Abstract
Background. Treatment options in oncology are rapidly advancing, and public payer systems are increasingly under pressure to adopt new but expensive cancer treatments. Cost-utility analyses (CUAs) are used to estimate the relative costs and effects of competing interventions, where health outcomes are measured using quality-adjusted life years (QALYs). Health state utility values (HSUVs) are used to reflect health-related quality of life or health status in the calculation of QALYs. To support reimbursement agencies in the appraisal of oncology drug submissions, which typically include a CUA component, we have proposed a systematic literature review of published HSUV estimates in the field of oncology. Methods. The following databases will be searched: MEDLINE, EMBASE, EconLit, and CINAHL. A team of reviewers, working independently and in duplicate, will evaluate abstracts and full-text publications for eligibility against broad inclusion criteria. Studies using a direct, indirect, or combination approach to eliciting preferences related to cancer or cancer treatments are eligible. Data extraction will capture details of study methodology, participants, health states, and corresponding HSUVs. We will summarize our findings with descriptive analyses at this stage. A pilot review in thyroid cancer is presented to illustrate the proposed methods. Discussion. This systematic review will generate a comprehensive summary of the oncology HSUV literature. As a component of the Health Utility Book (HUB) project, we anticipate that this work will assist both health economic modelers as well as critical reviewers in the development and appraisal of CUAs in oncology.
Keywords: medical oncology, health state utility values, systematic review
Reimbursement practices have evolved over time. Health technology assessment (HTA) represents a comprehensive approach to the evaluation of emerging and existing health care interventions. A major component of HTA submissions to public-payer drug plans are economic evaluations designed to compare competing interventions with respect to both clinical and economic consequences. Cost-utility analyses (CUAs) are a type of economic evaluation accepted by several major HTA bodies worldwide.1–3 These analyses are particularly useful for interventions or programs that not just extend life but also affect the patient’s health-related quality of life or health status. In a CUA, health state utility values (HSUVs) are used to estimate quality-adjusted life years (QALYs) and provide an estimate of both quantity and quality of life.4 Health states may be simple or complex, defined by several factors, including disease and treatment characteristics as well as functionality and limitations. By convention, full or perfect health is assigned the maximum utility score of 1, while death is assigned a utility score of 0. Health states with negative utilities are perceived as worse than death. While HSUV estimates are inherently subjective, the general rule is to weight more preferable health states with higher utility.
Investigators can measure HSUVs using different approaches. These can be through direct measurements, such as the visual analogue scale (VAS), or through preferences elicited using probabilities or willingness to tradeoff between quantity and quality of life methods, such as the standard gamble (SG) or time tradeoff (TTO) technique. Indirect methods using multi-attribute utility-based instruments, relatively short questionnaires that require only a few minutes to complete, provide an easier alternative to these direct measures. The term indirect measure is used as individual patients do not explicitly provide preferences for their health states but rather describe their state, which is then assigned a value according to a scoring algorithm that has been developed separately with the general public or patient groups. Investigators have used both direct and indirect methods in addressing HSUV for cancer treatments.5
Rapid progress in the field of oncology has given way to new therapies, and these treatments have made significant contributions to prolonging life expectancies or improving quality of life.6 However, these new cancer treatments often come at high costs.7,8 As part of the drug reimbursement process in many countries, manufacturers are required to submit dossiers containing CUAs. It is recommended that HSUV inputs for these CUAs be identified through systematic literature reviews.9 However, a cross-sectional review of 71 technology appraisals submitted to the National Institute for Health and Clinical Excellence found that out of 39 submissions that obtained HSUVs from published studies, only 31% adopted a systematic approach.10 Moreover, even when these reviews are commissioned, the review methods and findings may not be made explicit to reimbursement agencies. Thus, the complete evidence profile for a given health state may not be clear to reviewers and their ability to make a critical appraisal of model inputs may be limited.
There are several systematic reviews of HSUVs across the cancer spectrum.11–15 However, these reviews are targeted to answer a question in a particular area of oncology. To date, no central catalogue of cancer-related HSUVs has been established. Such a resource would dramatically enhance researchers’ abilities to select and evaluate the available health utility literature in a thorough and timely manner. In the interest of the comprehensive, accountable, and transparent evaluation of CUAs in the support of oncology drug reimbursement submissions, we have developed a systematic review protocol to identify and describe published health states and HSUVs across the spectrum of oncology research. This review is part of the Health Utility Book (HUB) as described by Xie et al.16
Objectives
The objective of this article is to present a study protocol for systematically identifying and describing the health utility literature in the field of oncology with respect to both the methods used and the estimates attained for cancer-related health states. These methods are illustrated with a pilot study in thyroid cancer.
Methods
This systematic review will be conducted in general accordance with published guidelines and good practices.17–22 Additional considerations have been made given the broad scope of the review and the anticipated volume of work. The traditional Patient, Intervention, Comparison, Outcome (PICO) statement, common to clinical research, is not typically amenable to reviews of HSUVs.17,18 Specifically, this review is not designed to collect information on any particular intervention or comparator. This protocol has been registered with the Prospective Register of Systematic Reviews (PROSPERO CRD42018095049).
Search Strategy
The scope of the search strategies for this review is purposefully broad. The databases to be queried include the following: MEDLINE via Ovid; EMBASE via Ovid; EconLit via EBSCOhost; and CINAHL via EBSCOhost. The search strategies were developed by reviewing published HSUV review recommendations, the strategies of published HSUV protocols, and published guidance from other sources such as the Canadian Agency for Drugs and Technologies in Health (CADTH). Specifically, we will use two categories of search terms: disease-specific queries and health state utility elicitation methodology-specific queries. To strengthen this search strategy, we added utility-based instruments as an additional search query. To validate the search strategy, we reviewed the reference lists of published systematic reviews of health utility studies and collected 28 citations (the validation set) to reflect a range of years, methods, and cancer types.23–50 The search strategies developed for the current review were tested to confirm that they successfully captured the citations included in the validation set. The strategies were then adapted to the other databases. Table 1 presents the search strategies in MEDLINE and EMBASE, which were searched separately.
Table 1.
Search Strategy for MEDLINE and EMBASE (via Ovid)
| Search | Query |
|---|---|
| Cancer-related search terms | |
| 1 | cancer*.mp. |
| 2 | exp Neoplasms/ |
| 3 | neoplasm*.mp. |
| 4 | exp Carcinoma/ |
| 5 | carcinoma*.mp. |
| 6 | exp Sarcoma/ |
| 7 | sarcoma*.mp. |
| 8 | exp Lymphoma/ |
| 9 | lymphoma*.mp. |
| 10 | exp Leukemia/ |
| 11 | leukemia*.mp. |
| 12 | myeloma.mp. |
| 13 | tumor*.mp. |
| 14 | tumour*.mp. |
| 15 | Or/1–14 |
| Health utility elicitations methods-related search terms | |
| 16 | (health adj3 (utilit* or status)).mp. |
| 17 | (utilit* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or weight)).mp. |
| 18 | (preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument* or scale* or quest*)).mp. |
| 19 | disutilit*.mp. |
| 20 | standard gamble*.mp. |
| 21 | (time trade off or time tradeoff or time trade-off).mp. |
| 22 | tto.ti,ab,kw. |
| 23 | visual analog* scale*.mp. |
| 24 | VAS.mp. |
| 25 | discrete choice experiment*.mp. |
| 26 | Rating scale.mp. AND (health adj3 (utilit* or status)).mp. |
| 27 | (Personal trade-off or PTO).mp AND (health adj3 (utilit* or status)).mp. |
| 28 | (multiattribute health status* or multi-attribute health status* or multiattribute utility* or multi-attribute utility*).mp. |
| Utility-based instrument search terms | |
| 29 | (hui or hui1 or hui2 or hui3).ti,ab,kw. |
| 30 | health utility index.mp. |
| 31 | (eq or euroqol or euro qol or eq5d or eq 5d or euroqual or euro qual or European Quality of Life 5-dimension or EQ-5D or EQ5D or EQ 5D).mp. |
| 32 | (sf6d or sf 6d or sf-6d or short form 6d or shortform 6d).mp. |
| 33 | (15-D or 15D).mp AND (health utility or health utilities or utility or utilities).mp |
| 34 | (AQoL or AQL or Assessment of Quality of Life).mp |
| 35 | (Patient ORiented Prostate Utility Scale or PORPUS).mp |
| 36 | (PROMIS or Patient-Reported Outcomes Measurement Information System).mp AND (health utility or health utilities or utility or utilities).mp |
| 37 | Or/16–36 |
| 38 | 15 and 37 |
Study Eligibility
Inclusion Criteria
A high-level set of inclusion criteria was selected for this review. To be eligible for inclusion, a publication must meet all the following:
The publication presents the methods of a primary HSUV study, such that a study uses a direct elicitation method (e.g., TTO), an indirect method (e.g., EQ-5D), or a combination of both to elicit preferences for health states from patients or nonpatients (e.g., general public, family, caregivers, or clinicians);
The study targets cancer, cancer treatments, and/or the cancer patient population, including nonpatient respondents; and
The study reports HSUV estimates.
This review is limited to the context of patients who have been diagnosed with cancer, though studies have been published for related populations, such as unaffected high-risk individuals. No restrictions have been specified for publication date or language. In order to provide sufficient information to be used in CUAs, this review focuses only on peer-reviewed studies, excluding grey literature (e.g., unpublished studies, dissertations, conference abstracts). We anticipate that several reviews and economic evaluations, which reference HSUV literature, will be identified with the proposed search strategies. Reviewers will identify these records and the reference lists of these publications will be cross-referenced with the final list of included studies to assess the comprehensiveness of our review.
Data Collection
Study Selection
Prior to screening, duplicate publications will be identified and excluded. The titles and abstracts of all publications identified by the search strategies will be screened according to the eligibility criteria. Where unclear, reviewers will carry the record forward to the full-text screening phase. We have adopted this sensitive approach based on research demonstrating limitations in evaluating study eligibility at the title and abstract levels.18 The full text publications of included abstracts will be retrieved and assessed for eligibility. Publications published in languages other than English will be reviewed by language-matched reviewers having a working knowledge of the language of publication. All screening will be conducted independently and in duplicate. The flow of information process, which documents the number of records retrieved as well as the number of inclusions and exclusions at each screening phase, will be summarized in a PRISMA flow diagram.51
Based on preliminary searches, it is anticipated that a large volume of records will be retrieved through the literature search. Thus, this review necessitates the participation of multiple reviewers whose availability may change over time. As a means of promoting consistency across reviewers, we will establish a training set of 150 records purposefully chosen to represent a broad range of eligible and ineligible studies. Prior to beginning abstract screening, new reviewers will complete the training set and review their results, including reasons for exclusion, against the answer key. The complete set of publications to be screened will be divided into blocks of 1000 records. Reviewers will be assigned one block of records at a time and, upon completion, will be assigned a new, previously unscreened block. Once all blocks have been screened in single, the blocks will be reassigned to satisfy the requirement of duplicate screening. Full-text screening will also be managed using a blocking approach.
Data Extraction and Management
A complete list of data extraction items is presented in Appendix A. All data will be extracted independently and in duplicate using a similar blocking approach as described in the screening process. The reviewers who complete data extraction will review and resolve discrepancies by discussion, with a third reviewer providing arbitration, as necessary. In the case of missing data, we will attempt to contact the corresponding authors for clarification. The data extraction form has been successfully piloted. All screening and data extraction will be maintained in Microsoft Excel workbooks, which include extensive standardized vocabulary to promote consistency and ease of data extraction and reconciliation.
Data Synthesis
A descriptive summary of the findings of this review will be presented, arranged by cancer type. Health state descriptions and corresponding HSUVs from each study will be presented along with a summary of the study methodologies and respondent characteristics. At present, this review is designed to gather and describe published HSUVs. Cognizant of the assumptions that must be made, particularly when HSUVs are derived through different methodologies, we will explore different quantitative evidence synthesis approaches that have been used to pool the HSUVs in the literature.52
Ethics and Dissemination
No ethics approval will be sought for the purpose of this review as no primary data collection will take place. All information will be identified from published studies. The completed review will be disseminated in a series of publications in peer-reviewed journals, arranged by cancer type, detailing the systematic review methodology as well as a summary of the findings. We are also in the process of seeking funding support to develop an online portal to disseminate the HSUVs identified through this review.
Pilot Review
Summary of Screening and Validation
To illustrate the systematic review process described here, we present the screening of a subset of records identified for thyroid cancer. According to data maintained by the World Health Organization (1970–2012) and the Cancer Incidence in Five Continents (1960–2007), the incidence of thyroid cancer has been increasing over the last several decades despite a falling mortality rate. These trends have been attributed to changes in the diagnosis, treatment, and exposure to risk factors.53 However, if current trends persist, it is suggested that thyroid cancer may be the fourth most common cancer in the United States by the year 2030.54 Despite this, our preliminary review suggested that there are relatively few published studies for health utilities for this indication.
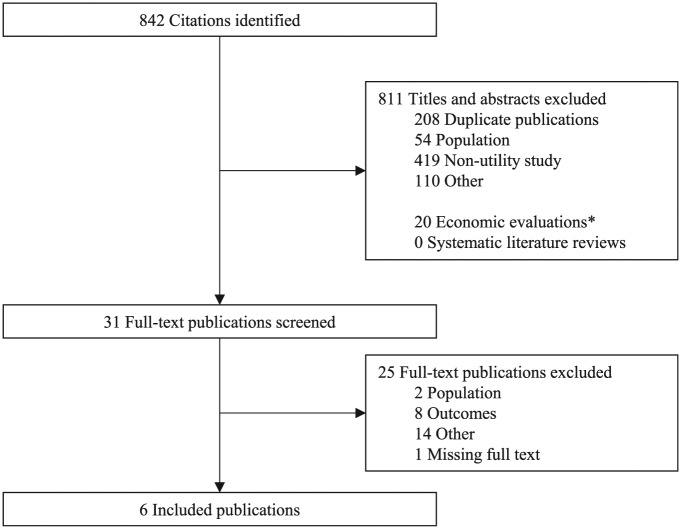
From the complete set of records identified with our search strategy (N = 52,551), we selected a subset that contained the phrase “thyroid” in the title or abstract (n = 842). From these 842 abstracts, 31 were reviewed at the full-text screening level, and six fulfilled all eligibility criteria. Additionally, 21 economic evaluations were identified.55–75 No systematic literature reviews related to health utilities for thyroid cancer were identified. The screening process is summarized in Figure 1.
Figure 1.
PRISMA flow of information diagram.
From the 21 economic evaluations, 35 unique citations for health utility inputs were identified. To validate the systematic review process, these were cross-referenced with the list of included studies. Of these 35 citations, 12 were in a clinical area outside of thyroid cancer, 10 lacked indexing or abstract keywords to indicate that health utility evidence was presented, 4 were published in a source not indexed in the included medical literature databases (i.e., books, websites), and 3 referenced an economic evaluation where no original health utility study was conducted. The remaining six citations were also identified through our search strategy in thyroid cancer and were reviewed for inclusion through our systematic review. However, only two of these citations were considered eligible for inclusion.62,76 While economic evaluations may contain bespoke health utility studies, they often lack indexing or keywords to identify them as a health utility study. Overall, the validation steps suggest that the literature search strategy and screening process adequately identified all relevant publications per the prespecified eligibility criteria.
Descriptive Synthesis
Characteristics of the six eligible studies are presented in Table 2. Most studies reported on health utilities collected using a single technique except for one that employed multiple methodologies (EQ-5D, SF-6D, HUI-2, HUI-3).77 Four studies used a cross-sectional design.62,76,78,79 Respondents varied across studies, with three recruiting patients,77,79,80 two recruiting clinicians,62,78 and one recruiting members of the general public.76 One health utility studied was conducted alongside a clinical trial.80 However, participant characteristics were generally poorly described in the included publications. All health states were either derived for the purpose of the study or relied on patient’s own health. Adverse events or toxicities were explicitly incorporated into the health state descriptions of only one paper.76 In the study by Choi and colleagues,78 the impact of several cancer diagnoses, including thyroid cancer, was reported as a disutility weight. These estimates were considered eligible for inclusion in this review. A summary of the published health utility estimates, arranged by respondent subgroups and scaling method, is presented in Table 3. The health state with the highest HSUV was obtained from clinicians using the TTO for “Disease-free after thyroid lobectomy” (0.99),62 while the lowest observed estimate was reported for “Stable disease with grade 3 diarrhea” (0.42, SD 0.29, 95% confidence interval: 0.36, 0.48) by the general public using the TTO.76 In both studies, health state descriptions were provided by investigators.
Table 2.
Characteristics of Included Studies of Health Utility in Thyroid Cancer
| Study | Scaling Method | Respondents | Mode of Administration | Source of Health State Descriptions | Country |
|---|---|---|---|---|---|
| Lubitz et al77 (2017) | EQ-5D, SF-6D, HUI-2, HUI-3 | Patients | Trained interviewer or mail; Subsequent surveys conducted online | Own health (implied) | USA |
| Choi et al78 (2013) | VAS | Clinicians | Initial rating unclear; intrarater reliability assessed by mail survey | Most common conditions in terms of histologic types and stages (ICD-10, C73) | South Korea |
| Esnaola et al62 (2001) | TTO | Clinicians | Unclear | Investigator-proposed | USA |
| Kent et al79 (2015) | SF-6D | Patients | Mail or telephone | Own health (implied) | USA |
| Fordham et al76 (2015) | TTO | General public | Face-to-face interviews | Vignettes designed through a qualitative study with patients and clinicians | United Kingdom |
| Borget et al80 (2015) | EQ-5D | Patients | Unclear | Own health (implied) | France |
Table 3.
Health States in Thyroid Cancer
| Author | Health State | Respondents | Scaling Method | Analysis N | Mean (SD); Median (Q1, Q3) |
|---|---|---|---|---|---|
| Lubitz et al77 (2017) | Preoperation | All participants | EQ-5D | 117 | 0.895 (0.103); 0.876 (0.82, 1) |
| SF-6D | 117 | 0.773 (0.125); 0.793 (0.66, 0.86) | |||
| HUI-2 | 117 | 0.875 (0.133); 0.917 (0.83, 0.95) | |||
| HUI-3 | 117 | 0.859 (0.185); 0.919 (0.79, 0.97) | |||
| Postoperation | All participants | EQ-5D | 117 | 0.882 (0.114, 95% CI: 0.665, 1) | |
| SF-6D | 117 | 0.748 (0.117, 95% CI: 0.548, 0.919) | |||
| HUI-2 | 117 | 0.873 (0.120, 95% CI: 0.647, 1) | |||
| HUI-3 | 117 | 0.843 (0.167, 95% CI: 0.518, 1) | |||
| 26 weeks postoperation | All participants | EQ-5D | 117 | 0.911 (0.107, 95% CI: 0.752, 1) | |
| SF-6D | 117 | 0.798 (0.122, 95% CI: 0.609, 0.922) | |||
| HUI-2 | 117 | 0.879 (0.111, 95% CI: 0.705, 1) | |||
| HUI-3 | 117 | 0.863 (0.136, 95% CI: 0.596, 1) | |||
| Choi et al78 (2013) | Thyroid cancer | All participants | VAS | 32 | 0.10 (Median)a |
| Oncologists | VAS | 12 | 0.11 (Median)a | ||
| Dermatologists | VAS | 8 | 0.08 (Median)a | ||
| Psychiatrists | VAS | 12 | 0.09 (Median)a | ||
| Esnaola et al62 (2001) | Disease-free after thyroid lobectomy | All participants | TTO | 15 | 0.99 |
| Disease-free after total thyroidectomy/radioiodine therapy | 15 | 0.95 | |||
| Disease-free after thyroid surgery/permanent complication | 15 | 0.88 | |||
| Disease-free after surgery for cervical recurrence | 15 | 0.95 | |||
| Systemic recurrence | 15 | 0.60 | |||
| Kent et al79 (2015) | Thyroid cancer | All participants | SF-6D | 386 | 0.70 (95% CI: 0.69, 0.71) |
| Fordham et al76 (2015) | Stable/no response | All participants | TTO | 100 | 0.80 (0.19, 95% CI: 0.77, 0.84) |
| Response to therapy | 0.86 (0.15, 95% CI: 0.83, 0.89) | ||||
| Progressive disease | 0.50 (0.28, 95% CI: 0.45, 0.56) | ||||
| Stable + Grade 3 diarrhea | 0.42 (0.29, 95% CI: 0.36, 0.48) | ||||
| Stable + Grade 3 fatigue | 0.72 (0.24, 95% CI: 0.67, 0.77) | ||||
| Stable + Grade 3 hand and foot syndrome | 0.52 (0.30, 95% CI: 0.46, 0.58) | ||||
| Stable + Grades 1 or 2 alopecia | 0.75 (0.21, 95% CI: 0.71, 0.79) | ||||
| Borget et al80 (2015) | At treatment assignment | THW | EQ-5D | 336 | 0.87 |
| Immediately before radioiodine administration | THW | 336 | 0.84 | ||
| 2 weeks | THW | 336 | 0.82 | ||
| 4 weeks | THW | 336 | 0.85 | ||
| 6 weeks | THW | 336 | 0.87 | ||
| 3 months | THW | 336 | 0.88 | ||
| 8 months | THW | 336 | 0.90 | ||
| At treatment assignment | rhTSH | 348 | 0.84 | ||
| Immediately before radioiodine administration | rhTSH | 348 | 0.85 | ||
| 2 weeks | rhTSH | 348 | 0.86 | ||
| 4 weeks | rhTSH | 348 | 0.86 | ||
| 6 weeks | rhTSH | 348 | 0.87 | ||
| 3 months | rhTSH | 348 | 0.86 | ||
| 8 months | rhTSH | 348 | 0.88 | ||
| At radioidine administration | THW | 336 | 0.833 (0.192) | ||
| rhTSH | 348 | 0.849 (0.173) | |||
| 3.7 GBq radioidine activity | 337 | 0.836 (0.184) | |||
| 1.1 GBq radioidine activity | 347 | 0.846 (0.182) |
CI, confidence interval; rhTSH, patients managed with recombinant human thyroid-stimulation hormone; THW, patients managed with thyroid hormone withdrawal; VAS, visual analogue scale.
Reported as disutility weights.
Discussion
Published studies that measure HSUVs are a main source of health utilities used in CUAs. These evaluations are an integral component of reimbursement submissions prepared by drug manufacturers seeking listing on public formularies. However, the selection of HSUVs, where multiple studies are available, is often left to the discretion of analysts. Thus, the lack of a systematic approach to the identification and use of published health utilities may lead to a reimbursement policy that does not reflect the preferences of the public. The current review applies a systematic approach to the identification of published HSUVs and thus affords a level of confidence to knowledge users who rely on valid information to complete economic evaluations and HTA appraisals.
Where a health utility estimate does not exist in the literature for a given condition or health state, it is common to use estimates derived for a similar condition. This was the case in several of the economic evaluations identified through our pilot review. However, it is outside the scope of our review to suggest indications that may be interchangeable.
While the proposed review is extensive in scope, there are limitations. Estimates of HSUVs coming from grey literature sources, including conference abstracts and other unpublished media, are not eligible for inclusion. According to our past experience, information provided in conference abstracts or media reports often is not sufficient to be used in CUAs. If the reporting in the grey literature changes in the future, we will revise our review to expand the search strategies and eligibility criteria accordingly. Despite this, the proposed review will culminate in a comprehensive summary of the evidence landscape for published HSUVs in oncology. Detailed study methodologies and respondent characteristics will be collected and summarized. Moreover, this review is the first component of the HUB project.16 The publication of this review protocol is in line with the HUB project team’s goal to maintain transparency and accountability.
Supplemental Material
Supplemental material, Zoratti_HUB_SLR_Protocol_-_Appendix_A_online_supp for Health Utility Book (HUB)–Cancer: Protocol for a Systematic Literature Review of Health State Utility Values in Cancer by Michael James Zoratti, Ting Zhou, Kelvin Chan, Oren Levine, Murray Krahn, Don Husereau, Tammy Clifford, Holger Schunemann, Gordon Guyatt and Feng Xie in MDM Policy & Practice
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michael James Zoratti  https://orcid.org/0000-0003-3069-1159
https://orcid.org/0000-0003-3069-1159
Kelvin Chan  https://orcid.org/0000-0002-2501-3057
https://orcid.org/0000-0002-2501-3057
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Michael James Zoratti, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Ting Zhou, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada; School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China.
Kelvin Chan, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Oren Levine, Department of Oncology, McMaster University, Hamilton, Ontario, Canada.
Murray Krahn, Toronto General Hospital, Toronto, Ontario, Canada.
Don Husereau, Faculty of Health Sciences, University of Ottawa, Ottawa, Ontario, Canada.
Tammy Clifford, Canadian Institutes for Health Research, Ottawa, Ontario, Canada.
Holger Schunemann, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Gordon Guyatt, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
Feng Xie, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada.
References
- 1. Canadian Agency for Drugs and Technology in Health. CADTH Common Drug Review. Submission Guidelines for the CADTH Common Drug Review. Ottawa: Canadian Agency for Drugs and Technology in Health; 2014. [Google Scholar]
- 2. Department of Health, Commonwealth of Australia. Procedure Guidance for Listing Medicines on the Pharmaceutical Benefits Scheme. Canberra: Department of Health, Commonwealth of Australia; 2018. [Google Scholar]
- 3. National Institute for Health and Care Excellence. Developing NICE Guidelines: The Manual. London: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 4. Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296(13):716–21. [DOI] [PubMed] [Google Scholar]
- 5. Blinman P, King M, Norman R, Viney R, Stockler MR. Preferences for cancer treatments: an overview of methods and applications in oncology. Ann Oncol. 2012;23(5):1104–10. [DOI] [PubMed] [Google Scholar]
- 6. Heymach J, Krilov L, Alberg Aet al. Clinical Cancer Advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020–44. [DOI] [PubMed] [Google Scholar]
- 7. Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol. 2016;17(1):39–47. [DOI] [PubMed] [Google Scholar]
- 8. Paying a high price for cancer drugs. Lancet. 2015;386(9992):404. [DOI] [PubMed] [Google Scholar]
- 9. Brazier J. Valuing health states for use in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):769–79. [DOI] [PubMed] [Google Scholar]
- 10. Tosh JC, Longworth LJ, George E. Utility values in National Institute for Health and Clinical Excellence (NICE) technology appraisals. Value Health. 2011;14(1):102–9. [DOI] [PubMed] [Google Scholar]
- 11. Tran AD, Fogarty G, Nowak AKet al. A systematic review and meta-analysis of utility estimates in melanoma. Br J Dermatol. 2018;178(2):384–93. [DOI] [PubMed] [Google Scholar]
- 12. Jeong K, Cairns J. Systematic review of health state utility values for economic evaluation of colorectal cancer. Health Econ Rev. 2016;6(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paracha N, Thuresson PO, Moreno SG, MacGilchrist KS. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:549–59. [DOI] [PubMed] [Google Scholar]
- 14. Meregaglia M, Cairns J. A systematic literature review of health state utility values in head and neck cancer. Health Qual Life Outcomes. 2017;15(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forsythe A, Brandt PS, Dolph M, Patel S, Rabe APJ, Tremblay G. Systematic review of health state utility values for acute myeloid leukemia. Clinicoecon Outcomes Res. 2018;10:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie F, Zoratti M, Chan Ket al. Toward a centralized, systematic approach to the identification, appraisal, and use of health state utility values for reimbursement decision making: introducing the Health Utility Book (HUB) [published online March 22, 2019]. Med Decis Making. doi: 10.1177/0272989X19837969 [DOI] [PubMed] [Google Scholar]
- 17. Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health. 2013;16(4):686–95. [DOI] [PubMed] [Google Scholar]
- 18. Papaioannou D, Brazier J, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature. NICE Decision Support Unit Technical Support Documents; London: National Institute for Health and Care Excellence; 2010. [PubMed] [Google Scholar]
- 19. Paisley S. Identification of evidence for key parameters in decision-analytic models of cost effectiveness: a description of sources and a recommended minimum search requirement. Pharmacoeconomics. 2016;34(6):597–608. [DOI] [PubMed] [Google Scholar]
- 20. Saramago P, Manca A, Sutton AJ. Deriving input parameters for cost-effectiveness modeling: taxonomy of data types and approaches to their statistical synthesis. Value Health. 2012;15(5):639–49. [DOI] [PubMed] [Google Scholar]
- 21. Kaltenthaler E, Tappenden P, Paisley S. Reviewing the evidence to inform the population of cost-effectiveness models within health technology assessments. Value Health. 2013;16(5):830–6. [DOI] [PubMed] [Google Scholar]
- 22. Zechmeister-Koss I, Schnell-Inderst P, Zauner G. Appropriate evidence sources for populating decision analytic models within health technology assessment (HTA): a systematic review of HTA manuals and health economic guidelines. Med Decis Making. 2014;34(3):288–99. [DOI] [PubMed] [Google Scholar]
- 23. Albertsen PC. Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. J Urol. 2005;173(2):565. [Google Scholar]
- 24. Albertsen PC, Nease RF, Jr, Potosky AL. Assessment of patient preferences among men with prostate cancer. J Urol. 1998;159(1):158–63. [DOI] [PubMed] [Google Scholar]
- 25. Bennet CL, Chapman G, Elstein ASet al. A comparison of perspectives on prostate cancer: analysis of utility assessments of patients and physicians. Eur Urol. 1997;32(Suppl. 3):86–8. [PubMed] [Google Scholar]
- 26. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391–400. [DOI] [PubMed] [Google Scholar]
- 27. Beusterien KM, Davies J, Leach Met al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beusterien KM, Szabo SM, Kotapati Set al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101(3):387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cappelli M, Surh L, Humphreys Let al. Measuring women’s preferences for breast cancer treatments and BRCA1/BRCA2 testing. Qual Life Res. 2001;10(7):595–607. [DOI] [PubMed] [Google Scholar]
- 30. Dominitz JA, Provenzale D. Patient preferences and quality of life associated with colorectal cancer screening. Am J Gastroenterol. 1997;92(12):2171–8. [PubMed] [Google Scholar]
- 31. Grann VR, Jacobson JS, Sundararajan V, Albert SM, Troxel AB, Neugut AI. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am. 1999;5(5):283–92. [PubMed] [Google Scholar]
- 32. Havrilesky LJ, Broadwater G, Davis DMet al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayman JA, Fairclough DL, Harris JR, Weeks JC. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15:1252–60. [DOI] [PubMed] [Google Scholar]
- 34. Hess LM, Malone DC, Reed PG, Skrepnek G, Weihs KL. Preferences of patients and oncologists for advanced ovarian cancer treatment-related health states. Health Outcomes Res Med. 2010;1(1):e51–e59. [Google Scholar]
- 35. Hornbrook MC, Wendel CS, Coons SJet al. Complications among colorectal cancer survivors: SF-6D preference-weighted quality of life scores. Med Care. 2011;49(3):321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hudgens S, Briggs A, Tremblay G, Forsythe A, Lloyd A. Comparison of methods to estimate health state utilities in metastatic breast cancer (MBC). Value Health. 2014;17(7):A557. [DOI] [PubMed] [Google Scholar]
- 37. Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res. 2003;114(1):1–5. [DOI] [PubMed] [Google Scholar]
- 38. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lloyd A, Van Hanswijck De, Jonge P, Doyle S, Cornes P. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health. 2008;11(7):1178–85. [DOI] [PubMed] [Google Scholar]
- 40. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. [DOI] [PubMed] [Google Scholar]
- 41. Nafees B, Patel C, Ray D, Gray E, Lau HJ, Lloyd AJ. An assessment of health-state utilities in metastatic breast cancer in the United Kingdom. Value Health. 2016;19(3):A157. [Google Scholar]
- 42. Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–7. [DOI] [PubMed] [Google Scholar]
- 44. Papatheofanis FJ. Utility evaluations for Markov states of lung cancer for PET-based disease management. Q J Nucl Med. 2000;44(2):186–90. [PubMed] [Google Scholar]
- 45. Saigal CS, Gornbein J, Nease R, Litwin MS. Predictors of utilities for health states in early stage prostate cancer. J Urol. 2001;166(3):942–6. [PubMed] [Google Scholar]
- 46. Saigal CS, Gornbein J, Reid K, Litwin MS. Stability of time trade-off utilities for health states associated with the treatment of prostate cancer. Qual Life Res. 2002;11(5):405–14. [DOI] [PubMed] [Google Scholar]
- 47. Shiroiwa T, Fukuda T, Tsutani K. Health utility scores of colorectal cancer based on societal preference in Japan. Qual Life Res. 2009;18(8):1095–103. [DOI] [PubMed] [Google Scholar]
- 48. Smith DS, Krygiel J, Nease RF, Jr, Sumner IW, 2nd, Catalona WJ. Patient preferences for outcomes associated with surgical management of prostate cancer. J Urol. 2002;167(5):2117–22. [PubMed] [Google Scholar]
- 49. Stein D, Joulain F, Naoshy Set al. Assessing health-state utility values in patients with metastatic colorectal cancer: a utility study in the United Kingdom and the Netherlands. Int J Colorectal Dis. 2014;29(10):1203–10. [DOI] [PubMed] [Google Scholar]
- 50. Volk RJ, Cantor SB, Cass AR, Spann SJ, Weller SC, Krahn MD. Preferences of husbands and wives for outcomes of prostate cancer screening and treatment. J Gen Intern Med. 2004;19(4):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peasgood T, Brazier J. Is meta-analysis for utility values appropriate given the potential impact different elicitation methods have on values? Pharmacoeconomics. 2015;33:1101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. La Vecchia C, Malvezzi M, Bosetti Cet al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–95. [DOI] [PubMed] [Google Scholar]
- 54. Rahib L, Smith BD, Aizenberg Ret al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 55. Venkatesh SP, Pasternak JD, Beninato Tet al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery. 2017;161(1):116–26. [DOI] [PubMed] [Google Scholar]
- 56. Al-Qurayshi ZK, Kandil E, Randolph GW. Cost-effectiveness of intraoperative nerve monitoring in avoidance of bilateral recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. Br J Surg. 2017;104(11):1523–31. [DOI] [PubMed] [Google Scholar]
- 57. Garcia AP, Palmer BJ, Parks NA, Liu TH. Routine prophylactic central neck dissection for low-risk papillary thyroid cancer is not cost-effective. Clin Endocrinol (Oxf). 2014;81(5):754–61. [DOI] [PubMed] [Google Scholar]
- 58. Zanocco KH, Heller M, Elaraj D, Sturgeon C. Cost effectiveness of intraoperative pathology examination during diagnostic hemithyroidectomy for unilateral follicular thyroid neoplasms. J Am Coll Surg. 2013;217(4):702–10. [DOI] [PubMed] [Google Scholar]
- 59. Heller MZ, Zanacco K, Zydowicz S, Elaraj D, Nayar R, Sturgeon C. Cost-effectiveness analysis of repeat fine-needle aspiration for thyroid biopsies read as atypia of undetermined significance. Surgery. 2012;152(3):423–30. [DOI] [PubMed] [Google Scholar]
- 60. Wang TS, Cheung K, Mehta P, Roman SA, Walker HD, Sosa JA. To stimulate or withdraw? A cost-utility analysis of recombinant human thyrotropin versus thyroxine withdrawal for radioiodine ablation in patients with low-risk differentiated thyroid cancer in the United States. J Clin Endocrinol Metab. 2010;95(4):1672–80. [DOI] [PubMed] [Google Scholar]
- 61. Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective. Eur J Endocrinol. 2006;155(3):405–14. [DOI] [PubMed] [Google Scholar]
- 62. Esnaola NF, Cantor SB, Sherman SI, Lee JE, Evans DB. Optimal treatment strategy in patients with papillary thyroid cancer: a decision analysis. Surgery. 2001;130(6):921–30. [DOI] [PubMed] [Google Scholar]
- 63. Zanocco K, Kaltman DJ, Wu JXet al. Cost effectiveness of routine laryngoscopy in the surgical treatment of differentiated thyroid cancer. Ann Surg Oncol. 2018;25(4):949–56. [DOI] [PubMed] [Google Scholar]
- 64. Lang BH, Wong CKH. Lobectomy is a more cost-effective option than total thyroidectomy for 1 to 4 cm papillary thyroid carcinoma that do not possess clinically recognizable high-risk features. Ann Surg Oncol. 2016;23(11):3641–52. [DOI] [PubMed] [Google Scholar]
- 65. Balentine CJ, Vanness DJ, Schneider DF. Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: considering the costs of surveillance. Surgery. 2018;163(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilson L, Huang W, Chen L, Ting J, Cao V. Cost effectiveness of lenvatinib, sorafenib and placebo in treatment of radioiodine-refractory differentiated thyroid cancer. Thyroid. 2017;27(8):1043–52. [DOI] [PubMed] [Google Scholar]
- 67. Huang W, Chen L, Cao Vet al. Cost effectiveness of lenvatinib, sorafenib, and placebo in treatment of radioiodine-refractory differentiated thyroid cancer. Value Health. 2016;19(3):A204. [DOI] [PubMed] [Google Scholar]
- 68. Tremblay G, Pelletier C, Copher R, Forsythe A, Majethia U. Cost-effectiveness analysis of lenvatinib as a treatment for radioactive iodine refractory differentiated thyroid cancer in the United States. Value Health. 2016;19(3):A151. [Google Scholar]
- 69. Stern S, Hilly O, Horowitz E, Leshno M, Feinmesser R. Is there justification for total thyroidectomy in low-risk papillary thyroid carcinoma? A decision-analysis model. World J Surg. 2015;39(11):2707–17. [DOI] [PubMed] [Google Scholar]
- 70. Erdal E, Sayman H, Turkmen Cet al. Cost-effectiveness of sorafenib for treatment of radioactive iodine (RAI)-refractory locally advanced/metastatic differentiated thyroid cancer (DTC) in Turkey. Value Health. 2015;18:A203–A204. [Google Scholar]
- 71. Vriens D, Adang EMM, Netea-Maier RTet al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodule. Eur J Nucl Med Mol Imaging. 2013;40(2):S239–S40. [Google Scholar]
- 72. Vega RBM, Kim J, Bussière Met al. Cost-effectiveness of proton therapy compared to photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119(24):4299–307. [DOI] [PubMed] [Google Scholar]
- 73. Borget I, Bonastre J, Catargi Bet al. Cost effectiveness of strategies of radioiodine ablation in thyroid carcinoma patients: results of the randomized phase III ESTIMABL study. Thyroid. 2012;(1):A63. [Google Scholar]
- 74. Zanocco KPZ, Dalal S, Elaraj D, Nayar R, Sturgeon C. On-site adequacy evaluation is not cost effective for experienced operators performing initial ultrasound-guided fine-needle aspiration of thyroid nodules. Thyroid. 2012;(1):A44. [Google Scholar]
- 75. Zanocco K, Sturgeon C. The cost-effectiveness of recombinant human thyroid stimulating hormone administration prior to remnant ablation for treatment of differentiated thyroid cancer. J Surg Res. 2012;172(2):239. [Google Scholar]
- 76. Fordham BA, Kerr C, de Freitas HMet al. Health state utility valuation in radioactive iodine-refractory differentiated thyroid cancer. Patient Prefer Adherence. 2015;9:1561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lubitz CC, De Gregorio L, Fingeret ALet al. Measurement and variation in estimation of quality of life effects of patients undergoing treatment for papillary thyroid carcinoma. Thyroid. 2017;27(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Choi KS, Parks JH, Lee KS. Disability weights for cancers in Korea. J Korean Med Sci. 2013;28(6):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Borget I, Bonastre J, Catargi Bet al. Quality of life and cost-effectiveness assessment of radioiodine ablation strategies in patients with thyroid cancer: results from the randomized phase III ESTIMABL trial. J Clin Oncol. 2015;33(26):2885–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Zoratti_HUB_SLR_Protocol_-_Appendix_A_online_supp for Health Utility Book (HUB)–Cancer: Protocol for a Systematic Literature Review of Health State Utility Values in Cancer by Michael James Zoratti, Ting Zhou, Kelvin Chan, Oren Levine, Murray Krahn, Don Husereau, Tammy Clifford, Holger Schunemann, Gordon Guyatt and Feng Xie in MDM Policy & Practice