
- 7. Oosterheert JJ, Bonten MJ, Schneider MMet al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ 2006; 333: 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hale AJ, Snyder GM, Ahern JWet al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med 2018; 13: 328–335. [DOI] [PubMed] [Google Scholar]
- 9. Kutob LF, Justo JA, Bookstaver PBet al. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48: 498–503. [DOI] [PubMed] [Google Scholar]
- 10. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for enterobacteriaceae bloodstream infections: fluoroquinolones versus beta-lactams. Int J Antimicrob Agents 2018; 51: 687–92. [DOI] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke Ret al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. 13th ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2018. [Google Scholar]
- 13. Chotiprasitsakul D, Han JH, Cosgrove SEet al. Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 2018; 66: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yahav D, Franceschini E, Koppel Fet al. Seven versus fourteen days of antibiotic therapy for uncomplicated gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin Infect Dis. Epub ahead of print 11 December 2018. DOI: 10.1093/cid/ciy1054. [DOI] [PubMed] [Google Scholar]
- 15. Tamma PD, Conley AT, Cosgrove SEet al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with enterobacteriaceae bacteremia. JAMA Intern Med 2019; 179: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]


