Abstract
Past studies have shown that lung angiogenic signaling may be abnormal in children with Down syndrome, but whether differences in circulating angiogenic proteins can identify pulmonary hypertension in children with Down syndrome is unknown. A prospective study of 78 children from birth to 21 years of age was conducted to evaluate clinical data, echocardiograms, and cardiac catheterizations. Four patient populations were enrolled, including children with Down syndrome who have pulmonary hypertension (Down syndrome + pulmonary hypertension, n = 12); control children without Down syndrome who have pulmonary hypertension (C + pulmonary hypertension, n = 15); children with Down syndrome without a known diagnosis of pulmonary hypertension (Down syndrome − pulmonary hypertension, n = 26); and children without Down syndrome or a known diagnosis of pulmonary hypertension (C − pulmonary hypertension, n = 25). Blood samples were collected at enrollment and concentrations for 11 proteins were evaluated. A classification tree was created to identify angiogenic peptide signals that may be associated with pulmonary hypertension in children with Down syndrome compared with controls. Findings identified elevated endostatin levels (>4.98 log10 pg/ml) were associated with Down syndrome. Platelet-derived growth factor AA levels (>2.51 log10 pg/ml) were higher in non-Down syndrome patients with pulmonary hypertension (C + pulmonary hypertension), whereas lower angiogenin (<5.428 log10 pg/ml) or lower angiogenin with elevated angiopoietin-1 levels (>3.59 log10 pg/ml) distinguished pulmonary hypertension in those with Down syndrome from the other groups. This study suggests that children with Down syndrome have high endostatin levels, but low levels of angiogenin levels in children with Down syndrome more often identified pulmonary hypertension than Down syndrome subjects without pulmonary hypertension or non-Down syndrome children. We speculate that these changes in circulating peptides support the concept of dysregulated angiogenesis in children with Down syndrome and pulmonary hypertension, which may further support potential utility as biomarkers for identifying subjects with Down syndrome at risk for pulmonary hypertension in this population.
Keywords: pulmonary arterial hypertension, trisomy 21, developmental lung disease
Introduction
Children with Down syndrome (DS), or trisomy 21, have an increased propensity to develop pulmonary hypertension (PH).1–3 The increased risk of developing PH has been attributed to the many and varied cardiopulmonary issues that patients with DS exhibit and include congenital heart disease (CHD), obstructive sleep apnea, recurrent respiratory infections, and intermittent hypoxia.3 Prior autopsy reports have revealed abnormalities in lung development in children with DS including alveolar simplification with decreased secondary septation and persistence of the double capillary network.4,5 Histologic evidence of hypertensive pulmonary arterial remodeling has accompanied these findings, further suggesting that impaired lung development contributes to the risk for PH.5,6 The over-expression of anti-angiogenic factors during critical periods of lung development in utero7 and during postnatal life may contribute to the early onset of PH in children with DS.
The dysregulation of angiogenic factors has been associated with the development of pulmonary vascular disease in children born prematurely, but has not yet been associated with PH in the DS population.8 Human chromosome 21 encodes several anti-angiogenic proteins, including amyloid protein precursor (APP), regulator of calcineurin-1 (RCAN-1), and endostatin (ES), all of which are elevated in trisomy 21.9–11 Further, early pulmonary artery remodeling was identified and correlated with over-expression of ES and APP in fetal DS lungs suggesting early abnormalities in pulmonary vascular adaptation occur in infants born with trisomy 21.7 Based on past clinical observations and studies of angiogenic signals in DS, we hypothesize that circulating angiogenic signaling proteins may be altered in children with DS who have PH, favoring an angiostatic milieu and that angiogenic factor levels may serve as biomarkers for PH in this population.
Methods
This study was approved by the Institutional Review Board of the University of Colorado School of Medicine. We analyzed data from 78 children, from birth to 21 years of age, who received care at the Children’s Hospital Colorado (altitude 1609 m) from 2005 to 2017. Informed, written consent was obtained from guardians and verbal assent obtained from all subjects where appropriate. Four patient populations were enrolled: (1) children with DS who have PH (DS + PH), (2) children with DS without a known diagnosis of PH (DS−PH), (3) children without DS who have PH (C + PH), and (4) children without DS or a known diagnosis of PH (C−PH). Demographic and clinical data were identified through electronic medical record review and data were entered into a Research Electronic Data Capture database hosted at the University of Colorado.12
Description of population enrolled
All children with PH were identified in the Children’s Hospital Colorado Pediatric Pulmonary Hypertension Clinic. All subjects were diagnosed by clinical and echocardiographic evidence of PH followed by right heart catheterization for confirmation. All patients with PH were naïve to pulmonary vasodilator therapy and were enrolled at the time of cardiac catheterization when the diagnosis of PH was confirmed. At the time of cardiac catheterization, all clinical details were noted and phlebotomy performed. Children with DS without PH were identified and enrolled through the Anna and John J. Sie Center for Down Syndrome (SCDS) Clinic at the Children’s Hospital of Colorado. Analyses of Colorado Department of Public Health and Environment comparative data of all children born in the state from 2000 to 2013 were compared to the patient population at one of the sites, Children’s Hospital Colorado. Data results indicate that SCDS clinic patients capture approximately 50.3% of the state of Colorado’s population of children with DS and provides support of a population based representation.13 Healthy, non-DS controls were identified as siblings of children followed by the SCDS or children followed at non-SCDS clinics at the Children’s Hospital Colorado for noncardiopulmonary reasons.
Clinical characterization of children with PH
PH was defined as a mean pulmonary arterial pressure (mPAP) greater than 20 mmHg or echocardiographic evidence of pulmonary artery pressure (PAP)/systemic artery pressure > 1/3, interventricular septal flattening, right ventricular (RV) dilation, or presence of RV hypertrophy in the absence of RV outflow obstruction (modified criteria from Mourani et al.14). Initial diagnosis was made at the time of enrollment which coincided with cardiac catheterization and phlebotomy for sample acquisition. Clinical data including demographics, PH World Health Organization (WHO) functional classification, PH WHO Group Classification, echocardiographic findings, and cardiac catheterization findings were recorded at the time of initial diagnosis. PH severity was determined using a semiquantitative scoring system modified from Mourani et al.14 and McLaughlin and McGoon15 (Supplemental Table 1). If severity varied between cardiac catheterization and echocardiographic data, catheterization data were utilized. Further, if echocardiographic data fit into multiple severity categories, the most severe was utilized.
Angiogenic factor analysis
Phlebotomy was performed at enrollment, with 3–5 ml of whole blood obtained in tubes containing ethylenediaminetetraacetic acid. Samples were centrifuged and supernatant extracted and stored at −80℃ freezer until ready for analysis. All samples were evaluated using the Luminex Performance Human Angiogenesis Panel A assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol and validated to the manufacturer’s standard curve.16 Analyte details and limits of detection for the assay are available in Supplemental Table 2.
Statistical analysis
Clinical characteristics were expressed as means and frequencies and were compared across groups using an analysis of variance or a chi-squared test, respectively. Angiogenic factor levels were compared across all four groups using Kruskal–Wallis tests and between those with PH using Mann–Whitney U-tests. Groups with truncated data based on values lower than detectable on the assay were excluded from analysis when appropriate. To evaluate angiogenic factors in multivariate fashion, a canonical discriminant analysis (CDA) was performed after standardizing the biomarker values. The CDA identifies the best linear combination of factors that discriminate between groups. In addition, a classification tree was created to incorporate all values (including those truncated) and to identify the most useful factors. The tree was pruned to ensure that six observations were included in each leaf. Data were analyzed by the statistical package SPSS 24.0 (IBM Corporation, New York) and SAS v9.4 (Cary, NC). All analyses were conducted at an α-level 0.05 significance.
Results
A total of 78 children were included in the final study cohorts (Table 1). Overall, 27 children with PH (DS = 12; non-DS = 15) were enrolled and sampled at the time of diagnosis via first cardiac catheterization. Fifty-one children (DS = 26; non-DS = 25) were enrolled as non-PH controls. Children with DS were younger (DS + PH = 6.13 years; DS − PH = 6.16 years) at the time of enrollment (Table 2) compared to those without DS (C + PH = 9.12 years; C − PH = 7.57 years).
Table 1.
Descriptive statistics of study participants.
| Characteristics | DS + PH | DS−PH | C + PH | C−PH |
|---|---|---|---|---|
| Total patients (n) | 12 | 26 | 15 | 25 |
| Sex (n, %) | ||||
| Male | 7 (58.3%) | 11 (42.3%) | 6 (40.0%) | 11 (44.0%) |
| Race/ethnicity (n, %) | ||||
| White, non-Hispanic | 9 (75.0%) | 16 (61.5%) | 10 (66.7%) | 17 (68.0%) |
| Hispanic/Latino | 2 (16.7%) | 8 (30.8%) | 3 (20.0%) | 1 (4.0%) |
| Black or African American, non-Hispanic | 0 (0.0%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) |
| Asian | 1 (8.3%) | 0 (0.0%) | 2 (13.3%) | 5 (20.0%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (8.0%) |
| Mean age at blood draw in years (SD) | 6.13 (6.30) | 6.16 (4.63) | 9.12 (5.80) | 7.57 (5.43) |
DS: Down syndrome; PH: pulmonary hypertension.
Table 2.
Pulmonary hypertension clinical information.
| Characteristics | DS + PH | C + PH | p-value |
|---|---|---|---|
| Total patients (n) | 12 | 15 | |
| Echo | |||
| Peak TR regurg velocity (mean, SD) | 3.7 (0.8) | 4.2 (0.7) | 0.08 |
| Estimated PA pressure (echo) (n) | |||
| Normal | 5 (42%) | 1 (7%) | |
| Less than 1/3 systemic | 0 (0.0%) | 0 (0.0%) | |
| 1/3 to 2/3 systemic | 1 (8%) | 5 (33%) | |
| Greater than 2/3 to systemic | 0 | 4 (27%) | |
| Suprasystemic | 2 (17%) | 4 (27%) | |
| Unknown | 4 (33%) | 1 (7%) | |
| Cath (mean, SD) | |||
| Age at cath | 6.1 (6.3) | 9.1 (5.8) | 0.22 |
| RAP | 6.8 (2.8) | 7.5 (3.8) | 0.59 |
| PCWP | 7.78 (1.6) | 8.1 (3.1) | 0.69 |
| PA pressure | 24.5 (4.7) | 56.5 (16.1) | <0.01 |
| Systemic arterial mean | 53.4 (7.4) | 59.5 (11.5) | 0.12 |
| PVRi | 5.5 (2.7) | 17.8 (11.3) | <0.01 |
| SVRi | 14.6 (4.1) | 16.9 (7.5) | 0.34 |
| Rp/Rs | 0.4 (0.1) | 1.1 (0.4) | <0.01 |
| Classification (n, %) | |||
| WHO Group I | 5 (42%) | 15 (100.0%) | |
| WHO Group II | 1 (8%) | 0 | |
| WHO Group III | 5 (42%) | 0 | |
| WHO Group IV | 0 | 0 | |
| WHO Group V | 1 (8%) | 0 | |
| WHO Functional Class at physical exam (n, %) | |||
| Class I | 6 (50%) | 3 (20%) | |
| Class II | 2 (17%) | 6 (40%) | |
| Class III | 1 (8%) | 5 (33%) | |
| Class IV | 0 | 1 (7%) | |
| Unknown | 3 (25%) | 0 | |
| Severity score (n, %) | |||
| Mild | 11 (92%) | 2 (13%) | |
| Moderate | 1 (8%) | 8 (54%) | |
| Severe | 0 (0.0%) | 5 (33%) | |
DS: Down syndrome; PA: pulmonary artery; PCWP: pulmonary capillary wedge pressure; PH: pulmonary hypertension; PVRi: pulmonary vascular resistance index; RAP: right atrial pressure; SVRi: systemic vascular resistance index; TR: tricuspid regurgitation; WHO: World Health Organization.
WHO Group Classification of PH in children with and without DS
All children with PH who did not have DS were categorized as WHO Group I pulmonary arterial hypertension (PAH) whereas 42% of children with DS were categorized as having Group I disease (Table 2). An equal percentage of the DS + PH group had disease attributed to WHO Group III (PH caused by lung disease or hypoxia). For children with DS classified as PAH (Group 1), the etiology was attributed to congenital heart defects (two with ventricular septal defects, two with atrio-ventricular septal defects, and one with atrial septal defect alone). For all those classified as PH caused by lung disease or hypoxia (Group 3), the etiology was due to sleep disordered breathing.
Mann–Whitney U-tests were performed to determine significance between the two groups with PH for both Tables 2 and 3.
Table 3.
WHO Group I pulmonary hypertension clinical information.
| Characteristics | DS + PH | C + PH | p-value |
|---|---|---|---|
| Total patients (n) | 5 | 14 | |
| General information | |||
| Age at diagnosis (mean, SD) | 5.24 (7.41) | 9.71 (5.53) | 0.17 |
| Sex (male) (n, %) | 2 (40%) | 5 (35.7%) | |
| Echo | |||
| Mean peak TR regurg velocity (mean, SD) | 3.68 (0.48) | 4.26 (0.75) | 0.17 |
| Estimated PA pressure (echo) (n, %) | |||
| Normal | 2 (40.0%) | 1 (7.1%) | |
| 1/3 to 2/3 systemic | 1 (20.0%) | 5 (35.7%) | |
| 2/3 systemic to systemic | 0 (0.0%) | 4 (28.6%) | |
| Suprasystemic | 1 (20.0%) | 4 (28.6%) | |
| Unknown | 1 (20.0%) | 0 (0.0%) | |
| Cath (mean, SD) | |||
| Age at cath | 5.24 (7.41) | 9.71 (5.53) | 0.17 |
| RAP | 7.20 (3.50) | 7.57 (3.91) | 0.85 |
| PCWP | 7.40 (1.67) | 8.14 (3.13) | 0.62 |
| PA pressure | 23.80 (4.82) | 58.29 (15.17) | <0.01 |
| Systemic arterial mean | 55.50 (4.20) | 59.50 (11.49) | 0.51 |
| PVRi | 6.17 (3.93) | 17.77 (11.29) | 0.04 |
| SVRi | 15.62 (4.43) | 16.90 (7.47) | 0.75 |
| Rp/Rs | 0.31 (0.11) | 1.06 (0.40) | <0.01 |
| WHO Functional Class at physical exam (n, %) | |||
| Class I | 1 (20.0%) | 2 (14.3%) | |
| Class II | 2 (40.0%) | 6 (42.9%) | |
| Class III | 1 (20.0%) | 5 (35.7%) | |
| Class IV | 0 (0.0%) | 1 (7.1%) | |
| Unknown | 1 (20.0%) | 0 (0.0%) | |
| Severity score (n, %) | |||
| Mild | 5 (100.0%) | 1 (7.1%) | |
| Moderate | 0 (0.0%) | 8 (57.1%) | |
| Severe | 0 (0.0%) | 5 (35.7%) | |
DS: Down syndrome; PA: pulmonary artery; PCWP: pulmonary capillary wedge pressure; PH: pulmonary hypertension; PVRi: pulmonary vascular resistance index; RAP: right atrial pressure; SVRi: systemic vascular resistance index; TR: tricuspid regurgitation; WHO: World Health Organization.
Comparison of clinical findings in children with PH
Children with DS and PH (DS + PH) appeared to have less severe PAP (SD) with cardiac catheterization data identifying lower mPAP (24.5 mmHg (4.7)) compared to C + PH (56.5 mmHg (16.1); p < 0.01; Table 2). Echocardiographic data similarly identified greater estimated PAP in the C + PH group with more than half having greater than 2/3 systemic estimated PA pressure (Table 2). These differences persisted when comparing children only with PH WHO Group I classification between DS + PH and C + PH (Table 3).
Half of the DS + PH group fit into WHO Functional Class I while only 20% of those in the C + PH group fit into this mild functional classification with 40% identified as functional class III or IV suggesting more severe limitations in the non-DS population (Table 2). A semiquantitative scoring system additionally reported more mild disease in the DS + PH group compared to the C + PH group with a majority of DS + PH categorized as mild, while 87% of C + PH were categorized as moderate or severe (Table 2).
Comparison of angiogenic factor levels across groups
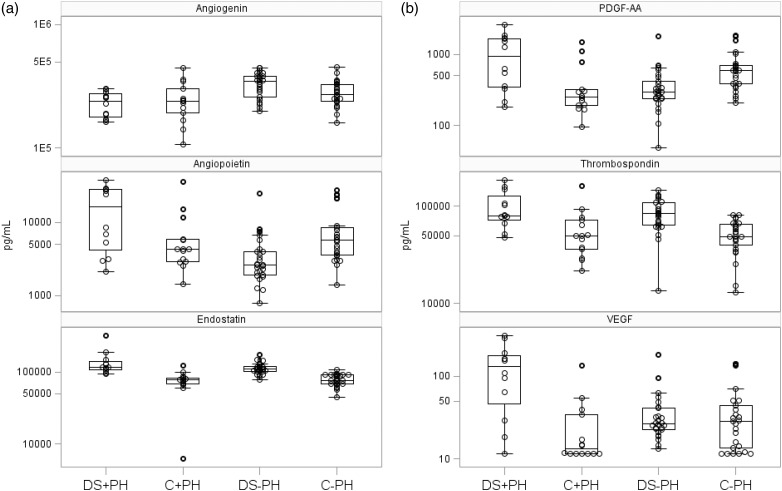
Median angiogenic factor levels were significantly different across all four groups with respect to angiogenin, angiopoietin-1, ES, platelet-derived growth factor (PDGF) AA, PDGF BB, thrombospondin, and vascular endothelial growth factor (VEGF) (Kruskal–Wallis test significance of p < 0.01 for all; Tables 4 and 5; Figs. 1a and 1b). As a sensitivity analysis, a direct comparison of angiogenic factor levels of ES, PDGF-AA, thrombospondin, and VEGF showed that the magnitude of differences was similar when restricting to WHO Group I PAH (Tables 4 and 5).
Table 5.
Median and interquartile range angiogenic factor levels (Only WHO Group I).
| DS + PH | nDS + PH | |
|---|---|---|
| Characteristics (pg/ml) | Median (IQR) | median (IQR) |
| n = 5 | n = 15 | |
| Angiogenin | 185,429 (165,439–277,281) | 240,874 (186,030–312,889) |
| Angiopoietin-1 | 26,724 (4177–28,812) | 4285 (2922–7335) |
| Endostatin | 131,409 (104,509–168,626) | 78,821 (68,041–83,678) |
| PDGF AA | 1637 (341–2204) | 248 (187–434) |
| PDGF BB | 4233 (807–4908) | 715 (406–1395) |
| Thrombospondin | 106,765 (77,596–168,509) | 50,019 (34,405–73,903) |
| VEGF | 162 (83–299) | 12 (5–34) |
DS: Down syndrome; FGF: fibroblast growth factor; PDGF: platelet-derived growth factor; PH: pulmonary hypertension; VEGF: vascular endothelial growth factor; WHO: World Health Organization.
Table 4.
Median and interquartile range angiogenic factor levels.
| Characteristics (pg/ml) | DS + PH | DS−PH | C + PH | C−PH | |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p-value | |
| Angiogenin | 240,363 (17,3391–279,465) | 346,426 (255,360–387,993) | 240,874 (186,030–312,889)) | 272,495 (238,048–330,439) | <0.01 |
| Angiopoietin-1 | 16,418 (3702–28,528) | 2623 (1892–4085) | 4285 (2922–7335) | 5740 (3529–8663) | <0.01 |
| Endostatin | 116,528 (106,351–144,936) | 109,650 (101,898–119,763) | 78,821 (68,041–83,678) | 77,305 (68,450–92,213) | <0.01 |
| FGF acidic | 0 (0–13) | 0 (0-0) | 0 (0–3) | 0 (0–0) | 0.18 |
| FGF basic | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.17 |
| PIGF | 0 (0–0) | 0 (0-0) | 0 (0–0) | 0 (0–0) | 0.21 |
| PDGF AA | 938 (335–1663) | 295 (237–434) | 284 (187–434) | 586 (371–716) | <0.01 |
| PDGF BB | 2101 (745–4678) | 767 (496–944) | 715 (406–1395) | 1654 (1107–2682) | <0.01 |
| Thrombospondin | 80,414 (70,021–139,634) | 83,812 (63,264–110,445) | 50,019 (34,405–73,903) | 48,986 (38,119–66,030) | <0.01 |
| VEGF | 131 (37–184) | 26 (21–40) | 12 (5–34) | 28 (12–47) | <0.01 |
| VEGF-D | 1 (0–1) | 0 (0–1) | 0 (1–1) | 0 (0–1) | 0.94 |
Fig. 1.
(a) Distribution of angiogenic peptide levels in study participants across DS and PH. Median is indicated by a line and the box extends to the 25th and 75th percentiles. Large circles designate means, whiskers extend to values within 1.5 interquartile range, and smaller points are values outside of 1.5 interquartile range. DS: Down syndrome; PDGF: platelet-derived growth factor; PH: pulmonary hypertension; VEGF: vascular endothelial growth factor. (b) Distribution of angiogenic peptide levels in study participants across DS and PH.
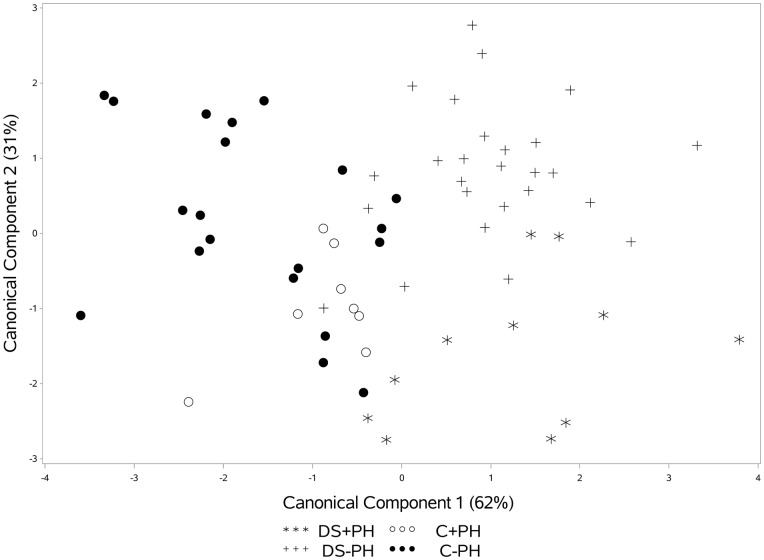
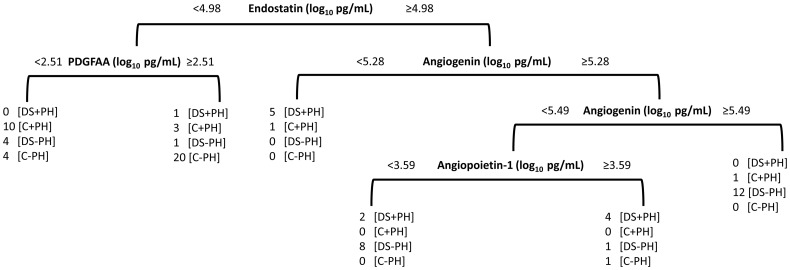
In addition to evaluating differences in angiogenic factor levels individually, a CDA was applied to evaluate the ability of all factors to discriminate between groups. The first two components explained 93% of the variability across the four groups. The first component identified differences between the groups with DS (DS + PH and DS−PH) and those without DS (C + PH and C−PH) and the second component separates groups with PH (DS + PH and C + PH) from those without PH (DS−PH, C−PH; Fig. 2). To identify which angiogenic factors are useful for discriminating between groups, a classification tree was created that simultaneously identified optimal cutoffs for each factor (Fig. 3). The classification tree identified four angiogenic factors that combined discriminated between groups. Non-DS controls tended to have low ES levels and PDGF-AA levels were useful for discriminating PH within this group (C + PH had high PDGF-AA and C−PH had low PDGF-AA). Interestingly, angiogenin and angiopoietin-1 were factors that were most useful for distinguishing PH in those with DS. Low levels of angiogenin and high levels of angiopoietin-1 distinguished DS + PH, whereas high levels of angiogenin or low angiogenin combined with low angiopoietin-1 distinguished DS-PH.
Fig. 2.
Canonical Discriminant Analysis for distinguish between DS and PH groups. Component 1 (high endostatin) separates DS from control while component 2 differentiates DS+PH from DS-PH (low values of angiogenin).
Fig. 3.
Classification tree for diagnosing PAH in children with DS. Tree was pruned to ensure that six observations were included in each leaf. ES best discriminates DS from control (C); PDGF AA best discriminates C+PH from C-PH; angiogenin and angiopoietin-I discerns DS+PH from DS-PH. DS: Down syndrome; PH: pulmonary hypertension.
Discussion
This study found that circulating levels of angiogenic factors are altered in children with DS and PH in comparison with subjects with DS without PH and non-DS controls. Specifically, we found that elevated levels of ES with increased thrombospondin, which have anti-angiogenic properties, and reductions of the pro-angiogenic factors angiogenin and angiopoietin-1, distinguished children with DS who have PH from those without PH. These findings suggest that dysregulated angiogenic factors may play a role in the development of pulmonary vascular disease in children with DS and may provide unique biomarkers to identify at-risk children with DS.
In addition, we report that elevated ES levels were found in DS subjects with and without PH, suggesting that measurement of circulating ES alone may not be sufficient to identify children with DS at risk for PH. ES is known to be elevated in children with DS; however, this study is the first to evaluate ES as a predictor of PH disease in this at-risk population. In non-DS adults with PAH, elevated ES levels predicted poor outcomes while a single, loss-of-function, missense variant in COL18A1, the gene encoding ES, was associated with reduced circulating levels of ES and improved outcomes.17 Circulating ES levels have also positively correlated with the development of PH in infants with bronchopulmonary dysplasia,18 onset of PH in adults with systemic sclerosis,19 and with NT-proBNP in adults with heart failure with preserved ejection fraction.20 Elevated anti-angiogenic factors, such as ES, may lead to diminished microvascular angiogenesis during cardiomyocyte adaptation to RV stress, contribute to impairments in RV function, and predict more severe outcomes in PH.21–23 An elevation of anti-angiogenic protein expression may put children with PH at additional risk of disease progression or severity due to this impaired cardiomyocyte adaptation.
Thrombospondin, an anti-angiogenic protein, was also elevated in children with DS compared to controls; however, it did not help differentiate those with PH. ES was significantly increased in the DS + PH cohort; however, this level alone was not significant enough to identify PH. Elevated ES (≥4.98 pg/ml) differentiates children with and without DS, while elevated ES and an angiogenin level below 5.49 pg/ml identify those with DS who have PH. Angiogenin, a potent pro-angiogenic factor, is known to be reduced in the amniotic fluid of developing fetuses with trisomy 21.24 Reduced levels of angiogenin and increased levels of anti-angiogenic factors noted in our study, may contribute to an impaired angiogenic response to myocardial stress, leading to a maladaptive myocardium in the presence of PH as has previously been suggested in adults with PAH.17,25 Our study suggests that these angiogenic proteins may serve as potential biomarkers for the diagnosis of PH in children with DS.
There are several potential limitations of this study. First, the groups without PH were not evaluated for the condition and as such no confirmation of disease negativity was available. Any future studies will need to assess for evidence of PH in control groups, in particular those with DS who are at risk. Second, this pilot study confirms an association with angiogenic factor abnormalities in children with DS who have pulmonary vascular disease, but further work is needed in larger cohorts to validate these observations. Third, a more direct comparison between WHO PH classification groups is challenged by sample size limitations. Fourth, this study did not evaluate all known angiogenic factor levels and, therefore, does not completely assess the global angiogenic milieu. Whether circulating peptide levels that reflect other anti-angiogenic factors of interest in children with DS, such as RCAN-1, APP, and TIMP3 are significantly elevated in children with DS and PH is unknown. Finally, the pro-angiogenic factors PDGF AA, PDGF BB, and VEGF appeared elevated in children with DS and PH, which may suggest a more complex relationship between pro- and anti-angiogenic factors in disease pathogenesis. As ES is a potent inhibitor of VEGF, the elevated levels of VEGF may not be serving a physiologic role or may be serving as a protective, compensatory, or reparative response along with elevated PDGF-AA.26 Larger proteomic studies may prove useful in further elucidating the association between angiogenic dysregulation and pulmonary vascular disease in children with DS. Improved understanding of this complex interplay between pro- and anti-angiogenic factors may additionally contribute to the development of novel therapeutics that target genes or proteins involved in vascular development.
Children with DS have multiple risk factors for developing PH including the presence of CHD with left to right intracardiac shunting, a high incidence of respiratory comorbidities, and as previously described, lung growth abnormalities. Despite this, our findings from this study sample suggest a milder PH phenotype in children with DS compared to those that do not have DS. While several studies have reported the increased incidence and risk of developing PH in children with DS, few have compared severity of disease.2,3,27 The variable etiologies for developing PH in this population may make direct comparisons of severity challenging, and our relatively small sample size of children with DS who have WHO Group I PAH may challenge this conclusion. Further, not all children were characterized with WHO functional classification, a more commonly accepted severity classification scheme.
We conclude that a high level of the anti-angiogenic factor ES, coupled with a low level of the pro-angiogenic factor angiogenin, may serve as a novel biomarker for the diagnosis of PH in children with DS. Prospective, large studies are required to confirm the utility of these proteins as useful biomarkers.
Supplemental Material
Supplemental material, Supplemental Material1 for Angiogenic profile identifies pulmonary hypertension in children with Down syndrome by Douglas Bush, Kristine Wolter-Warmerdam, Brandie D. Wagner, Csaba Galambos, D.Dunbar Ivy, Steven H. Abman, Deven McMorrow and Francis Hickey in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Material2 for Angiogenic profile identifies pulmonary hypertension in children with Down syndrome by Douglas Bush, Kristine Wolter-Warmerdam, Brandie D. Wagner, Csaba Galambos, D.Dunbar Ivy, Steven H. Abman, Deven McMorrow and Francis Hickey in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was supported by the Global Down Syndrome Foundation, NIH/NCATS Colorado CTSA Grant Number UL1 TR002535, the Colorado Clinical and Translational Sciences Institute Microgrant Number UL1 TR001082, with generous support from Kathleen Miller-Reed.
References
- 1.Espinola-Zavaleta N, Soto ME, Romero-Gonzalez A, et al. Prevalence of congenital heart disease and pulmonary hypertension in Down’s syndrome: an echocardiographic study. J Cardiovasc Ultrasound 2015; 23: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saji T. Clinical characteristics of pulmonary arterial hypertension associated with Down syndrome. Pediatr Int 2014; 56: 297–303. [DOI] [PubMed] [Google Scholar]
- 3.Bush D, Galambos C, Ivy DD, et al. Clinical characteristics and risk factors for developing pulmonary hypertension in children with Down syndrome. J Pediatr 2018; 202: 212–219. [DOI] [PubMed]
- 4.Cooney TP, Thurlbeck WM. Pulmonary hypoplasia in Down’s syndrome. N Engl J Med 1982; 307: 1170–1173. [DOI] [PubMed] [Google Scholar]
- 5.Bush D, Abman SH, Galambos C. Prominent intrapulmonary bronchopulmonary anastomoses and abnormal lung development in infants and children with Down syndrome. J Pediatr 2017; 180: 156–162. [DOI] [PubMed] [Google Scholar]
- 6.Chi TPL, Krovetz J. The pulmonary vascular bed in children with Down syndrome. J Pediatr 1975; 86: 533–538. [DOI] [PubMed] [Google Scholar]
- 7.Galambos C, Minic AD, Bush D, et al. Increased lung expression of anti-angiogenic factors in Down syndrome: potential role in abnormal lung vascular growth and the risk for pulmonary hypertension. PLoS One 2016; 11: e0159005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner BD, Babinec AE, Carpenter C, et al. Proteomic profiles associated with early echocardiogram evidence of pulmonary vascular disease in preterm infants. Am J Respir Crit Care Med 2018; 197: 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta PD, Capone G, Jewell A, et al. Increased amyloid beta protein levels in children and adolescents with Down syndrome. J Neurol Sci 2007; 254: 22–27. [DOI] [PubMed] [Google Scholar]
- 10.Zorick TS, Mustacchi Z, Bando SY, et al. High serum endostatin levels in Down syndrome: implications for improved treatment and prevention of solid tumours. Eur J Hum Genet 2001; 9: 811–814. [DOI] [PubMed] [Google Scholar]
- 11.Ryeom S, Folkman J. Role of endogenous angiogenesis inhibitors in Down syndrome. J Craniofac Surg 2009; 20: 595–596. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey F, Wolter-Warmerdam K, Hickey E, et al. Pediatric comorbidities and medical complications identified in children with Down syndrome. J Down Syndr Chr Abnorm 2017; 3: 1–7. [Google Scholar]
- 14.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008; 121: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 2006; 114: 1417–1431. [DOI] [PubMed] [Google Scholar]
- 16.Beane JD, Lee G, Zheng Z, et al. Clinical scale zinc finger nuclease-mediated gene editing of PD-1 in tumor infiltrating lymphocytes for the treatment of metastatic melanoma. Mol Ther 2015; 23: 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damico R, Kolb TM, Valera L, et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 191: 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Kim HS. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology 2014; 106: 55–61. [DOI] [PubMed] [Google Scholar]
- 19.Reiseter S, Molberg O, Gunnarsson R, et al. Associations between circulating endostatin levels and vascular organ damage in systemic sclerosis and mixed connective tissue disease: an observational study. Arthritis Res Ther 2015; 17: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barroso MC, Boehme P, Kramer F, et al. Endostatin a potential biomarker for heart failure with preserved ejection fraction. Arq Bras Cardiol 2017; 109: 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–D33. [DOI] [PubMed] [Google Scholar]
- 22.Sutendra G, Dromparis P, Paulin R, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med 2013; 91: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 23.Drake JI, Bogaard HJ, Mizuno S, et al. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 2011; 45: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demir A, Guclu S, Bige O, et al. Amniotic fluid angiogenin levels are decreased in pregnancies with fetal trisomy 21. Prenat Diagn 2011; 31: 1101–1103. [DOI] [PubMed] [Google Scholar]
- 25.Vogel-Claussen J, Skrok J, Shehata ML, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011; 258: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi N, Anand-Apte B, Lee M, et al. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J 1999; 18: 4414–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma M, Khera S, Sondhi V, et al. A study to determine the prevalence of pulmonary arterial hypertension in children with DS and congenital heart disease. Med J Armed Forces India 2013; 69: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Angiogenic profile identifies pulmonary hypertension in children with Down syndrome by Douglas Bush, Kristine Wolter-Warmerdam, Brandie D. Wagner, Csaba Galambos, D.Dunbar Ivy, Steven H. Abman, Deven McMorrow and Francis Hickey in Pulmonary Circulation
Supplemental material, Supplemental Material2 for Angiogenic profile identifies pulmonary hypertension in children with Down syndrome by Douglas Bush, Kristine Wolter-Warmerdam, Brandie D. Wagner, Csaba Galambos, D.Dunbar Ivy, Steven H. Abman, Deven McMorrow and Francis Hickey in Pulmonary Circulation