Abstract
Background: Metformin use reportedly reduces cancer risk and improves survival in lung cancer patients. This study aimed to investigate the effect of metformin use in patients with diabetes mellitus (DM) and lung cancer receiving epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy. Methods: A nationwide, population-based cohort study was conducted using the Taiwan National Health Insurance Research Database. From January 1, 2004, to December 31, 2012, a total of 373 metformin and 1260 non-metformin lung cancer cohorts with type 2 DM and EGFR-TKI treatment were studied. Results: Metformin use was significantly associated with a reduced risk of death (hazard ratio: 0.73, 95% confidence interval [CI]: 0.62-0.85, P < .001), as well as a significantly longer median progression-free survival (9.2 months, 95% CI: 8.6-11.7, vs 6.4 months, 95% CI: 5.9-7.2 months, P < .001) and median overall survival (33.4 months, 95% CI: 29.4-40.2, vs 25.4 months, 95% CI: 23.7-27.2 months, P < 0.001). Conclusions: In conclusion, metformin may potentially enhance the therapeutic effect and increase survival in type 2 DM patients with lung cancer receiving EGFR-TKI therapy.
Keywords: lung cancer, metformin, DM, EGFR, TKI
Introduction
Lung cancer is the most common cause of cancer death worldwide,1 and most patients have advanced-stage disease at diagnosis. Despite treatment-related advances, the prognosis of lung cancer remains poor with a 5-year survival rate of 4% in distant-stage disease.2,3 Epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy is a promising treatment for non–small cell lung cancer (NSCLC). Specific mutations in the tyrosine kinase domain of the EGFR gene are associated with favorable EGFR-TKI therapy-related clinical outcomes4 in patients with NSCLC. Most mutations are present in exons 18 to 21 of the EGFR gene,5 and are frequently observed in lung adenocarcinoma.6 EGFR mutations are found in <10% of non-Asian NSCLC patients7 and in 30% to 50% of East Asian patients.8 Missense mutations in exon 21 (L858R) and in-frame deletions within exon 19 are the most frequently occurring EGFR-TKI-sensitive mutations (80%) in patients with NSCLC.9 EGFR mutations in patients with lung cancer are associated with a favorable response to the administration of EGFR-TKIs,10 such as gefitinib,11 erlotinib,12 and afatinib,13 versus standard chemotherapy (CT).
Metfromin (N′,N′-dimethylbiguanide) has been a standard drug for the treatment of type 2 diabetes mellitus (T2DM) for more than 50 years. A lower cancer-related mortality has been noted in T2DM cancer patients with metformin use compared with those with sulfonylurea and insulin use.14 Recently, metformin was observed to decrease the incidence of lung cancer in T2DM patients15 and was associated with a decreased mortality in T2DM lung cancer patients receiving CT.16,17 The synergistic effect of metformin and EGFR-TKI was reported recently in a retrospective clinical study.18
In this study, we proposed that metformin use may enhance the effect of EGFR-TKI and prolong survival in T2DM patients with lung cancer receiving this therapy. A nationwide population-based study was conducted to determine the effect of metformin use in patients with T2DM and lung cancer receiving EGFR-TKI therapy.
Material and Methods
Data Source
The National Health Insurance (NHI) is a compulsory program for all Taiwan residents. The Taiwan National Health Insurance Research Database (NHIRD)—a comprehensive health care database that covers nearly the entire 23.7-million-strong population of this country—was used in our study. Data on patients’ characteristics, such as sex and date of birth, and information regarding admissions and outpatient visits, including date of admission, date of discharge, dates of visits, and up to 5 discharge diagnoses or 3 outpatient visit diagnoses, were collected. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used for diagnosis. All patients with “catastrophic illnesses” were also included in this database. The Ethics Review Board of Chang Gung Memorial Hospital, Chiayi Branch, Taiwan, approved this study (201600067B1). The data used in this study were analyzed anonymously in accordance with strict confidentially guidelines and regulations regarding personal electronic data protection. The requirement for informed consent was waived by the institutional review board.
Study Cohorts
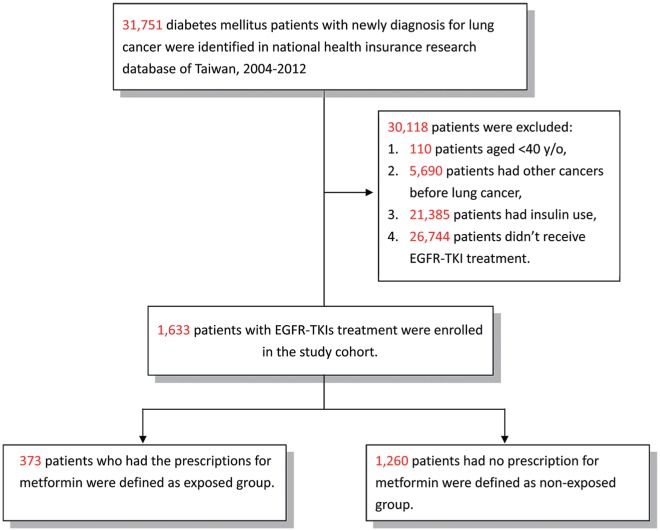
We identified all patients with DM (ICD-9-CM 249-250) and an initial and primary diagnosis of lung cancer (ICD-9-CM 162) between January 1, 2004, and December 31, 2012, from the NHIRD. Patients who underwent EGFR-TKI therapy (gefitinib or erlotinib) were included, while those diagnosed with other types of cancer, aged <40 years, or with insulin use were excluded. Since insulin is used in the treatment of type 1 DM, all the patients in our study were T2DM patients. The metformin cohort comprised patients who had taken metformin for more than 28 cumulative defined daily doses after a diagnosis of lung cancer and with the use of EGFR-TKI (Figure 1). All participants were followed-up till the end of 2013.
Figure 1.
Flowchart of the patient enrollment process of the metformin cohort and matched non-metformin cohort.
Demographic Variables and Comorbidities
Demographic variables, including age, sex, income for the estimation of insurance payment, and urbanization of the participants’ residential areas, were included. Monthly income was categorized as follows: ≤NT$15 840, NT$15 841 to $25 000, and ≥NT$25 000. The urbanization level was categorized as “very high,” “high,” “moderate,” or “low,” based on the population density.19 Hypertension (ICD-9-CM 401-405), coronary artery disease (ICD-9-CM 414-419), stroke (ICD-9-CM 430-438), renal insufficiency (ICD-9-CM 585, 586), chronic obstructive pulmonary disease (COPD; ICD9-CM 491, 492, 496), and smoking-related disorders (ICD9-CM 305.1, 491.2, 492.8, 496, 523.6, and V15.82) were included as comorbidities. Radiotherapy (RT), CT, or both (concurrent chemoradiotherapy [CCRT]) were also included in our study. EGFR-TKI responders were defined as patients who received EGFR-TKI therapy for more than 90 days; the remaining patients were defined as nonresponders.20 The CT regimens before EGFR-TKI therapy were also included in this study.
The EGFR-TKIs used were approved by the NHI in November 2007 (gefitinib) and June 2008 (erlotinib) for the treatment of stage IIIB or IV lung cancer as the second-line therapy for lung adenocarcinoma and the third-line therapy for NSCLC (erlotinib), and in June 2011 and November 2013, as the first-line therapy for lung adenocarcinoma with EGFR mutations (gefitinib and erlotinib, respectively), in Taiwan. The performance of imaging studies and the application of EGFR-TKI therapy every 3 months were requested in patients who received EGFR-TKI therapy. EGFR-TKI therapy was declined by the NHI once progressive disease was observed. Since the results of imaging studies were not available in the NHIRD, we alternatively defined progression-free survival (PFS) in our study as the interval from the beginning to the end of EGFR-TKI therapy. All patients receiving second-line gefitinib and erlotinib therapy in our study had adenocarcinoma, and those receiving third-line erlotinib therapy had NSCLC. Since data on the EGFR mutation status are not available in the NHIRD, EGFR-TKI responders were used as surrogates of EGFR mutations in our study. Overall survival (OS) was defined as the time from diagnosis to any cause of death or the time of censoring at the last follow-up.
Statistical Analysis
The differences in the demographic characteristics and comorbidities between the metformin and non-metformin cohorts were examined by the χ2 test. The hazard ratios (HRs) and 95% confidence intervals (CIs) of the risk of death for metformin users compared with the comparison cohort were examined by Cox proportional hazard regression analysis. Survival analysis was performed using Kaplan-Meier analysis and a log-rank test. A multivariate Cox proportional hazards model was used to determine the risk factors of mortality in patients with lung cancer and their adjusted HR within the metformin cohort. All analyses were conducted using SAS statistical software (Version 9.4; SAS Institute, Cary, NC).
Results
Differences in Demographic Characteristics and Comorbidities Between the Metformin and Non-Metformin Diabetes EGFR-TKI Lung Cancer Cohorts
A total of 1633 patients with T2DM and lung cancer, undergoing EGFR-TKI therapy from 2004 to 2012, were included in our study. Of these patients, 373 patients were enrolled in the metformin cohort and 1260 patients in the non-metformin cohort. The metformin cohort had a significantly higher presence of hypertension, less COPD, renal insufficiency, and smoking-related disorders than the nonuser cohort (Table 1). The metformin cohort also had a significantly higher proportion of patients without CT or/and RT, and with gefitinib use. In the non-metformin cohort, 103 of the 1260 patients received oral antidiabetic agents (Table S1, available online) after the diagnosis of lung cancer. The average years from the diagnosis of T2DM to the diagnosis of lung cancer in the metformin (6.7 ± 4.7 years) and non-metformin (6.9 ± 4.3 years) cohorts were not significantly different (P = .419).
Table 1.
Characteristics of NSCLC Patients Undergoing EGFR-TKI Therapy.
| Variables | Metformin Use |
|||
|---|---|---|---|---|
| User | Nonuser | P | Total | |
| Patients, n (%) | 373 (100.0%) | 1260 (100.0%) | 1633 (100.0%) | |
| Sex, n (%) | .8065 | |||
| Female | 196 (52.5%) | 653 (51.8%) | 849 (52.0%) | |
| Male | 177 (47.5%) | 607 (48.2%) | 784 (48.0%) | |
| Age (years), n (%) | ||||
| 40-64 | 129 (34.6%) | 428 (34.0%) | 557 (34.1%) | |
| ≥65 | 244 (65.4%) | 832 (66.0%) | 1076 (65.9%) | |
| Median (IQR) | 69.0 (62.0-76.0) | 70.0 (61.0-76.0) | 70.0 (62.0-76.0) | |
| Urbanization, n (%) | .1460 | |||
| Very high | 118 (31.6%) | 372 (29.5%) | 490 (30.0%) | |
| High | 168 (45.0%) | 517 (41.0%) | 685 (41.9%) | |
| Moderate | 54 (14.5%) | 232 (18.4%) | 286 (17.5%) | |
| Low | 33 (8.8%) | 139 (11.0%) | 172 (10.5%) | |
| Income (NT$), n (%) | .7835 | |||
| 0 | 143 (38.3%) | 448 (35.6%) | 591 (36.2%) | |
| 1-15 840 | 70 (18.8%) | 238 (18.9%) | 308 (18.9%) | |
| 15 841-25 000 | 111 (29.8%) | 397 (31.5%) | 508 (31.1%) | |
| ≥25 000 | 49 (13.1%) | 177 (14.0%) | 226 (13.8%) | |
| Comorbidities, n (%) | ||||
| Hypertension | 292 (78.3%) | 900 (71.4%) | .0088* | 1192 (73.0%) |
| Stroke | 89 (23.9%) | 363 (28.8%) | .0606 | 452 (27.7%) |
| CAD | 142 (38.1%) | 514 (40.8%) | .3459 | 656 (40.2%) |
| COPD | 111 (29.8%) | 501 (39.8%) | .0005* | 612 (37.5%) |
| Renal insufficiency | 8 (2.1%) | 66 (5.2%) | .0116* | 74 (4.5%) |
| Smoking-related disorder | 64 (17.2%) | 331 (26.3%) | .0003* | 395 (24.2%) |
| CT/RT, n (%) | .0091* | |||
| CCRT | 135 (36.2%) | 566 (44.9%) | 701 (42.9%) | |
| CT | 129 (34.6%) | 409 (32.5%) | 538 (32.9%) | |
| RT | 34 (9.1%) | 102 (8.1%) | 136 (8.3%) | |
| Without CT or RT | 75 (20.1%) | 183 (14.5%) | 258 (15.8%) | |
| EGFR-TKI, n (%) | .0248* | |||
| Gefitinib | 215 (57.6%) | 633 (50.2%) | 848 (51.9%) | |
| Erlotinib | 129 (34.6%) | 487 (38.7%) | 616 (37.7%) | |
| Both | 29 (7.8%) | 140 (11.1%) | 169 (10.3%) | |
| EGFR-TKI response, n (%) | .0534 | |||
| Responder | 239 (64.1%) | 737 (58.5%) | 976 (59.8%) | |
| Nonresponder | 134 (35.9%) | 523 (41.5%) | 657 (40.2%) | |
| CT regimens before EGFR-TKI, n (%) | .3316 | |||
| ≤1 | 261 (70.0%) | 848 (67.3%) | 1109 (67.9%) | |
| ≥2 | 112 (30.0%) | 412 (32.7%) | 524 (32.1%) | |
| Follow-up duration (months) | ||||
| Median (IQR) | 22.9 (14.9-36.1) | 21.2 (13.5-33.5) | 21.5 (13.9-34.3) | |
Abbreviations: NSCLC, non–small cell lung cancer; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; IQR, interquartile range; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; RT, radiotherapy; CCRT, concurrent chemoradiotherapy.
P < .05.
Clinical Variables and HRs of Death in Diabetes Patients With Lung Cancer Undergoing EGFR-TKI Therapy
The risks of death in the metformin user and nonuser cohorts were then compared with the different clinical variables. In the univariate analysis, a reduced risk of death was observed in the metformin users (HR: 0.69, 95% CI: 0.59-0.81, P < .001), those from very high urbanization areas (HR: 0.74, 95% CI: 0.60-0.92, P = .0056), and the EGFR-TKI responder group (HR: 0.34, 95% CI: 0.30-0.39, P < .001). An increased risk of death was observed in patients with male sex (HR: 1.60, 95% CI: 1.42-1.81, P < .001), a monthly income of 15 841 to 25 000 NT$ (HR: 1.20, 95% CI: 1.04-1.39, P = .0134), stroke (HR: 1.21, 95% CI: 1.06-1.38, P = .0042), smoking-related disorders (HR: 1.37, 95% CI: 1.19-1.57, P < .001), CCRT (HR: 1.23, 95% CI: 1.01-1.51, P = .044), and RT (HR: 1.65, 95% CI: 1.26-2.17, P < 0.001; Table 2).
Table 2.
Comparison of Hazard Ratios.
| Variable | Crude |
Adjusted |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Metformin use (ref: nonuser) | ||||||
| User | 0.69 | 0.59-0.81 | <.001* | 0.73 | 0.62-0.85 | <.001* |
| Sex (ref: female) | ||||||
| Male | 1.60 | 1.42-1.81 | <.001* | 1.41 | 1.24-1.61 | <.001* |
| Age (ref: 40-64 years) | ||||||
| ≥65 | 1.11 | 0.97-1.26 | .120 | 1.00 | 0.86-1.16 | .993 |
| Urbanization (ref: low) | ||||||
| Very high | 0.74 | 0.60-0.92 | .0056* | 0.78 | 0.62-0.98 | .0321* |
| High | 0.84 | 0.68-1.02 | .078 | 0.94 | 0.76-1.16 | .549 |
| Moderate | 0.91 | 0.73-1.14 | .408 | 0.97 | 0.77-1.21 | .777 |
| Income (NT$) (ref: 0) | ||||||
| 1-15 840 | 1.02 | 0.86-1.20 | .866 | 0.88 | 0.74-1.05 | .157 |
| 15 841-25 000 | 1.20 | 1.04-1.39 | .0134* | 1.12 | 0.95-1.32 | .172 |
| ≥25000 | 0.83 | 0.68-1.01 | .058 | 0.84 | 0.68-1.04 | .109 |
| Comorbidities (ref: without) | ||||||
| Hypertension | 1.00 | 0.88-1.15 | .955 | 1.09 | 0.94-1.26 | .235 |
| Stroke | 1.21 | 1.06-1.38 | .0042* | 1.16 | 1.01-1.33 | .0409* |
| CAD | 0.92 | 0.82-1.04 | .205 | 0.88 | 0.77-1.00 | .046 |
| COPD | 1.11 | 0.98-1.26 | .089 | 0.81 | 0.69-0.96 | .0124* |
| Renal insufficiency | 0.98 | 0.73-1.33 | .916 | 1.01 | 0.75-1.37 | .945 |
| Smoking-related disorder | 1.37 | 1.19-1.57 | <.001* | 1.36 | 1.14-1.63 | .0009* |
| CT/RT (ref: without CT or RT) | ||||||
| CCRT | 1.23 | 1.01-1.51 | .0444* | 1.06 | 0.85-1.31 | .627 |
| CT | 1.08 | 0.88-1.34 | .458 | 0.93 | 0.74-1.16 | .505 |
| RT | 1.65 | 1.26-2.17 | <.001* | 1.46 | 1.11-1.93 | .0075* |
| EGFR-TKI response (ref: nonresponder) | ||||||
| Responder | 0.34 | 0.30-0.39 | <.001* | 0.34 | 0.30-0.39 | <.001* |
| CT regimens before EGFR-TKI (ref: ≤1) | ||||||
| ≥2 | 1.09 | 0.96-1.23 | .184 | 0.89 | 0.78-1.01 | .072 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
P < .05.
After adjustment for metformin use, age, sex, urbanization, income, hypertension, stroke, CAD, COPD, smoking-related disorders, CT/RT, EGFR-TKI response, and the regimens used before EGFR-TKI therapy, a reduced risk of death was still observed in the metformin users (HR: 0.73, 95% CI: 0.62-0.85, P < .001), those from very high urbanization areas (HR: 0.78, 95% CI: 0.62-0.98, P = .0321), COPD (HR: 0.81, 95% CI: 0.69-0.96, P = .0124), and the EGFR-TKI responders (HR: 0.34, 95% CI: 0.30-0.39, P < .001). An increased risk of death was observed in patients with male sex (HR: 1.41, 95% CI: 1.24-1.61, P < .001), stroke (HR: 1.16, 95% CI: 1.01-1.33, P = .0409), smoking-related disorders (HR: 1.36, 95% CI: 1.14-1.63, P = .0009), and RT (HR: 1.46, 95% CI: 1.11-1.93, P = 0.0075; Table 2).
HRs of Death in Subpopulations Treated With Metformin
The risk of death after metformin use was then evaluated in subpopulations of diabetic lung cancer patients with EGFR-TKI therapy. All subpopulations of metformin users stratified by sex, age, hypertension, stroke, CAD, COPD, and renal insufficiency before EGFR-TKI treatment had a significantly reduced risk of death (Table 3). A significantly reduced risk of death was also observed in patients without smoking-related disorders, and patients with CCRT, CT, RT, gefitinib, or ≤1 CT regimen before EGFR-TKI therapy (Table 3).
Table 3.
Adjusted Hazard Ratios of Mortality in Subpopulations Treated With Metformin.
| Stratified Variables | Metformin |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| User |
Nonuser |
Reference: nonuser |
|||||||
| Patients | Death | % | Patients | Death | % | HRa | 95% CI | P | |
| Sex | |||||||||
| Female | 196 | 85 | 43.4% | 653 | 414 | 63.4% | 0.66 | 0.52-0.84 | .0006* |
| Male | 177 | 111 | 62.7% | 607 | 467 | 76.9% | 0.80 | 0.65-0.99 | .0442* |
| Age (years) | |||||||||
| 40-64 | 129 | 66 | 51.2% | 428 | 288 | 67.3% | 0.79 | 0.60-1.05 | .102 |
| ≥65 | 244 | 130 | 53.3% | 832 | 593 | 71.3% | 0.71 | 0.59-0.86 | .0006* |
| Hypertension | |||||||||
| Without | 81 | 45 | 55.6% | 360 | 258 | 71.7% | 0.70 | 0.50-0.97 | .0311* |
| With | 292 | 151 | 51.7% | 900 | 623 | 69.2% | 0.75 | 0.63-0.90 | .002* |
| Stroke | |||||||||
| Without | 284 | 150 | 52.8% | 897 | 615 | 68.6% | 0.75 | 0.63-0.90 | .0023* |
| With | 89 | 46 | 51.7% | 363 | 266 | 73.3% | 0.63 | 0.45-0.87 | .0047* |
| CAD | |||||||||
| Without | 231 | 128 | 55.4% | 746 | 519 | 69.6% | 0.76 | 0.62-0.92 | .0055* |
| With | 142 | 68 | 47.9% | 514 | 362 | 70.4% | 0.70 | 0.53-0.91 | .0083* |
| COPD | |||||||||
| Without | 262 | 137 | 52.3% | 759 | 520 | 68.5% | 0.74 | 0.61-0.89 | .0016* |
| With | 111 | 59 | 53.2% | 501 | 361 | 72.1% | 0.71 | 0.54-0.95 | .0195* |
| Renal insufficiency | |||||||||
| Without | 365 | 194 | 53.2% | 1194 | 838 | 70.2% | 0.74 | 0.63-0.87 | .0002* |
| With | 8 | 2 | 25.0% | 66 | 43 | 65.2% | 0.10 | 0.02-0.55 | .0085* |
| Smoking-related disorder | |||||||||
| Without | 309 | 156 | 50.5% | 929 | 634 | 68.2% | 0.72 | 0.60-0.86 | .0002* |
| With | 64 | 40 | 62.5% | 331 | 247 | 74.6% | 0.79 | 0.55-1.13 | .190 |
| CT/RT | |||||||||
| CCRT | 135 | 88 | 65.2% | 566 | 445 | 78.6% | 0.79 | 0.62-1.00 | .0457* |
| CT | 129 | 67 | 51.9% | 409 | 269 | 65.8% | 0.75 | 0.57-0.98 | .0383* |
| RT | 34 | 15 | 44.1% | 102 | 79 | 77.5% | 0.46 | 0.25-0.84 | .0118* |
| Without CT or RT | 75 | 26 | 34.7% | 183 | 88 | 48.1% | 0.79 | 0.50-1.25 | .308 |
| EGFR-TKI | |||||||||
| Gefitinib | 215 | 103 | 47.9% | 633 | 422 | 66.7% | 0.64 | 0.51-0.79 | <.001* |
| Erlotinib | 129 | 84 | 65.1% | 487 | 380 | 78.0% | 0.82 | 0.64-1.04 | .100 |
| Both | 29 | 9 | 31.0% | 140 | 79 | 56.4% | 0.44 | 0.21-0.94 | .0343* |
| EGFR-TKI response | |||||||||
| Responder | 239 | 91 | 38.1% | 737 | 434 | 58.9% | 0.62 | 0.49-0.78 | <.001* |
| Nonresponder | 134 | 105 | 78.4% | 523 | 447 | 85.5% | 0.85 | 0.69-1.06 | .143 |
| CT regimens before EGFR-TKI | |||||||||
| ≤1 | 261 | 116 | 44.4% | 848 | 542 | 63.9% | 0.67 | 0.54-0.82 | <.001* |
| Gefitinib (adenocarcinoma) | 194 | 72 | 37.1% | 574 | 340 | 59.2% | 0.59 | 0.46-0.77 | <.001* |
| Erlotinib (adenocarcinoma) | 67 | 44 | 65.7% | 274 | 202 | 73.7% | 0.81 | 0.57-1.14 | .223 |
| ≥2 | 112 | 80 | 71.4% | 412 | 339 | 82.3% | 0.87 | 0.68-1.12 | .290 |
| Gefitinib (adenocarcinoma) | 48 | 40 | 83.3% | 178 | 154 | 86.5% | 0.98 | 0.68-1.41 | .894 |
| Erlotinib (NSCLC) | 64 | 40 | 62.5% | 234 | 185 | 79.1% | 0.82 | 0.57-1.17 | .265 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; NSCLC, non-small-cell lung cancer.
HRs were adjusted for sex, age, urbanization, income, hypertension, stroke, CAD, COPD, renal insufficiency, smoking-related disorders, CT/RT, CT regimens used before EGFR-TKI therapy.
P < .05.
PFS and OS in the Metformin and Non-Metformin Diabetes Patients With Lung Cancer Undergoing EGFR-TKI Therapy
PFS and OS were also evaluated among the metformin users and nonusers. Metformin use was associated with a significantly longer median PFS (9.2 months, 95% CI: 8.6-11.7 vs 6.4 months, 95% CI: 5.9-7.2, P < .001; Figure 2), and OS (33.4 months, 95% CI: 29.4-40.2 vs 25.4 months, 95% CI: 23.7-27.2, P < .001; Figure 3).
Figure 2.
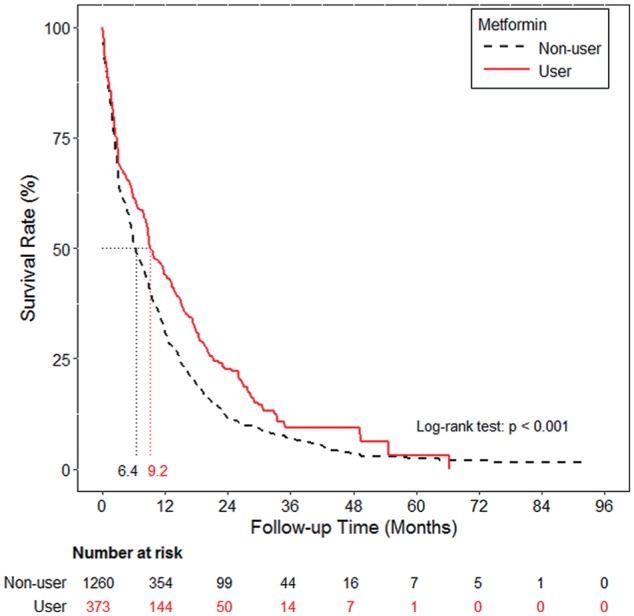
Progression-free survival curve of the metformin and non-metformin cohorts.
Figure 3.
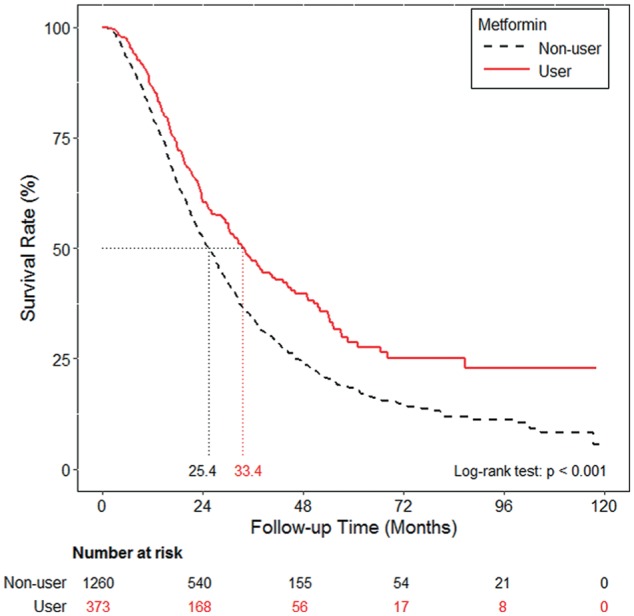
Overall survival curve of the metformin and non-metformin cohorts.
Discussion
In this retrospective, nationwide, longitudinal cohort study, we observed that metformin use was associated with a decreased risk of death, and prolonged PFS and OS in patients with T2DM and lung cancer receiving EGFR-TKI therapy.
The underlying mechanism of how metformin enhances the effect of EGFR-TKIs in lung cancer has been explored in several studies. Metformin increases the sensitivity of EGFR-TKI-resistant lung cancer cells to erlotinib or gefitinib through the inhibition of interleukin-6 signaling and reversal of epithelial-mesenchymal transition.21 Metformin has antitumor effects via the inhibition of the mTORC1 through either AMP kinase-dependent or independent signaling pathways,22 and also inhibits the PI3K/AKT/mTOR signaling pathway.23 Since the aberrant activation of the PI3K/AKT/mTOR signaling pathway is one of the mechanisms behind the acquired resistance to EGFR-TKI therapy in patients with adenocarcinoma and EGFR mutations,24 the use of metformin in combination with an EGFR-TKIs could produce a synergistic antitumor effect on lung cancer cells.
In our study, an increased risk of death was observed in patients with male sex, stroke, COPD, smoking-related disorders, and RT. These findings are similar to those of previous studies. Male sex has a negative impact on EGFR-TKI therapy outcomes compared with female sex.25 An increased risk of stroke was reported in patients with lung cancer.26 The administration of RT may imply a more advanced stage of lung cancer, such as the presence of brain or bone metastasis, and the prognosis of such patients is poor.27 Smoking is a risk factor of NSCLC and is associated with a lower response to EGFR-TKI therapy.28
As data on the EGFR mutation status is not available in the NHIRD, we alternatively used EGFR-TKI responders as surrogates for EGFR mutations.20,29 In the subgroup analysis, EGFR-TKI responders had a reduced risk of death in the metformin cohort, implying that metformin may have protective effects mainly in lung patients with EGFR mutations. The protective effect of metformin was also more prominent in patients with first-line or second-line gefitinib use, owing perhaps to the smaller number of patients with erlotinib use, or the different effects of metformin on gefitinib or erlotinib. The protective effect of metformin was also more prominent in patients who received CT, RT, or both, which is consistent with the findings of previous studies in which metformin enhanced the effect of CT30 and RT.31
DM is a poor prognostic factor in lung cancer patients.29,32 In our study, metformin users had a prolonged OS of up to 33.4 months. The OS in our study is similar to that observed in previous studies focusing on the first-line treatment of patients with EGFR mutation-positive advanced NSCLC (23.6 to 30.5 months).12,33 This implies that metformin use enhances the therapeutic effects of EGFR-TKI in patients with T2DM.
Our study has some limitations. Data on lung cancer stage, pathology, symptoms, physical status, smoking status, and genetic factors are not available in the NHIRD. The immortal bias could be a confounding factor in our study. In our study, patients in both the metformin and non-metformin cohorts received EGFR-TKI therapy after the diagnosis of lung cancer. We also reviewed our data and observed that no patients died in the non-metformin cohort within the 28 days immediately after the diagnosis of lung cancer. As a result, we assumed that the effects of immortal bias in our study could be minimal. Nevertheless, further prospective randomized controlled trials are needed to verify our findings.
Conclusions
In conclusion, our study showed that metformin use potentially enhances the therapeutic effect and decreases the mortality in T2DM patients with lung cancer receiving EGFR-TKI therapy. Our results suggest that in T2DM lung cancer patients with EGFR-TKI therapy, metformin could be the preferred oral hypoglycemic agent.
Supplemental Material
Supplemental material, Table_S1 for Metformin Prolongs Survival in Type 2 Diabetes Lung Cancer Patients With EGFR-TKIs by Ming-Szu Hung, Min-Chun Chuang, Yi-Chuan Chen, Chuan-Pin Lee, Tsung-Ming Yang, Pau-Chung Chen, Ying-Huang Tsai and Yao-Hsu Yang in Integrative Cancer Therapies
Acknowledgments
The authors would like to thank the Health Information and Epidemiology Laboratory (CLRPG6G0042) for the comments and assistance in the data analysis. This study was based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant (CLRPG6G0042) from Chang Gung Memorial Hospital, Chiayi Branch.
ORCID iDs: Ming-Szu Hung  https://orcid.org/0000-0002-5742-7932
https://orcid.org/0000-0002-5742-7932
Yao-Hsu Yang  https://orcid.org/0000-0002-8080-0504
https://orcid.org/0000-0002-8080-0504
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 2. Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523-529. [DOI] [PubMed] [Google Scholar]
- 3. Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e1S-e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch TJ, Bell DW, Sordella Ret al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [DOI] [PubMed] [Google Scholar]
- 5. Paez JG, Janne PA, Lee JCet al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500. [DOI] [PubMed] [Google Scholar]
- 6. Huang SF, Liu HP, Li LHet al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10:8195-8203. [DOI] [PubMed] [Google Scholar]
- 7. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257-262. [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Zhong WZ, Li LYet al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430-439. [DOI] [PubMed] [Google Scholar]
- 9. Gazdar AF, Shigematsu H, Herz J, Minna JD. Mutations and addiction to EGFR: the Achilles “heal” of lung cancers? Trends Mol Med. 2004;10:481-486. [DOI] [PubMed] [Google Scholar]
- 10. Sequist LV, Joshi VA, Janne PAet al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist. 2007;12:90-98. [DOI] [PubMed] [Google Scholar]
- 11. Mok TS, Wu YL, Thongprasert Set al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [DOI] [PubMed] [Google Scholar]
- 12. Zhou C, Wu YL, Chen Get al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26:1877-1883. [DOI] [PubMed] [Google Scholar]
- 13. Wu YL, Zhou C, Hu CPet al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-222. [DOI] [PubMed] [Google Scholar]
- 14. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254-258. [DOI] [PubMed] [Google Scholar]
- 15. Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13:143-148. [DOI] [PubMed] [Google Scholar]
- 16. Tan BX, Yao WX, Ge Jet al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103-5111. [DOI] [PubMed] [Google Scholar]
- 17. Chuang MC, Yang YH, Tsai YHet al. Survival benefit associated with metformin use in inoperable non-small cell lung cancer patients with diabetes: a population-based retrospective cohort study. PLoS One. 2018;13:e0191129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Yao W, Chu Qet al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett. 2015;369:97-102. [DOI] [PubMed] [Google Scholar]
- 19. Liu CY, Hung YT, Chuang YLet al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage. 2006;4:1-22. [Google Scholar]
- 20. Chang CH, Lee CH, Ho CC, Wang JY, Yu CJ. Gender-based impact of epidermal growth factor receptor mutation in patients with nonsmall cell lung cancer and previous tuberculosis. Medicine (Baltimore). 2015;94:e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L, Han R, Xiao Het al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20:2714-2726. [DOI] [PubMed] [Google Scholar]
- 22. Howell JJ, Hellberg K, Turner Met al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25:463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Sun H, Feng Met al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inhibiting PI3K/AKT/mTOR signaling. Gynecol Endocrinol. 2018;34:428-432. [DOI] [PubMed] [Google Scholar]
- 24. Gadgeel SM, Wozniak A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer. Clin Lung Cancer. 2013;14:322-332. [DOI] [PubMed] [Google Scholar]
- 25. Lee CK, Wu YL, Ding PNet al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33:1958-1965. [DOI] [PubMed] [Google Scholar]
- 26. Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42:3034-3039. [DOI] [PubMed] [Google Scholar]
- 27. D’Antonio C, Passaro A, Gori Bet al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Kang S, Fang Wet al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer. 2015;16:144-151.e1. [DOI] [PubMed] [Google Scholar]
- 29. Hung MS, Chen IC, Lee CPet al. Statin improves survival in patients with EGFR-TKI lung cancer: a nationwide population-based study. PLoS One. 2017;12:e0171137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sayed R, Saad AS, El Wakeel L, Elkholy E, Badary O. Metformin addition to chemotherapy in stage IV non-small cell lung cancer: an open label randomized controlled study. Asian Pac J Cancer Prev. 2015;16:6621-6626. [DOI] [PubMed] [Google Scholar]
- 31. Gash KJ, Chambers AC, Cotton DE, Williams AC, Thomas MG. Potentiating the effects of radiotherapy in rectal cancer: the role of aspirin, statins and metformin as adjuncts to therapy. Br J Cancer. 2017;117:210-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu L, Cao H, Zhang Tet al. The effect of diabetes mellitus on lung cancer prognosis: a PRISMA-compliant meta-analysis of cohort studies. Medicine (Baltimore). 2016;95:e3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maemondo M, Inoue A, Kobayashi Ket al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_S1 for Metformin Prolongs Survival in Type 2 Diabetes Lung Cancer Patients With EGFR-TKIs by Ming-Szu Hung, Min-Chun Chuang, Yi-Chuan Chen, Chuan-Pin Lee, Tsung-Ming Yang, Pau-Chung Chen, Ying-Huang Tsai and Yao-Hsu Yang in Integrative Cancer Therapies